Copyright
©The Author(s) 2025.
World J Orthop. May 18, 2025; 16(5): 106377
Published online May 18, 2025. doi: 10.5312/wjo.v16.i5.106377
Published online May 18, 2025. doi: 10.5312/wjo.v16.i5.106377
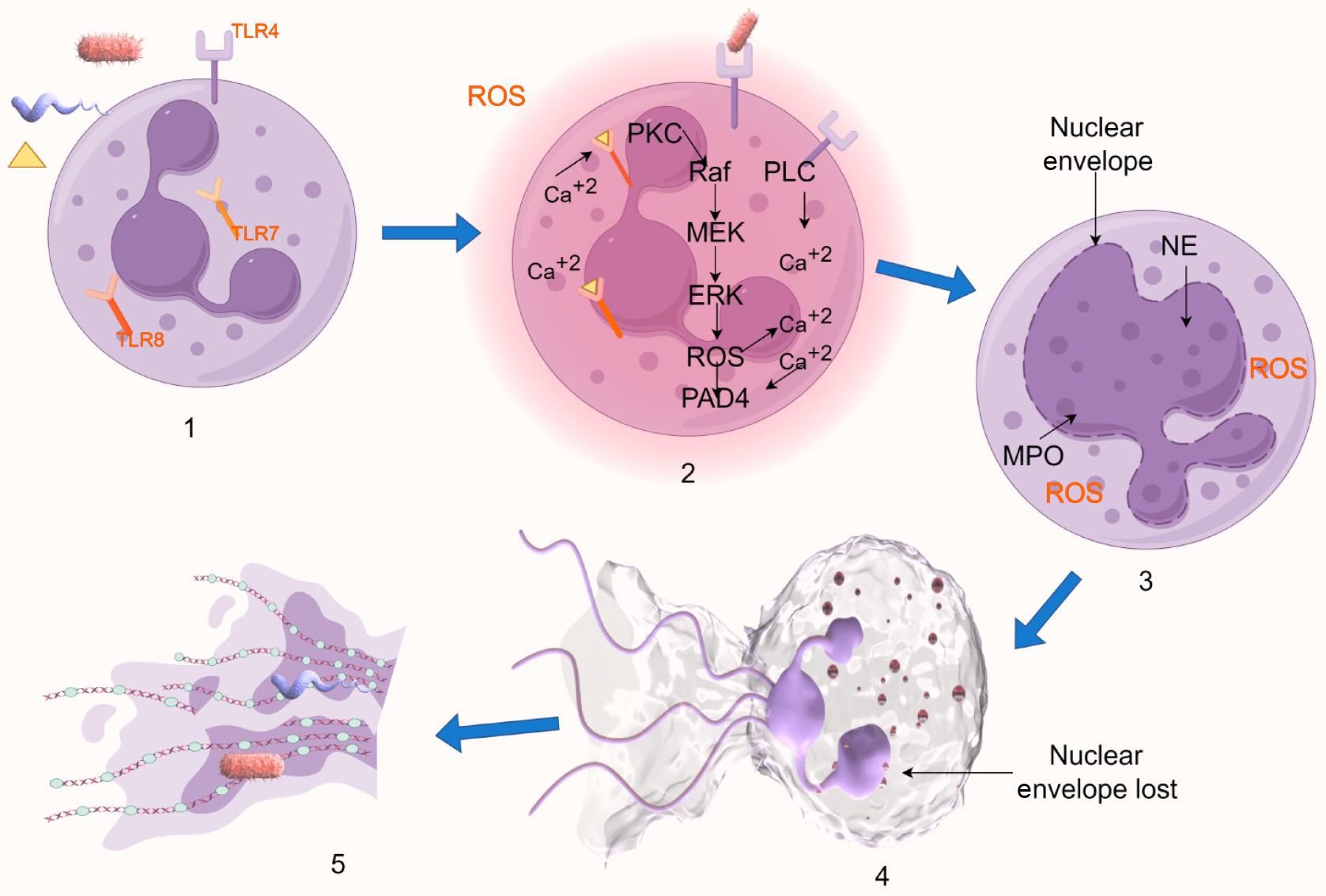
Figure 1 Schematic diagram of suicidal NETosis.
1: Receptor-mediated stimulus detection initiates the signaling cascade; 2: The Raf/MEK/ERK pathway activation and elevated calcium levels trigger gp91phox phosphorylation, enabling nicotinamide adenine dinucleotide phosphate oxidase assembly and reactive oxygen species generation; 3: Reactive oxygen species-dependent mechanisms facilitate nuclear translocation of azurophilic granule components (elastase and myeloperoxidase), inducing chromatin decondensation and nuclear morphological changes; 4: Nuclear envelope disintegration allows mixing of decondensed chromatin with cytoplasmic proteins; 5: Plasma membrane rupture results in the release of chromatin-protein complexes as extracellular traps. Created by Figdraw, ID: SPUAUc244c. TLR: Toll-like receptor; ROS: Reactive oxygen species; PKC: Protein kinase C; PAD4: Peptidyl arginine deiminases 4; NE: Neutrophil elastase; MPO: Myeloperoxidase.
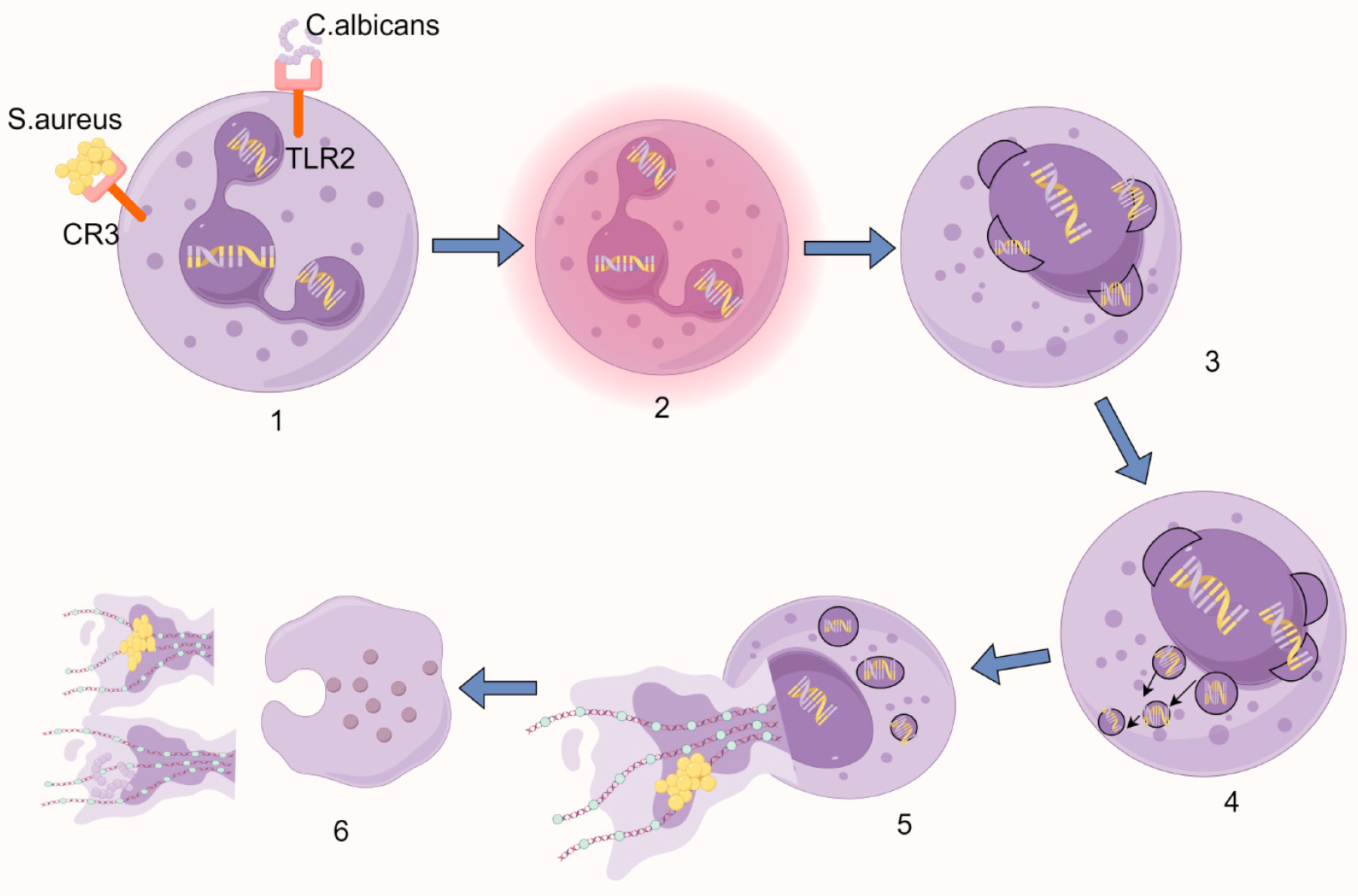
Figure 2 Schematic diagram of vital NETosis.
1: Pathogen recognition via surface receptors triggers cellular activation; 2: Nuclear lobulation is lost, accompanied by chromatin decondensation; 3: The nuclear envelope undergoes sequential disassembly - outer and inner membranes separate and vesiculate; 4: Nuclear-derived vesicles containing stretched chromatin filaments accumulate in the cytoplasm; 5: Cytoplasmic granules migrate toward the intact plasma membrane while maintaining membrane integrity; 6: Focal plasma membrane rupture enables extracellular DNA release, while selective granule exocytosis deposits antimicrobial proteins onto the expelled chromatin network. Created by Figdraw, ID: USWOWe861a. C. albicans: Candida albicans; S. aureus: Staphylococcus aureus; CR3: Complement receptor 3; TLR2: Toll-like receptor 2.
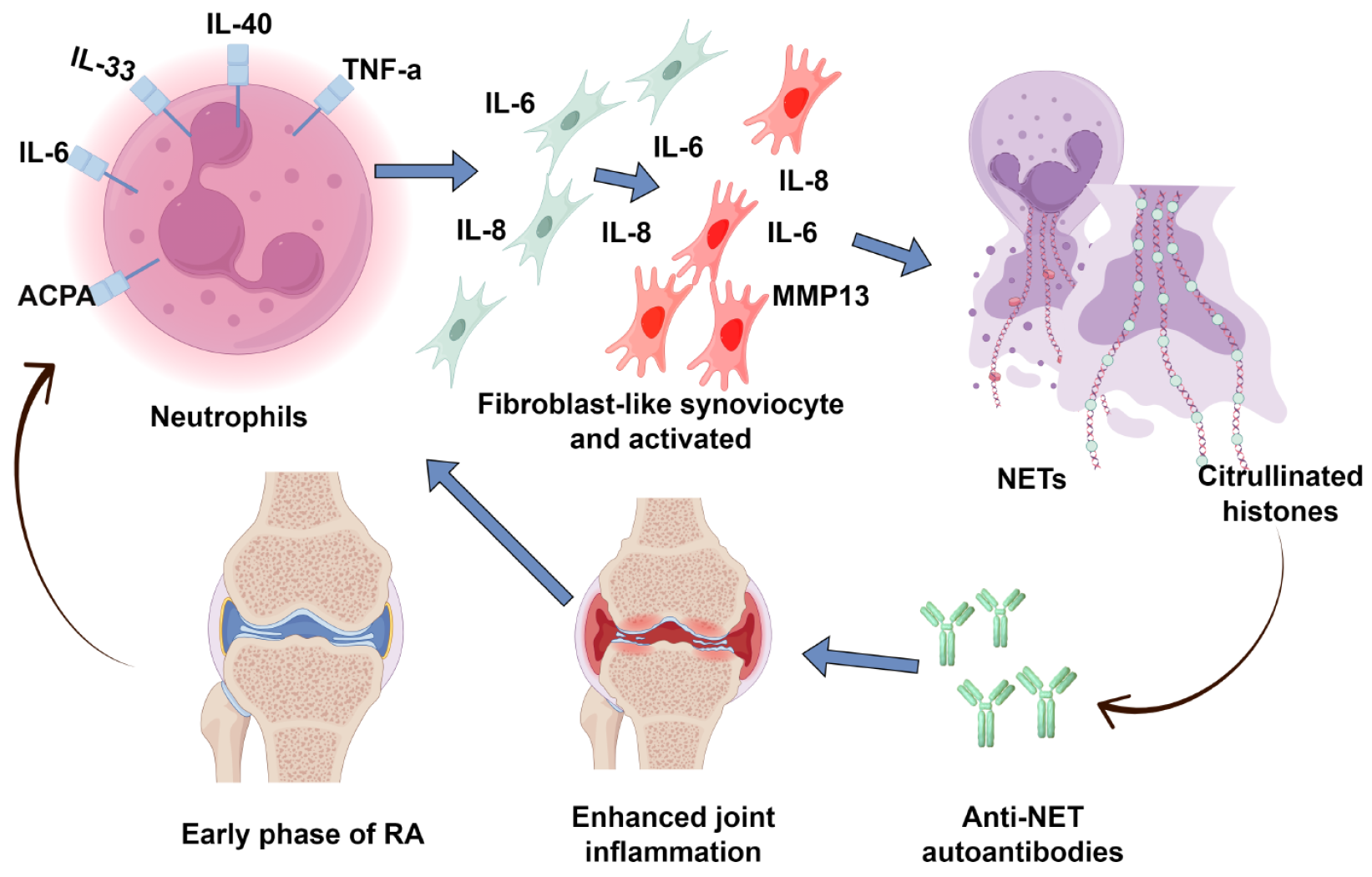
Figure 3 The role of neutrophil extracellular traps in the pathogenesis of rheumatoid arthritis.
In rheumatoid arthritis, circulating factors promote excessive neutrophil extracellular traps formation. This overproduction results in citrullinated histones (including citH2A, citH2B, and citH4) that serve as modified autoantigens. Within rheumatoid arthritis joints, these citrullinated neoepitopes trigger adaptive immune responses, driving the production of neutrophil extracellular trap-targeting autoantibodies. This self-perpetuating cycle of autoantigen presentation and autoantibody generation contributes to chronic synovial inflammation. Created by Figdraw, ID: IYTII99622. NETs: Neutrophil extracellular traps; IL: Interleukin; TNF: Tumor necrosis factor; MMP: Matrix metalloproteinase; ACPA: Anti-citrullinated protein antibody; RA: Rheumatoid arthritis.
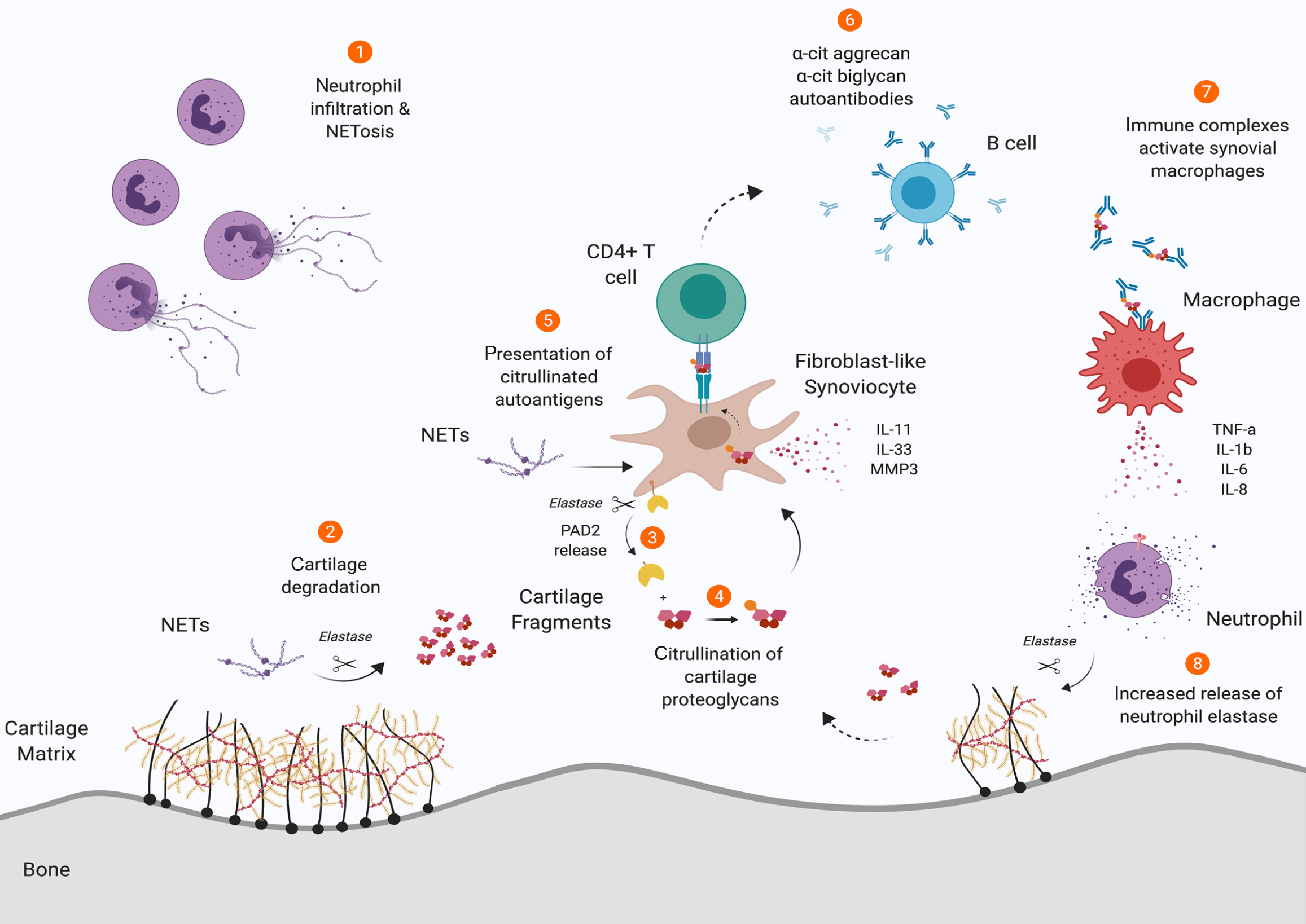
Figure 4 Schematic representation of the role of neutrophil elastase in synovial cartilage integrity in rheumatoid arthritis.
1: Neutrophils in rheumatoid synovium exhibit dysregulated NETosis; 2: Neutrophil-derived elastase within neutrophil extracellular traps degrades cartilage matrix, producing immunogenic fragments; 3: Membrane-bound peptidyl arginine deiminase 2 is liberated from fibroblast-like synoviocytes (FLSs) through elastase-mediated cleavage; 4: The liberated peptidyl arginine deiminases 2 catalyzes citrullination of cartilage fragments, which are subsequently internalized by FLSs; 5: FLSs present these citrullinated peptides to autoreactive CD4+ T cells; 6: Triggering B cell differentiation and production of autoantibodies targeting citrullinated proteoglycans; 7: The resulting immune complexes stimulate macrophage activation and secretion of proinflammatory mediators; 8: These cytokines further prime neutrophils for elastase release, establishing a self-amplifying inflammatory cascade that perpetuates cartilage destruction. Created by Figdraw, ID: TAASU77e88. NETs: Neutrophil extracellular traps; IL: Interleukin; TNF: Tumor necrosis factor; MMP: Matrix metalloproteinase.
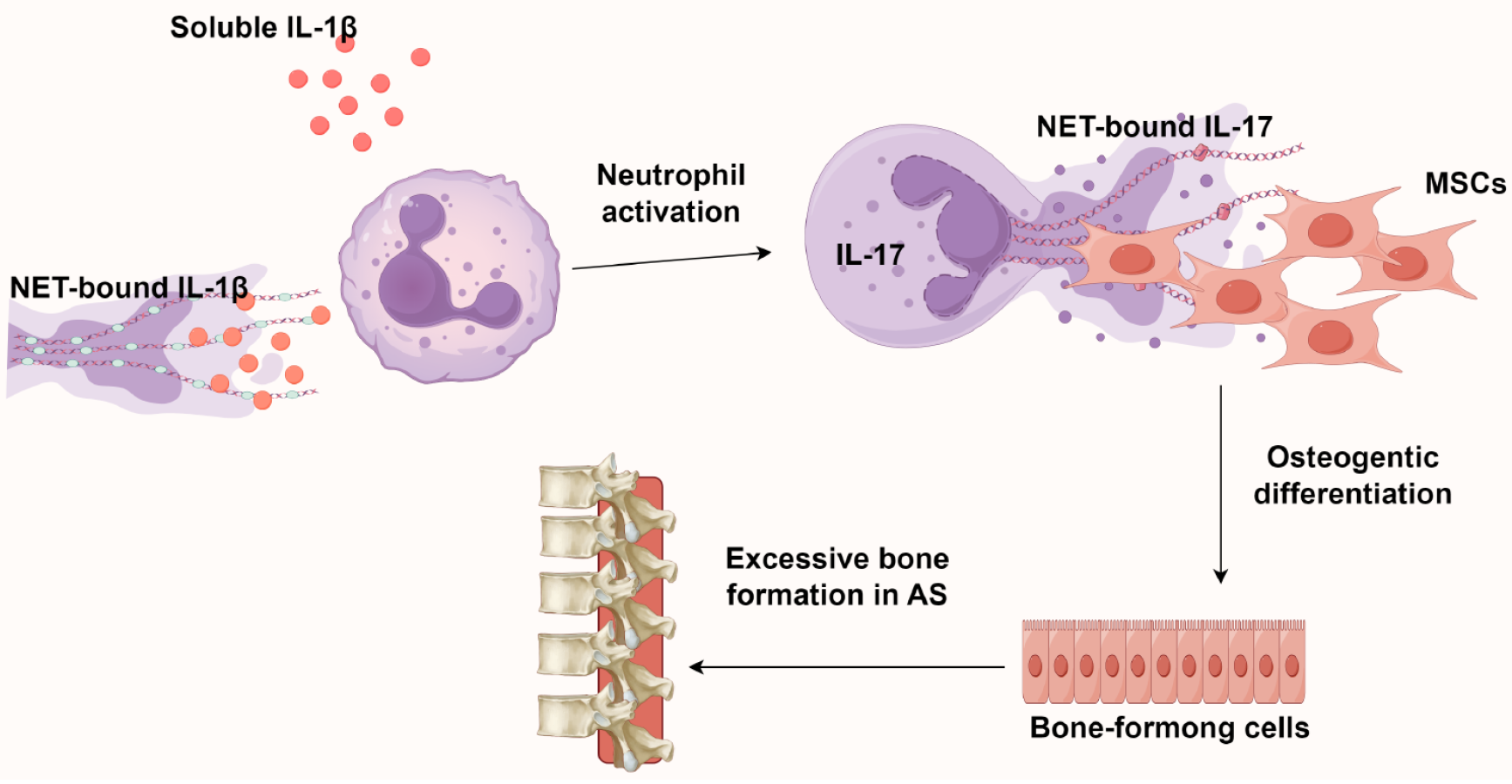
Figure 5 The role of neutrophil extracellular traps in the pathogenesis of ankylosing spondylitis.
The interleukin-17 (IL-17)/neutrophil extracellular traps axis directs mesenchymal stem cell osteogenesis, while IL-1β (from both serum and neutrophil extracellular traps) further stimulates neutrophilic IL-17 secretion. This reciprocal interaction establishes a pro-osteogenic inflammatory microenvironment that perpetuates both NETosis and aberrant bone formation. Created by Figdraw, ID: WRRPRa9680. NETs: Neutrophil extracellular traps; IL: Interleukin; MSCs: Mesenchymal stem cells; AS: Ankylosing spondylitis.
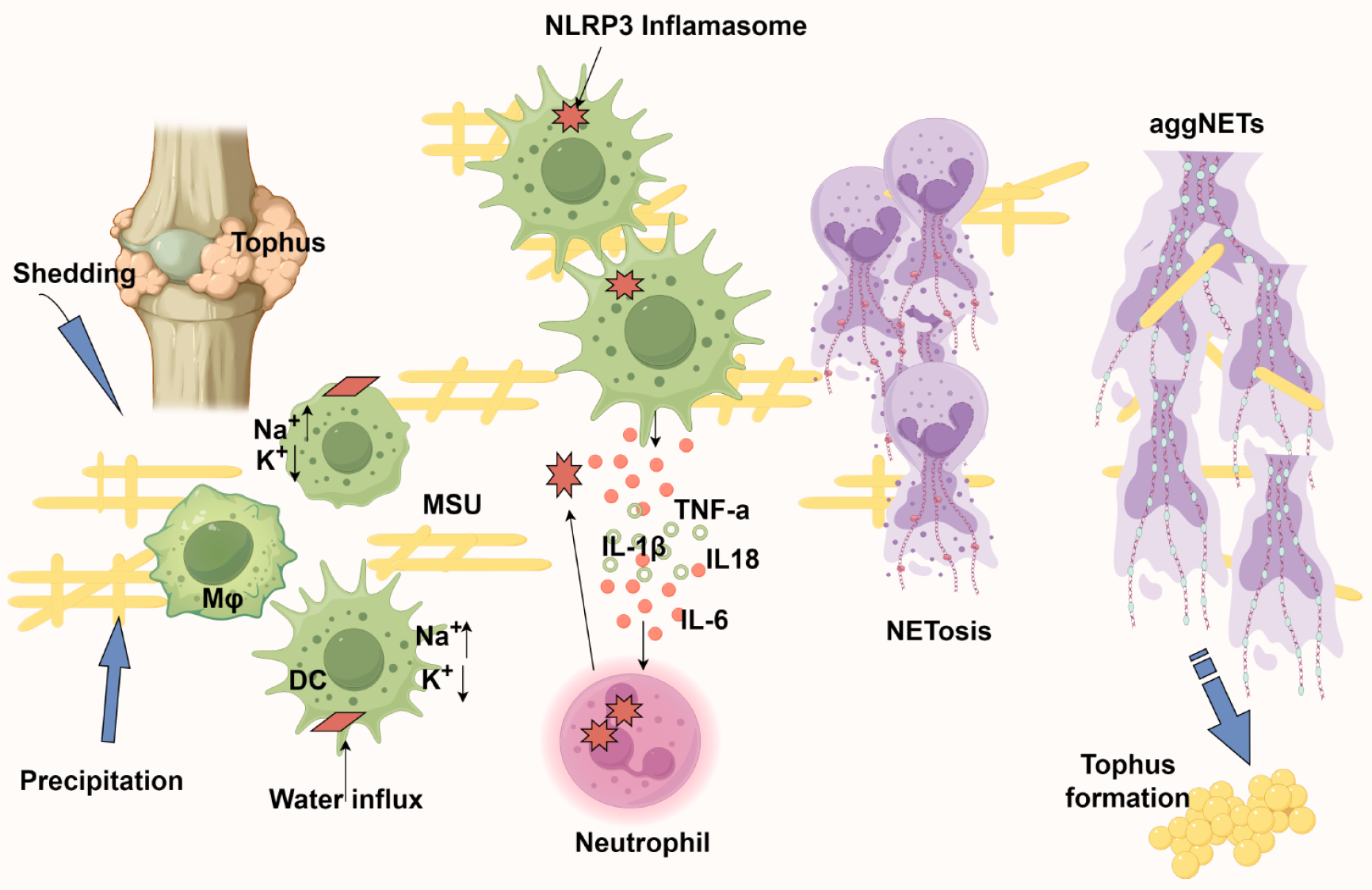
Figure 6 The neutrophil extracellular traps alleviate inflammation caused by monosodium urate crystals.
1: Monosodium urate crystals nucleate in joints either through de novo precipitation or release from existing tophi, subsequently being phagocytosed by antigen-presenting cells; 2: Intracellular crystal dissolution creates osmotic stress - sodium influx drives water entry, critically reducing potassium concentration below the threshold required for nucleotide-binding domain, leucine-rich repeat and pyrin domain-containing protein 3 inflammasome assembly; 3: Inflammasome activation triggers interleukin-18 (IL-18) and IL-1β release, establishing a chemokine gradient for neutrophil recruitment; 4: Infiltrating neutrophils amplify inflammation through proteinase 3-mediated pro-IL-1β processing while simultaneously engaging in crystal clearance via NETosis; 5: The resulting neutrophil extracellular trap (NET) structures serve dual roles: Physically encapsulating monosodium urate crystals to limit their bioavailability and forming aggregated NETs that proteolytically degrade inflammatory mediators; 6: Paradoxically, persistent aggregated NET deposition may nucleate new tophi, completing the pathogenic cycle. Created by Figdraw, ID: UAWAYbf41e. AggNETs: Aggregated neutrophil extracellular traps; NLRP3: Nucleotide-binding domain, leucine-rich repeat and pyrin domain-containing protein 3; DC: Dendritic cells; MSU: Monosodium urate; IL: Interleukin; TNF: Tumor necrosis factor.
- Citation: Sun GJ, Xu F, Jiao XY, Yin Y. Advances in research of neutrophil extracellular trap formation in osteoarticular diseases. World J Orthop 2025; 16(5): 106377
- URL: https://www.wjgnet.com/2218-5836/full/v16/i5/106377.htm
- DOI: https://dx.doi.org/10.5312/wjo.v16.i5.106377