Published online Apr 24, 2025. doi: 10.5306/wjco.v16.i4.104061
Revised: February 2, 2025
Accepted: February 25, 2025
Published online: April 24, 2025
Processing time: 107 Days and 22.4 Hours
Hepatocellular carcinoma (HCC) is the predominant form of primary liver cancer, accounting for 90% of all cases. Currently, early diagnosis of HCC can be achieved through serum alpha-fetoprotein detection, B-ultrasound, and computed tomo
Core Tip: The molecular mechanisms underlying hepatocellular carcinoma (HCC) development remain challenging to fully elucidate. The advent of high-throughput sequencing technologies has revealed that long non-coding RNAs (lncRNAs) play a pivotal role in gene regulation, contributing to cell proliferation, inhibiting apoptosis, promoting invasion and metastasis, and modulating metabolic processes. Dysregulation of lncRNAs is intricately linked to the progression of HCC, indicating their potential as prognostic markers and therapeutic targets. In this review, we systematically examine the research progress on lncRNA-mediated regulation of HCC cell death and discuss its potential therapeutic applications.
- Citation: Wang J, Liu ZX, Huang ZH, Wen J, Rao ZZ. Long non-coding RNA in the regulation of cell death in hepatocellular carcinoma. World J Clin Oncol 2025; 16(4): 104061
- URL: https://www.wjgnet.com/2218-4333/full/v16/i4/104061.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i4.104061
Hepatocellular carcinoma (HCC) ranks as the sixth most prevalent cancer globally and is responsible for the fourth highest number of cancer-related deaths. The 5-year survival rate for liver cancer patients worldwide stands at a mere 18%, with even lower rates observed in many Asian countries[1,2] Consequently, this imposes a substantial burden on both families and society. Common treatment modalities for HCC encompass hepatectomy, liver transplantation, ablative therapy, transarterial chemoembolization, radiation therapy, systematic anti-tumor therapy, and other approaches. Hepatectomy represents the primary curative option for early-stage liver cancer, while radiofrequency ablation and transcatheter chemoembolization serve as standard treatments in the disseminated stage of the disease[3,4]. However, due to the intricate nature and heterogeneity of this condition coupled with its often asymptomatic or atypical clinical presentation among patients with liver cancer, a significant proportion of patients lose their eligibility for surgery due to local progression upon diagnosis. However, remarkable progress has recently been made in advanced liver cancer treatment owing to the emergence of various targeted therapies and immunotherapeutic agents[5].
Since its approval by the United States Food and Drug Administration in 2007, the multi-targeted tyrosine kinase inhibitor sorafenib has transformed the therapeutic landscape for advanced HCC. For nearly a decade, sorafenib remained the sole systemic therapy option for this condition. However, clinical studies of IMbrave150 have demonstrated that the combination of atezolizumab and bevacizumab yields superior outcomes compared to sorafenib. Atezolizumab extends progression-free survival by 2-6 mo and reduces the risk of cancer progression by 41% compared to sorafenib alone. As a result, the combination of bevacizumab and atezolizumab has emerged as a current standard first-line treatment[6]. Immune checkpoint inhibitors (ICIs) are currently considered mainstream immunotherapy options for liver cancer[7]. In this therapeutic approach, immunotherapy primarily targets the immune microenvironment surrounding tumors. By employing ICIs, interactions between tumor cells and immune cell surfaces are obstructed while signal transduction is inhibited, consequently activating immune cell activity. This leads to autoreactive killing of cancer cells and tumor tissues, thereby achieving effective tumor eradication[8,9].
However, targeted therapy primarily focuses on aberrant genes within the body, such as EGFR gene, ALK gene, ROS-1 gene, etc. Targeted therapy directly acts on these targets and inhibits their pathways when abnormal mutations occur in the genes. This effectively restricts tumor growth and achieves anti-tumor objectives[10,11]. Thus, a thorough under
To elucidate the mechanism of lncRNA-mediated regulatory cell death in HCC cells, we conducted a literature search in the PubMed database. We used the keywords "HCC", "lncRNA", "necroptosis", "apoptosis", "ferroptosis", "pyroptosis", "autophagy", and "cuproptosis", restricting our search to articles published within the past five years.
By elucidating the mechanisms underlying necrosis, apoptosis, ferroptosis, and autophagy in HCC cells, as well as exploring the involvement of lncRNAs in RCD processes, this study highlights the pivotal regulatory role exerted by lncRNAs in HCC cells (Table 1). Furthermore, it delves into the potential utility of lncRNAs as biomarkers and discusses their prospective diagnostic and therapeutic applications in HCC.
| Type | Ref. | Year | Findings |
| Necroptosis | Peng et al[54] | 2022 | Ten candidate lncRNAs were obtained, from which a prognostic risk model was constructed. The patients with high-risk scores have lower survival rates |
| Chen et al[56] | 2022 | A 6-lncRNA prediction models have good prognostic value for liver cancer, and are worthy of clinical application | |
| Wang et al[57] | 2022 | A model containing four necrotic apoptoses-associated lncRNAs was constructed. The OS of low-risk patients is significantly longer than that of high-risk patients | |
| Chen et al[58] | 2024 | A positive correlation was observed between the five necrosis-associated lncRNA and the malignant phenotypes of HCC | |
| Apoptosis | Hussain et al[70] | 2023 | NEAT1 has the ability to inhibit the proliferation, migration and invasion of cancer cells in HCC and promote apoptosis |
| Yao et al[71] | 2021 | LncRNA CASC9 promotes proliferation, migration and inhibits apoptosis of hepatocellular carcinoma cells by down-regulating miR-424-5p | |
| Cai et al[72] | 2021 | LncRNA AIRN influences the proliferation and apoptosis of hepatocellular carcinoma cells by regulating STAT1 ubiquitination | |
| Fei et al[73] | 2020 | LncRNA ST8SIA6-AS1 promotes hepatocellular carcinoma cell proliferation and resistance to apoptosis by targeting miR-4656/HDAC11 axis | |
| Ferroptosis | Xu et al[83] | 2021 | A nine-lncRNA-based signature was identified as the ferroptosis-related prognostic model for HCC, independent of multiple clinicopathological parameters |
| Zhang et al[84] | 2022 | LncRNA HEPFAL accelerates ferroptosis in hepatocellular carcinoma by regulating SLC7A11 ubiquitination | |
| Pyroptosis | Wu et al[93] | 2023 | These prognosis-related lncRNAs, miRNAs, and PRGs formed eleven lncRNA-miRNA-mRNA regulatory axes |
| Autophagy | Braconi et al[101] | 2011 | The resistance of HCC to lenvatinib was influenced by the regulatory axis of HOTAIRM1-miR-34a-Beclin-1 |
| Cuproptosis | Li et al[108] | 2023 | The expression of copper poisoning gene CDKN2A was closely positively associated with lncRNA DDX 11-AS 1 |
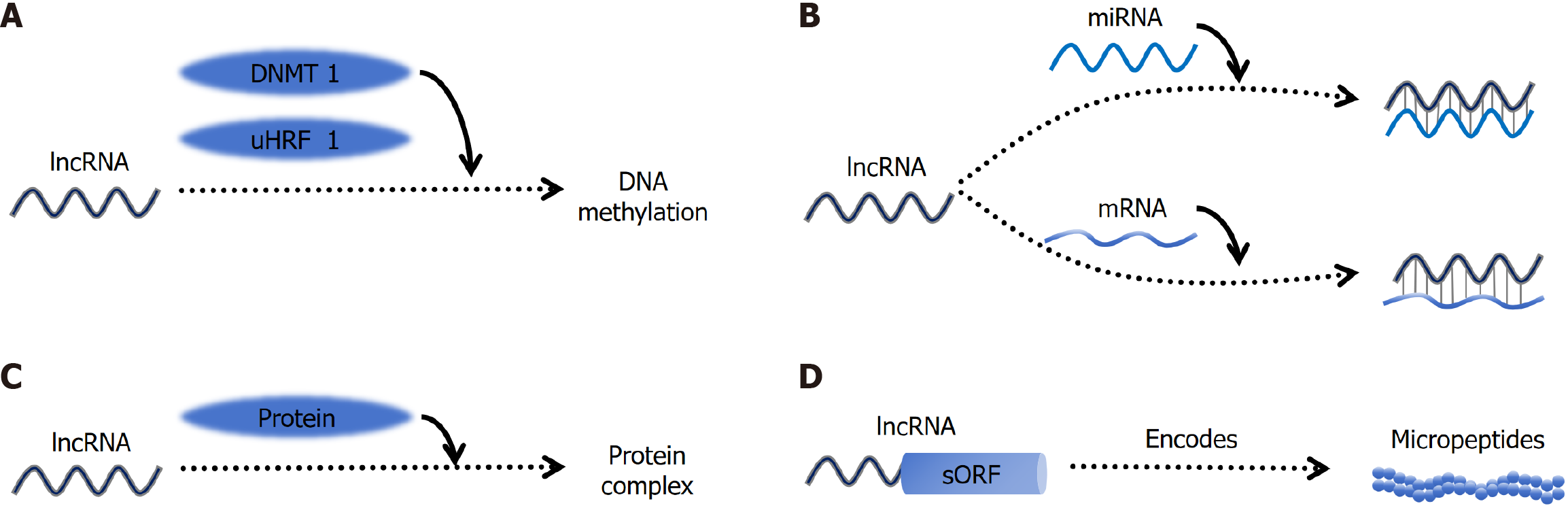
Extensive investigations into the human transcriptome have revealed that protein-coding sequences constitute only a minor fraction of the overall transcriptional output[22-24]. The most abundant type of RNA is lncRNA[25], which are characterized by a length exceeding 200 nucleotides and predominantly lack the capacity to encode or translate proteins[26]. The structure of lncRNAs is intricate and varied, encompassing linear, circular, Y-shaped, U-shaped, and other configurations. Moreover, lncRNAs tend to fold into complex secondary and tertiary structures, undergo modifications such as methylation and acetylation, interact with proteins, DNA, and other RNAs, thereby modulating the activities of multi-protein complexes and DNA targets[27].
Moreover, lncRNAs exhibit highly cell type-specific expression patterns[28,29]. Notably, the total number of lncRNAs surpasses that of protein-coding genes by approximately 4.5-fold[30]. Initially regarded as transcriptional by-products of RNA polymerase II with no discernible biological function due to their nuclear or cytoplasmic localization and absence of protein coding potential[31], recent studies have revealed that lncRNAs can act as competing endogenous RNAs (ceRNAs) capable of sequestering miRNA molecules, thereby modulating miRNA-mediated gene regulation through sponge-like mechanisms and consequently influencing cellular physiology[32]. LncRNAs function as molecular signals to modulate various signaling pathways, including p53, AKT, and Notch, as well as epigenetic regulation, DNA damage responses, and a wide range of biological processes such as tumor proliferation, metabolism, apoptosis, aerobic gly
Despite their dominance in the transcriptome, the functions of lncRNAs have remained largely unexplored. To date, only a limited number of studies have investigated their mechanisms, and research on lncRNA transmission mechanisms in HCC is particularly inadequate. Numerous international lncRNA databases with variations in species information, function, data classification, and sample reference have been established[37]. Currently, two highly recognized lncRNA databases are lncATLAS and LncRBase[38,39]. To accelerate lncRNA research, Montero et al[40] developed a genome-wide screening platform utilizing CasRX, which facilitates high-throughput mapping and integration of molecular and phenotypic data across various cancers, thereby enabling the inference of lncRNA functions. Extensive investigations into the role of lncRNAs in cancer development have revealed their potential as prognostic indicators, therapeutic targets, and diagnostic tools (Figure 1). Consequently, it is evident that lncRNAs play a crucial role in the growth dynamics, me
Numerous studies have demonstrated a strong correlation between aberrant expression or functionality of lncRNA and the development of various human diseases, including cancer and degenerative neurological disorders, which pose significant threats to human health[42-45]. Specifically, lncRNAs display aberrant sequence and spatial structures, deregulated expression levels, and atypical interactions with binding proteins. In the context of cancer, lncRNAs exert diverse functions through their interactions with biomolecules, chromatin remodeling processes, transcriptional regulation mechanisms, and post-transcriptional modifications[45-49].
Compared with other cancers, the mechanisms involving lncRNAs in HCC exhibit distinct characteristics. These unique features are primarily attributed to the specific biological properties of the liver, the etiology of HCC, and the tumor microenvironment. For instance, nonalcoholic steatohepatitis (NASH) and viral hepatitis are established risk factors for HCC. NASH is frequently associated with liver fibrosis, obesity, and metabolic syndrome. The lncRNA H19 influences liver fibrosis in NASH by acting as a ceRNA that sequesters miRNAs, thereby modulating the expression of genes involved in fibrosis[50]. Additionally, the lncRNA MALAT1 enhances hepatic steatosis and insulin resistance by stabilizing the nuclear SREBP-1c protein[51]. Consequently, it is imperative to investigate the role of lncRNAs in HCC progression.
LncRNAs regulate HCC cells through multiple mechanisms. Firstly, lncRNAs can bind to DNA, reshaping chromatin structure and inducing epigenetic modifications, thereby modulating the expression of target genes. Secondly, lncRNAs function as molecular sponges by interacting with mRNAs or miRNAs, thus regulating mRNA stability and translation, as well as the binding of miRNAs to their targets. Thirdly, lncRNAs can bind to proteins, influencing protein con
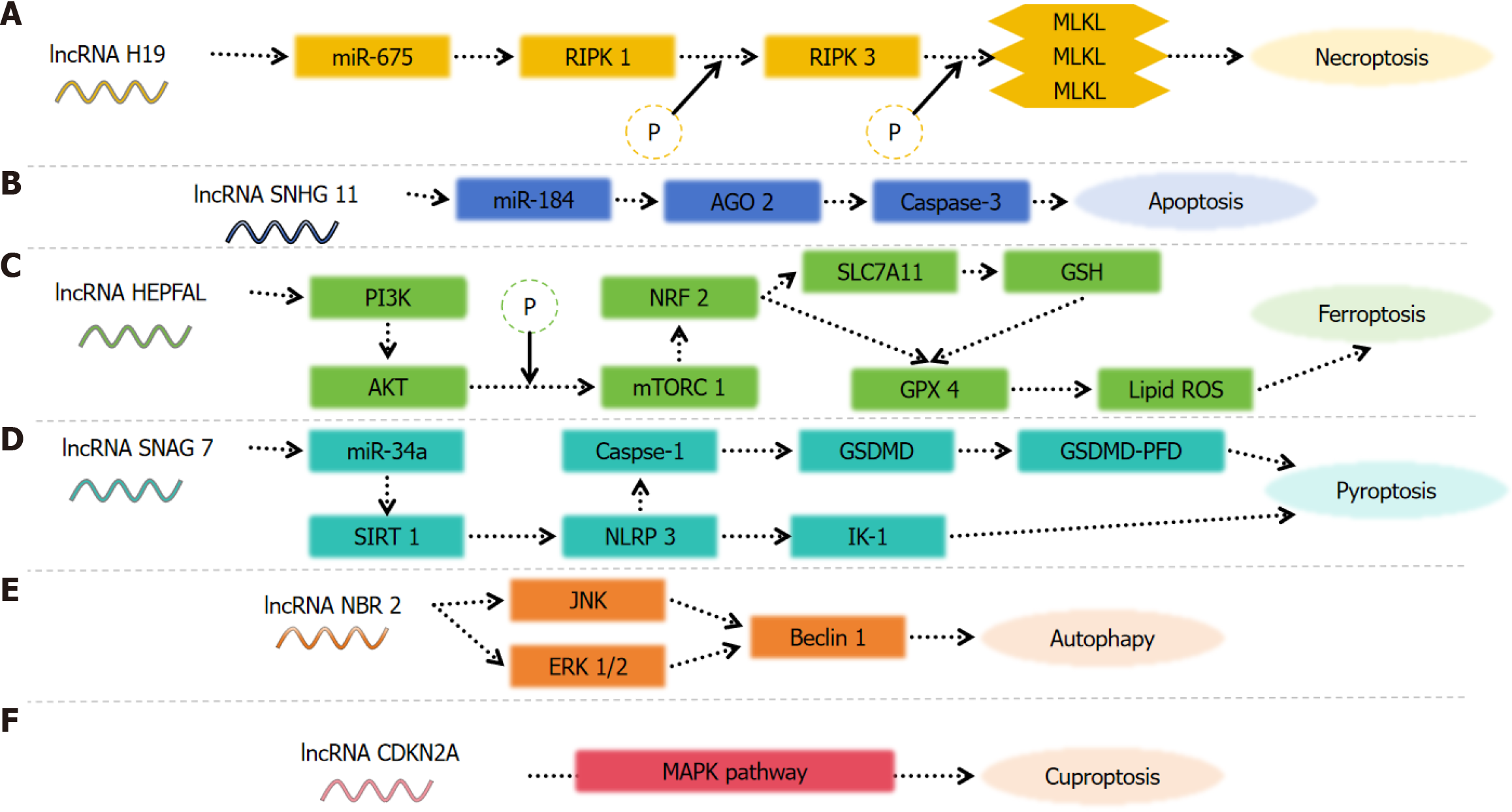
In recent years, programmed cell death has garnered significant attention in the field of cell death research. Necroptosis, a regulated form of cytolytic death rather than an accidental occurrence[52], plays a pivotal role in the regulation of tumorigenesis, cancer metastasis, and cancer immunity[53]. To investigate the prognostic value of necrosis-associated lncRNAs in HCC, Peng et al[54] constructed a prognostic model based on The Cancer Genome Atlas (TCGA) public database to validate the role of necrosis-associated lncRNAs in HCC. Through single-factor Cox regression analysis among 779 genes, they identified 58 LncRNAs (PRlncRNAs) associated with necrosis and further screened collinear factors using Lasso-Cox method. Ten candidate lncRNAs (AL031985.3, SREBF2-AS1, ZFPM2-AS1, KDM4A-AS1, AC026412.3, AC145207.5, DUXAP8, LINC01224, AC099850.4, MKLN1-AS) were selected accordingly. Subsequently, a prognostic risk model was established[54]. The roles of AL031985.3 and AC145207.5 in glycolytic-related prognostic models of liver cancer have been demonstrated[55]. However, it is important to note that the reliability of these findings should be further validated using other databases. A novel prognostic model incorporating six necrosis-associated lncRNAs (AL606489.1, NRAV, LINC02870, DUXAP8, "ZFPM2-AS1", AL031985.3) exhibits promising potential for accurately predicting the prognosis of HCC and warrants clinical application[56]. Another model comprising four necrotic lncRNAs (POLH-AS1, DUXAP8, AC131009.1, and TMCC1-AS1) demonstrates significantly prolonged overall survival in low-risk patients compared to high-risk patients[57]. Recent studies[58] have demonstrated that five necrosis-related lncRNAs (ZFPM2-AS1, AC0998
Although several necrosis-related lncRNA models have been proposed, their ability to enhance the efficacy of immunotherapy in HCC patients remains unproven. Therefore, future in vivo and in vitro experiments are essential for further confirmation.
Apoptosis is a tightly regulated form of programmed cell death. Properly functioning apoptosis eliminates cells damaged by infection and prevents the development of cancer. The extrinsic pathway of apoptosis is initiated by external stimuli through death receptors, including Fas receptor, DR4/DR5, TNF-R, and TNF-related apoptosis-inducing ligand receptors, which are present on various cell surfaces. The intrinsic pathway can be regulated or activated by internal stimuli such as DNA damage and oxidative stress. Proteins like Bax and Bcl-2, located on the mitochondrial membrane, serve as key mediators of the intrinsic apoptosis pathway. Additionally, there exists a perforin/granzyme-mediated pathway.
Apoptosis plays a crucial role in the tumorigenesis, proliferation, and metastasis of tumors. Tumor cells evade apoptosis, contributing to tumorigenesis[59,60]. The inhibition of apoptosis can result in uncontrolled cell proliferation and tumor expansion[61,62]. A well-studied lncRNA in cancer research is paraventular assembly transcript 1 (NEAT1), which is transcribed from the multiple endocrine neoplasia gene on chromosome 11q13.1[63,64]. Numerous studies have demonstrated the association between NEAT1 and various malignancies including HCC[65-68]. Knockdown of NEAT1 has been shown to enhance apoptosis and reduce proliferation of HCC cells. Furthermore, NEAT1 overexpression has been found to suppress pro-inflammatory cytokine production and protect cells by modulating apoptosis control mechanisms[69]. Overall, NEAT1 knockdown exhibits inhibitory effects on HCC cell proliferation, migration, and invasion[70].
Additionally, lncRNA CASC9 hinders miR-424-5p activity to promote HCC cell proliferation, invasion, and migration while suppressing apoptosis[71]. LncRNA AIRN regulates STAT1 ubiquitination to influence liver cancer cell growth and programmed cell death[72], whereas lncRNA ST8SIA6-AS1 targets miR-4656/HDAC11 axis to facilitate liver cancer cell proliferation as well as induce apoptosis[73].
Existing evidence supports multiple regulatory pathways involving lncRNAs as promising prognostic predictors and therapeutic targets for HCC. However, it should be noted that many studies have primarily focused on analyzing the impact of lncRNAs on HCC using in vitro models. Therefore, future comprehensive analyses incorporating in vivo animal experiments are warranted for a more thorough understanding of lncRNA regulation.
Ferroptosis, a distinctive form of programmed cell death discovered by Brent R. Stockwell's laboratory in 2012, is characterized by the lethal accumulation of iron-dependent lipid peroxides localized on the membrane[74-76]. The main features are changes in mitochondrial morphology, including the mitochondrial membrane becoming dense with accompanying smaller size, and outer membrane rupture and reduction or disappearance of mitochondrial cristae[77,78]. Inducing iron-mediated cell death in the context of malignancy has emerged as a highly promising approach that may synergize with cancer immunotherapy and effectively target resistant and metastatic cancers[79,80]. Glutathione peroxidase 4 (GPX4) is known to be a crucial regulator of iron-induced cell death, while sorafenib is recognized as one of the targeted drug options for advanced HCC[81]. Li et al[82] found that tumor cells can develop primary or secondary resistance to sorafenib, and HCG18 inhibits GPX4 through binding with miR-450b-5p, promoting iron-mediated apoptosis inhibited by GPX4 and overcoming sorafenib resistance in HCC. Xu et al[83] used Pearson assay to assess the correlation between differentially expressed lncRNAs in 374 HCC samples and 50 normal liver samples from the TCGA. They identified a signature based on nine lncRNAs (CTD-2033A16.3, CTD-2116N20.1, CTD-2510F5.4, DDX11-AS1, LINC00942, LINC01224, LINC01231, LINC01508 and ZFPM2-AS) which served as an independent prognostic model for liver cancer associated with iron-induced cell death irrespective of multiple clinicopathological parameters. Furthermore, in vitro and in vivo experiments have confirmed that the lncRNA HEPFAL can attenuate migration and invasion capabilities of liver cancer cells while accelerating apoptosis specifically in ferroptotic liver cancer cells through regulation of SLC7A11 ubiquitination[84].
Pyroptosis is an inflammatory form of programmed cell death characterized by cellular swelling and dissolution, accompanied by the release of a diverse array of pro-inflammatory factors. The underlying mechanism involves caspase-mediated cleavage of the substrate, leading to the formation of pores in the plasma membrane. This is followed by the subsequent release of inflammatory factors, ultimately resulting in membrane rupture and cell death[85]. As a regulated mode of cell death, pyrodeath exhibits accelerated kinetics and elicits a more robust inflammatory response compared to other forms of cell demise. Furthermore, inflammation triggered by pyrodeath can potentiate the functional attributes of tumor-infiltrating immune cells while inducing potent anti-tumor immunity[86,87]. Continuous exposure to inflammatory factors released upon pyrogenic activation poses an increased risk for cancer development in normal tissues and cells[88,89]. Recently conducted studies have demonstrated a close association between pyrodeath and tumor deve
Autophagy serves as the principal mechanism responsible for the degradation and recycling of diverse cellular com
Microtubule-associated protein 1 light chain 3-II (LC3-II) serves as a marker for autophagosome formation[105]. HULC promotes HCC cell metastasis and proliferation via the Beclin 1 and LC3 pathways[106]. In HCC cells transfected with HULC, LC3-II and Beclin 1 expression levels were elevated, while the level of p62 was markedly reduced. Additionally, an increased number of autophagic vesicles were observed, indicating that HULC overexpression enhances autophagosome formation in HCC cells.
Cuproptosis represents a novel form of programmed cell death that was first introduced in 2022, quickly garnering significant attention from researchers globally. The primary biological processes associated with cuproptosis are oxidative phosphorylation and the tricarboxylic acid (TCA) cycle. In vitro experiments identified five lncRNAs, FOXD2-AS1,
Liver cancer poses a significant threat to human health. Early diagnosis of liver cancer can markedly improve patient prognosis. In recent years, the introduction of novel diagnostic markers such as des-gamma-carboxy prothrombin (DCP) has enhanced the accuracy of liver cancer diagnosis; however, these markers still possess certain limitations. With the deepening research into lncRNAs, particularly the elucidation of their relationship with liver cancer, an increasing number of lncRNAs have been identified as potential biomarkers for early liver cancer detection. The combination of lncRNAs with established markers like alpha-fetoprotein and DCP may significantly enhance the efficacy of early liver cancer diagnosis.
The current challenge lies in the fact that while statistical analysis has identified numerous lncRNAs associated with liver cancer development, such as HULC, UCA1, CCAT1, MEG3, and NEAT1, their specific regulatory mechanisms remain complex and multifaceted. For instance, lncRNA NEAT1 is highly expressed in liver cancer tissues and exerts its effects through multiple pathways. It can influence necrosis by regulating key molecules like RIPK1 and RIPK3, and it can indirectly modulate the necroptosis pathway by acting as a molecular sponge for miRNAs. However, existing research on lncRNAs in liver cancer predominantly focuses on individual signaling pathways, with limited studies systematically investigating the role of a single lncRNA across multiple pathways to elucidate how they collectively regulate tumor progression, invasion, and metastasis. Future research should aim to accurately translate these findings into clinical applications, potentially targeting lncRNAs to inhibit liver cancer progression.
In addition, it is well established that certain lncRNAs not only exert regulatory functions in HCC patients but also play significant roles in the progression of other malignancies such as pancreatic, gastric, and breast cancers. Monitoring specific lncRNAs enables the prospective prediction of HCC development trends, including potential metastatic patterns. This would allow clinicians to enhance patient management strategies and improve prognostic outcomes for HCC patients.
The research on apoptosis has been ongoing for over 30 years; however, the effectiveness of therapeutic agents targeting apoptosis regulators, such as apoptosis-associated cystease or B-cell lymphoma-2 family proteins, in antitumor therapy is limited[109]. Targeting non-apoptotic cell death presents a promising strategy to enhance the efficacy of immunotherapy in HCC treatment. Nevertheless, it remains uncertain whether long-term induction of nonapoptotic RCD by anticidal drugs benefits HCC patients, as DAMPs released through nonapoptotic RCD may also trigger death in other normal cells[110]. Therefore, there is an urgent need to develop more specific cell death inducers that selectively act on HCC tumor cells with minimal impact on normal tissues.
| 1. | Craig AJ, von Felden J, Garcia-Lezana T, Sarcognato S, Villanueva A. Tumour evolution in hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2020;17:139-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 555] [Article Influence: 111.0] [Reference Citation Analysis (1)] |
| 2. | Ma Z, Chen M, Liu X, Cui H. Identification and verification of a prognostic autophagy-related gene signature in hepatocellular carcinoma. Sci Rep. 2024;14:3032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 3. | Singal AG, Llovet JM, Yarchoan M, Mehta N, Heimbach JK, Dawson LA, Jou JH, Kulik LM, Agopian VG, Marrero JA, Mendiratta-Lala M, Brown DB, Rilling WS, Goyal L, Wei AC, Taddei TH. AASLD Practice Guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma. Hepatology. 2023;78:1922-1965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 471] [Cited by in RCA: 707] [Article Influence: 353.5] [Reference Citation Analysis (23)] |
| 4. | Stefanini B, Ielasi L, Chen R, Abbati C, Tonnini M, Tovoli F, Granito A. TKIs in combination with immunotherapy for hepatocellular carcinoma. Expert Rev Anticancer Ther. 2023;23:279-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 82] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 5. | Ganesan P, Kulik LM. Hepatocellular Carcinoma: New Developments. Clin Liver Dis. 2023;27:85-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 227] [Article Influence: 113.5] [Reference Citation Analysis (0)] |
| 6. | Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL; IMbrave150 Investigators. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382:1894-1905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2542] [Cited by in RCA: 4633] [Article Influence: 926.6] [Reference Citation Analysis (2)] |
| 7. | Wang Z, Wang Y, Gao P, Ding J. Immune checkpoint inhibitor resistance in hepatocellular carcinoma. Cancer Lett. 2023;555:216038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 85] [Reference Citation Analysis (0)] |
| 8. | Li J, Xuan S, Dong P, Xiang Z, Gao C, Li M, Huang L, Wu J. Immunotherapy of hepatocellular carcinoma: recent progress and new strategy. Front Immunol. 2023;14:1192506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 9. | Zhou M, Liu B, Shen J. Immunotherapy for hepatocellular carcinoma. Clin Exp Med. 2023;23:569-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 10. | Wang Y, Deng B. Hepatocellular carcinoma: molecular mechanism, targeted therapy, and biomarkers. Cancer Metastasis Rev. 2023;42:629-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 157] [Article Influence: 78.5] [Reference Citation Analysis (0)] |
| 11. | Yang C, Zhang H, Zhang L, Zhu AX, Bernards R, Qin W, Wang C. Evolving therapeutic landscape of advanced hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2023;20:203-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 386] [Reference Citation Analysis (0)] |
| 12. | Donne R, Lujambio A. The liver cancer immune microenvironment: Therapeutic implications for hepatocellular carcinoma. Hepatology. 2023;77:1773-1796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 319] [Article Influence: 159.5] [Reference Citation Analysis (0)] |
| 13. | Liu Y, Xun Z, Ma K, Liang S, Li X, Zhou S, Sun L, Liu Y, Du Y, Guo X, Cui T, Zhou H, Wang J, Yin D, Song R, Zhang S, Cai W, Meng F, Guo H, Zhang B, Yang D, Bao R, Hu Q, Wang J, Ye Y, Liu L. Identification of a tumour immune barrier in the HCC microenvironment that determines the efficacy of immunotherapy. J Hepatol. 2023;78:770-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 310] [Article Influence: 155.0] [Reference Citation Analysis (0)] |
| 14. | Kotsari M, Dimopoulou V, Koskinas J, Armakolas A. Immune System and Hepatocellular Carcinoma (HCC): New Insights into HCC Progression. Int J Mol Sci. 2023;24:11471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 42] [Reference Citation Analysis (0)] |
| 15. | Verma S, Sahu BD, Mugale MN. Role of lncRNAs in hepatocellular carcinoma. Life Sci. 2023;325:121751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (1)] |
| 16. | Huang Z, Zhou JK, Peng Y, He W, Huang C. The role of long noncoding RNAs in hepatocellular carcinoma. Mol Cancer. 2020;19:77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 216] [Cited by in RCA: 358] [Article Influence: 71.6] [Reference Citation Analysis (0)] |
| 17. | Han TS, Hur K, Cho HS, Ban HS. Epigenetic Associations between lncRNA/circRNA and miRNA in Hepatocellular Carcinoma. Cancers (Basel). 2020;12:2622. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 121] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 18. | Chen X, Tang FR, Arfuso F, Cai WQ, Ma Z, Yang J, Sethi G. The Emerging Role of Long Non-Coding RNAs in the Metastasis of Hepatocellular Carcinoma. Biomolecules. 2019;10:66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 19. | Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, Alnemri ES, Altucci L, Amelio I, Andrews DW, Annicchiarico-Petruzzelli M, Antonov AV, Arama E, Baehrecke EH, Barlev NA, Bazan NG, Bernassola F, Bertrand MJM, Bianchi K, Blagosklonny MV, Blomgren K, Borner C, Boya P, Brenner C, Campanella M, Candi E, Carmona-Gutierrez D, Cecconi F, Chan FK, Chandel NS, Cheng EH, Chipuk JE, Cidlowski JA, Ciechanover A, Cohen GM, Conrad M, Cubillos-Ruiz JR, Czabotar PE, D'Angiolella V, Dawson TM, Dawson VL, De Laurenzi V, De Maria R, Debatin KM, DeBerardinis RJ, Deshmukh M, Di Daniele N, Di Virgilio F, Dixit VM, Dixon SJ, Duckett CS, Dynlacht BD, El-Deiry WS, Elrod JW, Fimia GM, Fulda S, García-Sáez AJ, Garg AD, Garrido C, Gavathiotis E, Golstein P, Gottlieb E, Green DR, Greene LA, Gronemeyer H, Gross A, Hajnoczky G, Hardwick JM, Harris IS, Hengartner MO, Hetz C, Ichijo H, Jäättelä M, Joseph B, Jost PJ, Juin PP, Kaiser WJ, Karin M, Kaufmann T, Kepp O, Kimchi A, Kitsis RN, Klionsky DJ, Knight RA, Kumar S, Lee SW, Lemasters JJ, Levine B, Linkermann A, Lipton SA, Lockshin RA, López-Otín C, Lowe SW, Luedde T, Lugli E, MacFarlane M, Madeo F, Malewicz M, Malorni W, Manic G, Marine JC, Martin SJ, Martinou JC, Medema JP, Mehlen P, Meier P, Melino S, Miao EA, Molkentin JD, Moll UM, Muñoz-Pinedo C, Nagata S, Nuñez G, Oberst A, Oren M, Overholtzer M, Pagano M, Panaretakis T, Pasparakis M, Penninger JM, Pereira DM, Pervaiz S, Peter ME, Piacentini M, Pinton P, Prehn JHM, Puthalakath H, Rabinovich GA, Rehm M, Rizzuto R, Rodrigues CMP, Rubinsztein DC, Rudel T, Ryan KM, Sayan E, Scorrano L, Shao F, Shi Y, Silke J, Simon HU, Sistigu A, Stockwell BR, Strasser A, Szabadkai G, Tait SWG, Tang D, Tavernarakis N, Thorburn A, Tsujimoto Y, Turk B, Vanden Berghe T, Vandenabeele P, Vander Heiden MG, Villunger A, Virgin HW, Vousden KH, Vucic D, Wagner EF, Walczak H, Wallach D, Wang Y, Wells JA, Wood W, Yuan J, Zakeri Z, Zhivotovsky B, Zitvogel L, Melino G, Kroemer G. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25:486-541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3672] [Cited by in RCA: 4477] [Article Influence: 639.6] [Reference Citation Analysis (0)] |
| 20. | Tang D, Kang R, Berghe TV, Vandenabeele P, Kroemer G. The molecular machinery of regulated cell death. Cell Res. 2019;29:347-364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1493] [Cited by in RCA: 1731] [Article Influence: 288.5] [Reference Citation Analysis (0)] |
| 21. | Koren E, Fuchs Y. Modes of Regulated Cell Death in Cancer. Cancer Discov. 2021;11:245-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 245] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 22. | Cabili MN, Trapnell C, Goff L, Koziol M, Tazon-Vega B, Regev A, Rinn JL. Integrative annotation of human large intergenic noncoding RNAs reveals global properties and specific subclasses. Genes Dev. 2011;25:1915-1927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2555] [Cited by in RCA: 2767] [Article Influence: 197.6] [Reference Citation Analysis (0)] |
| 23. | Derrien T, Johnson R, Bussotti G, Tanzer A, Djebali S, Tilgner H, Guernec G, Martin D, Merkel A, Knowles DG, Lagarde J, Veeravalli L, Ruan X, Ruan Y, Lassmann T, Carninci P, Brown JB, Lipovich L, Gonzalez JM, Thomas M, Davis CA, Shiekhattar R, Gingeras TR, Hubbard TJ, Notredame C, Harrow J, Guigó R. The GENCODE v7 catalog of human long noncoding RNAs: analysis of their gene structure, evolution, and expression. Genome Res. 2012;22:1775-1789. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3516] [Cited by in RCA: 3952] [Article Influence: 329.3] [Reference Citation Analysis (0)] |
| 24. | ENCODE Project Consortium. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489:57-74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14554] [Cited by in RCA: 12896] [Article Influence: 992.0] [Reference Citation Analysis (0)] |
| 25. | Ma L, Cao J, Liu L, Du Q, Li Z, Zou D, Bajic VB, Zhang Z. LncBook: a curated knowledgebase of human long non-coding RNAs. Nucleic Acids Res. 2019;47:D128-D134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 161] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 26. | Liu J, Ren L, Li S, Li W, Zheng X, Yang Y, Fu W, Yi J, Wang J, Du G. The biology, function, and applications of exosomes in cancer. Acta Pharm Sin B. 2021;11:2783-2797. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 351] [Cited by in RCA: 343] [Article Influence: 85.8] [Reference Citation Analysis (0)] |
| 27. | Zampetaki A, Albrecht A, Steinhofel K. Corrigendum: Long Non-coding RNA Structure and Function: Is There a Link? Front Physiol. 2019;10:1127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Mercer TR, Dinger ME, Sunkin SM, Mehler MF, Mattick JS. Specific expression of long noncoding RNAs in the mouse brain. Proc Natl Acad Sci U S A. 2008;105:716-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 880] [Cited by in RCA: 941] [Article Influence: 55.4] [Reference Citation Analysis (0)] |
| 29. | Yan X, Hu Z, Feng Y, Hu X, Yuan J, Zhao SD, Zhang Y, Yang L, Shan W, He Q, Fan L, Kandalaft LE, Tanyi JL, Li C, Yuan CX, Zhang D, Yuan H, Hua K, Lu Y, Katsaros D, Huang Q, Montone K, Fan Y, Coukos G, Boyd J, Sood AK, Rebbeck T, Mills GB, Dang CV, Zhang L. Comprehensive Genomic Characterization of Long Non-coding RNAs across Human Cancers. Cancer Cell. 2015;28:529-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 486] [Cited by in RCA: 531] [Article Influence: 53.1] [Reference Citation Analysis (0)] |
| 30. | Frankish A, Diekhans M, Jungreis I, Lagarde J, Loveland JE, Mudge JM, Sisu C, Wright JC, Armstrong J, Barnes I, Berry A, Bignell A, Boix C, Carbonell Sala S, Cunningham F, Di Domenico T, Donaldson S, Fiddes IT, García Girón C, Gonzalez JM, Grego T, Hardy M, Hourlier T, Howe KL, Hunt T, Izuogu OG, Johnson R, Martin FJ, Martínez L, Mohanan S, Muir P, Navarro FCP, Parker A, Pei B, Pozo F, Riera FC, Ruffier M, Schmitt BM, Stapleton E, Suner MM, Sycheva I, Uszczynska-Ratajczak B, Wolf MY, Xu J, Yang YT, Yates A, Zerbino D, Zhang Y, Choudhary JS, Gerstein M, Guigó R, Hubbard TJP, Kellis M, Paten B, Tress ML, Flicek P. GENCODE 2021. Nucleic Acids Res. 2021;49:D916-D923. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 826] [Article Influence: 206.5] [Reference Citation Analysis (0)] |
| 31. | Hussen BM, Azimi T, Abak A, Hidayat HJ, Taheri M, Ghafouri-Fard S. Role of lncRNA BANCR in Human Cancers: An Updated Review. Front Cell Dev Biol. 2021;9:689992. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 32. | Ma B, Wang S, Wu W, Shan P, Chen Y, Meng J, Xing L, Yun J, Hao L, Wang X, Li S, Guo Y. Mechanisms of circRNA/lncRNA-miRNA interactions and applications in disease and drug research. Biomed Pharmacother. 2023;162:114672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 101] [Reference Citation Analysis (0)] |
| 33. | Li S, Zhang S, Huang M, Hu H, Xie Y. U1RNP/lncRNA/Transcription Cycle Axis Promotes Tumorigenesis of Hepatocellular Carcinoma. Diagnostics (Basel). 2022;12:1133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 34. | Yao Q, Zhang X, Chen D. The emerging potentials of lncRNA DRAIC in human cancers. Front Oncol. 2022;12:867670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 35. | Fan N, Fu H, Feng X, Chen Y, Wang J, Wu Y, Bian Y, Li Y. Long non-coding RNAs play an important regulatory role in tumorigenesis and tumor progression through aerobic glycolysis. Front Mol Biosci. 2022;9:941653. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 36. | Liang W, Zhao Y, Meng Q, Jiang W, Deng S, Xue J. The role of long non-coding RNA in hepatocellular carcinoma. Aging (Albany NY). 2024;16:4052-4073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 37. | Pinkney HR, Wright BM, Diermeier SD. The lncRNA Toolkit: Databases and In Silico Tools for lncRNA Analysis. Noncoding RNA. 2020;6:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 38. | Mas-Ponte D, Carlevaro-Fita J, Palumbo E, Hermoso Pulido T, Guigo R, Johnson R. LncATLAS database for subcellular localization of long noncoding RNAs. RNA. 2017;23:1080-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 239] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 39. | Das T, Deb A, Parida S, Mondal S, Khatua S, Ghosh Z. LncRBase V.2: an updated resource for multispecies lncRNAs and ClinicLSNP hosting genetic variants in lncRNAs for cancer patients. RNA Biol. 2021;18:1136-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 40. | Montero JJ, Trozzo R, Sugden M, Öllinger R, Belka A, Zhigalova E, Waetzig P, Engleitner T, Schmidt-Supprian M, Saur D, Rad R. Genome-scale pan-cancer interrogation of lncRNA dependencies using CasRx. Nat Methods. 2024;21:584-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 41. | Xing C, Sun SG, Yue ZQ, Bai F. Role of lncRNA LUCAT1 in cancer. Biomed Pharmacother. 2021;134:111158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 215] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 42. | Kazemzadeh M, Safaralizadeh R, Orang AV. LncRNAs: emerging players in gene regulation and disease pathogenesis. J Genet. 2015;94:771-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 82] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 43. | Xiong XD, Ren X, Cai MY, Yang JW, Liu X, Yang JM. Long non-coding RNAs: An emerging powerhouse in the battle between life and death of tumor cells. Drug Resist Updat. 2016;26:28-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 44. | Shahzad U, Krumholtz S, Rutka JT, Das S. Noncoding RNAs in Glioblastoma: Emerging Biological Concepts and Potential Therapeutic Implications. Cancers (Basel). 2021;13:1555. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 45. | Fang Y, Fullwood MJ. Roles, Functions, and Mechanisms of Long Non-coding RNAs in Cancer. Genomics Proteomics Bioinformatics. 2016;14:42-54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 551] [Cited by in RCA: 777] [Article Influence: 86.3] [Reference Citation Analysis (0)] |
| 46. | Weinberg MS, Morris KV. Long non-coding RNA targeting and transcriptional de-repression. Nucleic Acid Ther. 2013;23:9-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 47. | Noviello TMR, Di Liddo A, Ventola GM, Spagnuolo A, D'Aniello S, Ceccarelli M, Cerulo L. Detection of long non-coding RNA homology, a comparative study on alignment and alignment-free metrics. BMC Bioinformatics. 2018;19:407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 48. | Peng L, Li EM, Xu LY. From start to end: Phase separation and transcriptional regulation. Biochim Biophys Acta Gene Regul Mech. 2020;1863:194641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 49. | Squillaro T, Peluso G, Galderisi U, Di Bernardo G. Long non-coding RNAs in regulation of adipogenesis and adipose tissue function. Elife. 2020;9:eLife.59053. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 50. | He Z, Yang D, Fan X, Zhang M, Li Y, Gu X, Yang M. The Roles and Mechanisms of lncRNAs in Liver Fibrosis. Int J Mol Sci. 2020;21:1482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 74] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 51. | Yan C, Chen J, Chen N. Long noncoding RNA MALAT1 promotes hepatic steatosis and insulin resistance by increasing nuclear SREBP-1c protein stability. Sci Rep. 2016;6:22640. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 151] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 52. | Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, Cuny GD, Mitchison TJ, Moskowitz MA, Yuan J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1:112-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1858] [Cited by in RCA: 2390] [Article Influence: 119.5] [Reference Citation Analysis (0)] |
| 53. | Seehawer M, Heinzmann F, D'Artista L, Harbig J, Roux PF, Hoenicke L, Dang H, Klotz S, Robinson L, Doré G, Rozenblum N, Kang TW, Chawla R, Buch T, Vucur M, Roth M, Zuber J, Luedde T, Sipos B, Longerich T, Heikenwälder M, Wang XW, Bischof O, Zender L. Necroptosis microenvironment directs lineage commitment in liver cancer. Nature. 2018;562:69-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 304] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 54. | Peng Y, Wu G, Qiu X, Luo Y, Zou Y, Wei X, Li A. Construction and validation of a necroptosis-related lncRNAs prognosis signature of hepatocellular carcinoma. Front Genet. 2022;13:916024. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 55. | Xia X, Zhang H, Xia P, Zhu Y, Liu J, Xu K, Yuan Y. Identification of Glycolysis-Related lncRNAs and the Novel lncRNA WAC-AS1 Promotes Glycolysis and Tumor Progression in Hepatocellular Carcinoma. Front Oncol. 2021;11:733595. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 56. | Chen M, Wu GB, Hua S, Zhao ZF, Li HJ, Luo M. Identification and validation of a prognostic model of necroptosis-related lncRNAs in hepatocellular carcinoma. Front Genet. 2022;13:907859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 57. | Wang W, Ye Y, Zhang X, Ye X, Liu C, Bao L. Construction of a Necroptosis-Associated Long Non-Coding RNA Signature to Predict Prognosis and Immune Response in Hepatocellular Carcinoma. Front Mol Biosci. 2022;9:937979. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 58. | Chen H, Hou G, Lan T, Xue S, Xu L, Feng Q, Zeng Y, Wang H. Identification and validation of a five-necroptosis-related lncRNAs signature for prognostic prediction in hepatocellular carcinoma. Heliyon. 2024;10:e37403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 59. | Morana O, Wood W, Gregory CD. The Apoptosis Paradox in Cancer. Int J Mol Sci. 2022;23:1328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 227] [Article Influence: 75.7] [Reference Citation Analysis (0)] |
| 60. | Kashyap D, Garg VK, Goel N. Intrinsic and extrinsic pathways of apoptosis: Role in cancer development and prognosis. Adv Protein Chem Struct Biol. 2021;125:73-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 188] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 61. | Sankari SL, Masthan KM, Babu NA, Bhattacharjee T, Elumalai M. Apoptosis in cancer--an update. Asian Pac J Cancer Prev. 2012;13:4873-4878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 62. | Xu X, Lai Y, Hua ZC. Apoptosis and apoptotic body: disease message and therapeutic target potentials. Biosci Rep. 2019;39:BSR20180992. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 223] [Cited by in RCA: 595] [Article Influence: 99.2] [Reference Citation Analysis (0)] |
| 63. | Mello SS, Sinow C, Raj N, Mazur PK, Bieging-Rolett K, Broz DK, Imam JFC, Vogel H, Wood LD, Sage J, Hirose T, Nakagawa S, Rinn J, Attardi LD. Neat1 is a p53-inducible lincRNA essential for transformation suppression. Genes Dev. 2017;31:1095-1108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 173] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 64. | Yu X, Li Z, Zheng H, Chan MT, Wu WK. NEAT1: A novel cancer-related long non-coding RNA. Cell Prolif. 2017;50:e12329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 216] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 65. | Klec C, Prinz F, Pichler M. Involvement of the long noncoding RNA NEAT1 in carcinogenesis. Mol Oncol. 2019;13:46-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 132] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 66. | Fang L, Sun J, Pan Z, Song Y, Zhong L, Zhang Y, Liu Y, Zheng X, Huang P. Long non-coding RNA NEAT1 promotes hepatocellular carcinoma cell proliferation through the regulation of miR-129-5p-VCP-IκB. Am J Physiol Gastrointest Liver Physiol. 2017;313:G150-G156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 82] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 67. | Xiong W, Huang C, Deng H, Jian C, Zen C, Ye K, Zhong Z, Zhao X, Zhu L. Oncogenic non-coding RNA NEAT1 promotes the prostate cancer cell growth through the SRC3/IGF1R/AKT pathway. Int J Biochem Cell Biol. 2018;94:125-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 68. | Luo M, Zhang L, Yang H, Luo K, Qing C. Long noncoding RNA NEAT1 promotes ovarian cancer cell invasion and migration by interacting with miR1321 and regulating tight junction protein 3 expression. Mol Med Rep. 2020;22:3429-3439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 69. | An H, Williams NG, Shelkovnikova TA. NEAT1 and paraspeckles in neurodegenerative diseases: A missing lnc found? Noncoding RNA Res. 2018;3:243-252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 90] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 70. | Hussain MS, Gupta G, Afzal M, Alqahtani SM, Samuel VP, Hassan Almalki W, Kazmi I, Alzarea SI, Saleem S, Dureja H, Singh SK, Dua K, Thangavelu L. Exploring the role of lncrna neat1 knockdown in regulating apoptosis across multiple cancer types: A review. Pathol Res Pract. 2023;252:154908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 71. | Yao J, Fu J, Liu Y, Qu W, Wang G, Yan Z. LncRNA CASC9 promotes proliferation, migration and inhibits apoptosis of hepatocellular carcinoma cells by down-regulating miR-424-5p. Ann Hepatol. 2021;23:100297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 72. | Cai H, Zheng Y, Wen Z, Yang Y, Yang S, Zhang Q. LncRNA AIRN influences the proliferation and apoptosis of hepatocellular carcinoma cells by regulating STAT1 ubiquitination. Arch Pharm Res. 2021;44:414-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 73. | Fei Q, Song F, Jiang X, Hong H, Xu X, Jin Z, Zhu X, Dai B, Yang J, Sui C, Xu M. LncRNA ST8SIA6-AS1 promotes hepatocellular carcinoma cell proliferation and resistance to apoptosis by targeting miR-4656/HDAC11 axis. Cancer Cell Int. 2020;20:232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 74. | Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, Morrison B 3rd, Stockwell BR. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4711] [Cited by in RCA: 11544] [Article Influence: 888.0] [Reference Citation Analysis (1)] |
| 75. | Zhou Q, Meng Y, Li D, Yao L, Le J, Liu Y, Sun Y, Zeng F, Chen X, Deng G. Ferroptosis in cancer: From molecular mechanisms to therapeutic strategies. Signal Transduct Target Ther. 2024;9:55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 230] [Cited by in RCA: 185] [Article Influence: 185.0] [Reference Citation Analysis (0)] |
| 76. | Dixon SJ, Olzmann JA. The cell biology of ferroptosis. Nat Rev Mol Cell Biol. 2024;25:424-442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 391] [Cited by in RCA: 314] [Article Influence: 314.0] [Reference Citation Analysis (0)] |
| 77. | Koskenkorva-Frank TS, Weiss G, Koppenol WH, Burckhardt S. The complex interplay of iron metabolism, reactive oxygen species, and reactive nitrogen species: insights into the potential of various iron therapies to induce oxidative and nitrosative stress. Free Radic Biol Med. 2013;65:1174-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 313] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 78. | Jiang X, Stockwell BR, Conrad M. Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol. 2021;22:266-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2184] [Cited by in RCA: 4097] [Article Influence: 1024.3] [Reference Citation Analysis (0)] |
| 79. | Rodriguez R, Schreiber SL, Conrad M. Persister cancer cells: Iron addiction and vulnerability to ferroptosis. Mol Cell. 2022;82:728-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 154] [Article Influence: 51.3] [Reference Citation Analysis (0)] |
| 80. | Wang W, Green M, Choi JE, Gijón M, Kennedy PD, Johnson JK, Liao P, Lang X, Kryczek I, Sell A, Xia H, Zhou J, Li G, Li J, Li W, Wei S, Vatan L, Zhang H, Szeliga W, Gu W, Liu R, Lawrence TS, Lamb C, Tanno Y, Cieslik M, Stone E, Georgiou G, Chan TA, Chinnaiyan A, Zou W. CD8(+) T cells regulate tumour ferroptosis during cancer immunotherapy. Nature. 2019;569:270-274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 769] [Cited by in RCA: 1891] [Article Influence: 315.2] [Reference Citation Analysis (0)] |
| 81. | Tian S, Cao Y, Wang J, Bi Y, Zhong J, Meng X, Sun W, Yang R, Gan L, Wang X, Li H, Wang R. The miR-378c-Samd1 circuit promotes phenotypic modulation of vascular smooth muscle cells and foam cells formation in atherosclerosis lesions. Sci Rep. 2021;11:10548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 82. | Li X, Li Y, Lian P, Lv Q, Liu F. Silencing lncRNA HCG18 regulates GPX4-inhibited ferroptosis by adsorbing miR-450b-5p to avert sorafenib resistance in hepatocellular carcinoma. Hum Exp Toxicol. 2023;42:9603271221142818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 37] [Reference Citation Analysis (0)] |
| 83. | Xu Z, Peng B, Liang Q, Chen X, Cai Y, Zeng S, Gao K, Wang X, Yi Q, Gong Z, Yan Y. Construction of a Ferroptosis-Related Nine-lncRNA Signature for Predicting Prognosis and Immune Response in Hepatocellular Carcinoma. Front Immunol. 2021;12:719175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 130] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 84. | Zhang B, Bao W, Zhang S, Chen B, Zhou X, Zhao J, Shi Z, Zhang T, Chen Z, Wang L, Zheng X, Chen G, Wang Y. LncRNA HEPFAL accelerates ferroptosis in hepatocellular carcinoma by regulating SLC7A11 ubiquitination. Cell Death Dis. 2022;13:734. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 101] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 85. | Humphries F, Shmuel-Galia L, Ketelut-Carneiro N, Li S, Wang B, Nemmara VV, Wilson R, Jiang Z, Khalighinejad F, Muneeruddin K, Shaffer SA, Dutta R, Ionete C, Pesiridis S, Yang S, Thompson PR, Fitzgerald KA. Succination inactivates gasdermin D and blocks pyroptosis. Science. 2020;369:1633-1637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 448] [Article Influence: 89.6] [Reference Citation Analysis (0)] |
| 86. | Zhang Z, Zhang Y, Xia S, Kong Q, Li S, Liu X, Junqueira C, Meza-Sosa KF, Mok TMY, Ansara J, Sengupta S, Yao Y, Wu H, Lieberman J. Gasdermin E suppresses tumour growth by activating anti-tumour immunity. Nature. 2020;579:415-420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 430] [Cited by in RCA: 1122] [Article Influence: 224.4] [Reference Citation Analysis (0)] |
| 87. | Wang Q, Wang Y, Ding J, Wang C, Zhou X, Gao W, Huang H, Shao F, Liu Z. A bioorthogonal system reveals antitumour immune function of pyroptosis. Nature. 2020;579:421-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 678] [Article Influence: 135.6] [Reference Citation Analysis (0)] |
| 88. | Xia X, Wang X, Cheng Z, Qin W, Lei L, Jiang J, Hu J. The role of pyroptosis in cancer: pro-cancer or pro-"host"? Cell Death Dis. 2019;10:650. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 306] [Cited by in RCA: 606] [Article Influence: 101.0] [Reference Citation Analysis (0)] |
| 89. | Karki R, Kanneganti TD. Diverging inflammasome signals in tumorigenesis and potential targeting. Nat Rev Cancer. 2019;19:197-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 468] [Article Influence: 78.0] [Reference Citation Analysis (0)] |
| 90. | Fang Y, Tian S, Pan Y, Li W, Wang Q, Tang Y, Yu T, Wu X, Shi Y, Ma P, Shu Y. Pyroptosis: A new frontier in cancer. Biomed Pharmacother. 2020;121:109595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 697] [Article Influence: 116.2] [Reference Citation Analysis (0)] |
| 91. | Jia Y, Wang X, Deng Y, Li S, Xu X, Qin Y, Peng L. Pyroptosis Provides New Strategies for the Treatment of Cancer. J Cancer. 2023;14:140-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 37] [Reference Citation Analysis (0)] |
| 92. | Vasudevan SO, Behl B, Rathinam VA. Pyroptosis-induced inflammation and tissue damage. Semin Immunol. 2023;69:101781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 126] [Article Influence: 63.0] [Reference Citation Analysis (0)] |
| 93. | Wu T, Li N, Luo F, Chen Z, Ma L, Hu T, Hong G, Li H. Screening prognostic markers for hepatocellular carcinoma based on pyroptosis-related lncRNA pairs. BMC Bioinformatics. 2023;24:176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 94. | Wang T, Yang Y, Sun T, Qiu H, Wang J, Ding C, Lan R, He Q, Wang W. The Pyroptosis-Related Long Noncoding RNA Signature Predicts Prognosis and Indicates Immunotherapeutic Efficiency in Hepatocellular Carcinoma. Front Cell Dev Biol. 2022;10:779269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 95. | Debnath J, Gammoh N, Ryan KM. Autophagy and autophagy-related pathways in cancer. Nat Rev Mol Cell Biol. 2023;24:560-575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 655] [Article Influence: 327.5] [Reference Citation Analysis (0)] |
| 96. | Qian H, Chao X, Williams J, Fulte S, Li T, Yang L, Ding WX. Autophagy in liver diseases: A review. Mol Aspects Med. 2021;82:100973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 218] [Article Influence: 54.5] [Reference Citation Analysis (0)] |
| 97. | Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011;147:728-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3678] [Cited by in RCA: 4842] [Article Influence: 345.9] [Reference Citation Analysis (0)] |
| 98. | Ke PY. Diverse Functions of Autophagy in Liver Physiology and Liver Diseases. Int J Mol Sci. 2019;20:300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 80] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 99. | Yazdani HO, Huang H, Tsung A. Autophagy: Dual Response in the Development of Hepatocellular Carcinoma. Cells. 2019;8:91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 70] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 100. | Zhou Y, Zhang X, Klibanski A. MEG3 noncoding RNA: a tumor suppressor. J Mol Endocrinol. 2012;48:R45-R53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 524] [Cited by in RCA: 605] [Article Influence: 46.5] [Reference Citation Analysis (0)] |
| 101. | Braconi C, Kogure T, Valeri N, Huang N, Nuovo G, Costinean S, Negrini M, Miotto E, Croce CM, Patel T. microRNA-29 can regulate expression of the long non-coding RNA gene MEG3 in hepatocellular cancer. Oncogene. 2011;30:4750-4756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 487] [Cited by in RCA: 545] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 102. | Yu S, Hou D, Chen P, Zhang Q, Lv B, Ma Y, Liu F, Liu H, Song EJ, Yang D, Liu J. Adenosine induces apoptosis through TNFR1/RIPK1/P38 axis in colon cancer cells. Biochem Biophys Res Commun. 2015;460:759-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 103. | Pu Z, Wu L, Guo Y, Li G, Xiang M, Liu L, Zhan H, Zhou X, Tan H. LncRNA MEG3 contributes to adenosine-induced cytotoxicity in hepatoma HepG2 cells by downregulated ILF3 and autophagy inhibition via regulation PI3K-AKT-mTOR and beclin-1 signaling pathway. J Cell Biochem. 2019;120:18172-18185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 104. | Gu D, Tong M, Wang J, Zhang B, Liu J, Song G, Zhu B. Overexpression of the lncRNA HOTAIRM1 promotes lenvatinib resistance by downregulating miR-34a and activating autophagy in hepatocellular carcinoma. Discov Oncol. 2023;14:66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 105. | Rajendran P, Renu K, Ali EM, Genena MAM, Veeraraghavan V, Sekar R, Sekar AK, Tejavat S, Barik P, Abdallah BM. Promising and challenging phytochemicals targeting LC3 mediated autophagy signaling in cancer therapy. Immun Inflamm Dis. 2024;12:e70041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 106. | Liu S, Huttad L, He G, He W, Liu C, Cai D, Chen H, Qiu J. Long noncoding RNA HULC regulates the NF-κB pathway and represents a promising prognostic biomarker in liver cancer. Cancer Med. 2023;12:5124-5136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 107. | Li D, Jin S, Chen P, Zhang Y, Li Y, Zhong C, Fan X, Lin H. Comprehensive analysis of cuproptosis-related lncRNAs for prognostic significance and immune microenvironment characterization in hepatocellular carcinoma. Front Immunol. 2022;13:991604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 108. | Li Y, Song K, Zheng W. The Cuproptosis-Related Long Noncoding RNA Signature Predicts Prognosis and Immune Cell Infiltration in Hepatocellular Carcinoma. J Oncol. 2023;2023:9557690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 109. | Carneiro BA, El-Deiry WS. Targeting apoptosis in cancer therapy. Nat Rev Clin Oncol. 2020;17:395-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 911] [Cited by in RCA: 1599] [Article Influence: 319.8] [Reference Citation Analysis (0)] |
| 110. | Bian M, Fan R, Yang Z, Chen Y, Xu Z, Lu Y, Liu W. Pt(II)-NHC Complex Induces ROS-ERS-Related DAMP Balance to Harness Immunogenic Cell Death in Hepatocellular Carcinoma. J Med Chem. 2022;65:1848-1866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 50] [Article Influence: 16.7] [Reference Citation Analysis (0)] |