Published online Nov 27, 2023. doi: 10.4240/wjgs.v15.i11.2513
Peer-review started: September 19, 2023
First decision: October 8, 2023
Revised: October 26, 2023
Accepted: November 3, 2023
Article in press: November 3, 2023
Published online: November 27, 2023
Processing time: 69 Days and 1.9 Hours
Accurate preoperative staging of gastric cancer (GC), a common malignant tumor worldwide, is critical for appropriate treatment plans and prognosis. Dynamic three-phase enhanced computed tomography (CT) scanning for preoperative staging of GC has limitations in evaluating tumor angiogenesis. CD34, a marker on vascular endothelial cell surfaces, is promising in evaluating tumor angiogenesis. We explored the value of their combination for preoperative staging of GC to improve the efficacy and prognosis of patients with GC.
To explore the evaluation value of CD34 expression + dynamic three-phase enhanced CT scanning in preoperative staging of GC.
Medical records of 106 patients with GC treated at the First People's Hospital of Lianyungang between February 2021 and January 2023 were retrospectively studied. All patients underwent three-phase dynamic contrast-enhanced CT scanning before surgery, and CD34 was detected in gastroscopic biopsy specimens. Using surgical and pathological results as the gold standard, the diagnostic results of three-phase dynamic contrast-enhanced CT scanning at different T and N stages were analyzed, and the expression of CD34-marked microvessel density (MVD) at different T and N stages was determined. The specificity and sensitivity of three-phase dynamic contrast-enhanced CT and CD34 in T and N staging were calculated; those of the combined diagnosis of the two were evaluated in parallel. Independent factors affecting lymph node metastasis were analyzed using multiple logistic regression.
The accuracy of three-phase dynamic contrast-enhanced CT scanning in diagnosing stages T1, T2, T3 and T4 were 68.00%, 75.00%, 79.41%, and 73.68%, respectively, and for diagnosing stages N0, N1, N2, and N3 were 75.68%, 74.07%, 85.00%, and 77.27%, respectively. CD34-marked MVD expression increased with increasing T and N stages. Specificity and sensitivity of three-phase dynamic contrast-enhanced CT in T staging were 86.79% and 88.68%; for N staging, 89.06% and 92.86%; for CD34 in T staging, 64.15% and 88.68%; and for CD34 in N staging, 84.38% and 78.57%, respectively. Specificity and sensitivity of joint diagnosis in T staging were 55.68% and 98.72%, and N staging were 75.15% and 98.47%, respectively, with the area under the curve for diagnosis improving accordingly. According to multivariate analysis, a longer tumor diameter, higher pathological T stage, lower differentiation degree, and higher expression of CD34-marked MVD were independent risk factors for lymph node metastasis in patients with GC.
With high accuracy in preoperatively determining the invasion depth and lymph node metastasis of GC, CD34 expression and three-phase dynamic contrast-enhanced CT can provide a reliable basis for surgical resection.
Core Tip: We evaluated the value of CD34 expression combined with dynamic three-phase enhanced computed tomography (CT) scanning in the preoperative staging and invasion evaluation of gastric cancer (GC). This study demonstrated that the diagnostic accuracy of dynamic three-phase enhanced CT scanning for the T stage and the N stage was 68.00%-79.41% and 74.07%-85.00% respectively, and the addition of CD34-marked microvessel density improved the diagnostic efficiency. This combination can be used as a reliable basis to preoperatively assess the invasion depth and lymph node metastasis of GC and provide guidance for surgical treatment.
- Citation: Liu H, Zhao KY. Application of CD34 expression combined with three-phase dynamic contrast-enhanced computed tomography scanning in preoperative staging of gastric cancer. World J Gastrointest Surg 2023; 15(11): 2513-2524
- URL: https://www.wjgnet.com/1948-9366/full/v15/i11/2513.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i11.2513
Gastric cancer (GC) is a commonly observed malignancy and a global health concern. Despite its gradually decreasing morbidity and mortality, the annual worldwide incidence of GC is greater than 1 million cases; thus, it is the fourth most frequently observed malignant tumor and the third leading cause of cancer-associated deaths[1,2]. Surgical treatment is required to control or cure GC. Even in the late stages of GC, the prognosis and symptoms of patients can be improved by surgery combined with other treatment schemes such as radiotherapy and chemotherapy[3].
Early and accurate preoperative evaluation can enable an ideal prognosis, which is essential for planning optimal treatment options, such as endoscopic mucosal resection, endoscopic submucosal dissection, and laparoscopic surgery[4]. Early diagnosis and accurate staging before surgery are critical for formulating reasonable treatment plans, selecting the optimal surgical method, and determining the prognosis[5]. Ordinary computed tomography (CT) has been adopted for the preoperative staging of GC, but its accuracy is controversial[6]. Three-phase dynamic contrast-enhanced CT scanning has improved the clarity of vascular images and helped effectively observe the degree of tumor invasion of the gastric wall with the injection of contrast media into patients[7].
CD34 is a transmembrane glycoprotein often expressed on the surface of hematopoietic stem cells and endothelial cells and is a marker of these cell types; it has been extensively used to evaluate the vascular system in tumors, that is, microvessel density (MVD)[8,9]. Similarly, the formation of new blood vessels influences the growth and progression of GC. One study has revealed higher MVD in diffuse GC than in intestinal-type GC and significantly lower MVD in highly/moderately differentiated GC than in poorly differentiated GC[10]. Compared to other microvascular markers, CD34 exhibits high specificity for endothelial cells, which indicates it is less likely to stain non-vascular cells, thus providing a more accurate MVD image[11]. Currently, CD34 has been adopted in the preoperative evaluation of colorectal cancer and the efficacy prediction of preoperative radiotherapy and chemotherapy[12,13]. In addition, several studies have indicated that a high MVD determined using CD34 staining is associated with poorer prognosis in patients with GC[14]. However, the application of CD34 expression combined with three-phase dynamic contrast-enhanced CT scanning in the preoperative staging and evaluation of GC invasion has rarely been studied.
Thus, in this study, preoperative CD34 detection and three-phase dynamic contrast-enhanced CT scans were performed in 106 patients with GC and compared with postoperative histopathology to determine the applicability of this scheme.
Medical records of 106 patients with GC treated at The First People's Hospital of Lianyungang between February 2021 and January 2023 were retrospectively studied. All patients underwent three-phase dynamic contrast-enhanced CT scanning before surgery, and CD34 was detected in gastroscopic biopsy specimens. Surgical and pathological results were used as gold standards. This study was conducted with permission from the Medical Ethics Committee of The First People's Hospital of Lianyungang.
The inclusion criteria were as follows: Patients who had received both CD34 detection and three-phase dynamic contrast-enhanced CT scanning, with a time difference between the two tests of less than 1 wk; patients whose lesions were obtained using surgical resection and sent for pathological diagnosis (the results were considered the gold standard); patients diagnosed with malignant GC; and patients with detailed medical data, namely medical records, past medical history, and laboratory and imaging examination results.
The exclusion criteria were as follows: Comorbidities with other malignant tumors, congenital malformations in the chest that would disrupt the imaging diagnosis, coagulation dysfunction, allergy to contrast media, pregnancy or lactation, and neoadjuvant therapy before testing.
CD34 is specifically expressed in microvascular endothelial cells, and its expression intensity is bound to the MVD; therefore, the MVD value can be adopted to represent CD34. The inclusion criterion was brownish-yellow microvascular endothelial cells. The procedure was as follows: A whole slice was browsed in a low-power field (× 100), three different fields of view in each slice were randomly selected and counted using a high-power lens (× 200), and the average value was obtained. The average value was the MVD in this case.
A 128-row, 256-slice Philips spiral CT scanner was used, and the parameters were set as follows: Detector, 0.625 × 128 rows; pitch, 0.993; tube voltage, 120 KV; tube current, 250 mA; spiral scanning, 3.367 s; and acquisition matrix, 512 × 512. Nonionic contrast medium was injected through an intravenous bolus injection using a high-pressure syringe (concentration: 350 mg/mL) at a dose of 80-100 mL and an injection speed of 3 mL/s. Scanning was performed from the liver to the kidney at 25, 55, and 180 s after contrast injection to acquire the arterial, portal, and delayed phases. Part of the data was transmitted to the post-processing workstation for processing and reconstruction using multiplanar reconstruction and other techniques to show the relationship between the lesions and adjacent blood vessels. The corresponding parameters were recorded.
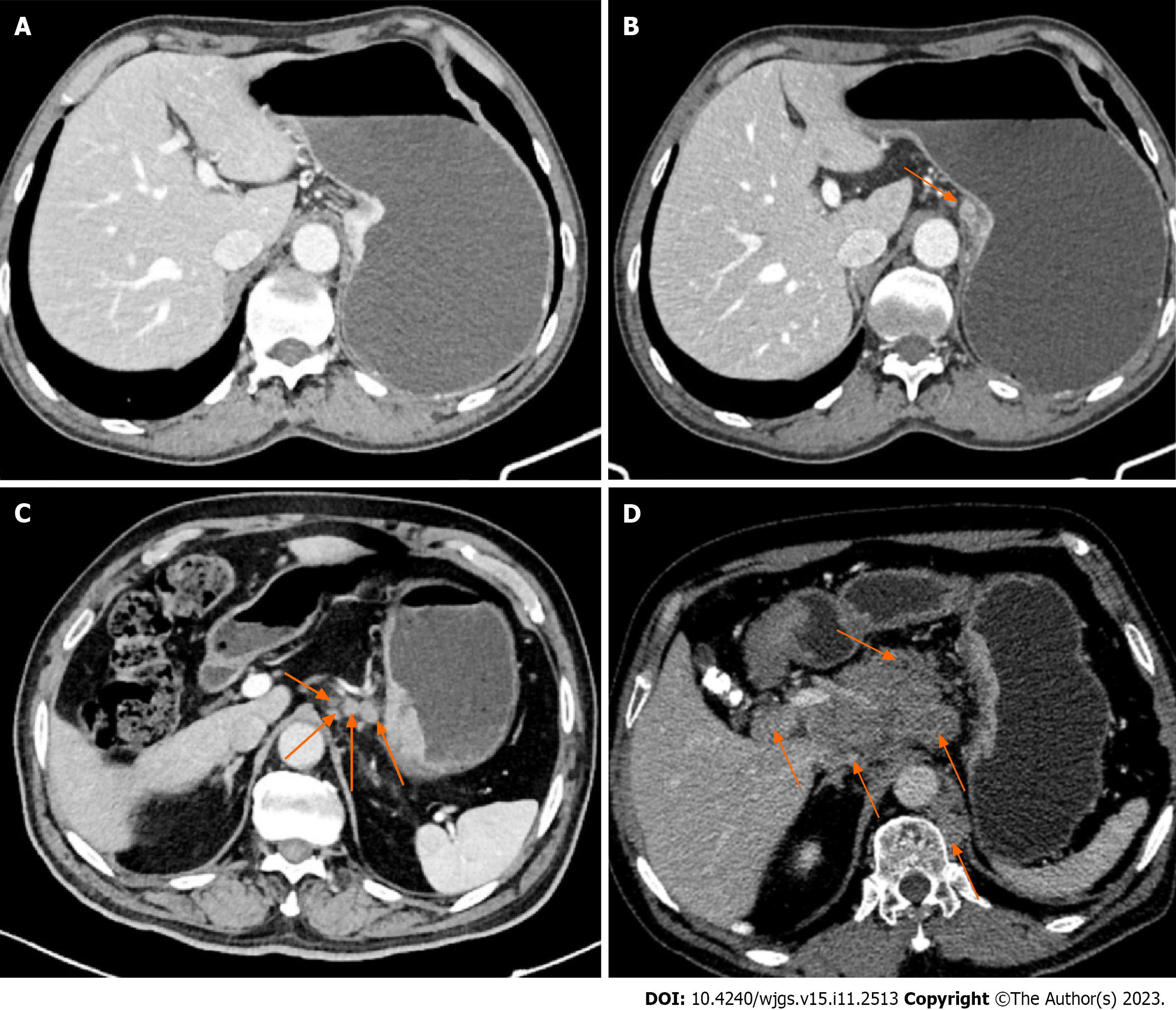
The diagnostic criteria of three-phase dynamic contrast-enhanced CT scanning for the T staging of patients with GC are as follows (Figure 1): T1, the tumor invades the lamina propria, muscularis mucosa, or submucosa; T2, the tumor invades the muscularis propria; T3, the tumor penetrates the subserous connective tissue but does not invade the visceral peritoneum or adjacent structures; and T4, the tumor invades the serosa (visceral peritoneum) or adjacent structures.
The following are the diagnostic criteria for three-phase dynamic contrast-enhanced CT scanning for the N staging of patients with GC (Figure 2). Positive criteria for lymph node metastasis: When the short axis diameter of abdominal lymph nodes was larger than 6 mm, and the size of gastric lymph nodes was larger than 8 mm, especially the nodes with a round shape and local necrosis in enhanced CT examination, the conclusion was a positive metastasis of lymph nodes. The N stage refers to the number of lymph nodes with metastases around the stomach. N0: No local lymph node metastasis; N1: 1-2 local lymph node metastases; N2: 3-6 local lymph node metastases; N3: 7 lymph node metastases or more.
With the final pathological diagnosis results of patients as the gold standard, the diagnostic results of the two separate diagnostic methods and the joint diagnosis for the pathological staging of patients with GC were evaluated. Joint diagnosis was conducted in parallel, and the specificity and sensitivity of each diagnostic method were calculated and compared to evaluate diagnostic efficacy.
All data were processed using SPSS software (version 20.0; SPSS Inc., Chicago, IL, United States). The measurement data are presented as the mean ± SD. Normally distributed data were compared between groups using the independent-samples T test and presented as t. Counting data are expressed as percentages (%), analyzed using the chi-square test, and expressed as χ2. Receiver operating characteristic curves were constructed to evaluate the diagnostic value of CD34 for T and N staging. Multivariate logistic regression analysis was conducted to analyze the independent factors for preoperative lymph node metastasis. Statistical significance was set at P < 0.05.
The patients’ general data are summarized in Table 1.
| n = 106 | ||
| Age (yr) | 57.5 ± 7.8 | |
| Gender | ||
| Male | 81 (76.42) | |
| Female | 25 (23.58) | |
| Tumor length (cm) | 5.02 ± 1.85 | |
| Pathological T staging | ||
| T1 | 25 (23.58) | |
| T2 | 28 (26.42) | |
| T3 | 34 (32.08) | |
| T4 | 19 (17.92) | |
| Pathological N staging | ||
| N0 | 37 (34.91) | |
| N1 | 27 (25.47) | |
| N2 | 20 (18.87) | |
| N3 | 22 (20.75) | |
| Tissue typing | ||
| Adenocarcinoma | 83 (78.30) | |
| Signet-ring cell carcinoma | 17 (16.04) | |
| Neuroendocrine carcinoma | 6 (5.66) | |
| Degree of differentiation | ||
| High differentiation | 32 (30.19) | |
| Moderate differentiation | 55 (51.89) | |
| Low differentiation | 19 (17.92) |
With pathological results as the gold standard, the diagnostic results of three-phase dynamic contrast-enhanced CT for stages T1-T4 are summarized in Table 2, and the expression of CD34-marked MVD in different T stages is summarized in Table 2.
| Pathological T staging | Three-phase dynamic contrast-enhanced CT in T staging | Expression of CD34-marked MVD | ||||
| T1 | T2 | T3 | T4 | Accuracy | ||
| T1 (n = 25) | 17 | 4 | 4 | 0 | 68.00 | 47.44 ± 10.22 |
| T2 (n = 28) | 4 | 21 | 3 | 0 | 75.00 | 63.41 ± 7.16 |
| T3 (n = 34) | 0 | 4 | 27 | 3 | 79.41 | 86.21 ± 8.36 |
| T4 (n = 19) | 0 | 2 | 3 | 14 | 73.68 | 103.71 ± 10.92 |
With pathological results as the gold standard, the diagnostic results of three-phase dynamic contrast-enhanced CT for N0-N3 are summarized in Table 3, and the expression of CD34-marked MVD in different N stages is summarized in Table 3.
| Pathological N staging | N staging of three-phase dynamic contrast-enhanced CT | Expression of CD34-marked MVD | ||||
| N0 | N1 | N2 | N3 | Accuracy | ||
| N0 (n = 37) | 28 | 6 | 3 | 0 | 75.68 | 52.43 ± 12.77 |
| N1 (n = 27) | 3 | 20 | 4 | 0 | 74.07 | 71.89 ± 10.13 |
| N2 (n = 20) | 0 | 1 | 17 | 2 | 85.00 | 86.83 ± 9.74 |
| N3 (n = 22) | 0 | 2 | 3 | 17 | 77.27 | 102.07 ± 11.27 |
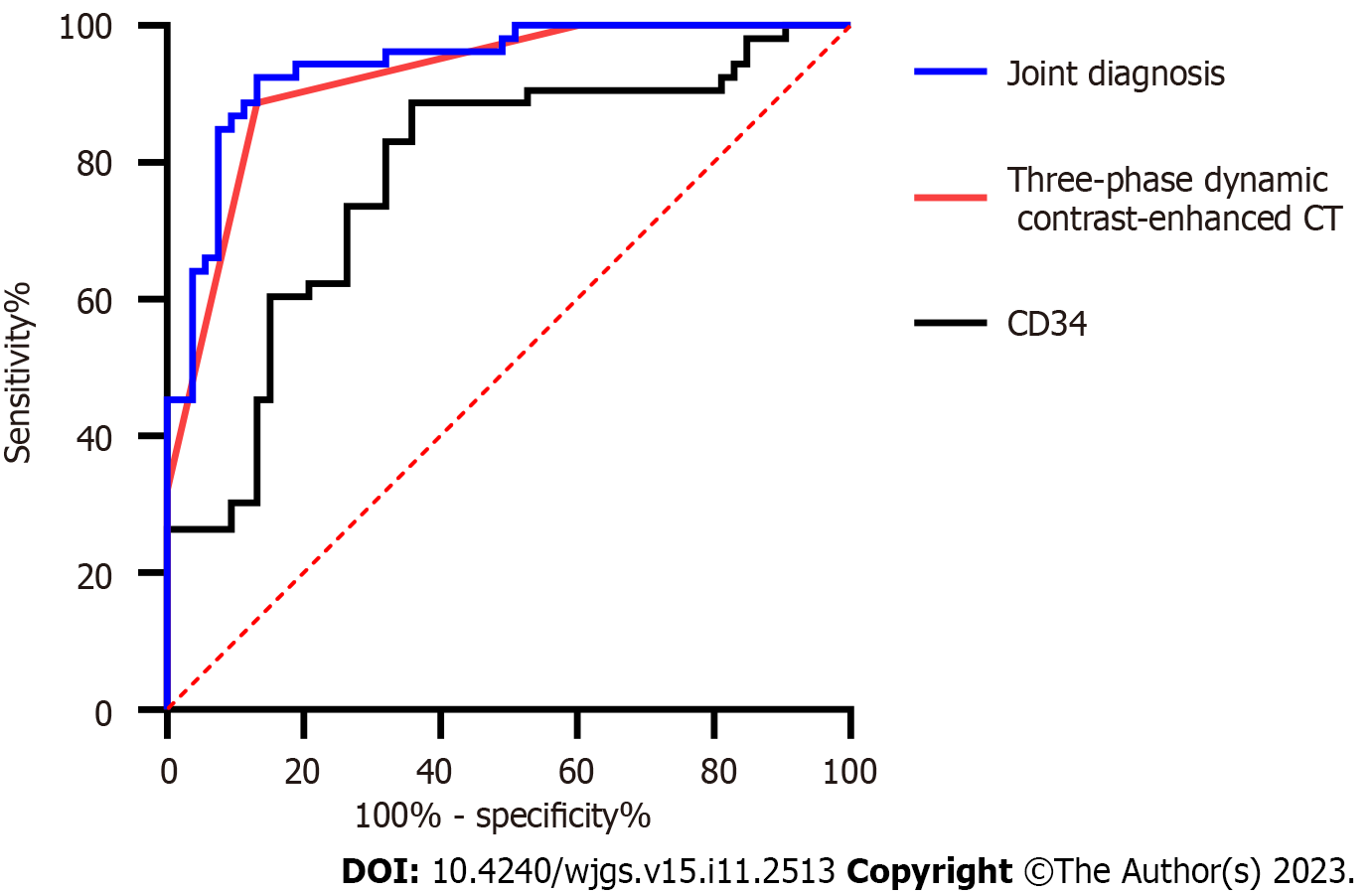
The sensitivity and specificity of three-phase dynamic contrast-enhanced CT in diagnosing stages T1-2 and T3-4 were calculated according to the results, and the sensitivity and specificity of CD34-marked MVD in diagnosing stages T1-2 and T3-4 were calculated according to the expression of CD34-marked MVD in T stages. The sensitivity and area under the curve (AUC) of the diagnosis improved through joint diagnosis (Figure 3, Table 4).
| Pathological T staging | Three-phase dynamic contrast-enhanced CT | CD34 | Joint diagnosis |
| AUC | 0.921 | 0.779 | 0.940 |
| 95%CI | 0.870-0.972 | 0.690-0.869 | 0.897-0.984 |
| Specificity (%) | 86.79 | 64.15 | 55.6 |
| Sensitivity (%) | 88.68 | 88.68 | 98.72 |
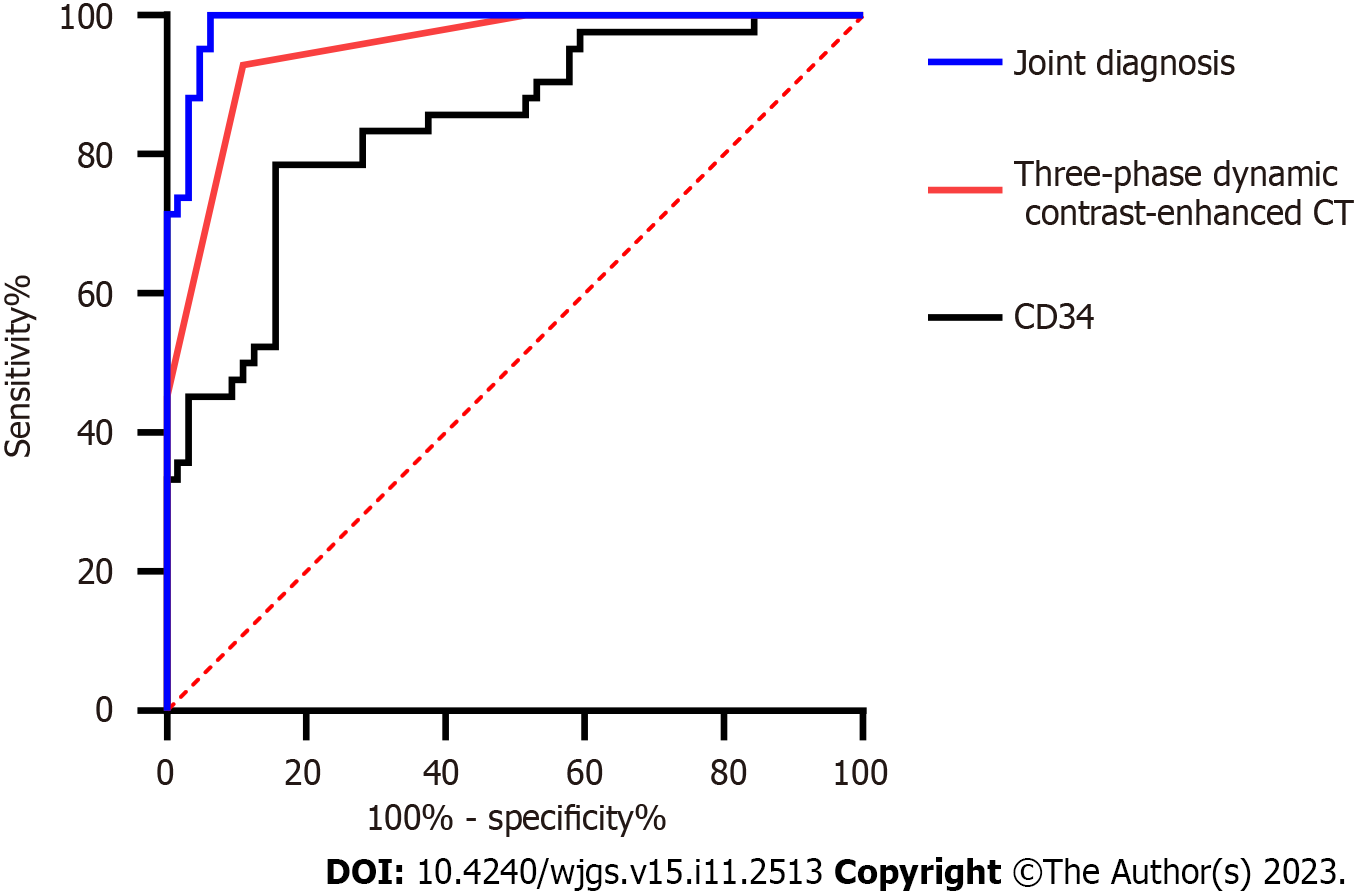
The sensitivity and specificity of three-phase dynamic contrast-enhanced CT in N staging were calculated according to these results. The sensitivity and specificity of CD34-marked MVD in diagnosing stages N0-1 and stages N1-2 were also calculated according to the expression of CD34-marked MVD in N stages. The sensitivity and AUC of the diagnosis improved through joint diagnosis (Figure 4, Table 5).
| Pathological N staging | Three-phase dynamic contrast-enhanced CT | CD34 | Joint diagnosis |
| AUC | 0.952 | 0.839 | 0.989 |
| 95%CI | 0.915-0.989 | 0.761-0.917 | 0.975-0.999 |
| Specificity (%) | 89.06 | 84.38 | 75.15 |
| Sensitivity (%) | 92.86 | 78.57 | 98.47 |
Patients were divided into metastatic (n = 69) and non-metastatic (n = 37) groups based on the occurrence of lymph node metastasis. According to the univariate analysis, the two groups were not significantly different in age, tumor length, pathological T staging, histological classification, degree of differentiation, CD34, or expression of CD34-marked MVD (P < 0.05; Table 6).
| Metastatic group (n = 69) | Non-metastatic group (n = 37) | χ2/t | P value | ||
| Age (yr) | 59.3 ± 7.6 | 54.2 ± 7.2 | 3.353 | 0.001 | |
| Gender | 0.687 | 0.407 | |||
| Male | 51 (73.91) | 30 (81.08) | |||
| Female | 18 (26.09) | 7 (18.92) | |||
| Tumor length (cm) | 3.73 ± 1.25 | 5.72 ± 1.75 | 6.124 | < 0.001 | |
| Pathological T staging | 21.296 | < 0.001 | |||
| T1 | 7 (10.14) | 18 (48.65) | |||
| T2 | 19 (27.54) | 9 (24.32) | |||
| T3 | 28 (40.58) | 6 (16.22) | |||
| T4 | 15 (21.74) | 4 (10.81) | |||
| Tissue typing | 6.226 | 0.046 | |||
| Adenocarcinoma | 49 (71.01) | 34 (91.89) | |||
| Signet-ring cell carcinoma | 15 (21.74) | 2 (5.41) | |||
| Neuroendocrine carcinoma | 5 (7.25) | 1 (2.70) | |||
| Degree of differentiation | 8.117 | 0.017 | |||
| High differentiation (n = 32) | 15 (21.74) | 17 (45.95) | |||
| Moderate differentiation (n = 55) | 38 (55.07) | 17 (45.95) | |||
| Low differentiation (n = 19) | 16 (23.19) | 3 (8.11) | |||
| Expression of CD34-labelled MVD | 84.57 ± 17.83 | 47.81 ± 14.93 | 10.686 | < 0.001 |
Indexes with notable differences in the univariate analysis were subjected to multivariate logistic regression analysis. According to multivariate logistic regression analysis, a longer tumor diameter, higher pathological T staging, lower differentiation degree, and higher expression of CD34-marked MVD were independent risk factors for lymph node metastasis in patients with GC (Table 7).
| B | S.E. | Wals | Sig. | Exp(B) | 95%CI for EXP (B) | ||
| Lower limit | Upper limit | ||||||
| Age (yr) | -0.071 | 0.097 | 0.526 | 0.468 | 0.932 | 0.770 | 1.128 |
| Long diameter of tumor | 1.367 | 0.527 | 6.728 | 0.009 | 3.923 | 1.397 | 11.019 |
| T staging | 2.544 | 0.714 | 12.697 | 0.001 | 12.73 | 3.141 | 51.586 |
| Tissue typing | 1.198 | 1.119 | 1.146 | 0.284 | 3.314 | 0.369 | 29.724 |
| Degree of differentiation | 2.417 | 0.921 | 6.882 | 0.009 | 11.209 | 1.842 | 68.194 |
| Expression of CD34-labelled MVD | 0.160 | 0.051 | 9.658 | 0.002 | 1.174 | 1.061 | 1.298 |
The pathogenesis of GC remains under investigation. The causes of malignant gastric tumors are complicated, among which Helicobacter pylori infection is the most likely. However, personal living habits and family genetic factors are also causes and mechanisms that should be considered[15,16]. Early diagnosis and treatment are the most important aspects of early stage GC, and preoperative staging is necessary. Improving the accuracy of preoperative clinical staging can help increase the accuracy treatment plan for patients with GC, including the timing of chemotherapy drugs. Postoperative pathological classification and the number of lymph node metastases are crucial for the prognosis of patients with malignant tumors, and the depth of tumor invasion is a key factor affecting the prognosis of patients with GC[17,18]. In the literature, according to Cox regression analysis[19], the main factors affecting the prognosis of patients were tumor stage, invasion depth, lymph node metastasis, distant metastasis, and tumor size. Therefore, infiltration and metastasis of GC tumors are the main causes of clinical treatment failure and death. Accordingly, it is crucial to identify the tumor as early as possible and to correctly understand the tumor stage, infiltration depth, and existence of lymph node metastasis in the clinical treatment of patients with tumors.
T staging represents the depth of infiltration, a crucial reference for the selection of surgery for patients[20]. In this study, the accuracy of three-phase dynamic contrast-enhanced CT in diagnosing stage T1 was 68.00%, which was lower than that in diagnosing stages T2-T4 (75.00%, 79.41%, and 73.68%, respectively). A likely reason for this result is that three-phase dynamic contrast-enhanced CT cannot clearly display the low-density zone of the submucosa, resulting in a conclusion that the tumor has invaded it, resulting in excessive T staging. Wang et al[21] also explored the diagnostic results of dynamic contrast-enhanced CT for the T staging of GC and found that its accuracy in diagnosing stages T2-T4 was higher than that in diagnosing stage T1, which is similar to the results of this study. These results suggest that three-phase dynamic contrast-enhanced CT is suitable for determining the depth of invasion in advanced GC. GC mainly metastasizes via the lymphatic pathway. Three-phase enhanced scanning of the stomach before surgery and multi-CT angiography can help determine the variation in the perigastric blood vessels and the swollen lymph nodes along the blood vessels, which is beneficial for the clearance of lymph nodes during surgery[22,23]. In this study, the accuracies of three-phase dynamic contrast-enhanced CT for N staging were 75.68%, 74.07%, 85.00%, and 77.27%. Misdiagnosis may occur because the aggregation and fusion of lymph nodes reduce the number of lymph nodes, and when perigastric adipose tissue decreases, lymph nodes cannot be displayed because the contrast is insufficient, which reduces the number of lymph nodes. Chen et al[24] revealed that the diagnostic accuracy of dynamic contrast-enhanced CT in the N staging of GC is not high (the total accuracy is 78%), similar to the results of this study.
The growth of any solid tumor depends on the process of angiogenesis; that is, the endothelial cells of the host proliferate, sprout to form new blood vessels, grow toward the tumor, and construct tumor blood supply channels to provide nutrition and transport metabolites[25]. The greater the number of microvessels in a tumor, the greater the possibility of tumor cells entering the blood circulation. As the most specific marker of endothelial cells, CD34 is strongly associated with angiogenesis. An increasing number of studies have found a close relationship among an increase in MVD, the risk of tumor metastasis, and a decrease in survival rate[26]. In this study, CD34-marked MVD increased with an increase in T and N stages, indicating that CD34-marked MVD also increased with an increase in infiltration depth and lymph node metastasis. This result may be because with the development of GC, the primary tumor penetrates into the gastric parietal layer (corresponding to the T stage), or the invasion of local lymph nodes (corresponding to the N stage) requires increased angiogenesis to support its growth, increasing CD34 expression. Furthermore, advanced T stage, indicating larger tumor size or extensive local spread, is often associated with increased vascularization. Larger tumors require more blood supply, which can lead to higher MVD. Shi et al[27] explored the relationship among CD34 and clinicopathological features of gastric adenocarcinoma and found a positive correlation among high MVD values and lymph node metastasis and TNM staging, a negative correlation with high MVD values with pathological grade, and no correlation with tumor size or a patient’s age or sex.
Finally, this study compared three-phase dynamic contrast-enhanced CT and CD34 in diagnosing stages T1-2 and T3-4 and stages N0-1 and N1-2. The specificity and sensitivity of the three-phase dynamic contrast-enhanced CT in T staging were 86.79% and 88.68%, respectively, and those for N staging were 89.06% and 92.86%, respectively. The specificity and sensitivity of CD34 in T staging were 64.15% and 88.68%, respectively, and those for N staging were 84.38% and 78.57%, respectively. Through parallel joint diagnosis, the sensitivity of T staging improved to 98.72%, and the sensitivity of N staging improved to 98.47%, with an improved AUC corresponding to the higher diagnostic value of joint diagnosis. According to multivariate analysis, a longer tumor diameter, higher pathological T stage, lower differentiation degree, and higher expression of CD34-marked MVD were independent risk factors for lymph node metastasis in patients with GC, suggesting an increase in attention to these risk factors before surgery.
Despite achieving our aim, this study still has limitations. First, there was an inevitable bias in this retrospective study. Second, the number of cases in this study was limited; therefore, further research should expand the sample size to support our results. Finally, three-phase dynamic contrast-enhanced CT is also affected by the experience of the doctor in analyzing the preoperative examination results, which may affect the accuracy of diagnosis.
In summary, CD34 expression combined with three-phase dynamic contrast-enhanced CT scanning has high accuracy in determining the invasion depth and lymph node metastasis of GC before surgery and can provide a reliable basis for surgical resection.
Gastric cancer (GC) is a commonly observed malignancy and the main cause of cancer-related deaths worldwide. However, the accuracy of traditional computed tomography (CT) in preoperative staging remains controversial. CD34 is an endothelial cell marker that can help evaluate microvessel density (MVD) and angiogenesis in tumors. The combination of CD34 expression and dynamic three-phase enhanced CT can improve the accuracy of preoperative staging.
Early diagnosis, accurate staging, and detection of lymph node metastasis are crucial for the prognosis and development of treatment plans for patients with GC. This study aimed to evaluate the value of CD34 expression combined with dynamic three-phase enhanced CT in the preoperative staging and invasion evaluation of GC to explore new methods to improve the accuracy of preoperative evaluation and postoperative treatment.
The main goal of this study was to evaluate the value of CD34 expression combined with dynamic three-phase enhanced CT in the preoperative staging and invasion evaluation of GC. According to a study of 106 patients with GC, CD34-marked MVD positively correlated with the T and N stages, and CD34 expression combined with CT showed high sensitivity in GC staging. Tumor diameter, T stage, degree of differentiation, and CD34-marked MVD were independent risk factors for lymph node metastasis. The results of this study provide a new method for the preoperative staging and treatment planning of GC. The combination of CD34 expression and CT can improve staging accuracy and contribute to the evaluation of infiltration.
In this study, the results of CD34 detection and dynamic three-phase enhanced CT scanning in 106 patients with GC were compared and analyzed with postoperative pathological results. The independent factors of preoperative lymph node metastasis were analyzed using multivariate logistic regression. The novelty of this study is that CD34 expression was combined with dynamic three-phase enhanced CT for diagnosis, and the accuracy of preoperative staging and invasion assessment of GC was improved through a comprehensive analysis of the two types of results. In addition, independent risk factors were determined using multivariate logistic regression analysis, which provided a scientific basis for clinical decision-making.
According to the results of this study, the combination of CD34 expression and dynamic three-phase enhanced CT showed a high sensitivity for preoperative staging of GC. Additionally, tumor diameter, T stage, degree of differentiation, and CD34-marked MVD were identified as independent risk factors for lymph node metastasis. Therefore, the combination of CD34 expression and dynamic three-phase-enhanced CT scanning can improve the accuracy of GC staging and help evaluate invasion. Although CD34 expression and dynamic three-phase enhanced CT scanning showed high sensitivity in this study, their specificity and accuracy require further evaluation.
This study highlights the critical role of angiogenesis in the development of GC and provides new theories and methods to improve preoperative staging and invasion evaluation of GC by combining CD34 expression with dynamic three-phase enhanced CT scanning. These new theories and methods will help deepen the understanding of the developmental mechanism of GC, guide clinical decision-making, and improve prognosis.
Further research should verify and expand our research results by exploring new biomarkers and imaging technologies, studying the mechanisms of GC development, and developing individualized treatment strategies. These efforts will further improve the diagnosis and treatment of GC, improving the prognosis and quality of life of patients.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Anaissi AKM, Brazil; Wirsik NM, Germany S-Editor: Qu XL L-Editor: A P-Editor:Zhao S
| 1. | Wei L, Sun J, Zhang N, Zheng Y, Wang X, Lv L, Liu J, Xu Y, Shen Y, Yang M. Noncoding RNAs in gastric cancer: implications for drug resistance. Mol Cancer. 2020;19:62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 279] [Cited by in RCA: 337] [Article Influence: 67.4] [Reference Citation Analysis (0)] |
| 2. | Thrift AP, El-Serag HB. Burden of Gastric Cancer. Clin Gastroenterol Hepatol. 2020;18:534-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 983] [Article Influence: 196.6] [Reference Citation Analysis (1)] |
| 3. | Chen J, Bu Z, Ji J. Surgical treatment of gastric cancer: Current status and future directions. Chin J Cancer Res. 2021;33:159-167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 4. | Gao YL, Zhang YH, Cao M. Preoperative evaluation of endoscopic submucosal dissection for early gastric cancer. Medicine (Baltimore). 2022;101:e30582. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 5. | Kim DJ, Hyung WJ, Park YK, Lee HJ, An JY, Kim HI, Kim HH, Ryu SW, Hur H, Kim MC, Kong SH, Kim JJ, Park DJ, Ryu KW, Kim YW, Kim JW, Lee JH, Yang HK, Han SU, Kim W; Korean Laparoendoscopic Gastrointestinal Surgery Study (KLASS) Group. Accuracy of preoperative clinical staging for locally advanced gastric cancer in KLASS-02 randomized clinical trial. Front Surg. 2022;9:1001245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Reference Citation Analysis (0)] |
| 6. | Wu CX, Zhu ZH. Diagnosis and evaluation of gastric cancer by positron emission tomography. World J Gastroenterol. 2014;20:4574-4585. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 62] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 7. | Tsurumaru D, Miyasaka M, Muraki T, Nishie A, Asayama Y, Oki E, Oda Y, Honda H. Histopathologic diversity of gastric cancers: Relationship between enhancement pattern on dynamic contrast-enhanced CT and histological type. Eur J Radiol. 2017;97:90-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 8. | Bhatia JK, Chaudhary T, Boruah D, Bharadwaj R. Study of angiogenesis in invasive breast carcinoma by morphometry and immunohistochemistry. Med J Armed Forces India. 2022;78:345-354. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Chabowski M, Nowak A, Grzegrzolka J, Piotrowska A, Janczak D, Dziegiel P. Comparison of Microvessel Density Using Nestin and CD34 in Colorectal Cancer. Anticancer Res. 2018;38:3889-3895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Tenderenda M, Rutkowski P, Jesionek-Kupnicka D, Kubiak R. Expression of CD34 in gastric cancer and its correlation with histology, stage, proliferation activity, p53 expression and apoptotic index. Pathol Oncol Res. 2001;7:129-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Paschoal JP, Bernardo V, Canedo NH, Ribeiro OD, Caroli-Bottino A, Pannain VL. Microvascular density of regenerative nodule to small hepatocellular carcinoma by automated analysis using CD105 and CD34 immunoexpression. BMC Cancer. 2014;14:72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Ruffolo C, Ferrara F, Trevellin E, Cataldo I, Fornasier C, Pozza A, Campo Dell'Orto M, Angriman I, Dei Tos AP, Bardini R, Massani M, Kotsafti A, Scarpa M. Can Vascular Endothelial Growth Factors and CD34 Expression Implement NICE (Narrow-Band Imaging International Colorectal Endoscopic) Classification in Colorectal Polypoid Lesion Diagnosis? Eur Surg Res. 2020;61:72-82. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 13. | Min BS, Choi YJ, Pyo HR, Kim H, Seong J, Chung HC, Rha SY, Kim NK. Cyclooxygenase-2 expression in pretreatment biopsy as a predictor of tumor responses after preoperative chemoradiation in rectal cancer. Arch Surg. 2008;143:1091-7; discussion 1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Tao X, Cheng L, Li Y, Ci H, Xu J, Wu S, Tao Y. Expression of CRYAB with the angiogenesis and poor prognosis for human gastric cancer. Medicine (Baltimore). 2019;98:e17799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Alipour M. Molecular Mechanism of Helicobacter pylori-Induced Gastric Cancer. J Gastrointest Cancer. 2021;52:23-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 153] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 16. | Ito M, Tanaka S, Chayama K. Characteristics and Early Diagnosis of Gastric Cancer Discovered after Helicobacter pylori Eradication. Gut Liver. 2021;15:338-345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 17. | Sorrentino L, De Ruvo N, Serra F, Salati M, Ricciardolo AA, Bonetti LR, Gelmini R. Role of poorly differentiated cluster in gastric cancer: is it a new prognosis factor? Scand J Gastroenterol. 2022;57:44-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Yi-Wen W, Long-Long L, Ming L, Hao L, Kong-Wang H. Stem cell-like circulating tumor cells indicate poor prognosis in gastric cancer. Arch Med Sci. 2022;18:1297-1307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 19. | Rugge M, Sonego F, Panozzo M, Baffa R, Rubio J Jr, Farinati F, Nitti D, Ninfo V, Ming SC. Pathology and ploidy in the prognosis of gastric cancer with no extranodal metastasis. Cancer. 1994;73:1127-1133. [PubMed] [DOI] [Full Text] |
| 20. | Zhu Z, Gong Y, Xu H. Clinical and pathological staging of gastric cancer: Current perspectives and implications. Eur J Surg Oncol. 2020;46:e14-e19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 21. | Wang J, Li X, Zhang Z, Jing C, Li J. Clinical Research of Combined Application of DCEUS and Dynamic Contrast-Enhanced MSCT in Preoperative cT Staging of Gastric Cancer. J Oncol. 2021;2021:9868585. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Ma D, Zhang Y, Shao X, Wu C, Wu J. PET/CT for Predicting Occult Lymph Node Metastasis in Gastric Cancer. Curr Oncol. 2022;29:6523-6539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 23. | Jin C, Jiang Y, Yu H, Wang W, Li B, Chen C, Yuan Q, Hu Y, Xu Y, Zhou Z, Li G, Li R. Deep learning analysis of the primary tumour and the prediction of lymph node metastases in gastric cancer. Br J Surg. 2021;108:542-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 24. | Chen CY, Hsu JS, Wu DC, Kang WY, Hsieh JS, Jaw TS, Wu MT, Liu GC. Gastric cancer: preoperative local staging with 3D multi-detector row CT--correlation with surgical and histopathologic results. Radiology. 2007;242:472-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 204] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 25. | Oya Y, Hayakawa Y, Koike K. Tumor microenvironment in gastric cancers. Cancer Sci. 2020;111:2696-2707. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 206] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 26. | Yang P, Yuan W, He J, Wang J, Yu L, Jin X, Hu Y, Liao M, Chen Z, Zhang Y. Overexpression of EphA2, MMP-9, and MVD-CD34 in hepatocellular carcinoma: Implications for tumor progression and prognosis. Hepatol Res. 2009;39:1169-1177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 27. | Shi JF, Xu SX, He P, Xi ZH. Expression of carcinoembryonic antigen-related cell adhesion molecule 1(CEACAM1) and its correlation with angiogenesis in gastric cancer. Pathol Res Pract. 2014;210:473-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |