Published online Sep 19, 2023. doi: 10.5498/wjp.v13.i9.665
Peer-review started: July 18, 2023
First decision: August 4, 2023
Revised: August 16, 2023
Accepted: August 25, 2023
Article in press: August 25, 2023
Published online: September 19, 2023
Processing time: 59 Days and 1.7 Hours
Gastric ulcer (GU) is a common digestive tract disease, and medical records of GU combined with depression are increasingly common. Currently, the risk factors and pathogenesis of GU complicated with depression remain unclear. Low im
To explore the immune function, gastrointestinal hormone level, and clinical sig
A retrospective analysis was conducted on 300 patients with GU combined with depression admitted to Guizhou Provincial People’s Hospital from January 2021 to June 2022 as the study subjects. According to the Hamilton Depression Scale (HAMD) score, patients were divided into mild-to-moderate (n = 210) and heavy (n = 90) groups. Basic data, immune function indices [immunoglobulin A (IgA), IgM, IgG, serum CD4+ and CD8+ percentage, and CD4+/CD8+ ratio], and gastro
There were no marked differences in sex, age, body mass index, abdominal distension, abdominal pain, belching, nausea, vomiting, or sleep disorders between the heavy and mild-to-moderate groups (P > 0.05). There was a marked difference in the family history of depression between the heavy and mild-to-moderate groups (P < 0.05). There were significant differences in serum IgA and IgM levels and serum CD4+, CD8+, and CD4+/CD8+ ratios between the heavy and mild-to-moderate groups (P < 0.05). Multivariate analysis showed that IgA, IgM, GAS, and CCK serum levels influenced the severity of GU with depression (P < 0.05). The AUC of the ROC curve for serum IgA level predicting GU with depression severity was 0.808 [95% confidence interval (CI): 0.760-0.857], the AUC of the serum IgM level was 0.757 (95%CI: 0.700-0.814), the AUC of the serum GAS level was 0.853 (95%CI: 0.810-0.897), the AUC of the serum CCK level was 0.762 (95%CI: 0.709-0.822), the AUC of immune function (IgA, IgM) and gastrointestinal hormone levels (GAS, CCK) for the prediction of GU with depression severity was 0.958 (95%CI: 0.933-0.976).
Important factors influencing GU complicated with depression are serum IgA, IgM, GAS, and CCK indicators. They can be used as indicators to predict the severity of GU complicated with depression.
Core Tip: The occurrence and severity of gastric ulcer (GU) combined with depression may be related to autoimmune dysfunction and gastrointestinal hormone levels. In this study, multivariate logistic regression analysis was used to influence the severity of concurrent depression in GU. The results suggest that impaired T cell function and gastrointestinal hormone disorders may directly affect the development and development of depression. In addition, the immune function indicators combined with gastrointestinal hormone levels predict high AUC, specificity and sensitivity, which has a very good re
- Citation: Yang YH, Cui DJ, Yang ZL, Yuan WQ, Huang B. Immune function, gastrointestinal hormone levels, and their clinical significance in patients with gastric ulcers complicated with depression. World J Psychiatry 2023; 13(9): 665-674
- URL: https://www.wjgnet.com/2220-3206/full/v13/i9/665.htm
- DOI: https://dx.doi.org/10.5498/wjp.v13.i9.665
With the continuous development of living standards, people’s eating and living habits have changed to varying degrees; however, poor living habits have had a greater impact on people’s health. Gastric ulcers (GU) are common digestive system diseases. The occurrence and continuous progression of the disease are related to the dynamic balance between gastric mucosal defense and invasion factors. Helicobacter pylori (H. pylori) infection, excessive gastric acid secretion, and adverse drug factors are all influencing factors that cause GU[1,2]. Gastric juice concentration in patients with GU stimulates the gastric mucosa, promotes changes in the gastric structure and environment, causes serious damage to the gastric mucosa, and causes long-term stay of pathogenic factors, resulting in the long-term treatment of the disease[3]. Clinical manifestations of GU include stomach and abdominal pain, heartburn, acid reflux, among others. The symptoms worsen after eating irritating foods or foods that are hard to digest. Smoking and alcohol consumption can also cause GU. Patients with severe GU often experience gastric perforation and gastric bleeding[4].
Recently, psychophysiological factors have been significant in the pathogenesis of gastrointestinal illnesses. GU are characterized by repeated attacks that can easily cause adverse severe psychological states. Some patients also experience anxiety and depression due to a lack of timely and effective treatment for physical illness, which makes depression increasingly prominent in GU patients[5]. Anti-H. pylori therapy, drugs controlling excessive gastric acid secretion, and protecting gastric mucosa are primarily used for the clinical treatment of GU. The impact of psychological factors on disease occurrence and progression in clinical practice is often overlooked, which makes it difficult to treat patients comprehensively[6]. Relevant data show that poor psychological status may increase the risk of digestive disorders[7]; however, there are few studies on patients with GU associated with depression. Exploring the factors influencing GU combined with depression is important for clinical treatment and the improvement of patients’ quality of life. Studies have shown that the occurrence of GU combined with depression may be related to abnormal autoimmune function and disorders of gastrointestinal hormone levels[8]; however, the specific mechanism is not clear. Due to the decrease in gas
A retrospective analysis was conducted to select 300 patients with GU complicated with depression admitted to Guizhou Provincial People’s Hospital from January 2021 to June 2022 as the study subjects. Inclusion criteria: (1) Age ≥ 18 years old; (2) All patients have nausea, stomach pain, and acid reflux symptoms; and (3) The basic information and laboratory indicators of all patients are complete. The exclusion criteria were as follows: (1) Patients with malignant tumors or chronic atrophic gastritis and other gastrointestinal illnesses; (2) Patients who had undergone gastrointestinal surgery in the past 3 mo; (3) Patients with organic disorders; and (4) Patients with autoimmune diseases and systemic inflammatory diseases.
Diagnostic criteria for GU[9]: GU was confirmed by endoscopy, and the pathological diagnosis of H. pylori infection was positive. Diagnostic criteria for depression[10]: Diagnostic criteria for depression in the Diagnostic and Statistical Manual of Mental Disorders were employed. The diagnosis was based on the patient’s low mood, slow thinking, reduced language, movements, suspected illness, insomnia, loss of interest, and related symptoms lasting more than 2 wk. The severity of the disease and the degree of social function impairment increase with the number of symptoms.
All patients on the day of admission, patients were routinely assessed using the Hamilton Depression Scale (HAMD)[11], And were graded according to the HAMD scale: A score of 7 points indicates no depression, > 8-19 points indicates depression, > 20-34 points indicates mild to moderate depression, and > 35 points indicates major depression. All en
Clinical data collection: (1) Basic information: Sex, age, body mass index (BMI), abdominal distension, abdominal pain, belching, nausea, vomiting, sleep disorders, and family history of depression; (2) Immune function index: The levels of serum immunoglobulin A (IgA), IgM, and IgG were detected by immunoturbidimetry (the kit was purchased from Lifotronic Technology Co., Ltd.). The percentages of serum CD4+ and CD8+ cells were detected using a Beckman CytoFLEX flow cytometer and a supporting kit (Beckman Coulter), and the ratio of CD4+/CD8+ cells was calculated; and (3) Gastrointestinal hormone indices: The levels of serum gastrin (GAS), cholecystokinin (CCK), and motilin (MTL) were detected by ELISA (the kit was purchased from MLBIO Enzyme Linked Biology).
Data analysis was performed using SPSS statistical software 24.0. The measurement data conforming to the normal distribution were expressed as mean ± SD and analyzed by t-test. Count data is expressed as frequency percentage (n%) by the χ2 test. Multivariate logistic regression analysis was used to explore factors influencing the severity of GU complicated with depression. The receiver operating characteristic curve (ROC) and area under the ROC curve (AUC) were used to analyze the value of the immune function index, gastrointestinal hormone index, and combined index in predicting the severity of GU complicated with depression, and the specificity and sensitivity were calculated. Test level: α = 0.05.
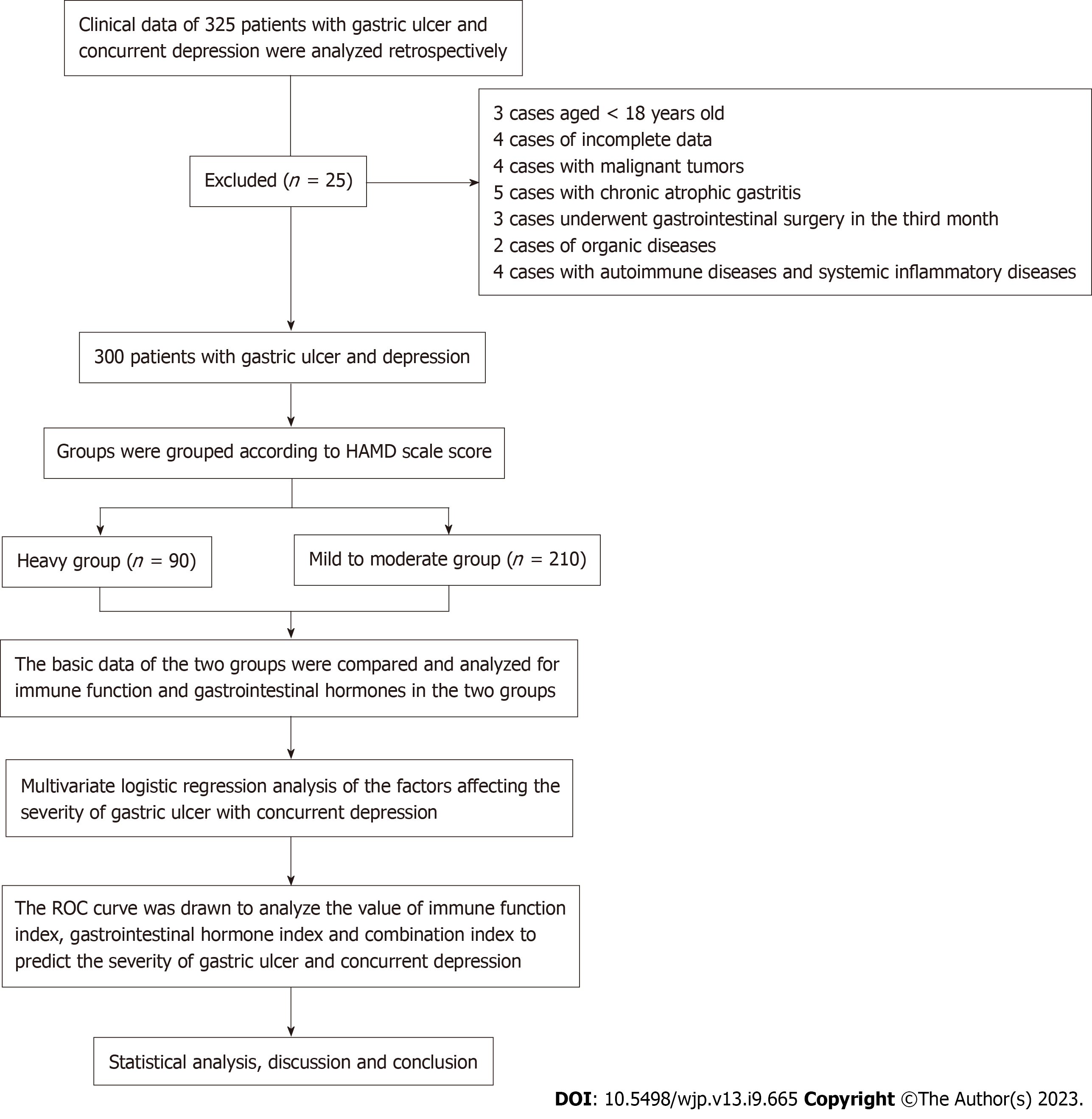
Figure 1 shows the number of patients, the number of patient inclusion, and the flow chart of the analysis method. There was no significant difference in gender, age, BMI, abdominal distension, abdominal pain, belching, nausea, vomiting, and sleep disorders between the heavy group and the mild to moderate group (P > 0.05). There was statistically significant difference in family history of depression between severe group and mild to moderate group (P < 0.05) (Table 1).
| Feature | Heavy group (n = 90) | Mild to moderate group (n = 210) | χ2/t value | P value |
| Gender (male/female) | 48/42 | 109/101 | 0.052 | 0.820 |
| Age (yr) | 45.29 ± 4.56 | 46.37 ± 5.44 | -1.773 | 0.078 |
| BMI (kg/m2) | 22.48 ± 2.17 | 22.19 ± 2.13 | 1.114 | 0.266 |
| Abdominal distension (Yes/No) | 51/39 | 102/108 | 1.652 | 0.199 |
| Abdominal pain (Yes/No) | 43/47 | 100/110 | 0.001 | 0.980 |
| Belching (Yes/No) | 40/50 | 107/103 | 1.068 | 0.301 |
| Nausea (Yes/No) | 38/52 | 98/112 | 0.502 | 0.479 |
| Vomiting (Yes/No) | 46/44 | 105/105 | ||
| Sleep disorder (Yes/No) | 47/43 | 85/125 | 3.528 | 0.060 |
| Family history of depression (Yes/No) | 45/45 | 48/162 | 21.699 | < 0.001 |
There were significant differences in serum IgA and IgM levels and serum CD4+, CD8+, and CD4+/CD8+ ratios between the heavy and mild-to-moderate groups (P < 0.05). There was no significant difference in serum IgG level between the two groups (P > 0.05) (Table 2).
| Index | Heavy group (n = 90) | Mild to moderate group (n = 210) | T value | P value |
| IgA (g/L) | 1.86 ± 0.42 | 2.54 ± 0.63 | -10.791 | < 0.001 |
| IgM (g/L) | 2.97 ± 0.62 | 3.59 ± 0.65 | -7.698 | < 0.001 |
| IgG (g/L) | 15.37 ± 3.48 | 15.42 ± 3.52 | -0.113 | 0.910 |
| CD4+ (%) | 32.16 ± 5.31 | 38.48 ± 5.49 | -9.218 | < 0.001 |
| CD8+ (%) | 29.37 ± 3.52 | 23.59 ± 3.13 | 6.800 | < 0.001 |
| CD4+/CD8+ ratio | 1.24 ± 0.27 | 1.67 ± 0.36 | -11.104 | < 0.001 |
Serum GAS and CCK levels were compared between the severe and mild-to-moderate groups (P < 0.05), and there was no difference in serum MTL levels between the two groups (P > 0.05) (Table 3).
| Group | n | GAS (pg/mL) | CCK (ng/L) | MTL (pg/mL) |
| Heavy group | 90 | 43.52 ± 5.79 | 154.49 ± 11.68 | 256.28 ± 13.12 |
| Mild to moderate group | 210 | 52.44 ± 6.23 | 167.25 ± 13.54 | 258.18 ± 20.47 |
| t value | -11.598 | -7.781 | -0.960 | |
| P value | < 0.001 | < 0.001 | 0.338 |
The above statistically significant indicators (family history of depression, IgA, IgM, CD4+, CD8+, CD4+/CD8+ ratio, GAS, and CCK) were used as independent variables, and GU combined with depression severity as the dependent variable (see Table 4). Multivariate analysis showed that serum IgA, IgM, GAS, and CCK levels influenced the severity of GU com
| Index | Code | Description of valuation |
| Family history of depression | X1 | 1 = Yes; 0 = No |
| IgA | X2 | Enter actual value |
| IgM | X3 | Enter actual value |
| CD4+ | X4 | Enter actual value |
| CD8+ | X5 | Enter actual value |
| CD4+/CD8+ ratio | X6 | Enter actual value |
| GAS | X7 | Enter actual value |
| CCK | X8 | Enter actual value |
| Index | β | SE | Wald χ2 | P value | OR (95%CI) |
| Family history of depression | 0.744 | 0.551 | 1.822 | 1.770 | 2.103 (0.714-6.193) |
| IgA | -2.703 | 0.600 | 20.313 | < 0.001 | 0.067 (0.021-0.217) |
| IgM | -1.509 | 0.487 | 9.586 | 0.002 | 0.221 (0.085-0.575) |
| CD4+ | 0.151 | 0.218 | 0.482 | 0.488 | 1.163 (0.759-1.782) |
| CD8+ | -0.286 | 0.290 | 0.969 | 0.325 | 0.751 (0.425-1.327) |
| CD4+/CD8+ ratio | -9.136 | 5.432 | 12.829 | 0.093 | 0.000 (0.000-4.525) |
| GAS | 0.267 | 0.050 | 28.147 | < 0.001 | 0.766 (0.694-0.845) |
| CCK | 0.093 | 0.024 | 15.137 | < 0.001 | 0.911 (0.869-0.955) |
| Constant | 52.073 | 10.637 | 23.963 | < 0.001 | - |
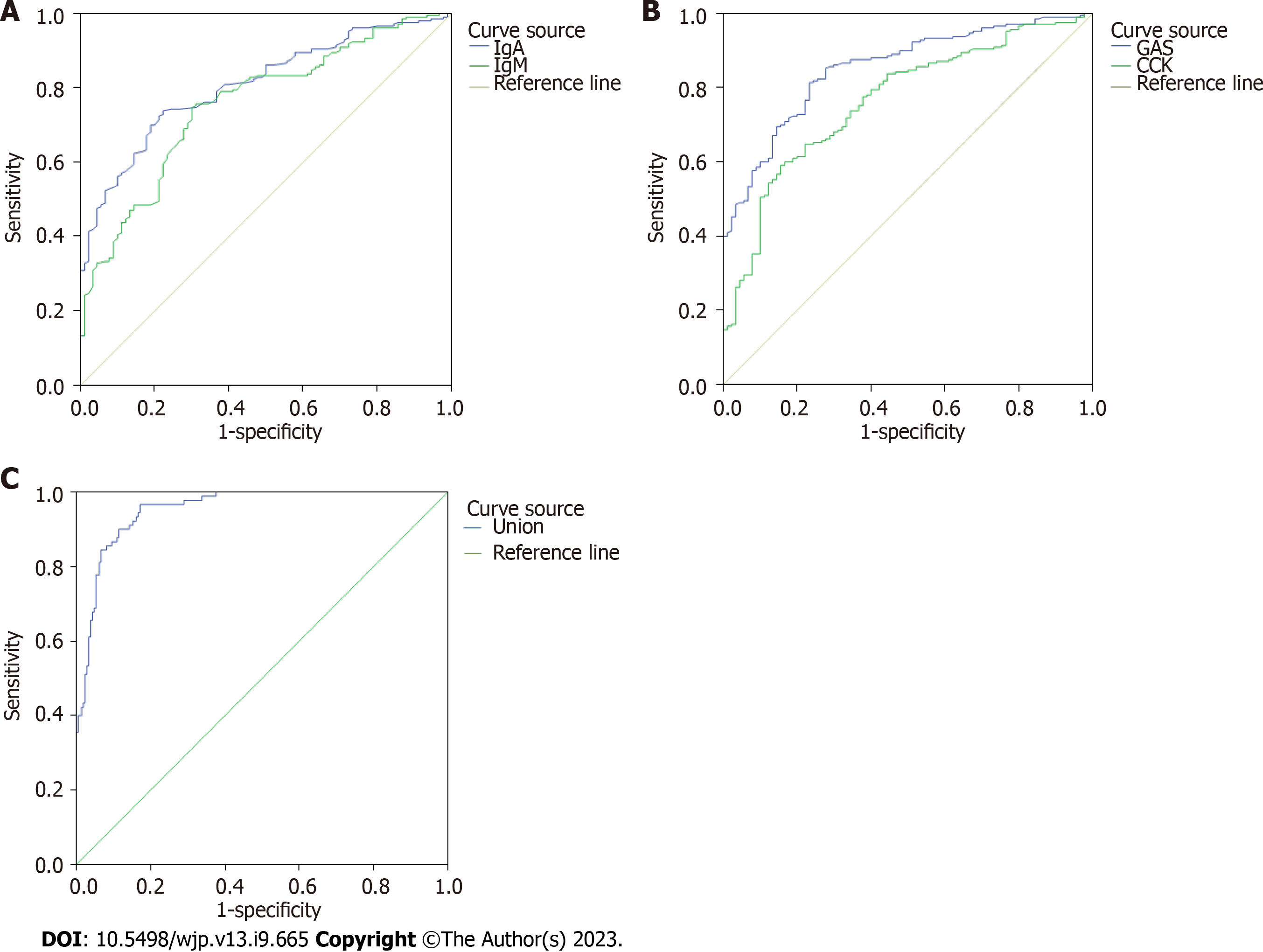
The AUC of the ROC curve of serum IgA level for predicting GU with depression severity was 0.808 [95% confidence interval (CI): 0.760-0.857], sensitivity was 0.738, and specificity was 0.778, The AUC of the serum IgM level was 0.757 (95%CI: 0.700-0.814), with a sensitivity of 0.748 and a specificity of 0.700 (Figure 2A).
The AUC of the ROC curve of serum GAS level predicting GU with depression severity was 0.853 (95%CI: 0.810-0.897), sensitivity was 0.814, and specificity was 0.767, The AUC of serum CCK level was 0.762 (95%CI: 0.709-0.822), with a sen
The AUC of immune function (IgA, IgM) combined with gastrointestinal hormone levels (GAS, CCK) predicting GU with depression severity was 0.958 (95%CI: 0.933-0.976), sensitivity was 0.967, and specificity was 0.829 (Figure 2C).
GU refers to the ulcer formed in the gastric angle, gastric antrum, cardia, and hiatal hernia. It is a gastric mucosal injury that is caused by various factors. It can be caused by H. pylori infection, drugs, heredity, diet, and other factors and is often accompanied by acid reflux, belching, abdominal distension, and upper abdominal pain symptoms[12]. The related study showed that the occurrence of GU is caused by the process of ‘attack factor’ or the weakening of ‘defense factor’; the “attack factor” includes gastric acid, pepsin, H. pylori, among others, and the “defense factor” includes gastric mucosa, gastric mucus, bicarbonate, among others[13]. It has been found that H. pylori infection is the most common cause of GU. H. pylori can damage the protective layer of the gastric mucosa, thus accelerating the occurrence of gastric mucosal lesions, easily causing various complications, and threatening the physical and mental health of patients[14]. GU com
Depression is a primary emotional and common mood disorder. Its incidence ranks second in the incidence of various diseases worldwide. Furthermore, it is found that patients with various chronic diseases have anxiety and depression, and the mortality rate of patients with heart disease complicated with depression is higher than that of patients with simple depression, so the treatment of patients with depression is necessary[16]. The National Institute of Mental Health showed that the prevalence of mental disorders in patients with somatic diseases is as high as 41%[17]. Therefore, while paying clinical attention to the diagnosis and treatment of physical diseases, we should also pay attention to mental and psychological interventions, as the number of patients with GU complicated with depression in China has increased annually[18]. Several factors affect the pathogenesis of GU, which is complicated with depression. Autoimmune dys
In this study, all selected patients were scored using the HAMD. Among them, 210 patients with mild to moderate GU complicated with depression, accounting for 70%, and 90 patients in the heavy group, accounting for 30%. Studies have shown that disease characteristics and severity of depression are influenced by a family history of affective disorder[19]. In this study, the mild-to-moderate and heavy groups had a family history of depression. This indicates that patients with a family history of depression may have more severe anhedonia and anxiety symptoms and that these mood disorders can potentially affect immune function and gastrointestinal hormone levels. The pathogenesis of GU is closely associated with immune deficiency[20]. Depression and anxiety can cause digestive system diseases. Long-term mental disorders overstretch the nervous system, leading to gastrointestinal hormone secretion disorders and excessive sensitivity to food stimulation. Patients with GU and depression have weakened gastrointestinal motility, delayed gastric emptying, and uncoordinated gastrointestinal motility, causing a series of complex syndromes such as fullness, belching, and abdominal pain[21]. There was no significant difference in gender, age, BMI, abdominal distension, abdominal pain, belching, nau
Immunoglobulin is produced by B lymphocytes as long as it is a specific binding antigen. Serum IgA, IgM, and IgG are important components of the body’s immune cell molecules, which can be consumed in large amounts in consumptive diseases, acute inflammation, and malignant tumors, resulting in a decline in immune function[22]. In this study, serum IgA and IgM levels in the moderate and heavy groups were lower than those in the mild and moderate groups, indicating that impaired B cell function and low immune function could aggravate the risk of infection, GU progression, and dep
The gastrointestinal hormone level is a sensitive index that reflects the function of the gastric mucosa. It plays an important role in regulating the function of the digestive system and is closely related to digestive system dysfunction[25]. GAS is the main hormone of the digestive tract that affects gastrointestinal transitional compound movement, pro
In this study, the ROC curves of immune function, gastrointestinal hormone levels, and the combined prediction curve of GU and depression severity were drawn. The results showed that the immune function and gastrointestinal hormone indices alone predicted low AUC, specificity, and sensitivity, and the combined prediction had the highest efficacy, indi
Limitations of this study: All the selected participants were admitted to our hospital, the reliability may be affected by the limitation of sample size. In the later stages, the sample size can be expanded clinically, a multicenter study can be conducted, and the mechanism of immune function and gastrointestinal hormone levels in GU complicated with dep
In summary, the important influencing factors of GU with depression were serum IgA, IgM, GAS, and CCK indices. Immune function and gastrointestinal hormone levels can be used as effective indicators to predict the severity of GU combined with depression. Clinically, targeted measures should be taken to address these causes and reduce the risk of GU concomitant with depression.
Psychophysiological factors play a significant role in the pathogenesis of gastrointestinal illnesses. Gastric ulcers (GU) are characterized by repeated attacks that can cause adverse severe psychological states. Some patients also experience anxiety and depression due to a lack of timely and effective treatment for physical diseases, which makes depression increasingly prominent in patients with GU.
The relevant risk factors and pathogenesis of GU concomitant with depression have not yet been fully elucidated. The occurrence and severity of GU complicated with depression may be related to autoimmune dysfunction and gastro
This study aimed to investigate the immune function, gastrointestinal hormone levels, and clinical significance of patients with GU combined with depression and to control disease progression in patients with GU complicated with depression.
This study used a retrospective approach to analyze two indexes of immune function and gastrointestinal hormones. Fur
Serum immunoglobulin A (IgA), IgM, gastrin (GAS), and cholecystokinin (CCK) levels were the influencing factors affecting the severity of GU combined with depression; the area under the receiver operating characteristic curve of immune function (IgA, IgM) and gastrointestinal hormone levels (GAS, CCK) for the prediction of GU with depression severity was 0.958 (95% confidence interval: 0.933-0.976).
The occurrence of GU complicated with depression is related to autoimmune dysfunction and disorders of gastro
Using a retrospective analysis approach, patients were divided into severe and mild-moderate groups according to the Hamilton Depression Scale. Basic data from both groups, including immune function and gastrointestinal hormone markers, were used to analyze the factors affecting the severity of GU and concurrent depression. To explore the value of immune function indices, gastrointestinal hormone indices and a combination of indices were used to predict the severity of GU and depression.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Psychiatry
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ahmed Tousif H, United States; Huang C, Taiwan S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Tarnawski AS, Ahluwalia A. The Critical Role of Growth Factors in Gastric Ulcer Healing: The Cellular and Molecular Mechanisms and Potential Clinical Implications. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 78] [Article Influence: 19.5] [Reference Citation Analysis (1)] |
| 2. | Kempenich JW, Sirinek KR. Acid Peptic Disease. Surg Clin North Am. 2018;98:933-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | Clarke K, Adler N, Agrawal D, Bhakta D, Sata SS, Singh S, Gupta A, Pahwa A, Pherson E, Sun A, Volpicelli F, Cho HJ. Indications for the Use of Proton Pump Inhibitors for Stress Ulcer Prophylaxis and Peptic Ulcer Bleeding in Hospitalized Patients. Am J Med. 2022;135:313-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 4. | Sivaram P, Sreekumar A. Preoperative factors influencing mortality and morbidity in peptic ulcer perforation. Eur J Trauma Emerg Surg. 2018;44:251-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | Levenstein S, Jacobsen RK, Rosenstock S, Jørgensen T. Mental vulnerability, Helicobacter pylori, and incidence of hospital-diagnosed peptic ulcer over 28 years in a population-based cohort. Scand J Gastroenterol. 2017;52:954-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Starup-Linde J, Langdahl B, Vestergaard P, Harsløf T. Incident peptic ulcers and concomitant treatment of direct oral anticoagulants and oral bisphosphonates-a real-world cohort study. Osteoporos Int. 2022;33:1323-1334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 7. | Wu Y, Murray GK, Byrne EM, Sidorenko J, Visscher PM, Wray NR. GWAS of peptic ulcer disease implicates Helicobacter pylori infection, other gastrointestinal disorders and depression. Nat Commun. 2021;12:1146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 111] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 8. | Hsu CC, Hsu YC, Chang KH, Lee CY, Chong LW, Lin CL, Shang CS, Sung FC, Kao CH. Depression and the Risk of Peptic Ulcer Disease: A Nationwide Population-Based Study. Medicine (Baltimore). 2015;94:e2333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Sung SY, Choi HH, Seo KJ. A Differential Diagnosis of Unusual Gastric Ulcer. Diagnostics (Basel). 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 10. | Parker G, Tavella G, Macqueen G, Berk M, Grunze H, Deckersbach T, Dunner DL, Sajatovic M, Amsterdam JD, Ketter TA, Yatham LN, Kessing LV, Bassett D, Zimmerman M, Fountoulakis KN, Duffy A, Alda M, Calkin C, Sharma V, Anand A, Singh MK, Hajek T, Boyce P, Frey BN, Castle DJ, Young AH, Vieta E, Rybakowski JK, Swartz HA, Schaffer A, Murray G, Bayes A, Lam RW, Bora E, Post RM, Ostacher MJ, Lafer B, Cleare AJ, Burdick KE, O'Donovan C, Ortiz A, Henry C, Kanba S, Rosenblat JD, Parikh SV, Bond DJ, Grunebaum MF, Frangou S, Goldberg JF, Orum M, Osser DN, Frye MA, McIntyre RS, Fagiolini A, Manicavasagar V, Carlson GA, Malhi GS. Revising Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, criteria for the bipolar disorders: Phase I of the AREDOC project. Aust N Z J Psychiatry. 2018;52:1173-1182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Babić E, Bevanda M, Karin M, Volarić M, Bogut A, Bevanda Glibo D, Bevanda D, Šutalo N. Anxiety, Depression and Personality Types in Patients with Inflammatory Bowel Disease: Comparisons with Peptic Ulcer and the General Population. Psychiatr Danub. 2021;33:48-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Graham DY. History of Helicobacter pylori, duodenal ulcer, gastric ulcer and gastric cancer. World J Gastroenterol. 2014;20:5191-5204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 202] [Cited by in RCA: 230] [Article Influence: 20.9] [Reference Citation Analysis (3)] |
| 13. | Shetty V, Ballal M, Balaraju G, Shetty S, Pai GC, Lingadakai R. Helicobacter pylori in Dyspepsia: Phenotypic and Genotypic Methods of Diagnosis. J Glob Infect Dis. 2017;9:131-134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Ramezani F, Shakib P, Soroush S, Rezaei F, Moradniani M, Delfani S. Frequency of Helicobacter pylori Infection in Patients with Peptic Ulcer Referred to the Endoscopy Departments of Khorramabad City Hospitals, Iran, During 2013-2016. Infect Disord Drug Targets. 2022;22:e020222200771. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 15. | Cogley C, Carswell C, Bramham K, Chilcot J. Chronic Kidney Disease and Severe Mental Illness: Addressing Disparities in Access to Health Care and Health Outcomes. Clin J Am Soc Nephrol. 2022;17:1413-1417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 16. | Roseman A, Kovacs AH. Anxiety and Depression in Adults with Congenital Heart Disease: When to Suspect and How to Refer. Curr Cardiol Rep. 2019;21:145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Choi KW, Kim YK, Jeon HJ. Comorbid Anxiety and Depression: Clinical and Conceptual Consideration and Transdiagnostic Treatment. Adv Exp Med Biol. 2020;1191:219-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 145] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 18. | Xiao H, Zhao Z, Zhang C, Wang J. Influence of Standardized Nursing Intervention Combined with Mindfulness Stress Reduction Training on the Curative Effect, Negative Emotion, and Quality of Life in Patients with Chronic Gastritis and Gastric Ulcer. Evid Based Complement Alternat Med. 2021;2021:2131405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 19. | van Dijk MT, Murphy E, Posner JE, Talati A, Weissman MM. Association of Multigenerational Family History of Depression With Lifetime Depressive and Other Psychiatric Disorders in Children: Results from the Adolescent Brain Cognitive Development (ABCD) Study. JAMA Psychiatry. 2021;78:778-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 58] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 20. | Bisgaard TH, Allin KH, Keefer L, Ananthakrishnan AN, Jess T. Depression and anxiety in inflammatory bowel disease: epidemiology, mechanisms and treatment. Nat Rev Gastroenterol Hepatol. 2022;19:717-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 281] [Article Influence: 93.7] [Reference Citation Analysis (0)] |
| 21. | Fond G, Loundou A, Hamdani N, Boukouaci W, Dargel A, Oliveira J, Roger M, Tamouza R, Leboyer M, Boyer L. Anxiety and depression comorbidities in irritable bowel syndrome (IBS): a systematic review and meta-analysis. Eur Arch Psychiatry Clin Neurosci. 2014;264:651-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 474] [Cited by in RCA: 405] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 22. | Li K, Xu Y, Hu Y, Liu Y, Chen X, Zhou Y. Effect of Enteral Immunonutrition on Immune, Inflammatory Markers and Nutritional Status in Gastric Cancer Patients Undergoing Gastrectomy: A Randomized Double-Blinded Controlled Trial. J Invest Surg. 2020;33:950-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 23. | Jacobse J, Li J, Rings EHHM, Samsom JN, Goettel JA. Intestinal Regulatory T Cells as Specialized Tissue-Restricted Immune Cells in Intestinal Immune Homeostasis and Disease. Front Immunol. 2021;12:716499. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 24. | Koemans WJ, van Dieren JM, van den Berg JG, Meijer GA, Snaebjornsson P, Chalabi M, Lecot F, Riedl R, Krijgsman O, Hofland I, Broeks A, Voncken FEM, Peppelenbosch MP, Sosef MN, van Sandick JW, Kodach LL. High CD8(+) tumour-infiltrating lymphocyte density associates with unfavourable prognosis in oesophageal adenocarcinoma following poor response to neoadjuvant chemoradiotherapy. Histopathology. 2021;79:238-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 25. | Mosiienko HP. [Role of gastrointestinal hormones in pathogenesis of functional diseases of the digestive system in adolescent]. Lik Sprava. 2008;47-50. [PubMed] |
| 26. | Li Y, Song Y. Diagnostic Value of Serum Gastrin and Epidermal Growth Factor to the Gastric Ulcer Complicated with Upper Gastrointestinal Hemorrhage. J Coll Physicians Surg Pak. 2020;30:1269-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Thornton LM, Andersen BL, Blakely WP. The pain, depression, and fatigue symptom cluster in advanced breast cancer: covariation with the hypothalamic-pituitary-adrenal axis and the sympathetic nervous system. Health Psychol. 2010;29:333-337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 144] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 28. | Lin K, Wang Y, Gong J, Tan Y, Deng T, Wei N. Protective effects of total flavonoids from Alpinia officinarum rhizoma against ethanol-induced gastric ulcer in vivo and in vitro. Pharm Biol. 2020;58:854-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |