Published online Sep 15, 2017. doi: 10.4251/wjgo.v9.i9.390
Peer-review started: December 14, 2016
First decision: March 22, 201
Revised: April 20, 2017
Accepted: July 14, 2017
Article in press: July 17, 2017
Published online: September 15, 2017
Processing time: 272 Days and 23.8 Hours
A 69-year-old woman from a kindred with familial atypical multiple mole melanoma and carrier of a germline mutation in CDKN2A, presented with abdominal pain caused by a solid-cystic pancreatic mass. The patient had an abdominal computed tomography three years before in which there was no evidence of pancreatic lesion. The endoscopic ultrasound guided fine needle aspiration showed adenocarcinoma with squamous component. After surgical resection the final diagnosis was adenosquamous pancreatic carcinoma (ASPC) arising in an intraductal papillar mucinous neoplasm (IPMN). Adenosquamous carcinomas are uncommon in the pancreas and have rarely been described in association with IPMNs. It has worse prognosis than the ordinary pancreatic ductal adenocarcinoma and some distinct features. We review the clinical, imaging, pathologic and molecular aspects of ASPC. Differential diagnosis with contamination, squamous metaplasia and pancreatic metastases from a distant squamous carcinoma is discussed. Besides, the case is an accelerated model of the adenoma (IPMN)-carcinoma sequence probably due to the CDKN2A germline mutation. Somatic CDKN2A mutations are common events in the early steps of sporadic pancreatic cancer, but germline mutation carriers have a significantly higher risk of pancreatic carcinoma.
Core tip: We present a rare case of adenosquamous pancreatic carcinoma (ASPC) arising in an intraductal papillar mucinous neoplasm in a CDKN2A germline mutation carrier from a kindred with familial atypical multiple mole melanoma. The case is an accelerated model of the adenoma-carcinoma sequence in pancreatic carcinogenesis. We discuss the differential diagnoses of squamous lesions in the pancreas and review the clinical and morphological features of ASPC.
- Citation: Martínez de Juan F, Reolid Escribano M, Martínez Lapiedra C, Maia de Alcantara F, Caballero Soto M, Calatrava Fons A, Machado I. Pancreatic adenosquamous carcinoma and intraductal papillary mucinous neoplasm in a CDKN2A germline mutation carrier. World J Gastrointest Oncol 2017; 9(9): 390-396
- URL: https://www.wjgnet.com/1948-5204/full/v9/i9/390.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v9.i9.390
Ductal adenocarcinoma is the most frequent malignant tumour of the pancreas, and with a life expectancy of 5% at 5 years, the prognosis has not improved in the last 20 years[1]. Surgical excision, the only potentially curative treatment, yields a 20% chance of 5-year survival[1]. Unfortunately, only 15%-20% of patients are candidates for surgical resection due to distant metastases or locally advanced disease at diagnosis[1]. Hence, much effort should be focused in recognizing premalignant lesions or early invasive carcinomas. Pancreatic cancer screening risks outweigh benefits in the general population, but it might benefit individuals at high risk of pancreatic cancer[2]. However, only 10% of the pancreatic cancers are considered to be caused by inherited germline mutations, sporadically occurring mutations being responsible for the vast majority[3]. Pancreatic adenosquamous carcinoma is a rare variant with even worse prognosis than adenocarcinoma with some distinct clinical, imaging and pathological features[4,5]. We present the case of a pancreatic cancer predisposing germinal mutation carrier who developed an adenosquamous carcinoma of the pancreas arising in an intraductal papillar mucinous neoplasm. The case illustrates the adenoma-carcinoma sequence in pancreatic cancer, probably accelerated due to the germline mutation.
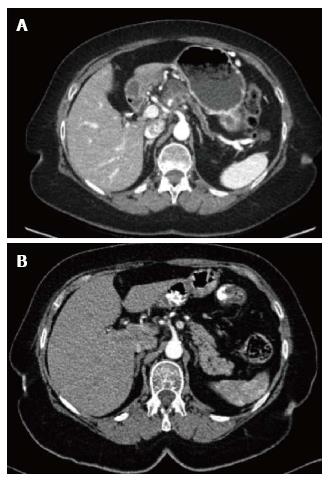
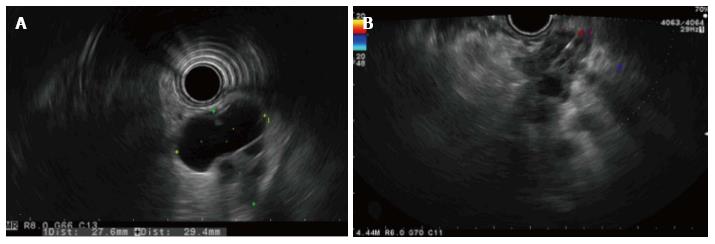
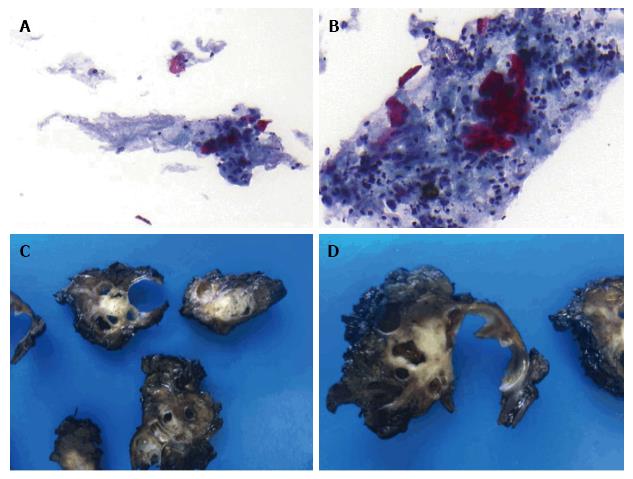
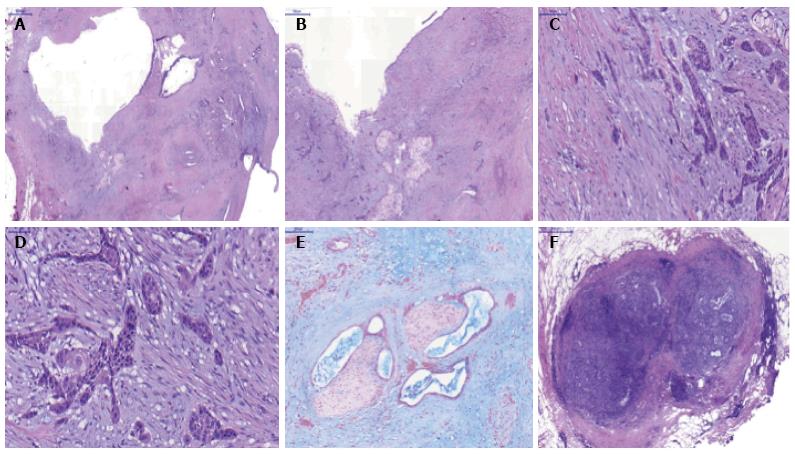
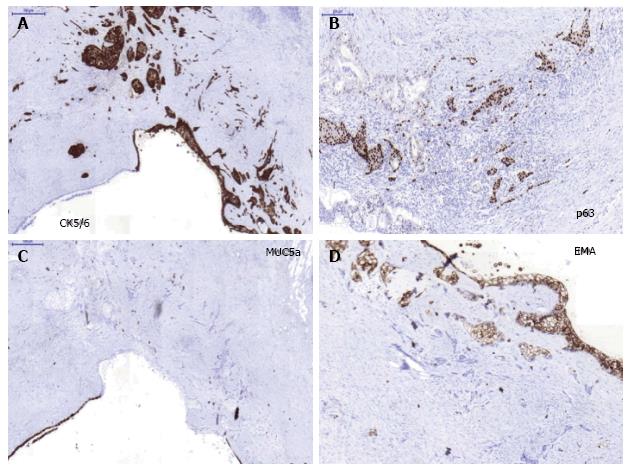
A 69-year-old woman attended the hospital with upper abdominal pain of growing intensity during two months. She had a cutaneous malignant melanoma resected in 1997 and was a carrier of a germline mutation in CDKN2A at codon 59 (GTG>GGG) from a kindred with Familial Atypical Mole Melanoma (FAMMM). The computed tomography (CT) revealed a 30-mm solid-cystic mass in the body of the pancreas, with ill-defined borders, pancreatic tail atrophy, splenic vessels encasement and partial superior mesenteric artery and vein involvement (Figure 1A). The celiac trunk and branches were not involved. A CT performed 3 years previously due to a self-limited diffuse abdominal pain, resulted in no evident pancreatic lesion (Figure 1B). Radial and linear endoscopic ultrasonography (EUS) (Figure 2) confirmed the CT findings and the transgastric EUS guided fine needle aspiration (EUS-FNA) showed the presence of malignant cells with both glandular and squamous differentiation (Figure 3A and B). The multidisciplinary oncology board considered the tumour borderline for surgical resection, hence neoadjuvant chemotherapy with FOLFIRINOX (folinic acid, 5-fluorouracil, irinotecan and oxaliplatin) was indicated. After the neoadjuvant treatment, pain was relieved and CT showed reduction in the tumor size and no superior mesenteric artery involvement, therefore the patient was considered for surgical treatment. Partial pancreatectomy including the body and the tail together with splenectomy was performed without complications. In the surgical specimen, the pathological study of the solid-cystic mass (Figure 3C and D) showed an intraductal papillary mucinous neoplasm (IPMN) in connection with adenocarcinoma and squamous cell carcinoma components (Figure 4). Squamous metaplasia was also observed. The squamous carcinoma represented one third of the whole malignant component, thus a final diagnosis of pancreatic adenosquamous carcinoma probably arising in an IPMN was rendered. The immunohistochemical study showed CK (34βE12/p63, CEA and CK 5/6 strong positivity in the squamous component. CK 7 and MUC5A revealed strong positivity in the adenocarcinoma area (Figure 5A-C). p53 and EMA were strongly positive in both components (Figure 5D). Ki-67 index was 25%. The retroperitoneal surgical margin was affected, and 10 out of 27 lymph nodes were metastatic, one of them located between cava and aorta. The final pathological stage was ypT3N1M1. Five months after surgery, the patient is currently ongoing maintenance chemotherapy.
Adenosquamous carcinomas may appear in various locations in the body (gastrointestinal tract[6,7] female reproductive organs[8], lung, bladder). Adenosquamous pancreatic carcinoma (ASPC) is rare, accounting for less than 5% of all pancreatic carcinomas in the largest series[4]. Areas of squamous differentiation are often seen in a pancreatic ductal adenocarcinoma (PDA), but the diagnosis of ASPC is conventionally accepted only if the squamous component is present in at least 30% of the total volume of the tumour. Exclusively squamous carcinomas of the pancreas are exceptional; moreover, they are usually regarded as metastases from a primary squamous carcinoma. ASPC symptoms are basically the same as PDA’s (mainly abdominal or back pain, but also jaundice, weight loss or diabetes depending on its relation with the biliary tract and the grade of exocrine and endocrine impairment caused). It may be seen more frequently in the head of the pancreas, but ASPC presents less likely in the head and more commonly in the body or tail than PDA[4]. It is slightly predominant in white males, around the seventh decade of life[4]. Respecting size, ASPC is usually bigger than PDA. The squamous component usually locates in the periphery of the lesion, whilst adenocarcinoma lays in the center. Extensive necrotic areas are more common in ASPC. Poor differentiation grade is frequent[4,5], and tends to grow perineurally. In the same way, vascular and lymph nodes involvement is frequently seen[5]. Rhabdoid components[9], osteoclast-like and pleomorphic giant cells, and acantholisis[10] have been described in some cases. Immunohistochemical (IHC) analysis show positivity for keratins (AE1/E3, CK1, CK5/6, CK7, and less frequently Cam 5.2 and CK20)[11], p63 (which may be helpful in identifying squamous differentiation in cases with acantholysis), overexpression of EGFR, and reduced or loss of E-cadherin expression[12]. p16 and Dpc4 expression are usually lost or reduced and nuclear p53[12], CA 19.9 and CEA are usually positive[12] similar to PDA. One study[13] used an IHC commercial assay[14] of molecular markers which are implicated in anti-tumor drug performance, showing TOPO2A, MRP1, BCRP and MGMT overexpression. There is not a definitive sign of ASPC in imaging tests, but some studies remark that it is frequently round or has a lobulated shape[15] with ill-defined borders[16], and characteristically demonstrates central necrotic areas[15]. It usually displays peripheral contrast enhancement in the arterial phase, which persists in the venous phase[17], and thrombus in the portal vein system is often present[15]. Opposite to PDA, lack of distal glandular atrophy and only mild pancreatic ductal dilatation[16] have been noted. Not surprisingly, in view of the aggressive morphologic features, prognosis is even poorer than PDA’s: In a large population based study comparing 415 cases of ASPC and 45693 of PDA, 2-year survival after surgery was 29% in ASPC patients and 36% in the PDA group (P < 0.0001)[4]. A matched case control study yielded a significantly worse median overall survival of 8.38 mo in ASPC patients compared with 15.75 mo in PDA patients (HR = 1.94; 95%CI: 1.07-3.51; P = 0.026)[18].
The etiology of the ASPC is unknown, but there are three hypotheses that try to explain the development of ASPC: (1) Squamous carcinoma metaplasia may be a consequence of metaplastic changes due to the microenvironmental conditions in the ductal lumen obstructed by an already existent PDA; (2) areas of squamous carcinoma might be present since the first steps of carcinogenesis originated at the same time that the adenocarcinoma line from a common tumor stem cell; and (3) two concomitant carcinomas (squamous and adenocarcinoma) collide and merge forming one tumor mass. There are no studies that demonstrate which, if any, is correct, but some studies find the same KRAS mutation in both tumor lines[12] suggesting a common origin, thus making the collision hypothesis less likely.
The molecular alterations in ASPC are similar to PDA[12], being the loss of p16 (the protein coded by the gene CDKN2A) a common event in the early steps of pancreatic carcinogenesis. Loss of p16 may be caused by genetic mutations or gene silencing due to epigenetic changes. Our patient, being a carrier of a germline mutation in CDKN2A, had a predisposition to pancreatic cancer. As a fact, it has been estimated that carriers of a germline CDKN2A mutation in kindreds with FAMMM, have a 38-fold higher risk of pancreatic cancer than the general population[3]. Nevertheless, many other genetic, epigenetic or environmental factors and interactions among them must influence, as not all the families with CDKN2A mutations have a familial history of pancreatic cancer[19]. There are some scientific societies[20-22] that have reached some consensus in screening special populations with markedly increased risk of pancreatic cancer, mainly with EUS or MRI. Notwithstanding this, there are still knowledge gaps, lack of standardization in procedures, and differences in availability of resources that hinder the implementation in daily clinical practice, so that this patient was not following a pancreatic cancer screening program.
There are few reports of ASPC arising or associated with IPMN[23,24]. IPMNs are premalignant lesions that are estimated to develop a carcinoma in 47% the main duct type, and in 17% the branch duct type[25]. It usually takes more than a decade to transform into a malignant invasive lesion, for those who eventually do[25]. Our patient had an abdominal CT three years before, in which no lesion was apparent in the pancreas. It could be argued that a lesion of less than 2 cm may have been overlooked; even if this was case, the sequence from premalignant to malignant was clearly accelerated, most probably due to the CDKN2A mutation.
The EUS-guided samples in our case showed plenty squamous component, not only in the cell aspirate but also in the cellular block, together with adenocarcinoma, therefore a diagnosis of ASPC was proposed. Preoperative diagnosis of ASPC is difficult: the finding of squamous component in the cytology should be interpreted cautiously, as it could represent contamination[26]. Furthermore, even if the specimen allows architectural study and thus contamination is likely ruled out, it is not possible to determine the proportion of squamous carcinoma in the whole malignant mass with the biopsy sampling. Hence, the preoperative diagnosis of ASPC may be suggested, but not definitive. The differential diagnosis when squamous component is found in a pancreatic neoplasia also includes ductal squamous metaplasia, mucoepidermoid carcinoma (which is characterized by the presence of squamoid intermediate cells and the absence of individual cell keratinization and keratin pearls) and pancreatoblastoma, which is rarely found in adults. In addition, in a patient with a previous history of malignant melanoma the possibility of de-differentiated melanoma with adenocarcinoma-like component should be also excluded[27].
In summary, this case illustrates the accelerated carcinogenesis in a genetically predisposed patient, with the premalignant ground (IPMN) and a rare malignant variant (ASPC), all together in one lesion. The imaging studies (CT) and especially EUS may help to detect this rare entity, although the definitive diagnosis requires surgical resection. Currently there is not a specific strategy for ASPC, but the advances in the molecular profiling and the new targeted therapies might change its management.
We acknowledge David Thivey for reviewing the language.
A 69-year-old woman with antecedents of familial melanoma and CDKN2A mutation carrier presented with a two months of abdominal pain.
The physical exam was normal.
Although the abdominal pain was unspecific, the personal, familial and genetic antecedents lead to the suspicion of pancreatic carcinoma. The relevant differential diagnosis is mainly pathological. When squamous component is found in a pancreatic tumor, ductal squamous metaplasia, adenosquamous carcinoma, mucoepidermoid carcinoma, pancreatoblastoma, and metastasis of a primary squamous carcinoma or a de-differentiated melanoma with adenocarcinoma -like component, should be considered.
All labs, including CA19.9 were within normal limits.
The computed tomography and endoscopic ultrasonography revealed a 30-mm solid-cystic mass in the body of the pancreas, with ill-defined borders, pancreatic tail atrophy, splenic vessels encasement and partial superior mesenteric artery and vein involvement.
Pancreatic adenosquamous carcinoma in an intraductal papillary mucinous neoplasm.
Neoadjuvant chemotherapy (FOLFIRINOX) and partial pancreatectomy followed by adjuvant chemotherapy.
Adenosquamous pancreatic carcinomas (ASPCs) are unfrequent have worse prognosis than pancreatic adenocarcinoma, and have rarely been described in a intraductal papillar mucinous neoplasm.
ASPC is a rare variant of pancreatic adenocarcinoma, characterized by the presence of squamous component in at least 30% of the tumoral mass. Intraductal papillary mucinous neoplasms (IPMN) are premalignant pancreatic cystic lesions which may need surgical resection. Cycline dependant kinase inhibitor 2A gene (CDKN2A) is a tumor suppressor gene that codes p16 or CDKN2A protein; mutations in this gene has been related to melanoma and pancreatic carcinoma, but also to head and neck, breast and lung cancers. Familial Atypical Mole Melanoma (FAMMM) includes families with a high tendency to develop malignant melanoma. Some of this families carry inherited mutations in CDKN2A.
ASPC is a rare variant of pancreatic carcinoma and may develop in intrapapillary mucinous neoplasms as the usual pancreatic adenocarcinoma. CDKN2A germinal mutation carriers bear a high risk of pancreatic carcinoma. Although evidence supporting pancreatic cancer screening is limited, the indication of a surveillance strategy might be discussed with the patient.
The authors report a rare case of ASPC arising in a FAMMM patient who is a carrier of CDKN2A germline mutation. Although it is well know that FAMMM patient has a high risk of occurring pancreatic carcinoma, ASPC arising in a background of IPMN has rarely been reported. Therefore the paper may enrich our knowledge on FAMMM associated pancreatic tumor.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Spain
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Gazouli M, Shi XY S- Editor: Gong ZM L- Editor: A E- Editor: Lu YJ
| 1. | Ducreux M, Cuhna AS, Caramella C, Hollebecque A, Burtin P, Goéré D, Seufferlein T, Haustermans K, Van Laethem JL, Conroy T. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26 Suppl 5:v56-v68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 905] [Cited by in RCA: 930] [Article Influence: 93.0] [Reference Citation Analysis (0)] |
| 2. | Lennon AM, Wolfgang CL, Canto MI, Klein AP, Herman JM, Goggins M, Fishman EK, Kamel I, Weiss MJ, Diaz LA. The early detection of pancreatic cancer: what will it take to diagnose and treat curable pancreatic neoplasia? Cancer Res. 2014;74:3381-3389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 171] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 3. | Grover S, Syngal S. Hereditary pancreatic cancer. Gastroenterology. 2010;139:1076-1080, 1080.e1-1080.e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 4. | Boyd CA, Benarroch-Gampel J, Sheffield KM, Cooksley CD, Riall TS. 415 patients with adenosquamous carcinoma of the pancreas: a population-based analysis of prognosis and survival. J Surg Res. 2012;174:12-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 123] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 5. | Voong KR, Davison J, Pawlik TM, Uy MO, Hsu CC, Winter J, Hruban RH, Laheru D, Rudra S, Swartz MJ. Resected pancreatic adenosquamous carcinoma: clinicopathologic review and evaluation of adjuvant chemotherapy and radiation in 38 patients. Hum Pathol. 2010;41:113-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 6. | Chen SB, Weng HR, Wang G, Yang JS, Yang WP, Liu DT, Chen YP, Zhang H. Primary adenosquamous carcinoma of the esophagus. World J Gastroenterol. 2013;19:8382-8390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 7. | Chen H, Shen C, Yin R, Yin Y, Chen J, Han L, Zhang B, Chen Z, Chen J. Clinicopathological characteristics, diagnosis, treatment, and outcomes of primary gastric adenosquamous carcinoma. World J Surg Oncol. 2015;13:136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Terada T. Adenosquamous carcinoma of the ovary arising from endometriosis: two case reports. Cases J. 2009;2:6661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 9. | Jamali M, Serra S, Chetty R. Adenosquamous carcinoma of the pancreas with clear cell and rhabdoid components. A case report. JOP. 2007;8:330-334. [PubMed] |
| 10. | Alwaheeb S, Chetty R. Adenosquamous carcinoma of the pancreas with an acantholytic pattern together with osteoclast-like and pleomorphic giant cells. J Clin Pathol. 2005;58:987-990. [PubMed] |
| 11. | Kardon DE, Thompson LD, Przygodzki RM, Heffess CS. Adenosquamous carcinoma of the pancreas: a clinicopathologic series of 25 cases. Mod Pathol. 2001;14:443-451. [PubMed] |
| 12. | Brody JR, Costantino CL, Potoczek M, Cozzitorto J, McCue P, Yeo CJ, Hruban RH, Witkiewicz AK. Adenosquamous carcinoma of the pancreas harbors KRAS2, DPC4 and TP53 molecular alterations similar to pancreatic ductal adenocarcinoma. Mod Pathol. 2009;22:651-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 13. | Borazanci E, Millis SZ, Korn R, Han H, Whatcott CJ, Gatalica Z, Barrett MT, Cridebring D, Von Hoff DD. Adenosquamous carcinoma of the pancreas: Molecular characterization of 23 patients along with a literature review. World J Gastrointest Oncol. 2015;7:132-140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 52] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 14. | Borazanci E, Millis SZ, Winkler J, Ramanathan RK, Von Hoff DD. Multiplatform profiling of rare pancreatic carcinoma subtypes to identify potential actionable targets. J Clin Oncol. 2014;32:abstr e15229 Available from: http://meetinglibrary.asco.org/content/135008-144. |
| 15. | Toshima F, Inoue D, Yoshida K, Yoneda N, Minami T, Kobayashi S, Ikdeda H, Matsui O, Gabata T. Adenosquamous carcinoma of pancreas: CT and MR imaging features in eight patients, with pathologic correlations and comparison with adenocarcinoma of pancreas. Abdom Radiol (NY). 2016;41:508-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 16. | Ding Y, Zhou J, Sun H, He D, Zeng M, Rao S. Contrast-enhanced multiphasic CT and MRI findings of adenosquamous carcinoma of the pancreas. Clin Imaging. 2013;37:1054-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Yin Q, Wang C, Wu Z, Wang M, Cheng K, Zhao X, Yuan F, Tang Y, Miao F. Adenosquamous carcinoma of the pancreas: multidetector-row computed tomographic manifestations and tumor characteristics. J Comput Assist Tomogr. 2013;37:125-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Imaoka H, Shimizu Y, Mizuno N, Hara K, Hijioka S, Tajika M, Kondo S, Tanaka T, Ogura T, Obayashi T. Clinical characteristics of adenosquamous carcinoma of the pancreas: a matched case-control study. Pancreas. 2014;43:287-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Goldstein AM, Chan M, Harland M, Hayward NK, Demenais F, Bishop DT, Azizi E, Bergman W, Bianchi-Scarra G, Bruno W, Calista D, Albright LA, Chaudru V, Chompret A, Cuellar F, Elder DE, Ghiorzo P, Gillanders EM, Gruis NA, Hansson J, Hogg D, Holland EA, Kanetsky PA, Kefford RF, Landi MT, Lang J, Leachman SA, MacKie RM, Magnusson V, Mann GJ, Bishop JN, Palmer JM, Puig S, Puig-Butille JA, Stark M, Tsao H, Tucker MA, Whitaker L, Yakobson E; Lund Melanoma Study Group; Melanoma Genetics Consortium (GenoMEL). Features associated with germline CDKN2A mutations: a GenoMEL study of melanoma-prone families from three continents. J Med Genet. 2007;44:99-106. [PubMed] |
| 20. | Canto MI, Harinck F, Hruban RH, Offerhaus GJ, Poley JW, Kamel I, Nio Y, Schulick RS, Bassi C, Kluijt I, Levy MJ, Chak A, Fockens P, Goggins M, Bruno M; International Cancer of Pancreas Screening (CAPS) Consortium. International Cancer of the Pancreas Screening (CAPS) Consortium summit on the management of patients with increased risk for familial pancreatic cancer. Gut. 2013;62:339-347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 546] [Cited by in RCA: 564] [Article Influence: 47.0] [Reference Citation Analysis (1)] |
| 21. | Syngal S, Brand RE, Church JM, Giardiello FM, Hampel HL, Burt RW. ACG Clinical Guideline: Genetic Testing and Management of Hereditary Gastrointestinal Cancer Syndromes. Am J Gastroenterol. 2015;110:223-262. [RCA] [DOI] [Full Text] [Cited by in Crossref: 957] [Cited by in RCA: 1090] [Article Influence: 109.0] [Reference Citation Analysis (0)] |
| 22. | Available from: http://www.pancreaticcancer.org.uk. |
| 23. | Okamura Y, Sugimoto H, Fujii T, Nomoto S, Takeda S, Nakao A. Adenosquamous carcinoma arising in an intraductal papillary mucinous neoplasm of the pancreas. Pancreas. 2010;39:945-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Matsuzaka S, Karasaki H, Ono Y, Ogata M, Oikawa K, Tamakawa S, Chiba S, Muraki M, Yokochi T, Funakoshi H. Tracking the Clonal Evolution of Adenosquamous Carcinoma, a Rare Variant of Intraductal Papillary Mucinous Neoplasm of the Pancreas. Pancreas. 2016;45:915-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Brosens LA, Hackeng WM, Offerhaus GJ, Hruban RH, Wood LD. Pancreatic adenocarcinoma pathology: changing “landscape”. J Gastrointest Oncol. 2015;6:358-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 34] [Reference Citation Analysis (0)] |
| 26. | Olson MT, Siddiqui MT, Ali SZ. The differential diagnosis of squamous cells in pancreatic aspirates: from contamination to adenosquamous carcinoma. Acta Cytol. 2013;57:139-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Agaimy A, Specht K, Stoehr R, Lorey T, Märkl B, Niedobitek G, Straub M, Hager T, Reis AC, Schilling B. Metastatic Malignant Melanoma With Complete Loss of Differentiation Markers (Undifferentiated/Dedifferentiated Melanoma): Analysis of 14 Patients Emphasizing Phenotypic Plasticity and the Value of Molecular Testing as Surrogate Diagnostic Marker. Am J Surg Pathol. 2016;40:181-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 96] [Article Influence: 10.7] [Reference Citation Analysis (0)] |