Published online Feb 15, 2016. doi: 10.4251/wjgo.v8.i2.222
Peer-review started: July 31, 2015
First decision: September 29, 2015
Revised: November 27, 2015
Accepted: December 9, 2015
Article in press: December 11, 2015
Published online: February 15, 2016
Processing time: 188 Days and 19.1 Hours
AIM: To investigate the prognostic role of invariant natural killer T (iNKT) cells and antibody-dependent cell-mediated cytotoxicity (ADCC) in wild type KRAS metastatic colorectal cancer (mCRC) patients treated with cetuximab.
METHODS: Forty-one KRAS wt mCRC patients, treated with cetuximab and irinotecan-based chemotherapy in II and III lines were analyzed. Genotyping of single nucleotide polymorphism (SNP)s in the FCGR2A, FCGR3A and in the 3’ untranslated regions of KRAS and mutational analysis for KRAS, BRAF and NRAS genes was determined either by sequencing or allelic discrimination assays. Enriched NK cells were obtained from lymphoprep-peripheral blood mononuclear cell and iNKT cells were defined by co-expression of CD3, TCRVα24, TCRVβ11. ADCC was evaluated as ex vivo NK-dependent activity, measuring lactate dehydrogenase release.
RESULTS: At basal, mCRC patients performing ADCC activity above the median level (71%) showed an improved overall survival (OS) compared to patients with ADCC below (median 16 vs 8 mo; P = 0.026). We did not find any significant correlation of iNKT cells with OS (P = 0.19), albeit we observed a trend to a longer survival after 10 mo in patients with iNKT above median basal level (0.382 cells/microliter). Correlation of OS and progression-free survival (PFS) with interesting SNPs involved in ADCC ability revealed not to be significant. Patients carrying alleles both with A in FCGR2A and TT in FCGR3A presented a trend of longer PFS (median 9 vs 5 mo; P = 0.064). Chemotherapy impacted both iNKT cells and ADCC activity. Their prognostic values get lost when we analysed them after 2 and 4 mo of treatment.
CONCLUSION: Our results suggest a link between iNKT cells, basal ADCC activity, genotypes in FCGR2A and FCGR3A, and efficacy of cetuximab in KRAS wt mCRC patients.
Core tip: A high number of invariant natural killer T (iNKT) cells and a high antibody-dependent cell-mediated cytotoxicity (ADCC) activity, evaluated before therapy, do correlate significantly with a longer overall survival in metastatic colorectal cancer patients treated with irinotecan-based chemotherapy and cetuximab in II and III lines. Chemotherapy impacted both iNKT cells and ADCC activity. The prognostic value of ADCC above the median basal level, get lost when we analysed those parameters after 2 and 4 mo of treatment. Correlation of overall survival and progression-free survival with interesting single nucleotide polymorphisms reported as involved in ADCC ability, either in the FCGR2A, FCGR3A or in the 3’ untranslated regions of KRAS gene, revealed not to be significant.
- Citation: Lo Nigro C, Ricci V, Vivenza D, Monteverde M, Strola G, Lucio F, Tonissi F, Miraglio E, Granetto C, Fortunato M, Merlano MC. Evaluation of antibody-dependent cell-mediated cytotoxicity activity and cetuximab response in KRAS wild-type metastatic colorectal cancer patients. World J Gastrointest Oncol 2016; 8(2): 222-230
- URL: https://www.wjgnet.com/1948-5204/full/v8/i2/222.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v8.i2.222
Colorectal cancer (CRC) is the third most common cancer worldwide, accounting for 940000 million new cases annually and nearly 500000 deaths each year. Metastatic colorectal cancer (mCRC) previously untreated patients have demonstrated substantial improvements, with a median overall survival time now reaching more than 24 mo, by the development of systemic chemotherapy, including molecular-targeted therapy[1].
The epidermal growth factor receptor (EGFR) signalling pathway is involved in cell differentiation, proliferation, migration, angiogenesis and apoptosis, all processes dysregulated in cancer cells.
Cetuximab is a chimeric immunoglobulin G1 (IgG1) monoclonal antibody (mAb) which binds EGFR with high affinity and inhibits ligand binding[2].
KRAS activating mutations have been reported in 40% of mCRC showing a negative effect on response to anti-EGFR antibodies[3,4]. Mutations in other downstream effectors of the EGFR signalling pathway, such as BRAF, NRAS and PI3kinase, might also impact the efficacy of monoclonal therapy. Thus, the absence of mutations in RAS appears to be a reliable marker for predicting the efficacy of cetuximab which was been restricted to mCRC patients with wild-type RAS[5]. Several studies supported the biological activity of cetuximab in advanced CRC. Cetuximab enhances response rate and progression-free survival (PFS) in first-line therapy in combination with Folfiri and Folfox regimen of chemotherapy[6,7]. However, some clinical studies have failed to show a significant correlation between EGFR expression and the response to cetuximab[8]. The proposed working mechanism of cetuximab is thought to include antibody-dependent cell-mediated cytotoxicity (ADCC)[9].
ADCC utilizes the response of innate immune cells to provide antitumor cytotoxicity triggered by the interaction of the Fc portion of the antibody with the Fc receptor on the immune cell. Immunotherapeutics that target natural killer (NK) cells, γδ T cells, macrophages and dendritic cells can, by augmenting the function of the immune response, enhance the antitumor activity of the antibodies[10].
Invariant CD1d-rescricted natural killer T (NKT) cells are T lymphocytes characterized by an invariant T-cell antigen receptor-chain rearrangement that co-express NK cell markers[11].
Molling et al[12] in 2007 demonstrated that a severe circulating invariant NKT (iNKT) cell deficiency was related to poor clinical outcome in head and neck squamous cell carcinoma patients, suggesting their critical contribution to antitumor immune responses. Furthermore, screening for iNKT cell levels may be useful for determining which patients can benefit from immunotherapeutic adjuvant therapies aimed at reconstitution of the circulating iNKT cell pool.
Whether ADCC is associated with EGFR expression and/or the mutational status of RAS and BRAF in CRC remains unclear. Seo et al[13] demonstrated that the ADCC activities were significantly associated with the cell surface expression levels of EGFR but not with the mutational status of KRAS and BRAF.
In this study we aimed to evaluate the prognostic and predictive value of cetuximab-mediated ADCC and circulating iNKT cells levels in mCRC and to analyse their correlation with EGFR level, mutational status of KRAS, NRAS, BRAF, PFS and overall survival (OS) in a prospective cohort of mCRC patients treated with cetuximab-based therapy.
A total of 41 mCRC patients were enrolled in this study from March 2008 to September 2014. Characteristics of the 41 patients are described in Table 1. An informed consent for tissue collection and use for scientific purpose was obtained from each patient enrolled in this study, approved by the local Ethical Committee and carried out in the respect to Helsinki Declaration. Inclusion criteria for mCRC patients were: Suitability for combination therapy including cetuximab with irinotecan-based chemotherapy in second and third lines and KRAS wild type (wt) status. Patients were evaluated for PFS, OS and response at the end of treatment with CT scan according to RECIST criteria[2]. Median follow-up was 25 mo (range 10-70).
| Number of patients | Rates | Median age (range) yr | |
| Gender | |||
| Male (M) | 23 | 56% | 67.5 (51-84) |
| Female (F) | 18 | 44% | 64.6 (49-83) |
| Primary tumour | |||
| Right colon | 7 | 17% | |
| Left colon | 21 | 51% | |
| Rectal | 13 | 32% | |
| Grade | |||
| G1/G2 | 27 | 65.8% | |
| G3 | 13 | 31.7% | |
| NA | 1 | 2.5% | |
| Metastasis | |||
| Liver only | 12 | 29.3% | |
| Liver plus other sites | 14 | 34.1% | |
| Extra-hepatic sites | 15 | 36.6% | |
| Response | |||
| Responders | |||
| CR | 4 | 9.8% | |
| PR | 12 | 29.3% | |
| SD | 8 | 19.5% | |
| Non-responders | |||
| PD | 17 | 41.4% | |
| Line of treatment | |||
| II | 33 | 8% | |
| III | 8 | 2% |
Genotyping of rs1801274 (A > G) in the FCGR2A, rs 396991 (T > G) in FCGR3A and rs61764370 in the 3’ untranslated regions (3’ UTR) of KRAS gene was done on genomic DNA isolated from whole peripheral blood samples using the EZ1 DNA Blood 200 Kit (Qiagen, Germany) according to the manufacturer’s instructions. Analyses were determined using the appropriate “allelic discrimination assay” from Life Technologies (Foster city, CA, United States): c_9077561_20 for rs1801274; c_25815666_10 for rs396991 and 1350086 for rs61764370 using the ABIPRISM 7000 Sequence Detection System (Applied Biosystems Foster City, CA, United States).
Mutational analyses for KRAS (codons 12-13-59-61-146), BRAF (codon 600) and NRAS (codons 12-13-59-61-117-146) genes were determined on patients’ DNA extracted from Formalin Fixed Paraffin Embedded (FFPE) tumor tissues archived at diagnosis in the Pathology Department of our Institution, by a standard protocol that included proteinase K treatment (EuroClone, Pero, IT).
KRAS and BRAF gene analyses were performed by pyrosequencing using PyroMark ID System (Biotage, Uppsala, Sweden), while a Real-Time PCR (OncoSreen NRAS; Relab, Jesi, Italy) was employed for NRAS gene using the Rotor-Gene 6000 (Corbett Research, Pty Ltd; Sydney, Australia) according to the manufacturer’s protocol.
Twelve milliliter peripheral blood samples were collected at start of therapy for all the 41 patients and ADCC and NK cells were evaluated at basal level. After 2 and 4 mo of treatment a second collection of blood was done in 30 and 23 patients respectively where ADCC and NK cells were longitudinally studied.
Enriched NK cells were obtained from lymphoprep-peripheral blood mononuclear cell pellets using the human NK Cell Isolation Kit (Miltenyi Biotec, Cologne, Germany). NK cells were defined as CD56+/CD3-; T cells as CD3+/CD56- and invariant NKT (iNKT) cells by co-expression of CD3, TCR Vα24, TCR Vβ11.
ADCC was evaluated as ex vivo NK-dependent activity with a standard lactate dehydrogenase (LDH) assay (Cytotox 96® non radioactive cytotoxicity assay, Promega, Madison, WI) as set up in our Laboratory[14].
Statistical analyses were performed using the GraphPad Prism 5 (San Diego, CA, United States) and SPSS version 13 (SPSS, Chicago, IL) programs. The association between ADCC median levels was analyzed using the Fisher’s exact test or the Pearson’s test when appropriate. OS analyses were based on the time from treatment start to death or last contact in which the survivors were censored. PFS analyses were based on the time from treatment start to first event; patients without an event were censored at their last follow-up. OS was calculated using the Kaplan-Meier method with log-rank test for statistical significance. A P-value < 0.05 was considered statistically significant.
Clinical characteristics of the 41 mCRC patients are detailed in Table 1. Genotyping analyses for single nucleotide polymorphisms are reported in Table 2. All patients were wt for KRAS gene. Determination of NRAS and BRAF mutations failed in 1 out the 41 patients, due to poor quality of DNA obtained from tumoral tissue. In particular we identified 6 mutations in NRAS gene (1 mutation in G12x-G13x; 1 in G61R; 1 in Q61K, 1 in Q61L and 2 in A59x-Q61H codons) and 1 mutation in BRAF gene (V600E).
| Gene | SNP | Genotype | Aminoacid change | Number of subjects (%) |
| FCGR2A | rs1801274 | A/A | H131H | 14 (34.1%) |
| A/G | H131R | 20 (48.8%) | ||
| G/G | R131R | 7 (17.1%) | ||
| FCGR3A | rs396991 | T/T | F158F | 14 (34.1%) |
| T/G | F158V | 21 (51.2%) | ||
| G/G | V158V | 6 (14.7%) | ||
| KRAS 3’ UTR | rs61764370 | T/T | --- | 32 (78%) |
| T/G | --- | 9 (22%) | ||
| G/G | --- | 0 (0%) |
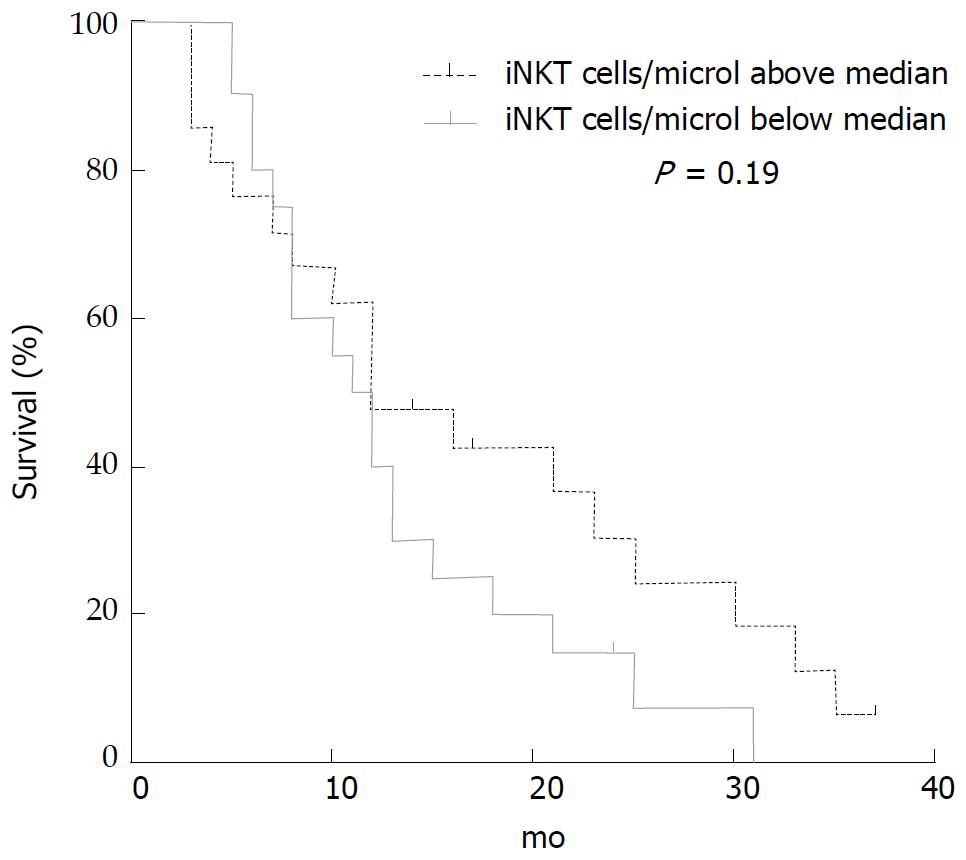
iNKT cells evaluated before treatment were analysed to seek correlation with OS and PFS either as number of cells/microliter or as % of T cells, since a low level of circulating iNKT cells has been reported to predict poor clinical outcome in patients with head and neck squamous cell carcinoma[12]. iNKT cells median value at basal determination, before treatment, was 0.382 cells/microliter. We did not find any significant correlation of iNKT cells with OS (P = 0.19), albeit we observed a trend to a longer survival after 10 mo in the population of patients (n = 21) with iNKT above median level (Figure 1).
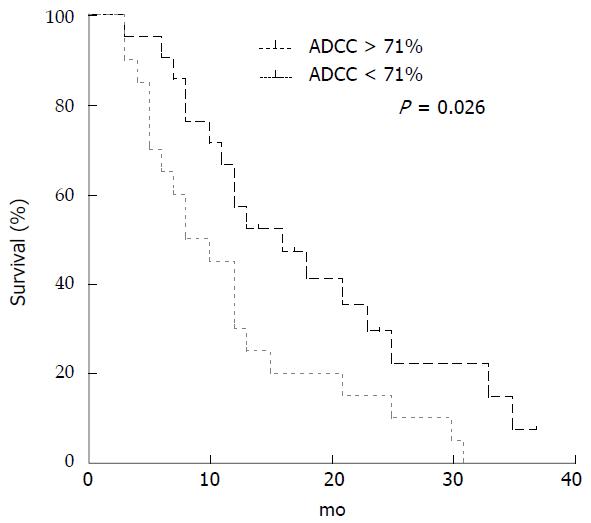
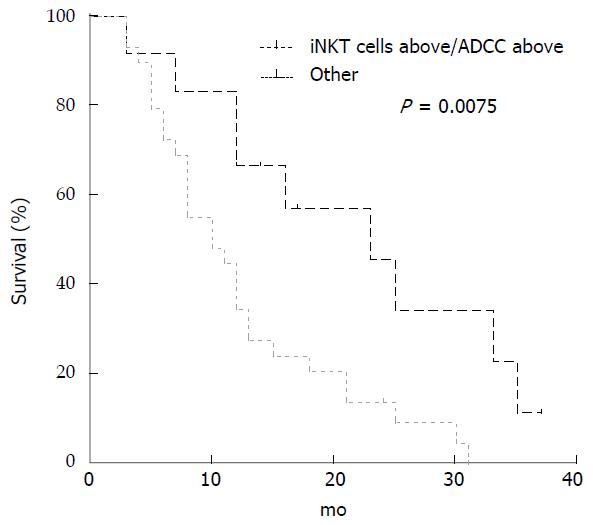
Median ADCC activity before treatment for all the 41 mCRC patients was 71% (range 10%-99%). Comparison between patients with ADCC above and below median value is reported in Table 3. There were no differences in the clinical characteristics between the two groups, although EGFR over-expression was more common in patients with ADCC activity above the median level (P = 0.052; Fisher’s exact test). Correlation with OS and PFS was evaluated. Median OS was 12 mo (range 3-37) and PFS was 6 mo (range 3-37). Patients performing ADCC activity above the median level showed an improved OS compared to patients with ADCC activity below this value (median 16 vs 8 mo; P = 0.026; Long-rank Mantel-Cox Test) (Figure 2). On the contrary, there was no difference in PFS between patients with ADCC below or above the median level (data not shown). When we stratified patients for both iNKT and ADCC activity at basal level, below and above the respective median level, we observed a better OS in patients having both values above the median level compared to all the other combinations (median 23 vs 10 mo; P = 0.0075; Long-rank Mantel-Cox Test) (Figure 3).
| ADCC < 71% | n = 20 | ADCC > 71% | n = 21 | P | |
| Gender | |||||
| Male (M) | 11 | 55% | 12 | 57% | 0.91 |
| Female (F) | 9 | 45% | 9 | 43% | |
| Primary tumour | |||||
| Right colon | 5 | 25% | 2 | 10% | 0.282 |
| Left colon | 8 | 40% | 13 | 62% | |
| Rectal | 7 | 35% | 6 | 29% | |
| Grade | |||||
| G1/G2 | 13 | 65% | 14 | 67% | 0.872 |
| G3 | 7 | 35% | 6 | 29% | |
| NA | 0 | 0% | 1 | 5% | |
| Metastasis | |||||
| Liver only and liver plus other sites | 12 | 60% | 14 | 67% | 0.651 |
| Extra-hepatic sites | 8 | 40% | 7 | 33% | |
| Response | |||||
| Responders | |||||
| CR | 3 | 15% | 1 | 5% | 0.362 |
| PR | 4 | 20% | 8 | 38% | |
| SD | 3 | 15% | 5 | 24% | |
| Non-responders | |||||
| PD | 10 | 50% | 7 | 33% | |
| Line of treatment | |||||
| II | 17 | 85% | 17 | 81% | 12 |
| III | 3 | 15% | 4 | 19% | |
| EGFR | |||||
| Neg; 1+; 2+ | 19 | 95% | 14 | 67% | 0.0522 |
| 3+ | 1 | 5% | 5 | 24% | |
| NA | 0 | 0% | 2 | 10% |
Correlation in terms of OS and PFS with each genotype, either rs1801274 (A > G) in the FCGR2A, rs396991 (T > G) in FCGR3A or rs61764370 in the 3’ UTR of KRAS gene reveal not to be significant (data not shown).
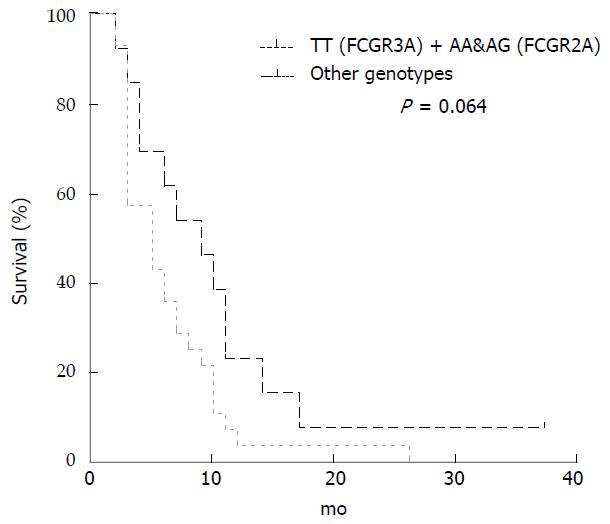
Patients carrying alleles both with A in FCGR2A (AA/AG genotypes) and TT in FCGR3A presented a longer PFS (median 9 vs 5 mo; P = 0.064; Long-rank Mantel-Cox Test) in comparison to all the other subgroups (Figure 4), although the difference was not significant.
Due to the limited number of patients we were not able to perform OS and PFS analyses according to all-RAS gene mutations. Nevertheless we observed that of ADCC activity, as median level, was not affected by the presence of a NRAS or a BRAF mutation (data not shown).
Both iNKT cells and ADCC activity were evaluated over time to seek for dynamic changes during treatment and to investigate the impact of therapy on patients’ ability to perform ADCC and their clinical outcome. iNKT cells median number decreased from 0.382 cells/microliter at basal, before treatment, to 0.193 after 2 mo and to 0.165 after 4 mo of treatment. Likewise, ADCC activity was longitudinally evaluated up to 2 and 4 mo and its median level fell down from of 71% to 45% at two withdrawal further analyses.
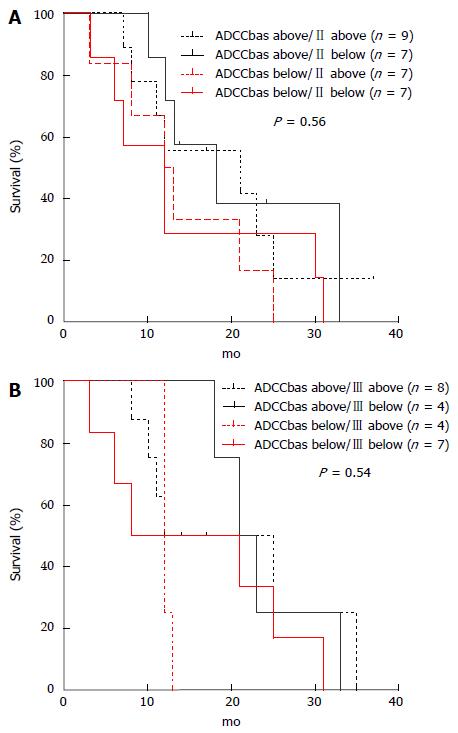
ADCC determination during treatment lost its prognostic value since there was no difference in OS between patients with ADCC activity above or below the median level after 2 and after 4 mo of treatment (data not shown). Variation of ADCC values was analyzed by stratifying patients on the basis of to the median values at basal level (71%, 41 patients), after 2 mo (45%, 30 patients) and after 4 mo (45%, 23 patients) of treatment. Combination of longitudinal values generated 4 groups of patients: the first included patients showing both ADCC activities above the median level [ADCCbas above/II (or III) above, where II means on blood drawn after 2 mo and III after 4 mo], the second group a decrease from a basal above median to a II or III determination below median [ADCCbas above/II (or III) below], the third group patients showing instead an increase from below at basal and above at II or III determination [ADCCbas below/II (or III) above] and the fourth group patients with both ADCC activities below the median level [ADCCbas below/II (or III) below].
We then analysed correlation with OS in the 4 groups. Patients performing ADCC activity above the median value both at basal level and either after 2 and/or 4 mo presented a trend in longer OS, albeit not significant (Figure 5).
When we focus on patients presenting ADCC values above the median levels in both determinations (basal and after 2 mo) we found that this 9 out of the 30 patients (30%) showed a higher OS compared to other patients (median 21 vs 13 mo, P = 0.5; Long-rank Mantel-Cox Test). After 4 mo, 8 patients out of 23 (35%) had both values above the median levels of ADCC activity, but their OS was not statistically different from that of the other patients (median 18.5 vs 15 mo; P = 0.42; Long-rank Mantel-Cox Test) (data not shown).
Treatment of mCRC requires a multidisciplinary approach and multiple treatment options are nowadays available[1]. Advances in the understanding of tumor biology have led to the development of EGFR-targeted therapies as mAbs. In fact, the EGFR-signalling pathway regulates important processes involved in cell differentiation, proliferation, migration, angiogenesis and apoptosis, all of which become deregulated in cancer cells. However, the mechanisms that mediate the therapeutic effect of these mAbs are still unclear.
Cetuximab is a chimeric monoclonal antibody that specifically targets EGFR with high affinity and prevents the ligand-mediated activation of the EGFR-dependent pathway. KRAS mutations occur in 35%-45% of mCRC and preclude responsiveness to EGFR-targeted therapy with cetuximab or panitumumab. Initial response rates of about 10% were seen with cetuximab monotherapy in patients with heavily pretreated mCRC. A phase II BOND study demonstrated the ability of cetuximab to circumvent irinotecan-based chemotherapy resistance[2]. Less than 20% patients displaying wild-type KRAS tumors achieve objective response. In fact, it subsequently became clear that tumors without mutations in codon 12 or 13 of the KRAS gene responded in 13%-17% of cases, whereas only 0.1%-2% of the KRAS mutant tumors did[15].
Alterations in other effectors downstream of the EGFR and deregulation of the PIK3CA/PTEN pathway have independently been found to give rise to resistance. Moreover, the PIK3CA gene is mutated in approximately 20% of CRCs. BRAF is the principal downstream effector of KRAS and its oncogenic V600E mutation is mutually exclusive with KRAS mutations in CRCs[4,16].
It has recently become clear that IgG1 mAb, like cetuximab, may have mechanisms of action other than the selective blockade of tumoral membrane receptors. Among them, the Fc region of the mAb may also trigger ADCC, binding via Fv regions the target cell to any of the Fc-γ receptors, i.e., CD16, CD32 and CD64, which are expressed, with different patterns, by cells of the innate immune system, namely monocytes, macrophages, granulocytes and NK. The contribution of the different cell types to the anti-tumor ADCC exerted in vivo by anti-EGFR mAbs is still debated. In general these cell are thought to play a relevant role controlling tumor growth and in preventing metastatic dissemination in humans[17,18].
In particular, NK cells have been suggested to be the major mediators of the ADCC-dependent therapeutic effect of cetuximab[19]. Moreover, invariant CD1d-restricted NKT cells has been reported to play an allegedly pivotal role in such responses via transactivation of immune effector cells. In particular, a severe circulating iNKT cell deficiency was related to poor clinical outcome in head and neck squamous cell carcinoma patients[12].
Thus, the number of iNKT and the level of ADCC activity exerted by NK cells from tumor patients in the presence of cetuximab might be useful prognostic or predictive parameters for response to treatment. With this in mind, we investigated 41 mCRC patients suitable for combination therapy including cetuximab with irinotecan-based chemotherapy in second and third lines and KRAS wild type.
Analyses were carried out at start of therapy for all the 41 patients and ADCC and iNKT cells were evaluated at basal level. After 2 and 4 mo of treatment additional determinations were done in 30 and 23 patients respectively where ADCC and NK cells were longitudinally studied.
Main aim of the project was to study ex-vivo the prognostic and predictive value of the number of iNKT cells and the level of cetuximab-mediated ADCC and to analyse their correlation with EGFR level, mutational status of KRAS, NRAS, BRAF and PFS and OS in our prospective cohort of mCRCs.
We did not find any significant correlation of iNKT cells at basal level with PFS nor with OS, albeit we observed a trend to a longer survival after 10 mo in the population of patients with iNKT above median level. Instead, patients performing, at basal determination, ADCC activity above the median level showed an improved OS compared to patients with ADCC activity below this value.
Moreover, if we combine iNKT number and ADCC basal level and we stratified patients for both determinations, as below or above the respective median level, we observed a better OS in patients having both values above the median level compared to all the other combinations. Of note, when we analysed the same parameters after 2 and 4 mo of treatment, levels of circulating T, NK, iNTK cells were significantly reduced. On the clinical side, we observed that cancer patients exhibited a lower capacity to perform ADCC as compared to the beginning of therapy; this observation has to be replaced in the global context of an immunosuppressed state of cancer patients and the immunosuppressive effect of chemotherapy and it is consistent with earlier reports[20].
Intriguing, during treatment, neither low level of iNKT nor low ADCC activity did correlate to prognosis. In our study this could be in apparent contrast with what observed by us at the beginning of therapy and also with what reported by others, which is patients with a severe numeric iNKT cell deficiency have a strikingly poor clinical outcome in response to chemo and radiotherapy[12].
On the other hand, we’re analysing NK levels and their activity in peripheral blood; we did not have the picture of the functional properties of tumor-infiltrating T and NK cells in patients. A reduced number of NK and iNKT cells in periphery might be “the other side of the coin” and may reflect an increased activity in the tumor infiltrates[21].
The impact of ADCC on the efficacy of cetuximab might also be influenced by the occurrence of polymorphic forms of genes coding receptors for the antibody Fc region. The most relevant polymorphisms regulating Fc:FcR interactions are phenylalanine (F) or valine (V) expression at position 158 of the Fc fragment[22]. In particular, differential response to therapeutic mAbs has been reported to correlate with specific polymorphisms in two of these genes: FCGR2A (H131R) and FCGR3A (V158F)[23]. However, previous studies exploring the relation between the FCGR polymorphisms and cetuximab efficacy in mCRC have demonstrated conflicting and have been mostly low-powered studies with small sample sizes[24].
More recently a variant allele in a let-7 microRNA complementary site within the 3’UTR of KRAS (rs61764370) has been correlated with clinical outcome in mCRC patients receiving cetuximab[25].
In our cohort of mCRCs, correlation in terms of OS and PFS with each genotype, either rs1801274 (A > G) in the FCGR2A, rs396991 (T > G) in FCGR3A or rs61764370 in the 3’ UTR of KRAS gene didn’t reveal to be significant. Interestingly enough, patients carrying alleles both with A in FCGR2A (AA/AG genotypes) and TT in FCGR3A presented a longer PFS, although the difference was not significant, probably due to the low number of patients.
For the same reason, we were not able to perform OS and PFS analyses according to all-RAS gene mutations. It is well know, in fact, that activating KRAS mutations are negative predictors of the response to cetuximab therapy in patients with mCRC, since cetuximab is widely considered to be unable to block the signal initiated by oncogenic KRAS[26,27].
Nevertheless we observed that of ADCC activity, as median level, was not affected by the presence of a NRAS or a BRAF mutation.
Seo et al[13] demonstrated cetuximab-mediated ADCC in human CRC cell lines and observed that ADCC activities for the tumor cells were higher in CRC patients with a high expression level of EGFR. Furthermore, the ADCC activity level was significantly associated with EGFR, but not with the KRAS/BRAF mutational status.
This has to be considered also in the light of the preclinical studies of nakadate and colleagues, who demonstrated that, in an ADCC assay, perforin-dependent target cell lysis was not affected by the KRAS mutation status. On the other hand, perforin-independent ADCC was observed only in CRC cells with wild-type KRAS, but not in cells with mutant KRAS. Their experiments also revealed that the Fas-Fas ligand (FasL) interaction was responsible for the induction of apoptosis and perforin-independent ADCC. Thus, their findings clearly suggested that ADCC is an important mode of action of cetuximab and that KRAS mutation impairs the therapeutic effect exerted by cetuximab-mediated ADCC. In our study, regrettably, the limited number of patients precluded any definitive confirmation of this in our clinical setting of mCRC patients[27]. Therefore, all together, our results seem to suggest a link between iNKT cells, basal ADCC activity, genotypes in FCGR2A and FCGR3A, and efficacy of cetuximab in KRAS wild-type mCRC patients.
The efficacy of monoclonal anti-EGFR antibodies, like cetuximab, has been proven in mCRC patients. It has been established clearly that response to anti-EGFR antibody treatment is only possible in selected patient groups. However, predictive factors for the efficacy of anti-EGFR therapy have still to be completely elucidated. A factor identified in multiple studies as essential for appropriate assessment of eligibility for cetuximab or panitumumab treatment is the absence of KRAS gene mutations. EGFR expression on the surface of cancer cells does not seem to have a decisive influence on the efficacy of the therapy. There are ongoing studies assessing the predictive value of the number of copies of the EGFR gene, mutations in the NRAS, PI3KCA, P53 and PTEN genes, concentration of EGFR ligands and polymorphisms in the EGF and EGFR, and the FCGR2A and FCGR3A, genes. In our study, we observed that combining iNKT number and ADCC basal level allowed to identify a group of mCRC patients, having both determinations above the respective median level and a longer OS. This combination looks like the best prognosticator in our population of patients. However, it has not as of yet been examined in large randomized prospective studies and hence should still be better elucidated before using as a basis for mCRC patient eligibility for cetuximab treatment.
We are grateful to the Fondazione Veronesi that granted Daniela Vivenza and Martino Monteverde with Post-Doctoral Fellowship Veronesi and to the Fondazione Cassa Risparmio of Cuneo for partially supporting the study.
The efficacy of monoclonal anti-epidermal growth factor receptor (EGFR) antibodies, like cetuximab, has been proven in metastatic colorectal cancer (mCRC) patients. It has been established clearly that response to anti-EGFR antibody treatment is only possible in selected patient groups. However, predictive factors for the efficacy of anti-EGFR therapy have still to be completely elucidated. A factor identified in multiple studies as essential for appropriate assessment of eligibility for cetuximab or panitumumab treatment is the absence of mutation in RAS genes. EGFR expression on the surface of cancer cells does not seem to have a decisive influence on the efficacy of the therapy. There are ongoing studies assessing the predictive value of the number of copies of the EGFR gene, mutations in the NRAS, PI3KCA, P53 and PTEN genes, concentration of EGFR ligands and polymorphisms in the EGF and EGFR, and the FCGR2A and FCGR3A genes.
In this study the authors aim to evaluate the prognostic and predictive value of cetuximab-mediated antibody-dependent cell-mediated cytotoxicity (ADCC) and circulating invariant natural killer T (iNKT) cells levels in mCRC; the authors shall analyse their correlation with EGFR level, mutational status of KRAS, NRAS, BRAF, progression free survival and overall survival in a prospective cohort of mCRC patients treated with cetuximab-based therapy.
The prognostic value of circulating iNKT cell and ADCC basal level reported here adds to previous notions and strengthens the hypothesis that human iNKT cells may also contribute to antitumor responses in cancer patients and to cetuximab efficacy. These results indicate also that the contribution of the indirect action of cetuximab may be relative high, compared with the direct anti-EGFR action, toward the clinical therapeutic effect.
In summary, the authors demonstrated here, in a prospective study, that a low level of circulating iNKT cells and a low ADCC activity before treatment in mCRC patients are significantly associated with poor survival. These data suggest that reconstitution of the iNKT cell pool (e.g., by adoptive transfer of ex vivo expanded autologous iNKT cells) provides a promising immunotherapeutic strategy for mCRC. Furthermore, screening for iNKT cells and ADCC levels in peripheral-blood samples might provide a noninvasive, straightforward prognostic parameter and may also be useful for determining which patients can benefit from cetuximab therapy.
The antibody-dependent cell-mediated cytotoxicity is a mechanism of cell-mediated immune defense whereby an effector cell of the immune system actively lyses a target cell, whose membrane-surface antigens have been bound by specific antibodies. It is one of the mechanisms of the adaptive immune response through which antibodies can act to limit and contain tumors; classical antibody-dependent cell-mediated cytotoxicity is mediated by NK cells, which express CD16 which is an Fc receptor. This receptor recognizes, and binds to, the Fc portion of an antibody, such as the IgG1 anti-EGFR cetuximab; cetuximab is a recombinant chimeric mAb composed of the variable regions of a murine anti-EGFR antibody and of the constant regions of a human IgG1 kappa immunoglobulin. It is indicated for the treatment of squamous cell carcinoma of the head and neck and of RAS wild type mCRC.
This manuscript contributes to shed light to monoclonal therapy response in mCRC patients.
P- Reviewer: Chen Z S- Editor: Qiu S L- Editor: A E- Editor: Lu YJ
| 1. | Kopetz S, Chang GJ, Overman MJ, Eng C, Sargent DJ, Larson DW, Grothey A, Vauthey JN, Nagorney DM, McWilliams RR. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol. 2009;27:3677-3683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 919] [Cited by in RCA: 1032] [Article Influence: 64.5] [Reference Citation Analysis (0)] |
| 2. | Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337-345. [PubMed] |
| 3. | Allegra CJ, Jessup JM, Somerfield MR, Hamilton SR, Hammond EH, Hayes DF, McAllister PK, Morton RF, Schilsky RL. American Society of Clinical Oncology provisional clinical opinion: testing for KRAS gene mutations in patients with metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy. J Clin Oncol. 2009;27:2091-2096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 893] [Cited by in RCA: 895] [Article Influence: 55.9] [Reference Citation Analysis (0)] |
| 4. | Sartore-Bianchi A, Di Nicolantonio F, Nichelatti M, Molinari F, De Dosso S, Saletti P, Martini M, Cipani T, Marrapese G, Mazzucchelli L. Multi-determinants analysis of molecular alterations for predicting clinical benefit to EGFR-targeted monoclonal antibodies in colorectal cancer. PLoS One. 2009;4:e7287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 186] [Cited by in RCA: 211] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 5. | De Roock W, Claes B, Bernasconi D, De Schutter J, Biesmans B, Fountzilas G, Kalogeras KT, Kotoula V, Papamichael D, Laurent-Puig P. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. Lancet Oncol. 2010;11:753-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1491] [Cited by in RCA: 1656] [Article Influence: 110.4] [Reference Citation Analysis (1)] |
| 6. | Van Cutsem E, Lenz HJ, Köhne CH, Heinemann V, Tejpar S, Melezínek I, Beier F, Stroh C, Rougier P, van Krieken JH. Fluorouracil, leucovorin, and irinotecan plus cetuximab treatment and RAS mutations in colorectal cancer. J Clin Oncol. 2015;33:692-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 564] [Cited by in RCA: 632] [Article Influence: 63.2] [Reference Citation Analysis (0)] |
| 7. | Bokemeyer C, Bondarenko I, Makhson A, Hartmann JT, Aparicio J, de Braud F, Donea S, Ludwig H, Schuch G, Stroh C. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2009;27:663-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1218] [Cited by in RCA: 1241] [Article Influence: 73.0] [Reference Citation Analysis (0)] |
| 8. | Maréchal R, De Schutter J, Nagy N, Demetter P, Lemmers A, Devière J, Salmon I, Tejpar S, Van Laethem JL. Putative contribution of CD56 positive cells in cetuximab treatment efficacy in first-line metastatic colorectal cancer patients. BMC Cancer. 2010;10:340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 9. | Iannello A, Ahmad A. Role of antibody-dependent cell-mediated cytotoxicity in the efficacy of therapeutic anti-cancer monoclonal antibodies. Cancer Metastasis Rev. 2005;24:487-499. [PubMed] |
| 10. | Kohrt HE, Houot R, Marabelle A, Cho HJ, Osman K, Goldstein M, Levy R, Brody J. Combination strategies to enhance antitumor ADCC. Immunotherapy. 2012;4:511-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 11. | Porcelli S, Yockey CE, Brenner MB, Balk SP. Analysis of T cell antigen receptor (TCR) expression by human peripheral blood CD4-8- alpha/beta T cells demonstrates preferential use of several V beta genes and an invariant TCR alpha chain. J Exp Med. 1993;178:1-16. [PubMed] |
| 12. | Molling JW, Langius JA, Langendijk JA, Leemans CR, Bontkes HJ, van der Vliet HJ, von Blomberg BM, Scheper RJ, van den Eertwegh AJ. Low levels of circulating invariant natural killer T cells predict poor clinical outcome in patients with head and neck squamous cell carcinoma. J Clin Oncol. 2007;25:862-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 158] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 13. | Seo Y, Ishii Y, Ochiai H, Fukuda K, Akimoto S, Hayashida T, Okabayashi K, Tsuruta M, Hasegawa H, Kitagawa Y. Cetuximab-mediated ADCC activity is correlated with the cell surface expression level of EGFR but not with the KRAS/BRAF mutational status in colorectal cancer. Oncol Rep. 2014;31:2115-2122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 14. | Monteverde M, Milano G, Strola G, Maffi M, Lattanzio L, Vivenza D, Tonissi F, Merlano M, Lo Nigro C. The relevance of ADCC for EGFR targeting: A review of the literature and a clinically-applicable method of assessment in patients. Crit Rev Oncol Hematol. 2015;95:179-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Karapetis CS, Khambata-Ford S, Jonker DJ, O’Callaghan CJ, Tu D, Tebbutt NC, Simes RJ, Chalchal H, Shapiro JD, Robitaille S. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359:1757-1765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2724] [Cited by in RCA: 2761] [Article Influence: 162.4] [Reference Citation Analysis (0)] |
| 16. | Laurent-Puig P, Cayre A, Manceau G, Buc E, Bachet JB, Lecomte T, Rougier P, Lievre A, Landi B, Boige V. Analysis of PTEN, BRAF, and EGFR status in determining benefit from cetuximab therapy in wild-type KRAS metastatic colon cancer. J Clin Oncol. 2009;27:5924-5930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 516] [Cited by in RCA: 525] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 17. | Imai K, Matsuyama S, Miyake S, Suga K, Nakachi K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: an 11-year follow-up study of a general population. Lancet. 2000;356:1795-1799. [PubMed] |
| 18. | Levy EM, Roberti MP, Mordoh J. Natural killer cells in human cancer: from biological functions to clinical applications. J Biomed Biotechnol. 2011;2011:676198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 135] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 19. | Lee SC, Srivastava RM, López-Albaitero A, Ferrone S, Ferris RL. Natural killer (NK): dendritic cell (DC) cross talk induced by therapeutic monoclonal antibody triggers tumor antigen-specific T cell immunity. Immunol Res. 2011;50:248-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 133] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 20. | Krawczyk PA, Kowalski DM. Genetic and immune factors underlying the efficacy of cetuximab and panitumumab in the treatment of patients with metastatic colorectal cancer. Contemp Oncol (Pozn). 2014;18:7-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Reichert TE, Strauss L, Wagner EM, Gooding W, Whiteside TL. Signaling abnormalities, apoptosis, and reduced proliferation of circulating and tumor-infiltrating lymphocytes in patients with oral carcinoma. Clin Cancer Res. 2002;8:3137-3145. [PubMed] |
| 22. | Taylor RJ, Chan SL, Wood A, Voskens CJ, Wolf JS, Lin W, Chapoval A, Schulze DH, Tian G, Strome SE. FcgammaRIIIa polymorphisms and cetuximab induced cytotoxicity in squamous cell carcinoma of the head and neck. Cancer Immunol Immunother. 2009;58:997-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 23. | Mellor JD, Brown MP, Irving HR, Zalcberg JR, Dobrovic A. A critical review of the role of Fc gamma receptor polymorphisms in the response to monoclonal antibodies in cancer. J Hematol Oncol. 2013;6:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 226] [Cited by in RCA: 269] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 24. | Kjersem JB, Skovlund E, Ikdahl T, Guren T, Kersten C, Dalsgaard AM, Yilmaz MK, Fokstuen T, Tveit KM, Kure EH. FCGR2A and FCGR3A polymorphisms and clinical outcome in metastatic colorectal cancer patients treated with first-line 5-fluorouracil/folinic acid and oxaliplatin +/- cetuximab. BMC Cancer. 2014;14:340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | Kjersem JB, Ikdahl T, Guren T, Skovlund E, Sorbye H, Hamfjord J, Pfeiffer P, Glimelius B, Kersten C, Solvang H. Let-7 miRNA-binding site polymorphism in the KRAS 3’UTR; colorectal cancer screening population prevalence and influence on clinical outcome in patients with metastatic colorectal cancer treated with 5-fluorouracil and oxaliplatin +/- cetuximab. BMC Cancer. 2012;12:534. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 26. | van Houdt WJ, Hoogwater FJ, de Bruijn MT, Emmink BL, Nijkamp MW, Raats DA, van der Groep P, van Diest P, Borel Rinkes IH, Kranenburg O. Oncogenic KRAS desensitizes colorectal tumor cells to epidermal growth factor receptor inhibition and activation. Neoplasia. 2010;12:443-452. [PubMed] |
| 27. | Nakadate Y, Kodera Y, Kitamura Y, Shirasawa S, Tachibana T, Tamura T, Koizumi F. KRAS mutation confers resistance to antibody-dependent cellular cytotoxicity of cetuximab against human colorectal cancer cells. Int J Cancer. 2014;134:2146-2155. [PubMed] |