Published online Jun 26, 2022. doi: 10.4330/wjc.v14.i6.372
Peer-review started: January 25, 2022
First decision: March 16, 2022
Revised: April 13, 2022
Accepted: May 14, 2022
Article in press: May 14, 2022
Published online: June 26, 2022
Processing time: 146 Days and 18.4 Hours
Coronary sinus (CS) imaging has recently gained importance due to increasing need for mapping and ablation of electrophysiological arrhythmias and left ventricular (LV) pacing during cardiac resynchronization therapy (CRT). Retr
To evaluate CS anatomy during levophase of routine coronary angiography to aid LV lead implantation during CRT.
In this prospective observational study, 164 patients undergoing routine coronary angiography for various indications (Chronic stable angina-44.5%, acute coronary syndrome- 39.5%, Dilated cardiomyopathy-11%, atypical chest pain-5%) were included. Venous phase (levophase) of left coronary injection was recorded in left anterior oblique - cranial and right anterior oblique -cranial views. Visibility of coronary veins, width and shape of CS ostium, angulations of proximal CS with body of CS were noted. Presence, size, take-off angle and tortuosity of posterolateral vein (PLV), anterior interventricular veins (AIV) and middle cardiac vein (MCV) were also noted.
During levophase, visibility grade (Muhlenbruch grade) for coronary veins was 3 in 74% and 2 in 26% of cases. Visibility of CS did not correlate with body mass index. The diameter of CS ostium was < 10 mm, 10-15 mm and > 15 mm in 48%, 42% and 10% of patients respectively. Proximal CS was tubular in 136 (83%) patients and funnel-shaped in 28 (17%) patients. Sharp take-off angulation between ostium and body of CS was seen in 16 (10%) patients. Two or more PLV were present in 8 patients while PLV was absent in 52 (32%) patients. Angle of take-off of PLV with body of CS was favourable (0°-45°) in 65 (40%) patients. The angle was 45°-90° in 36 patients and difficult take-off angle (> 90°) was seen in 8 patients. Length of PLV reached distal third of myocardium in 84 cases and middle third in 11 cases. There was no tortuosity in 79 cases, a single bend in 29 cases and more than 2 bends in 4 cases. Thirty nine (24%) patients had other veins supplying posterior/Lateral wall of LV. There was a single vein supplying lateral/posterior wall in 31 (19%) patients. Diameter of MCV and AIV was significantly larger in patients with absent PLV as compared to patients with a PLV.
Levophase study of left coronary injection is effective in visualization of the CS in almost all patients undergoing coronary angiography and may be an effective alternative to retrograde venogram in patients with LV dysfunction or LBBB.
Core Tip: In this prospective, observational study, we assessed venous phase of coronary angiogram (n = 164) with the intent to evaluate coronary sinus anatomy for purpose of left ventricular (LV) lead placement during cardiac resynchronization therapy. Levophase analysis showed excellent visibility of coronary sinus and its tributaries irrespective of body mass index. Shape of ostium & angulations within body of coronary sinus could be delineated reliably. Number, size, take off angle and any tortuosity within postero-lateral vein could be well identified. We found levophase study of coronary angiography an acceptable alternative to retrograde venography for LV lead placement assessment.
- Citation: Pradhan A, Bajaj V, Vishwakarma P, Bhandari M, Sharma A, Chaudhary G, Chandra S, Sethi R, Narain VS, Dwivedi S. Study of coronary sinus anatomy during levophase of coronary angiography. World J Cardiol 2022; 14(6): 372-381
- URL: https://www.wjgnet.com/1949-8462/full/v14/i6/372.htm
- DOI: https://dx.doi.org/10.4330/wjc.v14.i6.372
Imaging of the coronary venous system is usually overshadowed by that of the coronary arteries. However, imaging of coronary venous system has gained importance in recent past, as many cardiac interventions currently use the coronary sinus e.g., mapping and ablation of various arrhythmias, left ventricular (LV) pacing, targeted drug delivery, and stem cell therapy. The coronary sinus (CS) is the largest cardiac venous structure and is frequently cannulated during electrophysiologic and interventional procedures. Thus, detailed knowledge of its normal anatomy and anomalies is of paramount importance to avoid any complications[1,2].
The cardiac veins are classified, according to the region they drain viz. the CS and its tributaries, the anterior cardiac veins, and the thebesian veins[3-6]. The anterior cardiac veins are the primary venous return for the anterior wall of the right ventricle. They include three or four small veins which drain directly into the right atrium either separately or after forming a common venous trunk before emp
The major tributaries of the CS include: (1) The anterior interventricular vein; (2) The great cardiac vein (GCV); (3) The left marginal vein and posterior vein; and (4) the middle cardiac vein or posterior interventricular vein. The CS is variable in size. It varies 45 to 63 mm in length, and the size of the ostium ranges from 4 mm × 5 mm to 9 mm × 16 mm[7-10].
Cardiac resynchronization therapy (CRT) involves LV pacing in patients with LV systolic dysfunction to improve synchronization of LV conduction. It yields the best results when the LV lead is implanted in area of latest mechanical activation, with the lateral and posterior branches being the usual target veins for this procedure[3]. Hence, CS imaging can play an important role in planning LV lead implantation strategy and help identify suitable veins in area of interest. The most co mmonly employed technique to visualize CS anatomy is occlusive retrograde venography. In this study, we evaluated venous phase (levophase) of coronary angiography to assess its utility in studying CS anatomy.
All patients above 18 years of age undergoing coronary angiography for various indications between November 2017 and October 2018 were considered for inclusion. Patients who were in cardiogenic shock, had congestive heart failure, had eGFR < 50 mL/min, those having severe left main disease or complex congenital heart disease were excluded from the study.
Our study included patients who were undergoing invasive coronary angiography for different reasons such as acute coronary syndromes or stable angina. A written informed consent was taken from all patients in our study. Baseline characteristics were recorded in all patients which included age, sex, height, weight, NYHA functional class, cardiovascular risk factors like diabetes mellitus, hypertension, smoking, prior history of coronary artery disease (CAD) and left ventricular ejection fraction (LVEF).
Patients underwent a coronary angiogram from either the radial route using a 5F tiger catheter or femoral route with a 5F/6F Judkins left diagnostic catheter. A non-ionic, low osmolarity, iodide contrast medium - Iohexol (Omnipaque, GE Healthcare, Chicago, Illinois, United States) was used through manual injection in all cases. After canulating the left coronary ostium, a left anterior oblique (LAO) caudal view was recorded to detect patients with severe left main disease or any abnormality affecting the safety of the procedure. Further coronary angiogram was done with necessary angiographic view as per detected lesions. LAO with cranial angulation (LAO 40 degrees, cranial 30 degrees) and right anterior oblique (RAO) with cranial angulation (RAO 30 degrees, cranial 30 degrees) views were recorded for all patients. These are the views in which venous anatomy was studied. Venous phase (levo-phase) of injection was recorded beyond the initial 5-10 seconds to visualise the coronary venous anatomy in these LAO- cranial and RAO-cranial views. Recording was done on cine mode at rate of 15 frames per second. The rest of the coronary angiogram and all other procedure including intervention, post-procedure care was done as per routine. In some cases, the coronary venous anatomy could also be seen in other views taken during the study. When this revealed additional information, they were also included in our analysis.
Visibility of the coronary veins was classified on the 0-3 point scale described by Muhlenbruch et al[10] (Table 1).
| Grade | Visibility |
| 0 | Not visible |
| 1 | Visible but with discontinuities |
| 2 | Visible but with irregular borders |
| 3 | Visible with vascular borders perfectly defined |
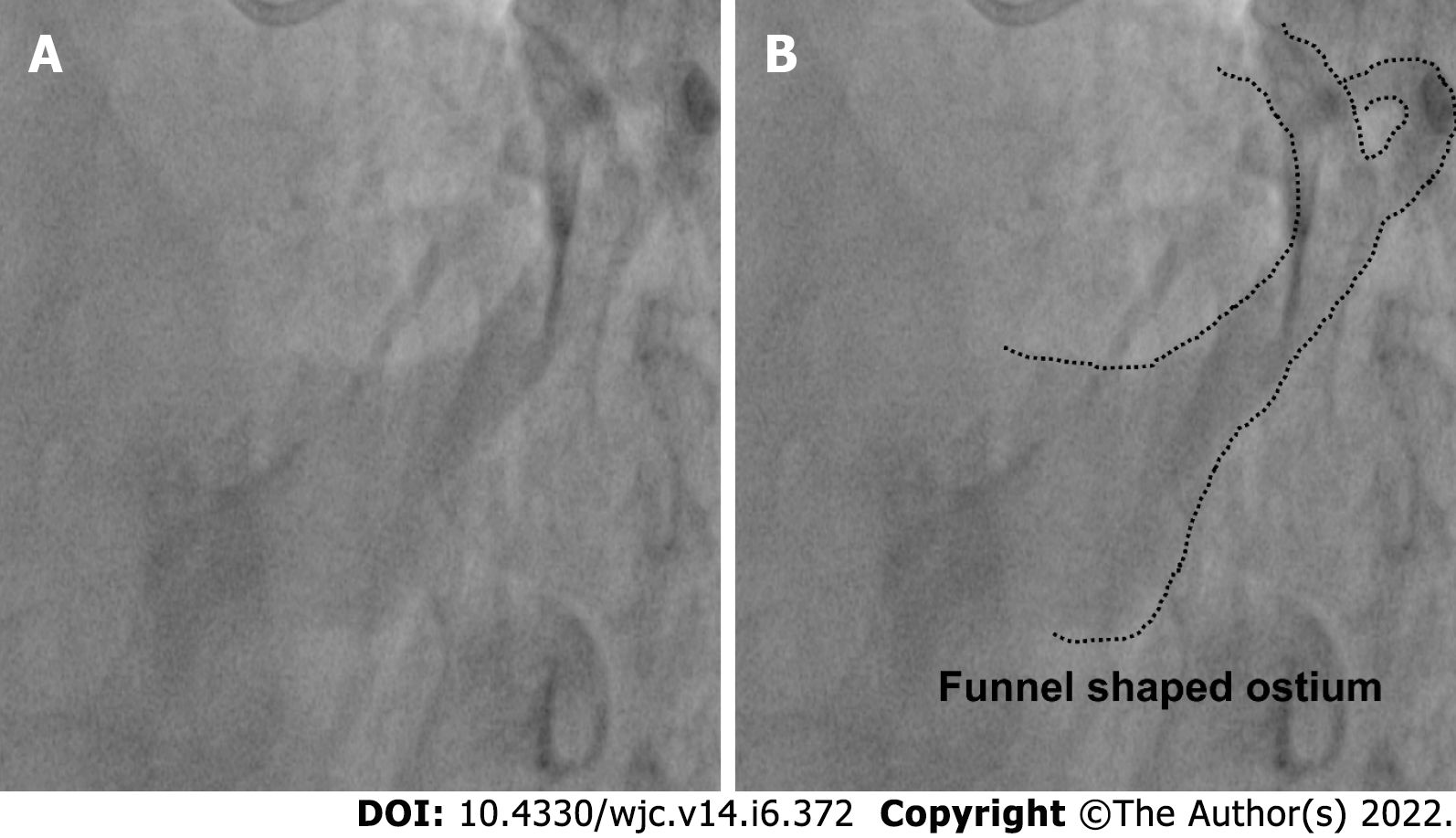
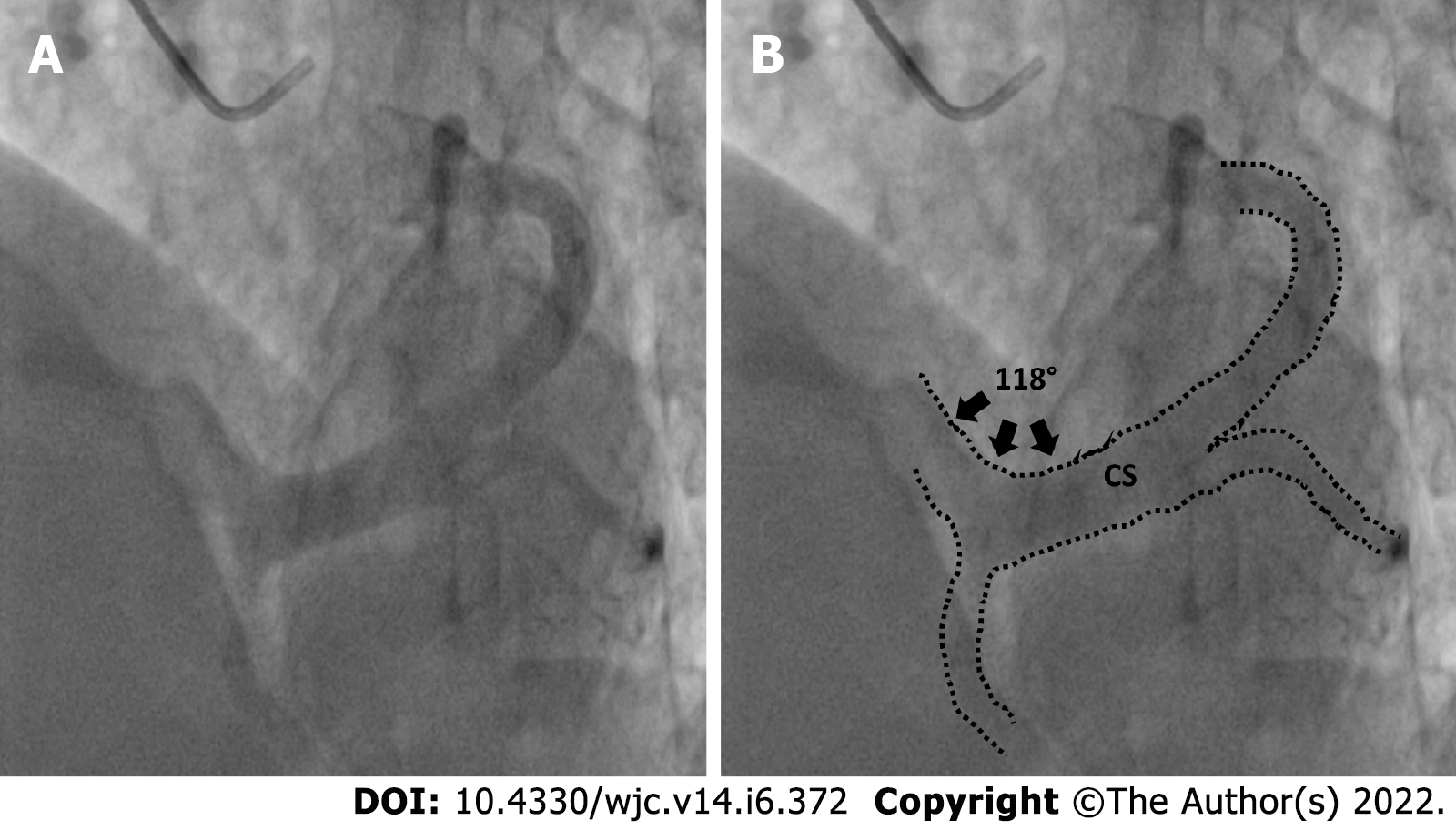
Measurements were taken on the CAAS QCA software based on pixel size and comparison with the catheter diameter. Width of the coronary sinus ostia was measured. Shape of the coronary sinus: tubular, funnel-shaped or other was recorded (Figure 1). The proximal portion of the coronary sinus was studied subjectively for factors that affect sheath selection such as angulation and presence of a thebesian valve. Superior angulation of the proximal portion of the coronary sinus with the body of the coronary sinus was measured in the LAO cranial view and classified into 3 groups: (Figure 2) Group 1: 0°-30°; Group 2: 31°-60° and Group 3: > 60°. The presence of posterior angulation of the coronary sinus ostia with the body of the coronary sinus was recorded in the RAO cranial view. LAO cranial view was studied for the presence of a Thebesian valve. A Thebesian valve was detected by noting an upward direction of outflow into the right atrium (RA) from the coronary sinus, and by a smooth inferior border of the outflow jet.
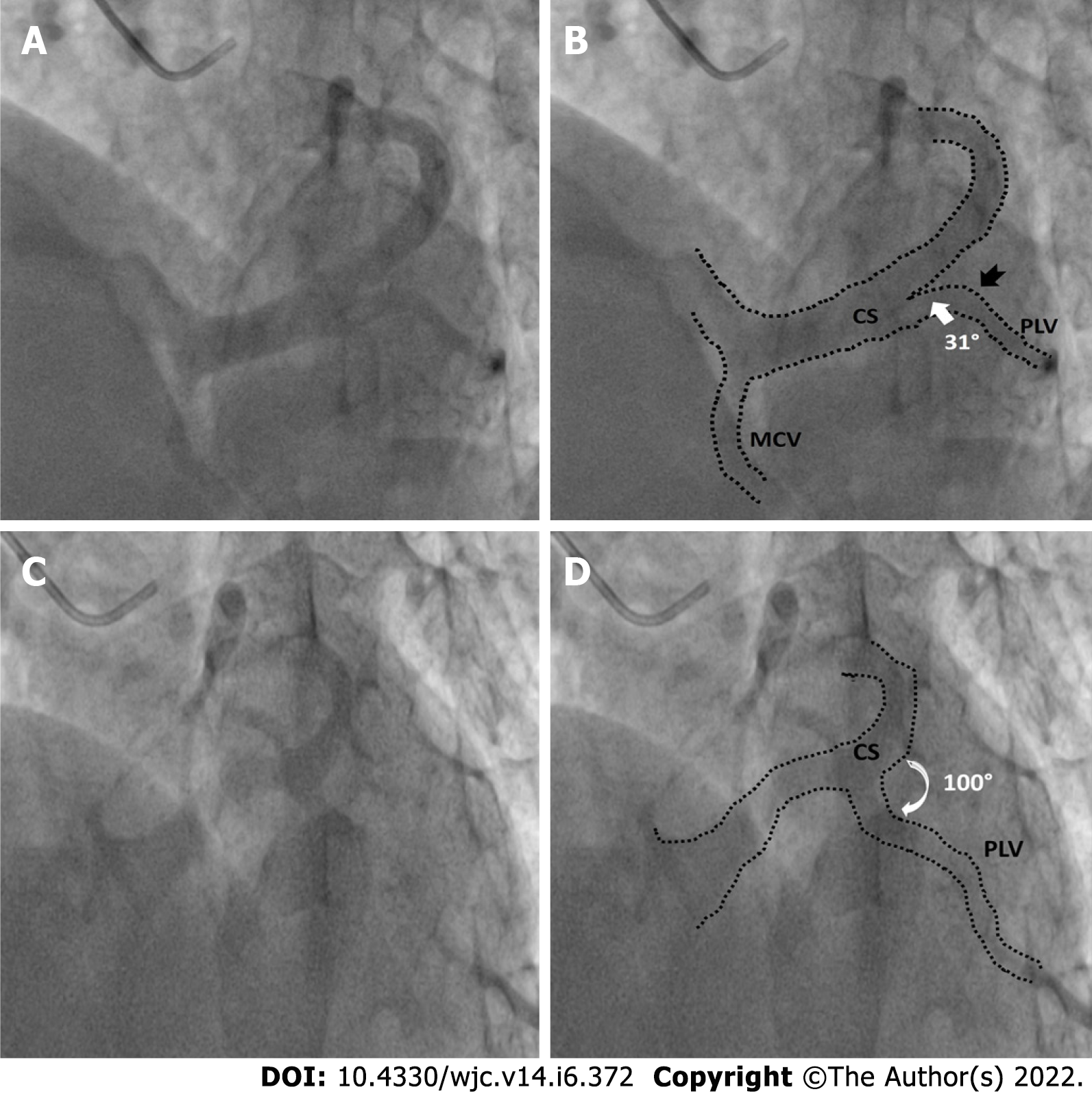
Other anomalies in venous anatomy such as posterior lateral vein (PLV) draining near the coronary sinus ostia or directly into the RA, left superior vena cava (SVC) were also recorded when relevant. Number of suitable (> 2 mm diameter) PLVs were noted. Veins with a diameter of > 2 mm measured 2cms distal to its ostium were considered favourable. Veins tapering or dividing into venules of less than 2 mm size before running 2 cms as seen in two recorded views were classified as unfavourable. The diameter of the PLV, measured at 2 cms distal to its ostia with the coronary sinus was noted. In cases with > 1 PLV, the diameter of the largest PLV was recorded. The angle made by the PLV with body of CS was also noted in the LAO cranial view and classified into 3 groups: (Figure 3) - Easy take-off: (0° to 45°); Medium difficulty: 45° to 90°; Difficult take-off: > 90°.
When a PLV was absent, the incidence of other tributaries draining the posterior-lateral part of the LV was studied. Diameter of the anterior interventricular vein (AIV) and the middle cardiac vein (MCV) was recorded in all cases. Comparisons were made of the diameters of the AIV and MCV in patients with and without a PLV. Tortuosity of the PLV most suitable for the purpose of CRT LV lead was studied, and the number of bends were also recorded.
Demographic data were reported as percentages, mean and median were calculated when applicable. Chi square test and the student t-test were used for statistical analysis. P value < 0.05 was considered statistically significant. All data were analyzed using the Statistical Package for the Social Sciences (IBM SPSS; Chicago, IL, United States) program, version 20.
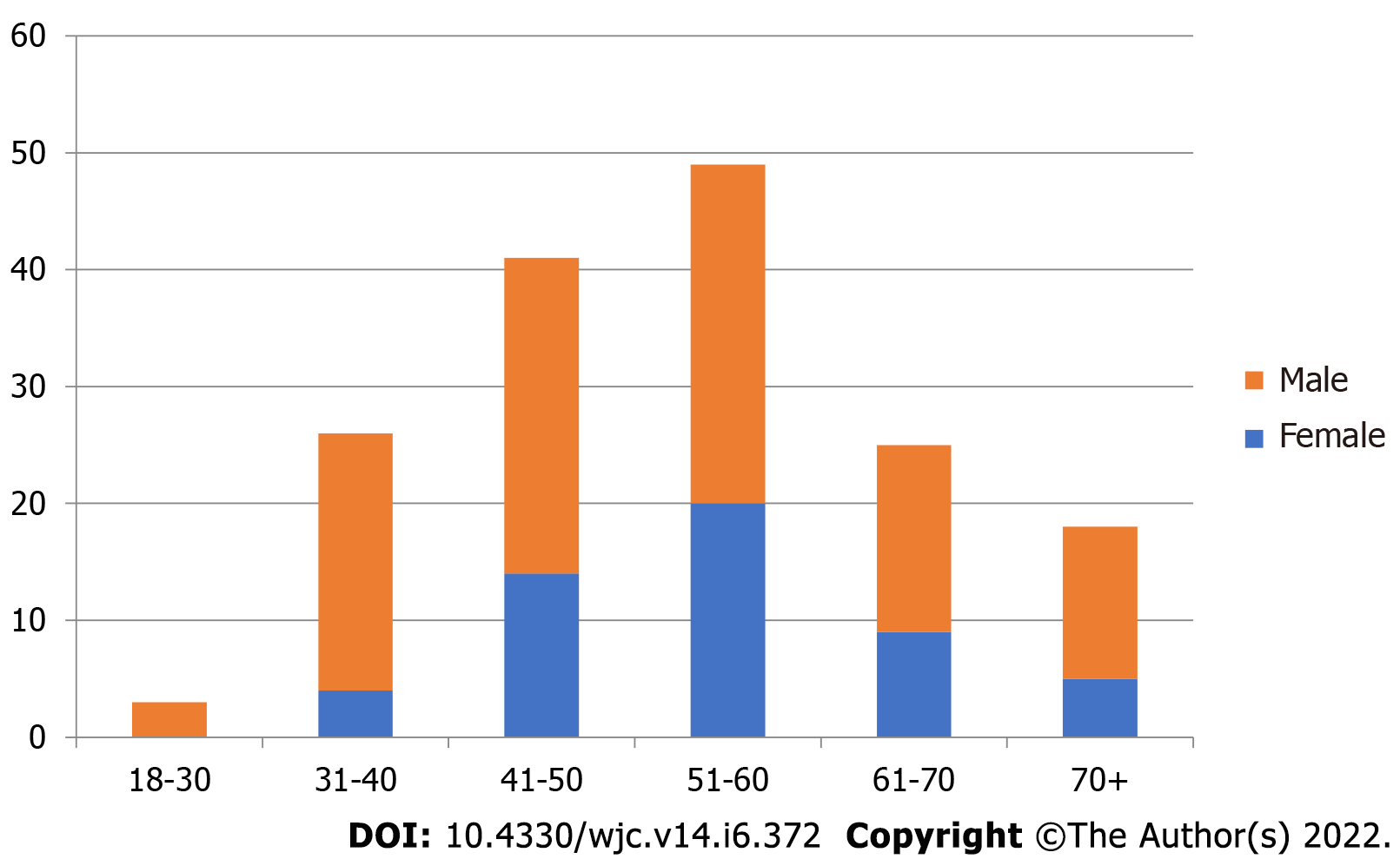
A total of 168 patients undergoing coronary angiography for various indications between November 2017 and October 2018 were included in our study. Coronary sinus could not be assessed in four patients due to a suboptimal study (inadequate volume of dye given, too short cine time) and were excluded from analysis. Data of the remaining 164 patients was analysed. 116 (72%) were male and the mean age of the study population was 53.3 years (Figure 4). The various indications for coronary angiography were Chronic Stable Angina in 73 (44.5%) cases, Acute Coronary Syndrome in 65 (39.5%) patients [43 with Non-ST elevation Myocardial infarction, 22 With ST-elevation myocardial infarction], Dilated cardiomyopathy/ischemic cardiomyopathy was present in 11 (7%) patients. Angiography was performed for the evaluation of atypical chest pain in 09 (5%) patients, 2 patients had VT in a structurally normal heart, 3 patients underwent routine angiography prior to valve replacement, and 1 patient had PSVT with angina. The LVEF of patients ranged from 22%-76%. Eleven patients (7%) had an EF of less than 35%. 93 patients (57%) had an EF of 50% and above. The body mass index (BMI) in 53% of patients ranged from 18-24, 6% had a BMI of 30 or above and 14% had a BMI of less than 18. The visibility of CS did not correlate with BMI (P = 0.69). The visibility score was 3 in 121 (74%) cases, 2 in 43 (26%) cases, < 2 in 0 cases. Access was via right radial artery in 134 (89%) patients, right femoral artery in 16 (11%) patients. The mean contrast medium volume used was 65.2 ± 26.6 mL. Among complications, two patients developed grade 1 radial hematoma, and none had any complications related to femoral arterial access. No contrast-induced nephropathy was seen in our study.
The diameter of CS ostia ranged from 2 mm-18 mm.The diameter was < 10 mm in 80 (48%) of the patients, 10-15 mm in 74 (42%) of patients and > 15 mm in 10% patients. Ostial stenosis (diameter < 2 mm) was present in 1 case with a co-existing left SVC. The shape of proximal portion of CS was tubular in 136 (83%) patients and funnel-shaped in 28 (17%) patients (Figure 1). The superior Angle between body of CS and ostia (take-off angle) in LAO cranial view was 0°-30° (horizontal) in 102 (63%) patients, 31°-60° (intermediate) in 45 (27%) patients and > 60° (sharp angulation) in 16 (10%) patients (Figure 2). Significant posterior indentation in RAO cranial view was seen in 5 patients. Presence of a thebesian valve (directing inferior border of flow into RA superiorly as seen in LAO cranial view) was present in 21 cases. Two or more PLV were present in 8 patients while PLV was absent in 52 (32%) patients. The diameter of the largest PLV was 4.1 mm. Angle of take-off of PLV with body of CS was favourable in 65 (40%) patients (0°-45°). The angle was 45°-90° in 36 patients and difficult take-off angle (> 90°) was seen in 8 patients. Extension or length of largest PLV (from base to apex) was assessed in RAO cranial view. Base to apex length was equally divided into 3 equal parts-proximal, mid and distal one third. It reached distal third in 84 patients, up to mid third in 11 patients and remained in proximal third in 5 patients. No bends were seen in PLV in 79 cases. There was single bend in 29 cases and more than 2 bends in 4 cases. 39 (24%) patients had other veins supplying posterior/lateral wall of LV. There was a single vein supplying lateral/posterior wall in 31 (19%) patients.
Diameter of the middle cardiac vein (MCV) was significantly larger in patients with absent PLV, compared to patients with a PLV. The mean diameter of MCV in patients without a PLV (n = 52) was 4.9 mm (SD 1.7), the mean diameter of patients with a PLV (n = 112) was 3.3 mm (SD 1.5), P = 0.000291. Similarly, diameter of the anterior inter-ventricular vein (AIV) was larger in patients with absent PLV compared to patients with a PLV. Mean diameter of AIV in patients without PLV was 3.3 mm (SD 1.2), the mean diameter of AIV in patients with a PLV was 2.6 mm (SD 1.3). However, the difference did not reach statistical significance (P = 0.076).
Of the 13 cases in which no suitable vein was visible, aberrant origin of left circumflex coronary artery (LCX) via the right sinus was detected in retrospect in 1 patient. Five patients had severe CAD involving the LCX, one patient had severe disease involving both the distal right coronary artery (RCA) and the LCX. The MCV was larger than 6 mm in 3 patients, The AIV was > 5 mm in 1 patient. No cause could be found in 2 patients.
Severe CAD involving the arterial supply of the posterior lateral wall of LV was present in 23 out of 50 (46%) patients with an absence of PLV, compared to 18 out of 100 (18%) patients with a PLV (Relative Risk (RR) = 2.55; P value 0.0086). Other findings included separate opening of the PLV into the RA in 1 patient. PLV opening near the ostia of the coronary sinus seen in 6 patients.
In our observational study of levophase of coronary angiograms, we analysed coronary venous anatomy for the purpose of cardiac resynchronization therapy (CRT) LV lead placement. Coronary sinus lead placement for resynchronisation therapy is required at all ages, and younger patients of dilated cardiomyopathy are a common subgroup. There is no sex predilection for dilated cardiomyopathy. In our study the median age was 56 years. Sex ratio was 2.5:1 (male:female). Age and sex distribution of the study is characteristic of the patient population seeking treatment at our tertiary care centre. This phenomenon is known to skew data of studies conducted in developing countries. There is a strong referral bias; female patients are more likely not to reach a tertiary care centre for treatment. The sex ratio in similar studies carried out in the western countries were 0.8:1 in a study of retrograde balloon occlusion venography by Mischke et al, and 1.2:1 in a study of levo-phase coronary angiograpy by Gilard et al[11,12]. Similarly there was no gender bias in anatomical studies of the coronary venous anatomy on human cadavers.
Nearly all cases in our study underwent coronary angiography for the evaluation and treatment of coronary artery disease. Only 11 patients had severe LV dysfunction which is present in all cases requiring CRT. Although the branching pattern can be assumed to be unaffected by the development of cardiomyopathy, there can be significant change in angle of opening of coronary ostium in cases with significant enlargement of left heart and normal sized RA and RV. Our study had too few cases of cardiomyopathy to be able to detect such a difference. The coronary sinus was adequately visualized in all cases. The borders were sharply delineated in a majority (visibility scale 3 in 74%). In the rest of the cases the borders were hazy but sufficient for the purpose of guiding the planning of LV lead implantation. Surprisingly, obesity (BMI) did not affect visibility. Because we obtained the CS anatomy in levophase in patients undergoing coronary angiography only in two views without any extreme angulation, insignificant amount of additional radiation exposure was anticipated.
The visibility of the venous system in our study was comparable to that attained by 360 slice CT study conducted by Chunjuan Sun et al[13]. In our study we identified a separate opening of the PLV into the RA in 1 case, and a suitable PLV opening close to the CS ostia in 6 cases. These are visualized better antegrade than retrograde as selective hooking will cause them not to be opacified. Meisel et al[14] in their study on retrograde CS venography obtained optimum anatomical information in 67% of cases, compared to 92% in our study.
It is believed that retrograde occlusive venography better visualises the CS anatomy but it has its own lacunae. Some physicians use hyperaemic agents to aid visualization of CS in levophase. Arbelo E et al[15] compared occlusive retrograde venography with hyperaemic venous return angiography (levophase of coronary angiography). They used 200 µg of intracoronary Nitroglycerine or 60 µg of Adenosine to increase the coronary flow and thus the venous return in coronary sinus. The anatomical information obtained by both methods was adequate (100% vs 97.5%, respectively). Although occlusive retrograde venography is the most co mmonly employed technique, it has its own drawbacks. First, in the absence of venous anastamoses, veins with a posterior origin may not be visualized. Sometimes balloon does not provide a completely occlusive barrier, and 2 injections (distal and proximal) are then required to highlight the venous anatomy. In addition, for the opacification of the AIV, contrast medium has to be injected with the balloon inflated more distally in the GCV. This may lead to complications such as dissection of CS and heart blocks[15].
We utilized 2 orthogonal views viz. LAO Cranial (40°, 30°), and RAO Cranial (30°, 30°) to view the Coronary Sinus. The LAO Cranial view showed the coronary ostia clearly. This view visualizes the proximal portion of CS without foreshortening and we can also see its angulation with main body of CS. Posterior vein, PLV, lateral vein were also well differentiated in this view along with the take-off angle of the PLV with the body of the CS. However, the distal portion of the Coronary Sinus was foreshortened in this view, making it hard to differentiate an anterolateral vein from an AIV. Presence of a thebesian valve was detected by noting an upward direction of the outflow jet into the RA. The inferior border of the outflow is smooth. We found evidence of a thebesian valve in 21 cases. An obstructive thebesian valve may require an Amplatz guiding catheter to cannulate the CS ostium. Mischke K et al[11] in their study of coronary venous anatomy found evidence of a thebesian valve in 11 out of 100 cases. These figures are much less than those found in anatomical studies carried out by Randhawa et al[16] (thebesian valves seen in 50 dissections) and by Noheria et al[17] (309 valves seen in 643 dissections). Small sized valves visible on direct examination are of little clinical significance[15,16].
RAO cranial view showed the ostia and the proximal portion of the CS end on. The proximal portion was however foreshortened. Posterior indentation of the proximal portion of the CS before joining the main CS body was studied in this view. We found this in 5 (3%) of 164 cases. This view was most useful for showing the length of the vein, from the base of the heart (AV groove) to the apex. In our study, we divided this base to apex distance into 3 equal lengths: Proximal, mid, and distal; 81% of the PLVs extended upto the distal one third.
In some cases, the coronary venous anatomy could also be seen in other views taken during the study. The RAO caudal view was very useful in showing the branches of the MCV, which were not visible in our 2 cranial views. Having more views also helped in showing the PLV in greater detail. More bends could be seen when more views were available.
The optimal site for LV lead placement is the posterior lateral wall of the LV. Of the 164 patients in our study, 151 (92%) had a suitable vein draining the posterior lateral wall of the LV. In 112 cases this was the PLV; in another 39, other veins drained this area. A critically stenosed LCX or a super-dominant RCA were strongly associated with failure to visualize an adequate vein. In one case in which the PLV was not visualized, the patient was found to have an anomalous LCX. If dye had been injected into this anomalous vein (instead of into the LAD) a PLV would probably be seen. The PLV opacifies if dye is injected into the region (posterior lateral wall of the LV) that it drains. In most cases the artery supplying the area drained by the PLV is the LCX, in some it is a super-dominant RCA. If the LCX has flow limiting stenosis, an anomalous origin, or if there is a super-dominant RCA, the posterior lateral part of the LV does not recieve enough dye and likely remains hidden.
We found 4 cases with a very large MCV or AIV (6 mm). Patients without a PLV were found to have on an average 1.5 mm larger MCV than patients with a visible PLV (P > 0.05). The AIV was found to be on an average 0.7 mm larger in cases without a PLV, compared to cases with a PLV; but this did not reach statistical significance. An absent PLV was found in 52 cases in our study. In 39 of these cases, there was another vein supplying the posterior-lateral wall of the LV. The number of cases in which the PLV is absent is far greater in our study than in a similar study using levo-phase coronary angiography by Gilard et al[12] or in the anatomical studies on human cadavers by Randhawa et al[16] and by Noheria et al[17]. We did not count small veins (unsuited for CRT LV lead placement) in our study. Veins with a diameter of > 2 mm measured 2cms distal to its ostium were considered favourable. The larger number of cases with no PLV seen in our study, is explained when this is considered. When a PLV was present, it was generally straight with 62% having no bends. The take-off angle was generally favourable, with an unfavourable take-off seen in only 7% patients.
Non availability of a suitable branch draining the lateral or posterior LV wall is an indication for epicardial lead placement by thoracotomy. In our study, we were unable to visualize an adequate vein in 13/164 cases, although the number of cases in which it is truly absent may be lower.
We evaluated only 2 levo-phase views of left coronary angiograms in our study. Use of more views may better identify the venous anatomy. We did not study the venous tree in right coronary angiograms. Although this is not required in most cases, our study shows that visualization from left injections is poor when circumflex territory is not perfused by the left coronary artery. The technique increases total quantity of dye, and radiation during coronary angiogram, and this may be significant in very sick patients in poor general condition. We excluded such patients from our study.
We did not compare our results with retrograde occlusive venography because it may increase the contrast and radiation dose. We also did not use a hyperaemic agent (e.g., nitroglycerine or adenosine) to increase coronary flow and resultant venous return in CS as used in some studies because it might cause hypotension and/or bradycardia in some patients. In addition, adenosine use may not be feasible in patients with bronchial asthma and severe obstructive pulmonary disease.
Levo-phase study of left coronary injection is effective in visualization of the coronary sinus in almost all patients undergoing coronary angiography. Visualization of postero-lateral veins is usually adequate in LAO cranial view, but the veins of interest are not well visualized in patients with occluded left circumflex artery and also in cases with very large right coronary and small left coronary artery. These patients may require levo-phase study of RCA or selective coronary sinus ostium cannulation. But broadly talking, levo-phase study of left coronary angiogram in LAO cranial view may be an useful extension of the standard coronary angiogram protocol, especially in patients with LV dysfunction or LBBB.
Coronary sinus imaging is gaining importance due to improvement in electrophysiology techniques and wide spread use of left ventricular lead as a part of Cardiac resynchronization therapy.
Although standard technique is retrograde venogram, it has its own challenges.
To evaluate the feasibility of levophase of routine coronary angiography for studying coronary anatomy.
We conducted a prospective observational in patients undergoing routine coronary angiography. In two angiographic views (left anterior oblique & right anterior oblique with cranial angulation), we evaluated the levophase for coronary sinus anatomy including ostial uptake, size, branches and angulation.
The levophase coronary angiography achieved good visibility in almost all patients. Most patients had a tubular coronary sinus ostia with size < 10 mm. Sharp take off between ostia and body as well tortuosity were seen only in a minority.
Levophase coronary angiogram evaluation for coronary sinus anatomy is safe and highly effective.
Large randomized studies are need to further substantiate the results.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: American College of Cardiology, Society for Cardiovascular Angiography and Interventions, Asian Pacific Society of Interventional Cardiology; European Society of Cardiology, Heart Failure Association of European Society of Cardiology, Cardiological Society of India.
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Papafaklis M, Greece; Teragawa H, Japan S-Editor: Wang LL L-Editor: A P-Editor: Wang LL
| 1. | Abraham WT. Cardiac resynchronization therapy: a review of clinical trials and criteria for identifying the appropriate patient. Rev Cardiovasc Med. 2003;4 Suppl 2:S30-S37. [PubMed] |
| 2. | Sanders P, Jaïs P, Hocini M, Haïssaguerre M. Electrical disconnection of the coronary sinus by radiofrequency catheter ablation to isolate a trigger of atrial fibrillation. J Cardiovasc Electrophysiol. 2004;15:364-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 3. | Singh JP, Houser S, Heist EK, Ruskin JN. The coronary venous anatomy: a segmental approach to aid cardiac resynchronization therapy. J Am Coll Cardiol. 2005;46:68-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 119] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 4. | Ortale JR, Gabriel EA, Iost C, Márquez CQ. The anatomy of the coronary sinus and its tributaries. Surg Radiol Anat. 2001;23:15-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 69] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 5. | Loukas M, Bilinsky S, Bilinsky E, el-Sedfy A, Anderson RH. Cardiac veins: a review of the literature. Clin Anat. 2009;22:129-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 6. | Ho SY, Sánchez-Quintana D, Becker AE. A review of the coronary venous system: a road less travelled. Heart Rhythm. 2004;1:107-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 48] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Saremi F, Krishnan S. Cardiac conduction system: anatomic landmarks relevant to interventional electrophysiologic techniques demonstrated with 64-detector CT. Radiographics. 2007;27:1539-65; discussion 1566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 63] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | El-Maasarany S, Ferrett CG, Firth A, Sheppard M, Henein MY. The coronary sinus conduit function: anatomical study (relationship to adjacent structures). Europace. 2005;7:475-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 51] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Silver MA, Rowley NE. The functional anatomy of the human coronary sinus. Am Heart J. 1988;115:1080-1084. [PubMed] [DOI] [Full Text] |
| 10. | Mühlenbruch G, Koos R, Wildberger JE, Günther RW, Mahnken AH. Imaging of the cardiac venous system: comparison of MDCT and conventional angiography. AJR Am J Roentgenol. 2005;185:1252-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Mischke K, Knackstedt C, Mühlenbruch G, Schimpf T, Neef P, Zarse M, Plisiene J, Stanzel S, Eickholt C, Fache K, Frechen D, Spüntrup E, Hanrath P, Kelm M, Schauerte P. Imaging of the coronary venous system: retrograde coronary sinus angiography versus venous phase coronary angiograms. Int J Cardiol. 2007;119:339-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Gilard M, Mansourati J, Etienne Y, Larlet JM, Truong B, Boschat J, Blanc JJ. Angiographic anatomy of the coronary sinus and its tributaries. Pacing Clin Electrophysiol. 1998;21:2280-2284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 70] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Sun C, Pan Y, Wang H, Li J, Nie P, Wang X, Ma H, Huo F. Assessment of the coronary venous system using 256-slice computed tomography. PLoS One. 2014;9:e104246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Meisel E, Pfeiffer D, Engelmann L, Tebbenjohanns J, Schubert B, Hahn S, Fleck E, Butter C. Investigation of coronary venous anatomy by retrograde venography in patients with malignant ventricular tachycardia. Circulation. 2001;104:442-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 106] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 15. | Arbelo A, Garci-quintana A, Cabellaro G. Usefulness of hyperemic venous return angiography for studying coronary anatomy prior to cardiac resynchronization device implantation. Rev EspCardiol. 2008;61:936-944. |
| 16. | Randhawa A, Saini A, Aggarwal A, Rohit MK, Sahni D. Variance in coronary venous anatomy: a critical determinant in optimal candidate selection for cardiac resynchronization therapy. Pacing Clin Electrophysiol. 2013;36:94-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Noheria A, DeSimone CV, Lachman N, Edwards WD, Gami AS, Maleszewski JJ, Friedman PA, Munger TM, Ha mmill SC, Hayes DL, Packer DL, Asirvatham SJ. Anatomy of the coronary sinus and epicardial coronary venous system in 620 hearts: an electrophysiology perspective. J Cardiovasc Electrophysiol. 2013;24:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |