Published online May 27, 2023. doi: 10.4331/wjbc.v14.i3.62
Peer-review started: March 14, 2023
First decision: April 7, 2023
Revised: April 16, 2023
Accepted: May 15, 2023
Article in press: May 15, 2023
Published online: May 27, 2023
Processing time: 71 Days and 22.7 Hours
Erythrocyte alloantibodies are mainly produced after immune stimulation, such as blood transfusion, pregnancy, and transplantation, and are the leading causes of severe hemolytic transfusion reactions and difficulty in blood grouping and matching. Therefore, antibody screening is critical to prevent and improve red cell alloantibodies. Routine tube assay is the primary detection method of antibody screening. Recently, erythrocyte-magnetized technology (EMT) has been increasingly used in clinical practice. This study intends to probe the application and efficacy of the conventional tube and EMT in red blood cell alloantibody titration to provide a reference for clinical blood transfusion.
To investigate the application value of conventional tube and EMT in red blood cell alloantibody titration and enhance the safety of blood transfusion practice.
A total of 1298 blood samples were harvested from blood donors at the Department of Blood Transfusion of our hospital from March 2021 to December 2022. A 5 mL blood sample was collected in tubing, which was then cut, and the whole blood was put into a test tube for centrifugation to separate the serum. Different red blood cell blood group antibody titers were simultaneously detected using the tube polybrene test, tube antiglobulin test (AGT), and EMT screening irregular antibody methods to determine the best test method.
Simultaneous detection was performed through the tube polybrene test, tube AGT and EMT screening irregular antibodies. It was discovered that the EMT screening irregular antibody method could detect all immunoglobulin G (IgG) and immunoglobulin M (IgM) irregular antibodies, and the results of manual tube AGT were satisfactory, but the operation time was lengthy, and the equipment had a large footprint. The EMT screening irregular antibody assay was also conducted to determine its activity against type O Rh (D) red blood cells, and the outcomes were satisfactory. Furthermore, compared to the conventional tube method, the EMT screening irregular antibody method was more cost-effective and had significantly higher detection efficiency.
With a higher detection rate, the EMT screening irregular antibody method can detect both IgG and IgM irregular antibodies faster and more effectively than the conventional tube method.
Core Tip: Irregular antibody screening has long been a routine blood test for blood donors in numerous developed countries. However, only a few blood stations in China have tried using a saline medium for this type of screening. Monoclonal anti-A (B) is a standard reagent for ABO blood grouping, but false positive or false negative reactions can occur, reducing the accuracy of the test. With the improvement of diagnostic techniques and medical levels, the erythrocyte-magnetized technology (EMT) screening irregular antibody method has been gradually applied in a range of clinical settings. This study analyzed blood samples from voluntary blood donors to explore the application value and effect of the conventional tube method and EMT in red blood cell alloantibody titration, with the goal of providing valuable references to improve the safety of clinical blood transfusion.
- Citation: He XH, Yan H, Wang CY, Duan XY, Qiao JJ, Guo XJ, Zhao HB, Ren D, Li JS, Zhang Q. Comparison of the conventional tube and erythrocyte-magnetized technology in titration of red blood cell alloantibodies. World J Biol Chem 2023; 14(3): 62-71
- URL: https://www.wjgnet.com/1949-8454/full/v14/i3/62.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v14.i3.62
Antibody screening aims to detect the presence of antibodies other than the ABO blood group in patients' serum or plasma by serum determination. This includes autoantibodies, drug antibodies, and allospecific antibodies to red blood cell blood groups[1,2]. Red blood cell alloantibodies, which are blood group antibodies other than anti-A and anti-B, are referred to as irregular antibodies. These antibodies are mainly produced after immune stimulation, such as blood transfusion, pregnancy, and transplantation, and are the primary cause of severe hemolytic transfusion reactions, difficulties in blood grouping, and complex matching[3-5]. Therefore, improving the screening rate of red blood cell alloantibodies is crucial, as it has significant implications for the safety of clinical blood transfusion.
Although many developed countries have been including irregular antibody screening in routine blood testing for blood donors, only a few blood stations in China have experimented with irregular antibody screening using saline media[6]. False positive or negative reactions sometimes occur using monoclonal anti-A (B) as a standard ABO blood grouping reagent[7]. The Collies automatic blood grouping system has recently been utilized in clinical practice employing erythrocyte-magnetized technology (EMT), an international advanced technique. This technique magnetizes red blood cells, causing rapid sedimentation of red blood cells under the attraction of a magnetic field, thus replacing the serological centrifuge[8]. Irregular antibody screening using this instrument has been reported to be an indirect antiglobulin test (AGT) method that combines solid-phase packaging antiglobulin with EMT. The plasma to be tested was incubated with ready-to-use irregular antibody screening red blood cells, which can be sensitized by irregular antibodies in the plasma. In the presence of a magnetic field, magnetized red blood cells migrate to the bottom of the microplate and react with antiglobulin, forming an evenly distributed red blood cell layer[9,10]. Earlier studies have demonstrated that EMT can effectively mitigate the impact of centrifugation on experimental results, making the results more reliable and saving the costs associated with centrifuge calibration and maintenance[11,12].
Based on this, this study analyzed blood samples from voluntary blood donors to explore the application value and effect of the conventional tube and EMT in red blood cell alloantibody titration, hoping to provide valuable references for the safety of clinical blood transfusion.
We randomly selected 1298 blood donors from the Department of Blood Transfusion at our hospital between March 2021 and December 2022. The donors were between 18 and 55, with 612 males and 686 females. Among the donors, 897 (69%) were between 25 and 40. We separated serum from 5 mL of whole blood using centrifugation.
Reagents and instruments: Anti-human globulin cards (Jiangsu Libo Pharmaceutical Biotechnology Co., Ltd., batch number: 202208001), red blood cell antibody screening cell kits (batch number: 20187012, 20187014), and red blood cell antibody identification spectrum cells (REAGENS, batch number: 726000, 733000) were provided by Shanghai Blood Biomedicine Co., Ltd. Special centrifuge for blood immunology 2005-2 Zhuhai Bezo Biotechnology Co., Ltd. Polybrene came from reagents and instruments company of Zhuhai Bezo Biotechnology Co., Ltd. Collies 3 (QWALYS3) automatic blood analyzer (DIAGAST, France), Deakin (EvO-2) immunoassay sample adding system (TECAN, Switzerland). Shanghai Medical Equipment Co., Ltd. Digital explicit water bath, Heidolph oscillator (Titramax101) made in Germany, and HeShi centrifuge with hanging micro reaction plates in the German obstetrics department.
Eleven known irregular antibodies with known specificity against D, E, C, c, e, S, Fy', JK', M, N, and phosphatidylinositol antibody were prepared by the Institute of Blood Transfusion Technology, Taiyuan, China.
The TSFT puncture kit is mainly composed of low ionic medium, polybrene application solution, neutralization solution, and positive control (1gG anti-D), which are provided by Shanghai Blood Bio-Pharmaceutical Co., Ltd. and Zhuhai Bezo Biotechnology Co., Ltd., respectively.
The following procedures were used to screen and identify antibodies in blood donors using the all-tube method: Equal amounts of sera from 10 blood donors were mixed and identified for cell reactions with antibodies using the tube method Treponema pallidum particle test for antibody screening. If a positive reaction was observed, antibody screening was repeated on the 10 original samples before mixing using the traditional AGT. For those who tested positive, irregular antibodies were identified using antibody identification cells, and an absorption and diffusion test was performed if necessary to determine antibody specificity.
The tube polybrene test was conducted as follows[13]: One drop (50 μL) of reagent red blood cells was mixed with two drops (100 μL) of serum or plasma and incubated at room temperature for 15 s at 3000 rpm. The results were double-checked and recorded. Agglutination indicated a positive reaction, while the absence of agglutination indicated a negative reaction.
The tube AGT method was performed as follows[14]: After observing the results using the above methods, the red blood cell serum or plasma mixture was incubated at 37 °C for 30 min. The red blood cells were then washed three times with saline and finally, the packed red blood cells were suspended with two drops (100 mL) of antiglobulin and centrifuged. The outcomes were evaluated according to the same criteria as the upper water tube polybrene test method.
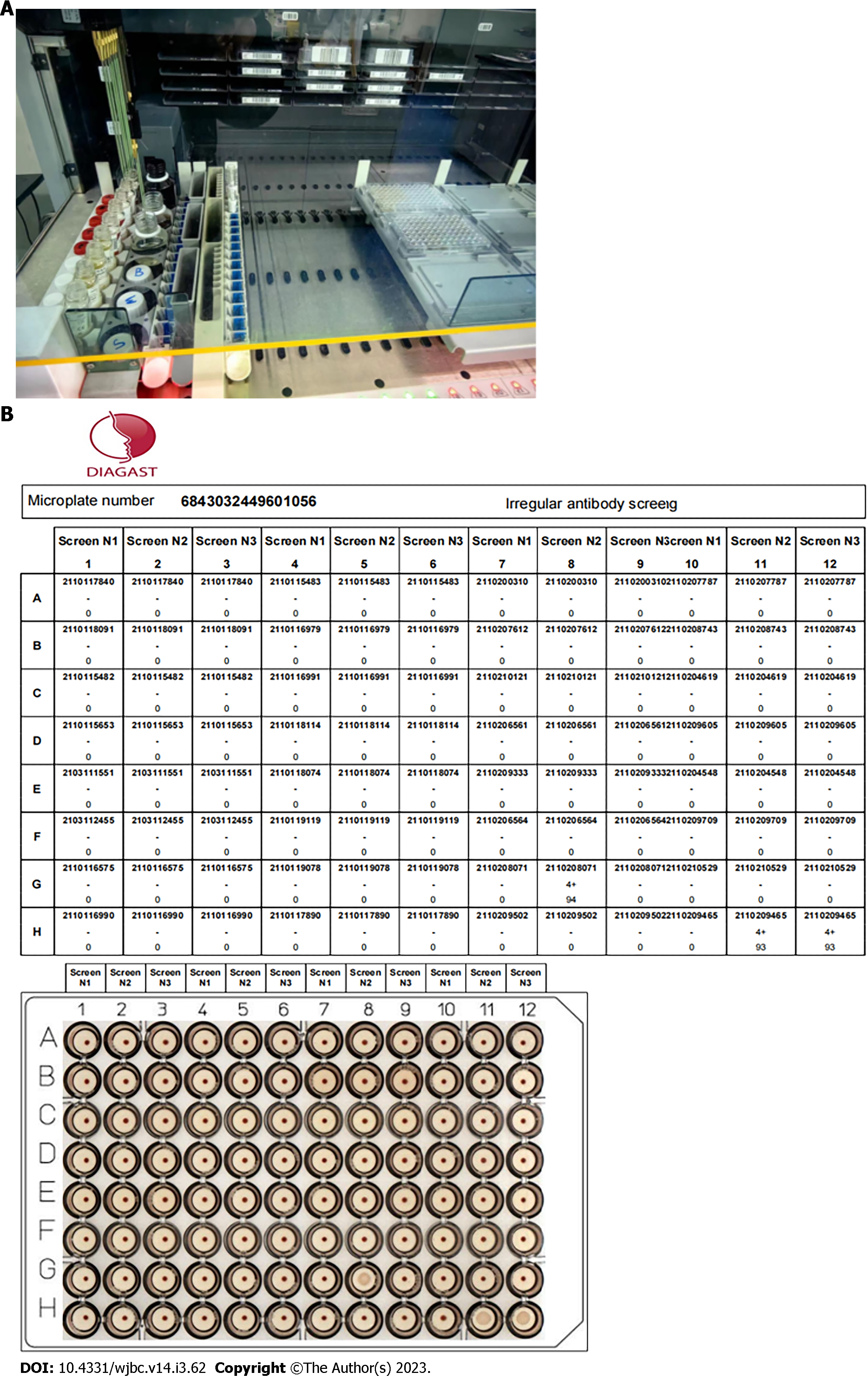
EMT screening for irregular antibodies[15]: With the use of the Collies automatic blood grouping system, with a special program matched with the original reagent, the instrument loads a 96-well ScreenLys microplate coated with antiglobulin, adds SONL isolation solution, diluent, 151 L magnetized screening cells, and 15 μL specimen plasma, completes the closed incubation at 37 °C after sample addition according to the set program, and completes the irregular antibody screening determination of the specimen by magnetization shaking, photography, and interpretation. Figure 1 shows the test results.
Antibody potency assay: The irregular antibody was diluted by doubling with antibody diluent and reacted with the reagent red blood cells with corresponding positive antigens, and its titer was measured. The titer endpoint is the highest dilution with agglutination ≥ 1 +. These reagent red blood cells were also reacted with normal donor sera as a negative control test.
All data from this study were processed and analyzed using IBM SPSS 21.0 software (SPSS Inc., Chicago, IL, United States), and a t-test was harnessed to compare groups. If P < 0.05, statistical significance was confirmed.
A total of 1298 random blood donors were screened using a commercial kit, and the polybrene test performed irregular antibody detection. The results revealed that 18 cases were positive, with a positive rate of 1.39%. Further identification showed the presence of Rh, MN, and other blood group system antibodies, as detailed in Table 1.
| Antibody specificity | Number of cases | Percentage of irregular antibodies (%) | IgG classification | Potency |
| Anti-D | 7 | 38.91 | IgG | 2-64 |
| Anti-E | 2 | 11.11 | IgG | 16-32 |
| Anti-cE | 1 | 5.56 | IgG | 8 |
| Anti-C | 1 | 5.56 | IgG | 16 |
| Anti-PI | 4 | 22.22 | IgM or IgG + IgM | 8-16 |
| Anti-M | 1 | 5.56 | IgG | 4-32 |
| Anti-N | 1 | 5.56 | IgG | 8.32 |
| Anti-Lea | 1 | 5.56 | IgG | 4-16 |
A total of 11 known irregular antibody titers were detected in parallel by tube polybrene test, tube AGT, and EMT screening irregular antibody methods, respectively, while negative quality control tests were performed. It was found that all immunoglobulin G (IgG) and immunoglobulin M (IgM) irregular antibodies could be detected by the EMT screening irregular antibody method, and the manual tube AGT results in the control group were satisfactory, but the operation time was long, and the equipment occupied a large space (such as tubes and racks), as shown in Table 2.
| Test method | Specificity of irregular antibodies | |||||||||||
| D | E | C | c | e | S | Fyb | Jka | M | N | P1 | ||
| Tube polybrene test | Potency | 512 | 256 | 128 | 64 | 32 | 16 | 32 | 64 | 64 | 64 | 32 |
| Score | 57 | 53 | 46 | 40 | 33 | 30 | 34 | 40 | 38 | 37 | 35 | |
| Tube AGT method | Potency | 256 | 64 | 64 | 32 | 16 | 16 | 8 | 32 | 16 | 16 | 32 |
| Score | 52 | 59 | 40 | 32 | 28 | 29 | 20 | 30 | 28 | 28 | 32 | |
| EMT screening irregular antibody method | Potency | 512 | 256 | 128 | 64 | 16 | 16 | 32 | 64 | 64 | 64 | 32 |
| Score | 56 | 54 | 64 | 41 | 31 | 29 | 33 | 40 | 38 | 36 | 34 | |
The 18 positive samples selected from the blood donor spectrum were detected in parallel by the tube polybrene test, tube ACT, and EMT screening irregular antibody method. It was discovered that the reaction pattern of all sera and commercial screening cells or spectrum cells was consistent.
The physical examination of blood donors discovered macroscopically significant severe hemolysis and lipemia samples. In equal proportion, the supernatant sera from these samples were mixed with low concentrations (titer 1:8) of IgG anti-D and AB sera (negative samples). In addition, their activities against type O Rh (D) red blood cells were determined by EMT screening irregular antibodies. The results indicated that the staining failed, but the cell membrane permeability remained normal, suggesting that the cells were active.
The consumption cost of screening irregular antibodies in the aforementioned blood donors was analyzed using the tube polybrene test, tube ACT and EMT screening methods. The cost was calculated by dividing the cost of each method by the number of blood donors tested. The outcomes showed that the EMT screening irregular antibody method cost less than 0.5 RMB per blood donor, while the tube polybrene test and tube AGT methods cost more than 5 RMB and 5.5 RMB per blood donor, respectively. These findings demonstrated that the EMT screening method was cost-effective and had advanced detection technology. Additionally, it allowed for sample concentration, resulting in improved work efficiency (see Table 3 for details).
| Methods | Cost (CNY/Donor) |
| EMT screening irregular antibody method | < 0.5 |
| Tube polybrene test | > 5 |
| Tube AGT method | > 5.5 |
At present, blood transfusion therapy, as a clinical treatment, is still irreplaceable in the treatment of some diseases. Although some advanced treatment options have significantly reduced the dependence on blood products in the treatment after being applied in clinical practice, hemolytic transfusion reactions and cross-matching difficulties caused by blood group antibodies other than ABO, that is, irregular antibodies, also occur from time to time[16,17]. Blood group antibodies destroy mismatched red blood cells in transfused blood or shorten their lifespan, producing hemolytic transfusion reactions, which may affect treatment outcomes or endanger the patient's life. Studies have unveiled that neonatal hemolytic disease caused by prenatal irregular blood group antibodies in pregnant women, especially Rh-HDN, has severe symptoms and often leads to serious harm[18,19]. Furthermore, monoclonal hyperactivity-A (B) reagent gas reportedly reacts with most red blood cells with high sensitivity and specificity[20]. Malformed hemolytic transfusion reactions caused by ABO blood group incompatibility are typically uncommon. In contrast, adverse transfusion reactions, neonatal hemolysis, and difficulty in blood grouping caused by irregular antibodies to ABO blood group accidents of red blood cells occasionally occur[21,22]. In addition, it has been internationally reported that monoclonal reagents can lead to false agglutination or false negative phenomenon during ABO blood group detection, and there have been corresponding reports in China in recent years[23]. Therefore, blood grouping alone is insufficient before transfusion, and antibody screening is necessary to ensure blood transfusion safety.
In recent years, with the development of medical technology, automatic blood grouping has been more and more widely used in the blood transfusion departments of major hospitals and blood collection institutions at all levels. It also plays a vital role in blood grouping tests, irregular antibody screening tests, and clinical cross-matching tests[24]. EMT is a new technology based on red blood cell magnetization, which has been maturely applied in immunodiagnosis, cell separation, protein purification, and nucleic acid extraction, and has also improved the automation rate of laboratories[25,26]. Based on this, this study aimed to investigate the effect of the conventional tube and EMT in red blood cell alloantibody titration, hoping to provide some help for clinically safe blood transfusion.
This study collected 5 mL of whole blood from 1298 blood donors. The serum was separated by centrifugation. Different red blood cell blood group antibody titers were detected in parallel using the tube polybrene test, tube AGT, and EMT screening irregular antibody. Usually, voluntary blood donor samples are centrally collected. In order to save labor and reagents, 5-10 samples can be mixed for primary screening, and positive samples can be reexamined one by one to detect antibody-positive samples. However, because a few irregular antibodies with low titer in the mixed plasma still have the possibility of missed detection, the mixed plasma primary screening method is not suitable for the pretransfusion test of patients. In addition, it was found that all irregular antibodies of IgG and IgM nature were detected by EMT screening irregular antibody method. Nevertheless, the tube polybrene test method and tube AGT method did not detect irregular antibodies of low concentration, which may be related to the lack of sensitivity of IgM active blood group antibodies in low concentrations and at room temperature in the polybrene method and AGT.
Red cell alloantibodies are classified based on their properties as macromolecular IgM or small molecular IgG[27]. There are many methods to detect red blood cell antibodies, but standard tube methods can only detect IgM antibodies in most cases[28,29]. The results showed that the EMT screening irregular antibody method had clear advantages in determining the activity of IgG O Rh (D) red blood cell antibodies. In recent years, the use of traditional in vitro AGT has become more popular in China[30]. The findings in this paper indicate that the EMT screening irregular antibody method, the in vitro polybrene test method, and the in vitro AGT method can be utilied for routine screening of irregular antibodies in blood donors. Among these methods, the EMT screening irregular antibody method is more rapid, convenient, efficient, and less expensive.
In summary, compared with the in vitro polybrene test method and in vitro AGT method, the Collies automatic blood group system based on EMT for irregular antibody screening has the characteristics of more robust antibody specificity, more convenient operation, more accurate results, and complete lake source, which is suitable for large-scale screening of irregular antibodies in blood stations and is worthy of being widely popularized in clinical practice. Of course, more sensitive test techniques help to improve the antibody detection rate, but there may still be some antibodies that are not easy. In daily testing, the recorded history of checking past antibodies should be listed as a part of routine compatibility tests. Hospitals at all levels should actively understand the patient's blood transfusion history, pregnancy history, and current history and perform blood group and irregular antibody detection in advance so that the matching blood components can be selected for patients in time to ensure the safety and effectiveness of clinical blood transfusion.
Compared with the conventional tube method, the EMT-based Collies automatic blood group system for irregular antibody screening can more accurately screen its irregular antibody and has more substantial antibody specificity. In addition, it is more convenient to operate, with more accurate results, less cost consumed, and other characteristics, and is worthy of broader promotion in clinical practice.
Magnetic red cell immunoseparation is a biomedical technique for separating and detecting small numbers of targeted cells. It has the advantages of high sensitivity, high precision, and easy operation and has been widely applied in the field of in vitro diagnostics and therapy.
Conventional separation methods can be time-consuming and involve complicated procedures, while magnetic red cell immunoseparation has the advantages of ease of use, high sensitivity, and precision. Therefore, this technology has been widely applied in in vitro diagnostics and therapy, attracting much attention from researchers and medical professionals.
This study aims to explore the application value of conventional test tubes and erythrocyte-magnetized technology (EMT) in red blood cell alloantibody titration to improve the safety of clinical blood transfusion.
Parallel detection of antibody titers for different red blood cell blood groups using in vitro polyene test, tube antiglobulin test (AGT) and EMT screening for irregular antibodies.
The irregular antibody method for EMT screening could detect all immunoglobulin G and immunoglobulin M irregular antibodies, and the operation time is shorter than manual tube AGT. Furthermore, the EMT screening irregular antibody test was performed to detect its activity on O-type R (D) red blood cells, and the results reflected that it was normal. In addition, compared to the conventional tube method, the EMT screening method for irregular antibodies had lower costs and significantly higher detection efficiency.
Compared with traditional in vitro methods, EMT screening for irregular antibodies has lower costs and significantly higher detection efficiency.
The broad application prospects of red blood cell magnetization technology in medicine are evident. Technological advancements will further expand the application scope of this technology. For example, red blood cell magnetization technology can diagnose diseases like early cancer and cardiovascular diseases. In addition, this technology can be used for in vivo and in vitro research on the movement, interaction, and molecular processes of cells and pathogenic microorganisms.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Biochemistry and molecular biology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mesa F, Spain; Yerke LM, United States S-Editor: Fan JR L-Editor: A P-Editor: Cai YX
| 1. | Fox T, Geppert J, Dinnes J, Scandrett K, Bigio J, Sulis G, Hettiarachchi D, Mathangasinghe Y, Weeratunga P, Wickramasinghe D, Bergman H, Buckley BS, Probyn K, Sguassero Y, Davenport C, Cunningham J, Dittrich S, Emperador D, Hooft L, Leeflang MM, McInnes MD, Spijker R, Struyf T, Van den Bruel A, Verbakel JY, Takwoingi Y, Taylor-Phillips S, Deeks JJ; Cochrane COVID-19 Diagnostic Test Accuracy Group. Antibody tests for identification of current and past infection with SARS-CoV-2. Cochrane Database Syst Rev. 2022;11:CD013652. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 40] [Reference Citation Analysis (0)] |
| 2. | Strand V, Balsa A, Al-Saleh J, Barile-Fabris L, Horiuchi T, Takeuchi T, Lula S, Hawes C, Kola B, Marshall L. Immunogenicity of Biologics in Chronic Inflammatory Diseases: A Systematic Review. BioDrugs. 2017;31:299-316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 246] [Cited by in RCA: 247] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 3. | Shastry S, Chenna D, Basavarajegowda A, Das S, Chaudhary RK. Red blood cell alloimmunization among recipients of blood transfusion in India: A systematic review and meta-analysis. Vox Sang. 2022;117:1057-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 4. | Ngoma AM, Mutombo PB, Ikeda K, Nollet KE, Natukunda B, Ohto H. Red blood cell alloimmunization in transfused patients in sub-Saharan Africa: A systematic review and meta-analysis. Transfus Apher Sci. 2016;54:296-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Wong K, Lai WK, Jackson DE. HLA Class II regulation of immune response in sickle cell disease patients: Susceptibility to red blood cell alloimmunization (systematic review and meta-analysis). Vox Sang. 2022;117:1251-1261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | Alshehri AA, Jackson DE. Non-Invasive Prenatal Fetal Blood Group Genotype and Its Application in the Management of Hemolytic Disease of Fetus and Newborn: Systematic Review and Meta-Analysis. Transfus Med Rev. 2021;35:85-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Boateng LA, Ngoma AM, Bates I, Schonewille H. Red Blood Cell Alloimmunization in Transfused Patients With Sickle Cell Disease in Sub-Saharan Africa; a Systematic Review and Meta-Analysis. Transfus Med Rev. 2019;33:162-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 8. | Fortin PM, Hopewell S, Estcourt LJ. Red blood cell transfusion to treat or prevent complications in sickle cell disease: an overview of Cochrane reviews. Cochrane Database Syst Rev. 2018;8:CD012082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Estcourt LJ, Fortin PM, Hopewell S, Trivella M, Doree C, Abboud MR. Interventions for preventing silent cerebral infarcts in people with sickle cell disease. Cochrane Database Syst Rev. 2017;5:CD012389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Fisher SA, Brunskill SJ, Doree C, Gooding S, Chowdhury O, Roberts DJ. Desferrioxamine mesylate for managing transfusional iron overload in people with transfusion-dependent thalassaemia. Cochrane Database Syst Rev. 2013;CD004450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | Estcourt LJ, Kimber C, Hopewell S, Trivella M, Doree C, Abboud MR. Interventions for preventing silent cerebral infarcts in people with sickle cell disease. Cochrane Database Syst Rev. 2020;4:CD012389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Cirillo P, Gold AK, Nardi AE, Ornelas AC, Nierenberg AA, Camprodon J, Kinrys G. Transcranial magnetic stimulation in anxiety and trauma-related disorders: A systematic review and meta-analysis. Brain Behav. 2019;9:e01284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 108] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 13. | Lee YF, Hsu TW, Liang CS, Yeh TC, Chen TY, Chen NC, Chu CS. The Efficacy and Safety of Tube Feeding in Advanced Dementia Patients: A Systemic Review and Meta-Analysis Study. J Am Med Dir Assoc. 2021;22:357-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 14. | Segel GB, Lichtman MA. Direct antiglobulin ("Coombs") test-negative autoimmune hemolytic anemia: a review. Blood Cells Mol Dis. 2014;52:152-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 15. | Selvaraj EA, Mózes FE, Jayaswal ANA, Zafarmand MH, Vali Y, Lee JA, Levick CK, Young LAJ, Palaniyappan N, Liu CH, Aithal GP, Romero-Gómez M, Brosnan MJ, Tuthill TA, Anstee QM, Neubauer S, Harrison SA, Bossuyt PM, Pavlides M; LITMUS Investigators. Diagnostic accuracy of elastography and magnetic resonance imaging in patients with NAFLD: A systematic review and meta-analysis. J Hepatol. 2021;75:770-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 207] [Article Influence: 51.8] [Reference Citation Analysis (0)] |
| 16. | Stussi G, Huggel K, Lutz HU, Schanz U, Rieben R, Seebach JD. Isotype-specific detection of ABO blood group antibodies using a novel flow cytometric method. Br J Haematol. 2005;130:954-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 87] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 17. | Nadarevic T, Colli A, Giljaca V, Fraquelli M, Casazza G, Manzotti C, Štimac D, Miletic D. Magnetic resonance imaging for the diagnosis of hepatocellular carcinoma in adults with chronic liver disease. Cochrane Database Syst Rev. 2022;5:CD014798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | McQuilten ZK, Crighton G, Brunskill S, Morison JK, Richter TH, Waters N, Murphy MF, Wood EM. Optimal Dose, Timing and Ratio of Blood Products in Massive Transfusion: Results from a Systematic Review. Transfus Med Rev. 2018;32:6-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 19. | Saadah NH, van Hout FMA, Schipperus MR, le Cessie S, Middelburg RA, Wiersum-Osselton JC, van der Bom JG. Comparing transfusion reaction rates for various plasma types: a systematic review and meta-analysis/regression. Transfusion. 2017;57:2104-2114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 20. | Dodd JM, Windrim RC, van Kamp IL. Techniques of intrauterine fetal transfusion for women with red-cell isoimmunisation for improving health outcomes. Cochrane Database Syst Rev. 2010;CD007096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Lin Y, Stanworth S, Birchall J, Doree C, Hyde C. Recombinant factor VIIa for the prevention and treatment of bleeding in patients without haemophilia. Cochrane Database Syst Rev. 2011;CD005011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Pecoraro V, Negro A, Pirotti T, Trenti T. Estimate false-negative RT-PCR rates for SARS-CoV-2. A systematic review and meta-analysis. Eur J Clin Invest. 2022;52:e13706. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 67] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 23. | Kohli M, Schiller I, Dendukuri N, Dheda K, Denkinger CM, Schumacher SG, Steingart KR. Xpert(®) MTB/RIF assay for extrapulmonary tuberculosis and rifampicin resistance. Cochrane Database Syst Rev. 2018;8:CD012768. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 138] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 24. | Severe Covid-19 GWAS Group; Ellinghaus D, Degenhardt F, Bujanda L, Buti M, Albillos A, Invernizzi P, Fernández J, Prati D, Baselli G, Asselta R, Grimsrud MM, Milani C, Aziz F, Kässens J, May S, Wendorff M, Wienbrandt L, Uellendahl-Werth F, Zheng T, Yi X, de Pablo R, Chercoles AG, Palom A, Garcia-Fernandez AE, Rodriguez-Frias F, Zanella A, Bandera A, Protti A, Aghemo A, Lleo A, Biondi A, Caballero-Garralda A, Gori A, Tanck A, Carreras Nolla A, Latiano A, Fracanzani AL, Peschuck A, Julià A, Pesenti A, Voza A, Jiménez D, Mateos B, Nafria Jimenez B, Quereda C, Paccapelo C, Gassner C, Angelini C, Cea C, Solier A, Pestaña D, Muñiz-Diaz E, Sandoval E, Paraboschi EM, Navas E, García Sánchez F, Ceriotti F, Martinelli-Boneschi F, Peyvandi F, Blasi F, Téllez L, Blanco-Grau A, Hemmrich-Stanisak G, Grasselli G, Costantino G, Cardamone G, Foti G, Aneli S, Kurihara H, ElAbd H, My I, Galván-Femenia I, Martín J, Erdmann J, Ferrusquía-Acosta J, Garcia-Etxebarria K, Izquierdo-Sanchez L, Bettini LR, Sumoy L, Terranova L, Moreira L, Santoro L, Scudeller L, Mesonero F, Roade L, Rühlemann MC, Schaefer M, Carrabba M, Riveiro-Barciela M, Figuera Basso ME, Valsecchi MG, Hernandez-Tejero M, Acosta-Herrera M, D'Angiò M, Baldini M, Cazzaniga M, Schulzky M, Cecconi M, Wittig M, Ciccarelli M, Rodríguez-Gandía M, Bocciolone M, Miozzo M, Montano N, Braun N, Sacchi N, Martínez N, Özer O, Palmieri O, Faverio P, Preatoni P, Bonfanti P, Omodei P, Tentorio P, Castro P, Rodrigues PM, Blandino Ortiz A, de Cid R, Ferrer R, Gualtierotti R, Nieto R, Goerg S, Badalamenti S, Marsal S, Matullo G, Pelusi S, Juzenas S, Aliberti S, Monzani V, Moreno V, Wesse T, Lenz TL, Pumarola T, Rimoldi V, Bosari S, Albrecht W, Peter W, Romero-Gómez M, D'Amato M, Duga S, Banales JM, Hov JR, Folseraas T, Valenti L, Franke A, Karlsen TH. Genomewide Association Study of Severe Covid-19 with Respiratory Failure. N Engl J Med. 2020;383:1522-1534. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1512] [Cited by in RCA: 1389] [Article Influence: 277.8] [Reference Citation Analysis (0)] |
| 25. | Ilic D, Djulbegovic M, Jung JH, Hwang EC, Zhou Q, Cleves A, Agoritsas T, Dahm P. Prostate cancer screening with prostate-specific antigen (PSA) test: a systematic review and meta-analysis. BMJ. 2018;362:k3519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 223] [Cited by in RCA: 364] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 26. | Nelson HD, Fu R, Cantor A, Pappas M, Daeges M, Humphrey L. Effectiveness of Breast Cancer Screening: Systematic Review and Meta-analysis to Update the 2009 U.S. Preventive Services Task Force Recommendation. Ann Intern Med. 2016;164:244-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 366] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 27. | Macedo ACL, Prestes GDS, Colonetti T, Candido ACR, Uggioni MLR, Gomes AC, Silva FR, Ceretta LB, Grande AJ, da Rosa MI. A systematic review and meta-analysis of the accuracy of SARS-COV-2 IGM and IGG tests in individuals with COVID-19. J Clin Virol. 2022;148:105121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 28. | Zhang ZL, Hou YL, Li DT, Li FZ. Diagnostic efficacy of anti-SARS-CoV-2 IgG/IgM test for COVID-19: A meta-analysis. J Med Virol. 2021;93:366-374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 29. | Rio-Aige K, Azagra-Boronat I, Castell M, Selma-Royo M, Collado MC, Rodríguez-Lagunas MJ, Pérez-Cano FJ. The Breast Milk Immunoglobulinome. Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 72] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 30. | Jarne-Borràs M, Miró-Mur F, Anunciación-Llunell A, Alijotas-Reig J. Antiphospholipid antibodies in women with recurrent embryo implantation failure: A systematic review and meta-analysis. Autoimmun Rev. 2022;21:103101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |