Published online Sep 27, 2022. doi: 10.4240/wjgs.v14.i9.887
Peer-review started: June 4, 2022
First decision: August 1, 2022
Revised: August 19, 2022
Accepted: September 8, 2022
Article in press: September 8, 2022
Published online: September 27, 2022
Processing time: 109 Days and 21.5 Hours
Cholesterol gallstones are very common in hepatobiliary surgery and have been studied to a certain extent by doctors worldwide for decades. However, the mechanism of cholesterol gallstone formation is not fully understood, so there is currently no completely effective drug for the treatment and prevention of cholesterol gallstones. The formation and development of cholesterol gallstones are caused by a variety of genetic and environmental factors, among which genetic susceptibility, intestinal microflora disorders, impaired gallbladder mo
Core Tip: Cholesterol gallstone disease is very common. At present, some new progress has been made in the research on the pathogenesis of cholesterol gallstones, and we have also gained a new understanding of this disease. Here, we discuss the latest research progress of genetic susceptibility, intestinal microflora disorders, impaired gallbladder motility, and immune disorders in the formation of cholesterol gallstones and some new drug targets.
- Citation: Jiao JY, Zhu XJ, Zhou C, Wang P. Research progress on the immune microenvironment of the gallbladder in patients with cholesterol gallstones. World J Gastrointest Surg 2022; 14(9): 887-895
- URL: https://www.wjgnet.com/1948-9366/full/v14/i9/887.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v14.i9.887
Gallstones occur in about 20% of adults in western countries and are one of the most common diseases of hepatobiliary surgery[1]. In past research studies[2], we found that more than 90% of gallstones are mainly composed of cholesterol, called cholesterol gallstones.
Normally, mixed micelles are composed of cholesterol, phospholipids (mainly phosphatidylcholine), and bile salts in bile. Under the action of mixed micelles, bile is thermodynamically stable and cho
In past studies, we found that risk factors for cholesterol gallstones comprise both unmodifiable and modifiable factors. Non-modifiable factors include age, sex, race, and genetic factors. Modifiable factors include the following: metabolic syndrome features such as diabetes[5], insulin resistance, and obesity[6]; dietary habits such as high-calorie and low-fiber diets[7]; intestinal damage such as colectomy[8]; Crohn’s disease; drug factors such as octreotide[9], lipid-lowering drugs, and hormones; and impaired gallbladder motility.
More than 20% of patients with cholesterol gallstones develop symptoms, such as biliary colic, during their lifetime and are at risk of developing cholecystitis, gallbladder cancer[10] and pancreatitis[11]. To date, surgery is the best way to treat cholesterol gallstone patients when they develop these symptoms or complications, but it comes with heavy economic and social burdens[12]. Therefore, it is urgent and important to treat and prevent cholesterol gallstones by studying the pathogenesis of gallstones and taking corresponding intervention measures for specific pathogenic links.
In this review, we focus on the important roles of genetic susceptibility, intestinal microflora disorders, and impaired gallbladder motility. We also discuss some strategies for the treatment and prevention of cholesterol gallstones, which inhibit some of the pathogenic aspects of cholesterol gallstones.
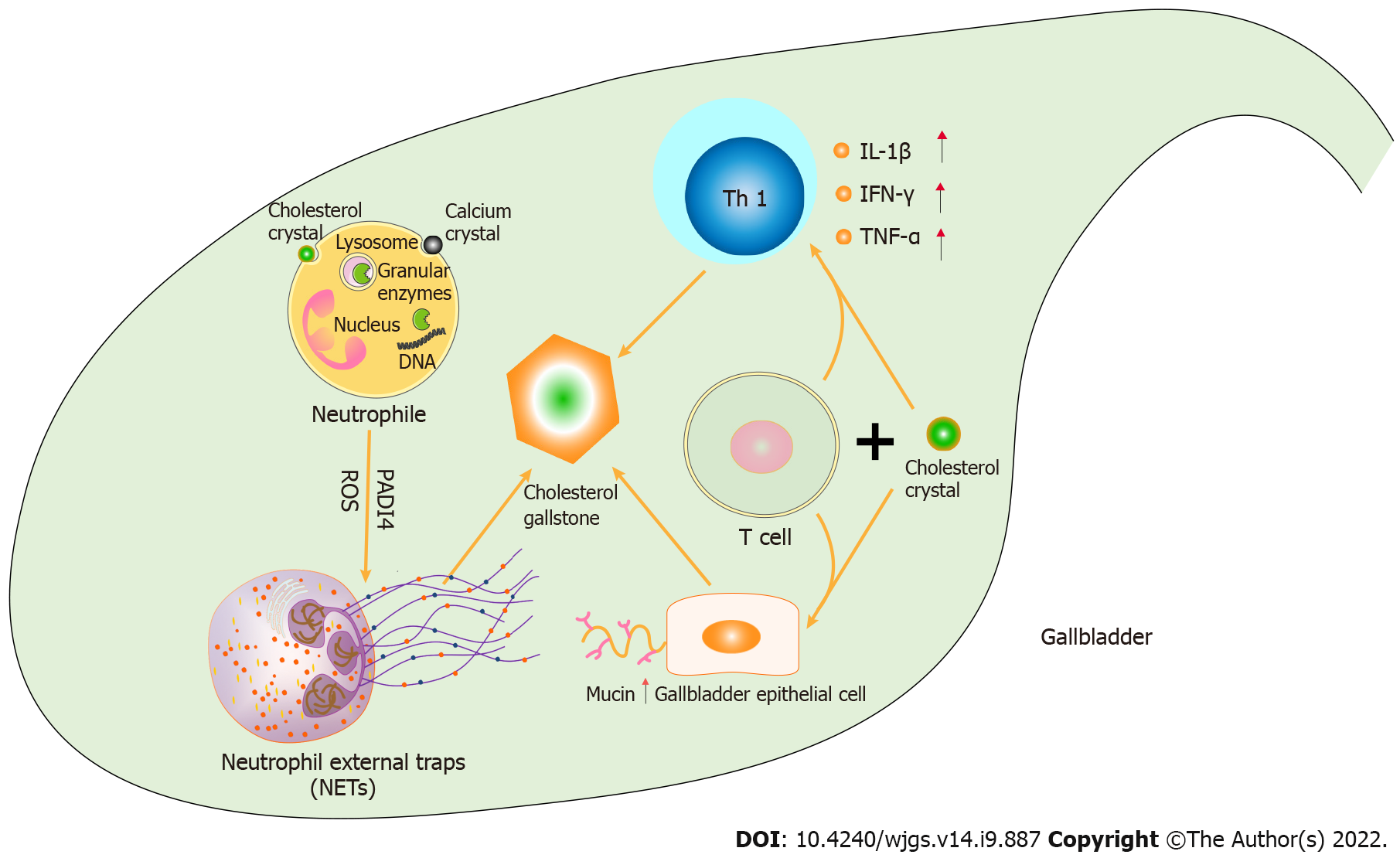
Immune disorders play a crucial role in the formation and development of cholesterol gallstones. First, low concentrations of various immunoglobulins including IgA, IgG, and IgM were contained in bile[13]. Among them, IgM is the most effective Ig in promoting the formation of cholesterol gallstones in supersaturated bile, while IgG is less effective and IgA is the least effective[14-16]. In addition, the formation of cholesterol gallstones is closely related to mucin (MUC) gel accumulation in human and animal models, and MUC gel accumulation occurs before cholesterol gallstone formation and is an important cause of cholesterol gallstone formation[17-22]. At the same time, MUC may be positively correlated with the calcification of cholesterol gallstones[23]. Some MUC genes are expressed in human bile duct epithelial cells such as MUC1, MUC2, MUC3, MUC4, MUC5AC, MUC5B, and MUC6[24], and the expression of these MUC genes and the production and secretion of MUC are regulated by inflammatory mediators in the immune system[25-27]. Cholesterol secretion can also be promoted by inflammatory mediators, which promote liver lipid metabolism and secretion, lead to bile cholesterol supersaturation, and promote cholesterol gallstone formation. For example, in mice, the formation of cholesterol gallstones can be promoted by the administration of lipopolysaccharide (LPS) or pro-inflammatory cytokines [interleukin (IL)-1, tumor necrosis factor (TNF)], because these result in elevated serum cholesterol levels and increase the production of 3-hydroxy-3-methylglutarate mono-acyl-coenzyme A reductase (HMG-CoA reductase)[28-30]. In addition, cholesterol catabolism can be inhibited by LPS, which reduces the production of cholesterol 7 alpha-hydroxylase (CYP7A1), CYP7B1, or CYP27A1 protein, leading to bile supersaturation and cholesterol gallstone formation[31,32]. Recent studies have found that immune factors can also influence the formation of cholesterol gallstones by influencing the movement of gallbladder contraction. Interstitial Cajal-like cells (ICLCs) are widespread in the gallbladder and bile duct and play a significant role in the regulation of gallbladder contractile motion. The density of ICLCs in the gallbladder is significantly reduced in patients with cholelithiasis, suggesting that decreased gallbladder contraction and cholesterol gallstone formation are closely associated with reduced ICLCs. Ursodeoxycholic acid protects ICLCs in the gallbladder from apoptosis by inhibiting the TNF-α/caspase 8/caspase 3 pathway[33], thereby protecting the contractile activity of the gallbladder and ultimately inhibiting the formation of cholesterol gallstones. These objective results indicate that immune disorders play a crucial role in the formation and development of cholesterol gallstones.
The role of adaptive immunity in cholesterol gallstone formation was analyzed by giving Helicobacter pylori (H. pylori)-infected and uninfected homozygous mice, as well as homozygous immunodeficient Rag mice, a lithogenic diet in a former study. Lymphocyte metastasis studies were also performed to determine which cell subsets are responsible for cholesterol gallstone formation[34]. H. pylori usually causes disease by inducing a pro-inflammatory immune response mediated by T-assisted type 1[35,36]. When fed the lithogenic diet for 2 mo, more cholesterol gallstones were found in non-immunodeficient mice than in Rag mice. There was a statistically significant increase in cholesterol gallstone prevalence in H. pylori-infected mice compared with uninfected mice. In addition, T lymphocyte transfer to Rag mice significantly increased the prevalence of cholesterol gallstones, while B lymphocyte transfer did not significantly increase cholesterol gallstones. A detailed description of the association between adaptive immunity and cholesterol gallstone formation was provided in this study, which suggested that T cells are an important link in the formation of cholesterol gallstones in mice (Figure 1).
The vital role of neutrophil external traps (NETs) in cholesterol gallstone formation and development was expounded upon in a recent study[37]. By fluorescence microscopy, patchy extracellular DNA (ecDNA), large ecDNA aggregates, and strong neutrophil elastase activity were found in both human and porcine cholesterol gallstones. In previous reports, obesity is related to the release of ecDNA into plasma in mice and humans[38], and ecDNA in peripheral circulation has contact with the risk of metabolic syndrome[39], both of which are risk factors for cholesterol gallstones. Upon contact with neutrophils, cholesterol or calcium crystals are ingested by neutrophils. This process of pinocytosis causes the granular enzymes in lysosomes to leak and bind to the DNA in the cytoplasm, ultimately decondensed chromatin and externalizing to form NETs. Cholesterol crystals and calcium crystals in the bile of the gallbladder are aggregated to form cholesterol gallstones by the “glue” role of NETs. Meanwhile, the formation of NETs is dependent on the activity of peptidyl arginine deiminase type 4 and the production of reactive oxygen species. In addition, this study confirmed that the formation and development of cholesterol gallstones can be effectively reduced by the inhibition of NET formation or neutrophils. The results of this study verify that the formation of NETs is the key link in the formation of cholesterol gallstones caused by the accumulation of crystals in bile, and the formation of neutrophils and NETs may be new targets for the prevention and treatment of cholesterol gallstones (Figure 1).
Together, these findings suggest that immune dysfunction is also an important link in the formation and development of cholesterol gallstones. Targeting immune disorders in the pathogenesis of cholesterol gallstones will be a new hotspot in the treatment and prevention of cholesterol gallstones in the future.
Bacteria are present in the bile, cholesterol gallstones, and even gallbladder tissue of patients with cholesterol gallstones[1]; however, the role of these bacteria in cholesterol gallstone formation is not fully understood. A lower incidence of cholesterol gallstones in germ-free mice was found in one of the earliest studies[40]. Another study showed that mice infected with enterohepatic H. pylori had an increased risk of cholesterol gallstones[41]. A recent study comparing the biliary microbiota of lithiasis and non-lithiasis groups found that the Alcaligenaceae reached higher relative abundance in lithiasis samples[42]. In this family, Alcaligenes recti are reportedly involved in the metabolism of various bile acids. These findings suggest that cholesterol gallstone formation appears to be related to intestinal microbiome dysregulation. With the abundance and diversity of intestinal flora decreased, the number of Firmicutes decreased, and the ratio of Firmicutes to Bacteroidetes decreased in mice with gallstones[43]. In addition, the intestinal bacteria phylum Proteobacteria were significantly increased, while Faecalibacterium, Lachnospira, and Roseburia were significantly decreased[44]. The number of Gram-positive fecal anaerobes in the cecum was increased in patients with gallstones compared with those without gallstones, and 7α-dehydroxylation activity was also increased, which seemed to explain the increased concentration of hydrophobic secondary bile acid deoxycholic acid in patients with gallstones[45].
Enrichment of Desulfovibrionales has been found in patients with metabolic syndrome and obesity associated with cholesterol gallstones[46], but the specific link between the bacteria and cholesterol gallstones has not been clarified. A recent study found that the abundance of Desulfovibrionales in the feces of cholesterol gallstone patients and cholesterol gallstone-susceptible mice was significantly higher than that in the non-gallstone population, and that the transplantation of intestinal flora from cholesterol gallstone patients into cholesterol gallstone-resistant mice resulted in a statistically significant increase in cholesterol gallstone prevalence[47]. The production of secondary bile acids will be promoted by a large number of Desulfovibrionales rich in the cecum, and the hydrophobicity of bile acids will therefore increase, resulting in increased absorption of intestinal cholesterol and easy to cause cholesterol gallstones. In addition, the intestinal lipid absorption process is regulated by CD36. The expression of CD36 can be induced by Desulfovibrionales; thus, the intestinal lipid absorption is enhanced, which may also lead to the formation of cholesterol gallstones[48]. On the other hand, hydrogen sulfide, a metabolite of Desulfovibrionales, can induce farnesoid X receptor and inhibit the expression of CYP7A1. The expression of cholesterol transporter ATP-binding cassette transporter G5/G8 (ABCG5/ABCG8) in the mouse liver was also induced by Desulfovibrionales, which promoted cholesterol secretion in the biliary tract. This study shows that cholesterol gallstone formation is promoted by intestinal Desulfovibrionales, which influences bile acid and cholesterol metabolism, further supporting the important role of intestinal microbiome imbalance in cholesterol gallstone formation.
In addition to these two mechanisms, there are other factors that contribute to the formation of cholesterol gallstones, such as genetic factors and gallbladder dyskinesia[49]. Indigenous populations in North and South America are reported to be at highest risk of gallstones in the world. Prevalence rates are lower in Asian populations and lowest in African populations[1]. A study of 43141 twins with gallstone disease in Sweden showed that about 25% of gallstones were caused by a genetic susceptibility[50]. These objective results suggest that gallstone risk and genetic susceptibility are inextricably linked.
Lipid composition in the biliary tract is regulated by complex ATP-binding cassette (ABC) tran
Mutations and variants of ABCB4 inhibit the secretion of phospholipids from the liver to the bile ducts, resulting in a decrease or deficiency of phospholipids in bile and the formation of cholesterol gallstones, known as low phospholipid-associated cholelithiasis. A recent study compared the chemical composition of fresh gallbladder bile between ABCB4 knockout and wild-type mice and found cholesterol supersaturation and the presence of cholesterol crystals in gallbladder bile in the former but not in the latter. The results of this study demonstrate the critical role of ABCB4 in phospholipid transport and the important role of ABCB4 mutations in the formation of cholesterol gallstones[54]. A strong association between gallstone disease and ABCG8 was shown in a genome-wide association study (GWAS) involving 280 patients with gallstones and 360 controls in 2007[55]. ABCG8 is responsible for transporting cholesterol into the biliary tract and intestinal lumen, and its association with cholesterol gallstones is attributed to a familiar variant that causes guanine at position 55 to become cytosine, resulting in the replacement of aspartic acid, the amino acid residue at position 19 of the transporter, by histidine (ABCG8D19H, RS11887534). ABCG8D19H constitutes a functional acquisition mutation, which increases the transport activity of ABCG8 by three-fold, increases the hepatic cholesterol discharge into the biliary tract, increases the absolute cholesterol saturation in bile, and ultimately leads to the occurrence of cholesterol gallstones[55-57].
In 2016, four new gallstones susceptibility loci, namely SULT2A1, TM4SF4, GCKR, and CYP7A1, were identified in a large GWAS (there were 8720 gallstones patients and 55152 people who did not have gallstones in the discovery set, and 6489 gallstones patients and 62797 people who did not have gallstones in the validation set), and the association between ABCG8 and gallstones were confirmed[58]. The metabolism of cholesterol into bile acid in the liver is mainly regulated by cholesterol CYP7A1, and its reduced function may lead to the formation and development of cholesterol gallstones by reducing the catabolism of cholesterol into bile acid[59]. The transport of cholesterol from the intestinal lumen into intestinal cells and from bile into liver cells is in the charge of Niemann-Pick C1-like protein 1 (NPC1L1). Reduced activity of the NPC1L1 gene leads to reduced uptake of cholesterol from the lumen to intestinal cells and from bile to liver cells, resulting in increased cholesterol content in the biliary tract, increased absolute cholesterol saturation in the biliary tract, and increased risk of cholesterol gallstone formation[60].
According to a 2019 study, six new gallstone-related or highly related variants were associated with blood cholesterol levels (HNF4A, HNF1A, FUT2, FADS2, MARCH 8, and JMJD1C)[61]. However, the association between these variants and cholesterol gallstone formation and development is unclear. In the future, GWASs will find more new cholesterol-gallstones related variants, and further studies are needed to determine the molecular basis behind these variants[62].
Whatever mechanism causes cholesterol gallstones to form, these processes are slow. Cholesterol gallstones cannot form if the gallbladder is completely emptied several times a day. Therefore, the total or partial extension of bile storage due to impaired gallbladder movement seems to be another important condition for cholesterol gallstone formation. Insufficient gallbladder motility contributes to cholesterol gallstone formation and is impaired under many risk factors for cholesterol gallstone formation, such as pregnant women, obese patients, and their rapid weight loss, diabetes mellitus, and patients receiving total parenteral nutrition[63]. A recent study showed that 78 of 959 patients (8%) who underwent laparoscopic Roux-en-Y gastric bypass (RYGB) or sleeve gastrectomy developed symptomatic gallstone disease within 24 mo[64]. In patients without gallstones before RYGB surgery, ursodeoxycholic acid treatment reduced the occurrence of symptomatic gallstone disease compared with placebo[65]. On an empty stomach, bile drained from the liver is stored in the gallbladder. After eating, bile is discharged by the gallbladder into the duodenum and small intestine. The motor function of the smooth muscle of the gallbladder is mainly regulated by cholecystokinin (CCK), a key gastrointestinal hormone. The release of CCK is mainly caused by the stimulation of dietary lipids and proteins. Insufficient gallbladder contraction during fasting is caused by reduced gallbladder stimulation. Patients using the somatostatin analog octreotide may develop cholesterol gallstones because postprandial CCK release and gallbladder contraction was inhibited by octreotide[9]. Injection of CCK in patients receiving total parenteral nutrition, or the addition of dietary fat to promote the release of CCK in the gastrointestinal tract of people who lose weight quickly, enhances the ability of their gallbladder to contract and prevents the formation of cholesterol gallstones[66,67]. Mice with reduced CCK or damaged CCK-1 receptor genes had slower small bowel movement[68,69], suggesting that CCK not only promotes contraction of gallbladder smooth muscle but also speeds up intestinal transport through a CCK-1 receptor signaling cascade. Loss of the CCK-1 receptor gene in mice led to reduced gallbladder contraction and reduced intestinal transport, which in turn led to cholestasis and increased intestinal cholesterol absorption, ultimately increasing the risk of gallstone formation[69]. In addition, ICLCs are widespread in the gallbladder and bile duct and play a significant role in the regulation of gallbladder contractile motion[70,71]. Previous studies have found that the density of ICLCs in the gallbladder is significantly reduced in patients with cholesterol gallstones, suggesting that decreased gallbladder contraction and cholesterol gallstone formation are closely associated with reduced ICLCs[72-74].
Cholesterol gallstones are common in hepatobiliary surgery and their incidence is increasing. At present, surgery is the preferred treatment for symptomatic cholesterol gallstones disease, but there is still a lack of primary prevention drugs for cholesterol gallstones. The pathogenesis of cholesterol gallstones is extremely complex. We identified the modifiable factors in the pathogenesis of cholesterol gallstones through research to provide strategies for the prevention of cholesterol gallstones disease in high-risk groups. At the same time, more emphasis should be placed on the prevention of cholesterol gallstones, which seems to be a better option than cholecystectomy.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gupta R, India; Hori T, Japan S-Editor: Zhang H L-Editor: Filipodia P-Editor: Zhang H
| 1. | Wang Y, Qi M, Qin C, Hong J. Role of the biliary microbiome in gallstone disease. Expert Rev Gastroenterol Hepatol. 2018;12:1193-1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 2. | Lammert F, Gurusamy K, Ko CW, Miquel JF, Méndez-Sánchez N, Portincasa P, van Erpecum KJ, van Laarhoven CJ, Wang DQ. Gallstones. Nat Rev Dis Primers. 2016;2:16024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 590] [Cited by in RCA: 516] [Article Influence: 57.3] [Reference Citation Analysis (0)] |
| 3. | Rudling M, Laskar A, Straniero S. Gallbladder bile supersaturated with cholesterol in gallstone patients preferentially develops from shortage of bile acids. J Lipid Res. 2019;60:498-505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 4. | Dosch AR, Imagawa DK, Jutric Z. Bile Metabolism and Lithogenesis: An Update. Surg Clin North Am. 2019;99:215-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 5. | Wang F, Wang J, Li Y, Yuan J, Yao P, Wei S, Guo H, Zhang X, Yang H, Wu T, He M. Gallstone Disease and Type 2 Diabetes Risk: A Mendelian Randomization Study. Hepatology. 2019;70:610-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 6. | Camilleri M, Malhi H, Acosta A. Gastrointestinal Complications of Obesity. Gastroenterology. 2017;152:1656-1670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 156] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 7. | Di Ciaula A, Garruti G, Frühbeck G, De Angelis M, de Bari O, Wang DQ, Lammert F, Portincasa P. The Role of Diet in the Pathogenesis of Cholesterol Gallstones. Curr Med Chem. 2019;26:3620-3638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 89] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 8. | Mark-Christensen A, Brandsborg S, Laurberg S, Johansen N, Pachler JH, Thorlacius-Ussing O, Kjær MD, Qvist N, Preisler L, Hillingsø J, Rosenberg J, Jepsen P. Increased Risk of Gallstone Disease Following Colectomy for Ulcerative Colitis. Am J Gastroenterol. 2017;112:473-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Moschetta A, Stolk MF, Rehfeld JF, Portincasa P, Slee PH, Koppeschaar HP, Van Erpecum KJ, Vanberge-Henegouwen GP. Severe impairment of postprandial cholecystokinin release and gall-bladder emptying and high risk of gallstone formation in acromegalic patients during Sandostatin LAR. Aliment Pharmacol Ther. 2001;15:181-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 51] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Barahona Ponce C, Scherer D, Brinster R, Boekstegers F, Marcelain K, Gárate-Calderón V, Müller B, de Toro G, Retamales J, Barajas O, Ahumada M, Morales E, Rojas A, Sanhueza V, Loader D, Rivera MT, Gutiérrez L, Bernal G, Ortega A, Montalvo D, Portiño S, Bertrán ME, Gabler F, Spencer L, Olloquequi J, Fischer C, Jenab M, Aleksandrova K, Katzke V, Weiderpass E, Bonet C, Moradi T, Fischer K, Bossers W, Brenner H, Hveem K, Eklund N, Völker U, Waldenberger M, Fuentes Guajardo M, Gonzalez-Jose R, Bedoya G, Bortolini MC, Canizales-Quinteros S, Gallo C, Ruiz-Linares A, Rothhammer F, Lorenzo Bermejo J. Gallstones, Body Mass Index, C-Reactive Protein, and Gallbladder Cancer: Mendelian Randomization Analysis of Chilean and European Genotype Data. Hepatology. 2021;73:1783-1796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 11. | Boxhoorn L, Voermans RP, Bouwense SA, Bruno MJ, Verdonk RC, Boermeester MA, van Santvoort HC, Besselink MG. Acute pancreatitis. Lancet. 2020;396:726-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 585] [Article Influence: 117.0] [Reference Citation Analysis (0)] |
| 12. | Everhart JE, Ruhl CE. Burden of digestive diseases in the United States Part III: Liver, biliary tract, and pancreas. Gastroenterology. 2009;136:1134-1144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 511] [Cited by in RCA: 582] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 13. | Reynoso-Paz S, Coppel RL, Mackay IR, Bass NM, Ansari AA, Gershwin ME. The immunobiology of bile and biliary epithelium. Hepatology. 1999;30:351-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 84] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 14. | Harvey PR, Upadhya GA, Strasberg SM. Immunoglobulins as nucleating proteins in the gallbladder bile of patients with cholesterol gallstones. J Biol Chem. 1991;266:13996-14003. [PubMed] |
| 15. | Harvey PR, Upadhya GA. A rapid, simple high capacity cholesterol crystal growth assay. J Lipid Res. 1995;36:2054-2058. [PubMed] |
| 16. | Upadhya GA, Harvey PR, Strasberg SM. Effect of human biliary immunoglobulins on the nucleation of cholesterol. J Biol Chem. 1993;268:5193-5200. [PubMed] |
| 17. | Lee SP, LaMont JT, Carey MC. Role of gallbladder mucus hypersecretion in the evolution of cholesterol gallstones. J Clin Invest. 1981;67:1712-1723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 285] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 18. | Lammert F, Wang DQ, Wittenburg H, Bouchard G, Hillebrandt S, Taenzler B, Carey MC, Paigen B. Lith genes control mucin accumulation, cholesterol crystallization, and gallstone formation in A/J and AKR/J inbred mice. Hepatology. 2002;36:1145-1154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | LaMont JT, Smith BF, Moore JR. Role of gallbladder mucin in pathophysiology of gallstones. Hepatology. 1984;4:51S-56S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 69] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Lee KT, Liu TS. Mucin gene expression in gallbladder epithelium. J Formos Med Assoc. 2002;101:762-768. [PubMed] |
| 21. | Wang HH, Afdhal NH, Gendler SJ, Wang DQ. Targeted disruption of the murine mucin gene 1 decreases susceptibility to cholesterol gallstone formation. J Lipid Res. 2004;45:438-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Wang HH, Afdhal NH, Gendler SJ, Wang DQ. Evidence that gallbladder epithelial mucin enhances cholesterol cholelithogenesis in MUC1 transgenic mice. Gastroenterology. 2006;131:210-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Hu FL, Chen HT, Guo FF, Yang M, Jiang X, Yu JH, Zhang FM, Xu GQ. Biliary microbiota and mucin 4 impact the calcification of cholesterol gallstones. Hepatobiliary Pancreat Dis Int. 2021;20:61-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 24. | Andrianifahanana M, Moniaux N, Batra SK. Regulation of mucin expression: mechanistic aspects and implications for cancer and inflammatory diseases. Biochim Biophys Acta. 2006;1765:189-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 123] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 25. | Zen Y, Harada K, Sasaki M, Tsuneyama K, Katayanagi K, Yamamoto Y, Nakanuma Y. Lipopolysaccharide induces overexpression of MUC2 and MUC5AC in cultured biliary epithelial cells: possible key phenomenon of hepatolithiasis. Am J Pathol. 2002;161:1475-1484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 77] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 26. | Ikeda H, Sasaki M, Ishikawa A, Sato Y, Harada K, Zen Y, Kazumori H, Nakanuma Y. Interaction of Toll-like receptors with bacterial components induces expression of CDX2 and MUC2 in rat biliary epithelium in vivo and in culture. Lab Invest. 2007;87:559-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 27. | Finzi L, Barbu V, Burgel PR, Mergey M, Kirkwood KS, Wick EC, Scoazec JY, Peschaud F, Paye F, Nadel JA, Housset C. MUC5AC, a gel-forming mucin accumulating in gallstone disease, is overproduced via an epidermal growth factor receptor pathway in the human gallbladder. Am J Pathol. 2006;169:2031-2041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 28. | Feingold KR, Pollock AS, Moser AH, Shigenaga JK, Grunfeld C. Discordant regulation of proteins of cholesterol metabolism during the acute phase response. J Lipid Res. 1995;36:1474-1482. [PubMed] |
| 29. | Hardardóttir I, Moser AH, Memon R, Grünfeld C, Feingold KR. Effects of TNF, IL-1, and the combination of both cytokines on cholesterol metabolism in Syrian hamsters. Lymphokine Cytokine Res. 1994;13:161-166. [PubMed] |
| 30. | Feingold KR, Hardardottir I, Memon R, Krul EJ, Moser AH, Taylor JM, Grunfeld C. Effect of endotoxin on cholesterol biosynthesis and distribution in serum lipoproteins in Syrian hamsters. J Lipid Res. 1993;34:2147-2158. [PubMed] |
| 31. | Feingold KR, Spady DK, Pollock AS, Moser AH, Grunfeld C. Endotoxin, TNF, and IL-1 decrease cholesterol 7 alpha-hydroxylase mRNA levels and activity. J Lipid Res. 1996;37:223-228. [PubMed] |
| 32. | Memon RA, Moser AH, Shigenaga JK, Grunfeld C, Feingold KR. In vivo and in vitro regulation of sterol 27-hydroxylase in the liver during the acute phase response. potential role of hepatocyte nuclear factor-1. J Biol Chem. 2001;276:30118-30126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 65] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 33. | Wan JF, Chu SF, Zhou X, Li YT, He WB, Tan F, Luo P, Ai QD, Wang Q, Chen NH. Ursodeoxycholic acid protects interstitial Cajal-like cells in the gallbladder from undergoing apoptosis by inhibiting TNF-α expression. Acta Pharmacol Sin. 2018;39:1493-1500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 34. | Maurer KJ, Rao VP, Ge Z, Rogers AB, Oura TJ, Carey MC, Fox JG. T-cell function is critical for murine cholesterol gallstone formation. Gastroenterology. 2007;133:1304-1315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 35. | Whary MT, Morgan TJ, Dangler CA, Gaudes KJ, Taylor NS, Fox JG. Chronic active hepatitis induced by Helicobacter hepaticus in the A/JCr mouse is associated with a Th1 cell-mediated immune response. Infect Immun. 1998;66:3142-3148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 70] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 36. | Fox JG, Beck P, Dangler CA, Whary MT, Wang TC, Shi HN, Nagler-Anderson C. Concurrent enteric helminth infection modulates inflammation and gastric immune responses and reduces helicobacter-induced gastric atrophy. Nat Med. 2000;6:536-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 359] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 37. | Muñoz LE, Boeltz S, Bilyy R, Schauer C, Mahajan A, Widulin N, Grüneboom A, Herrmann I, Boada E, Rauh M, Krenn V, Biermann MHC, Podolska MJ, Hahn J, Knopf J, Maueröder C, Paryzhak S, Dumych T, Zhao Y, Neurath MF, Hoffmann MH, Fuchs TA, Leppkes M, Schett G, Herrmann M. Neutrophil Extracellular Traps Initiate Gallstone Formation. Immunity. 2019;51:443-450.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 123] [Article Influence: 20.5] [Reference Citation Analysis (1)] |
| 38. | Nishimoto S, Fukuda D, Higashikuni Y, Tanaka K, Hirata Y, Murata C, Kim-Kaneyama JR, Sato F, Bando M, Yagi S, Soeki T, Hayashi T, Imoto I, Sakaue H, Shimabukuro M, Sata M. Obesity-induced DNA released from adipocytes stimulates chronic adipose tissue inflammation and insulin resistance. Sci Adv. 2016;2:e1501332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 196] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 39. | Celec P, Janovičová Ĺ, Gurecká R, Koborová I, Gardlík R, Šebeková K. Circulating extracellular DNA is in association with continuous metabolic syndrome score in healthy adolescents. Physiol Genomics. 2021;53:309-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 40. | Frey C, Thorpe C, Abrams G. Gallstone formation in the germ-free mouse. Am J Surg. 1968;115:75-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 41. | Maurer KJ, Ihrig MM, Rogers AB, Ng V, Bouchard G, Leonard MR, Carey MC, Fox JG. Identification of cholelithogenic enterohepatic helicobacter species and their role in murine cholesterol gallstone formation. Gastroenterology. 2005;128:1023-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 115] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 42. | Feng R, Zhang T, Kayani MUR, Wang Z, Shen Y, Su KL, Bielike K, Chen L. Patients with Primary and Secondary Bile Duct Stones Harbor Distinct Biliary Microbial Composition and Metabolic Potential. Front Cell Infect Microbiol. 2022;12:881489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 43. | Wang Q, Jiao L, He C, Sun H, Cai Q, Han T, Hu H. Alteration of gut microbiota in association with cholesterol gallstone formation in mice. BMC Gastroenterol. 2017;17:74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (2)] |
| 44. | Wu T, Zhang Z, Liu B, Hou D, Liang Y, Zhang J, Shi P. Gut microbiota dysbiosis and bacterial community assembly associated with cholesterol gallstones in large-scale study. BMC Genomics. 2013;14:669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 162] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 45. | Thomas LA, Veysey MJ, Murphy GM, Russell-Jones D, French GL, Wass JA, Dowling RH. Octreotide induced prolongation of colonic transit increases faecal anaerobic bacteria, bile acid metabolising enzymes, and serum deoxycholic acid in patients with acromegaly. Gut. 2005;54:630-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 39] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 46. | Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, Liang S, Zhang W, Guan Y, Shen D, Peng Y, Zhang D, Jie Z, Wu W, Qin Y, Xue W, Li J, Han L, Lu D, Wu P, Dai Y, Sun X, Li Z, Tang A, Zhong S, Li X, Chen W, Xu R, Wang M, Feng Q, Gong M, Yu J, Zhang Y, Zhang M, Hansen T, Sanchez G, Raes J, Falony G, Okuda S, Almeida M, LeChatelier E, Renault P, Pons N, Batto JM, Zhang Z, Chen H, Yang R, Zheng W, Yang H, Wang J, Ehrlich SD, Nielsen R, Pedersen O, Kristiansen K. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3971] [Cited by in RCA: 4832] [Article Influence: 371.7] [Reference Citation Analysis (1)] |
| 47. | Hu H, Shao W, Liu Q, Liu N, Wang Q, Xu J, Zhang X, Weng Z, Lu Q, Jiao L, Chen C, Sun H, Jiang Z, Gu A. Gut microbiota promotes cholesterol gallstone formation by modulating bile acid composition and biliary cholesterol secretion. Nat Commun. 2022;13:252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 138] [Article Influence: 46.0] [Reference Citation Analysis (3)] |
| 48. | Petersen C, Bell R, Klag KA, Lee SH, Soto R, Ghazaryan A, Buhrke K, Ekiz HA, Ost KS, Boudina S, O'Connell RM, Cox JE, Villanueva CJ, Stephens WZ, Round JL. T cell-mediated regulation of the microbiota protects against obesity. Science. 2019;365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 270] [Article Influence: 54.0] [Reference Citation Analysis (1)] |
| 49. | Sun H, Warren J, Yip J, Ji Y, Hao S, Han W, Ding Y. Factors Influencing Gallstone Formation: A Review of the Literature. Biomolecules. 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 89] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 50. | Katsika D, Grjibovski A, Einarsson C, Lammert F, Lichtenstein P, Marschall HU. Genetic and environmental influences on symptomatic gallstone disease: a Swedish study of 43,141 twin pairs. Hepatology. 2005;41:1138-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 175] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 51. | Gerloff T, Stieger B, Hagenbuch B, Madon J, Landmann L, Roth J, Hofmann AF, Meier PJ. The sister of P-glycoprotein represents the canalicular bile salt export pump of mammalian liver. J Biol Chem. 1998;273:10046-10050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 719] [Cited by in RCA: 673] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 52. | Smit JJ, Schinkel AH, Oude Elferink RP, Groen AK, Wagenaar E, van Deemter L, Mol CA, Ottenhoff R, van der Lugt NM, van Roon MA. Homozygous disruption of the murine mdr2 P-glycoprotein gene leads to a complete absence of phospholipid from bile and to liver disease. Cell. 1993;75:451-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1080] [Cited by in RCA: 1045] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 53. | Yu L, Hammer RE, Li-Hawkins J, Von Bergmann K, Lutjohann D, Cohen JC, Hobbs HH. Disruption of Abcg5 and Abcg8 in mice reveals their crucial role in biliary cholesterol secretion. Proc Natl Acad Sci U S A. 2002;99:16237-16242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 558] [Cited by in RCA: 547] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 54. | Wang HH, Portincasa P, Liu M, Wang DQ. Genetic Analysis of ABCB4 Mutations and Variants Related to the Pathogenesis and Pathophysiology of Low Phospholipid-Associated Cholelithiasis. Genes (Basel). 2022;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 55. | Buch S, Schafmayer C, Völzke H, Becker C, Franke A, von Eller-Eberstein H, Kluck C, Bässmann I, Brosch M, Lammert F, Miquel JF, Nervi F, Wittig M, Rosskopf D, Timm B, Höll C, Seeger M, ElSharawy A, Lu T, Egberts J, Fändrich F, Fölsch UR, Krawczak M, Schreiber S, Nürnberg P, Tepel J, Hampe J. A genome-wide association scan identifies the hepatic cholesterol transporter ABCG8 as a susceptibility factor for human gallstone disease. Nat Genet. 2007;39:995-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 236] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 56. | Berge KE, von Bergmann K, Lutjohann D, Guerra R, Grundy SM, Hobbs HH, Cohen JC. Heritability of plasma noncholesterol sterols and relationship to DNA sequence polymorphism in ABCG5 and ABCG8. J Lipid Res. 2002;43:486-494. [PubMed] |
| 57. | Acalovschi M, Ciocan A, Mostean O, Tirziu S, Chiorean E, Keppeler H, Schirin-Sokhan R, Lammert F. Are plasma lipid levels related to ABCG5/ABCG8 polymorphisms? Eur J Intern Med. 2006;17:490-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 46] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 58. | Joshi AD, Andersson C, Buch S, Stender S, Noordam R, Weng LC, Weeke PE, Auer PL, Boehm B, Chen C, Choi H, Curhan G, Denny JC, De Vivo I, Eicher JD, Ellinghaus D, Folsom AR, Fuchs C, Gala M, Haessler J, Hofman A, Hu F, Hunter DJ, Janssen HL, Kang JH, Kooperberg C, Kraft P, Kratzer W, Lieb W, Lutsey PL, Darwish Murad S, Nordestgaard BG, Pasquale LR, Reiner AP, Ridker PM, Rimm E, Rose LM, Shaffer CM, Schafmayer C, Tamimi RM, Uitterlinden AG, Völker U, Völzke H, Wakabayashi Y, Wiggs JL, Zhu J, Roden DM, Stricker BH, Tang W, Teumer A, Hampe J, Tybjærg-Hansen A, Chasman DI, Chan AT, Johnson AD. Four Susceptibility Loci for Gallstone Disease Identified in a Meta-analysis of Genome-Wide Association Studies. Gastroenterology. 2016;151:351-363.e28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 59. | Qayyum F, Lauridsen BK, Frikke-Schmidt R, Kofoed KF, Nordestgaard BG, Tybjærg-Hansen A. Genetic variants in CYP7A1 and risk of myocardial infarction and symptomatic gallstone disease. Eur Heart J. 2018;39:2106-2116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 60. | Lauridsen BK, Stender S, Tybjærg-Hansen A. Genetic Variation in NPC1L1 and Risk of Gallstone Disease. J Am Coll Cardiol. 2015;66:1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 61. | Gellert-Kristensen H, Dalila N, Fallgaard Nielsen S, Grønne Nordestgaard B, Tybjaerg-Hansen A, Stender S. Identification and Replication of Six Loci Associated With Gallstone Disease. Hepatology. 2019;70:597-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 62. | Krawczyk M, Müllenbach R, Weber SN, Zimmer V, Lammert F. Genome-wide association studies and genetic risk assessment of liver diseases. Nat Rev Gastroenterol Hepatol. 2010;7:669-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 63. | van Erpecum KJ, Venneman NG, Portincasa P, Vanberge-Henegouwen GP. Review article: agents affecting gall-bladder motility--role in treatment and prevention of gallstones. Aliment Pharmacol Ther. 2000;14 Suppl 2:66-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 64. | Haal S, Guman MSS, Bruin S, Schouten R, van Veen RN, Fockens P, Dijkgraaf MGW, Hutten BA, Gerdes VEA, Voermans RP. Risk Factors for Symptomatic Gallstone Disease and Gallstone Formation After Bariatric Surgery. Obes Surg. 2022;32:1270-1278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 65. | Haal S, Guman MSS, Boerlage TCC, Acherman YIZ, de Brauw LM, Bruin S, de Castro SMM, van Hooft JE, van de Laar AWJM, Moes DE, Schouten M, Schouten R, van Soest EJ, van Veen RN, de Vries CEE, Fockens P, Dijkgraaf MGW, Gerdes VEA, Voermans RP. Ursodeoxycholic acid for the prevention of symptomatic gallstone disease after bariatric surgery (UPGRADE): a multicentre, double-blind, randomised, placebo-controlled superiority trial. Lancet Gastroenterol Hepatol. 2021;6:993-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 66. | Sitzmann JV, Pitt HA, Steinborn PA, Pasha ZR, Sanders RC. Cholecystokinin prevents parenteral nutrition induced biliary sludge in humans. Surg Gynecol Obstet. 1990;170:25-31. [PubMed] |
| 67. | Gebhard RL, Prigge WF, Ansel HJ, Schlasner L, Ketover SR, Sande D, Holtmeier K, Peterson FJ. The role of gallbladder emptying in gallstone formation during diet-induced rapid weight loss. Hepatology. 1996;24:544-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 60] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 68. | Wang HH, Liu M, Portincasa P, Tso P, Wang DQ. Lack of endogenous cholecystokinin promotes cholelithogenesis in mice. Neurogastroenterol Motil. 2016;28:364-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 69. | Wang DQ, Schmitz F, Kopin AS, Carey MC. Targeted disruption of the murine cholecystokinin-1 receptor promotes intestinal cholesterol absorption and susceptibility to cholesterol cholelithiasis. J Clin Invest. 2004;114:521-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 67] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 70. | Pasternak A, Gajda M, Gil K, Matyja A, Tomaszewski KA, Walocha JA, Kulig J, Thor P. Evidence of interstitial Cajal-like cells in human gallbladder. Folia Histochem Cytobiol. 2012;50:581-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 71. | Ahmadi O, Nicholson Mde L, Gould ML, Mitchell A, Stringer MD. Interstitial cells of Cajal are present in human extrahepatic bile ducts. J Gastroenterol Hepatol. 2010;25:277-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 72. | Pasternak A, Gil K, Gajda M, Tomaszewski KA, Matyja A, Walocha JA. Interstitial cajal-like cell: a new player in cholelithiasis? Am J Gastroenterol. 2014;109:603-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 73. | Franks I. Gallbladder: Loss of interstitial Cajal-like cells in the gallbladder might contribute to gallstone formation. Nat Rev Gastroenterol Hepatol. 2012;9:689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 74. | Pasternak A, Gil K, Matyja A, Gajda M, Sztefko K, Walocha JA, Kulig J, Thor P. Loss of gallbladder interstitial Cajal-like cells in patients with cholelithiasis. Neurogastroenterol Motil. 2013;25:e17-e24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |