Published online Aug 15, 2016. doi: 10.4251/wjgo.v8.i8.615
Peer-review started: March 28, 2016
First decision: May 16, 2016
Revised: May 24, 2016
Accepted: June 1, 2016
Article in press: June 3, 2016
Published online: August 15, 2016
Processing time: 134 Days and 5.2 Hours
AIM: To investigate the role of the Wnt/β-catenin pathway in pancreatic neuroendocrine neoplasms (PanNENs).
METHODS: Tissue microarrays containing 88 PanNENs were immunohistochemically labeled with antibodies to β-catenin, E-cadherin, adenomatous polyposis coli (APC), chromogranin and synaptophysin. One case had only metastatic tumors resected, whereas others (n = 87) received pancreatectomy with or without partial hepatectomy. Pathology slides, demographic, clinicopathologic, and follow up data were reviewed. Patients’ demographics, clinicopathologic features, and immunohistochemical results from 87 primary tumors were compared between patients with low stage (stage I/II) and high stage (stage III/IV) tumors. In addition, correlation of immunohistochemical results from primary tumors with disease-specific survival (DSS) was evaluated.
RESULTS: Strong membranous β-catenin staining in the primary tumor was observed in all 13 stage III/IV PanNENs as compared to 47% (35/74) of stage I/II tumors (P < 0.01). However, the strong membranous β-catenin staining was unassociated with tumor grade or DSS. Decreased membranous β-catenin staining was associated with decreased membranous E-cadherin labeling. Nuclear β-catenin staining was seen in 15% (2/13) of stage III/IV PanNENs as compared to 0% (0/74) of stage I/II tumors (P = 0.02). The case with metastasectomy also only showed nuclear β-catenin staining. Two of the three cases with nuclear β-catenin staining were familial adenomatous polyposis (FAP) patients. Lack of APC expression was seen in 70% (57/81) of the cases, including the 3 cases with nuclear β-catenin staining. Expression of E-cadherin and APC in primary tumor was not correlated with tumor grade, tumor stage, or disease specific survival.
CONCLUSION: The Wnt/β-catenin pathway was altered in some PanNENs, but did not Impact DSS. PanNENs in FAP patients demonstrated nuclear β-catenin accumulation and loss of APC.
Core tip: Dysregulation of the Wnt/β-catenin pathway is present in some pancreatic neuroendocrine neoplasms (PanNENs). However, compared to other malignancies, this signaling pathway may have different functions in PanNENs as strong membranous β-catenin expression is more frequently present in stage III/IV tumors and membranous expression of β-catenin and E-cadherin does not have a prognostic importance in this setting. Additionally, PanNENs arising in patients with familial adenomatous polyposis (FAP) demonstrate abnormal nuclear β-catenin accumulation, and PanNENs may be one of the extraintestinal manifestations of FAP.
- Citation: Weiss V, Dueber J, Wright JP, Cates J, Revetta F, Parikh AA, Merchant NB, Shi C. Immunohistochemical analysis of the Wnt/β-catenin signaling pathway in pancreatic neuroendocrine neoplasms. World J Gastrointest Oncol 2016; 8(8): 615-622
- URL: https://www.wjgnet.com/1948-5204/full/v8/i8/615.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v8.i8.615
Although rare, pancreatic neuroendocrine neoplasms (PanNENs) are the second most common malignancy in the pancreas, accounting for 1%-2% of pancreatic tumors[1,2]. Most PanNENs are well-differentiated, with a 10 year survival rate of approximately 40%. Based on mitotic count and Ki67 labeling index, the World Health Organization (WHO) 2010 has classified PanNENs into three grades: Grade 1 with Ki67 ≤ 2.0% and mitotic rate < 2 mitoses/10 high power fields (HPFs), grade 2 with Ki67 3%-20% or mitotic rate 2-20 mitoses/HPFs, and grade 3 with Ki67 > 20% or mitotic rate > 20 mitoses/HPFs[3]. Grade 1 and 2 tumors are considered as well-differentiated neuroendocrine tumors, while grade 3 PanNENs include well-differentiated tumors with Ki67 > 20% and poorly differentiated neuroendocrine carcinomas[4].
PanNENs, like many other tumors, are thought to evolve following cellular dysregulation and proliferation. One of the pathways known to be involved in normal cell growth, proliferation, differentiation, and apoptosis is the Wnt/β-catenin signaling pathway. It is then conceivable that dysregulation of this critical homeostatic pathway may lead to tumor growth and proliferation. In fact, most colonic adenocarcinomas arise from tubular adenomas through dysregulation of the Wnt/β-catenin pathway[5]. Gastrointestinal well-differentiated neuroendocrine tumors, colonic glandular-neuroendocrine mixed tumors, and pancreatic acinar cell tumors have all been reported to have mutations and/or altered expression in the Wnt/β-catenin pathway[6-10]. A subset of ileal neuroendocrine neoplasms have been shown to have adenomatous polyposis coli (APC) mutations or loss of heterozygosity[11]. However, it is unclear whether this pathway plays a significant role in pancreatic endocrine tumorigenesis. In this study, we assessed the expression of β-catenin, E-cadherin and APC in PanNENs.
This study was approved by the Vanderbilt Institutional Review Board. Tissue microarrays (TMAs) were constructed from the resection specimens of 88 PanNEN patients who underwent pancreatectomy only (n = 84), pancreatecomy with partial hepatectomy (n = 3), and metastasectomy (partial hepatectomy) only (n = 1) at Vanderbilt University Medical Center from 01/1998 to 08/2012. The TMAs included 2-6 tumor and 1-2 normal pancreatic tissue cores from each patient studied. Demographic and clinicopathologic information was abstracted from the electronic medical record. Pathologic data were also recorded upon reviewing of original hematoxylin and eosin-stained slides. The American Joint Committee on Cancer TNM staging system 7th edition was used to divide PanNENs into four stages: Stage I(T1-2N0M0), stage II (T3N0M0 or T1-3N1M0), stage III (T4N0-1M0), and stage IV (T1-4N0-1M1). Stage I and II PanNENs were grouped into low stage disease, whereas stage III and IV high stage disease. Patient outcomes were confirmed using the social security death index.
Immunohistochemical labeling for β-catenin, E-cadherin, APC, chromogranin and synaptophysin was performed. Details of the antibodies used in this study are provided in Table 1.
| Antibody | Source | Dilution |
| β-catenin | BD Bioscience CAT# 610154 Mouse monoclonal (San Jose, CA) | 0.7361111111 |
| E-cadherin | BD Bioscience CAT# 610182 Mouse Monoclonal (San Jose, CA) | 0.5972222222 |
| APC | Abcam (ab15270) Rabbit Polyclonal (Cambridge, MA) | 0.1805555556 |
| Chromogranin | Abcam (ab15160) Rabbit polyclonal (Cambridge, MA) | 0.3888888889 |
| Synaptophysin | Abcam (ab32127) Rabbit monoclonal (Cambridge, MA) | 0.3888888889 |
Unstained slides were first deparaffinized by routine methods. Antigen retrieval was performed as follows: Slides were heated in citrate buffer (pH 6.0) at 100 °C for 20 min or in EDTA (pH 9.0) at 98 °C for 20 min, followed by a 10-min cool down to room temperature. All slides were then quenched with 0.03% (v/v) H2O2 with NaN3 for 5 min. Slides were blocked for 20 min with serum-free protein block (Dako, Carpinteria, CA), followed by application of primary antibodies. Slides were then incubated with Evision + HRP-labeled polymer (Dako) for 30 min, followed by 5 min incubation with DAB (Dako).
Immunohistochemical stains were reviewed by two pathologists (VW and CS). Membranous βcatenin and E-cadherin staining was scored as negative (0), weak (1), moderate (2) or strong (3). Scores from replicate cores were averaged. Those with an average score > 2 were considered strong labeling, and those with ≤ 2 as decreased. Nuclear β-catenin and cytoplasmic APC staining was scored as positive and negative (in at least 10% of the tumor cells). Immunohistochemical results from primary tumor were used for statistical analysis.
Immunohistochemical labeling for β-catenin, E-cadherin and APC was compared between PanNENs and normal pancreas using the Fisher’s exact test. Immunohistochemical results were also correlated with clinicopathologic parameters using standard bivariate methods. Kaplan-Meier disease-specific survival (DSS) curves were plotted and compared by Cox proportional hazards regression using the Stata® software package (v13, StataCorp, College Station, TX). All reported P values were derived from two-tailed hypothesis tests.
The 88 patients included 46 females and 42 males. The demographics and clinicopathological features of the 87 cases with primary tumor resected are listed in Table 2. While 85% (74/87) of the tumors were WHO grade 1 or 2 well-differentiated tumors, 15% (13/87) were WHO grade 3 well-differentiated tumors with Ki67 > 20%. Poorly differentiated neuroendocrine carcinomas including small cell carcinomas and large cell neuroendocrine carcinomas were excluded from this study as they are known to have very different molecular and biologic features from well-differentiated tumors[3,4]. Most patients (74/87, 85%) had low stage tumors (stage I/II). High stage tumors were associated with large tumor size, high tumor grade, infiltrative growth pattern and lymphovascular invasion, but did not show associations with age, gender, syndrome, tumor functionality, perineural invasion and tumor necrosis (Table 2).
| Low stage (n = 74) | High stage (n = 13) | Total (n = 87) | |
| Average age (range) | 56 (32-81 yr) | 50 (35-77 yr) | 55 (32-81 yr) |
| Male/female | 34/40 | 7/6 | 41/46 |
| Syndromic (%) | 11/74 (15) | 4/13 (31) | 15/87 (17) |
| Functional (%) | 15/74 (20) | 0/13 (0) | 15/87 (17) |
| Tumor sizea (mean ± SEM) | 2.8 ± 0.3 cm | 4.4 ± 0.5 cm | 3.0 ± 0.2 cm |
| Tumor gradea (%) | |||
| Grade 1/2 | 67/74 (91) | 7/13 (54) | 74/87 (85) |
| Grade 3 | 7/74 (9) | 6/13 (46) | 13/87 (15) |
| Infiltrative growth patterna | 21/74 (28) | 8/13 (58) | 29/87 (33) |
| LVIb | 20/74 (28) | 12/13 (92) | 32/87 (37) |
| PNI | 9/74 (12) | 3/13 (25) | 12/87 (14) |
| Necrosis | 8/74 (11) | 3/13 (25) | 11/87 (13) |
| Strong membranous β-cateninb | 35/74 (47) | 12/13 (92) | 48/87 (55) |
| Nuclear β-catenina | 0/74 (0) | 2/13 (15) | 3/87 (3) |
| Strong membranous E-cadherin (%) | 30/74 (41) | 8/13 (62) | 38/87 (44) |
| APC | 22/67 (33) | 2/13 (15) | 24/80 (30) |
| Death from diseaseb | 7/74 (10) | 6/13 (50) | 13/87 (15) |
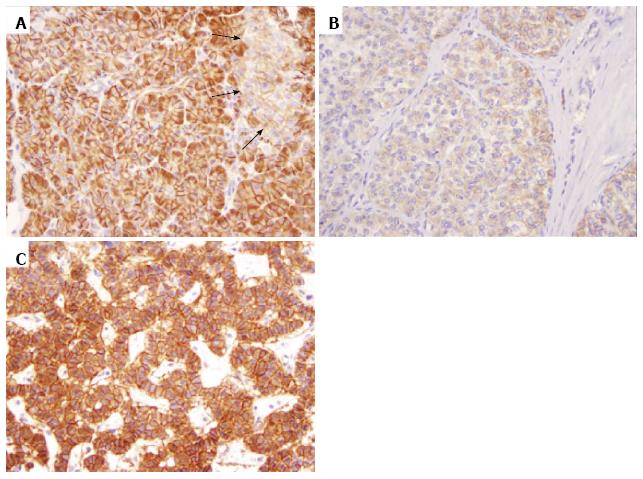
To explore whether the Wnt/β-catenin pathway was altered in PanNENs, TMAs were labeled with antibodies to β-catenin, E-cadherin and APC. Membranous β-catenin labeling was strong in the exocrine pancreas, but was weak in normal pancreatic islets (Figure 1A). Over half of the PanNENs studied (48/87, 55%) displayed strong membranous staining for β-catenin in the primary tumor (Figure 1B and C, Table 2). Interestingly, all 13 stage III/IV PanNENs strongly expressed membranous β-catenin in primary tumor, compared to only 47% (35/74) of stage I/II tumors (Table 2, P < 0.01). There was no correlation between membranous β-catenin staining and tumor grade.
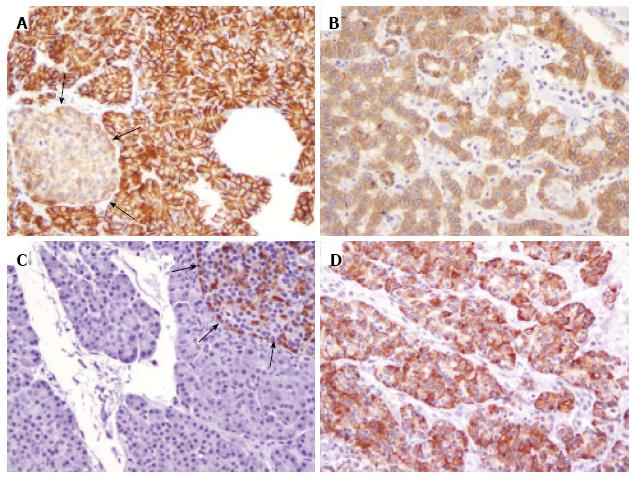
As expected, most primary tumors (85/87, 98%) were negative for nuclear β-catenin labeling (Table 2). However, when stratified by tumor stage, nuclear β-catenin staining was more often present in tumors of high stage (2/13, 15% vs 0/74, 0% of stage I/II tumors; P = 0.02). In addition, the case with metastasectomy only (stage IV) also showed nuclear β-catenin staining. Histologically, each of the 3 tumors with nuclear β-catenin labeling showed the typical morphologic features of well-differentiated neuroendocrine tumors (Figure 2A and D). Additional immunohistochemical labeling was subsequently performed to rule out solid-pseudopapillary neoplasms (SPNs), a pancreatic tumor characterized by nuclear accumulation of β-catenin and loss of membranous E-cadherin. Each expressed both neuroendocrine markers (synaptophysin and chromogranin, Figure 2B and C) and E-cadherin (membranous, Figure 2E), consistent with neuroendocrine neoplasms.
Careful review of the clinical history disclosed that two of the three cases with nuclear βcatenin expression were from FAP patients. One was a fifty-year-old male who presented with a stage IV PanNEN and multiple colonic adenocarcinomas arising in the setting of polyposis at the initial diagnosis. Sequencing the APC gene demonstrated a germline 853delT mutation. The other was a forty-year-old female who presented with a stage IV PanNEN. She had undergone total colectomy for FAP at 22 years of age. APC gene sequencing demonstrated a germline R564X (1690C < T) mutation. Immunohistochemical staining of the PanNENs from both patients showed loss of APC expression (Figure 2F).
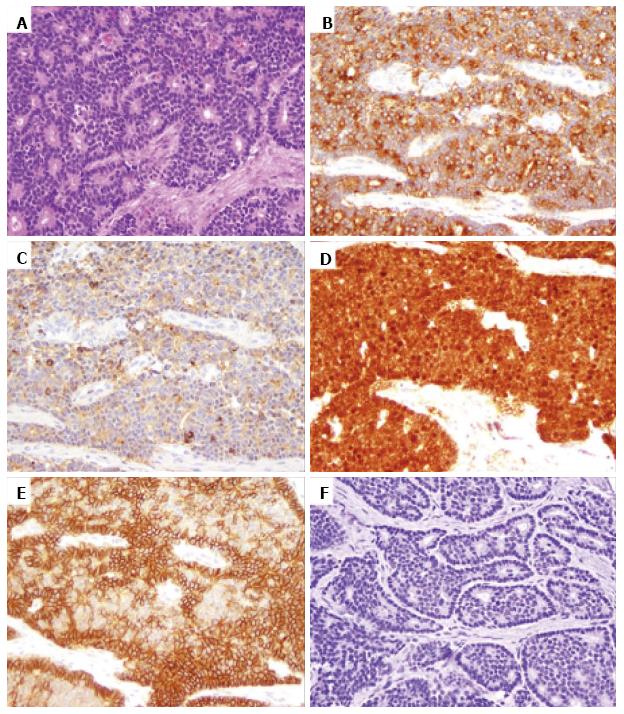
Similar to β-catenin, membranous E-cadherin labeling was frequently strong in pancreatic acinar cells, but weak in pancreatic islets (Figure 3A). Strong membranous E-cadherin expression in the primary tumor was observed in 44% (38/87) of PanNENs (Figure 2E), whereas decreased membranous E-cadherin expression was present in more than half of the cases (Figure 3B). Complete loss of membranous E-cadherin expression was only observed in one case (1/87, 11%), which also showed decreased membranous β-catenin labeling and complete loss of APC expression. However, nuclear β-catenin accumulation was not seen in the tumor. No associations between membranous E-cadherin expression and tumor grade or stage were observed in this series. Noteworthy is that tumors with decreased membranous β-catenin also showed decreased membranous E-cadherin staining compared to cases with strong membranous β-catenin staining (P < 0.01).
Cytoplasmic APC labeling was not seen in the exocrine pancreas, but was detected in most of the pancreatic islets (Figure 3C). While 86% (30/35) of normal pancreatic islets in this study clearly expressed APC, only 30% (24/80) of the primary tumors retained APC expression (Figure 3D, P < 0.01). Of note, all 3 cases with nuclear β-catenin staining demonstrated loss of cytoplasmic APC expression (Figure 2F). There was no correlation between APC and β-catenin expression (P > 0.05). APC expression was not correlated with tumor stage or grade (P > 0.05).
Median follow-up for the cases with resected primary tumor was 47 mo (range, 0.3-163 mo) during which 13 patients (15%) died of progressive disease (Table 2). Univariate analysis showed that high tumor stage, tumor functionality, large tumor size, high tumor grade, infiltrative growth pattern, and lymphovascular and perineural invasion were all associated with decreased DSS. In multivariate survival analysis, stage III/IV disease demonstrated a significantly worse DSS (hazard ratio = 10.4, P < 0.05) compared to stage I/II disease. When controlling for tumor stage and WHO grade, none of the immunohistochemical markers showed prognostic significance.
In the current study we investigated expression of select Wnt/β-catenin pathway components in PanNENs. Approximately half of PanNENs displayed strong expression of β-catenin at the plasma membrane. As expected, strong membranous expression of βcatenin correlated with strong membranous E-cadherin. In many other malignancies, decreased membranous β-catenin and E-cadherin expression predicts poor prognosis. We observed that in PanNENs, strong membranous β-catenin expression was frequently present in stage III/IV tumors and that membranous expression of β-catenin and E-cadherin did not have a prognostic importance in this setting. These data suggest that the role of the Wnt/βcatenin signaling pathway in PanNENs is different than in other neoplasms such as gastric and colon adenocarcinomas[5,12-15].
APC mutations have been implicated in the development of enterochromaffin cell neuroendocrine neoplasms, and are seen in 23% of these types of tumors arising in the midgut[11]. However, alterations in APC have not been well characterized in neuroendocrine neoplasms of the foregut. We report that most PanNENs show loss of APC expression, which may increase β-catenin signaling capacity for processes such as cell-cycle, apoptosis, and differentiation. Other described mutations in PanNENs may also lead to increased β-catenin signaling. For example, studies of MEN-1 deficient PanNENs (sporadic and familial) and pulmonary neuroendocrine tumors suggest that MEN1 mutations lead to increased β-catenin signaling[16,17]. Canonical β-catenin signaling is characterized by β-catenin accumulation within nuclei, where it regulates gene expression. However, we detected nuclear β-catenin in only 3 of 88 tumors and did not observe differences in β-catenin expression between non-syndromic and MEN1-associated PanNENs (data not shown). Nevertheless, strong membranous β-catenin expression was observed in 55% of PanNENs, which was not present in normal pancreatic islets. In a mouse model, knockout of β-catenin expression has been shown to suppress tumorigenesis and growth of Men1-deficient PNENs[17]. Therefore, βcatenin may represent a potential therapeutic target in these tumors.
The three tumors in our study with strong nuclear β-catenin expression also showed loss of APC expression (two of these tumors were from FAP patients). FAP is defined by mutations in the APC gene on chromosome 5q21[18]. APC binds βcatenin, targeting it for degradation. Therefore, when APC is mutated, β-catenin accumulates in the nucleus and activates gene transcription. Loss of APC expression, as well as nuclear accumulation of β-catenin, suggest that the APC gene is biallelically inactivated in these two FAP-associated tumors, and that PanNENs may be another extra-intestinal manifestation in patients with FAP. Prior to this investigation, an association between PanNENs and FAP had not been described.
Abnormal nuclear β-catenin accumulation has been demonstrated in other FAP-associated tumors, such as desmoid-type fibromatosis. Nuclear β-catenin expression was also recently described in intestinal neuroendocrine tumors from FAP patients[19]. Abnormal nuclear β-catenin accumulation has also been demonstrated in Sertoli cell tumors of the testis[20] and hepatoblastomas[21] arising in FAP patients. Pancreatoblastoma[22] and adrenocortical carcinoma[23] also show nuclear and cytoplasmic staining for βcatenin, while papillary thyroid carcinoma[24] has been found to have cytoplasmic labeling in FAP patients.
Abnormal immunolabeling for β-catenin in PanNENs in patients with FAP also has important implications in the pathological diagnosis of these neoplasms. In the pancreas, SPNs almost always harbor β-catenin gene mutations, and these neoplasms characteristically show nuclear β-catenin expression. SPN is one of the primary differential diagnoses for PanNENs, since these tumors can have a solid growth pattern; conversely, PanNENs can ocassionally show pseudopapilllary areas. Additionally, the cells of both can be round-to-oval, with uniform nuclei[25]. PanNENs and SPNs of the pancreas both express CD56 and synaptophysin. Differentiating these tumors is often based on the presence of nuclear β-catenin in SPN. Our results suggest that, at least for patients with FAP, nuclear β-catenin labeling should not be used as a primary diagnostic criterion for differentiating these lesions, as both neoplasms will have nuclear staining. Instead, other immunostains should be performed. For example, SPNs, but not PanNENs, will express CD10, show loss of membranous E-cadherin, and be negative for chromogranin[26].
In summary, dyregulation of the Wnt/β-catenin pathway is present in some PanNENs. However, this signaling pathway may have different functions in PanNENs as compared to other malignancies. Additionally, PanNENs arising in patients with FAP demonstrate abnormal nuclear β-catenin accumulation, and PanNENs may be one of extraintestinal manifestations of FAP.
Alterations in the Wnt/β-catenin signaling pathway may lead to tumor growth and proliferation. For example, most colonic adenocarcinomas arise from tubular adenomas through dysregulation of the Wnt/β-catenin pathway. In addition, reduced membranous expression of β-catenin and E-cadherin is associated with a poor prognosis in most malignancies.
Dysregulation of the Wnt/β-catenin signaling pathway is seen in some neuroendocrine tumors of the gut.
Dysregulation of the Wnt/β-catenin pathway is present in some pancreatic neuroendocrine neoplasms (PanNENs). However, compared to other malignancies, this signaling pathway may have different functions in PanNENs as membranous expression of β-catenin and E-cadherin does not have a prognostic importance in this setting. Additionally, PanNENs arising in patients with familial adenomatous polyposis (FAP) demonstrate abnormal nuclear β-catenin accumulation, and PanNENs may be one of the extraintestinal manifestations of FAP.
βcatenin may represent a potential therapeutic target in PanNENs as strong membranous β-catenin expression was observed in 55% of PanNENs and knockout of β-catenin expression has been shown to suppress tumorigenesis and growth in a mouse model. Nuclear β-catenin accumulation is seen in some PanNENs, especially those from FAP patients, which should be differentiated from solid pseudopapillary neoplasm, a low-malignant pancreatic tumor with similar morphology and expressing nuclear β-catenin. In addition, screening for PanNEN may be needed in patients with FAP.
This is a nice study.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Barreto S, Li XL S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Milan SA, Yeo CJ. Neuroendocrine tumors of the pancreas. Curr Opin Oncol. 2012;24:46-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 2. | Klimstra DS. Nonductal neoplasms of the pancreas. Mod Pathol. 2007;20 Suppl 1:S94-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 124] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 3. | Bosman FT, Carneiro F, Hruban RH, Theise ND (Eds). WHO Classification of Tumours of the Digestive System. IARC: Lyon 2010; . |
| 4. | Basturk O, Yang Z, Tang LH, Hruban RH, Adsay V, McCall CM, Krasinskas AM, Jang KT, Frankel WL, Balci S. The high-grade (WHO G3) pancreatic neuroendocrine tumor category is morphologically and biologically heterogenous and includes both well differentiated and poorly differentiated neoplasms. Am J Surg Pathol. 2015;39:683-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 341] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 5. | Brabletz T, Jung A, Reu S, Porzner M, Hlubek F, Kunz-Schughart LA, Knuechel R, Kirchner T. Variable beta-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proc Natl Acad Sci USA. 2001;98:10356-10361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 807] [Cited by in RCA: 857] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 6. | Fujimori M, Ikeda S, Shimizu Y, Okajima M, Asahara T. Accumulation of beta-catenin protein and mutations in exon 3 of beta-catenin gene in gastrointestinal carcinoid tumor. Cancer Res. 2001;61:6656-6659. [PubMed] |
| 7. | Li Y, Yau A, Schaeffer D, Magliocco A, Gui X, Urbanski S, Waghray R, Owen D, Gao ZH. Colorectal glandular-neuroendocrine mixed tumor: pathologic spectrum and clinical implications. Am J Surg Pathol. 2011;35:413-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 8. | Li CC, Xu B, Hirokawa M, Qian Z, Yoshimoto K, Horiguchi H, Tashiro T, Sano T. Alterations of E-cadherin, alpha-catenin and beta-catenin expression in neuroendocrine tumors of the gastrointestinal tract. Virchows Arch. 2002;440:145-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Wood LD, Klimstra DS. Pathology and genetics of pancreatic neoplasms with acinar differentiation. Semin Diagn Pathol. 2014;31:491-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 10. | Kim JT, Li J, Jang ER, Gulhati P, Rychahou PG, Napier DL, Wang C, Weiss HL, Lee EY, Anthony L. Deregulation of Wnt/β-catenin signaling through genetic or epigenetic alterations in human neuroendocrine tumors. Carcinogenesis. 2013;34:953-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 11. | Bottarelli L, Azzoni C, Pizzi S, D’Adda T, Silini EM, Bordi C, Rindi G. Adenomatous polyposis coli gene involvement in ileal enterochromaffin cell neuroendocrine neoplasms. Hum Pathol. 2013;44:2736-2742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Pelosi G, Scarpa A, Puppa G, Veronesi G, Spaggiari L, Pasini F, Maisonneuve P, Iannucci A, Arrigoni G, Viale G. Alteration of the E-cadherin/beta-catenin cell adhesion system is common in pulmonary neuroendocrine tumors and is an independent predictor of lymph node metastasis in atypical carcinoids. Cancer. 2005;103:1154-1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Salon C, Moro D, Lantuejoul S, Brichon Py Py, Drabkin H, Brambilla C, Brambilla E. E-cadherin-beta-catenin adhesion complex in neuroendocrine tumors of the lung: a suggested role upon local invasion and metastasis. Hum Pathol. 2004;35:1148-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Tan C, Qiao F, Wei P, Chi Y, Wang W, Ni S, Wang Q, Chen T, Sheng W, Du X. DIXDC1 activates the Wnt signaling pathway and promotes gastric cancer cell invasion and metastasis. Mol Carcinog. 2016;55:397-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 15. | Samowitz WS, Slattery ML, Sweeney C, Herrick J, Wolff RK, Albertsen H. APC mutations and other genetic and epigenetic changes in colon cancer. Mol Cancer Res. 2007;5:165-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 118] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 16. | Jiang X, Cao Y, Li F, Su Y, Li Y, Peng Y, Cheng Y, Zhang C, Wang W, Ning G. Targeting β-catenin signaling for therapeutic intervention in MEN1-deficient pancreatic neuroendocrine tumours. Nat Commun. 2014;5:5809. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 17. | Veschi S, Lattanzio R, Aceto GM, Curia MC, Magnasco S, Angelucci D, Cama A, Piantelli M, Battista P. Alterations of MEN1 and E-cadherin/β-catenin complex in sporadic pulmonary carcinoids. Int J Oncol. 2012;41:1221-1228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Aoki K, Taketo MM. Adenomatous polyposis coli (APC): a multi-functional tumor suppressor gene. J Cell Sci. 2007;120:3327-3335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 353] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 19. | Estrella JS, Taggart MW, Rashid A, Abraham SC. Low-grade neuroendocrine tumors arising in intestinal adenomas: evidence for alterations in the adenomatous polyposis coli/β-catenin pathway. Hum Pathol. 2014;45:2051-2058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Xiao GQ, Granato RC, Unger PD. Bilateral Sertoli cell tumors of the testis-a likely new extracolonic manifestation of familial adenomatous polyposis. Virchows Arch. 2012;461:713-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Inukai T, Furuuchi K, Sugita K, Uno K, Ooi A, Sasaki F, Hamada J, Moriuchi T, Nakazawa S. Nuclear accumulation of beta-catenin without an additional somatic mutation in coding region of the APC gene in hepatoblastoma from a familial adenomatous polyposis patient. Oncol Rep. 2004;11:121-126. [PubMed] |
| 22. | Abraham SC, Wu TT, Klimstra DS, Finn LS, Lee JH, Yeo CJ, Cameron JL, Hruban RH. Distinctive molecular genetic alterations in sporadic and familial adenomatous polyposis-associated pancreatoblastomas: frequent alterations in the APC/beta-catenin pathway and chromosome 11p. Am J Pathol. 2001;159:1619-1627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 171] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 23. | Gaujoux S, Pinson S, Gimenez-Roqueplo AP, Amar L, Ragazzon B, Launay P, Meatchi T, Libé R, Bertagna X, Audebourg A. Inactivation of the APC gene is constant in adrenocortical tumors from patients with familial adenomatous polyposis but not frequent in sporadic adrenocortical cancers. Clin Cancer Res. 2010;16:5133-5141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 24. | Lee S, Hong SW, Shin SJ, Kim YM, Rhee Y, Jeon BI, Moon WC, Oh MR, Lim SK. Papillary thyroid carcinoma associated with familial adenomatous polyposis: molecular analysis of pathogenesis in a family and review of the literature. Endocr J. 2004;51:317-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Meriden Z, Shi C, Edil BH, Ellison T, Wolfgang CL, Cornish TC, Schulick RD, Hruban RH. Hyaline globules in neuroendocrine and solid-pseudopapillary neoplasms of the pancreas: a clue to the diagnosis. Am J Surg Pathol. 2011;35:981-988. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Tanaka Y, Notohara K, Kato K, Ijiri R, Nishimata S, Miyake T, Fukunaga M, Horisawa M, Nakatani Y. Usefulness of beta-catenin immunostaining for the differential diagnosis of solid-pseudopapillary neoplasm of the pancreas. Am J Surg Pathol. 2002;26:818-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |