Published online Apr 24, 2024. doi: 10.5306/wjco.v15.i4.482
Peer-review started: November 22, 2023
First decision: January 12, 2024
Revised: January 22, 2024
Accepted: February 28, 2024
Article in press: February 28, 2024
Published online: April 24, 2024
Processing time: 147 Days and 18.4 Hours
This comprehensive review delves into the current updates and challenges associated with the management of low-grade gliomas (LGG), the predominant primary tumors in the central nervous system. With a general incidence rate of 5.81 per 100000, gliomas pose a significant global concern, necessitating advance
Core Tip: Our manuscript explores the dynamic landscape of glioma treatment, emphasizing the urgent need for innovative therapies to combat this prevalent central nervous system malignancy. We delve into the promising realm of immunotherapies, highlighting novel agents like zotiraciclib, pembrolizumab, and lerapolturev, offering insights into their mecha
- Citation: Lucke-Wold B, Rangwala BS, Shafique MA, Siddiq MA, Mustafa MS, Danish F, Nasrullah RMU, Zainab N, Haseeb A. Focus on current and emerging treatment options for glioma: A comprehensive review. World J Clin Oncol 2024; 15(4): 482-495
- URL: https://www.wjgnet.com/2218-4333/full/v15/i4/482.htm
- DOI: https://dx.doi.org/10.5306/wjco.v15.i4.482
Gliomas represent the most prevalent primary tumors in the central nervous system (CNS) across various age groups[1,2]. Gliomas have a general incidence rate of 5.81 per 100000 people, with older individuals having a threefold higher frequency than young children. Gliomas account for 29%-35% of all central nervous system tumors in the adolescent and young adult demographic (ages 15-39 years), with an incidence of 3.41 per 100000[3-5]. Gliomas continue to be a global concern, emphasizing the vital need to improve treatment techniques for lowering both mortality and morbidity, elevating it to the top of the neuro-oncology priority list[6,7].
Clinical care, therapeutic response, and outcomes differ significantly between pediatric and adult glioma patients. Children with high-grade gliomas (HGGs) have poor prognosis, with frequently limited long-term survival ranging from months to a few years after diagnosis[8,9]. In contrast, pediatric patients with low-grade gliomas (LGG) have good overall survival (OS)[10,11], despite significant tumor- and treatment-related morbidity[12] (Table 1). The increased likelihood of malignant transformation, which is extremely rare in children) adds to a less favorable prognosis in adults with low-grade gliomas[13,14].
| Grade | Name | Description and characteristics |
| I | Pilocytic astrocytoma | Well-differentiated, often cystic, slow-growing, generally benign |
| II | Diffuse astrocytoma | Infiltrative, moderately cellular, tends to recur, can progress to higher grades |
| II | Oligodendroglioma | Composed of oligodendrocyte-like cells, often associated with 1p/19q co-deletion |
| II | Mixed oligoastrocytoma | Combination of features of oligodendroglioma and diffuse astrocytoma |
| III | Anaplastic astrocytoma | Higher grade astrocytoma with increased cellularity and mitotic activity |
| III | Anaplastic oligodendroglioma | Higher grade oligodendroglioma with increased cellularity and atypia |
| III | Anaplastic oligoastrocytoma | Higher grade mixed tumor with features of both anaplastic astrocytoma and anaplastic oligodendroglioma |
| IV | Glioblastoma | Highly aggressive, necrosis, endothelial proliferation, molecular heterogeneity |
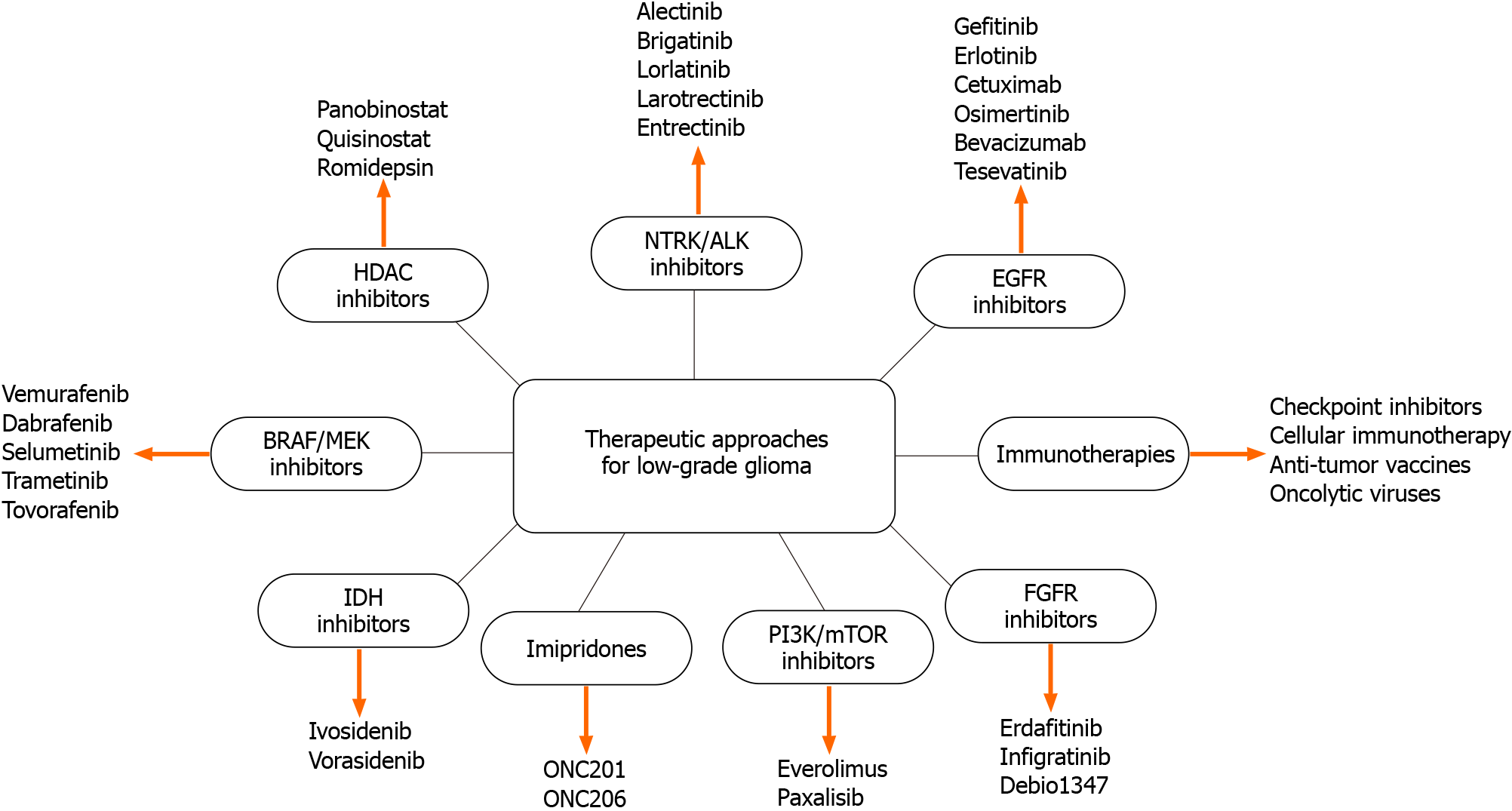
Despite advancements in surgery, radiotherapy, and chemotherapy for LGG, the disease remains incurable and often progresses to secondary malignant transformation. Immunotherapeutic strategies have demonstrated success in various cancers, including lung, skin, colon, and blood-related cancers (Figure 1). Given that low-grade gliomas, particularly in younger patients, exhibit slower growth compared to high-grade gliomas, there is a suggestion that immunotherapies may be more effective due to the healthier immune systems of younger individuals, potentially leading to better treatment responses. Immunotherapies, including Zotiraciclib and Lerapolturev, exert their effects through distinct mechanism (Table 2).
| Ref. | Completion year | Demographics | Study phase | Identifier | Experimental drug | Sample size | Primary endpoint/outcomes | Results for primary outcome |
| BRAF/MEK inhibitors | ||||||||
| Nicolaides et al[107], 2020 | 2023 | Pediatrics | Phase 2 | NCT01748149 (Ongoing Trial) | Vemurafenib | 40 | Safety and pharmacokinetics | Not yet reported |
| Hargrave et al[108], 2019 | 2020 | Pediatrics | Phase 1/2a | NCT01677741 | Dabrafenib | 32 | Objective response rates and safety | Objective response rate was 44% and 91% experienced adverse effects |
| Kaley et al[109], 2018 | 2016 | Adults | Phase 2 | NCT01524978 | Vemurafenib | 24 | Confirmed objective response rate, PFS, OS and safety | Confirmed objective response rate was 25% and median PFS was 5.5 months |
| FGFR inhibitors | ||||||||
| Lassman et al[110], 2022 | 2018 | Adults | Phase 2 | NCT01975701 | Infigratinib | 26 | 6-month PFS | 6-month PFS rate was 16.0% |
| Bahleda et al[111], 2019 | 2017 | Adults | Phase 1 | NCT01703481 | Erdafitinib | 187 | Safety | Most common treatment-related adverse events were hyperphosphatemia, dry mouth, and asthenia, generally grade 1/2 severity |
| HDAC inhibitors | ||||||||
| Wood et al[112], 2018 | 2018 | Pediatrics | Phase 1 | ACTRN12609000978268 | Panobinostat | 9 | Safety and pharmacokinetics | 2 patients experienced Grade 3-4 thrombocytopenia, 1 experienced Grade 3 anemia, and 2 experienced Grade 3 neutropenia |
| Imipridone | ||||||||
| Arrillaga-Romany et al[113], 2020 | 2023 | Phase 2 | NCT02525692 (Ongoing Trial) | ONC201 | 89 | 6-month PFS | Not yet reported | |
| PI3K/mTOR inhibitors | ||||||||
| Wen et al[114], 2022 | 2023 | Adults | Phase 2 | NCT03522298 | Paxalisib | 32 | Safety and pharmacokinetics | Well-tolerated with adverse events consistent with other PI3K inhibitors |
| Wen et al[115], 2020 | 2020 | Adults | Phase 1 | NCT01547546 | GDC-0084 | 47 | Safety and pharmacokinetics | Well-tolerated with adverse events consistent with other PI3K inhibitors |
| Franz et al[116], 2015 | 2014 | Adults/Pediatrics | Phase 1/2 | NCT00411619 | Enviroximes | 28 | 6-month change in the volume of sub ependymal giant-cell astrocytoma | Statistically significant reduction in the volume of the primary sub ependymal giant-cell astrocytoma at 6 months |
| NTRK/ALK inhibitors | ||||||||
| NCT02637687[117] | 2026 | Pediatrics | Phase 1/2 | NCT02637687 (Ongoing Trial) | Larotrectinib | 155 | Objective response rates | Not yet reported |
| NCT02576431[118] | 2025 | Adults/Pediatrics | Phase 2 | NCT02576431 (Ongoing Trial) | Larotrectinib | 204 | Objective response rates, PFS, OS, Safety | Not yet reported |
| Desai et al[119], 2022 | 2025 | Adults/Pediatrics | Phase 1/2 | NCT02650401 (Ongoing Trial) | Entrectinib | 69 | Maximum Tolerated Dose and Objective response rates | Not yet reported |
| IDH inhibitors | ||||||||
| NCT05588141[120] | 2029 | Adults | Phase 1/2 | NCT05588141 (Ongoing Trial) | Zotiraciclib | 96 | 12-months PFS | Not yet reported |
| Mellinghoff et al[121], 2023 | 2027 | Adults | Phase 3 | NCT04164901 | Vorasidenib | 340 | PFS | Significantly improved PFS |
| Mellinghoff et al[122], 2019 | 2024 | Adults | Phase 1 | NCT03343197 | AG-120, AG881 | 49 | 2-hydroxyglutarate concentrationin resectedtumors | decreased tumorcell proliferationand immune cellactivation |
| EGFR inhibitors | ||||||||
| Weller et al[123], 2017 | 2016 | Adults | Phase 3 | NCT01480479 | Rindopepimut/Temozolomide | 745 | OS | Median OS was 20.1 months in the Rindopepimut group versus 20.0 months in the control group |
| Lassman et al[124], 2023 | 2022 | Adults | Phase 3 | NCT02573324 | Depatuxizumab mafodotin | 691 | OS | No OS benefit for depatux-m in treating EGFR-amp newly diagnosed GBM |
Zotiraciclib, a potent CDK9 inhibitor, exhibits efficacy against glioblastoma by suppressing transcription and disrupting cellular energy production. Preclinical studies, both in vitro and in vivo, have revealed its synergistic effect with temozolomide. In clinical trials, Zotiraciclib demonstrated the ability to cross the blood-brain barrier and suppress CDK9 activity in tumor tissues[15]. This promising mechanism, targeting multiple glioblastoma survival pathways, positions Zotiraciclib as a potential therapeutic breakthrough[16-19].
A two-stage, two-arm, randomized phase 1 clinical trial further investigated the potential of zotiraciclib in recurrent high-grade gliomas. This study included a comprehensive evaluation of pharmacokinetics, patient-reported outcomes, and a detailed examination of rapid-onset neutropenia. Despite this observed neutropenia, a thorough analysis concluded that it did not compromise patient safety, allowing the research and development of this novel CDK9 inhibitor to progress[19].
Immunotherapy has garnered significant interest as a potential treatment for glioblastoma (GBM). Nevertheless, a recent clinical study focusing on recurrent glioblastoma and employing PD-1 immune checkpoint inhibitors revealed that a minority of patients (8%) exhibited noticeable improvements in their condition[20]. The mechanistic underpinnings of the variability in response patterns remain unclear.
Enhanced T cell infiltration in the tumor microenvironment and elevated mutational burdens in various cancer types have been associated with improved responses to anti-PD-1 therapy[21-23]. However, GBM presents a more immunosuppressive tumor microenvironment and a lower burden of somatic mutations than melanomas or non-small cell lung cancer[24]. Immunosuppression in GBM is facilitated by the expression of PD-1 ligands (PD-L1/2) in tumor cells, leading to T cell exhaustion and apoptosis. The binding of PD-1 to the surface of cytotoxic T cells hampers their ability to mount an effective anti-tumor response. PD-1 inhibitor therapy disrupts this immune checkpoint, reinforcing the immune response against tumors[23].
PD-1 inhibitors, such as pembrolizumab and nivolumab, have gained attention for glioblastoma treatment. However, recent clinical studies have revealed variable responses, necessitating deeper understanding of the underlying mechanisms. Glioblastoma’s immunosuppressive microenvironment and lower mutation burden compared to other cancers pose challenges. Molecular-tailored strategies hold promise for optimizing patient selection for immunotherapy, although further testing is required to validate their efficacy[25].
Lerapolturev, a viral immunotherapy, operates via a unique mechanism. As a polio-rhinovirus chimera, it induces persistent type-I interferon-dominant inflammation in glioblastoma, leading to polyfunctional antitumor CD8+ T-cell responses. Clinical trials involving Lerapolturev for recurrent adult glioblastoma demonstrated a 16% survival rate of at least 36 months, with a manageable safety profile[26-29].
In pediatric high-grade gliomas, Lerapolturev showed promise, with no grade 3 or 4 toxicity observed in early trials. The safety of treatment at this dose allows for further trials, including patients as young as 9 years of age. Ongoing research is crucial to understand the immunological factors influencing the efficacy of Lerapolturev in pediatric versus adult high-grade gliomas[30]. Our group’s previous research in adults gained additional support from the inclusion of patients as young as 9 years old, including one individual with WHO grade 3 glioma[30]. Moreover, pediatric high-grade gliomas typically exhibit significantly different molecular profiles compared to adult high-grade gliomas[31]. However, whether immunological factors affecting viral immunotherapies, such as Lerapolturev, vary between pediatric and adult high-grade gliomas remains uncertain[30].
Depatuxizumab (formerly ABT-806) is a humanized monoclonal antibody developed against epidermal growth factor receptor variant III (EGFRvIII) that also binds to wild-type EGFR at elevated levels[32,33]. The antibody-drug conjugate (ADC) Depatuxizumab mafodotin (formerly ABT-414) connects the depatux to the cytotoxic payload monomethyl auristatin F (MMAF or mafodotin). Upon binding to activated EGFR, ADC is internalized, degraded in acidic compart
Despite promising preclinical and early clinical data, depatux-m has proven ineffective in treating GBM. This disappointing outcome may result from the emergence of resistant clones over time, negating any overall survival benefit[38]. Limited penetration of depatux-m into large tumors and challenges in reaching intracranial tumors[39], especially in the non-enhancing tumor region, underscore crucial lessons for future studies involving large molecules[38]. Safety concerns with depatux-m were reversible, with adverse events, such as sensitivity to light and thrombocytopenia, being the most frequently observed.
Entrectinib, approved by both the United States Food and Drug Administration and European Medicines Agency for tumors containing TRK or ROS1 fusions[40], encounters a challenge in treating brain neoplasms due to the blood-brain barrier (BBB)[35]. Effective targeted therapies for leptomeningeal disseminated tumors depend on their ability to penetrate this barrier. Although entrectinib, designed to cross the BBB, has demonstrated promise with a 79% objective response rate in various solid tumors, including CNS tumors, information on its cerebrospinal fluid penetrance in brain tumor patients is currently lacking[41].
The potential therapeutic efficacy of entrectinib, a selective pan TRK inhibitor, has been explored in patients with leptomeningeal disseminated pediatric high-grade gliomas (pHGG) harboring NTRK or ROS1 fusions[42,43]. The STARTRK-NG trial reported positive radiographic responses in four pHGG patients treated with entrectinib, indicating promise for CNS tumors[44,45]. This study investigated the in vitro sensitivity of pHGG cell models to entrectinib and suggested potential combination therapies[46]. The need for further studies to understand resistance mechanisms is emphasized, along with the generally well-tolerated nature of entrectinib. The observed CNS penetrance of entrectinib in a gliosarcoma patient has been discussed, highlighting its ability to cross the blood-brain barrier[47]. The text also considers the combination of entrectinib with radiotherapy and suggests the importance of intrathecal therapy in cases of leptomeningeal dissemination[41]. This conclusion underscores the need for comprehensive investigations and prospective clinical studies to establish the role of entrectinib and potential combination therapies in pHGG with ROS1/NTRK fusions.
ONC201, an oral small-molecule imipridone anticancer therapy, has demonstrated early clinical success in patients with diffuse intrinsic pontine glioma (DIPG)[48] and recurrent H3K27M-mutant diffuse midline gliomas[49]. Investigations across various cancer types have shown that ONC201-induced apoptosis in cancer cells, independent of p53, occurs through an atypical integrated stress response involving the expression of the antitumor protein TRAIL. This mechanism has shown promise in hematological[50], colorectal[51], breast[52], uterine cancers[53], and glioblastoma[54]. A sustained positive response was observed in a patient with secondary glioblastoma carrying an H3.3K27M mutation, prompting further exploration in patients with similar mutations, including those with DIPG[54].
Studies have discussed the therapeutic benefits of combining ONC201, a dopamine receptor D2 antagonist[55], with the blood-brain barrier-penetrant PI3K/Akt inhibitor, paxalisib, for treating DIPG. Mechanistic insights indicate that ONC201, by decreasing tyrosine hydroxylase expression, exhibits global DRD2 antagonism, with ClpP identified as a crucial target that causes mitochondrial dysfunction and oxidative stress[56]. The combination of paxalisib shows promising results in preclinical and preliminary clinical trials, leading to symptom resolution and tumor regression. Challenges related to immunologically cold tumor microenvironments in DIPG have been acknowledged, but potential changes in the epigenetic landscape and metabolic plasticity following ONC201 treatment may enhance immunogenicity[57,58]. The observed link between H3K27M mutations, metabolic changes, and the immune response highlights the complexity of DIPG treatment, presenting a potential avenue for the effective administration of therapy for glioblastoma[59].
Radiation therapy (RT) is a successful management method for pediatric low-grade gliomas using both initial and salvage treatment approaches. Historically, RT was the chosen initial therapy for quickly progressing or unresectable tumors, with 10-year progression-free survival (PFS) and OS rates of 70% and 80%, respectively[60-62]. Furthermore, RT has been used as an adjuvant therapy, particularly when surgery is limited to partial resection or biopsy, particularly for tumors in the optic system, hypothalamus, deep midline tissues, and brainstem[63,64]. Adjuvant RT is suggested in cases of partial resection because PFS is greatly reduced[65,66]. However, there is a lack of agreement on its use, which is attributable in part to the paucity of randomized prospective studies[67,68].
For older children who have not responded to numerous systemic medications, RT is preferred as part of the care plan. Historically, postponing RT was motivated by concerns about RT-related toxicities such as cognitive impairment[69,70], endocrine dysfunction[71], secondary malignancies[72], vascular damage[72,73], and growth abnormalities[74]. The severity of these symptoms is directly related to the location of the tumor and the patient’s age, particularly in patients under the age of 10[69,72].
An institutional evaluation covering a median follow-up of 11 years found 8-year PFS and OS rates of 83% and 100%, respectively[75]. Overall neurocognitive performance did not deteriorate in the trial; however, significant cognitive impairment was noted in young children (under 7 years old) and in patients who received high doses to the left temporal lobe or hippocampus. Higher dosages to the hypothalamus or pituitary caused endocrine disruption, and two patients developed Moya disease. The 5-year PFS and OS rates in a recently published prospective research including 174 pediatric patients with LGG who received proton treatment were 84% and 92%, respectively, with a median follow-up of 4.4 years[76]. Four patients experienced severe late toxicities, including brainstem necrosis, symptomatic vasculopathy, radiation retinopathy, and fatal secondary cancers. While acknowledging the relevance of radiation-related damage, it is vital to emphasize that recent research has yielded promising outcomes. The extended latency of toxicity should be considered in light of the rapid developments in the field[77].
Concerns about RT-related toxicity originate mostly from long-term data collected from studies conducted during the 1970s and the 1990s using 2-dimensional RT methods that did not allow for accurate radiation dose administration. Significant technical progress has been achieved in reducing the radiation dose that reaches the normal structures surrounding the tumor. This began with the use of 3-dimensional conformal external beam RT (3D-CRT) and progressed in the 2000s with the advent of intensity-modulated RT (IMRT). Significantly, the introduction of proton therapy has reduced radiation exit dosage[78,79], contributing to its growing role in pediatric patients. Several studies have suggested that proton therapy might improve both patient quality of life and the cost-effectiveness of pediatric brain tumor treatment[80,81].
The primary objective of glioma treatment is to strike a balance between preserving the patient’s quality of life and improving PFS and OS[82,83]. The choice between oncological and surgical treatment depends on factors such as tumor size, location, and individual patient characteristics, including age and comorbidities[82,84,85]. Patients aged > 40 years at diagnosis, those with incomplete resection, and those with wild-type isocitrate dehydrogenase (IDH) status are typically considered to be at increased risk. The conventional treatment approach involves cytoreductive surgery to achieve gross total resection (GTR), followed by a combination of chemotherapy and/or radiation therapy[86,87].
The prognosis for gliomas, encompassing both LGG and HGG, is significantly influenced by the extent of surgical resection (EOSR) (Table 3). In LGG, EOSR is measured by the percentage of the FLAIR signal that is excised, whereas in HGG, it is determined by the removal of the percentage of enhancing tissue and the necrotic center. Extensive research on EOSR in LGG consistently shows that achieving GTR significantly improves survival rates, particularly among younger patients, classifying them as low-risk individuals compared to those who undergo only partial resection[82-84].
| Ref. | Study origin | Study design | Total number of patients | Supratotal resection sample | Male, % | Age atresection | Permanent neurological deficits | Progression-free survival | Overall survival |
| Gajjar et al[63], 1997 | United States | Cohort study | 142 | 48 (68/142) | 61 | 7 median (0.17-19) | Not reported | 70 ± 5 at 4 years | 90 ± 3 at 4 years |
| Fisher et al[67], 2008 | United States | Cohort study | 278 | 19 (52/278) | 58 | 9.1 ± 0.3 | Not reported | 55 ± 3 at 5 years | 87 ± 2 at 5 years |
| Wisoff et al[125], 2010 | United States | Prospective trial | 518 | 64 (332/518) | 54 | 7.9 median (0.6-20.5) | Not reported | 78 ± 2 at 8 years | 96 ± 0.9 at 8 years |
| Yordanova et al[93], 2011 | France | Case series | 15 | 100.00 | 53.3 | 36.4 (24-59) | 2, 13.3 | 73.3 at 38 months | 100 at study end |
| Youland et al[11], 2013 | United States | Retrospective cohort | 351 | 67 (235/351) | 55 | 10.9 (0.05-19.6) | Not reported | 75.8 at 5 years | 94.9 at 5 years |
| Lima et al[126], 2015 | France | Case series | 21 | 19.0 (4/21) | 28.57 | 35 (18-57) | 0, 0 | 100 at study end | 100 at study end |
| Duffau et al[127], 2016 | France | Cohort study | 16 | 100.00 | 43.75 | 41.3 (26-63) | 0, 0 | 50 relapse rate (avg 70 months) | 100 at study end |
| Lima et al[92], 2017 | France | Two-center prospective study | 19 | 26.3 (5/19) | 42.1 | 31.2 (19-51) | 0, 0 | 100 at study end | 100 at study end |
| Rossi et al[86], 2019 | Italy | Case series | 449 | 32 (145/449) | 53.1 | 37.9 (median 36.5) | 1, 0.69 (SupTR group) | Not reported | Not reported |
| Ng et al[128], 2020 | France | Case series | 74 | 28 (21/74) | 41.89 | 35.7 (18-66) | 0, 0 | Not reported | 100 at 5 years |
| Ng et al[129], 2020 | France | Case series | 47 | 26 (12/47) | 34.04 | 39.2 ± 11.3 | 0, 0 | Not reported | 100 at study end |
| Goel et al[130], 2021 | India | Cohort study | 74 | 34 (25/74) | 62.16 | 33 (21-55) | 0, 0 | 98.7 at 2 years | 100 at study end |
| Rossi et al[94], 2021 | Italy | Case series | 319 | 35 (110/319) | 61.1 | 38.9 (18-75) | 6, 1.9 | 94 at 92 months (SupTR group) | 100 at 80 months (SupTR group) |
| Ius et al[131], 2022 | United States, Canada, France, and Italy | Four center retrospective review | 267 | 9 (24/267) | 41.9 | 39.2 (18-71) | 8, 3.1 | Not reported | 100 at 100 months (SupTR) |
Similarly, investigations into EOSR in HGG consistently demonstrated a strong correlation between the extent of resection and survival outcomes, assuming no surgery-related neurological morbidities[88-90]. In most studies, the surgical goal is unequivocally defined as achieving GTR or complete tumor removal, typically amounting to 100% resection[91].
Recent clinical investigations have explored the concept of subtotal resection for gliomas[92]. This surgical approach aims to achieve GTR while simultaneously eliminating the FLAIR signal surrounding the necrotic and enhancing tumor mass in high-grade gliomas. Low-grade gliomas involve complete removal of the FLAIR signal along with additional radiographic extraction of the normal brain tissue adjacent to the tumor. Subtotal resection in LGG surgery was confirmed by observing that the resection cavity exceeded the initial FLAIR volume on the postoperative MRI at the three-month mark. Subtotal resection is considered justifiable when minimal neurological risks are involved, with the aim of eliminating invasive cells near the radiographic boundary[93-95]. Evidence from clinical case series of glioblastoma multiforme and HGG presents a conflicting picture, as performing supra total resection may entail an increased risk of neurological function decline, despite potential improvements in PFS and OS[92,96]. Additionally, there has been increased focus on the utilization of laser interstitial thermal (LIT) treatment for brain tumors. Recent trials investigating LIT have shown that achieving a greater level of ablation, including subtotal ablation, can lead to improved progression-free survival and overall survival outcomes in patients with HGG[97,98].
Intraoperative radiotherapy with a single high radiation dose administered following tumor resection, intraoperative radiotherapy (IORT), a novel and non-conventional form of radiotherapy, can eradicate any remaining tumor cells[99]. A wide range of cancers, including breast, pancreatic, lung, and colon cancers, have been treated with IORT[100-102]. The lack of a discernible increase in survival in IORT treatment reports for primary malignant gliomas has been ascribed to angle errors, low electrons, and small electron cones, which result in inadequate coverage of the target volume[103]. A mobile IORT unit, INTRABEAM (Zeiss, Oberkochen, Germany), can deliver an equal dose of low-energy radiation in all directions within a tumor cavity, along with spherical irradiation. According to research, IORT with low-energy X-rays increases glioblastoma patients’ survival rates without causing new problems[104].
Cancer vaccines targeting high-grade gliomas, predating coronavirus disease 2019, are gaining momentum. Strategies include peptide-based vaccines, dendritic cells, viral vectors, and personalized neoantigen vaccines. They are also being explored for the treatment of LGG. For IDH-mutant LGG, adjuvants such as poly (I:C) and poly-ICLC enhance immune responses, collectively reflecting a determined push for glioma immunotherapy[105]. To bolster the weak immune response in LGGs, synthetic double-stranded RNA molecules, such as polyinosinic acid homopolymers annealed to a polycytidylic acid homopolymer, have demonstrated potential[106]. They mimic viral infections and promote the release of interferon type 1 and other immune-boosting substances. Safely used as adjuvants with dendritic cells or peptide vaccines, they enhance therapeutic responses[106,107].
In conclusion, advancements in LGG treatment span immunotherapies, targeted therapies, radiation, surgery, and vaccine strategies. Immunotherapies like Zotiraciclib and Lerapolturev show promise, while targeted therapies such as Entrectinib and ONC201/Paxalisib combination demonstrate early success. Radiation therapy, evolving with proton therapy, remains crucial, and surgical approaches aim to achieve gross total resection. Cancer vaccines including synthetic RNA adjuvants have emerged. The evolving landscape underscores a shift toward personalized and targeted therapies, with ongoing research being essential for refining strategies and improving outcomes in LGG treatment.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Chu SH, China; Shibata Y, Japan S-Editor: Liu JH L-Editor: A P-Editor: Yuan YY
| 1. | Braunstein S, Raleigh D, Bindra R, Mueller S, Haas-Kogan D. Pediatric high-grade glioma: current molecular landscape and therapeutic approaches. J Neurooncol. 2017;134:541-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 105] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 2. | Stylli SS, Luwor RB, Ware TM, Tan F, Kaye AH. Mouse models of glioma. J Clin Neurosci. 2015;22:619-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 3. | Diwanji TP, Engelman A, Snider JW, Mohindra P. Epidemiology, diagnosis, and optimal management of glioma in adolescents and young adults. Adolesc Health Med Ther. 2017;8:99-113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 4. | Lim-Fat MJ, Das S. Unique molecular, clinical, and treatment aspects of gliomas in adolescents and young adults: a review. J Neurosurg. 2023;139:1619-1627. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 5. | Mughal ZUN, Ahmad TKF, Haseeb A, Shafique MA, Ahmdon OEA, Mahgoub AMA. Dabrafenib and trametinib as a promising treatment option for pediatric population with low-grade gliomas that have BRAF V600E mutation; a breakthrough in the field of neuro-oncology. IJS Global Health. 2024;7:e0395. [DOI] [Full Text] |
| 6. | Bush NA, Chang SM, Berger MS. Current and future strategies for treatment of glioma. Neurosurg Rev. 2017;40:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 391] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 7. | Naeem A, Aziz N, Nasir M, Rangwala HS, Fatima H, Mubarak F. Accuracy of MRI in Detecting 1p/19q Co-deletion Status of Gliomas: A Single-Center Retrospective Study. Cureus. 2024;16:e51863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 8. | Eisenstat DD, Pollack IF, Demers A, Sapp MV, Lambert P, Weisfeld-Adams JD, Burger PC, Gilles F, Davis RL, Packer R, Boyett JM, Finlay JL. Impact of tumor location and pathological discordance on survival of children with midline high-grade gliomas treated on Children's Cancer Group high-grade glioma study CCG-945. J Neurooncol. 2015;121:573-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Bavle A, Chintagumpala M. Pediatric high-grade glioma: a review of biology, prognosis, and treatment. J Radiat Oncol. 2018;7:7-15. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Sievert AJ, Fisher MJ. Pediatric low-grade gliomas. J Child Neurol. 2009;24:1397-1408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 179] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 11. | Youland RS, Khwaja SS, Schomas DA, Keating GF, Wetjen NM, Laack NN. Prognostic factors and survival patterns in pediatric low-grade gliomas over 4 decades. J Pediatr Hematol Oncol. 2013;35:197-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Ris MD, Beebe DW. Neurodevelopmental outcomes of children with low-grade gliomas. Dev Disabil Res Rev. 2008;14:196-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Rees JH. Low-grade gliomas in adults. Curr Opin Neurol. 2002;15:657-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Grier JT, Batchelor T. Low-grade gliomas in adults. Oncologist. 2006;11:681-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 102] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 15. | Su YT, Chen R, Wang H, Song H, Zhang Q, Chen LY, Lappin H, Vasconcelos G, Lita A, Maric D, Li A, Celiku O, Zhang W, Meetze K, Estok T, Larion M, Abu-Asab M, Zhuang Z, Yang C, Gilbert MR, Wu J. Novel Targeting of Transcription and Metabolism in Glioblastoma. Clin Cancer Res. 2018;24:1124-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 16. | Hofmeister CC, Berdeja JG, Vesole DH, Suvannasankha A, Parrott T, Abonour R. TG02, an oral CDK9-inhibitor, in combination with carfilzomib demonstrated objective responses in carfilzomib refractory multiple myeloma patients. Blood. 2015;126:3052. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Nechaev S, Adelman K. Pol II waiting in the starting gates: Regulating the transition from transcription initiation into productive elongation. Biochim Biophys Acta. 2011;1809:34-45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 207] [Cited by in RCA: 207] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 18. | Loyer P, Trembley JH, Katona R, Kidd VJ, Lahti JM. Role of CDK/cyclin complexes in transcription and RNA splicing. Cell Signal. 2005;17:1033-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 121] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 19. | Wu J, Yuan Y, Long Priel DA, Fink D, Peer CJ, Sissung TM, Su YT, Pang Y, Yu G, Butler MK, Mendoza TR, Vera E, Ahmad S, Bryla C, Lindsley M, Grajkowska E, Mentges K, Boris L, Antony R, Garren N, Siegel C, Lollo N, Cordova C, Aboud O, Theeler BJ, Burton EM, Penas-Prado M, Leeper H, Gonzales J, Armstrong TS, Calvo KR, Figg WD, Kuhns DB, Gallin JI, Gilbert MR. Phase I Study of Zotiraciclib in Combination with Temozolomide for Patients with Recurrent High-grade Astrocytomas. Clin Cancer Res. 2021;27:3298-3306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 20. | Filley AC, Henriquez M, Dey M. Recurrent glioma clinical trial, CheckMate-143: the game is not over yet. Oncotarget. 2017;8:91779-91794. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 254] [Cited by in RCA: 284] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 21. | Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, Biedrzycki B, Donehower RC, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Duffy SM, Goldberg RM, de la Chapelle A, Koshiji M, Bhaijee F, Huebner T, Hruban RH, Wood LD, Cuka N, Pardoll DM, Papadopoulos N, Kinzler KW, Zhou S, Cornish TC, Taube JM, Anders RA, Eshleman JR, Vogelstein B, Diaz LA Jr. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372:2509-2520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6096] [Cited by in RCA: 7233] [Article Influence: 723.3] [Reference Citation Analysis (0)] |
| 22. | Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, Miller ML, Rekhtman N, Moreira AL, Ibrahim F, Bruggeman C, Gasmi B, Zappasodi R, Maeda Y, Sander C, Garon EB, Merghoub T, Wolchok JD, Schumacher TN, Chan TA. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6065] [Cited by in RCA: 6330] [Article Influence: 633.0] [Reference Citation Analysis (0)] |
| 23. | Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, West AN, Carmona M, Kivork C, Seja E, Cherry G, Gutierrez AJ, Grogan TR, Mateus C, Tomasic G, Glaspy JA, Emerson RO, Robins H, Pierce RH, Elashoff DA, Robert C, Ribas A. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568-571. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4180] [Cited by in RCA: 5234] [Article Influence: 523.4] [Reference Citation Analysis (0)] |
| 24. | Alexandrov LB, Kim J, Haradhvala NJ, Huang MN, Tian Ng AW, Wu Y, Boot A, Covington KR, Gordenin DA, Bergstrom EN, Islam SMA, Lopez-Bigas N, Klimczak LJ, McPherson JR, Morganella S, Sabarinathan R, Wheeler DA, Mustonen V; PCAWG Mutational Signatures Working Group, Getz G, Rozen SG, Stratton MR; PCAWG Consortium. The repertoire of mutational signatures in human cancer. Nature. 2020;578:94-101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2218] [Cited by in RCA: 2226] [Article Influence: 445.2] [Reference Citation Analysis (0)] |
| 25. | Zhao J, Chen AX, Gartrell RD, Silverman AM, Aparicio L, Chu T, Bordbar D, Shan D, Samanamud J, Mahajan A, Filip I, Orenbuch R, Goetz M, Yamaguchi JT, Cloney M, Horbinski C, Lukas RV, Raizer J, Rae AI, Yuan J, Canoll P, Bruce JN, Saenger YM, Sims P, Iwamoto FM, Sonabend AM, Rabadan R. Immune and genomic correlates of response to anti-PD-1 immunotherapy in glioblastoma. Nat Med. 2019;25:462-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 498] [Cited by in RCA: 641] [Article Influence: 106.8] [Reference Citation Analysis (0)] |
| 26. | Brown MC, Mosaheb MM, Mohme M, McKay ZP, Holl EK, Kastan JP, Yang Y, Beasley GM, Hwang ES, Ashley DM, Bigner DD, Nair SK, Gromeier M. Viral infection of cells within the tumor microenvironment mediates antitumor immunotherapy via selective TBK1-IRF3 signaling. Nat Commun. 2021;12:1858. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 27. | Mosaheb MM, Dobrikova EY, Brown MC, Yang Y, Cable J, Okada H, Nair SK, Bigner DD, Ashley DM, Gromeier M. Genetically stable poliovirus vectors activate dendritic cells and prime antitumor CD8 T cell immunity. Nat Commun. 2020;11:524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 28. | Brown MC, Holl EK, Boczkowski D, Dobrikova E, Mosaheb M, Chandramohan V, Bigner DD, Gromeier M, Nair SK. Cancer immunotherapy with recombinant poliovirus induces IFN-dominant activation of dendritic cells and tumor antigen-specific CTLs. Sci Transl Med. 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 178] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 29. | Gromeier M, Alexander L, Wimmer E. Internal ribosomal entry site substitution eliminates neurovirulence in intergeneric poliovirus recombinants. Proc Natl Acad Sci USA. 1996;93:2370-2375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 257] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 30. | Thompson EM, Landi D, Brown MC, Friedman HS, McLendon R, Herndon JE 2nd, Buckley E, Bolognesi DP, Lipp E, Schroeder K, Becher OJ, Friedman AH, McKay Z, Walter A, Threatt S, Jaggers D, Desjardins A, Gromeier M, Bigner DD, Ashley DM. Recombinant polio-rhinovirus immunotherapy for recurrent paediatric high-grade glioma: a phase 1b trial. Lancet Child Adolesc Health. 2023;7:471-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 24] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 31. | Mackay A, Burford A, Carvalho D, Izquierdo E, Fazal-Salom J, Taylor KR, Bjerke L, Clarke M, Vinci M, Nandhabalan M, Temelso S, Popov S, Molinari V, Raman P, Waanders AJ, Han HJ, Gupta S, Marshall L, Zacharoulis S, Vaidya S, Mandeville HC, Bridges LR, Martin AJ, Al-Sarraj S, Chandler C, Ng HK, Li X, Mu K, Trabelsi S, Brahim DH, Kisljakov AN, Konovalov DM, Moore AS, Carcaboso AM, Sunol M, de Torres C, Cruz O, Mora J, Shats LI, Stavale JN, Bidinotto LT, Reis RM, Entz-Werle N, Farrell M, Cryan J, Crimmins D, Caird J, Pears J, Monje M, Debily MA, Castel D, Grill J, Hawkins C, Nikbakht H, Jabado N, Baker SJ, Pfister SM, Jones DTW, Fouladi M, von Bueren AO, Baudis M, Resnick A, Jones C. Integrated Molecular Meta-Analysis of 1,000 Pediatric High-Grade and Diffuse Intrinsic Pontine Glioma. Cancer Cell. 2017;32:520-537.e5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 669] [Cited by in RCA: 777] [Article Influence: 97.1] [Reference Citation Analysis (0)] |
| 32. | Jungbluth AA, Stockert E, Huang HJ, Collins VP, Coplan K, Iversen K, Kolb D, Johns TJ, Scott AM, Gullick WJ, Ritter G, Cohen L, Scanlan MJ, Cavenee WK, Old LJ. A monoclonal antibody recognizing human cancers with amplification/overexpression of the human epidermal growth factor receptor. Proc Natl Acad Sci USA. 2003;100:639-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 143] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 33. | Gan HK, Burgess AW, Clayton AH, Scott AM. Targeting of a conformationally exposed, tumor-specific epitope of EGFR as a strategy for cancer therapy. Cancer Res. 2012;72:2924-2930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 119] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 34. | Doronina SO, Bovee TD, Meyer DW, Miyamoto JB, Anderson ME, Morris-Tilden CA, Senter PD. Novel peptide linkers for highly potent antibody-auristatin conjugate. Bioconjug Chem. 2008;19:1960-1963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 125] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 35. | Brastianos PK, Ippen FM, Hafeez U, Gan HK. Emerging Gene Fusion Drivers in Primary and Metastatic Central Nervous System Malignancies: A Review of Available Evidence for Systemic Targeted Therapies. Oncologist. 2018;23:1063-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 36. | Birrer MJ, Moore KN, Betella I, Bates RC. Antibody-Drug Conjugate-Based Therapeutics: State of the Science. J Natl Cancer Inst. 2019;111:538-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 292] [Article Influence: 58.4] [Reference Citation Analysis (0)] |
| 37. | Gan HK, van den Bent M, Lassman AB, Reardon DA, Scott AM. Antibody-drug conjugates in glioblastoma therapy: the right drugs to the right cells. Nat Rev Clin Oncol. 2017;14:695-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 82] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 38. | Marin BM, Porath KA, Jain S, Kim M, Conage-Pough JE, Oh JH, Miller CL, Talele S, Kitange GJ, Tian S, Burgenske DM, Mladek AC, Gupta SK, Decker PA, McMinn MH, Stopka SA, Regan MS, He L, Carlson BL, Bakken K, Burns TC, Parney IF, Giannini C, Agar NYR, Eckel-Passow JE, Cochran JR, Elmquist WF, Vaubel RA, White FM, Sarkaria JN. Heterogeneous delivery across the blood-brain barrier limits the efficacy of an EGFR-targeting antibody drug conjugate in glioblastoma. Neuro Oncol. 2021;23:2042-2053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 39. | Gan HK, Parakh S, Lassman AB, Seow A, Lau E, Lee ST, Ameratunga M, Perchyonok Y, Cao D, Burvenich IJG, O'Keefe GJ, Rigopoulos A, Gomez E, Maag D, Scott AM. Tumor volumes as a predictor of response to the anti-EGFR antibody drug conjugate depatuxizumab mafadotin. Neurooncol Adv. 2021;3:vdab102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 40. | Cocco E, Scaltriti M, Drilon A. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat Rev Clin Oncol. 2018;15:731-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 644] [Cited by in RCA: 1008] [Article Influence: 168.0] [Reference Citation Analysis (0)] |
| 41. | Doebele RC, Drilon A, Paz-Ares L, Siena S, Shaw AT, Farago AF, Blakely CM, Seto T, Cho BC, Tosi D, Besse B, Chawla SP, Bazhenova L, Krauss JC, Chae YK, Barve M, Garrido-Laguna I, Liu SV, Conkling P, John T, Fakih M, Sigal D, Loong HH, Buchschacher GL Jr, Garrido P, Nieva J, Steuer C, Overbeck TR, Bowles DW, Fox E, Riehl T, Chow-Maneval E, Simmons B, Cui N, Johnson A, Eng S, Wilson TR, Demetri GD; trial investigators. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1-2 trials. Lancet Oncol. 2020;21:271-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 574] [Cited by in RCA: 1134] [Article Influence: 189.0] [Reference Citation Analysis (0)] |
| 42. | Drilon A, Siena S, Ou SI, Patel M, Ahn MJ, Lee J, Bauer TM, Farago AF, Wheler JJ, Liu SV, Doebele R, Giannetta L, Cerea G, Marrapese G, Schirru M, Amatu A, Bencardino K, Palmeri L, Sartore-Bianchi A, Vanzulli A, Cresta S, Damian S, Duca M, Ardini E, Li G, Christiansen J, Kowalski K, Johnson AD, Patel R, Luo D, Chow-Maneval E, Hornby Z, Multani PS, Shaw AT, De Braud FG. Safety and Antitumor Activity of the Multitargeted Pan-TRK, ROS1, and ALK Inhibitor Entrectinib: Combined Results from Two Phase I Trials (ALKA-372-001 and STARTRK-1). Cancer Discov. 2017;7:400-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 498] [Cited by in RCA: 606] [Article Influence: 75.8] [Reference Citation Analysis (0)] |
| 43. | Farago AF, Le LP, Zheng Z, Muzikansky A, Drilon A, Patel M, Bauer TM, Liu SV, Ou SH, Jackman D, Costa DB, Multani PS, Li GG, Hornby Z, Chow-Maneval E, Luo D, Lim JE, Iafrate AJ, Shaw AT. Durable Clinical Response to Entrectinib in NTRK1-Rearranged Non-Small Cell Lung Cancer. J Thorac Oncol. 2015;10:1670-1674. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 161] [Cited by in RCA: 178] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 44. | Alvarez-Breckenridge C, Miller JJ, Nayyar N, Gill CM, Kaneb A, D'Andrea M, Le LP, Lee J, Cheng J, Zheng Z, Butler WE, Multani P, Chow Maneval E, Ha Paek S, Toyota BD, Dias-Santagata D, Santagata S, Romero J, Shaw AT, Farago AF, Yip S, Cahill DP, Batchelor TT, Iafrate AJ, Brastianos PK. Clinical and radiographic response following targeting of BCAN-NTRK1 fusion in glioneuronal tumor. NPJ Precis Oncol. 2017;1:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 45. | Walter AW, Kandula VVR, Shah N. Larotrectinib imaging response in low-grade glioma. Pediatr Blood Cancer. 2020;67:e28002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 46. | Mayr L, Guntner AS, Madlener S, Schmook MT, Peyrl A, Azizi AA, Dieckmann K, Reisinger D, Stepien NM, Schramm K, Laemmerer A, Jones DTW, Ecker J, Sahm F, Milde T, Pajtler KW, Blattner-Johnson M, Strbac M, Dorfer C, Czech T, Kirchhofer D, Gabler L, Berger W, Haberler C, Müllauer L, Buchberger W, Slavc I, Lötsch-Gojo D, Gojo J. Cerebrospinal Fluid Penetration and Combination Therapy of Entrectinib for Disseminated ROS1/NTRK-Fusion Positive Pediatric High-Grade Glioma. J Pers Med. 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 47. | Fischer H, Ullah M, de la Cruz CC, Hunsaker T, Senn C, Wirz T, Wagner B, Draganov D, Vazvaei F, Donzelli M, Paehler A, Merchant M, Yu L. Entrectinib, a TRK/ROS1 inhibitor with anti-CNS tumor activity: differentiation from other inhibitors in its class due to weak interaction with P-glycoprotein. Neuro Oncol. 2020;22:819-829. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 71] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 48. | Duchatel RJ, Mannan A, Woldu AS, Hawtrey T, Hindley PA, Douglas AM, Jackson ER, Findlay IJ, Germon ZP, Staudt D, Kearney PS, Smith ND, Hindley KE, Cain JE, André N, La Madrid AM, Nixon B, De Iuliis GN, Nazarian J, Irish K, Alvaro F, Eisenstat DD, Beck A, Vitanza NA, Mueller S, Morris JC, Dun MD. Preclinical and clinical evaluation of German-sourced ONC201 for the treatment of H3K27M-mutant diffuse intrinsic pontine glioma. Neurooncol Adv. 2021;3:vdab169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 49. | Venneti S, Kawakibi AR, Ji S, Waszak SM, Sweha SR, Mota M, Pun M, Deogharkar A, Chung C, Tarapore RS, Ramage S, Chi A, Wen PY, Arrillaga-Romany I, Batchelor TT, Butowski NA, Sumrall A, Shonka N, Harrison RA, de Groot J, Mehta M, Hall MD, Daghistani D, Cloughesy TF, Ellingson BM, Beccaria K, Varlet P, Kim MM, Umemura Y, Garton H, Franson A, Schwartz J, Jain R, Kachman M, Baum H, Burant CF, Mottl SL, Cartaxo RT, John V, Messinger D, Qin T, Peterson E, Sajjakulnukit P, Ravi K, Waugh A, Walling D, Ding Y, Xia Z, Schwendeman A, Hawes D, Yang F, Judkins AR, Wahl D, Lyssiotis CA, de la Nava D, Alonso MM, Eze A, Spitzer J, Schmidt SV, Duchatel RJ, Dun MD, Cain JE, Jiang L, Stopka SA, Baquer G, Regan MS, Filbin MG, Agar NYR, Zhao L, Kumar-Sinha C, Mody R, Chinnaiyan A, Kurokawa R, Pratt D, Yadav VN, Grill J, Kline C, Mueller S, Resnick A, Nazarian J, Allen JE, Odia Y, Gardner SL, Koschmann C. Clinical Efficacy of ONC201 in H3K27M-Mutant Diffuse Midline Gliomas Is Driven by Disruption of Integrated Metabolic and Epigenetic Pathways. Cancer Discov. 2023;13:2370-2393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 86] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 50. | Ishizawa J, Kojima K, Chachad D, Ruvolo P, Ruvolo V, Jacamo RO, Borthakur G, Mu H, Zeng Z, Tabe Y, Allen JE, Wang Z, Ma W, Lee HC, Orlowski R, Sarbassov dos D, Lorenzi PL, Huang X, Neelapu SS, McDonnell T, Miranda RN, Wang M, Kantarjian H, Konopleva M, Davis RE, Andreeff M. ATF4 induction through an atypical integrated stress response to ONC201 triggers p53-independent apoptosis in hematological malignancies. Sci Signal. 2016;9:ra17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 158] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 51. | Wagner J, Kline CL, Zhou L, Khazak V, El-Deiry WS. Anti-tumor effects of ONC201 in combination with VEGF-inhibitors significantly impacts colorectal cancer growth and survival in vivo through complementary non-overlapping mechanisms. J Exp Clin Cancer Res. 2018;37:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 52. | Greer YE, Porat-Shliom N, Nagashima K, Stuelten C, Crooks D, Koparde VN, Gilbert SF, Islam C, Ubaldini A, Ji Y, Gattinoni L, Soheilian F, Wang X, Hafner M, Shetty J, Tran B, Jailwala P, Cam M, Lang M, Voeller D, Reinhold WC, Rajapakse V, Pommier Y, Weigert R, Linehan WM, Lipkowitz S. ONC201 kills breast cancer cells in vitro by targeting mitochondria. Oncotarget. 2018;9:18454-18479. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 53. | Fang Z, Wang J, Clark LH, Sun W, Yin Y, Kong W, Pierce SR, West L, Sullivan SA, Tran AQ, Prabhu VV, Zhou C, Bae-Jump V. ONC201 demonstrates anti-tumorigenic and anti-metastatic activity in uterine serous carcinoma in vitro. Am J Cancer Res. 2018;8:1551-1563. [PubMed] |
| 54. | Arrillaga-Romany I, Chi AS, Allen JE, Oster W, Wen PY, Batchelor TT. A phase 2 study of the first imipridone ONC201, a selective DRD2 antagonist for oncology, administered every three weeks in recurrent glioblastoma. Oncotarget. 2017;8:79298-79304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 121] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 55. | Madhukar NS, Khade PK, Huang L, Gayvert K, Galletti G, Stogniew M, Allen JE, Giannakakou P, Elemento O. A Bayesian machine learning approach for drug target identification using diverse data types. Nat Commun. 2019;10:5221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 140] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 56. | Pruss M, Dwucet A, Tanriover M, Hlavac M, Kast RE, Debatin KM, Wirtz CR, Halatsch ME, Siegelin MD, Westhoff MA, Karpel-Massler G. Dual metabolic reprogramming by ONC201/TIC10 and 2-Deoxyglucose induces energy depletion and synergistic anti-cancer activity in glioblastoma. Br J Cancer. 2020;122:1146-1157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 57. | Chung C, Sweha SR, Pratt D, Tamrazi B, Panwalkar P, Banda A, Bayliss J, Hawes D, Yang F, Lee HJ, Shan M, Cieslik M, Qin T, Werner CK, Wahl DR, Lyssiotis CA, Bian Z, Shotwell JB, Yadav VN, Koschmann C, Chinnaiyan AM, Blüml S, Judkins AR, Venneti S. Integrated Metabolic and Epigenomic Reprograming by H3K27M Mutations in Diffuse Intrinsic Pontine Gliomas. Cancer Cell. 2020;38:334-349.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 108] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 58. | Allen JE, Krigsfeld G, Mayes PA, Patel L, Dicker DT, Patel AS, Dolloff NG, Messaris E, Scata KA, Wang W, Zhou JY, Wu GS, El-Deiry WS. Dual inactivation of Akt and ERK by TIC10 signals Foxo3a nuclear translocation, TRAIL gene induction, and potent antitumor effects. Sci Transl Med. 2013;5:171ra17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 283] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 59. | Harutyunyan AS, Krug B, Chen H, Papillon-Cavanagh S, Zeinieh M, De Jay N, Deshmukh S, Chen CCL, Belle J, Mikael LG, Marchione DM, Li R, Nikbakht H, Hu B, Cagnone G, Cheung WA, Mohammadnia A, Bechet D, Faury D, McConechy MK, Pathania M, Jain SU, Ellezam B, Weil AG, Montpetit A, Salomoni P, Pastinen T, Lu C, Lewis PW, Garcia BA, Kleinman CL, Jabado N, Majewski J. H3K27M induces defective chromatin spread of PRC2-mediated repressive H3K27me2/me3 and is essential for glioma tumorigenesis. Nat Commun. 2019;10:1262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 244] [Cited by in RCA: 232] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 60. | Taveras JM, Mount LA, Wood EH. The value of radiation therapy in the management of glioma of the optic nerves and chiasm. Radiology. 1956;66:518-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 87] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 61. | Erkal HS, Serin M, Cakmak A. Management of optic pathway and chiasmatic-hypothalamic gliomas in children with radiation therapy. Radiother Oncol. 1997;45:11-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 52] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 62. | Cappelli C, Grill J, Raquin M, Pierre-Kahn A, Lellouch-Tubiana A, Terrier-Lacombe MJ, Habrand JL, Couanet D, Brauner R, Rodriguez D, Hartmann O, Kalifa C. Long-term follow up of 69 patients treated for optic pathway tumours before the chemotherapy era. Arch Dis Child. 1998;79:334-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 87] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 63. | Gajjar A, Sanford RA, Heideman R, Jenkins JJ, Walter A, Li Y, Langston JW, Muhlbauer M, Boyett JM, Kun LE. Low-grade astrocytoma: a decade of experience at St. Jude Children's Research Hospital. J Clin Oncol. 1997;15:2792-2799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 148] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 64. | Armstrong GT, Conklin HM, Huang S, Srivastava D, Sanford R, Ellison DW, Merchant TE, Hudson MM, Hoehn ME, Robison LL, Gajjar A, Morris EB. Survival and long-term health and cognitive outcomes after low-grade glioma. Neuro Oncol. 2011;13:223-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 166] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 65. | Oh KS, Hung J, Robertson PL, Garton HJ, Muraszko KM, Sandler HM, Hamstra DA. Outcomes of multidisciplinary management in pediatric low-grade gliomas. Int J Radiat Oncol Biol Phys. 2011;81:e481-e488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 66. | Mishra KK, Puri DR, Missett BT, Lamborn KR, Prados MD, Berger MS, Banerjee A, Gupta N, Wara WM, Haas-Kogan DA. The role of up-front radiation therapy for incompletely resected pediatric WHO grade II low-grade gliomas. Neuro Oncol. 2006;8:166-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 67. | Fisher PG, Tihan T, Goldthwaite PT, Wharam MD, Carson BS, Weingart JD, Repka MX, Cohen KJ, Burger PC. Outcome analysis of childhood low-grade astrocytomas. Pediatr Blood Cancer. 2008;51:245-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 118] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 68. | Packer RJ, Sutton LN, Atkins TE, Radcliffe J, Bunin GR, D'Angio G, Siegel KR, Schut L. A prospective study of cognitive function in children receiving whole-brain radiotherapy and chemotherapy: 2-year results. J Neurosurg. 1989;70:707-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 244] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 69. | Merchant TE, Conklin HM, Wu S, Lustig RH, Xiong X. Late effects of conformal radiation therapy for pediatric patients with low-grade glioma: prospective evaluation of cognitive, endocrine, and hearing deficits. J Clin Oncol. 2009;27:3691-3697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 289] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 70. | Brauner R, Malandry F, Rappaport R, Zucker JM, Kalifa C, Pierre-Kahn A, Bataini P, Dufier JL. Growth and endocrine disorders in optic glioma. Eur J Pediatr. 1990;149:825-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 53] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 71. | Tsang DS, Murphy ES, Merchant TE. Radiation Therapy for Optic Pathway and Hypothalamic Low-Grade Gliomas in Children. Int J Radiat Oncol Biol Phys. 2017;99:642-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 72. | Bowers DC, Mulne AF, Reisch JS, Elterman RD, Munoz L, Booth T, Shapiro K, Doxey DL. Nonperioperative strokes in children with central nervous system tumors. Cancer. 2002;94:1094-1101. [PubMed] [DOI] [Full Text] |
| 73. | Armstrong GT, Liu Q, Yasui Y, Huang S, Ness KK, Leisenring W, Hudson MM, Donaldson SS, King AA, Stovall M, Krull KR, Robison LL, Packer RJ. Long-term outcomes among adult survivors of childhood central nervous system malignancies in the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2009;101:946-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 389] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 74. | Greenberger BA, Pulsifer MB, Ebb DH, MacDonald SM, Jones RM, Butler WE, Huang MS, Marcus KJ, Oberg JA, Tarbell NJ, Yock TI. Clinical outcomes and late endocrine, neurocognitive, and visual profiles of proton radiation for pediatric low-grade gliomas. Int J Radiat Oncol Biol Phys. 2014;89:1060-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 145] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 75. | Indelicato DJ, Rotondo RL, Uezono H, Sandler ES, Aldana PR, Ranalli NJ, Beier AD, Morris CG, Bradley JA. Outcomes Following Proton Therapy for Pediatric Low-Grade Glioma. Int J Radiat Oncol Biol Phys. 2019;104:149-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 100] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 76. | Bitterman DS, MacDonald SM, Yock TI, Tarbell NJ, Wright KD, Chi SN, Marcus KJ, Haas-Kogan DA. Revisiting the Role of Radiation Therapy for Pediatric Low-Grade Glioma. J Clin Oncol. 2019;37:3335-3339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 77. | Harrabi SB, Bougatf N, Mohr A, Haberer T, Herfarth K, Combs SE, Debus J, Adeberg S. Dosimetric advantages of proton therapy over conventional radiotherapy with photons in young patients and adults with low-grade glioma. Strahlenther Onkol. 2016;192:759-769. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 78. | Takizawa D, Mizumoto M, Yamamoto T, Oshiro Y, Fukushima H, Fukushima T, Terunuma T, Okumura T, Tsuboi K, Sakurai H. A comparative study of dose distribution of PBT, 3D-CRT and IMRT for pediatric brain tumors. Radiat Oncol. 2017;12:40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 79. | Yock TI, Bhat S, Szymonifka J, Yeap BY, Delahaye J, Donaldson SS, MacDonald SM, Pulsifer MB, Hill KS, DeLaney TF, Ebb D, Huang M, Tarbell NJ, Fisher PG, Kuhlthau KA. Quality of life outcomes in proton and photon treated pediatric brain tumor survivors. Radiother Oncol. 2014;113:89-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 88] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 80. | Verma V, Mishra MV, Mehta MP. A systematic review of the cost and cost-effectiveness studies of proton radiotherapy. Cancer. 2016;122:1483-1501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 166] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 81. | Jakola AS, Skjulsvik AJ, Myrmel KS, Sjåvik K, Unsgård G, Torp SH, Aaberg K, Berg T, Dai HY, Johnsen K, Kloster R, Solheim O. Surgical resection versus watchful waiting in low-grade gliomas. Ann Oncol. 2017;28:1942-1948. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 212] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 82. | Bogdańska MU, Bodnar M, Piotrowska MJ, Murek M, Schucht P, Beck J, Martínez-González A, Pérez-García VM. A mathematical model describes the malignant transformation of low grade gliomas: Prognostic implications. PLoS One. 2017;12:e0179999. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 83. | Aghi MK, Nahed BV, Sloan AE, Ryken TC, Kalkanis SN, Olson JJ. The role of surgery in the management of patients with diffuse low grade glioma: A systematic review and evidence-based clinical practice guideline. J Neurooncol. 2015;125:503-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 127] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 84. | Opoku-Darko M, Lang ST, Artindale J, Cairncross JG, Sevick RJ, Kelly JJP. Surgical management of incidentally discovered diffusely infiltrating low-grade glioma. J Neurosurg. 2018;129:19-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 85. | Kurdi M, Moshref RH, Katib Y, Faizo E, Najjar AA, Bahakeem B, Bamaga AK. Simple approach for the histomolecular diagnosis of central nervous system gliomas based on 2021 World Health Organization Classification. World J Clin Oncol. 2022;13:567-576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (1)] |
| 86. | Rossi M, Ambrogi F, Gay L, Gallucci M, Conti Nibali M, Leonetti A, Puglisi G, Sciortino T, Howells H, Riva M, Pessina F, Navarria P, Franzese C, Simonelli M, Rudà R, Bello L. Is supratotal resection achievable in low-grade gliomas? Feasibility, putative factors, safety, and functional outcome. J Neurosurg. 2019;132:1692-1705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 87. | Hervey-Jumper SL, Berger MS. Maximizing safe resection of low- and high-grade glioma. J Neurooncol. 2016;130:269-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 328] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 88. | Brown TJ, Brennan MC, Li M, Church EW, Brandmeir NJ, Rakszawski KL, Patel AS, Rizk EB, Suki D, Sawaya R, Glantz M. Association of the Extent of Resection With Survival in Glioblastoma: A Systematic Review and Meta-analysis. JAMA Oncol. 2016;2:1460-1469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 819] [Cited by in RCA: 718] [Article Influence: 79.8] [Reference Citation Analysis (0)] |
| 89. | Barnett GH, Voigt JD, Alhuwalia MS. A Systematic Review and Meta-Analysis of Studies Examining the Use of Brain Laser Interstitial Thermal Therapy versus Craniotomy for the Treatment of High-Grade Tumors in or near Areas of Eloquence: An Examination of the Extent of Resection and Major Complication Rates Associated with Each Type of Surgery. Stereotact Funct Neurosurg. 2016;94:164-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 90. | Kreatsoulas D, Damante M, Gruber M, Duru O, Elder JB. Supratotal Surgical Resection for Low-Grade Glioma: A Systematic Review. Cancers (Basel). 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 91. | de Leeuw CN, Vogelbaum MA. Supratotal resection in glioma: a systematic review. Neuro Oncol. 2019;21:179-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 78] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 92. | Lima GLO, Dezamis E, Corns R, Rigaux-Viode O, Moritz-Gasser S, Roux A, Duffau H, Pallud J. Surgical resection of incidental diffuse gliomas involving eloquent brain areas. Rationale, functional, epileptological and oncological outcomes. Neurochirurgie. 2017;63:250-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 93. | Yordanova YN, Duffau H. Supratotal resection of diffuse gliomas - an overview of its multifaceted implications. Neurochirurgie. 2017;63:243-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 94. | Rossi M, Gay L, Ambrogi F, Conti Nibali M, Sciortino T, Puglisi G, Leonetti A, Mocellini C, Caroli M, Cordera S, Simonelli M, Pessina F, Navarria P, Pace A, Soffietti R, Rudà R, Riva M, Bello L. Association of supratotal resection with progression-free survival, malignant transformation, and overall survival in lower-grade gliomas. Neuro Oncol. 2021;23:812-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 81] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 95. | Incekara F, Koene S, Vincent AJPE, van den Bent MJ, Smits M. Association Between Supratotal Glioblastoma Resection and Patient Survival: A Systematic Review and Meta-Analysis. World Neurosurg. 2019;127:617-624.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 96. | Kaisman-Elbaz T, Xiao T, Grabowski MM, Barnett GH, Mohammadi AM. The Impact of Extent of Ablation on Survival of Patients With Newly Diagnosed Glioblastoma Treated With Laser Interstitial Thermal Therapy: A Large Single-Institutional Cohort. Neurosurgery. 2023;93:427-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 97. | Shah AH, Semonche A, Eichberg DG, Borowy V, Luther E, Sarkiss CA, Morell A, Mahavadi AK, Ivan ME, Komotar RJ. The Role of Laser Interstitial Thermal Therapy in Surgical Neuro-Oncology: Series of 100 Consecutive Patients. Neurosurgery. 2020;87:266-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 76] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 98. | Schueller P, Micke O, Palkovic S, Schroeder J, Moustakis C, Bruns F, Schuck A, Wassmann H, Willich N. 12 years' experience with intraoperative radiotherapy (IORT) of malignant gliomas. Strahlenther Onkol. 2005;181:500-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 99. | Vaidya JS, Joseph DJ, Tobias JS, Bulsara M, Wenz F, Saunders C, Alvarado M, Flyger HL, Massarut S, Eiermann W, Keshtgar M, Dewar J, Kraus-Tiefenbacher U, Sütterlin M, Esserman L, Holtveg HM, Roncadin M, Pigorsch S, Metaxas M, Falzon M, Matthews A, Corica T, Williams NR, Baum M. Targeted intraoperative radiotherapy versus whole breast radiotherapy for breast cancer (TARGIT-A trial): an international, prospective, randomised, non-inferiority phase 3 trial. Lancet. 2010;376:91-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 530] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 100. | Nishioka A, Ogawa Y, Miyatake K, Tadokoro M, Nogami M, Hamada N, Kubota K, Kariya S, Kohsaki T, Saibara T, Okabayashi T, Hanazaki K. Safety and efficacy of image-guided enzyme-targeting radiosensitization and intraoperative radiotherapy for locally advanced unresectable pancreatic cancer. Oncol Lett. 2014;8:404-408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 101. | Zhou GX, Zeng TW, Wang LY, Ma L. Analysis of the long-term effect of intraoperative radiotherapy (IORT) for non-small cell lung carcinoma (NSCLC). Zhongguo Zhongliu Linchuang. 2007;4:65-70. [DOI] [Full Text] |
| 102. | Nemoto K, Ogawa Y, Matsushita H, Takeda K, Takai Y, Yamada S, Kumabe T. Intraoperative radiation therapy (IORT) for previously untreated malignant gliomas. BMC Cancer. 2002;2:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 103. | Fangusaro J, Onar-Thomas A, Poussaint TY, Wu S, Ligon AH, Lindeman N, Banerjee A, Packer RJ, Kilburn LB, Goldman S. LGG-02. A phase II Prospective Trial Of Selumetinib In Children With Recurrent/Progressive pediatric low-grade glioma (pLGG) with a focus upon optic pathway/hypothalamic tumors and visual acuity outcomes: a pediatric brain tumor consortium (PBTC) study, PBTC-029B. Neuro-Oncology. 2019;21:ii98-ii99. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 104. | De Waele J, Verhezen T, van der Heijden S, Berneman ZN, Peeters M, Lardon F, Wouters A, Smits ELJM. A systematic review on poly(I:C) and poly-ICLC in glioblastoma: adjuvants coordinating the unlocking of immunotherapy. J Exp Clin Cancer Res. 2021;40:213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 68] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 105. | Trippett T, Toledano H, Campbell Hewson Q, Verschuur A, Langevin AM, Aerts I, Howell L, Gallego S, Rossig C, Smith A, Patel D, Pereira LR, Cheeti S, Musib L, Hutchinson KE, Devlin C, Bernardi R, Geoerger B. Cobimetinib in Pediatric and Young Adult Patients with Relapsed or Refractory Solid Tumors (iMATRIX-cobi): A Multicenter, Phase I/II Study. Target Oncol. 2022;17:283-293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 106. | Cantwell-Dorris ER, O'Leary JJ, Sheils OM. BRAFV600E: implications for carcinogenesis and molecular therapy. Mol Cancer Ther. 2011;10:385-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 333] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 107. | Nicolaides T, Nazemi KJ, Crawford J, Kilburn L, Minturn J, Gajjar A, Gauvain K, Leary S, Dhall G, Aboian M, Robinson G, Long-Boyle J, Wang H, Molinaro AM, Mueller S, Prados M. Phase I study of vemurafenib in children with recurrent or progressive BRAF(V600E) mutant brain tumors: Pacific Pediatric Neuro-Oncology Consortium study (PNOC-002). Oncotarget. 2020;11:1942-1952. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 108. | Hargrave DR, Bouffet E, Tabori U, Broniscer A, Cohen KJ, Hansford JR, Geoerger B, Hingorani P, Dunkel IJ, Russo MW, Tseng L, Dasgupta K, Gasal E, Whitlock JA, Kieran MW. Efficacy and Safety of Dabrafenib in Pediatric Patients with BRAF V600 Mutation-Positive Relapsed or Refractory Low-Grade Glioma: Results from a Phase I/IIa Study. Clin Cancer Res. 2019;25:7303-7311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 141] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 109. | Kaley T, Touat M, Subbiah V, Hollebecque A, Rodon J, Lockhart AC, Keedy V, Bielle F, Hofheinz RD, Joly F, Blay JY, Chau I, Puzanov I, Raje NS, Wolf J, DeAngelis LM, Makrutzki M, Riehl T, Pitcher B, Baselga J, Hyman DM. BRAF Inhibition in BRAF(V600)-Mutant Gliomas: Results From the VE-BASKET Study. J Clin Oncol. 2018;36:3477-3484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 260] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 110. | Lassman AB, Sepúlveda-Sánchez JM, Cloughesy TF, Gil-Gil MJ, Puduvalli VK, Raizer JJ, De Vos FYF, Wen PY, Butowski NA, Clement PMJ, Groves MD, Belda-Iniesta C, Giglio P, Soifer HS, Rowsey S, Xu C, Avogadri F, Wei G, Moran S, Roth P. Infigratinib in Patients with Recurrent Gliomas and FGFR Alterations: A Multicenter Phase II Study. Clin Cancer Res. 2022;28:2270-2277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 62] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 111. | Bahleda R, Italiano A, Hierro C, Mita A, Cervantes A, Chan N, Awad M, Calvo E, Moreno V, Govindan R, Spira A, Gonzalez M, Zhong B, Santiago-Walker A, Poggesi I, Parekh T, Xie H, Infante J, Tabernero J. Multicenter Phase I Study of Erdafitinib (JNJ-42756493), Oral Pan-Fibroblast Growth Factor Receptor Inhibitor, in Patients with Advanced or Refractory Solid Tumors. Clin Cancer Res. 2019;25:4888-4897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 187] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 112. | Wood PJ, Strong R, McArthur GA, Michael M, Algar E, Muscat A, Rigby L, Ferguson M, Ashley DM. A phase I study of panobinostat in pediatric patients with refractory solid tumors, including CNS tumors. Cancer Chemother Pharmacol. 2018;82:493-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 113. | Arrillaga-Romany I, Kurz SC, Tarapore R, Sumrall A, Butowski NA, Harrison RA, De Groot JF, Chi AS, Shonka NA, Umemura Y, Odia Y. Single-agent ONC201 in recurrent H3 K27M-mutant diffuse midline glioma. J Clin Oncol. 2020;38. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 114. | Wen PY, De Groot JF, Battiste J, Goldlust SA, Garner JS, Friend J, Simpson JA, Damek D, Olivero A, Cloughesy TF. Paxalisib in patients with newly diagnosed glioblastoma with unmethylated MGMT promoter status: Final phase 2 study results. J Clin Oncol. 2022;. [DOI] [Full Text] |
| 115. | Wen PY, Cloughesy TF, Olivero AG, Morrissey KM, Wilson TR, Lu X, Mueller LU, Coimbra AF, Ellingson BM, Gerstner E, Lee EQ, Rodon J. First-in-Human Phase I Study to Evaluate the Brain-Penetrant PI3K/mTOR Inhibitor GDC-0084 in Patients with Progressive or Recurrent High-Grade Glioma. Clin Cancer Res. 2020;26:1820-1828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 116. | Franz DN, Agricola K, Mays M, Tudor C, Care MM, Holland-Bouley K, Berkowitz N, Miao S, Peyrard S, Krueger DA. Everolimus for subependymal giant cell astrocytoma: 5-year final analysis. Ann Neurol. 2015;78:929-938. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 117] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 117. | National Library of Medicine (U.S.). (2024, February) A Study to Test the Safety and Efficacy of the Drug Larotrectinib for the Treatment of Tumors With NTRK-fusion in Children (SCOUT) [Identifier: NCT02637687] Available from: https://clinicaltrials.gov/study/NCT02637687. |
| 118. | National Library of Medicine (U.S.). (2024, February) A Study to Test the Effect of the Drug Larotrectinib in Adults and Children With NTRK-fusion Positive Solid Tumors (NAVIGATE) [Identifier: NCT02576431] Available from: https://clinicaltrials.gov/study/NCT02576431. |
| 119. | Desai AV, Robinson GW, Gauvain K, Basu EM, Macy ME, Maese L, Whipple NS, Sabnis AJ, Foster JH, Shusterman S, Yoon J, Weiss BD, Abdelbaki MS, Armstrong AE, Cash T, Pratilas CA, Corradini N, Marshall LV, Farid-Kapadia M, Chohan S, Devlin C, Meneses-Lorente G, Cardenas A, Hutchinson KE, Bergthold G, Caron H, Chow Maneval E, Gajjar A, Fox E. Entrectinib in children and young adults with solid or primary CNS tumors harboring NTRK, ROS1, or ALK aberrations (STARTRK-NG). Neuro Oncol. 2022;24:1776-1789. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 68] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 120. | National Library of Medicine (U.S.). (2023, November) Study of Zotiraciclib for Recurrent High-Grade Gliomas with Isocitrate Dehydrogenase 1 or 2 (IDH1 or IDH2) Mutations [Identifier: NCT05588141] Available from: https://clinicaltrials.gov/study/NCT05588141. |
| 121. | Mellinghoff IK, van den Bent MJ, Blumenthal DT, Touat M, Peters KB, Clarke J, Mendez J, Yust-Katz S, Welsh L, Mason WP, Ducray F, Umemura Y, Nabors B, Holdhoff M, Hottinger AF, Arakawa Y, Sepulveda JM, Wick W, Soffietti R, Perry JR, Giglio P, de la Fuente M, Maher EA, Schoenfeld S, Zhao D, Pandya SS, Steelman L, Hassan I, Wen PY, Cloughesy TF; INDIGO Trial Investigators. Vorasidenib in IDH1- or IDH2-Mutant Low-Grade Glioma. N Engl J Med. 2023;389:589-601. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 403] [Cited by in RCA: 343] [Article Influence: 171.5] [Reference Citation Analysis (0)] |
| 122. | Mellinghoff IK, Cloughesy TF, Wen PY, Taylor JW, Maher EA, Arrillaga I, Peters KB, Choi C, Ellingson BM, Lin AP, Thakur SB. A phase I, open label, perioperative study of AG-120 and AG-881 in recurrent IDH1 mutant, low-grade glioma: Results from cohort 1. J Clin Oncol. 2019;37. [RCA] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 123. | Weller M, Butowski N, Tran DD, Recht LD, Lim M, Hirte H, Ashby L, Mechtler L, Goldlust SA, Iwamoto F, Drappatz J, O'Rourke DM, Wong M, Hamilton MG, Finocchiaro G, Perry J, Wick W, Green J, He Y, Turner CD, Yellin MJ, Keler T, Davis TA, Stupp R, Sampson JH; ACT IV trial investigators. Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): a randomised, double-blind, international phase 3 trial. Lancet Oncol. 2017;18:1373-1385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 546] [Cited by in RCA: 786] [Article Influence: 98.3] [Reference Citation Analysis (0)] |
| 124. | Lassman AB, Pugh SL, Wang TJC, Aldape K, Gan HK, Preusser M, Vogelbaum MA, Sulman EP, Won M, Zhang P, Moazami G, Macsai MS, Gilbert MR, Bain EE, Blot V, Ansell PJ, Samanta S, Kundu MG, Armstrong TS, Wefel JS, Seidel C, de Vos FY, Hsu S, Cardona AF, Lombardi G, Bentsion D, Peterson RA, Gedye C, Bourg V, Wick A, Curran WJ, Mehta MP. Depatuxizumab mafodotin in EGFR-amplified newly diagnosed glioblastoma: A phase III randomized clinical trial. Neuro Oncol. 2023;25:339-350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 75] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 125. | Wisoff JH, Sanford RA, Heier LA, Sposto R, Burger PC, Yates AJ, Holmes EJ, Kun LE. Primary neurosurgery for pediatric low-grade gliomas: a prospective multi-institutional study from the Children's Oncology Group. Neurosurgery. 2011;68:1548-54; discussion 1554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 171] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 126. | Lima GL, Duffau H. Is there a risk of seizures in "preventive" awake surgery for incidental diffuse low-grade gliomas? J Neurosurg. 2015;122:1397-1405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 127. | Duffau H. Long-term outcomes after supratotal resection of diffuse low-grade gliomas: a consecutive series with 11-year follow-up. Acta Neurochir (Wien). 2016;158:51-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 175] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 128. | Ng S, Herbet G, Moritz-Gasser S, Duffau H. Return to Work Following Surgery for Incidental Diffuse Low-Grade Glioma: A Prospective Series With 74 Patients. Neurosurgery. 2020;87:720-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 56] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 129. | Ng S, Herbet G, Lemaitre AL, Cochereau J, Moritz-Gasser S, Duffau H. Neuropsychological assessments before and after awake surgery for incidental low-grade gliomas. J Neurosurg. 2020;135:871-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 130. | Goel A, Shah A, Vutha R, Dandpat S, Hawaldar A. Is "En Masse" Tumor Resection a Safe Surgical Strategy for Low-Grade Gliomas? Feasibility Report on 74 Patients Treated Over Four Years. Neurol India. 2021;69:406-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 131. | Ius T, Ng S, Young JS, Tomasino B, Polano M, Ben-Israel D, Kelly JJP, Skrap M, Duffau H, Berger MS. The benefit of early surgery on overall survival in incidental low-grade glioma patients: A multicenter study. Neuro Oncol. 2022;24:624-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |