Published online Jun 15, 2017. doi: 10.4251/wjgo.v9.i6.251
Peer-review started: Janaury 12, 2017
First decision: March 7, 2017
Revised: April 3, 2017
Accepted: May 22, 2017
Article in press: May 24, 2017
Published online: June 15, 2017
Processing time: 153 Days and 7.6 Hours
To evaluate a step up approach: Taking macrobiopsies and performing excision biopsies in patients with suspected rectal cancer in which biopsies taken though the flexible endoscope showed benign histology.
Patients with a rectal neoplasm who underwent flexible endoscopy and biopsies were included. In case of benign biopsies rigid rectoscopy and macrobiopsies were employed. If this failed to prove malignancy, transanal endoscopic microsurgery (TEM) was used in a final effort to establish a certain preoperative diagnosis. The preoperative results were compared with the findings after surgical excision and follow up to calculate the reliability of this algorithm.
One hundred and thirty-two patients were included. One hundred and ten patients with a carcinoma and 22 with an adenoma. Seventy-five of 110 carcinomas were proven malignant after flexible endoscopy. With the addition of rigid endoscopy and taking of macrobiopsies, this number increased to 89. Performing TEM excision biopsies further enlarged the number of proven malignancies to 100.
The step-up approach includes taking macrobiopsies through the rigid rectoscope and performing excision biopsies using transanal endoscopic microsurgery in addition to flexible endoscopy. This approach, reduced the number of missed preoperative malignant diagnoses from 32% to 9%.
Core tip: Increasing the number of biopsies taken through a flexible endoscope, taking macrobiopsies and performing excision biopsies with transanal endoscopic microsurgery can reduce the number of missed preoperative malignant diagnoses in patients with rectal cancer.
- Citation: Bökkerink GMJ, van der Wilt GJ, de Jong D, van Krieken HHJM, Bleichrodt RP, de Wilt JHW, Bremers AJA. Value of macrobiopsies and transanal endoscopic microsurgery in the histological work-up of rectal neoplasms: A retrospective study. World J Gastrointest Oncol 2017; 9(6): 251-256
- URL: https://www.wjgnet.com/1948-5204/full/v9/i6/251.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v9.i6.251
Adequate pre-treatment histological sampling is of paramount importance for the optimal treatment of rectal neoplasms. A wide spectrum of surgical and neoadjuvant treatments is available. In case of benign disease, surgical excision alone, will suffice. For a majority of the malignant tumors however, a combination of neoadjuvant therapy and total mesorectal excision is indicated to optimize local control[1-5]. High complete response rates after chemoradiation therapy have led to the development of organpreserving strategies[6-8].
Although the oncological benefits of neoadjuvant treatments are evident, the acute toxicity and long term side effects of chemoradiation therapy are considerable. Therefore, administration of neoadjuvant chemoradiation therapy requires definite proof of malignancy. As the diagnosis of malignancy based on imaging alone may be erroneous because of the risk of overstaging MRI based imaging, these neoadjuvant treatments require histological evidence of malignancy before treatment can commence.
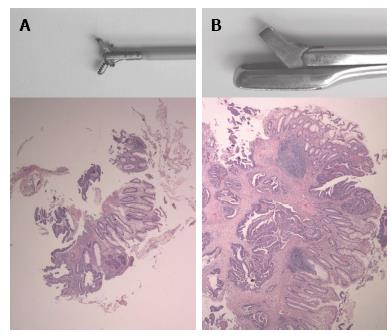
A preoperative histological diagnosis is usually obtained by taking biopsies through a flexible endoscope. Flexible endoscopy offers a high tumor detection rate[9] and the possibility to take biopsies. However, from limited evidence available, sensitivity for malignancy on these biopsies is suboptimal at best[10-12]. The most important reason for this is that biopsies taken through flexible endoscopes are small and sometimes too superficial to demonstrate high grade neoplasia[13]. In case of superficial biopsies, the diagnosis of malignancy relies solely on tissue structure and atypical appearance of cells (Figure 1). One way to overcome this problem is to take more biopsies. Indeed, several authors demonstrated a correlation between sensitivity and the number of biopsies taken from a suspected lesion. When 3 or 4 biopsies were taken, the sensitivity for invasive growth varied between 50% and 86%[10-12]. By taking up to 10 biopsies, the sensitivity increased to 78% to 100% (Table 1).
| Number of biopsies | ≤ 2 | 3 | 4 | 5 | 6 | 7 | 8 | ≥ 9 | |
| Marshall (1993) | Sens | 68.3 | 78.3 | 78.3 | 78.3 | ||||
| n = 701 | 70 | 70 | 70 | 70 | |||||
| Colleypriest (2009) | Sens | 80% | 86% | 86% | 88% | 98% | 100% | 98% | 100% |
| n = 217 | Not specified | ||||||||
| Dabos (2011) | Sens | 50% | 72% | 70% | 76% | 88% | 91% | 100% | |
| n = 149 | Not specified | ||||||||
| Current study | Sens | 40% | 30% | 76% | 75% | 83% | 50% | 91% | 72% |
| n = 113 | 7 | 12 | 21 | 17 | 14 | 16 | 13 | 13 |
Another way to increase the sensitivity of pre-treatment histological sampling for the detection of malignancy is to increase the volume and depth of the biopsy. Although considered old-fashioned by many clinicians, rigid rectoscopy is an easy, cost effective, fast and well-tolerated tool for examination of the rectum[14], that enables the endoscopist to take so-called “macrobiopsies”. Macrobiopsies are 2-10 times larger in three dimensions and approximately 50 times larger in volume than those obtained with flexible rectoscopy. The rigidity of the biopsy forceps also enables the endoscopist to push the forceps against the tumor so that deeper layers of the rectal wall can be included in the biopsy, and to “palpate” the lesion and take the biopsies from the firmer parts of the lesion selectively. For these reasons, rigid rectoscopy may perform better with respect to sampling error than flexible endoscopy.
Sometimes, even macrobiopsies may fail to demonstrate invasive growth. In an ultimate effort to obtain sufficient histological confirmation of malignancy without interfering with the optimal treatment strategy, transanal endoscopic microsurgery (TEM) may be used in these cases to perform an excision biopsy. TEM is an invasive way to obtain a histological diagnosis. However, it does have the advantage that it can sometimes be used as a definitive treatment for low risk T1 carcinomas.
Although there are sound theoretical grounds to expect that rigid rectoscopy and TEM can boost the sensitivity of the pre-treatment histological work-up for suspected rectal cancer, this has never been empirically investigated. The aim of this article, therefore, is to assess the accuracy, therapeutic value and tolerability of taking additional macrobiopsies and performing excision biopsies with TEM in patients with suspected rectal cancer: a step-up approach.
All patients who underwent biopsy through a flexible endoscope, as part of the work-up for surgery of a rectal neoplasm, between January 2005 and January 2011 in the Radboud University Nijmegen Medical Center, Nijmegen, The Netherlands were analyzed. Patient selection was based on the database of surgical procedure in our hospital. All patients who underwent surgical excision of a rectal neoplasm [local excision; transanal endoscopic microsurgery or total or partial mesorectal excision: Abdomino perineal resection or (low) anterior resetion] where selected. The medical records of all patients were reviewed for demographic characteristics and for endoscopy, pathology and surgical reports.
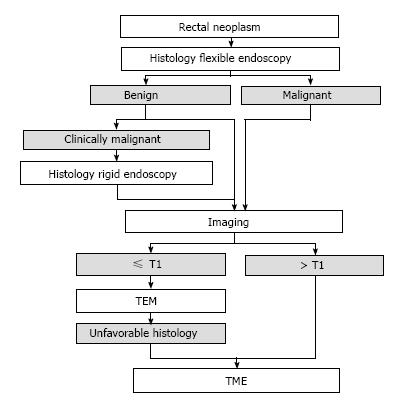
This is a retrospective analysis of the diagnostic and therapeutic step-up algorithm, which was followed during the study period. This algorithm is shown in Figure 2. Macrobiopsies were taken through the rigid sigmoidoscope in case of benign histology after flexible endoscopy and persisting clinical or radiological suspicion for malignancy, macrobiopsies were taken through rigid rectoscopy. TEM was performed in case of a benign or cT1 tumor on endorectal ultrasound (ERUS).
Flexible endoscopes were the CF140S 70 cm sigmoidoscope and CF 140 I colonoscope (Olympus, Tokyo, Japan). For flexible endoscopy, a 2.2 mm radial jaw biopsy forceps was used (Boston Scientific, Natick, United States) (Figure 1). For colonoscopy complete bowel preparation was used. Sedation and analgesia given upon request. During colonoscopy multiple biopsies were taken from any suspicious lesions. A 250 mm × 18 mm disposable rectoscopy tube, Heine, Herrsching, Germany was used for rigid rectoscopy. Biopsies were taken with a Franital biopsy forceps with a 5 mm × 10 mm bite (Figure 1). Bowel preparation before rigid and flexible sigmoidoscopy consisted of a single soap enema. All procedures were performed by, or under direct supervision of, consultant level surgeons or gastroenterologists.
TEM-surgery was performed by one of the authors (AB) as first described by Buess[15] using the stereo-optic Wolf rectoscope (Wolf, Knittlingen, Germany).
The additional yield of taking macrobiopsies and performing excision biopsies was analyzed by comparing all biopsies with the definitive excision specimen. The differences in sensitivity between the number of samples taken through the flexible endoscope was tested with the χ2 test for trends.
One hundred and thirty-two patients (82 males and 50 females) underwent flexible endoscopy with biopsies as part of the work-up for a rectal neoplasm (tumor located below 15 cm from the anal verge). Median age was 63 years (range: 27-92).
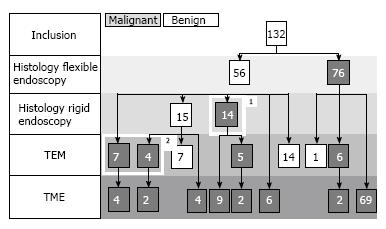
The histological work-up of all 132 patients is shown in Figure 3. At final pathology 110 patients had an adenocarcinoma, of which 75 (68%) were detected with flexible endoscopy only. The other 22 patients had a villous adenoma. One of the tumors, classified as malignant based on biopsies taken through the flexible endoscope (snare polypectomy), showed benign histology after (transanal) resection.
The number of biopsies was documented for 113 patients and varies from 1 to 14, with a median of 4 biopsies (Table 1). There was a significant correlation between the number of biopsies and a correct histological diagnosis (P = 0.020; 2-sided χ2 test for trends). Taking 4 or more biopsies resulted in a significant higher sensitivity than taking 3 or less (P = 0.004; 2-sided χ2 test for trends). Prior probability of malignancy was 83.3% in this group. Sensitivity and specificity were 68% and 95% respectively. A malignant result is useful with a posterior probability of malignancy of 99% (95%CI: 92%-100%). Benign histology after flexible endoscopy is clearly inconclusive, leaving a posterior probability of malignancy of 62% (95%CI: 55%-69%).
In 29 of the 56 patients who were diagnosed with a benign tumor after flexible endoscopy, additional rigid endoscopy was performed. With this addition, 14 previously undetected carcinomas were diagnosed. In this selected group of 29 patients who underwent rigid endoscopy, prior probability of malignancy was 75.9%. Sensitivity and specificity were 64% and 100% respectively, which makes a malignant histology after rigid endoscopy useful with a posterior probability of malignancy of 100% (95%CI: 68%-100%). Benign histology after rigid endoscopy is leaves a posterior probability of malignancy of 53% (95%CI: 41%-68%). The remaining 27 patients did not undergo additional macrobiopsies taken through a rigid endoscope because there was no clinical suspicion of malignancy and endorectal ultrasound did not show invasion deeper than the submucosa (clinical benign or clinical T1). Further management was not dependent on histology analysis, since these lesions were regarded as indication for TEM for complete removal.
A total of 44 patients underwent TEM (Figure 3), 32 patients after benign biopsies (combined flexible and rigid), 12 after malignant biopsies (clinical and radiological T1). With this addition, another 11 invasive carcinomas were detected. The number of detected carcinomas increased from 89 out of 110 (81%) to 100 out of 110 (91%).
Histology after TEM showed 18 adenomas, 4 in situ carcinomas, and 22 carcinomas. After TEM, 10 patients underwent a completion TME because of unfavorable histological findings. The excision specimen of one of these 10 patients was perforated at the former local excision site. One patient with an ypT3 tumor was unfit to undergo a total mesorectal excision and was treated with short course radiotherapy and TEM after a 6 wk interval. No major complications were observed nor preoperative perforations or conversions to laparotomy after TEM in this group. One patient with postoperative rectal blood loss needed transfusion.
A total of 79 patients received neoadjuvant treatment in 4 different schemes according to tumor stage and general condition. Thirty-eight patients received 5 Gy × 5 Gy in the week prior to surgery according to protocol for T2 and T3 tumors. Thirty Patients with a radiologically involved circumferential resection margin received neoadjuvant chemoradiation therapy (25 Gy × 2 Gy with concomitant capecitabine) and delayed surgery after 8 wk. Eleven patients whose general condition did not allow chemoradiation therapy (CRT) and who required tumor regression received 5 Gy × 5 Gy (n = 9) or long course radiotherapy (24 Gy × 2 Gy) (n = 2) and delayed surgery as decided by a multidisciplinary team.
Forty-four patients underwent TEM, 53 underwent a LAR and a further 34 underwent APR, 1 patient with MSH6 mutation underwent a subtotal colectomy with LAR. After TEM 10 patients underwent a completion TME. Definitive histology after resection showed 18 adenomas, 4 in situ carcinomas, 101 carcinomas and 9 complete responses after neoadjuvant treatment.
In the present study we demonstrated that macrobiopsies obtained through a rigid endoscope and excision biopsies by TEM are valuable additional tools to obtain a correct preoperative histological diagnosis in a significant number of patients with suspected rectal cancer.
Over time, flexible endoscopy has replaced rigid rectoscopy because of its superior (videoscopic) visualization of the entire colon, better mobility and deeper intubation[16-20] and subsequently a good tumor detection rate[9]. However, when it comes to the diagnostic sensitivity to detect malignancy in rectal tumors, our results are in accordance with the literature and confirm the disappointing overall performance of flexible endoscopy. The proportion of false negative biopsies after flexible endoscopy alone was 32%. This can be explained by the number of biopsies taken in our study. With a median number of biopsies of 4, a sensitivity of 70% can be expected.
Increasing the number of biopsies with flexible endoscopy can increase the number of detected malignancies in the group of suspicious rectal neoplasms (Table 1). However, increasing the number of biopsies through flexible endoscopy, as suggested by some authors[10-12], was not our main strategy to increase diagnostic sensitivity, because these biopsies are often too superficial to show high grade neoplasia[13]. Our algorithm included rigid endoscopy and TEM as additional steps.
In terms of accuracy, the selected group of patients with false negative biopsies after flexible endoscopy, 14 additional patients with a malignancy were identified with rigid endoscopy, and with TEM, another 11 patients. In total, 100 of 110 malignancies could be diagnosed preoperatively. This means that the proportion of carcinomas of which the malignant nature would have been proven in time was 32% with flexible endoscopy alone and was reduced to 9% in the evaluated algorithm. This is a significant reduction with high therapeutic value.
Regarding procedure-related morbidity, both rigid endoscopy and TEM were well-tolerated. In our experience, TEM did not cause an increase in positive circumferential resection margins (CRM) in TME as determined by standardized pathological evaluation according to Quircke[21].
With the current treatment options for patients with rectal cancer, optimal preoperative histological diagnosis is essential. Besides the combinations with radical surgery, multimodality organ sparing treatments are becoming more and more accepted. Short-term results show high percentages of pathologic complete response[6,22] and acceptable oncological outcome[6,7], adequate histological sampling seems of paramount importance for these new treatment strategies, not only before but also after (chemo)radiation therapy.
In the present study we demonstrated that macrobiopsies obtained through a rigid endoscope and excision biopsies by TEM are valuable additional tools in obtaining a correct preoperative histological diagnosis in a significant number of patients with suspected rectal cancer. Prospective trials are needed to compare the yield of these strategies to increasing the amount of biopsies through flexible endoscopy. Evidence-based recommendations for guidelines regarding the histological work-up of rectal neoplasms can be based on those trials.
Histological sampling is one of the key components of the work-up for rectal neoplasms. For neoadjuvant and radical surgical treatments histological proof of invasive growth is mandatory. It can be difficult to obtain this proof with flexible endoscopy only. There are only a few publications available in which the sensitivity for malignancy of biopsies taken through a flexible endoscope is discussed. The aim of this study was to evaluate a step up approach: Taking macrobiopsies and performing excision biopsies in patients with suspected rectal cancer in which biopsies taken though the flexible endoscope showed benign histology.
An important subject in current rectal cancer research is the evaluation of organ sparing treatment techniques. Adequate pre-treatment histological sampling is of paramount importance for this treatment technique.
Other studies evaluating the value of macrobiopsies and excision biopsies are not available in literature. More studies with larger populations need to be done to confirm the results from this study.
This study can motivate the reader to take macrobiopsies and perform excision biopsies in daily practice.
Excision biopsy: Transanal local excision of (a part of) a rectal malignancy with the intention to assess its histology; Macrobiopsy: Large biopsy taken through a rigid recto- or sigmoidoscope.
Bökkerink et al describe the use of macrobiopsies in the diagnosis of rectal cancer.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: The Netherlands
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Cathomas G S- Editor: Qi Y L- Editor: A E- Editor: Lu YJ
| 1. | van Gijn W, Marijnen CA, Nagtegaal ID, Kranenbarg EM, Putter H, Wiggers T, Rutten HJ, Påhlman L, Glimelius B, van de Velde CJ. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. 2011;12:575-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1138] [Cited by in RCA: 1345] [Article Influence: 96.1] [Reference Citation Analysis (0)] |
| 2. | Sebag-Montefiore D, Stephens RJ, Steele R, Monson J, Grieve R, Khanna S, Quirke P, Couture J, de Metz C, Myint AS. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): a multicentre, randomised trial. Lancet. 2009;373:811-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1219] [Cited by in RCA: 1111] [Article Influence: 69.4] [Reference Citation Analysis (2)] |
| 3. | Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, Daban A, Bardet E, Beny A, Ollier JC. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355:1114-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1993] [Cited by in RCA: 2040] [Article Influence: 107.4] [Reference Citation Analysis (0)] |
| 4. | Gérard JP, Conroy T, Bonnetain F, Bouché O, Chapet O, Closon-Dejardin MT, Untereiner M, Leduc B, Francois E, Maurel J. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: results of FFCD 9203. J Clin Oncol. 2006;24:4620-4625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731-1740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4342] [Cited by in RCA: 4460] [Article Influence: 212.4] [Reference Citation Analysis (1)] |
| 6. | Verseveld M, de Graaf EJ, Verhoef C, van Meerten E, Punt CJ, de Hingh IH, Nagtegaal ID, Nuyttens JJ, Marijnen CA, de Wilt JH. Chemoradiation therapy for rectal cancer in the distal rectum followed by organ-sparing transanal endoscopic microsurgery (CARTS study). Br J Surg. 2015;102:853-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 138] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 7. | Garcia-Aguilar J, Renfro LA, Chow OS, Shi Q, Carrero XW, Lynn PB, Thomas CR, Chan E, Cataldo PA, Marcet JE. Organ preservation for clinical T2N0 distal rectal cancer using neoadjuvant chemoradiotherapy and local excision (ACOSOG Z6041): results of an open-label, single-arm, multi-institutional, phase 2 trial. Lancet Oncol. 2015;16:1537-1546. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 249] [Cited by in RCA: 296] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 8. | Bökkerink GM, de Graaf EJ, Punt CJ, Nagtegaal ID, Rütten H, Nuyttens JJ, van Meerten E, Doornebosch PG, Tanis PJ, Derksen EJ. The CARTS study: Chemoradiation therapy for rectal cancer in the distal rectum followed by organ-sparing transanal endoscopic microsurgery. BMC Surg. 2011;11:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 9. | Lin JS, Piper MA, Perdue LA, Rutter CM, Webber EM, O’Connor E, Smith N, Whitlock EP. Screening for Colorectal Cancer: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2016;315:2576-2594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 598] [Cited by in RCA: 558] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 10. | Dabos KJ, Gousi T, Navrozoglou A, Kodogeorgou E, Papadopoulos M. Optimal number of biopsies to correctly identify colorectal cancer during colonoscopy. Scand J Gastroenterol. 2011;46:247-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 11. | Colleypriest BJ, Marden PF, Linehan JD. What is the optimal number of biopsies to diagnose a tumor found during colonoscopy? J Clin Gastroenterol. 2009;43:1012-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Marshall JB, Diaz-Arias AA, Barthel JS, King PD, Butt JH. Prospective evaluation of optimal number of biopsy specimens and brush cytology in the diagnosis of cancer of the colorectum. Am J Gastroenterol. 1993;88:1352-1354. [PubMed] |
| 13. | Gondal G, Grotmol T, Hofstad B, Bretthauer M, Eide TJ, Hoff G. Biopsy of colorectal polyps is not adequate for grading of neoplasia. Endoscopy. 2005;37:1193-1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Takahashi T, Zarate X, Velasco L, Mass W, Garcia-Osogobio S, Jimenez R, Tellez O, Ponce-de-Leon S. Rigid rectosigmoidoscopy: still a well-tolerated diagnostic tool. Rev Invest Clin. 2003;55:616-620. [PubMed] |
| 15. | Buess G, Hutterer F, Theiss J, Böbel M, Isselhard W, Pichlmaier H. [A system for a transanal endoscopic rectum operation]. Chirurg. 1984;55:677-680. [PubMed] |
| 16. | Wilking N, Petrelli NJ, Herrera-Ornelas L, Walsh D, Mittelman A. A comparison of the 25-cm rigid proctosigmoidoscope with the 65-cm flexible endoscope in the screening of patients for colorectal carcinoma. Cancer. 1986;57:669-671. [PubMed] |
| 17. | Vellacott KD, Hardcastle JD. An evaluation of flexible fibreoptic sigmoidoscopy. Br Med J (Clin Res Ed). 1981;283:1583-1586. [PubMed] |
| 18. | Marks G, Boggs HW, Castro AF, Gathright JB, Ray JE, Salvati E. Sigmoidoscopic examinations with rigid and flexible fiberoptic sigmoidoscopes in the surgeon’s office: a comparative prospective study of effectiveness in 1,012 cases. Dis Colon Rectum. 1979;22:162-168. [PubMed] |
| 19. | Bohlman TW, Katon RM, Lipshutz GR, McCool MF, Smith FW, Melnyk CS. Fiberoptic pansigmoidoscopy. An evaluation and comparison with rigid sigmoidoscopy. Gastroenterology. 1977;72:644-649. [PubMed] [DOI] [Full Text] |
| 20. | Winnan G, Berci G, Panish J, Talbot TM, Overholt BF, McCallum RW. Superiority of the flexible to the rigid sigmoidoscope in routine proctosigmoidoscopy. N Engl J Med. 1980;302:1011-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 73] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Quirke P, Durdey P, Dixon MF, Williams NS. Local recurrence of rectal adenocarcinoma due to inadequate surgical resection. Histopathological study of lateral tumour spread and surgical excision. Lancet. 1986;2:996-999. [PubMed] [DOI] [Full Text] |
| 22. | Garcia-Aguilar J, Shi Q, Thomas CR, Chan E, Cataldo P, Marcet J, Medich D, Pigazzi A, Oommen S, Posner MC. A phase II trial of neoadjuvant chemoradiation and local excision for T2N0 rectal cancer: preliminary results of the ACOSOG Z6041 trial. Ann Surg Oncol. 2012;19:384-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 216] [Article Influence: 15.4] [Reference Citation Analysis (0)] |