Published online Oct 27, 2023. doi: 10.4240/wjgs.v15.i10.2247
Peer-review started: July 3, 2023
First decision: August 17, 2023
Revised: August 22, 2023
Accepted: September 4, 2023
Article in press: September 4, 2023
Published online: October 27, 2023
Processing time: 115 Days and 19 Hours
The preoperative total bilirubin-albumin ratio (TBAR) and fibrinogen-albumin ratio (FAR) have been proven to be valuable prognostic factors in various cancers.
To detect the prognostic value of TBAR and FAR in ampullary adenocarcinoma (AC) patients who underwent curative pancreaticoduodenectomy.
AC patients who underwent curative pancreaticoduodenectomy in the National Cancer Center of China between 1998 and 2020 were retrospectively reviewed. The prognostic cutoff values of TBAR and FAR were determined through the best survival separation model. Then, a novel prognostic score combining TBAR and FAR was calculated and validated through the logistic regression analysis and Cox regression analysis.
A total of 188 AC patients were enrolled in the current study. The best cutoff values of TBAR and FAR for predicting overall survival were 1.7943 and 0.1329, respectively. AC patients were divided into a TBAR-low group (score = 0) vs a TBAR-high group (score = 1) and a FAR-low group (score = 0) vs a FAR-high group (score = 1). The total score was calculated as a novel prognostic factor. Multivariable logistic regression analysis revealed that a high score was an independent protective factor for recurrence [score = 1 vs score = 0: Odds ratio (OR) = 0.517, P = 0.046; score = 2 vs score = 0 OR = 0.236, P = 0.038]. In addition, multivariable survival analysis also demonstrated that a high score was an independent protective factor in AC patients (score = 2 vs score = 0: Hazard ratio = 0.230, P = 0.046).
A novel prognostic score based on preoperative TBAR and FAR has been demonstrated to have good predictive power in AC patients who underwent curative pancreaticoduodenectomy. However, more studies with larger samples are needed to validate this conclusion.
Core Tip: Considering that effective prognostic predictors are still lacking for ampullary carcinoma, we conducted a retrospective study to elucidate the prognostic value of total bilirubin-albumin ratio (TBAR) and fibrinogen-albumin ratio (FAR) in ampullary adenocarcinoma (AC) patients who underwent curative pancreaticoduodenectomy. We found that the novel prognostic score based on the preoperative TBAR and FAR was an independent predictor for tumor recurrence and an independent protective factor for overall survival in AC patients who underwent curative pancreaticoduodenectomy.
- Citation: Zhang XJ, Fei H, Sun CY, Li ZF, Li Z, Guo CG, Zhao DB. Novel prognostic score based on the preoperative total bilirubin-albumin ratio and fibrinogen-albumin ratio in ampullary adenocarcinoma. World J Gastrointest Surg 2023; 15(10): 2247-2258
- URL: https://www.wjgnet.com/1948-9366/full/v15/i10/2247.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i10.2247
Ampullary adenocarcinoma (AC) is an uncommon malignant digestive adenocarcinoma and accounts for approximately 0.2% of all digestive malignancies[1]. Radical surgery is the only potential curative treatment for AC patients[2]. It has been reported that the 5-year survival rate of AC patients after surgery is 45%, and postoperative recurrence and meta
Preoperative total bilirubin (TB) is a critical biomarker for the diagnosis of biliary obstruction and is present at the first diagnosis for the majority of AC patients. In addition, preoperative albumin has been widely used for defining immune and nutritional statuses[4]. Recently, several studies have proven that the preoperative fibrinogen-albumin ratio (FAR) is a significant prognostic biomarker in multiple tumor types, including lung cancer[5], hepatocellular carcinoma[6], colorectal cancer[7], gastric cancer[8], bladder cancer[9], and rectal cancer[10]. However, whether the relationship of preoperative TB, fibrinogen, and albumin could be used as prognostic factors is still debated. Therefore, in the current study, we aimed to explore the relationship among the preoperative TB-albumin ratio (TBAR), FAR, and long-term prognosis in AC patients based on a high-volume retrospective cohort in the National Cancer Center of China.
AC patients who received curative surgery at the National Cancer Center of China between 1998 and 2020 were retro
The TBAR was calculated as preoperative TB (μmol/L) divided by preoperative albumin (g/L). The FAR was calculated as preoperative fibrinogen (g/L) divided by preoperative albumin (g/L).
The postoperative follow-up was performed through telephone review, clinic visits, and the death registration system. The last follow-up time was December 2021. None of the patients was lost to follow-up. The median follow-up time was 36 mo.
All categorical variables are expressed as frequencies (percentages), and continuous variables are expressed as medians (interquartile ranges). The χ2 test or Fisher’s exact test was used for comparison of categorical variable groups, and the Mann-Whitney U test was used for comparison of continuous variable groups. The cutoff values of TBAR and FAR were calculated by the best survival separation model through the “Survival” and “Survminer” packages in R software (version 4.0.3). The corresponding P value and hazard ratio (HR) were calculated one by one with each TBAR and FAR value as the cutoff value by setting a cycle. When the P value was the smallest, the cutoff TBAR and FAR values were the best survival separation cutoff values. The correlations between preoperative carbohydrate antigen 199 (CA199), carc
In total, 188 AC patients were enrolled in the study, with an overall male:female ratio of 1.47. Overall, nearly a quarter of patients had lymph node metastasis. Since all patients underwent open pancreaticoduodenectomy and the surgical trauma was relatively large, nearly half of the patients received intraoperative blood transfusion. In addition, a total of 54 patients (28.72%) received postoperative adjuvant therapy. The detailed baseline clinicopathologic characteristics of AC patients in the current study are illustrated in Table 1.
| Variables | Total (n = 188) | Score-0 (n = 88) | Score-1 (n = 84) | Score-2 (n = 16) | P value |
| Gender, n (%) | 0.166 | ||||
| Male | 112 (59.574) | 58 (65.909) | 47 (55.952) | 7 (43.750) | |
| Female | 76 (40.426) | 30 (34.091) | 37 (44.048) | 9 (56.250) | |
| Age, median (IQR) | 58 (50, 64) | 58 (51, 64) | 59 (50, 66) | 53 (45, 64) | 0.449 |
| Differentiation, n (%) | 0.052 | ||||
| Poor | 73 (38.830) | 34 (38.636) | 36 (42.857) | 3 (18.750) | |
| Moderate | 70 (37.234) | 38 (43.182) | 27 (32.143) | 5 (31.250) | |
| Well | 45 (23.936) | 16 (18.182) | 21 (25.000) | 8 (50.000) | |
| Tumor size, n (%) | 0.479 | ||||
| ≤ 2 cm | 94 (50.000) | 43 (48.864) | 45 (53.571) | 6 (37.500) | |
| > 2 cm | 94 (50.000) | 45 (51.136) | 39 (46.429) | 10 (62.500) | |
| AST (U/L), median (IQR) | 57 (35, 112) | 72 (48, 126) | 50 (23, 94) | 30 (20, 72) | < 0.001 |
| ALT (U/L), median (IQR) | 87 (36, 150) | 105 (66, 186) | 54 (29, 117) | 45 (18, 70) | < 0.001 |
| CEA (ng/mL), median (IQR) | 2.75 (1.88, 3.81) | 3.06 (1.85, 4.48) | 2.58 (1.92, 3.78) | 2.24 (1.94, 2.97) | 0.193 |
| CA199 (U/mL), median (IQR) | 55.80 (18.83, 184.30) | 60.84 (27.41, 162.30) | 55.80 (13.57, 191.40) | 35.46 (20.32, 72.94) | 0.444 |
| Perioperative transfusion, n (%) | 0.226 | ||||
| No | 96 (51.064) | 45 (51.136) | 46 (54.762) | 5 (31.250) | |
| Yes | 92 (48.936) | 43 (48.864) | 38 (45.238) | 11 (68.750) | |
| T stage, n (%) | 0.088 | ||||
| I | 36 (19.149) | 10 (11.364) | 21 (25.000) | 5 (31.250) | |
| II | 57 (30.319) | 28 (31.818) | 23 (27.381) | 6 (37.500) | |
| III | 95 (50.532) | 50 (56.818) | 40 (47.619) | 5 (31.250) | |
| Examined lymph nodes, median (IQR) | 11 (7, 18) | 12 (8, 18) | 11 (6, 19) | 9 (4, 10) | 0.044 |
| Examined lymph node, n (%) | 0.035 | ||||
| < 12 | 107 (56.915) | 47 (53.409) | 46 (54.762) | 14 (87.500) | |
| ≥ 12 | 81 (43.085) | 41 (46.591) | 38 (45.238) | 2 (12.500) | |
| Lymph node metastasis, n (%) | 0.402 | ||||
| No | 140 (74.468) | 63 (71.591) | 63 (75.000) | 14 (87.500) | |
| Yes | 48 (25.532) | 25 (28.409) | 21 (25.000) | 2 (12.500) | |
| TNM stage, n (%) | None | ||||
| I | 82 (43.617) | 32 (36.364) | 40 (47.619) | 10 (62.500) | |
| II | 76 (40.426) | 37 (42.045) | 33 (39.286) | 6 (37.500) | |
| III | 30 (15.957) | 19 (21.591) | 11 (13.095) | 0 (0.000) | |
| Vessel invasion, n (%) | None | ||||
| No | 141 (75.000) | 57 (64.773) | 68 (80.952) | 16 (100.000) | |
| Yes | 47 (25.000) | 31 (35.227) | 16 (19.048) | 0 (0.000) | |
| Postoperative complications, n (%) | 0.353 | ||||
| No | 110 (58.511) | 49 (55.682) | 49 (58.333) | 12 (75.000) | |
| Yes | 78 (41.489) | 39 (44.318) | 35 (41.667) | 4 (25.000) | |
| Adjuvant treatment, n (%) | 0.235 | ||||
| No | 134 (71.277) | 59 (67.045) | 61 (72.619) | 14 (87.500) | |
| Yes | 54 (28.723) | 29 (32.955) | 23 (27.381) | 2 (12.500) | |
| Fibrinogen (g/L), median (IQR) | 3.68 (3.12, 4.22) | 3.71 (3.20, 4.02) | 3.74 (3.15, 4.42) | 3.06 (2.67, 3.96) | 0.153 |
| Albumin (g/L), median (IQR) | 37.10 (31.40, 41.20) | 39.00 (35.40, 41.40) | 35.70 (27.60, 41.70) | 4.13 (3.82, 4.47) | < 0.001 |
| Total bilirubin (μmol/L), median (IQR) | 38.70 (12.82, 173.60) | 161.90 (63.10, 228.80) | 15.31 (8.96, 24.58) | 1.32 (0.75, 1.99) | < 0.001 |
| FAR, median (IQR) | 0.106 (0.086, 0.150) | 0.095 (0.086, 0.109) | 0.134 (0.084, 0.195) | 0.687 (0.174, 0.764) | < 0.001 |
| TBAR, median (IQR) | 0.538 (0.205, 2.325) | 0.266 (0.162, 0.580) | 1.807 (0.386, 3.303) | 3.694 (2.742, 5.027) | < 0.001 |
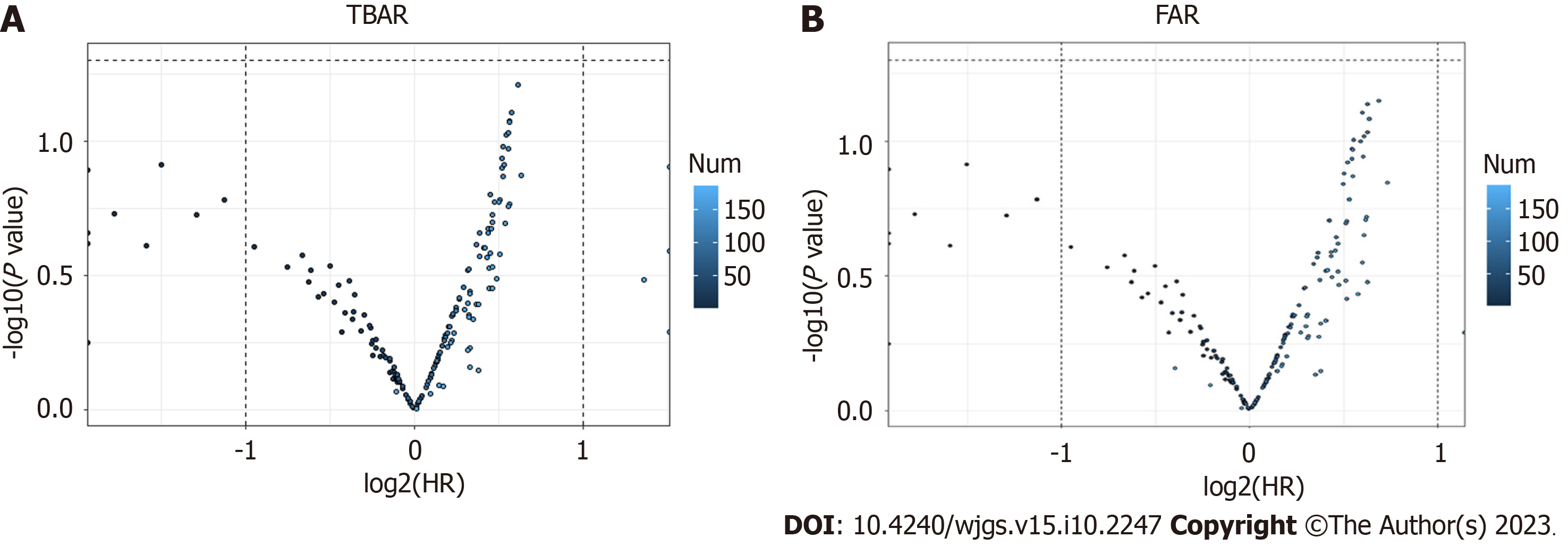
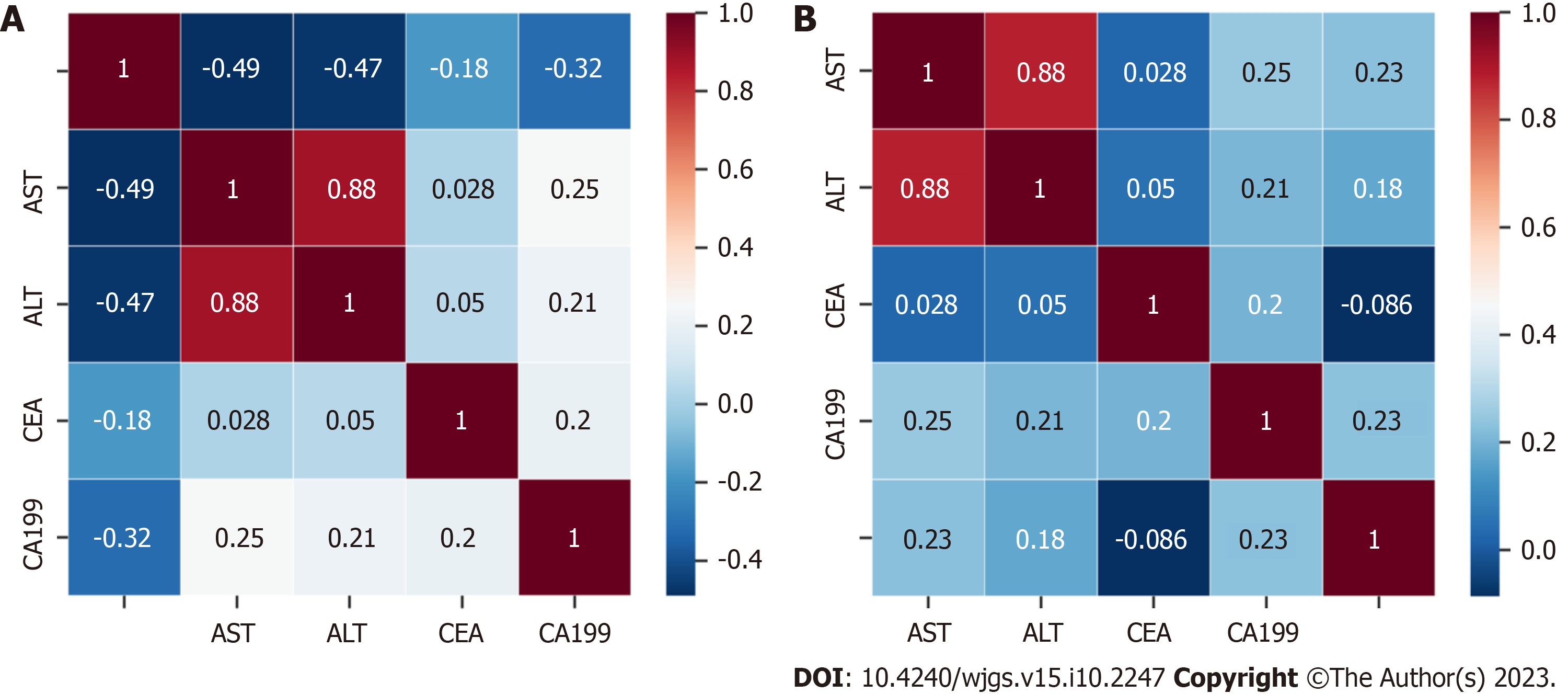
The cutoff values of TBAR and FAR were calculated through the best survival separation model. In the current study, the cutoff value of TBAR was 1.7943, while the cutoff value of FAR was 0.1329 (Figure 1). Furthermore, we conducted correlations between preoperative CA199, CEA, ALT, AST, TBAR, and FAR. The results demonstrated that FAR was significantly correlated with preoperative AST, ALT, and CA199, while TBAR was signi
In the current study, we defined TBAR-low and FAR-low as score 0 and TBAR-high and FAR-high as score 1. Directly after, we calculated the total score and classified all the patients into the score-0 group, score-1 group, and score-2 group. By comparing the baseline clinicopathologic data, we found that there were no significant differences among the three groups in tumor stage, tumor differentiation degree, tumor size, CA199 level, CEA level, or postoperative adjuvant the
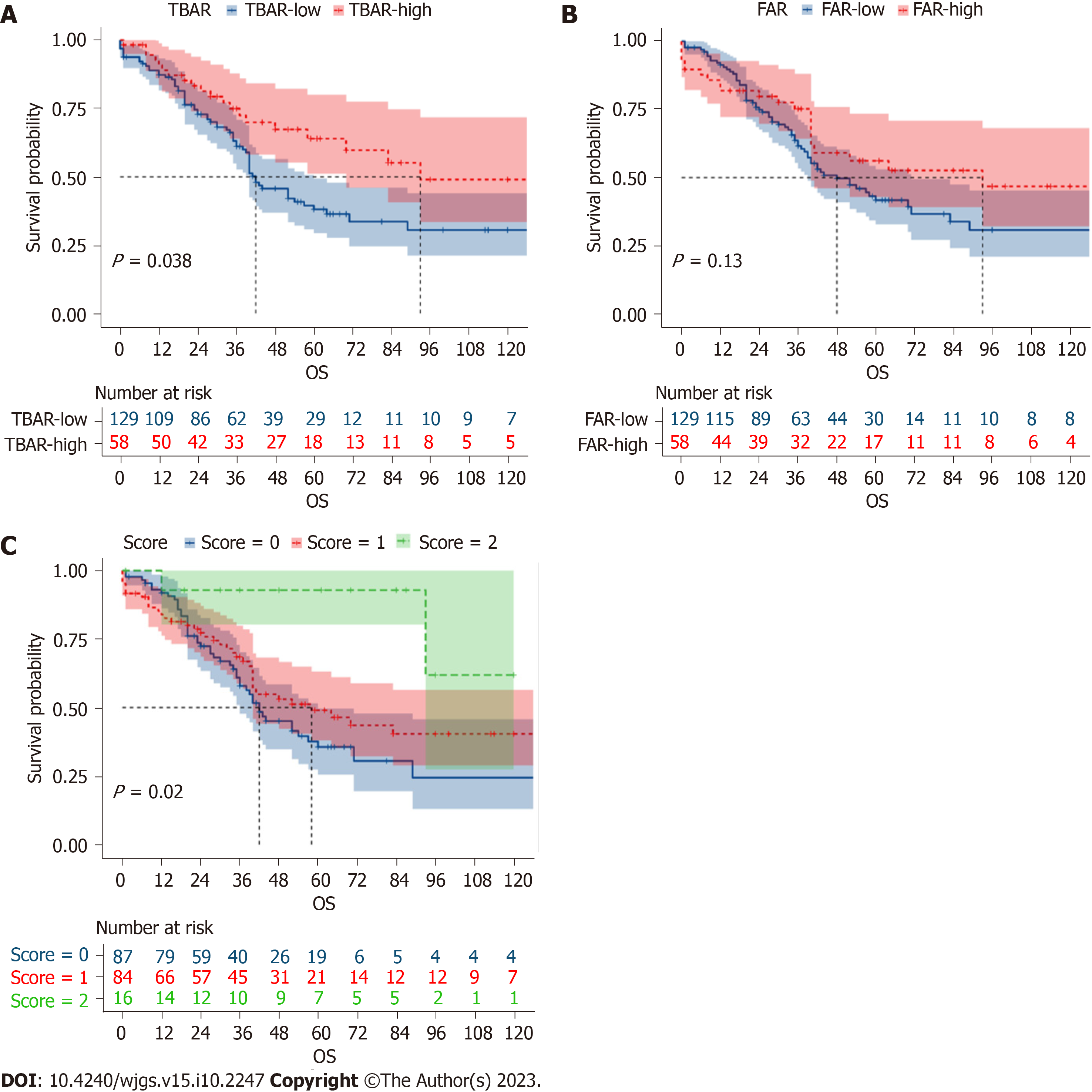
The 1-year, 3-year, and 5-year overall survival (OS) rates were 91.3%, 63.6%, and 41.7%, respectively. According to the univariable survival analysis, the TBAR-high group, FAR-high group, and score-high group had better prognoses (Figure 3). In the multivariable logistic regression analysis, score 1 [odds ratio (OR) = 0.517; 95%CI: 0.270-0.988; P = 0.046) and score 2 (OR = 0.236; 95%CI: 0.060-0.922; P = 0.038] were independent protective factors for tumor recurrence (Table 2). However, in the multivariable survival analysis, a high score was not an independent factor for recurrence-free survival (RFS) (P > 0.05) (Supplementary Table 3). Furthermore, we conducted multivariable OS analysis and found that only the score-2 group was an independent protective factor for OS (HR = 0.230; 95%CI: 0.054-0.972; P = 0.046) (Table 3).
| Variables | Univariable analysis | Multivariable analysis | ||||
| OR | 95%CI | P value | OR | 95%CI | P value | |
| Gender | ||||||
| Male | Reference | |||||
| Female | 1.176 | 0.656-2.110 | 0.586 | |||
| Age | ||||||
| < 60 | Reference | |||||
| ≥ 60 | 0.747 | 0.414-1.347 | 0.332 | |||
| Differentiation | ||||||
| Poor | Reference | |||||
| Moderate | 1.150 | 0.596-2.216 | 0.677 | |||
| Well | 0.599 | 0.279-1.286 | 0.189 | |||
| Tumor size | ||||||
| ≤ 2 cm | Reference | |||||
| > 2 cm | 0.879 | 0.496-1.561 | 0.661 | |||
| Perioperative transfusion | ||||||
| No | Reference | |||||
| Yes | 0.619 | 0.347-1.103 | 0.104 | |||
| Operation time | ||||||
| ≤ 3 h | ||||||
| > 3 h and ≤ 6 h | ||||||
| > 6 h | ||||||
| T stage | ||||||
| I | Reference | Reference | ||||
| II | 1.197 | 0.506-2.833 | 0.683 | 0.815 | 0.321-2.064 | 0.665 |
| III | 2.051 | 0.930-4.521 | 0.075 | 0.743 | 0.132-4.184 | 0.736 |
| Examined lymph nodes | ||||||
| < 12 | Reference | |||||
| ≥ 12 | 1.359 | 0.761-2.427 | 0.300 | |||
| Lymph node metastasis | ||||||
| No | Reference | Reference | ||||
| Yes | 2.735 | 1.384-5.406 | 0.004 | 1.224 | 0.425-3.524 | 0.707 |
| TNM stage | ||||||
| I | Reference | Reference | ||||
| II | 1.560 | 0.823-2.956 | 0.173 | 1.574 | 0.289-8.574 | 0.600 |
| III | 6.005 | 2.300-15.676 | < 0.001 | 4.149 | 0.491-35.043 | 0.191 |
| Vessel invasion | ||||||
| No | Reference | Reference | ||||
| Yes | 2.601 | 1.313-5.151 | 0.006 | 1.353 | 0.616-2.970 | 0.451 |
| Postoperative complications | ||||||
| No | Reference | |||||
| Yes | 0.696 | 0.387-1.250 | 0.225 | |||
| Adjuvant treatment | ||||||
| No | Reference | |||||
| Yes | 1.689 | 0.893-3.192 | 0.107 | |||
| CEA | 1.071 | 0.978-1.172 | 0.139 | |||
| CA199 | 1.000 | 1.000-1.000 | 0.824 | |||
| TBAR | 0.988 | 0.895-1.090 | 0.807 | |||
| FAR | 0.504 | 0.182-1.393 | 0.186 | |||
| Score | ||||||
| 0 | Reference | Reference | ||||
| 1 | 0.469 | 0.255-0.863 | 0.015 | 0.517 | 0.270-0.988 | 0.046 |
| 2 | 0.167 | 0.045-0.630 | 0.008 | 0.236 | 0.060-0.922 | 0.038 |
| Variables | Univariate analysis | Multivariate analysis | ||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Gender | ||||||
| Male | Reference | |||||
| Female | 1.080 | 0.711-1.639 | 0.719 | |||
| Age | ||||||
| < 60 | Reference | |||||
| ≥ 60 | 1.198 | 0.788-1.822 | 0.398 | |||
| Differentiation | ||||||
| Poor | Reference | Reference | ||||
| Moderate | 0.873 | 0.548-1.391 | 0.568 | 0.981 | 0.600-10603 | 0.938 |
| Well | 0.596 | 0.344-1.034 | 0.066 | 0.979 | 0.501-1.911 | 0.949 |
| Tumor size | ||||||
| ≤ 2 cm | Reference | |||||
| > 2 cm | 1.139 | 0.756-1.716 | 0.534 | |||
| Perioperative transfusion | ||||||
| No | Reference | |||||
| Yes | 0.861 | 0.570-1.298 | 0.474 | |||
| T stage | ||||||
| I | Reference | Reference | ||||
| II | 0.927 | 0.485-1.769 | 0.817 | 0.792 | 0.375-1.672 | 0.541 |
| III | 2.128 | 1.211-3.741 | 0.009 | 1.731 | 0.474-6.322 | 0.407 |
| Examined lymph nodes | ||||||
| < 12 | Reference | |||||
| ≥ 12 | 1.344 | 0.881-2.051 | 0.171 | |||
| Lymph node metastasis | ||||||
| No | Reference | Reference | ||||
| Yes | 2.203 | 1.407-3.447 | 0.001 | 1.704 | 0.851-3.412 | 0.132 |
| TNM stage | ||||||
| I | Reference | Reference | ||||
| II | 1.965 | 1.230-3.137 | 0.005 | 0.886 | 0.272-2.888 | 0.841 |
| III | 3.030 | 1.711-5.368 | < 0.001 | 0.918 | 0.208-4.057 | 0.910 |
| Vessel invasion | ||||||
| No | Reference | |||||
| Yes | 1.410 | 0.885-2.248 | 0.148 | |||
| Postoperative complications | ||||||
| No | Reference | |||||
| Yes | 1.343 | 0.886-2.035 | 0.164 | |||
| Adjuvant treatment | ||||||
| No | Reference | |||||
| Yes | 0.946 | 0.590-1.514 | 0.816 | |||
| CEA | 1.001 | 0.999-1.004 | 0.359 | |||
| CA199 | 1.000 | 1.000-1.000 | 0.585 | |||
| TBAR | 0.966 | 0.880-1.061 | 0.470 | |||
| FAR | 0.748 | 0.359-1.558 | 0.438 | |||
| Score | ||||||
| 0 | Reference | Reference | ||||
| 1 | 0.787 | 0.518-1.196 | 0.262 | 0.890 | 0.577-1.373 | 0.599 |
| 2 | 0.176 | 0.043-0.726 | 0.016 | 0.230 | 0.054-0.972 | 0.046 |
Most previous studies separately analyzed the prognostic value of preoperative TB, fibrinogen, and albumin for solid tumors, and the results from different cohorts are still controversial. After inclusion of these predictors, we found that TBAR combined with the FAR was an independent predictor for tumor recurrence and an independent protective factor for OS in AC patients. These peripheral blood biomarkers could be used as prognostic factors and are novel, reliable, eco
The hemostatic system can regulate angiogenesis in various ways. Fibrinogen is an extracellular matrix protein composed of three polypeptide chains with fibrinogen alpha, beta, and gamma. Recent studies have shown that the FAR is associated with prognosis in several solid malignancies, including pancreatic neuroendocrine neoplasms[11] and gastrointestinal stromal tumors[12]. FAR is considered a feasible and predictive biomarker for prognosis in patients. In addition, Zhang and Xiao[13] revealed that the FAR was significantly associated with deeper tumor invasion and increased regional lymph node involvement. However, Zhao et al[14] showed that fibrinogen-derived fibrinostatin has antiangiogenic activity by inhibiting endothelial cell proliferation, adhesion, migration and tubule formation. By inducing apoptosis and inhibiting epithelial-mesenchymal transition, alpha may suppress lung adenocarcinoma cell growth and metastasis[15]. Nevertheless, the specific mechanism of the relationship between the FAR and prognosis still needs to be investigated further.
Bilirubin is one of the routine biochemical testing items, which originates primarily from circulating hemoglobin and is the most important product of hemoglobin decomposition. It has antioxidant properties and can protect cells against oxidative stress as an endogenous antioxidant[16]. Sun et al[17] found that decreases in serum bilirubin levels are asso
Albumin is a secretory protein produced only by functional hepatocytes, and it reflects the chronic nutritional state, which is commonly used for nutritional status assessment. The nutritional state of cancer patients was associated with unfavorable outcomes in malignant tumor patients. Our score is a composite indicator containing representative results of biochemical analysis, which can reflect the nutritional and inflammatory status, liver function and tumor effect. FAR in conjunction with TBAR has the potential to assist clinicians in the stratification of patients. Nevertheless, further investigation is still needed for the application of the novel score in clinical practice.
The traditional Child-Pugh grade used to assess liver function faces several inherent limitations. Therefore, Johnson et al[22] developed a simple model based on albumin and bilirubin levels alone, the ALBI grade, which can stratify patients into three risk categories and objectively prognosticate survival. A meta-analysis demonstrated that high pretreatment ALBI is closely associated with poor prognosis in hepatocellular cancer[23]. The ALBI grade also showed improved discriminatory ability in pancreatic cancer[24] and colorectal cancer[25]. Our new score adds the blood coagulation index and broadens the use of AC. In addition, the score is easily calculated compared with the ALBI grade and increases ver
High scores were independent factors for RFS in the univariable survival analysis but not in the multivariable analysis. Although there were some differences in RFS, these were not statistically significant. Furthermore, we conducted multivariable OS analysis and found that only the score-2 group was an independent protective factor for OS. Such conflicting results might be related to several factors. First, patients with higher scores might be more likely to detect recurrence earlier and receive more aggressive treatment as a result. Consequently, this might not significantly affect RFS but could emerge as an independent prognostic factor for OS. Second, the number of patients in the score-high groups was relatively small, and it might be challenging to detect statistically significant differences in RFS. Third, the inclusion of other prognostic factors in the model might have resulted in multicollinearity or confounding effects, which could attenuate the initial observed relationship between the score-high groups and RFS. These results emphasize the need for further research, potentially involving larger patient cohorts to comprehensively understand the underlying mechanisms and relationships between the scoring system and different survival outcomes.
During tumor growth, tumor markers are produced and released into the blood circulation, which can be used for the diagnosis and monitoring of tumors. However, sensitive tumor markers are still lacking for predicting survival and treatment outcomes in AC[26]. In recent years, liquid biopsies have been increasingly emphasized. Circulating tumor DNA (ctDNA) derived from tumors is being used in analyzing prognosis and tumor burden in some solid tumors. Patel et al[27] found that higher levels of total ctDNA were an independent prognostic factor for worse survival for patients with advanced pancreatic ductal adenocarcinoma. However, this new technique can be limited by the small amounts of circulating biomarkers, and not everyone has detectable ctDNA levels[28]. To date, liquid biopsy is a complement to tissue biopsy, and more research studies are needed for the improvement of clinical applications. They are not considered to be perfect predictors for the above reasons. Therefore, our new scoring system contained three common indicators and showed great predictive power in AC patients who underwent curative pancreaticoduodenectomy. The novel score is expected to become a new biomarker for identifying poor prognosis in patients with AC.
There were limitations to this study. First, this was a single-center retrospective study with a relatively small sample, and multicentric prospective studies with large samples are needed in the future. Second, we did not perform a cohort verification of the results, and external validation is needed. Third, we did not investigate whether the postoperative peripheral blood biomarkers could be used as prognostic factors and analyzed the dynamic changes in the three indi
The novel prognostic score based on the preoperative TBAR and FAR was an independent predictor for tumor recurrence and an independent protective factor for OS in AC patients who underwent curative pancreaticoduodenectomy. How
Whether the relationship of preoperative total bilirubin, fibrinogen, and albumin could be used as prognostic factors is still debated.
The present study attempted to explore the prognostic value of total bilirubin-albumin ratio (TBAR) and fibrinogen-albumin ratio (FAR) in ampullary adenocarcinoma (AC) patients who underwent curative pancreaticoduodenectomy.
This study aimed to investigate whether there was an association between the novel prognostic score and poor oncologic outcomes in ampullary carcinoma.
The clinicopathological data of ampullary carcinoma patients who underwent surgery from January 1998 to January 2020 were analyzed. Then, a novel prognostic score combining TBAR and FAR was calculated and validated through logistic regression analysis and Cox regression analysis.
Multivariable logistic regression analysis revealed that a high score was an independent protective factor for recurrence. In addition, multivariable survival analysis also demonstrated that a high score was an independent protective factor in AC patients.
We found that the novel prognostic score based on the preoperative TBAR and FAR has good predictive power in AC patients who underwent curative pancreaticoduodenectomy.
There are several limitations in this retrospective study, and more studies with larger samples are needed to validate this conclusion.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Aoki H, Japan; Arigami T, Japan S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Zimmermann C, Wolk S, Aust DE, Meier F, Saeger HD, Ehehalt F, Weitz J, Welsch T, Distler M. The pathohistological subtype strongly predicts survival in patients with ampullary carcinoma. Sci Rep. 2019;9:12676. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 2. | de Rooij T, van Hilst J, Bosscha K, Dijkgraaf MG, Gerhards MF, Groot Koerkamp B, Hagendoorn J, de Hingh IH, Karsten TM, Lips DJ, Luyer MD, Molenaar IQ, van Santvoort HC, Tran TCK, Busch OR, Festen S, Besselink MG; Dutch Pancreatic Cancer Group. Minimally invasive versus open pancreatoduodenectomy (LEOPARD-2): study protocol for a randomized controlled trial. Trials. 2018;19:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 87] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 3. | Wu CL, Chao YJ, Yang TM, Chen YL, Chang KC, Hsu HP, Shan YS, Lai MD. Dual role of CD44 isoforms in ampullary adenocarcinoma: CD44s predicts poor prognosis in early cancer and CD44ν is an indicator for recurrence in advanced cancer. BMC Cancer. 2015;15:903. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Lin JX, Tang YH, Wang JB, Lu J, Chen QY, Cao LL, Lin M, Tu RH, Huang CM, Li P, Zheng CH, Xie JW. Blood parameters score predicts long-term outcomes in stage II-III gastric cancer patients. World J Gastroenterol. 2019;25:6258-6272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Yuan C, Huang M, Wang H, Jiang W, Su C, Zhou S. Pretreatment Fibrinogen-Albumin Ratio (FAR) Associated with Treatment Response and Survival in Advanced Non-Small Cell Lung Cancer Patients Treated with First-Line Anti-PD-1 Therapy Plus Platinum-Based Combination Chemotherapy. Cancer Manag Res. 2022;14:377-386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Mai RY, Bai T, Luo XL, Wu GB. Preoperative fibrinogen-to-albumin ratio predicts the prognosis of patients with hepatocellular carcinoma subjected to hepatectomy. BMC Gastroenterol. 2022;22:261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 7. | Ying HQ, Sun F, Liao YC, Cai D, Yang Y, Cheng XX. The value of circulating fibrinogen-to-pre-albumin ratio in predicting survival and benefit from chemotherapy in colorectal cancer. Ther Adv Med Oncol. 2021;13:17588359211022886. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Lin GT, Ma YB, Chen QY, Zhong Q, Zheng CH, Li P, Xie JW, Wang JB, Lin JX, Huang CM. Fibrinogen-Albumin Ratio as a New Promising Preoperative Biochemical Marker for Predicting Oncological Outcomes in Gastric Cancer: A Multi-institutional Study. Ann Surg Oncol. 2021;28:7063-7073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | Chen J, Hao L, Zhang S, Zhang Y, Dong B, Zhang Q, Han C. Preoperative Fibrinogen-Albumin Ratio, Potential Prognostic Factors for Bladder Cancer Patients Undergoing Radical Cystectomy: A Two-Center Study. Cancer Manag Res. 2021;13:3181-3192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Lu S, Liu Z, Zhou X, Wang B, Li F, Ma Y, Wang W, Ma J, Wang Y, Wang H, Fu W. Preoperative Fibrinogen-Albumin Ratio Index (FARI) is a Reliable Prognosis and Chemoradiotherapy Sensitivity Predictor in Locally Advanced Rectal Cancer Patients Undergoing Radical Surgery Following Neoadjuvant Chemoradiotherapy. Cancer Manag Res. 2020;12:8555-8568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Deng S, Fan Z, Xia H, Gong Y, Qian Y, Huang Q, Cheng H, Jin K, Xiao Z, Luo G, Yu X, Liu C. Fibrinogen/Albumin Ratio as a Promising Marker for Predicting Survival in Pancreatic Neuroendocrine Neoplasms. Cancer Manag Res. 2021;13:107-115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Li R, Song S, He X, Shi X, Sun Z, Li Z, Song J. Relationship Between Fibrinogen to Albumin Ratio and Prognosis of Gastrointestinal Stromal Tumors: A Retrospective Cohort Study. Cancer Manag Res. 2020;12:8643-8651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Zhang Y, Xiao G. Prognostic significance of the ratio of fibrinogen and albumin in human malignancies: a meta-analysis. Cancer Manag Res. 2019;11:3381-3393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 14. | Zhao C, Su Y, Zhang J, Feng Q, Qu L, Wang L, Liu C, Jiang B, Meng L, Shou C. Fibrinogen-derived fibrinostatin inhibits tumor growth through anti-angiogenesis. Cancer Sci. 2015;106:1596-1606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Tiscia GL, Margaglione M. Human Fibrinogen: Molecular and Genetic Aspects of Congenital Disorders. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 16. | Jeon C, Lee JY, Lee SJ, Jung KJ, Kimm H, Jee SH. Bilirubin and risk of ischemic heart disease in Korea: a two-sample Mendelian randomization study. Epidemiol Health. 2019;41:e2019034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Sun H, He B, Nie Z, Pan Y, Lin K, Peng H, Xu T, Chen X, Hu X, Wu Z, Wu D, Wang S. A nomogram based on serum bilirubin and albumin levels predicts survival in gastric cancer patients. Oncotarget. 2017;8:41305-41318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 18. | Temme EH, Zhang J, Schouten EG, Kesteloot H. Serum bilirubin and 10-year mortality risk in a Belgian population. Cancer Causes Control. 2001;12:887-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 156] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 19. | Feng L, Gu S, Wang P, Chen H, Chen Z, Meng Z, Liu L. Pretreatment values of bilirubin and albumin are not prognostic predictors in patients with advanced pancreatic cancer. Cancer Med. 2018;7:5943-5951. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Aoyama T, Ju M, Machida D, Komori K, Tamagawa H, Tamagawa A, Maezawa Y, Kano K, Hara K, Segami K, Hashimoto I, Nagasawa S, Nakazono M, Oshima T, Yukawa N, Rino Y. Clinical Impact of Preoperative Albumin-Bilirubin Status in Esophageal Cancer Patients Who Receive Curative Treatment. In Vivo. 2022;36:1424-1431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Lee JE, Choi SY, Lee MH, Lim S, Min JH, Hwang JA, Lee S, Kim JH. Differentiating between benign and malignant ampullary strictures: a prediction model using a nomogram based on CT imaging and clinical findings. Eur Radiol. 2022;32:7566-7577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 22. | Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL, O'Beirne J, Fox R, Skowronska A, Palmer D, Yeo W, Mo F, Lai P, Iñarrairaegui M, Chan SL, Sangro B, Miksad R, Tada T, Kumada T, Toyoda H. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33:550-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1962] [Cited by in RCA: 2009] [Article Influence: 200.9] [Reference Citation Analysis (0)] |
| 23. | Xu YX, Wang YB, Tan YL, Xi C, Xu XZ. Prognostic value of pretreatment albumin to bilirubin ratio in patients with hepatocellular cancer: A meta-analysis. Medicine (Baltimore). 2019;98:e14027. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 24. | Yagyu T, Saito H, Sakamoto T, Uchinaka EI, Morimoto M, Amisaki M, Watanabe J, Tokuyasu N, Honjo S, Ashida K, Fujiwara Y. Preoperative Albumin-Bilirubin Grade as a Useful Prognostic Indicator in Patients With Pancreatic Cancer. Anticancer Res. 2019;39:1441-1446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 25. | Watanabe D, Fujii H, Yamada Y, Matsuhashi N, Makiyama A, Iihara H, Takahashi T, Kiyama S, Kobayashi R, Yoshida K, Suzuki A. Association of albumin-bilirubin score in patients with colorectal cancer receiving later-line chemotherapy with regorafenib. Int J Clin Oncol. 2021;26:1257-1263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 26. | Kawaida H, Kono H, Amemiya H, Hosomura N, Watanabe M, Saito R, Maruyama S, Shimizu H, Furuya S, Akaike H, Kawaguchi Y, Sudo M, Matusda M, Itakura J, Shindo H, Takahashi EI, Takano S, Fukasawa M, Satou T, Nakazawa T, Enomoto N, Fujii H, Ichikawa D. Stratification of Prognosis in Patients With Ampullary Carcinoma After Surgery by Preoperative Platelet-to-lymphocyte Ratio and Conventional Tumor Markers. Anticancer Res. 2019;39:6923-6929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Patel H, Okamura R, Fanta P, Patel C, Lanman RB, Raymond VM, Kato S, Kurzrock R. Clinical correlates of blood-derived circulating tumor DNA in pancreatic cancer. J Hematol Oncol. 2019;12:130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 72] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 28. | Nikanjam M, Kato S, Kurzrock R. Liquid biopsy: current technology and clinical applications. J Hematol Oncol. 2022;15:131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 428] [Reference Citation Analysis (0)] |