Published online Jun 15, 2023. doi: 10.4239/wjd.v14.i6.883
Peer-review started: March 14, 2023
First decision: April 7, 2023
Revised: April 16, 2023
Accepted: April 24, 2023
Article in press: April 24, 2023
Published online: June 15, 2023
Processing time: 92 Days and 23.6 Hours
Diabetic nephropathy (DN) is a microangiopathy of type 2 diabetes mellitus (T2DM), which can damage the kidney through various ways and mechanisms due to the nature of the disease, involving the renal interstitium and glomeruli. However, in the early stage of the disease, patients only showed kidney volume increase and glomerular hyperthyroidism, and typical symptoms that are difficult to arouse individual attention were noticed.
To observe the expression of serum retinol-binding protein (RBP) and urinary N-acetyl-β-D-glucosaminidase (NAG) in patients with DN, and to analyze their value in disease prediction, so as to provide new targets for early diagnosis and treatment of DN.
The baseline data of 50 T2DM patients treated in our hospital between January 2021 and December 2022 were retrospectively reviewed and included in group A. The baseline data of 50 patients with type 2 DN admitted to our hospital during the same period were collected and included in group B. The baseline data and serum RBP and urine NAG expression were compared between the two groups to analyze their value in the early prediction of DN.
There was no significant difference in age, gender, duration of diabetes, combined hyperlipidemia and combined hypertension between the two groups (P > 0.05); the expression of urinary NAG and serum RBP in group B was higher than that in group A, and the difference was statistically significant (P < 0.05); a multiple logistic regression model was established, and the results showed that urinary NAG and serum RBP were related to the presence or absence of injury in diabetic patients, and overexpression of urinary NAG and serum RBP may be risk factors for renal injury in T2DM patients (OR > 1, P < 0.05); receiver operating curve curve was plotted, and the results showed that the area under the curve of urinary NAG and serum RBP expression alone and in combination for predicting DN was > 0.80, and the predictive value was satisfactory; bivariate Spearman linear correlation analysis showed that there was a positive correlation between urinary NAG and serum RBP expression in patients with DN (r = 0.566, P = 0.000).
The increased expression of urinary NAG and serum RBP may be the risk factors leading to the progression of T2DM to DN. The possibility of DN can be considered in patients with urinary NAG and serum RBP overexpression by examining the expression of urinary NAG and serum RBP in patients with T2DM in clinical practice.
Core Tip: Retinol-binding protein (RBP) can combine with thyroid transporters to form polymer complexes, and activated RBP can free in plasma, pass through glomerular filtration, and be absorbed and decomposed by renal tubules. N-acetyl-β-D-glucosaminidase is a high molecular glycoprotein acidic hydrolase, which is an intracellular lysosomal enzyme mainly present in body fluids, organ tissues and blood cells of the body, and has a high expression especially in the proximal renal tubules, thus being clinically used as an important indicator for the evaluation of renal tubular function.
- Citation: Lin ZH, Dai SF, Zhao JN, Jiang Y. Application of urinary N-acetyl-β-D-glucosaminidase combined with serum retinol-binding protein in early detection of diabetic nephropathy. World J Diabetes 2023; 14(6): 883-891
- URL: https://www.wjgnet.com/1948-9358/full/v14/i6/883.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i6.883
Diabetic nephropathy (DN) is a microangiopathy of type 2 diabetes mellitus (T2DM), which is caused by many factors such as hemodynamics, glucose metabolism mechanism, oxidative stress, resulting in relative or absolute lack of insulin in the body. Patients mainly have persistent elevated blood glucose, nutritional metabolism disorders. DN can damage the kidney through various ways and mechanisms due to the nature of the disease, involving the renal interstitium, glomeruli, resulting in pathological changes in the kidney, such as glomerulosclerosis, but the initial manifestations of patients are only increased kidney volume, glomerular hyperfunction, not easy to appear the typical symptoms that attract individual attention, only in patients with edema, proteinuria caused detection, but at this time the disease has progressed to the irreversible stage, the best time of treatment is missed, the prognosis of patients is mostly unsatisfactory[1-3]. Therefore, it is particularly important to find new clinical biochemical factors or examination methods to help the early detection of patients with clinical DN to guide the development of early intervention means and improve the prognosis of patients. It has been reported that tubular injury is earlier than glomerular injury in patients with DN, suggesting that tubular injury-related indicators are more significant for guiding the early detection of DN[4-6]. Urine N-acetyl-β-D-glucosaminidase (NAG) is a hot indicator in the diagnosis and treatment of kidney-related diseases at present, and it has more research value in reflecting kidney injury, especially tubular injury[7-9]. Retinol-binding protein (RBP) is a transporter of retinol in blood and has significant value in the assessment of proximal tubular reabsorption function and glomerular filtration performance[10-12]. Based on the biological mechanism of the above two indicators in the body, consider whether they can be used as early diseases in patients with DN. In view of this, this study will focus on observing the expression of serum RBP and urinary NAG in patients with DN, and analyze the value of the two indicators in disease prediction, providing a new target for early diagnosis and treatment of patients with DN.
Retrospective analysis was performed to collect the baseline data of 50 patients with T2DM admitted to our hospital between January 2021 and December 2022, and were included in group A. Within the group, there were 30 males and 20 females; the mean age was (43.12 ± 5.02) years. Baseline data were collected from 50 patients with type 2 DN admitted to our hospital during the same period and included in Group B, Within the group, there were 28 males and 22 females; the mean age was (43.25 ± 5.12) years.
Inclusion criteria: (1) The diagnosis of T2DM refers to the contents in the [Chinese Guidelines for the Prevention and Treatment of Type 2 Diabetes (2013 Edition)][13], which is clinically confirmed by oral glucose tolerance test; (2) Patients with DN refer to the contents in [the Expert Consensus on the Clinical Diagnosis of Diabetic Kidney Disease in Chinese Adults][14]; (3) No other related diseases of the kidney, such as acute and chronic nephritis; and (4) The relevant treatments involved in this study are properly preserved.
Exclusion criteria: (1) Combined endocrine diseases, such as thyroid disease; (2) Combined tumor, tuberculosis and other cachexia; (3) Patients with kidney damage caused by other reasons, such as long-term drug history; and (4) Patients with low compliance caused by combined psychological or mental disorders, who cannot successfully cooperate with the study.
According to the study objectives and methods, a statistical table of general data was designed, which mainly included duration of diabetes, gender, age, combined hyperlipidemia and combined hypertension. Participants in this study all came from the same region.
5 mL of fasting venous blood was collected at a rate of 3500 r/min with a radius of 15 cm, and the supernatant was obtained after centrifugation for 5 min. Serum RBP was measured by immunoturbidimetry (Beckman AU5800 automatic biochemical analyzer). Patients were asked to randomly obtain 5 mL of morning midstream urine, centrifuged at 1500 r/min, and the supernatant was obtained after 10 min of centrifugation to detect urinary NAG by colorimetry (kit produced by Beijing Jiuqiang Company). All the above operations were carried out in strict accordance with the instructions of relevant instruments, reagents.
Data processing was performed using SPSS 24.0 software, and all measurement data were tested for normality by Shapiro-Wilk test, and data that conformed to the normal distribution were expressed as mean ± SD, and comparisons between groups were performed using the independent samples t-test; “%” was used for enumeration data and expressed as χ2 Test, correlation analysis was performed using bivariate Spearman line, and logistic regression analysis was used to test the relationship between urinary NAG and serum RBP expression and patients with DN; receiver operating curve (ROC) was plotted to test the value of urinary NAG and serum RBP in predicting DN, evaluated by area under the curve (AUC), AUC ≤ 0.50: No predictive value; 0.50 < AUC ≤ 0.70: Low predictive value; 0.70 < AUC ≤ 0.90: Moderate predictive value; AUC > 0.90: High predictive value; P < 0.05 was considered statistically significant.
There was no significant difference in age, gender, duration of diabetes, combined hyperlipidemia and combined hypertension between the two groups (P > 0.05); urinary NAG and serum RBP expression in group B were higher than those in group A, and the difference was statistically significant (P < 0.05, Table 1).
| Indicators | Group A (n = 50) | Group B (n = 50) | Statistical value | P value |
| Gender, n (%) | χ2 = 0.164 | 0.685 | ||
| Male | 30 (60.00) | 28 (56.00) | ||
| Female | 20 (40.00) | 22 (44.00) | ||
| Age (mean ± SD, yr) | 43.12 ± 5.02 | 43.25 ± 5.12 | t = 0.128 | 0.898 |
| Duration of diabetes (mean ± SD, yr) | 2.1 5 ± 0.52 | 2.23 ± 0.55 | t = 0.747 | 0.457 |
| Combined hyperlipidemia, n (%) | χ2 = 0.164 | 0.685 | ||
| Yes | 20 (40.00) | 22 (44.00) | ||
| No | 30 (60.00) | 28 (56.00) | ||
| Combined hypertension, n (%) | χ2 = 0.170 | 0.680 | ||
| Yes | 18 (36.00) | 20 (40.00) | ||
| No | 32 (64.00) | 30 (60.00) | ||
| Urine NAG (mean ± SD, U/L) | 14.05 ± 2.20 | 19.45 ± 3.68 | t = 8.906 | < 0.001 |
| Serum RBP (mean ± SD, mg/L) | 43.56 ± 5.50 | 84.98 ± 15.70 | t = 17.606 | < 0.001 |
Serum RBP and urine NAG of the included subjects were used as covariates, and the conditions of the included subjects were used as dependent variables (1 = DN, 0 = T2DM). After binary regression analysis, all the data in 2.1 were included to establish a multiple logistic regression model. The results showed that urine NAG and serum RBP were related to the presence or absence of injury in diabetic patients, and overexpression of urine NAG and serum RBP may be risk factors for renal injury in T2DM patients (OR > 1, P < 0.05, Table 2).
| Variable | B | SE | Wals | P value | OR | 95%CI | |
| Upper limit | Lower limit | ||||||
| Constant | -9.366 | 1.808 | 26.839 | 0.000 | 0.000 | - | - |
| Urine NAG (U/L) | 0.568 | 0.111 | 26.338 | 0.000 | 1.765 | 1.421 | 2.192 |
| Serum RBP (mg/L) | 0.346 | 0.109 | 9.996 | 0.002 | 1.413 | 1.141 | 1.751 |
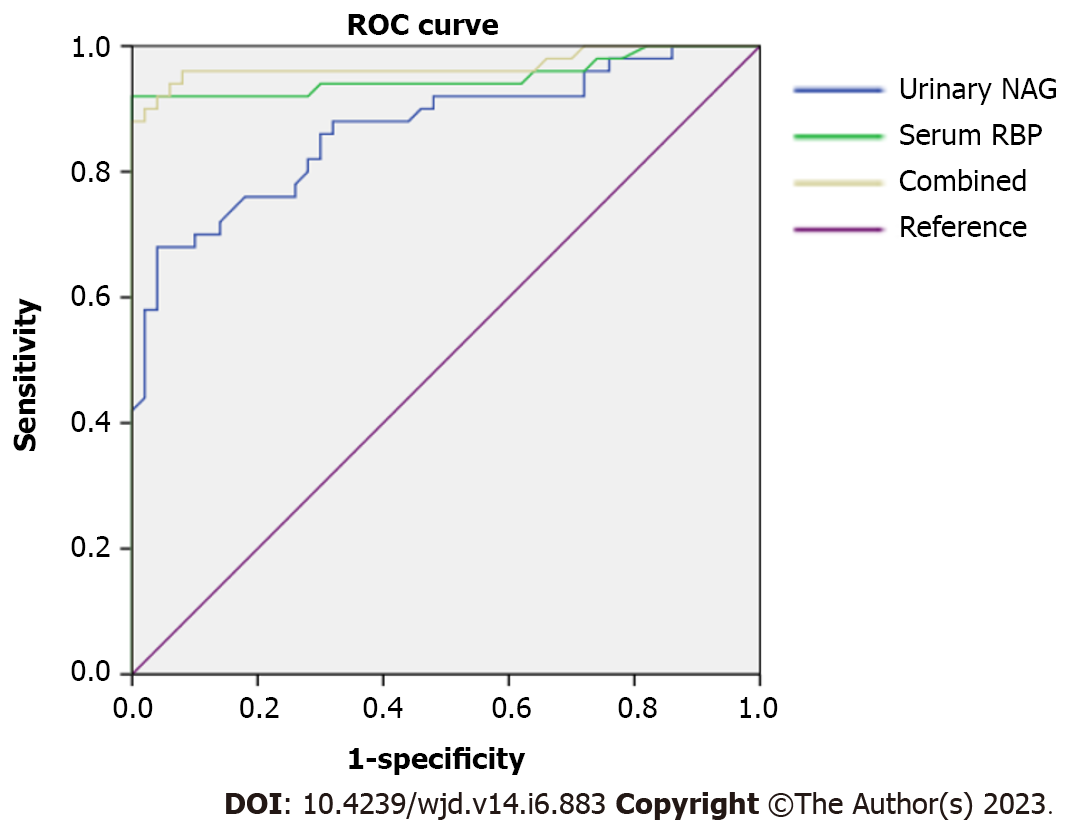
Urinary NAG and serum RBP expression of the included subjects were used as test variables, and the conditions of the included subjects were used as state variables (1 = DN, 0 = T2DM) to draw ROC curves (Figure 1), and the results showed that the AUC of urinary NAG and serum RBP expression alone and in combination in predicting DN were > 0.80, with satisfactory predictive value (Table 3).
| Indicators | AUC | 95%CI of AUC | SE | P value | Cut-off value | Specificity | Sensitivity | Youden index |
| Urine NAG | 0.867 | 0.796-0.939 | 0.036 | 0.000 | 11.855 (U/L) | 0.980 | 0.860 | 0.840 |
| Serum RBP | 0.951 | 0.902-1.000 | 0.025 | 0.000 | 39.620 (mg/L) | 0.980 | 0.780 | 0.640 |
| Combined diagnosis | 0.974 | 0.936-1.000 | 0.020 | 0.000 | - | 0.980 | 0.940 |
Bivariate Spearman linear correlation analysis showed a positive correlation between urinary NAG and serum RBP expression in patients with DN (r = 0.566, P = 0.000).
DN is a common complication in patients with T2DM. Urinary albumin, creatinine, blood urea nitrogen and other indicators have been used to assess whether diabetic patients have kidney damage. However, since kidney has self-compensation effect, indicators do not show significant changes in early stage renal impairment, the sensitivity of these indicators is low, and the above indicators can detect abnormalities only when the collective kidney has been damaged. However, irreversible damage has occurred in the body kidney at this stage, resulting in difficulty in the early detection of DN[15-17]. In view of this, many clinical reports have pointed out that inflammatory response, polyol metabolic pathway, abnormal changes in renal hemodynamics, oxidative stress and other mechanisms are related to the occurrence and disease progression of patients with DN, in the process of occurrence and progression of DN, there are renal tubular reabsorption dysfunction, glomerular filtration changes, and abnormal changes of multiple molecules in blood and urine. Thus, whether other indicators in serum or urine can be used as early detection of patients with clinical DN[18-20].
RBP is a carrier protein synthesized and secreted by stem cells, which is mainly synthesized by carbohydrates and a polypeptide chain, and has a very short half-life, which is necessary to help vitamin A transport on hepatocytes to epithelial cells. In many plasma, RBP can bind to thyroid transporter to form a polymer complex. Activated RBP can be free in plasma and filtered by glomeruli, where most of RBP is absorbed and decomposed by the proximal renal tubules for normal use by tissues, and only a few is excreted in the urine, so the level detected in serum or urine is extremely low under healthy conditions[21-23]. The changes of RBP content suggest the pathological changes of renal tubules and glomeruli. Under the action of induction factors, RBP can stimulate oxidative stress in the body and increase the damage of oxygen free radicals to the vascular endothelium[24-26].
NAG is a large lysosomal molecule present in tubular epithelial cells and does not efficiently pass through the glomerular filtration membrane[27-29]. NAG is a high molecular glycoprotein acid hydrolase, an intracellular lysosomal enzyme mainly present in body fluids, organ tissues and blood cells, especially highly expressed in the proximal renal convoluted tubules, and is clinically used as an important indicator for tubular function assessment[30-32]. In a healthy state, cause NAG has a large molecular weight and cannot normally pass through glomerular filtration, the renal tubules in the early stage of DN can still absorb the excessive proteinuria of glomerular filtration. Urine albumin in this stage is normal, but the expression of NAG increases, which may be due to the strengthening of reabsorption by the renal proximal convoluted tubule, the high protein content in the renal proximal convoluted tubule stimulating the reabsorption system, activation of mitochondrial lysosomal enzyme, and increased lysosomal enzyme density, the large release of lytic enzyme and the leakage of lysosomal enzyme[33-35]. The results of this study showed that compared with the data of age, gender, duration of diabetes, combined hyperlipidemia and combined hypertension in the two groups, the expression of urinary NAG and serum RBP in group B was higher than that in group A, suggesting that the expression of serum RBP and urinary NAG may be the cause of disease progression to DN in patients with T2DM.
In order to further verify the above conjecture, logistic regression model was used in this study. The results showed that urinary NAG and serum RBP were related to the occurrence of injury in diabetic patients. Urinary NAG and serum RBP overexpression may be risk factors of renal injury in T2DM patients, and ROC curve was drawn, the results showed that the AUC of urinary NAG and serum RBP expression alone and in combination in predicting DN was > 0.80, and the predictive value was satisfactory, suggesting that urinary NAG and serum RBP overexpression are the key to lead to the progression of disease to DN in T2DM patients. The possible reasons for analysis may be: (1) When the renal tubules are damaged, the glomerular filtration decreased, the renal hemodynamics change, when the free RBP passes through the renal tubules, its ability to absorb and decompose the free RBP is limited, resulting in a large number of RBP retention, so the RBP in the serum shows a high expression state[36-38]; and (2) When the renal tubules degenerate, necrosis, damage and fall off, the NAG in the cells enters the urine with the exfoliated and necrotic cells, so a high level of NAG can be measured in the urine[39,40]. In addition, the pathways for obtaining urine NAG and serum RBP were relatively easy, the combination of urine NAG and serum RBP as early evaluation indicators of DN was based on two pathways of urine and blood, which was more reliable than the indicators in pure blood or urine. In this study, bivariate Spearman linear correlation analysis was also used, and the results showed that there was a positive correlation between urinary NAG and serum RBP expression in patients with DN, which may be due to the fact that both indicators are closely related to renal function, so the change of one of the indicators will certainly be cited another indicator changes, but the relationship between the two indicators lacks clinical demonstration support, and the reliability of the study needs to be further explored in the future.
In summary, elevated expression of urinary NAG and serum RBP may be risk factors leading to disease progression to DN in patients with T2DM, and the possibility of DN can be considered in patients with urinary NAG and serum RBP overexpression by examining urinary NAG and serum RBP expression in patients with T2DM in clinical practice.
Diabetic nephropathy (DN) is a microangiopathy of type 2 diabetes mellitus (T2DM), which can damage the kidney through various ways and mechanisms due to the nature of the disease, involving the renal interstitium and glomeruli. However, in the early stage of the disease, patients only showed kidney volume increase and glomerular hyperthyroidism, and typical symptoms that are difficult to arouse individual attention were noticed. The symptoms were only noticed when the patients developed edema and proteinuria. At this time, the disease has progressed to an irreversible stage, and the best treatment timing should be taken. Therefore, finding new clinical biochemical factors or examination methods to help early detection of clinical DN patients is particularly important to guide the development of early intervention measures and improve the prognosis of patients.
This study provided new targets for early diagnosis and treatment of DN.
This study aimed to observe the expression of serum retinol-binding protein (RBP) and urinary N-acetyl-β-D-glucosaminidase (NAG) in patients with DN.
Total 50 T2DM patients were retrospectively reviewed and included in group A. The baseline data of 50 patients with type 2 DN during the same period were collected and included in group B. The baseline data and serum RBP and urine NAG expression were compared between the two groups to analyze their value in the early prediction of DN.
The increased expression of urinary NAG and serum RBP may be the risk factors leading to the progression of T2DM to DN.
The possibility of DN can be considered in patients with urinary NAG and serum RBP overexpression by examining the expression of urinary NAG and serum RBP in patients with T2DM in clinical practice.
This study showed that urine NAG combined with serum RBP had good application prospects in the early detection of DN. Future studies can further expand the research sample size and improve the diagnostic accuracy of urinary NAG combined with serum RBP.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Defeudis G, Italy; Mohan V, India S-Editor: Wang JL L-Editor: A P-Editor: Fan JR
| 1. | Schrauben SJ, Shou H, Zhang X, Anderson AH, Bonventre JV, Chen J, Coca S, Furth SL, Greenberg JH, Gutierrez OM, Ix JH, Lash JP, Parikh CR, Rebholz CM, Sabbisetti V, Sarnak MJ, Shlipak MG, Waikar SS, Kimmel PL, Vasan RS, Feldman HI, Schelling JR; CKD Biomarkers Consortium and the Chronic Renal Insufficiency Cohort (CRIC) Study Investigators. Association of Multiple Plasma Biomarker Concentrations with Progression of Prevalent Diabetic Kidney Disease: Findings from the Chronic Renal Insufficiency Cohort (CRIC) Study. J Am Soc Nephrol. 2021;32:115-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 97] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 2. | Shinjo T, Ishikado A, Hasturk H, Pober DM, Paniagua SM, Shah H, Wu IH, Tinsley LJ, Matsumoto M, Keenan HA, Van Dyke TE, Genco RJ, King GL. Characterization of periodontitis in people with type 1 diabetes of 50 years or longer duration. J Periodontol. 2019;90:565-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 3. | Fan Y, Yi Z, D'Agati VD, Sun Z, Zhong F, Zhang W, Wen J, Zhou T, Li Z, He L, Zhang Q, Lee K, He JC, Wang N. Comparison of Kidney Transcriptomic Profiles of Early and Advanced Diabetic Nephropathy Reveals Potential New Mechanisms for Disease Progression. Diabetes. 2019;68:2301-2314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 96] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 4. | Li J, Ye WF, Xu AJ, Zhang MR, Feng W. [Relationship between serum Sirt1 and inflammation, oxidative stress, short-term progression of albuminuria in patients with diabetic kidney disease]. Linchuang He Shiyan Yixue Zazi. 2022;24:274-277. |
| 5. | Wang Z, Jiang Y, Chen P, Wang J, Zhang X, Huang B, Zhou X, Shi S, Liu L, Lv J, Zhang H. The level of urinary C4d is associated with disease progression in IgA nephropathy with glomerular crescentic lesions: a cohort study. Nephrol Dial Transplant. 2022;37:2119-2127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Nicot G, Lachâtre G, Gonnet C, Dupuy JL, Valette JP. Rapid assay of N-acetyl-beta-D-glucosaminidase isoenzymes in urine by ion-exchange chromatography. Clin Chem. 1987;33:1796-1800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 7. | Liu L, Zhang HF, Yang HX, Yuan BJ, Liu X. [Levels and significance of eGFR, urinary NAG and serum NGAL in patients with type 2 diabetic nephropathy]. Zhongguo Linchuang Yisheng Zazhi. 2019;47:52-55. [DOI] [Full Text] |
| 8. | Jiang ZM, Wang AG, Jiang XL. [Application value of urinary NAG/Crea, TRF detection in early diagnosis of diabetic nephropathy]. Neimenggu Yixueyuan Xuebao. 2021;43:188-192. |
| 9. | Gao J, Xiao M, Li D, Bing Y, Wei P, Dong J, Jing H, Chen XM. [Evaluation Value of Urinary and Renal Function Indexes in Early Renal Injury of Diabetic Nephropathy]. Yixue Linchuang Yanjiu. 2019;36:843-845. [DOI] [Full Text] |
| 10. | Kong CW, Feng J, Sun YL. [Value of cystatin C, retinol binding protein and endothelin-1 in the early diagnosis of type 2 diabetic nephropathy]. Xinjiang Yikedaxue Xuebao. 2020;43:458-461. [DOI] [Full Text] |
| 11. | Lin LY, Jin YJ, Yao XY, Teng YQ, Zhao TT, Jin QS, Zhang DD, Shang HX. [Relationship between serum retinol binding protein, stromal cell derived factor-1 and renal function in patients with diabetic nephropathy]. Guoji Waikexue Zazhi. 2021;48:184-189. [DOI] [Full Text] |
| 12. | Wang F, Jin XW. [Effect of combined detection of RBP and CysC in early diagnosis of diabetic nephropathy]. Jianyan Yixue Yu Linchuang. 2020;17:1278-1281. [DOI] [Full Text] |
| 13. | Chinese Diabetes Society; Chinese Medical Association. [Chinese Guidelines for the Prevention and Treatment of Type 2 Diabetes (2013 Edition)]. Zhonghua Neifenmi Daixie Zazhi. 2014;30:893-942. [DOI] [Full Text] |
| 14. | Tong NW. [Expert consensus on clinical diagnosis of diabetic nephropathy in Chinese adults]. Zhonghua Neifenmi Daixie Zazhi. 2015;31:385. |
| 15. | Li Z, Chi H, Zhu W, Yang G, Song J, Mo L, Zhang Y, Deng Y, Xu F, Yang J, He Z, Yang X. Cadmium induces renal inflammation by activating the NLRP3 inflammasome through ROS/MAPK/NF-κB pathway in vitro and in vivo. Arch Toxicol. 2021;95:3497-3513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 66] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 16. | Wang JY, Ai Q. [Application value of serum cystatin C and retinol binding protein in early type 2 diabetic nephropathy]. Zhongguo Shiyan Zhenduanxue. 2020;24:1788-1791. [DOI] [Full Text] |
| 17. | Chang J. [Diagnostic Value of Serum Rbp and Urinary Microalbumin in Early Nephropathy of Type 2 Diabetes Mellitus]. Zhongguo Quankeyixue. 2020;23:132-133. |
| 18. | Tönnies T, Stahl-Pehe A, Baechle C, Castillo K, Yossa R, Holl RW, Rosenbauer J. Diabetic nephropathy and quality of life among youths with long-duration type 1 diabetes: A population-based cross-sectional study. Pediatr Diabetes. 2019;20:613-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Jiang S, Fang J, Yu T, Liu L, Zou G, Gao H, Zhuo L, Li W. Novel Model Predicts Diabetic Nephropathy in Type 2 Diabetes. Am J Nephrol. 2020;51:130-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 20. | Karim N, Rahman A, Chanudom L, Thongsom M, Tangpong J. Mangosteen Vinegar Rind from Garcinia mangostana Prevents High-Fat Diet and Streptozotocin-Induced Type II Diabetes Nephropathy and Apoptosis. J Food Sci. 2019;84:1208-1215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 21. | Yang DM, Chen HB, Liu ZM, Chen H, Li YF. [The Study of the Relationship Between Cystatin C, Retinol Binding Protein, Free Fatty Acid and the Development of Type 2 Diabetic Nephropathy]. Biaoji Mianyi Fenxi Yu Linchuang. 2020;27:1022-1025, 1032. [DOI] [Full Text] |
| 22. | Guo Y, Chen Y, Cui Y. [Clinical significance of urinary N-Acetylgluconsaminidase in elderly patients with non-dialysis chronic kidney disease associated with hypoproteinemia]. Shiyong Laonian Yixue. 2019;33:858-861. [DOI] [Full Text] |
| 23. | Lu LL, Xie B, Zhang JD, Ding CC. [The value of the combination of urine m-ALB, α1-MG, NAG and serum CysC in the diagnosis of early renal damage in gestational diabetes]. Zhongguo Fuyou Baojian. 2019;34:432-434. [DOI] [Full Text] |
| 24. | Costa C, Scabini S, Kaimal A, Kasozi W, Cusato J, Kafufu B, Borderi M, Mwaka E, Di Perri G, Lamorde M, Calcagno A, Castelnuovo B. Calcaneal Quantitative Ultrasonography and Urinary Retinol-Binding Protein in Antiretroviral-Treated Patients With Human Immunodeficiency Virus in Uganda: A Pilot Study. J Infect Dis. 2020;222:263-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Gong H, Huang L, Zhang QH, Li T, Shi XY. [Diagnostic efficacy of urine microalbumin,β2-microglobulin and N-acetyl-beta-glucosaminidase levels for renal injury in patients with decompensated liver cirrhosis]. Shiyong Ganzangbing Zazhi. 2022;25:681-684. |
| 26. | Yao Q, Li Y. Study of decreased serum levels of retinol binding protein 4 in major depressive disorder. J Psychiatr Res. 2020;129:24-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Huang YL, Li N. [Clinical value of serum RBP, urine KIM-1 and urine NAG in diagnosis and prognosis of diabetic nephropathy]. Jianyan Yixue Yu Linchuang. 2021;18:1701-1704. [DOI] [Full Text] |
| 28. | Wang Y, Liu CL, Yang M. [Evaluation of urine N-acetyl-β-D-glucosaminidase in renal tubular interstitial lesions of IgA nephropathy]. Guoji Miniaoxitong Zazhi. 2020;40:112-115. [DOI] [Full Text] |
| 29. | Wang YC, Wang QY. [Clinical value of combined detection of urinary NAG RBP and β2-MG in diagnosis of early renal injury in patients with hepatitis B cirrhosis]. Zhongguo Yaowu Yu Linchuang. 2021;21:3671-3674. [DOI] [Full Text] |
| 30. | Wang HF, Dong C, Li CM. [Clinical value of combined detection of serum β2- microglobulin, urinary microalbumin and urinary N- acetyl-β-glucosaminidase in the diagnosis of early renal injury due to hypertension]. Shaanxi Yixue Zazhi. 2020;49:897-899. [DOI] [Full Text] |
| 31. | Zhang JP, Li F, Liu WJ, Wang N, Gao S. [The diagnostic value of serum CysC, RBP, urine NAG and GAL in patients with early diabetic nephropathy]. Jianyan Yixue Yu Linchuang. 2020;17:640-642, 646. [DOI] [Full Text] |
| 32. | Zhang JY, Tang YM, Yang X, Yang WX. [Value of urinaryα1-microglobulin and N-acetyl-β-D-glucosaminidase/urinary creatinine in monitoring early renal injury in patients with chronic hepatitis B virus-related liver diseases]. Linchuang Gandanbing Zazhi. 2022;38:322-327. [DOI] [Full Text] |
| 33. | Mahmoud AA, Mostafa NM, Mesbah O, Sabry OM, Al-Barshomy SM. Study of Urinary N-Acetyl-Beta-D-Glucosaminidase as a biomarker of Diabetic Nephropathy. Egy J Hosp Med. 2021;. [DOI] [Full Text] |
| 34. | Huang R, Bai X, Li X, Wang X, Zhao L. Retinol-Binding Protein 4 Activates STRA6, Provoking Pancreatic β-Cell Dysfunction in Type 2 Diabetes. Diabetes. 2021;70:449-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 35. | Zhang S, Albrecht T, Rodriguez-Niño A, Qiu J, Schnuelle P, Peters V, Schmitt CP, van den Born J, Bakker SJL, Lammert A, Krämer BK, Yard BA, Hauske SJ. Carnosinase concentration, activity, and CNDP1 genotype in patients with type 2 diabetes with and without nephropathy. Amino Acids. 2019;51:611-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 36. | Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, Cannon CP, Capuano G, Chu PL, de Zeeuw D, Greene T, Levin A, Pollock C, Wheeler DC, Yavin Y, Zhang H, Zinman B, Meininger G, Brenner BM, Mahaffey KW; CREDENCE Trial Investigators. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N Engl J Med. 2019;380:2295-2306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2826] [Cited by in RCA: 3950] [Article Influence: 658.3] [Reference Citation Analysis (0)] |
| 37. | Fan XJ, Gu JC, Chen XH. [Relationship between serum UA, Cys C, urinary RBP and mAlb in patients with type 2 diabetic nephropathy]. Hainan Yixue. 2021;32:1645-1647. [DOI] [Full Text] |
| 38. | DAI JW, Tang XL. [Correlation between levels of CysC, RBP, Hcy and CRP in serum and stage of diabetic nephropathy]. Guoji Miniaoxitong Zazhi. 2020;40:385-388. [DOI] [Full Text] |
| 39. | ALTamimi JZ, AlFaris NA, Al-Farga AM, Alshammari GM, BinMowyna MN, Yahya MA. Curcumin reverses diabetic nephropathy in streptozotocin-induced diabetes in rats by inhibition of PKCβ/p(66)Shc axis and activation of FOXO-3a. J Nutr Biochem. 2021;87:108515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 40. | Bagos PG. Urinary N-Acetyl-β-d-glucosaminidase (uNAG) as an Indicative Biomarker of Early Diabetic Nephropathy in Patients with Diabetes Mellitus (T1DM, T2DM): A Systematic Review and Meta-Analysis. Diabetology. 2021;2. [DOI] [Full Text] |