METHODOLOGY OF EXERCISE STRESS ECHOCARDIOGRAPHY
Exercise tests on a treadmill
Patients are questioned about their symptoms, past cardiovascular medical history, and risk factors for coronary artery disease. After an explanation and preparation for the procedure by a cardiovascular technician, a classic 12-lead ECG is obtained. The Bruce protocol, by routine, is performed. The criteria for test interruption are fatigue, angina with increasing intensity, dizziness, ST-segment depression greater than 3 mm, complex ventricular arrhythmias, systolic blood pressure greater than 240 mmHg or diastolic blood pressure greater than 130 mmHg or a blood pressure drop greater than 20 mmHg during exercise. The test result is considered positive for myocardial ischemia when ST-segment depression occurs, with a horizontal or down-sloping displacement greater than 1 mm measured 0.08 s after the J point. The ECG exercise treadmill test result is considered inconclusive when there are baseline ST-T changes (left bundle branch block, the digitalis effect, or left ventricular hypertrophy) or when the patient does not reach 85% of the theoretical maximum age-adjusted heart rate. The test result is considered negative for myocardial ischemia when the patient’s heart rate exceeds 85% of the theoretical maximum age-adjusted heart rate and the previously mentioned changes did not occur.
Exercise stress echocardiography on a treadmill
We started performing exercise stress echocardiography (ESE) with an evaluation of cardiac function during exercise more than 20 years ago. The method was published and is also used by other groups[4,5].
Patients undergo symptom-limited treadmill exercise tests following the standard Bruce protocol. A modified Bruce protocol can be used for the assessment of non-coronary artery disease, allowing an easier evaluation of Doppler parameters than that offered by the classical Bruce protocol. Standard 12-channel electrocardiographic monitoring is performed. Blood pressure, heart rate, and 12-lead electrocardiography are performed at baseline and at each stage of the exercise protocol. Before starting an exercise test, a baseline echocardiogram is performed in the left lateral decubitus position for an initial assessment, with 2D and M-mode image acquisition in at least four planes, i.e., parasternal long axis, parasternal short axis, apical four-chamber, and apical two-chamber, with Doppler parameters being evaluated and stored based on the patient’s condition. Two-dimensional echocardiographic images are obtained from the parasternal (long axis and short axis) and apical (four-chamber and two-chamber) views, in the standing position at rest, during exercise (Figure 1) and at peak exercise, in the immediate postexercise period, and during the recovery period. Peak and immediately postexercise imaging acquisition is obtained using a continuous image-capturing system. After the test, those frames with the best image quality in each view are selected. The acquired digitized images are reviewed and compared in digital side-by-side quad-screen format within the echocardiographic equipment. After the exercise test is stopped, when appropriate, the patient is quickly placed in the left lateral decubitus position, and images are reacquired in the same plane. When relevant, for example, for the detection and evaluation of intraventricular gradients (IVGs) in HCM or in symptomatic athletes, the patient remains standing after finishing the stress test, and echocardiography is carried out in this position. For the evaluation of left ventricular regional wall motion abnormalities, we use the model that divides the left ventricle into 17 segments[7]. Ischemic changes are considered when the segments develop hypokinesia, akinesia or dyskinesia. However, when an akinetic segment becomes dyskinetic, it is not considered to be ischemic. Most groups that use exercise echocardiography in treadmill exercise acquire echocardiographic images only before and after exercise[6].
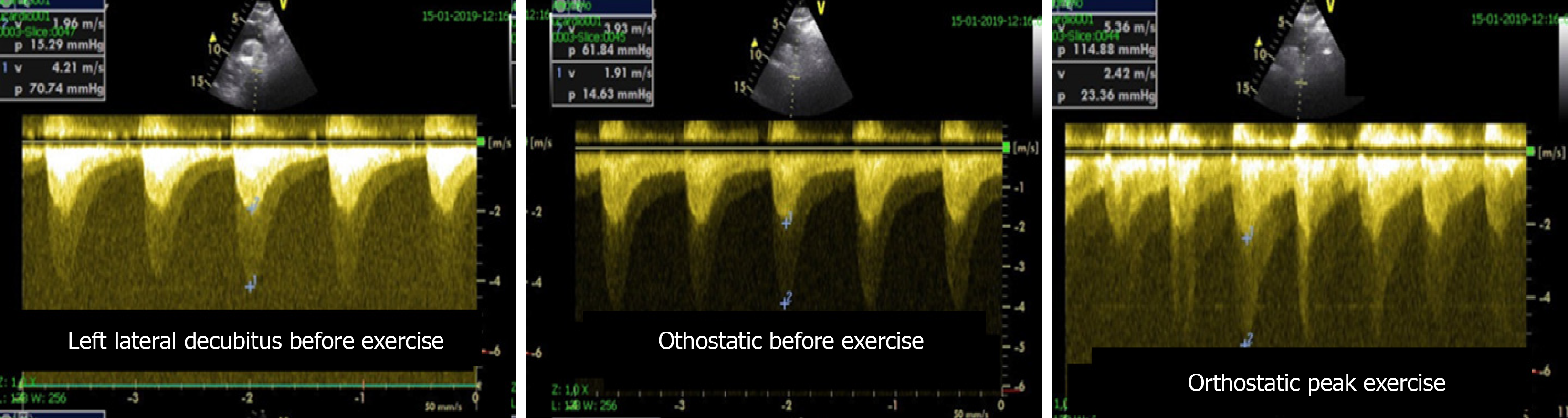
Figure 1 Echocardiographic data acquisition with the patient in the orthostatic position during exercise on a treadmil.
Citation: Cotrim C, João I, Fazendas P, Almeida AR, Lopes L, Stuart B, Cruz I, Caldeira D, Loureiro MJ, Morgado G, Pereira H. Clinical applications of exercise stress echocardiography in the treadmill with upright evaluation during and after exercise. Cardiovasc Ultrasound 2013; 11: 26 [PMID: 23875614 DOI: 10.1186/1476-7120-11-26] Copyright © The Author (s) 2013. Published by BMC part of Springer Nature.
APPLICATIONS OF ESE
Ischemia detection
Stress echocardiography has demonstrated substantial clinical relevancy in ischemia detection because of its high sensitivity and specificity[8,9] both in patients without antecedents of prior intervention and in those previously submitted to percutaneous coronary intervention[10] or coronary artery bypass graft surgery[11]. Women have a higher rate of false-positive results with exercise electrocardiographic testing. In this group, stress echocardiography has been shown to be an accurate method for ischemia detection[12]. In patients with left ventricular hypertrophy, stress echocardiography has a sensitivity of 84% and a specificity of 75% for the detection of ischemia[13], substantiating its use in clinical practice. Detection of ischemia with exercise electrocardiographic testing is not possible in patients with left bundle bunch block. ESE may also be limited in these patients because of the paradoxical motion of the interventricular septum. In a series of 30 patients with left bundle branch block, Pellika and colleagues showed that ESE had 60% sensitivity for ischemia detection, compared to 88% sensitivity with dobutamine stress echocardiography (DSE)[14]. The prognostic accuracy of myocardial perfusion and stress echocardiography appeared similar. Although these conclusions are based on a small sample study, DSE was preferred to ESE for this subgroup of patients in our center. Detection of ischemia, as well as its magnitude, has obvious prognostic implications. In a study with more than 500 patients, Marwick et al[15] demonstrated that the use of stress echocardiography to detect ischemia provides additional prognostic information. The comparative advantage of exercise echocardiography with image acquisition during treadmill exercise was clearly demonstrated by the Peteiro et al[16,17], who showed an increased diagnostic accuracy with this methodology compared with evaluation only before and after exercise. Those results confirm our preliminary results from a previous small study. In comparison with other widely available imaging techniques, ESE has some advantages, including greater safety, with only one adverse event in every 7000 exams compared to one adverse event in every 700 dobutamine stress echocardiograms[18]. This issue was addressed by the international practice guidelines[19], reserving drug-induced stress echocardiography for those unable to perform an exercise stress test. Another important advantage of ESE is that the procedure does not involve radiation[20,21].
Evaluation of patients with suspected or confirmed pulmonary hypertension, including patients with mitral stenosis.
The evaluation of pulmonary artery systolic pressure at rest using echocardiography is common and of great clinical importance. A diagnosis of pulmonary arterial hypertension is based on a mean pulmonary artery pressure > 25 mmHg at rest[22]. Although usual assessment is generally carried out at rest, the clinical utility of determining pulmonary artery systolic pressure during exercise has been demonstrated in various clinical situations, such as heart failure, rheumatologic disease, mitral stenosis and mitral regurgitation[23-27].
In one study using our methodology[23], we included 56 patients with mitral stenosis and determined the gradient between the right ventricle and right atrium using continuous wave Doppler in left lateral decubitus (LLD) before exercise testing, in the orthostatic position at peak workload before termination of the test, and in LLD in the first 60 s of the recovery period. The mean gradient between the left atrium and left ventricle was also determined at different stages of the test in patients with mitral stenosis. In patients with mitral stenosis, the effect of access to gradient values obtained at peak workload on clinical management was compared to the effect if this parameter had been evaluated only in the immediate recovery period. In this group of patients, basing the decision to treat on pulmonary artery systolic pressure > 60 mmHg at peak workload resulted in 10 patients (18% of those with this pathology) being referred for valvuloplasty or valve replacement. These patients would have continued with medical therapy if the decision had been based on the values obtained during the recovery period.
In the same study’s 42 patients with scleroderma, basing the decision for right heart catheterization in patients with systemic sclerosis on the gradient between the right ventricle and right atrium at peak exercise resulted in 13 more patients (30% of those with this pathology) being referred for this procedure than if it had been based on the values obtained only during the recovery period, i.e., as ESE is usually performed in most centers.
In patients with a previous history of thromboembolic pulmonary disease, with mild or moderate pulmonary hypertension or with unclear cause for symptoms, we have used ESE to help in clinical evaluations and decision-making. In one published case[28], right ventricular dilatation occurred that was not seen before exercise and that was detected only during exercise. The right ventricle after exercise was also normal.
It is often difficult to obtain adequate tricuspid regurgitation signals for the measurement of the gradient between the right ventricle and right atrium, potentially leading to its underestimation. Therefore, air-blood-saline contrast during exercise has been utilized to improve the Doppler signal in several clinical situations. We have concluded that the utilization of contrast should probably be limited to patients with a poor tricuspid regurgitation jet signal, to obviate the high number of false positives results that occur. Therefore, contrast should be an aid to obtain a measurable gradient but should not be used routinely in patients submitted to this type of study.
Intraventricular obstruction induced by exercise in athletes with “positive screening” in medical evaluations for participation in sports
The development of IVGs during exercise is a rare finding and occurs usually in association with left ventricular hypertrophy[29,30]. The development of IVGs during exercise has been described in athletes[31]. The clinical meaning of this observation and the most suitable exercise technique (upright exercise vs semisupine exercise vs lying supine after exercise) to trigger IVGs remain unknown. Supine exercise and lying supine after exercise are less technically demanding but also less physiological than upright exercise.
Importantly, in usual daily life, after exercise, individuals generally do not lay supine, as practiced in postexercise treadmill stress echocardiography in most cardiology departments. Therefore, we have conducted other studies in which we searched for IVGs during ESE, in a standing position after exercise, in athletes who screened positive according to the Guidelines of the European Society of Cardiology[32] and who also had an echocardiogram considered normal at rest. In that investigation[33], 139 athletes (135 were amateur, and four were professional; 30 were female), with a mean age of 22 ± 9.9 years (age range from 9 to 56 years old), were included. The athletes participated in the following sports: athletics (58), soccer (51), tennis (7), basketball (5), handball (5), swimming (5) and other (8).
As stated, all athletes screened positive: 112 had symptoms (chest pain, dizziness, or syncope) or a positive exercise ECG treadmill test (11 athletes). Of the 27 asymptomatic athletes, four had a history of sudden death in the family, three had slight mitral valve prolapse without mitral regurgitation, 18 had alterations in their ECG, and three had ventricular premature beats on ECG. All the athletes had a normal resting echocardiogram, i.e., no left ventricular hypertrophy or significant valve pathology.
Of all 139 athletes, 52 (37.4%) developed IVGs (group 1), and 87 (62.6%) did not develop IVGs (group 2) (we considered only IVGs greater than 50 mmHg).
Of the 52 athletes in group 1, 23 (63%) developed systolic anterior motion (SAM) of the mitral valve associated with significant IVG during exercise (Figure 2).
Figure 2 Systolic anterior movement of the mitral valve and significant intraventricular gradient detected at peak exercise.
Citation: Cotrim C, João I, Fazendas P, Almeida AR, Lopes L, Stuart B, Cruz I, Caldeira D, Loureiro MJ, Morgado G, Pereira H. Clinical applications of exercise stress echocardiography in the treadmill with upright evaluation during and after exercise. Cardiovasc Ultrasound 2013; 11: 26 [PMID: 23875614 DOI: 10.1186/1476-7120-11-26] Copyright © The Author (s) 2013. Published by BMC part of Springer Nature.
In group 1, IVGs were present in all athletes after exercise in the standing position. In seven of these athletes, IVGs were detected only at this timepoint.
Small IVGs are a common phenomenon. Three mechanisms have been proposed by Yotti[34] regarding their significant increase during exercise: (1) Increase in nonobstructive physiological gradients; (2) End-systolic obstruction secondary to ventricular cavity obliteration; and (3) Midsystolic obstruction caused by SAM of the mitral valve with restriction of ejection. However, for SAM to occur, there must be some alteration in the geometry of the ventricular chamber or in the mitral valve apparatus. This was not the case in our athletes, although it has been demonstrated that IVGs can be caused by maneuvers that change loading conditions in structurally normal hearts[35] that participating in sports can bring about such changes. In a study with children[36], IVGs induced by exercise were considered normal; however, the clinical data for the population were not contextually clear[37], and further studies are needed to better clarify the clinical meaning of these gradients.
In our study, the athletes had normal echocardiograms, indicating that a morphological study of their hearts would probably reveal no abnormalities[38,39]. The phenomenon that we detected before, during and after exercise testing, in the standing position – IVG associated with mitral valve SAM at the end of and after exercise – could well have been responsible for the positive screening in the athletes in group 1. Medical examinations of these athletes were carried out mostly because of symptoms arising from intense effort; for most of the athletes, we did not reproduce the symptoms during exercise, but we detected an anomaly in cardiac function[31] that, in our opinion, may explain the symptoms. The abnormality, however, was only detectable before (two athletes), during (41 athletes), and after exercise in the orthostatic position (52 athletes). These phenomena are not among the diagnoses that contraindicate participation in competitive sports according to the recommendations of the 36th Bethesda conference[40] and the European Society of Cardiology[32]. It is possible that the phenomena observed in these athletes could be among the causes of sudden death in cases where anatomopathological examination reveals no abnormalities, and we accordingly advised the athletes to suspend their participation in sports and referred them to a sports medicine center for further assessments. In our opinion, the cases described in which significant abnormalities in cardiac function were found before, during, and more clearly after exercise (Figures 3 and 4) in the standing position suggests that this methodology should be applied to the athletes who have symptoms related to exercise and no structural abnormalities. We emphasize that this phenomenon has almost been excluded as a normal response to exercise in healthy adults[41]. The results of this study underscore the importance that the literature attributes to searching for IVGs as a possible cause of symptoms related to exercise in athletes and in pediatric populations[42,43].
Figure 3 Intraventricular gradient present in the orthostatic position before exercise in an athlete, decreased during the initial phase of exercise testing.
Citation: Cotrim C, João I, Fazendas P, Almeida AR, Lopes L, Stuart B, Cruz I, Caldeira D, Loureiro MJ, Morgado G, Pereira H. Clinical applications of exercise stress echocardiography in the treadmill with upright evaluation during and after exercise. Cardiovasc Ultrasound 2013; 11: 26. [PMID: 23875614 DOI: 10.1186/1476-7120-11-26] Copyright © The Author (s) 2013. Published by BMC part of Springer Nature.
Figure 4 Intraventricular gradient increased during the last portion of the exercise test and after exercise in the orthostatic position.
Obstruction suddenly disappeared after placing the athlete in decubitus. Citation: Cotrim C, João I, Fazendas P, Almeida AR, Lopes L, Stuart B, Cruz I, Caldeira D, Loureiro MJ, Morgado G, Pereira H. Clinical applications of exercise stress echocardiography in the treadmill with upright evaluation during and after exercise. Cardiovasc Ultrasound 2013; 11: 26. [PMID: 23875614 DOI: 10.1186/1476-7120-11-26] Copyright © The Author (s) 2013. Published by BMC part of Springer Nature.
The athletes in whom these findings were detected should also undergo long-term follow-up to accurately assess the clinical significance of the findings.
To the best of our knowledge, our group provided the first report on the importance of the standing position before and after exercise to detect IVGs in symptomatic athletes without left ventricular hypertrophy. Our study of the evaluation of IVGs with continuous wave Doppler also constitutes a new step in the use of stress echo as a diagnostic tool beyond is initial use for coronary heart disease[44] and in the use of a new stressor: the standing position[33,45].
Considering our experience, ESE, with evaluation in an upright position before, during, and after exercise, should possibly be part of a new diagnostic algorithm whenever athletes have a positive screening on a medical evaluation. This is especially true if the athletes have symptoms.
Monitoring beta-blocker use
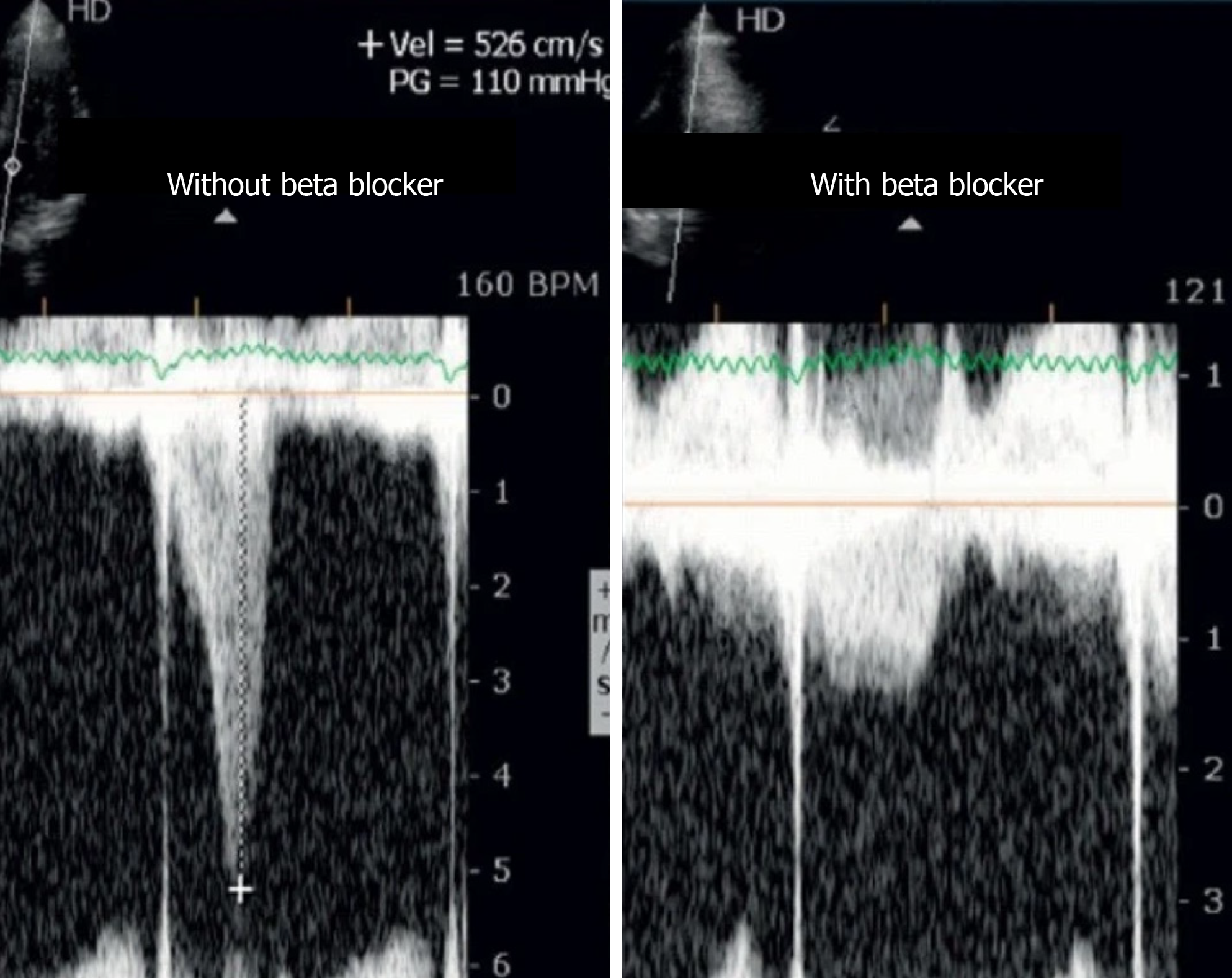
Because most of the athletes evaluated in the previous study were treated with beta-blockers, we conducted an open-label, prospective, nonrandomized study to provide proof-of-concept that ESE can guide tailored treatment in athletes with positive screenings[46] on medical evaluations for participation in sports and who develop IVG and mitral valve SAM on exertion. Of the 52 athletes who developed IVGs, 35 repeated ESE while taking beta-blockers. Thirty-three had exercise-related symptoms or a positive exercise ECG. Thirty athletes (85%) showed improvement on the follow-up ESE, with a significant reduction in gradients (Figure 5) and SAM.
Figure 5 Intraventricular gradient in an athlete assessed before and on beta-blocker therapy.
Citation: Cotrim C, João I, Fazendas P, Almeida AR, Lopes L, Stuart B, Cruz I, Caldeira D, Loureiro MJ, Morgado G, Pereira H. Clinical applications of exercise stress echocardiography in the treadmill with upright evaluation during and after exercise. Cardiovasc Ultrasound 2013; 11: 26. [PMID: 23875614 DOI: 10.1186/1476-7120-11-26] Copyright © The Author (s) 2013. Published by BMC part of Springer Nature.
We concluded that for athletes with positive screening - mostly by symptoms - on medical evaluations for participation in sports and IVG on exertion, treatment with beta-blockers prevented the occurrence of IVGs and SAM or significantly reduced their magnitude. These changes were associated with a significant reduction in heart rate at peak exercise and reflect clinical improvement, which occurred in 85% of the study population.
Exercise echocardiography represents a useful tool to evaluate athletes who screen positive and have a normal rest echocardiogram, allowing identification for beta-blocker therapy. The findings of this study represented a proof-of-concept of tailored beta-blocker therapy, driven by exercise Doppler echocardiography results, in symptomatic athletes and were also observed by other authors[47].
Intraventricular gradients in patients with cardiac X syndrome
The development of IVGs during DSE has been reported and is commonly associated with symptoms during the study[48,49]. The occurrence of IVGs during ESE is rare[29,30]. In a group of 10 patients who developed IVGs during DSE, we performed ESE and found a small IVG in only one patient. A 23-year-old male with angina and a positive treadmill test, a structurally normal heart, and normal coronary angiography underwent an ESE, and during the study, we unexpectedly detected a 102 mmHg IVG[31] and SAM of the mitral valve. A similar case was reported by Lau et al[30] and was treated successfully with beta-blockers. After this first case, we conducted a study for IVG detection during ESE in patients with angina, positive stress electrocardiography, normal coronary arteries, and a normal echocardiogram (cardiac X syndrome)[50]. This study included 91 patients, 44 of whom were women. All patients had angina, positive exercise ECG treadmill tests (four patients had only ischemia in a myocardial perfusion study), normal resting echocardiogram - no left ventricular hypertrophy - and no coronary artery disease on coronary angiography.
Of the whole group, 33 patients (36%) developed IVGs, and 58 patients (64%) did not develop IVGs as defined previously by the authors. In the first group, the IVG at peak exercise was 86±34 mmHg (ranging from 30 to 165 mmHg), and 23 patients (70%) developed SAM during exercise, associated with IVGs. No patients developed segmental wall abnormalities. The results of our study, in which 36% of the patients with normal coronary angiograms and positive treadmill exercise tests developed IVGs, suggest that ST-segment depression may be related with the development of IVGs during exercise, which is possibly involved in the genesis of electrocardiographic changes. The possible association between cardiac X syndrome and IVG development during exercise has previously been described[51,52], however, some of the patients from these studies had arterial hypertension and left ventricular hypertrophy, more frequently developed IVGs[29] and were therefore excluded based on the definition of cardiac X syndrome[53].
In our study population, we found a great number of patients who developed SAM of the mitral valve in association with IVGs, in contrast to the findings of other authors. We hypothesize that we can detect SAM in a greater number of patients because we use echo throughout the entire treadmill exercise protocol and in the standing position after exercise[45]. The magnitude of the IVGs that we have detected in our patients is also greater for the same reason.
We conclude that a relevant number of patients with cardiac X syndrome develop significant IVGs during exercise. The authors believe that this phenomenon may constitute a new entity that should be included as a factor in the heterogeneous group of patients with angina, ST-depression during treadmill exercise tests and normal coronary arteriography. Because of these results, we believe that ESE should be part of a new diagnostic algorithm whenever patients with angina are suspected of having cardiac X syndrome.
Evaluation of patients with HCM
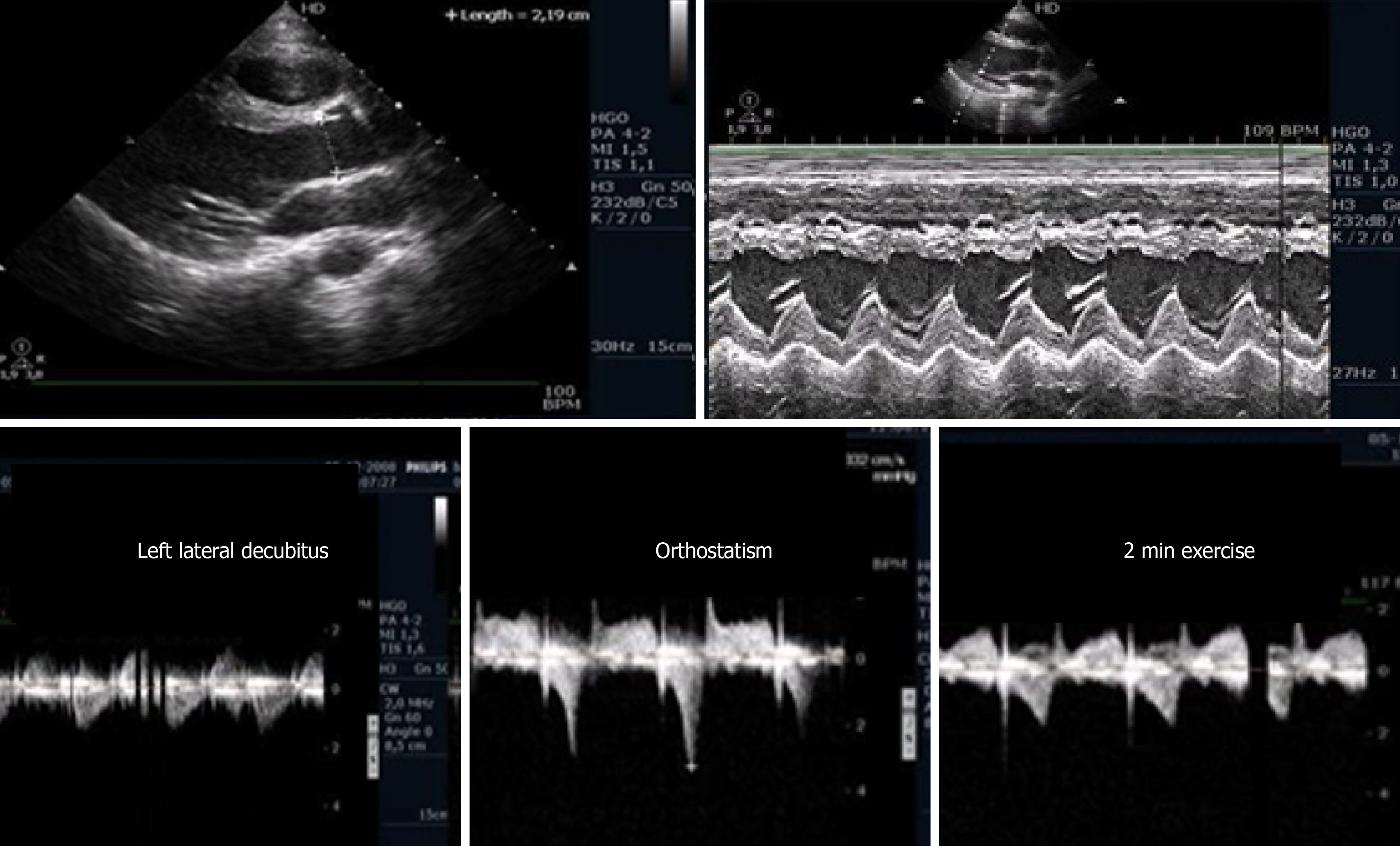
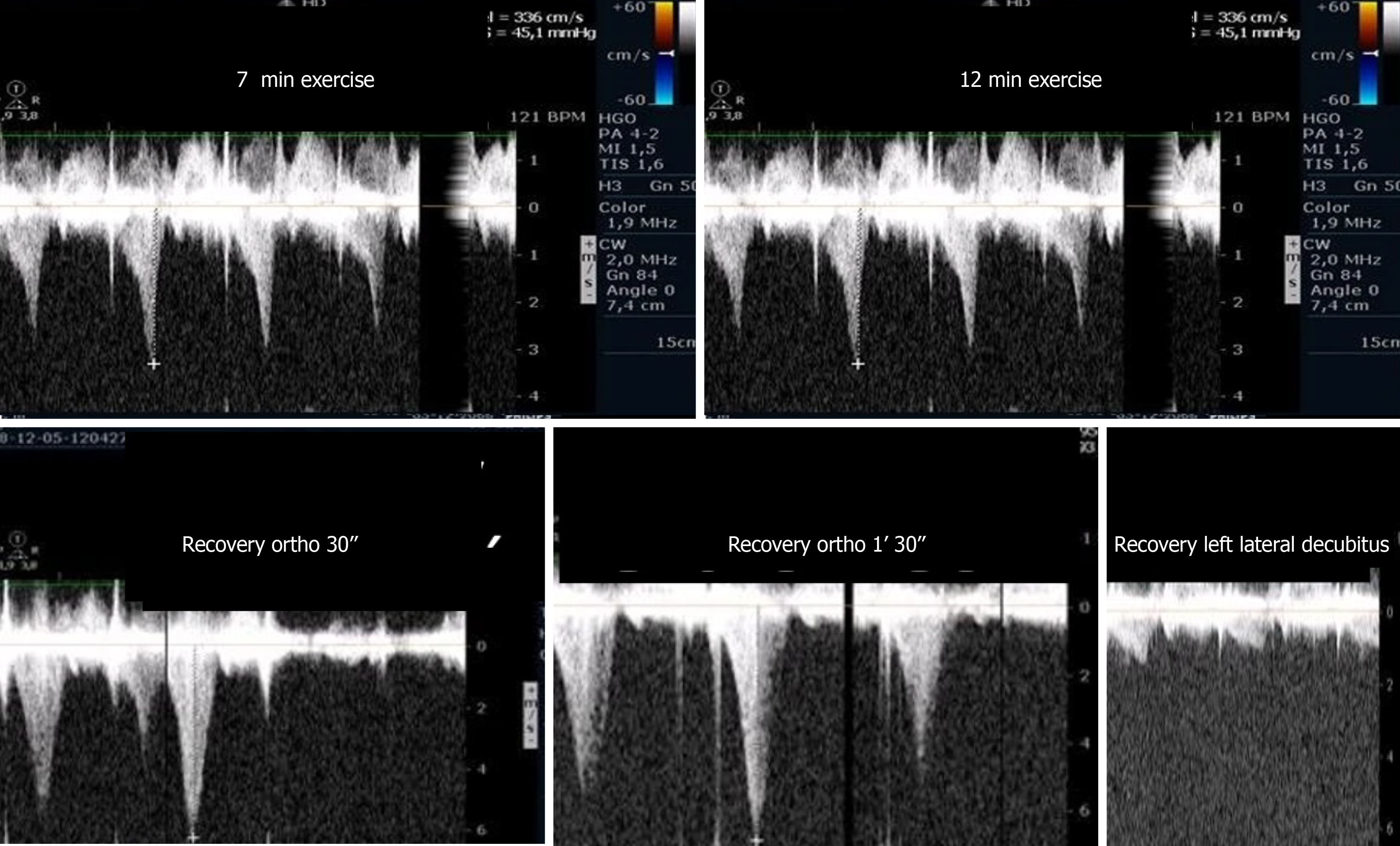
In our centers, ESE with image acquisition during treadmill exercise (a representation of regular exercise) is also commonly used for the evaluation of patients with HCM, enabling assessment of the outflow gradient during physiologic exercise and in recovery period in LLD[5]. In patients with obstructive HCM under resting conditions, it has been demonstrated that obstruction may increase after the change in position from supine to standing. The LVOT gradient increases in the orthostatic position and continues to increase at peak exercise, but after exercise, the gradient decreases rapidly when measured in LLD, indicating that the assessment of IVGs during the recovery period in the supine position does not reflect changes during effort. The LVOT gradient measured during the recovery period in the supine position does not reflect patients’ daily activities (in their daily lives, patients simply do not assume a supine position after an effort) nor the pathophysiology of this condition[54-58]. We unexpectedly found, in one patient, that after exercise, the IVG continued to increase if the patient remained in the standing position. Based on this observation, a new study was conducted[45] in 17 patients diagnosed with HCM based on echocardiographic findings of a nondilated hypertrophic left ventricle in the absence of diseases known to cause ventricular hypertrophy, including 11 patients with obstructive HCM due to an LVOT gradient greater than 30 mmHg under resting conditions and six patients with nonobstructive HCM. Three patients without resting obstruction developed IVGs during exercise; one patient developed a gradient only during the recovery period in the orthostatic position ; two patients had neither resting nor exercise-induced obstruction. All patients with obstructions exhibited increased IVGs in orthostatic recovery. These findings differ from those reported in other studies in which the participants assume the supine position immediately after exercise[59,60]. The importance of the standing position in this group of patients and in patients with other conditions as an additional and new stressor with important clinical application has been underscored by our group[61] and by other investigators[62-65]. Reflecting on our experience and referencing the literature, we believe that the standing position is the only way these patients can be correctly evaluated, and the mechanisms of their symptoms understood. The clinical importance of upright evaluation during and particularly after exercise was recently underscored by Dimitrow et al[66] and Petkow Dimitrow et al[67], and we think that laying patients in a supine position after any type of exercise is meaningless from the clinical point of view because this does not usually happen in real life. We recommend[67] that future guideline of scientific societies should clearly state one uniform methodology to be employed by all groups that study and treat these types of patients, with the aim of having a common language that can be used in the future.
OTHER USES OF EXERCISE STRESS ECHOCARDIOGRAPHY WITH ECHOCARDIOGRAPHIC EVALUATION ALSO DURING EXERCISE IN TREADMILL
Aortic stenosis
Most of the recommendations in the guidelines do not have an extensive evidence base, underscoring the need for more clinical investigations in this area. In valvular heart disease, exercise testing is preferred over pharmacological stress testing because it provides insights regarding exertional symptoms and blood pressure variations[27]. Supine bicycle exercise is recommended because Doppler information can be obtained during the different stages of exercise[19] rather than during post-treadmill imaging when substantial and rapid changes in heart rate and loading conditions occur. We and the Peteiro group[4,68,69] disagree with those recommendations, and we conduct echocardiography during treadmill exercise. Additionally, supine exercise is not as physiologic as is treadmill exercise, the equipment is not nearly as widely available as is a treadmill, and the maximum VO2 attained is at least 10% lower with a bicycle[70]. We routinely use ESE for the evaluation of patients with asymptomatic aortic stenosis. With the literature as support[24,71-73], we utilize ESE to better understand patients, adding information regarding exercise testing[74] and consider surgery accordingly with recommendations.
About one third[71-74] of asymptomatic patients with severe aortic stenosis submitted to exercise tests are in fact symptomatic, but complications do not usually occur in this group of patients. In selected symptomatic patients with aortic stenosis, we employ ESE to better clarify the mechanism of symptoms. As an example, we have published the case of a symptomatic patient in whom symptoms were due to the development of a significant IVG[75] due to SAM of the mitral valve and were treated with beta-blockers. Patients with severe aortic stenosis in whom there is proof that the symptoms have another cause should be considered, more aptly, symptomatic aortic stenosis patients, with symptoms due to other causes (false symptomatic aortic stenosis).
Mitral regurgitation and aortic regurgitation
The assessment of regurgitant mitral flow, with ESE, is mandatory in valvular heart disease in patients with symptoms and mild-moderate mitral regurgitation at rest and in patients with severe mitral regurgitation who have no symptoms[76]. Mitral regurgitation should also be evaluated when studying patients with HCM or heart failure with preserved ejection fraction or pulmonary edema with unknown etiology. Finally, but importantly, mitral regurgitation should also be evaluated in patients with coronary disease to determine if there is an increase in severity or a new appearance of mitral regurgitation[77]. In all these conditions, the presence of mitral regurgitation may contribute to risk stratification and may provide a potential explanation for clinical data susceptible to specific interventions. In aortic regurgitation, as in mitral regurgitation, the evaluation of the inotropic reserve (increase of 5% in the ejection fraction with exercise) can be used to indicate valve surgery in patients with compromised left ventricular function.
Prosthetic heart valves
A significant percentage of patients with aortic valve disease and mitral valve disease who undergo surgery are given a prosthesis[24]. Most prosthetic valves are inherently stenotic, as the effective orifice area is often too small in relation to the body surface, a phenomenon classified as valve prosthesis-patient mismatch[78]. In clinical practice, it is common for normal and abnormal prostheses to produce similar gradients at rest, and therefore, ESE may be valuable for confirming or excluding the presence of prosthetic valve dysfunction or mismatch. This is particularly true for disagreements between symptoms and the hemodynamic profile evaluated by Doppler echocardiography at rest[79-81]. According to Picano et al[27], a disproportionate increase in the transvalvular gradient (greater than 20 mmHg for an aortic prosthesis or greater than 12 mmHg for a mitral prosthesis) generally indicates severe prosthesis dysfunction or mismatch.
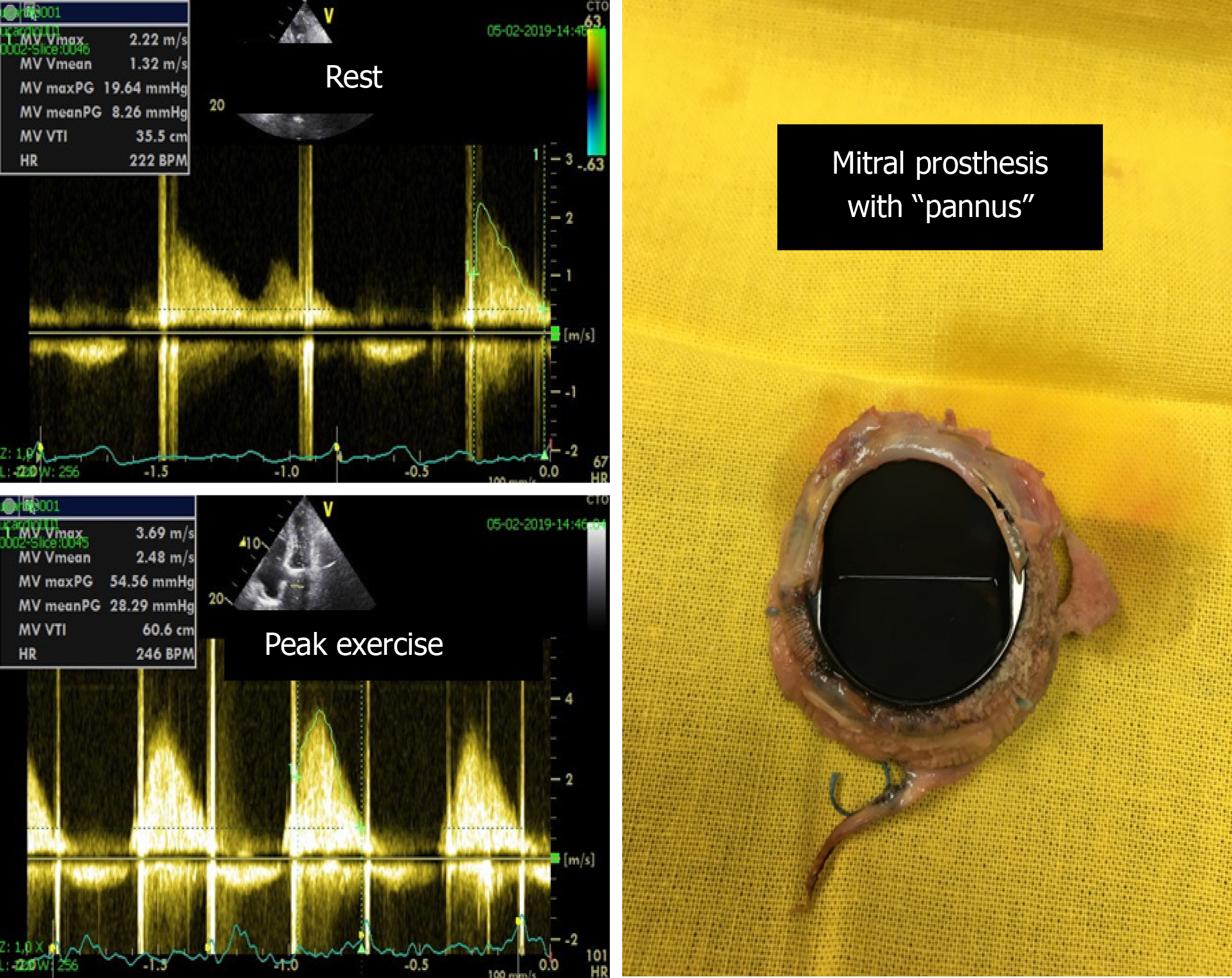
We use ESE for patients with valve prostheses whenever there exists a discrepancy[82] between the gradients evaluated via echocardiography and the presence of the symptoms (Figure 6). Evidence-based gradient cut-offs are needed for clinical decision-making. Until such cut-offs are established, we associate exercise Doppler parameters with clinical and exercise test data.
Figure 6 Significant increase in the mean gradient (from 8 mmHg to 28 mmHg) and appearance of severe symptoms with exercise in one patient with a mechanical mitral prosthesis with “pannus”.
Congenital heart disease and children
ESE is an imaging technique that has been almost exclusively used to evaluate and elucidate cardiac abnormalities in adult patients. Though it may have the potential to offer the same benefit in the pediatric population, it remains an underexplored field, perhaps due to concerns about its safety and applicability in younger children. Given its diagnostic accuracy, ability to assess cardiac reserve and lack of radiation, ESE is increasing in importance as a helpful tool in the clinical assessment of pediatric patients[83].
The usage of ESE in patients with congenital heart disease has been limited despite its potential for broader applications in different clinical scenarios[84]. In patients with congenital heart disease, measuring pulmonary systolic pressure and ventricular function both at rest and during exercise by echocardiography may be essential for determining the etiology of exercise intolerance in these individuals and for better treatment timing.
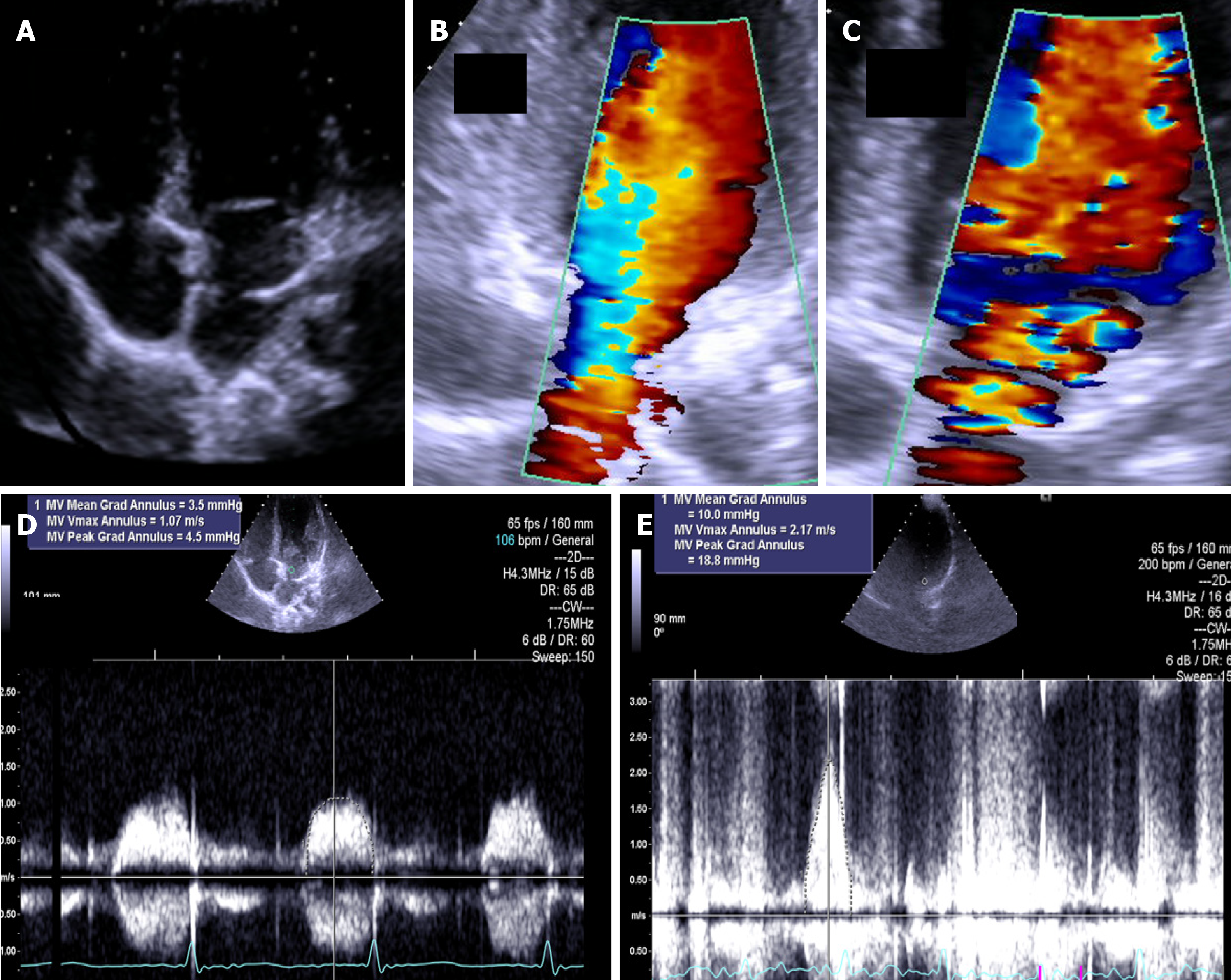
The presence of any stenotic lesion evaluated by echocardiography can also be studied during exercise, and the data obtained can be used for better clinical decision-making. We performed ESE in one patient with congenital mitral stenosis[3] and evaluated the mean mitral gradient before and at peak exercise and verified the absence of pulmonary hypertension. The exercise Doppler data in conjunction with the exercise data and clinical data led us to keep the patient in close clinical follow-up. The same result was obtained for a young 14-year-old boy, a soccer player, with cor triatriatum sinister who had only a small increase in the mean gradient with exercise (Figure 7).
Figure 7 The exercise Doppler data in conjunction with the exercise data and clinical data led us to keep the patient in close clinical follow-up.
A: Intraauricular septum in “cor triatriatrium”; B: Color flow before exercise; C: Color flow at peak exercise; D: CW flow before exercise; E: CW flow at peak exercise.
ESE can also be used to screen for residual narrowing in patients after repair of the coarctation of the aorta by detecting a significant diastolic gradient in the descending aorta during exercise provocation (Figure 8). This evaluation is also feasible with the method that we use to evaluate aortic flow during exercise. A significant increase in the diastolic gradient[85] was used as a guide for patient treatment decisions.
Figure 8 Aortic gradient evaluated in a patient previously treated with a stent. Based on the exercise stress echocardiography results, the patient was treated again.
Our group is increasingly using ESE to investigate intraventricular gradients in symptomatic children with normal hearts[33,42,43] or with HCM[86].
We believe that ESE can and should influence clinical decision-making for congenital heart disease patients if pediatric and adult cardiologists with experience in ESE start collaborating.
ESE and diastolic heart failure
We employ ESE in the treadmill evaluation of E/e’ as a measure of left ventricular filling pressure. This method has been validated against invasively measured LV filling pressure using simultaneous exercise echocardiography-catheterization[87]. The addition of E/e’ during exercise echocardiography improved the sensitivity of the diagnosis of heart failure with preserved heart function compared with that for a resting assessment alone, and the specificity can be improved if the gradient between the right ventricle and right atrium increases above the normal range with exercise. The independent prognostic value of exercise E/e’ has also been documented. We recommend that diastolic stress exercise echocardiography should be considered for patients with unexplained exertional dyspnea and normal diastolic filling pressure on resting echocardiography. The addition of a diastolic assessment to exercise echocardiography improves test sensitivity in patients with dyspnea, and there have been sufficient data that have led us to integrate diastolic exercise testing into our clinical practice.
THE LATEST ADVANCES IN ESE AND THE FUTURE
The exercise stress echo protocol has remained unchanged for more than 40 years[1] and focuses primarily on imaging regional wall motion abnormalities. In recent years, a significant evolution has occurred, and the technique was further exploited through the inclusion of four variables with recognized clinical, diagnostic, and prognostic importance with ESE, representing the new standard method. The variables are summarized in the ABCD protocol[88]. The four parameters converge conceptually, logistically, and methodologically: (1) Regional wall motion abnormalities[89]; (2) Comets or B-lines measured by lung ultrasound[90]; (3) Left ventricular contractile reserve assessed as the stress/rest ratio of elastance, also called force (systolic arterial pressure by cuff sphygmomanometer/ end-systolic volume from 2D)[91]; and (4) Coronary flow velocity reserve in the left anterior descending coronary artery (with color-Doppler-guided pulsed wave Doppler)[92]. This new way to conduct stress echo allows a functional assessment of epicardial coronary artery stenosis (wall motion), lung water (lung comets), myocardial function (left ventricular contractile reserve) and coronary small vessels (coronary flow velocity reserve in the mid or distal left anterior descending artery). In this new “ABCD” protocol, A stands for asynergy (ischemic vs nonischemic heart), B stands for B-lines/lung comets (wet vs dry lung), C stands for contractile reserve (weak vs strong heart), and D stands for Doppler flow evaluation (warm vs cold heart because the increase in blood flow increases the local temperature of the myocardium). From the technical and training points of view, B-lines/lung comets are the easiest to evaluate; on the other hand, the acquisition and analysis of left ventricular contractile reserve requires a higher level of competency. Wall motion evaluation is an even more challenging skill to obtain, and coronary flow velocity reserve is the hardest to evaluate. More recently[93], the protocol was amended with E, for EKG-based heart rate reserve (HRR, defined as peak/rest HR < 1.62), which also provides prognostic information, and ABCDE was born. This protocol has been used in the last 5 years in the SE2020 study[94-96], demonstrating utility in defining diagnosis and prognosis criteria with the new data acquired and evaluated with this methodology. This protocol will be now expanded in the new SE2030 study[97], with selected patients by further evaluating flows (mitral regurgitation flow) gradients (intraventricular or valvular gradient), left atrium volume , pulmonary circulation , and right ventricular function . These new steps should be viewed as diagnostic tools that can be used for each patient, according to the clinical condition and question to be answered, to obtain essential information for helping cardiologists design the most appropriate tests for each patient.
ETHICAL AND SAFETY ISSUES RELATED TO CARDIAC IMAGING: THE INSURMOUNTABLE ARGUMENT FOR THE PREFERENTIAL USE OF ESE IN CARDIOVASCULAR PATIENTS
Cardiac imaging methods
When addressing the ethical implications of the diagnostic options for heart disease, the main options include scintigraphy, CT angiography and catheterization, in which the individual is exposed to radiation; magnetic resonance imaging, which may require general anesthesia and intravenous administration of contrast and/or vasodilators; and stress echocardiography, which can be performed using exercise or pharmacological administration.
Regarding pharmacological stress echocardiography, it presents an increased risk of side effects, such as arrhythmias and anaphylactic reactions of greater or lesser severity, related to the administration of dobutamine or dipyridamole and thus requires an ethical review regarding the information to be made available to patients and obtaining informed consent. Regarding stress echocardiography, the European Association of Cardiovascular Imaging and the American Society of Echocardiography emphasize that this is the test of choice for most indications, reinforcing that any patient able to exercise should be tested with an exercise modality because doing so preserves the integrity of the electromechanical response and provides valuable information about functional status. The performance of echocardiography at the time of exercise also allows establishing links between symptoms, cardiovascular workload, wall motion abnormalities and hemodynamic responses, such as pulmonary pressure and transvalvular flows and gradients[98].
In addition to these advantages of exercise echocardiography, it is a radiation-free procedure, significantly reducing the associated ethical implications. Conversely, diagnostic options using radiation present a set of ethical implications that should be considered, namely, patients’ right to adequate and complete information, including risks, benefits and alternatives, on the diagnostic procedures available[99].
One of the main risks clearly associated with radiation exposure is the possibility of developing cancer.
According to Brody et al[100], there is clear evidence, observed in fundamental cellular processes, that even at in low-dose range of a few tens of mSv, it is scientifically plausible to assume that the risk of cancer will increase in direct proportion to the dose absorbed in organs and tissues. This may explain why cancer rates remain stubbornly high despite the great advances in its prevention and treatment and may, in the United States and soon, surpass heart disease as the main cause of death. One of the explanatory reasons points to the use of radiation in clinical practice, with exposure to medical radiation increasing sixfold between 1980 and 2006, largely due to the use of computed tomography because the radiation dose from this diagnostic technique is 100 to 1000 times higher than the radiation dose from a conventional radiograph[101].
Informed consent and communication quality
Considering the risks associated with radiation, information and informed consent are some of the ethical aspects to be highlighted. However, the information to be made available to patients involves some challenges, such as defining the exposure dose above which radiation is considered to pose an increased risk of developing radiation exposure-related diseases and determining what type of information should be given; although it is clear that this information should include the type and nature of the procedure, the risks, benefits and possible alternatives, as well as the risks of refusing a certain diagnostic test, it is not easy to define this information because these data vary according to a set of factors, such as patient age and sex[102].
As Picano[103] underscored, these difficulties seem to lead health professionals to develop strategies for risk communication to obtain informed consent to perform diagnostic tests with radiation exposure; such strategies include (1) Not mentioning the risk; (2) Underestimating the risk; and (3) Specifying the risk in detail. Regarding the first strategy, even for interventions with fluoroscopic control, there is no explicit or implicit mention of long-term risks. The risk exists and can be substantial, but it is still not heard by patients or spoken by doctors. Thus, patients’ right to information is eclipsed by two forces: efficiency and paternalism. In the second strategy, underestimating risk, obtaining informed consent is part of the “standard” practice of the team, and patients are not exposed to radiation without a consent form being signed. However, information on the actual effects and dose of radiation to be administered is not made available. Finally, the third strategy, full disclosure of risks, addresses the radiation dose to which patients will be exposed as well as the incidence of cancer and/or genetic diseases associated with it, especially if the test is performed for inclusion in a research study.
Regarding the pediatric population, children with congenital or acquired heart disease may be exposed to relatively high cumulative doses of ionizing radiation, resulting from imaging procedures requested, including radiographs, fluoroscopic procedures, such as diagnostic or interventional cardiac catheterizations, electrophysiology tests, CT angiography and nuclear cardiology tests, which in total and because the radiation dose is cumulative, represent an increased risk of cancer throughout life[104].
These children represent a vulnerable population group, considering the continuing need for medical care, and this requires greater ethical and professional responsibility, which underlies the fact that children do not have full decision-making ability nor adequate information about the various available care-related options they consider most suitable for themselves. Thus, health professionals ultimately have the obligation to make decisions that protect the well-being and life of this population and included among these is the decision regarding the diagnostic and therapeutic methods selected, considering the immediate and long-term effects on quality of life[105].
In addition to the previously mentioned ethical implications, such as the right to reliable and complete information and informed consent, which includes the risk of radiation, another ethical aspect is now being recognized, namely, the impact of medical radiation on the planet. This adds a new dimension to the ethical discussion about the best diagnostic approach, namely, the environmental impact, in addition to the medical benefits, costs, and long-term risks of radiation[20,21].
In conclusion, although we are aware of the life-long risks associated with exposure to ionizing radiation and although we recognize the vital role – both diagnostic and therapeutic – of medical imaging procedures, it is only prudent to perform such procedures using the lowest possible radiation doses while also ensuring their diagnostic value[106].