Published online Sep 27, 2022. doi: 10.4240/wjgs.v14.i9.1037
Peer-review started: March 18, 2022
First decision: May 12, 2022
Revised: May 25, 2022
Accepted: August 14, 2022
Article in press: August 14, 2022
Published online: September 27, 2022
Processing time: 188 Days and 8.1 Hours
Acute lung injury (ALI) after liver transplantation (LT) may lead to acute respiratory distress syndrome, which is associated with adverse postoperative outcomes, such as prolonged hospital stay, high morbidity, and mortality. Therefore, it is vital to maintain hemodynamic stability and optimize fluid management. However, few studies have reported cardiac output-guided (CO-G) management in pediatric LT.
To investigate the effect of CO-G hemodynamic management on early post
A total of 130 pediatric patients scheduled for elective living donor LT were enrolled as study participants and were assigned to the control group (65 cases) and CO-G group (65 cases). In the CO-G group, CO was considered the target for hemodynamic management. In the control group, hemodynamic management was based on usual perioperative care guided by central venous pressure, continuous invasive arterial pressure, urinary volume, etc. The primary outcome was early postoperative ALI. Secondary outcomes included other early post
The incidence of early postoperative ALI was 27.7% in the CO-G group, which was significantly lower than that in the control group (44.6%) (P < 0.05). During the surgery, the incidence of postreperfusion syndrome was lower in the CO-G group (P < 0.05). The level of intraoperative positive fluid transfusions was lower and the rate of dobutamine use before portal vein opening was higher, while the usage and dosage of epinephrine during portal vein opening and vasoactive inotropic score after portal vein opening were lower in the CO-G group (P < 0.05). Compared to the control group, serum inflammatory factors (interleukin-6 and tumor necrosis factor-α), cardiac troponin I, and N-terminal pro-brain natriuretic peptide were lower in the CO-G group after the operation (P < 0.05).
CO-G hemodynamic management in pediatric living-donor LT decreases the incidence of early postoperative ALI due to hemodynamic stability through optimized fluid management and ap
Core Tip: This is the first randomized controlled trial to evaluate the effect of cardiac output (CO)-guided hemodynamic therapy in pediatric liver recipients. In this study, hemodynamic parameters, including CO, stroke volume index, stroke volume variation, and the maximum increase in the speed of intraventricular pressure (dp/dtmax) obtained through the pressure recording analytical method monitoring were used to guide intraoperative hemodynamic management. The incidence of postoperative acute liver injury was significantly lower in the interventional group. Moreover, the inflammatory factors (interleukin-6 and tumor necrosis factor-α), cardiac troponin I, and N-terminal pro-brain natriuretic peptide levels decreased faster in the intervention group.
- Citation: Dou XJ, Wang QP, Liu WH, Weng YQ, Sun Y, Yu WL. Effect of cardiac output - guided hemodynamic management on acute lung injury in pediatric living donor liver transplantation. World J Gastrointest Surg 2022; 14(9): 1037-1048
- URL: https://www.wjgnet.com/1948-9366/full/v14/i9/1037.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v14.i9.1037
Pediatric liver transplantation (LT) is a life-saving procedure for children with end-stage liver disease caused by biliary atresia or progressive familial intrahepatic cholestasis[1]. The number of LTs performed globally has been reported to be 4-9 per million people < 18 years, with a 10-year survival rate of > 80%[1-3]. The incidence of post-LT acute lung injury (ALI) has been reported to vary between 34.2% and 77.8%[4,5]. ALI may lead to acute respiratory distress syndrome (ARDS), which is associated with adverse postoperative outcomes, such as prolonged hospital stay, high morbidity, and mortality[6]. ARDS is often caused by hemodynamic instability during surgery, which results in liver hypoperfusion and ischemia-reperfusion injury, exaggerating the inflammatory process[7]. Additionally, hemodynamic instability accompanied by excessive administration of fluids and blood products leads to fluid imbalance during LT. Clinical studies have demonstrated that intraoperative fluid overload is the primary risk factor for postoperative pulmonary complications (PPCs)[8]. Effective fluid management strategies can reduce the occurrence of PPCs[9].
In the early stages after LT, ALI may prolong the intubation time and increase the risk of systemic infectious complications. Prolonged mechanical ventilation due to refractory respiratory failure is an extremely morbid event and a marker of poor recipient recovery that predisposes a recipient to long-term ventilator dependency and predicts further complications. Several factors are involved in the onset of postoperative ALI, among which intraoperative hemodynamic instability and fluid overload are the most important[10].
Pediatric patients with poor oxygen reserve capacity are vulnerable to ischemia and hypoxia, leading to ALI. Therefore, it is vital to maintain hemodynamic stability and optimize fluid management. A study on pediatric kidney transplantation showed that the use of the cardiac output-guided (CO-G) algorithm led to excellent renal results, with a trend toward less fluids in favor of norepinephrine[11]. However, few studies have reported CO-G management in pediatric LT. CO monitoring is extremely difficult and limited due to the anatomical characteristics and biomaterial technology in pediatric liver transplant patients. The pressure recording analytical method (PRAM) is a minimally invasive hemodynamic monitoring method that calculates hemodynamic parameters, with the advantages of being invasive, not requiring calibration, and suitable for pediatric patients weighing < 20 kg compared to other devices[12]. In this study, a randomized controlled trial was designed to evaluate the effect of CO-G algorithm management on reducing ALI events after pediatric LT and intraoperative hemodynamic stability with PRAM.
This was a randomized controlled trial conducted at Tianjin First Central Hospital. This study was approved by the Ethics Committee of Tianjin First Center Hospital in China (Approval Number: 2019N180KY), and written informed consent was obtained from eligible guardians. The clinical trial registration number is ChiCTR1900026016. The inclusion criteria were as follows: (1) Pediatric liver recipients 5-24 mo of age; (2) American Society of Anesthesiologists physical status III or IV; and (3) Living donation. The exclusion criteria were as follows: (1) Contraindications to arterial puncture and cannulation; (2) Preoperative incomplete data; (3) Preoperative severe cardiac, renal, and other viral organ failure before LT; and (4) Sepsis and/or pulmonary complications, including pneumonia, atelectasis, pulmonary edema, pleural effusion, and ARDS within 2 wk before surgery. Every case of transplantation passed the ethical review and approval of the Tianjin First Center Hospital.
Patients enrolled in this study were routinely monitored for heart rate (HR), non-invasive blood pressure, pulse oximetry, and electrocardiography. Anesthesia was induced using scopolamine (0.01 mg/kg), midazolam (0.15 mg/kg), etomidate (0.15 mg/kg), fentanyl (2-5 μg/kg), and vecuronium (0.2 mg/kg) to maintain analgesia, muscle relaxation, and sedation. After intubation, mechanical ventilation was performed with a fraction of inspired oxygen (FiO2) of 50%-60%, tidal volume of 8-10 mL/kg, respiratory rate of 20-28/min, an inspiration-to-expiration ratio of (1.0:1.5)-2.0 min, an inspiration-to-expiration ratio of (1.0:1.5)-2.0, and a postapneic end-tidal carbon dioxide pressure of 30-35 mmHg (1 mmHg = 0.133 kPa). Anesthesia maintenance included intravenous infusion of propofol (9-15 mg/kg/h), intermittent intravenous fentanyl (1-3 μg/kg), and intravenous infusion of atracurium besylate (1-2 μg/kg/h).
The operative procedure was performed using both the caval replacement and piggyback techniques. Reperfusion of the liver graft started with opening of the portal vein, followed by opening of the artery. After arterial reperfusion, the bile duct was connected to the recipient’s bile duct (choledocho-choledochostomy) or to a small bowel loop (hepaticojejunostomy). A back table biopsy of the donor liver was performed before implantation.
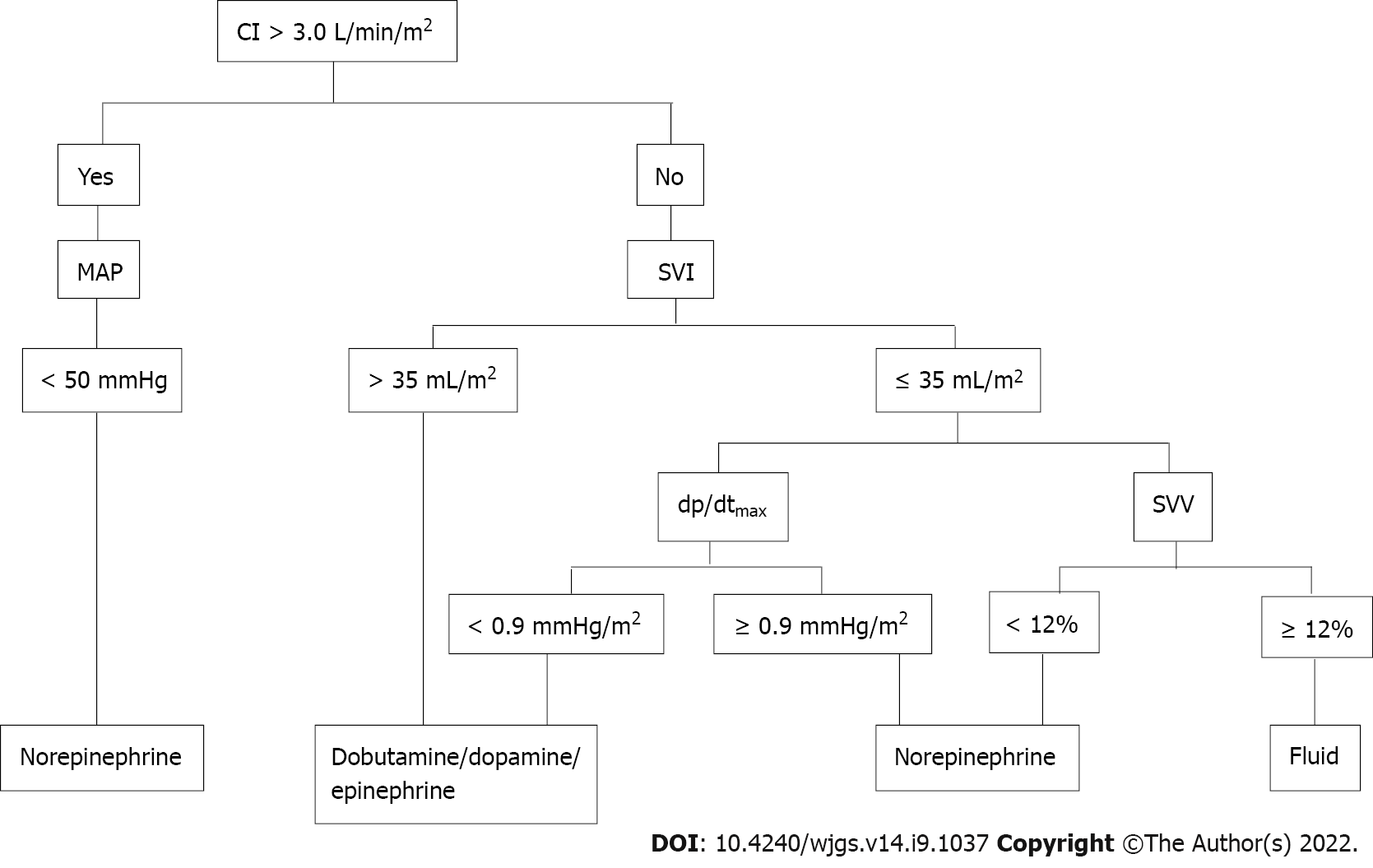
The central venous pressure (CVP) was monitored continuously with a three-lumen central venous catheter placed using ultrasound-guided right internal jugular vein puncture and arterial pressure was monitored invasively in both groups using a catheter placed in the radial artery. The mean arterial blood pressure (MAP), HR, cardiac index (CI), stroke volume index (SVI), stroke volume variation (SVV), and left ventricular contractility index, which is the maximum increase in the speed of intraventricular pressure (dp/dtmax), were continuously monitored through PRAM (Most Care monitoring system; Vytech Healthcare, Padova, Italy) via a pressure catheter (Pulsion Medical Systems, Munich, Germany) in the CO-G group.
Hemodynamic management included fluid transfusion and use of vasopressors and/or inotropes: (1) Fluid management protocol: In the control group, fluid management was implemented mainly according to CVP, urine volume, bleeding, etc. CVP was maintained at a level of 6-12 mmHg, and the urine volume at ≥ 20 mL/h. If the urine volume was < 20 mL/h and/or CVP < 6 mmHg, 4% albumin or crystalloid was infused to expand the volume; if the urine volume was < 20 mL/h and/or CVP > 12 mmHg, 0.5 g/kg furosemide was also administered to decrease fluid load. In the CO-G group, fluid was infused at a rate of 10 mL/kg/h to maintain SVV at 12%-15%. If SVV was > 12%, 4% albumin or crystalloid was administered in combination with CI, SVI, and other parameters; and (2) Vasopressor and/or inotrope protocol: In the control group, if MAP was < 50 mmHg, norepinephrine or dopamine was pumped intravenously, and if MAP fell rapidly below 30 mmHg after the opening of the portal vein, rehydration and/or epinephrine of 1-5 mg/kg was administrated. In the CO-G group, the admi
Venous blood (3 mL) was collected from the right internal jugular catheter and placed into vacuum tubes containing sodium heparin. Blood samples were collected at four time points: Immediately before the induction of general anesthesia (baseline, T0), at the end of surgery (T1), 1 d after surgery (T2), and 3 d after surgery (T3). The samples were then placed in dry tubes and centrifuged. The serum was removed and stored at -80 °C until analysis. The levels of serum inflammatory factors interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), cardiac troponin I (cTnI), and N-terminal pro-brain natriuretic peptide precursor (NT-pro-BNP) were analyzed at four time points. Wuhan Huamei Biological Technology Company (Wuhan, China) was used to construct the reaction standard curves. The protein levels were calculated by comparing the optical density values of the samples with the standard curve.
The following patients and preoperative variables were assessed: Patient characteristics, including age, weight, pediatric end-stage liver disease, and graft characteristics, including graft mass, graft-to-recipient body weight ratio, cold ischemia time of the graft, and preoperative laboratory test results. The intraoperative hemodynamic parameters included baseline values, the maximum and minimum values of HR, MAP, CVP, and the incidence of postreperfusion syndrome (PRS, defined as a sudden drop in MAP of ≥ 30% within 1-5 min of reperfusion)[13], and hemodynamic management, including transfusion of red blood cells, fresh frozen plasma, and fluids (colloids and crystalloids), usage of vasopressor or inotrope agents, and vasoactive drug score (VIS) [VIS = dopamine dose (μg/kg/min) + dobutamine dose (μg/kg/min) + 100 × epinephrine dose (μg/kg/min) + 10000 × vasopressin dose (μg/kg/min) + 100 × norepinephrine dose (μg/kg/h) + 100 × milrinone dose (μg/kg/min)][14]. The postoperative variables included the occurrence of ALI and pulmonary complications in the first week after surgery, duration of mechanical ventilation, intense care unit (ICU) stay, incidence of readmission to the ICU for pulmonary complications, hospital stay, and in-hospital mortality.
ALI was defined according to the following criteria[15]: (1) Acute onset; (2) PaO2/FiO2 < 300; (3) Pulmonary artery wedge pressure < 18 mmHg without clinical evidence of left atrial hypertension; and (4) Bilateral infiltrates on chest radiography.
The primary outcome was early postoperative ALI. The secondary outcomes included early PPCs, ICU stay, readmission to the ICU for pulmonary complications, hospital stay, in-hospital mortality, and intraoperative hemodynamic stability.
Sample size: The incidence of ALI in children after LT in the control and intervention groups was 50% and 25%, respectively, based on previous reports[3,4]. The α-error was set to 0.05, β-error to 80%, and the ratio to 1:1. PASS 15 (NCSS, LLC. Kaysville, UT, United States) was used to calculate the sample size, and the results showed that at least 58 patients should be included per group, with an expected dropout rate of 10%.
Randomization and blinding: Pediatric patients were randomly assigned to the CO-G hemodynamic therapy algorithm (CO-G group) and the control group by a computer-generated random number system and individually sealed in envelopes. One investigator created computer-generated randomization codes and enrolled participants in accordance with the approved study protocol (Chi
Outcome analyses were performed using SPSS software package (SPSS; IBM. Corp., Armonk, NY, United States). The Kolmogorov-Smirnov test was used to analyze the distribution of the data. The results are presented as the mean (SD), median (second quartile, third quartile), or number of patients. The patient characteristics and perioperative variables were compared using an independent t-test or Fisher’s exact test, as appropriate. Changes in the above variables in the group over time were analyzed using repeated ANOVA, followed by an appropriate post hoc test. Categorical data were compared using the chi-squared test or Fisher’s exact method. The results were evaluated within a 95% reliability index (P < 0.05).
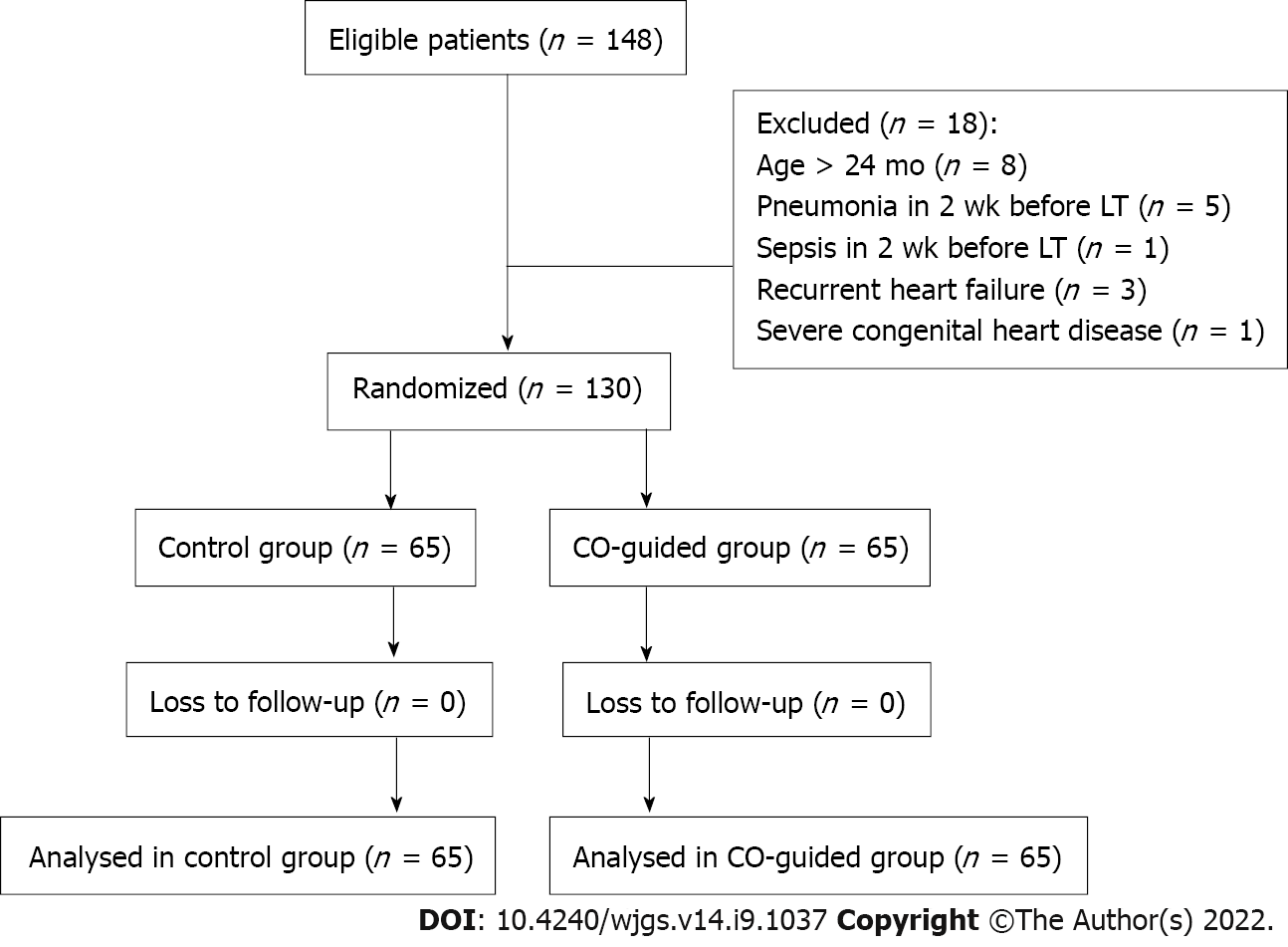
A total of 148 patients were screened from December 2019 to October 2020, and 130 patients were enrolled and analyzed in this study. Among whom, 65 patients were randomly allocated to the CO-G group and 65 to the control group (Figure 2, Table 1). The patient characteristics were similar between the study groups (Table 1).
| Variables | Control group (n = 65) | CO-G group (n = 65) | P value |
| Age, mo | 7.5 (5.9, 9.6) | 7.0 (6.0, 8.5) | 0.390 |
| Gender (boy/girl), n | 31/34 | 33/32 | 0.726 |
| Weight of receptor, kg | 7.5 (6.5, 9.0) | 7.4 (6.5, 8.0) | 0.383 |
| Mass of graft, g | 220.5 ± 40.7 | 218.8 ± 39.5 | 0.736 |
| GRWR, % | 3.10 ± 0.76 | 3.03 ± 0.76 | 0.631 |
| Pretransplant PELD score | 16.5 ± 3.2 | 17.2 ± 3.5 | 0.549 |
| Pretransplant INR, IU | 1.77 ± 0.86 | 1.91 ± 0.67 | 0.300 |
| Pretransplant PTA, % | 57.5 ± 20.7 | 51.4 ± 20.2 | 0.095 |
| Pretransplant PT, s | 20.2 ± 9.9 | 21.5 ± 8.7 | 0.454 |
| Pretransplant WBC, 109/L | 13.3 ± 6.3 | 12.2 ± 5.6 | 0.331 |
| Pretransplant hemoglobin, g/L | 90.4 ± 13.6 | 86.8 ± 12.8 | 0.116 |
| Pretransplant platelets, 1012/L | 194.3 ± 87.0 | 207.3 ± 72.1 | 0.355 |
| Pretransplant albumin, g/L | 34.1 ± 4.4 | 35.6 ± 5.9 | 0.088 |
| Pretransplant total bilirubin, μmol/L | 271.6 ± 128.3 | 282.9 ± 122.4 | 0.607 |
| Pretransplant creatinine, μmol/L | 12.7 ± 3.5 | 11.8 ± 3.0 | 0.099 |
| Graft cold ischemia time, min | 65.9 ± 25.7 | 60.2 ± 14.8 | 0.081 |
| Anhepatic time, min | 44.4 ± 11.5 | 47.1 ± 15.8 | 0.267 |
| Operation time, min | 545.0 ± 44.9 | 559.5 ± 49.6 | 0.083 |
| Mechanical ventilation after operation, h | 3.00 (2.25, 4.50) | 2.75 (2.00, 3.88) | 0.789 |
The incidence of early postoperative ALI was 27.7% in the CO-G group, which was lower than that in the control group (44.6%) (P < 0.05) (Table 2). There were no significant differences in other pulmonary complications and ICU stay, readmission to the ICU for pulmonary complications, hospital stay, and in-hospital mortality (Table 2).
| Control group (n = 65) | CO-G group (n = 65) | P value | |
| Primary outcomes | |||
| ALI, n (%) | 29 (44.6) | 18 (27.7) | 0.045 |
| Others | |||
| Pneumonia, n (%) | 12 (18.5) | 8 (12.3) | 0.634 |
| Atelectasis, n (%) | 18 (27.7) | 12 (18.5) | 0.687 |
| ARDS, n (%) | 6 (9.2) | 4 (6.2) | 0.742 |
| Refractory heart failure, n (%) | 3 (4.6) | 1 (1.5) | 0.612 |
| Readmission to ICU for pulmonary complications, n (%) | 3 (4.6) | 2 (3.1) | 1.000 |
| ICU stay, d | 2 (2, 3) | 2 (2, 3) | 0.200 |
| Hospital stay, d | 28 (22, 39) | 27 (20, 37) | 0.450 |
| In-hospital mortality, n (%) | 2 (3.1) | 0 | 0.476 |
Compared to the control group, intraoperative fluid transfusion (865.5 ± 153.1 mL vs 1222.7 ± 381.9 mL, P < 0.001), and positive fluid balance (598.8 ± 320.7 mL vs 1021.4 ± 467.9 mL, P < 0.001) were lower in the CO-G group. The utilization of dobutamine before portal vein opening was higher, whereas the usage and dosage of epinephrine during portal vein opening and VIS after portal vein opening [2 (2-3) vs 3 (2-7), P < 0.05] were lower in the CO-G group. The peak value of CVP was lower (9.46 ± 1.66 mmHg vs 11.64 ± 2.1 mmHg, P < 0.001) while the bottom value of MAP was higher (43.3 ± 7.4 mmHg vs 34.9 ± 5.5 mmHg, P < 0.001) in CO-G group. The incidence of PRS in the CO-G group was lower than that in the control group (33.8% vs 53.8%, P = 0.022) (Table 3).
| Control group (n = 65) | CO-G group (n = 65) | P value | |
| Preoperative hemodynamic parameters | |||
| HR, bpm/min | 110 ± 12 | 108 ± 11 | 0.325 |
| MAP, mmHg | 60.3 ± 8.0 | 61.6 ± 9.5 | 0.382 |
| CVP, cmH2O | 6.08 ± 1.37 | 5.79 ± 1.44 | 0.241 |
| Intraoperative hemodynamic parameters | |||
| HRH, bpm/min | 123 ± 15 | 125 ± 18 | 0.317 |
| HRL, bpm/min | 82 ± 8 | 86 ± 8 | 0.003 |
| MAPH, mmHg | 72.3 ± 8.8 | 71.7 ± 10.4 | 0.531 |
| MAPL, mmHg | 34.9 ± 5.5 | 43.3 ± 7.4 | < 0.001 |
| CVPH, cmH2O | 11.64 ± 2.1 | 9.46 ± 1.66 | < 0.001 |
| CVPL, cmH2O | 4.17 ± 1.49 | 3.55 ± 1.34 | 0.013 |
| Intraoperative hemodynamic events | |||
| PRS, n (%) | 35 (53.8) | 22 (33.8) | 0.022 |
| Malignant ventricular arrhythmia, n (%) | 3 (5) | 2 (3.1) | 1.000 |
| Cardiac arrest, n (%) | 1 (1.5) | 0 | 1.000 |
| Intraoperative hemodynamic management | |||
| Intraoperative blood transfusions, U | 2.5 (2, 3) | 2.0 (1.5, 2.5) | 0.821 |
| Intraoperative frozen plasma transfusions, mL | 0 (0, 200) | 0 (0, 110) | 0.751 |
| Intraoperative fluid transfusions, mL | 1222.7 ± 381.9 | 865.5 ± 153.1 | < 0.001 |
| Intraoperative bleeding volume, mL | 300 (200, 500) | 300 (200, 400) | 0.543 |
| Intraoperative urinary volume, mL | 300 (277.5, 400) | 400 (200, 510) | 0.416 |
| Positive fluid balance, mL | 1021.4 ± 467.9 | 598.8 ± 320.7 | < 0.001 |
| VIS before portal vein opening | 2 (2, 5) | 3 (2, 6.25) | 0.565 |
| During portal vein opening | |||
| Bolus injection of epinephrine, n (%) | 30 (46.2) | 18 (27.7) | 0.029 |
| Bolus dosage of epinephrine, μg | 3 (2, 5) | 2.5 (1.75, 4.25) | 0.030 |
| VIS after portal vein opening | 3 (2, 7) | 2 (2, 3) | 0.049 |
In both groups, the levels of inflammatory factors (IL-6 and TNF-α) and cTnI increased during the operation, decreased gradually during the following 3 d postoperatively, and returned to preoperative levels (Table 4). The NT-proBNP levels showed the same trend (Table 4). For group comparisons, at T1 and T2, the values of IL-6, TNF-α, and cTnI were significantly lower in the CO-G group (Table 4). At T1, T2, and T3, the NT-proBNP levels were significantly lower in the CO-G group (Table 4).
| IL-6 (pg/mL) | TNF-α (pg/mL) | cTnI (ug/L) | NT-proBNP (ng/L) | ||
| Control group (n = 65) | T0 | 78.9 ± 23.2 | 87.5 ± 25.6 | 0.032 ± 0.015 | 556.6 ± 251.2 |
| T1 | 170.4 ± 42.3b | 175.3 ± 43.1b | 0.383 ± 0.166b | 1012.4 ± 568.8b | |
| T2 | 126.2 ± 33.6b | 129.5 ± 35.2b | 0.182 ± 0.067b | 866.0 ± 283.6b | |
| T3 | 80.7 ± 23.2 | 92.8 ± 26.8 | 0.030 ± 0.011 | 667.4 ± 247.7 | |
| CO-G group (n = 65) | T0 | 80.6 ± 22.5 | 83.2 ± 23.8 | 0.029 ± 0.012 | 562.2 ± 195.8 |
| T1 | 145.5 ± 34.5a,b | 156.7 ± 36.1a,b | 0.255 ± 0.128a,b | 876.7 ± 268.2a,b | |
| T2 | 108.6 ± 24.9a,b | 115.5 ± 25.6a,b | 0.116 ± 0.070a,b | 594.0 ± 163.3a,b | |
| T3 | 78.6 ± 21.9 | 86.2 ± 22.6 | 0.028 ± 0.011 | 462.6 ± 154.5a,b |
To the best of our knowledge, this is the first randomized controlled trial to evaluate the effect of CO-guided hemodynamic therapy in pediatric liver recipients. In this study, hemodynamic parameters, including CO, SVV, SVI, and dp/dtmax, obtained through PRAM monitoring were used to guide intraoperative hemodynamic management. The incidence of postoperative ALI was significantly lower in the interventional group than in the control group. Moreover, the inflammatory factors of IL-6, TNF-α, and cTnI decreased faster in the intervention group than in the control group.
The incidence of ALI in the control group was 44.6%, which was close to that used in the sample size calculation (50%). These results are similar to those of previous studies. Hong et al[4] reported that the rate of ALI was 34.6% in adult LT, while Yao et al[5] showed that the incidence of ALI in a rat LT model was 77.8%. CO-G interventions significantly decreased ALI occurrence after pediatric LT. This might be due to more stable hemodynamic parameters, which can mitigate ischemia-reperfusion injury, as well as optimized vasopressor use and fluid management in the CO-G group.
Inflammatory lung liver interactions, and the activation of nuclear factor-kappa B in particular, may be implicated in the pathogenesis of permeability-type pulmonary edema[16]. It is well accepted known that the inflammatory response is involved in the progression of ALI and that cytokines, such as TNF-α, IL-1β, and IL-6, play important roles in the massive inflammatory response that is a hallmark feature of ALI[17]. In contrast, IL-4 and IL-10 seem to exert protective roles[18].
Therefore, in the present study, we selected TNF-α and IL-6, which are typical factors that reflect inflammation and oxidative stress in the lungs. The results showed that the inflammatory factors mentioned above were elevated from the end of the operation and returned to preoperative levels 3 d after surgery. Compared with the control group, TNF-α and IL-6 levels were significantly lower from the end of the operation to 1 d after surgery in the CO-G group, indicating that CO-G hemodynamic therapy can attenuate lung inflammation during LT.
Several triggering conditions, including bleeding, blood transfusion, and ischemia-reperfusion, can exaggerate the inflammatory process of ALI. Among them, liver ischemia-reperfusion may be the most notable factor. The greatest hemodynamic disturbance in LT is defined as PRS, which occurs during reperfusion of the donated liver after unclamping of the portal vein. PRS is characterized by marked decreases of > 30% in MAP lasting > 1 min within 5 min after reperfusion and occurring with an incidence of 12.1%-42%[19]. A dramatic drop in blood pressure and myocardial inhibition are manifestations, but are also risk factors for PRS[20]. It is noteworthy that the intraoperative stabilization of arterial pressure through the preventive use of vasopressors during the reperfusion phase is capable of decreasing the incidence of PRS[21]. In our study, the incidence of PRS in the CO-G group was lower than that in the control group, which was attributed to the appropriate cardiotonic and optimized vasopressor by the continuous monitoring of CO.
In our study, the use of dobutamine before portal vein opening was higher than that in the control group, whereas the usage and dosage of epinephrine during portal vein opening and VIS after portal vein opening were lower in the CO-G group. CO-G hemodynamic therapy can reduce hemodynamic fluctuations and prevent the occurrence of PRS by continuously monitoring the intraoperative CO, which can consistently summarize cardiac function, and aid to the appropriate administration of vasopressors and inotropes.
Myocardial injury commonly occurs in LT[22], which leads to arrhythmias and myocardial depression, severely affecting circulatory stability and aggravating ischemia-reperfusion injury. cTnI is currently recognized as a sensitive and specific gold standard for reflecting the degree of myocardial injury, and mildly elevated cTnI levels (≥ 0.04 ng/mL) are strongly associated with postoperative mortality[23]. Sheng et al[24] demonstrated that intraoperative cTnI elevation (≥ 0.07 ng/mL) was a significant prognostic risk factor in ALI after pediatric living-donor LT for children with biliary atresia. NT-proBNP is an early and reliable predictor of myocardial dysfunction onset[25]. BNP levels positively correlated with left ventricular systolic function and required inotropic support[26].
In our study, we analyzed cTnI and NT-pro-BNP levels to identify myocardial injury and cardiac dysfunction. The results showed that cTnI and NT-pro-BNP levels were elevated from the end of the operation and returned to preoperative levels 3 d after surgery. NT-pro-BNP level was lower at 3 d after surgery than at the preoperative level. Compared to the control group, the values of cTnI were significantly lower at the end of surgery and 1 d after surgery in the CO-G group. In the CO-G group, the NT-pro-BNP values from the end of surgery to 3 d after surgery were all lower than those in the control group, indicating that CO-G hemodynamic therapy can attenuate myocardial injury and cardiac volume load, which could be helpful in circulatory stability and attenuation of pulmonary edema.
Intraoperative fluid overload can exacerbate pulmonary edema and heart failure, thereby increasing the duration of postoperative mechanical ventilation, pulmonary infection, and mortality. Previous intraoperative volume management is often achieved through empirical rehydration and CVP-directed management; CVP is a pressure-based index that cannot accurately reflect volume status, and CVP-directed fluid management can result in volume overload[27,28]. Compared to pressure-monitoring metrics, volume-monitoring metrics better reflect volume status to guide hemodynamic management, and SVV < 12% and PPV < 13% are more accurate in predicting fluid responsiveness[29]. Shin et al[30] showed that the sensitivity of SVV for monitoring blood volume changes during the neohepatic period of LT was 89%, with a specificity of 80%, which was significantly better than that of CVP. In addition, studies have shown that CO-G fluid management reduces postoperative complications by 20% to 30% compared with any infusion strategy[31]. In this study, CO-directed fluid management combined with SVI and SVV showed that intraoperative fluid transfusion and maximum CVP were significantly lower in the CO-G group than in the control group. The incidence of postoperative ALI was also significantly lower, suggesting that CO-G hemodynamic management can reduce fluid overload, decrease the occurrence of pulmonary edema, stabilize cardiopulmonary function, control CVP, and reduce the occurrence of ALI.
As this was a single center study, a multicenter study with other monitoring indicators is needed for further analysis.
CO-G hemodynamic management in pediatric living donor LT can decrease the incidence of early postoperative ALI due to hemodynamic stability through optimized fluid management and appropriate administration of vasopressors and inotropes achieved by continuous monitoring of CO.
Acute lung injury (ALI) post-liver transplantation (LT) may lead to acute respiratory distress syndrome, which is associated with adverse postoperative outcomes, such as prolonged hospital stay, high morbidity, and mortality. Therefore, it is vital to maintain hemodynamic stability and optimize fluid management. However, few studies have reported cardiac output-guided (CO-G) management in pediatric LT.
In this study, a randomized controlled trial was designed to evaluate the effect of CO-G algorithm management on reducing ALI events after pediatric LT and intraoperative hemodynamic stability with pressure recording analytical method (PRAM).
To investigate the effect of CO-G hemodynamic management in pediatric living donor LT on early postoperative ALI and its influence on hemodynamic stability during surgery.
A total of 130 pediatricians scheduled for elective living donor LT were enrolled as study participants and were assigned to the control group (65 cases) and CO-G group (65 cases). In the CO-G group, CO was considered the target for hemodynamic management. In the control group, hemodynamic management was based on usual perioperative care guided by central venous pressure, continuous invasive arterial pressure, urinary volume, etc. The primary outcome was early postoperative ALI. Secondary outcomes included other early postoperative pulmonary complications, readmission to the intense care unit (ICU) for pulmonary complications, ICU stay, hospital stay, and in-hospital mortality.
The incidence of early postoperative ALI was 27.7% in the CO-G group, which was significantly lower than that in the control group (44.6%) (P < 0.05). During the surgery, the incidence of postreperfusion syndrome was lower in the CO-G group (P < 0.05). The level of intraoperative positive fluid transfusions was lower and the rate of dobutamine use before portal vein opening was higher, while the usage and dosage of epinephrine when portal vein opening and vasoactive inotropic score after portal vein opening were lower in the CO-G group (P < 0.05). Compared to the control group, the serum inflammatory factors interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), cardiac troponin I (cTnI), and N-terminal-pro hormone BNP in the CO-G group were lower after the operation (P < 0.05).
CO-G hemodynamic management in pediatric living-donor LT decreased the incidence of early postoperative ALI, which is considered to benefit from hemodynamic stability through optimized fluid management and appropriate administration of vasopressors and inotropes by continuous monitoring of CO.
This is the first randomized controlled trial to evaluate the effect of CO-G hemodynamic therapy in pediatric liver recipients. In this study, hemodynamic parameters, including CO, stroke volume index, stroke volume variation, and the maximum increase in the speed of intraventricular pressure (dp/dtmax), obtained through the PRAM monitoring were used to guide intraoperative hemodynamic management. The incidence of postoperative ALI was significantly lower in the interventional group. Moreover, the inflammatory factors of IL-6, TNF-α, cTnI, decreased faster in the intervention group.
Provenance and peer review: Unsolicited article; Externally peer reviewed
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sugawara Y, Japan; Yasuhiko S, Japan S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Bourdeaux C, Brunati A, Janssen M, de Magnée C, Otte JB, Sokal E, Reding R. Liver retransplantation in children. A 21-year single-center experience. Transpl Int. 2009;22:416-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 2. | Fischler B, Baumann U, D'Agostino D, D'Antiga L, Dezsofi A, Debray D, Durmaz O, Evans H, Frauca E, Hadzic N, Jahnel J, Loveland J, McLin V, Ng VL, Nobili V, Pawłowska J, Sharif K, Smets F, Verkade HJ, Hsu E, Horslen S, Bucuvalas J. Similarities and Differences in Allocation Policies for Pediatric Liver Transplantation Across the World. J Pediatr Gastroenterol Nutr. 2019;68:700-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Qin T, Fu J, Verkade HJ. The role of the gut microbiome in graft fibrosis after pediatric liver transplantation. Hum Genet. 2021;140:709-724. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Hong SK, Hwang S, Lee SG, Lee LS, Ahn CS, Kim KH, Moon DB, Ha TY. Pulmonary complications following adult liver transplantation. Transplant Proc. 2006;38:2979-2981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 65] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 5. | Yao W, Luo G, Zhu G, Chi X, Zhang A, Xia Z, Hei Z. Propofol activation of the Nrf2 pathway is associated with amelioration of acute lung injury in a rat liver transplantation model. Oxid Med Cell Longev. 2014;2014:258567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 6. | Chi XJ, Cai J, Luo CF, Cheng N, Hei ZQ, Li SR, Luo GJ. Relationship between the expression of Toll-like receptor 2 and 4 in mononuclear cells and postoperative acute lung injury in orthotopic liver transplantation. Chin Med J (Engl). 2009;122:895-899. [PubMed] |
| 7. | Nastos C, Kalimeris K, Papoutsidakis N, Tasoulis MK, Lykoudis PM, Theodoraki K, Nastou D, Smyrniotis V, Arkadopoulos N. Global consequences of liver ischemia/reperfusion injury. Oxid Med Cell Longev. 2014;2014:906965. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 224] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 8. | Lin YH, Cai ZS, Jiang Y, Lü LZ, Zhang XJ, Cai QC. Perioperative risk factors for pulmonary complications after liver transplantation. J Int Med Res. 2010;38:1845-1855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Carrier FM, Chassé M, Wang HT, Aslanian P, Iorio S, Bilodeau M, Turgeon AF. Restrictive fluid management strategies and outcomes in liver transplantation: a systematic review. Can J Anaesth. 2020;67:109-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Feltracco P, Carollo C, Barbieri S, Pettenuzzo T, Ori C. Early respiratory complications after liver transplantation. World J Gastroenterol. 2013;19:9271-9281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 80] [Cited by in RCA: 94] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 11. | Voet M, Nusmeier A, Lerou J, Luijten J, Cornelissen M, Lemson J. Cardiac output-guided hemodynamic therapy for adult living donor kidney transplantation in children under 20 kg: A pilot study. Paediatr Anaesth. 2019;29:950-958. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Romagnoli S, Bevilacqua S, Lazzeri C, Ciappi F, Dini D, Pratesi C, Gensini GF, Romano SM. Most Care®: a minimally invasive system for hemodynamic monitoring powered by the Pressure Recording Analytical Method (PRAM). HSR Proc Intensive Care Cardiovasc Anesth. 2009;1:20-27. [PubMed] |
| 13. | Bezinover D, Kadry Z, McCullough P, McQuillan PM, Uemura T, Welker K, Mastro AM, Janicki PK. Release of cytokines and hemodynamic instability during the reperfusion of a liver graft. Liver Transpl. 2011;17:324-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 14. | McIntosh AM, Tong S, Deakyne SJ, Davidson JA, Scott HF. Validation of the Vasoactive-Inotropic Score in Pediatric Sepsis. Pediatr Crit Care Med. 2017;18:750-757. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 183] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 15. | Sapru A, Curley MA, Brady S, Matthay MA, Flori H. Elevated PAI-1 is associated with poor clinical outcomes in pediatric patients with acute lung injury. Intensive Care Med. 2010;36:157-163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 16. | Christman JW, Sadikot RT, Blackwell TS. The role of nuclear factor-kappa B in pulmonary diseases. Chest. 2000;117:1482-1487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 253] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 17. | Bhatia M, Zemans RL, Jeyaseelan S. Role of chemokines in the pathogenesis of acute lung injury. Am J Respir Cell Mol Biol. 2012;46:566-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 198] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 18. | Yoshidome H, Kato A, Edwards MJ, Lentsch AB. Interleukin-10 inhibits pulmonary NF-kappaB activation and lung injury induced by hepatic ischemia-reperfusion. Am J Physiol. 1999;277:L919-L923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Rudnick MR, Marchi LD, Plotkin JS. Hemodynamic monitoring during liver transplantation: A state of the art review. World J Hepatol. 2015;7:1302-1311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 20. | Siniscalchi A, Gamberini L, Laici C, Bardi T, Ercolani G, Lorenzini L, Faenza S. Post reperfusion syndrome during liver transplantation: From pathophysiology to therapy and preventive strategies. World J Gastroenterol. 2016;22:1551-1569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 72] [Cited by in RCA: 92] [Article Influence: 10.2] [Reference Citation Analysis (1)] |
| 21. | Ryu HG, Jung CW, Lee HC, Cho YJ. Epinephrine and phenylephrine pretreatments for preventing postreperfusion syndrome during adult liver transplantation. Liver Transpl. 2012;18:1430-1439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 22. | Vilchez-Monge AL, Garutti I, Jimeno C, Zaballos M, Jimenez C, Olmedilla L, Piñeiro P, Duque P, Salcedo M, Asencio JM, Lopez-Baena JA, Maruszewski P, Bañares R, Perez-Peña JM. Intraoperative Troponin Elevation in Liver Transplantation Is Independently Associated With Mortality: A Prospective Observational Study. Liver Transpl. 2020;26:681-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Botto F, Alonso-Coello P, Chan MT, Villar JC, Xavier D, Srinathan S, Guyatt G, Cruz P, Graham M, Wang CY, Berwanger O, Pearse RM, Biccard BM, Abraham V, Malaga G, Hillis GS, Rodseth RN, Cook D, Polanczyk CA, Szczeklik W, Sessler DI, Sheth T, Ackland GL, Leuwer M, Garg AX, Lemanach Y, Pettit S, Heels-Ansdell D, Luratibuse G, Walsh M, Sapsford R, Schünemann HJ, Kurz A, Thomas S, Mrkobrada M, Thabane L, Gerstein H, Paniagua P, Nagele P, Raina P, Yusuf S, Devereaux PJ, McQueen MJ, Bhandari M, Bosch J, Buckley N, Chow CK, Halliwell R, Li S, Lee VW, Mooney J, Furtado MV, Suzumura E, Santucci E, Leite K, Santo JA, Jardim CA, Cavalcanti AB, Guimaraes HP, Jacka MJ, McAlister F, McMurtry S, Townsend D, Pannu N, Bagshaw S, Bessissow A, Duceppe E, Eikelboom J, Ganame J, Hankinson J, Hill S, Jolly S, Lamy A, Ling E, Magloire P, Pare G, Reddy D, Szalay D, Tittley J, Weitz J, Whitlock R, Darvish-Kazim S, Debeer J, Kavsak P, Kearon C, Mizera R, O'Donnell M, McQueen M, Pinthus J, Ribas S, Simunovic M, Tandon V, Vanhelder T, Winemaker M, McDonald S, O'Bryne P, Patel A, Paul J, Punthakee Z, Raymer K, Salehian O, Spencer F, Walter S, Worster A, Adili A, Clase C, Crowther M, Douketis J, Gangji A, Jackson P, Lim W, Lovrics P, Mazzadi S, Orovan W, Rudkowski J, Soth M, Tiboni M, Acedillo R, Garg A, Hildebrand A, Lam N, Macneil D, Roshanov PS, Srinathan SK, Ramsey C, John PS, Thorlacius L, Siddiqui FS, Grocott HP, McKay A, Lee TW, Amadeo R, Funk D, McDonald H, Zacharias J, Cortés OL, Chaparro MS, Vásquez S, Castañeda A, Ferreira S, Coriat P, Monneret D, Goarin JP, Esteve CI, Royer C, Daas G, Choi GY, Gin T, Lit LC, Sigamani A, Faruqui A, Dhanpal R, Almeida S, Cherian J, Furruqh S, Afzal L, George P, Mala S, Schünemann H, Muti P, Vizza E, Ong GS, Mansor M, Tan AS, Shariffuddin II, Vasanthan V, Hashim NH, Undok AW, Ki U, Lai HY, Ahmad WA, Razack AH, Valderrama-Victoria V, Loza-Herrera JD, De Los Angeles Lazo M, Rotta-Rotta A, Sokolowska B, Musial J, Gorka J, Iwaszczuk P, Kozka M, Chwala M, Raczek M, Mrowiecki T, Kaczmarek B, Biccard B, Cassimjee H, Gopalan D, Kisten T, Mugabi A, Naidoo P, Naidoo R, Rodseth R, Skinner D, Torborg A, Urrutia G, Maestre ML, Santaló M, Gonzalez R, Font A, Martínez C, Pelaez X, De Antonio M, Villamor JM, García JA, Ferré MJ, Popova E, Garutti I, Fernández C, Palencia M, Díaz S, Del Castillo T, Varela A, de Miguel A, Muñoz M, Piñeiro P, Cusati G, Del Barrio M, Membrillo MJ, Orozco D, Reyes F, Sapsford RJ, Barth J, Scott J, Hall A, Howell S, Lobley M, Woods J, Howard S, Fletcher J, Dewhirst N, Williams C, Rushton A, Welters I, Pearse R, Ackland G, Khan A, Niebrzegowska E, Benton S, Wragg A, Archbold A, Smith A, McAlees E, Ramballi C, Macdonald N, Januszewska M, Stephens R, Reyes A, Paredes LG, Sultan P, Cain D, Whittle J, Del Arroyo AG, Sun Z, Finnegan PS, Egan C, Honar H, Shahinyan A, Panjasawatwong K, Fu AY, Wang S, Reineks E, Blood J, Kalin M, Gibson D, Wildes T; Vascular events In noncardiac Surgery patIents cOhort evaluatioN (VISION) Writing Group, on behalf of The Vascular events In noncardiac Surgery patIents cOhort evaluatioN (VISION) Investigators; Appendix 1. The Vascular events In noncardiac Surgery patIents cOhort evaluatioN (VISION) Study Investigators Writing Group; Appendix 2. The Vascular events In noncardiac Surgery patIents cOhort evaluatioN Operations Committee; Vascular events In noncardiac Surgery patIents cOhort evaluatioN VISION Study Investigators. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology. 2014;120:564-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 529] [Cited by in RCA: 648] [Article Influence: 58.9] [Reference Citation Analysis (0)] |
| 24. | Sheng M, Lin Y, Weng Y, Xu R, Sun Y, Yu W, Du H. Predictive Value of Intraoperative Troponin I Elevation in Pediatric Living Donor Liver Transplant Recipients With Biliary Atresia. Transplantation. 2017;101:2385-2390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Papanikolaou J, Makris D, Mpaka M, Palli E, Zygoulis P, Zakynthinos E. New insights into the mechanisms involved in B-type natriuretic peptide elevation and its prognostic value in septic patients. Crit Care. 2014;18:R94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 26. | Klouche K, Pommet S, Amigues L, Bargnoux AS, Dupuy AM, Machado S, Serveaux-Delous M, Morena M, Jonquet O, Cristol JP. Plasma brain natriuretic peptide and troponin levels in severe sepsis and septic shock: relationships with systolic myocardial dysfunction and intensive care unit mortality. J Intensive Care Med. 2014;29:229-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 27. | Feng ZY, Xu X, Zhu SM, Bein B, Zheng SS. Effects of low central venous pressure during preanhepatic phase on blood loss and liver and renal function in liver transplantation. World J Surg. 2010;34:1864-1873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 28. | Rogiers X, Berrevoet F, Troisi R. Comments on Bonney et al. "Outcomes on right liver lobe transplantation: a match pair analysis" (Transpl. Int. 2008; 21: 1045-1051). Transpl Int. 2009;22:588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 29. | Marik PE, Cavallazzi R, Vasu T, Hirani A. Dynamic changes in arterial waveform derived variables and fluid responsiveness in mechanically ventilated patients: a systematic review of the literature. Crit Care Med. 2009;37:2642-2647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 852] [Cited by in RCA: 788] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 30. | Shin YH, Ko JS, Gwak MS, Kim GS, Lee JH, Lee SK. Utility of uncalibrated femoral stroke volume variation as a predictor of fluid responsiveness during the anhepatic phase of liver transplantation. Liver Transpl. 2011;17:53-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 31. | Weinberg L, Banting J, Churilov L, McLeod RL, Fernandes K, Chao I, Ho T, Ianno D, Liang V, Muralidharan V, Christophi C, Nikfarjam M. The effect of a surgery-specific cardiac output-guided haemodynamic algorithm on outcomes in patients undergoing pancreaticoduodenectomy in a high-volume centre: a retrospective comparative study. Anaesth Intensive Care. 2017;45:569-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |