Published online Jan 27, 2024. doi: 10.4240/wjgs.v16.i1.59
Peer-review started: October 30, 2023
First decision: November 8, 2023
Revised: December 6, 2023
Accepted: December 18, 2023
Article in press: December 18, 2023
Published online: January 27, 2024
Processing time: 86 Days and 22.2 Hours
Severe acute pancreatitis (SAP), a condition with rapid onset, critical condition and unsatisfactory prognosis, poses a certain threat to human health, warranting optimization of relevant treatment plans to improve treatment efficacy.
To evaluate the efficacy and safety of computerized tomography-guided the
Forty-two SAP patients admitted to The Second Affiliated Hospital of Fujian Medical University from June 2020 to June 2023 were selected. On the basis of routine treatment, 20 patients received SS therapy (control group) and 22 patients were given CT-TPPCD plus SS intervention (research group). The efficacy, safety (pancreatic fistula, intra-abdominal hemorrhage, sepsis, and organ dysfunction syndrome), abdominal bloating and pain relief time, bowel recovery time, hospital stay, inflammatory indicators (C-reactive protein, interleukin-6, and pro
Compared with the control group, the research group had a markedly higher total effective rate, faster abdominal bloating and pain relief and bowel recovery, shorter hospital length of stay, fewer complications, and lower posttreatment inflammatory indices and APACHE-II scores.
CT-TPPCD in combination with SS is effective for SAP patients, which can reduce complications, accelerate symptom resolution, inhibit inflammation, and improve patient condition, with promising prospects for clinical promotion.
Core Tip: Severe acute pancreatitis (SAP) is a severe acute manifestation of pancreatitis, which may lead to disease deterioration due to local and systemic infections. Therefore, an effective and safe intervention method is urgently needed to optimize the management of SAP patients. This study suggests that computerized tomography-guided therapeutic percutaneous puncture catheter drainage combined with somatostatin for SAP patients has high clinical efficacy and safety, providing a novel option for the clinical management optimization of such patients.
- Citation: Zheng XL, Li WL, Lin YP, Huang TL. Computerized tomography-guided therapeutic percutaneous puncture catheter drainage-combined with somatostatin for severe acute pancreatitis: An analysis of efficacy and safety. World J Gastrointest Surg 2024; 16(1): 59-66
- URL: https://www.wjgnet.com/1948-9366/full/v16/i1/59.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i1.59
Pancreatitis, an inflammatory disease occurring in the pancreatic tissue, is classified as either acute or chronic and is associated with high morbidity and mortality, imposing a socioeconomic burden[1,2]. The pathogenesis of this disease involves early protease activation, activation of nuclear factor kappa-B-related inflammatory reactions, and infiltration of immune cells[3]. Severe acute pancreatitis (SAP) is a serious condition involving systemic injury and subsequent possible organ failure, accounting for 20% of all acute pancreatitis cases[4]. SAP is also characterized by rapid onset, critical illness and unsatisfactory prognosis and is correlated with serious adverse events such as systemic inflammatory response syn
Somatostatin (SS), a peptide hormone that can be secreted by endocrine cells and the central nervous system, is in
In light of the limited studies on the efficacy and safety of SS plus CT-TPPCD in SAP treatment, this study performed a relevant analysis to improve clinical outcomes in SAP patients.
Forty-two SAP patients admitted to The Second Affiliated Hospital of Fujian Medical University between June 2020 and June 2023 were selected. In addition to routine treatment, 20 patients in the control group received SS treatment, and the rest 22 patients in the research group received CT-TPPCD plus SS intervention. The inclusion criteria were as follows: Diagnosis of SAP[15]; presence of seroperitoneum and intraperitoneal abscess as indicated by imaging examination; presence of symptoms such as nausea, vomiting and abdominal pain; treatment-naive SAP patients; no contraindications to the medication used in this study; and complete medical records. The exclusion criteria were as follows: Malignant tumors, autoimmune disease, cardiovascular disorders, coagulation dysfunction, etc.; mental illness; previous history of abdominal surgery; severe organ dysfunction; serious abnormality of basic gastrointestinal function; and lactating or pregnant women.
Both groups received routine treatment, primarily including fasting, anti-infection, maintenance of water and electrolyte balance, pain relief, and reduction of gastrointestinal hypertension. In addition, the control group was treated with SS intravenously, with the SS dose gradually adjusted according to the patient’s condition.
Based on the above measures, the research group was supplemented with CT-TPPCD. First, the patient was examined with a CT. The feasibility of catheter drainage was indicated by the presence of peripancreatic effusion, pancreatic necrosis, a pseudocyst, and local infection. A puncture was performed after precise positioning by CT, routine dis
Statistics on efficacy, adverse events [pancreatic fistula (PF), intra-abdominal hemorrhage (IAH), sepsis, and organ dysfunction syndrome], postoperative abdominal bloating and pain relief, bowel recovery time, hospital length of stay, inflammatory indices [C-reactive protein (CRP), interleukin-6 (IL-6) and procalcitonin (PCT)], and Acute Physiological and Chronic Health Evaluation (APACHE) II score were collected for comparative analyses. Among them, the efficacy is assessed as follows: Cure refers to the disappearance of clinical symptoms and signs and the return of laboratory indicators and imaging tests to normal; improvement is indicated by relieved clinical symptoms and signs and an incomplete recovery from complications such as infection, inflammation and false abscess shown by auxiliary examination; if the patient's clinical symptoms and signs did not improve or worsen, it was considered ineffectiveness. Second, CRP, IL-6, and PCT were all determined by enzyme-linked immunosorbent assays (ELISAs). Before detection, 5 mL of fasting elbow venous blood was extracted from each patient, and the serum was separated as a sample for detection. Finally, APACHE-II, including acute physiology (the sum of Glasgow Coma Scale score and various physiological variable scores, with a score range of 0-60), age (0-6 points), and chronic health subscales (0-5 points), was used to evaluate physical health status; on a scale of 0-71, the score is directly proportional to the severity of SAP.
The GraphPad Prism 7.0 software package was used for data analyses, and the level of statistical significance was P < 0.05. Categorical variables [described as the number of samples (percentage), n (%)] and continuous variables (represented by SD ± SEM) were compared by the χ2 test and the independent sample t test, respectively.
The two groups were similar in age, sex, course of disease, body mass index, classification of intra-abdominal hypertension, type of pancreatitis, and other general data (P > 0.05) (Table 1).
| Indicators | Control group (n = 20) | Research group (n = 22) | χ2/t | P value |
| Age | 37.20 ± 8.76 | 36.68 ± 10.61 | 0.172 | 0.864 |
| Sex | 0.877 | 0.349 | ||
| Male | 15 (75.00) | 19 (86.36) | ||
| Female | 5 (25.00) | 3 (13.64) | ||
| Course of disease (h) | 6.00 ± 2.49 | 6.82 ± 2.72 | ||
| BMI (kg/m2) | 21.76 ± 2.54 | 22.58 ± 2.68 | ||
| Intra-abdominal hypertension | 0.479 | 0.787 | ||
| I | 12 (60.00) | 13 (59.09) | ||
| II | 5 (25.00) | 7 (31.82) | ||
| III | 3 (15.00) | 2 (9.09) | ||
| Types of pancreatitis | 0.557 | 0.757 | ||
| Hyperlipidemic pancreatitis | 16 (80.00) | 18 (81.82) | ||
| Biliary | 3 (150.00) | 2 (9.09) | ||
| Alcoholic | 1 (5.00) | 2 (9.09) |
The efficacy was comparatively analyzed to evaluate the effect of the two treatments on the therapeutic efficacy of SAP patients. The total effective rate was 90.91% in the research group, which was significantly higher than the 65.00% in the control group (P < 0.05) (Table 2).
| Indicators | Control group (n = 20) | Research group (n = 22) | χ2 | P value |
| Cure | 8 (40.00) | 12 (54.55) | ||
| Improvement | 5 (25.00) | 8 (36.36) | ||
| Ineffectiveness | 7 (35.00) | 2 (9.09) | ||
| Effective rate | 13 (65.00) | 20 (90.91) | 4.177 | 0.041 |
Adverse events were counted in both groups to assess the impact of the two treatments on patient safety. The total incidence of adverse events such as PF, IAH, sepsis, and organ dysfunction syndrome was 22.73% in the research group and 55.00% in the control group, with a significant intergroup difference (P < 0.05) (Table 3).
| Indicators | Control group (n = 20) | Research group (n = 22) | χ2 | P value |
| Pancreatic fistula | 3 (15.00) | 1 (4.55) | ||
| Intra-abdominal hemorrhage | 3 (15.00) | 2 (9.09) | ||
| Sepsis | 2 (10.00) | 1 (4.55) | ||
| Organ dysfunction syndrome | 3 (15.00) | 1 (4.55) | ||
| Total | 11 (55.00) | 5 (22.73) | 4.627 | 0.032 |
The abdominal bloating and pain relief time, bowel recovery time, and hospital stay of both groups were recorded to evaluate the influence of the two treatments on SAP patients’ recovery. The research group was found to have significantly faster abdominal bloating and pain relief and bowel recovery and shorter hospital stays than the control group (P < 0.05) (Figure 1).
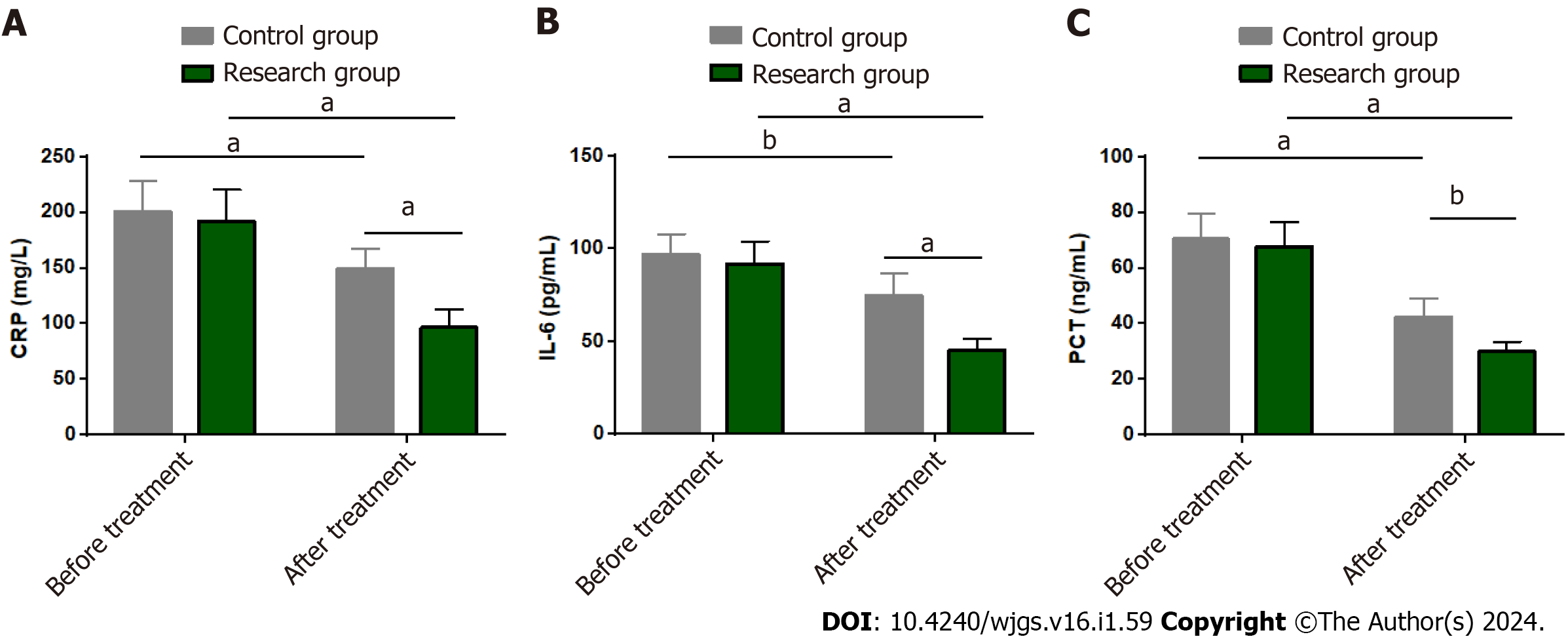
The effects of the two treatments on the serum inflammatory response of SAP patients were assessed by measuring CRP, IL-6, and PCT levels by ELISA. The levels of the above inflammatory factors were not significantly different between the groups before treatment (P > 0.05). CRP, IL-6 and PCT all decreased significantly after treatment (P < 0.05), with lower levels in the research group than in the control group (P < 0.05) (Figure 2).
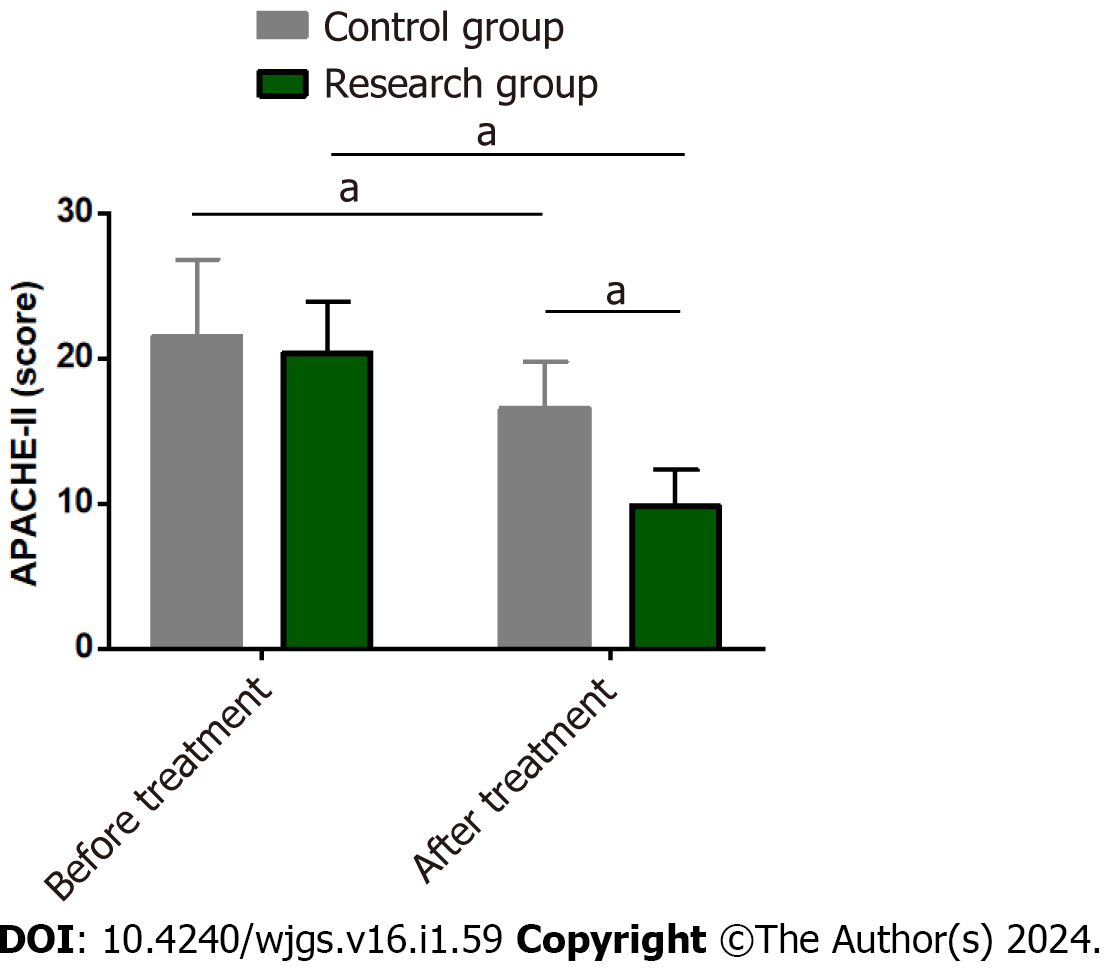
The APACHE-II score was tested in both groups to compare the effects of the two treatment modalities on disease severity in SAP patients. The pretreatment APACHE-II score was similar between the two groups (P > 0.05). A marked reduction in the APACHE-II score was observed in both groups after treatment (P < 0.05), with an even lower score in the research group (P < 0.05) (Figure 3).
Pancreatitis, a condition in which the pancreas itself digests abnormally, is mainly manifested by damage to the pan
Our research results showed a significantly higher total effective rate in the research group (90.91%) compared with the control group (65.00%), suggesting that SS plus CT-TPPCD is beneficial for therapeutic effect enhancement. The therapeutic mechanism of SS in SAP is related to its inhibition of insulin and glucagon secretion, which reduces the secretion of the pancreas and gallbladder and favors gastrointestinal absorption and nutritional function[20,21]. After statistical analysis of the incidence of PF, IAH, sepsis and organ dysfunction, the total incidence of the above adverse events was found to be markedly lower in the research group (22.73%) than in the control group (55.00%), indicating that SS plus CT-TPPCD can better guarantee the postoperative safety of SAP patients. Ai et al[22] reported that CT-TPPCD reduced mortality and the risk of inflammation-related complications in SAP patients, similar to our research results. In the study by Ganaie et al[23], CT-TPPCD is also shown to be clinically effective and safe for SAP pancreatic effusion management, both in patients with coinfection and symptomatic pancreatic effusion. The effects of the two treatments on patient postoperative recovery were evaluated from the aspects of abdominal bloating and pain relief time, bowel recovery time, and hospital stay. It was found that the research group had significantly better performance in the above aspects, suggesting that SS plus CT-TPPCD is conducive to promoting postoperative recovery in SAP patients. On the other hand, CRP is a protein that reflects the acute stage of a disease and is abnormally elevated in the setting of infection, tissue damage, etc[24]. IL-6, as an acute-phase reactive lymphocyte factor, not only activates the body's defense response and immunosuppression but also predicts the severity of SAP[25]. While PCT, an inflammatory factor closely related to secondary organ injury, is also associated with systemic inflammatory response syndrome[26]. Therefore, the above three inflammatory indices were detected to evaluate the influence of the two treatments on SAP patients. The research group showed markedly reduced- posttreatment CRP, IL-6, and PCT levels that were lower than the pretreatment levels and those of the control group, demonstrating the ability of SS plus CT-TPPCD to effectively inhibit serum inflammatory reactions in SAP patients. Previous evidence has shown that the downregulation of CRP, IL-6 and PCT levels can reflect treatment effectiveness in SAP patients, consistent with our findings[27]. Huang et al[28] also pointed out that lowering IL-6 levels was helpful to prevent SAP. Finally, the APACHE-II score of the research group decreased significantly after treatment and was lower than that of the control group, indicating that SS plus CT-TPPCD can significantly inhibit disease severity in SAP patients.
In summary, CT-TPPCD combined with SS is effective and safe in the treatment of SAP, which not only accelerates postoperative abdominal bloating and pain relief and intestinal recovery but also inhibits disease progression and improves patient health by reducing the levels of inflammatory indicators such as CRP, IL-6 and PCT. Our findings can provide a new choice for the clinical management optimization of SAP patients.
Severe acute pancreatitis (SAP) accounts for 20% of all acute pancreatitis cases and poses a more serious threat to human health, so it is necessary to provide timely intervention to patients to improve their outcomes and ensure a certain therapeutic effect.
In view of the limited studies on the efficacy and safety of somatostatin (SS) combined with computerized tomography-guided therapeutic percutaneous puncture catheter drainage (CT-TPPCD) in the treatment of SAP, this study aims to supplement the gaps in this area and provide reliable clinical guidance.
To analyze the efficacy and safety of CT-TPPCD combined with SS in the treatment of SAP.
Forty-two SAP patients were included, including 20 cases (control group) treated with SS intervention and 22 cases (research group) with CT-TPPCD + SS intervention. Comparative analyses were conducted from the following perspectives: Efficacy, safety (pancreatic fistula, intraperitoneal hemorrhage, sepsis, and organ dysfunction syndrome), abdominal bloating and pain relief time, intestinal recovery time, length of hospital stay, inflammatory indicators (C-reactive protein, interleukin-6, and procalcitonin), and Acute Physiology and Chronic Health Evaluation (APACHE) II score.
The research group showed a higher total effective rate than the control group, with faster relief of abdominal bloating and pain and intestinal recovery, shorter length of hospital stay, and fewer adverse events, all with statistical significance. In addition, lower levels of inflammation indexes and APACHE II scores were determined in the research group after treatment, significantly lower than the baseline and those of the control group.
CT-TPPCD plus SS is highly effective and safe in the treatment of SAP patients, contributing to fast inhibition of patients' disease and effective alleviation of serum inflammatory responses, which is worthy of clinical promotion.
The negative impact of SAP on patients should not be underestimated, and it is necessary to improve clinical efficacy from the perspective of treatment optimization. This study proposes that SS combined with CT-TPPCD is significantly superior to SS alone in the treatment of SAP, which is of great significance for improving the clinical outcome of SAP patients and provides new clinical basis and insights.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Carli ALE, Australia; Tsilidis KK, United Kingdom S-Editor: Fan JR L-Editor: A P-Editor: Zhang YL
| 1. | Peery AF, Crockett SD, Murphy CC, Lund JL, Dellon ES, Williams JL, Jensen ET, Shaheen NJ, Barritt AS, Lieber SR, Kochar B, Barnes EL, Fan YC, Pate V, Galanko J, Baron TH, Sandler RS. Burden and Cost of Gastrointestinal, Liver, and Pancreatic Diseases in the United States: Update 2018. Gastroenterology. 2019;156:254-272.e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 776] [Cited by in RCA: 1078] [Article Influence: 179.7] [Reference Citation Analysis (1)] |
| 2. | Habtezion A, Gukovskaya AS, Pandol SJ. Acute Pancreatitis: A Multifaceted Set of Organelle and Cellular Interactions. Gastroenterology. 2019;156:1941-1950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 194] [Article Influence: 32.3] [Reference Citation Analysis (1)] |
| 3. | Mayerle J, Sendler M, Hegyi E, Beyer G, Lerch MM, Sahin-Tóth M. Genetics, Cell Biology, and Pathophysiology of Pancreatitis. Gastroenterology. 2019;156:1951-1968.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 231] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 4. | Garg PK, Singh VP. Organ Failure Due to Systemic Injury in Acute Pancreatitis. Gastroenterology. 2019;156:2008-2023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 372] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 5. | Saeed SA. Acute pancreatitis in children: Updates in epidemiology, diagnosis and management. Curr Probl Pediatr Adolesc Health Care. 2020;50:100839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | Ge P, Luo Y, Okoye CS, Chen H, Liu J, Zhang G, Xu C. Intestinal barrier damage, systemic inflammatory response syndrome, and acute lung injury: A troublesome trio for acute pancreatitis. Biomed Pharmacother. 2020;132:110770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 123] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 7. | Ampofo E, Nalbach L, Menger MD, Laschke MW. Regulatory Mechanisms of Somatostatin Expression. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 8. | Herszényi L, Mihály E, Tulassay Z. [Somatostatin and the digestive system. Clinical experiences]. Orv Hetil. 2013;154:1535-1540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Shamsi BH, Chatoo M, Xu XK, Xu X, Chen XQ. Versatile Functions of Somatostatin and Somatostatin Receptors in the Gastrointestinal System. Front Endocrinol (Lausanne). 2021;12:652363. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 10. | Wang G, Liu Y, Zhou SF, Qiu P, Xu L, Wen P, Wen J, Xiao X. Effect of Somatostatin, Ulinastatin and Gabexate on the Treatment of Severe Acute Pancreatitis. Am J Med Sci. 2016;351:506-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 11. | Tang WF, Wang YG, Zhu L, Wan MH, Chen GY, Xia Q, Ren P, Huang X. Effect of somatostatin on immune inflammatory response in patients with severe acute pancreatitis. J Dig Dis. 2007;8:96-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Pluemvitayaporn T, Pongpanumaspaisan T, Kittithamvongs P, Kunakornsawat S, Sirivitayaphakorn P, Piyaskulkaew C, Pruttikul P. Does computed tomography-guided percutaneous catheter drainage is effective for spinal tuberculous abscess: a midterm results. Spinal Cord Ser Cases. 2022;8:19. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 13. | Liu T, Sun S, Gao H, Gao Y, Xu Q, Liu X, Miao Y, Wei J. CT-guided percutaneous catheter drainage of pancreatic postoperative collections. Minim Invasive Ther Allied Technol. 2020;29:269-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 14. | Baudin G, Chassang M, Gelsi E, Novellas S, Bernardin G, Hébuterne X, Chevallier P. CT-guided percutaneous catheter drainage of acute infectious necrotizing pancreatitis: assessment of effectiveness and safety. AJR Am J Roentgenol. 2012;199:192-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 15. | Gliem N, Ammer-Herrmenau C, Ellenrieder V, Neesse A. Management of Severe Acute Pancreatitis: An Update. Digestion. 2021;102:503-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 68] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 16. | Petrov MS, Yadav D. Global epidemiology and holistic prevention of pancreatitis. Nat Rev Gastroenterol Hepatol. 2019;16:175-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 526] [Article Influence: 87.7] [Reference Citation Analysis (0)] |
| 17. | Ismail OZ, Bhayana V. Lipase or amylase for the diagnosis of acute pancreatitis? Clin Biochem. 2017;50:1275-1280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 122] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 18. | Li HC, Fan XJ, Chen YF, Tu JM, Pan LY, Chen T, Yin PH, Peng W, Feng DX. Early prediction of intestinal mucosal barrier function impairment by elevated serum procalcitonin in rats with severe acute pancreatitis. Pancreatology. 2016;16:211-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Schietroma M, Pessia B, Carlei F, Mariani P, Sista F, Amicucci G. Intestinal permeability and systemic endotoxemia in patients with acute pancreatitis. Ann Ital Chir. 2016;87:138-144. [PubMed] |
| 20. | Pittaluga A, Roggeri A, Vallarino G, Olivero G. Somatostatin, a Presynaptic Modulator of Glutamatergic Signal in the Central Nervous System. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 21. | Cantone MC, Dicitore A, Vitale G. Somatostatin-Dopamine Chimeric Molecules in Neuroendocrine Neoplasms. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Ai XB, Qian XP, Pan WS, Xu J, Wu LQ, Zhang WJ, Wang A. Ultrasound-guided percutaneous catheter drainage in early treatment of severe acute pancreatitis. World J Emerg Med. 2010;1:45-48. [PubMed] |
| 23. | Ganaie KH, Choh NA, Parry AH, Shaheen FA, Robbani I, Gojwari TA, Singh M, Shah OJ. The effectiveness of image-guided percutaneous catheter drainage in the management of acute pancreatitis-associated pancreatic collections. Pol J Radiol. 2021;86:e359-e365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Gelain ME, Bonsembiante F. Acute Phase Proteins in Marine Mammals: State of Art, Perspectives and Challenges. Front Immunol. 2019;10:1220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 25. | Rao SA, Kunte AR. Interleukin-6: An Early Predictive Marker for Severity of Acute Pancreatitis. Indian J Crit Care Med. 2017;21:424-428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 26. | Davies J. Procalcitonin. J Clin Pathol. 2015;68:675-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 90] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 27. | Gao N, Yan C, Zhang G. Changes of Serum Procalcitonin (PCT), C-Reactive Protein (CRP), Interleukin-17 (IL-17), Interleukin-6 (IL-6), High Mobility Group Protein-B1 (HMGB1) and D-Dimer in Patients with Severe Acute Pancreatitis Treated with Continuous Renal Replacement Therapy (CRRT) and Its Clinical Significance. Med Sci Monit. 2018;24:5881-5886. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 28. | Huang Z, Ma X, Jia X, Wang R, Liu L, Zhang M, Wan X, Tang C, Huang L. Prevention of Severe Acute Pancreatitis With Cyclooxygenase-2 Inhibitors: A Randomized Controlled Clinical Trial. Am J Gastroenterol. 2020;115:473-480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |