Published online Dec 15, 2015. doi: 10.4251/wjgo.v7.i12.455
Peer-review started: June 27, 2015
First decision: July 25, 2015
Revised: October 10, 2015
Accepted: November 3, 2015
Article in press: November 4, 2015
Published online: December 15, 2015
Processing time: 170 Days and 11.1 Hours
Gastric cancer still is a major concern as the third most common cancer worldwide, despite declining rates of incidence in many Western countries. Helicobacter pylori (H. pylori) is the major cause of gastric carcinogenesis, and its infection insults gastric mucosa leading to the occurrence of atrophic gastritis which progress to intestinal metaplasia, dysplasia, early gastric cancer, and advanced gastric cancer consequently. This review focuses on multiple factors including microbial virulence factors, host genetic factors, and environmental factors, which can heighten the chance of occurrence of gastric adenocarcinoma due to H. pylori infection. Bacterial virulence factors are key components in controlling the immune response associated with the induction of carcinogenesis, and cagA and vacA are the most well-known pathogenic factors. Host genetic polymorphisms contribute to regulating the inflammatory response to H. pylori and will become increasingly important with advancing techniques. Environmental factors such as high salt and smoking may also play a role in gastric carcinogenesis. It is important to understand the virulence factors, host genetic factors, and environmental factors interacting in the multistep process of gastric carcinogenesis. To conclude, prevention via H. pylori eradication and controlling environmental factors such as diet, smoking, and alcohol is an important strategy to avoid H. pylori-associated gastric carcinogenesis.
Core tip:Helicobacter pylori (H. pylori) is an important etiologic agent in gastric carcinogenesis. Here, we summarize not only recently investigated mechanisms of virulence factors, host genetic factors, and environmental factors, but also potential prevention. The best preventive methods in H. pylori-induced carcinogenesis may be achieved through H. pylori eradication, dietary, or lifestyle modifications, as well as a better understanding of molecular pathogenesis.
-
Citation: Ahn HJ, Lee DS.
Helicobacter pylori in gastric carcinogenesis. World J Gastrointest Oncol 2015; 7(12): 455-465 - URL: https://www.wjgnet.com/1948-5204/full/v7/i12/455.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v7.i12.455
Gastric cancer remains the third leading cause of cancer death worldwide[1,2]. Although the incidence rates in the United States and many Western countries have declined significantly, the prevalence remains high in Eastern Europe, Central, and South America, and especially in East Asia, where up to 24.18 cases of gastric cancer per 100000 adults were estimated in 2012[3].
Because nearly 40% of patients never report tumor-related symptoms before diagnosis, most gastric cancer cases are advanced-type upon initial presentation, for which prognosis remains poor[4]. Thus, prevention may be the most promising strategy for cancer control.
Despite the fact that the molecular pathways of gastric carcinogenesis remain unclear[5], there are numerous factors that have been associated with gastric carcinogenesis, such as genetic background[6,7], behavioral factors (e.g., alcohol, smoking, diet)[8,9], and Helicobacter pylori (H. pylori). Most importantly, H. pylori is the most crucial etiologic agent for gastric adenocarcinoma[10,11], which is involved in 90% of all gastric malignancies[12].
Here, we review the recently investigated mechanisms of H. pylori-induced gastric carcinogenesis, focusing not only on epidemiological factors, bacterial virulence factors, host factors, or other environmental factors, but also on preventive management and future directions.
H. pylori is a gram-negative microaerophilic bacterium that infects nearly 50% of the world’s population. It has been found in every population premeditated, although the incidence varies with age, childhood socio-economic status, education level, living environment, occupation, and geographic regions, in that the incidence is higher in developing countries and much of East Asia[13-15].
In 1994, H. pylori was categorized as a class I (definite) carcinogen by the International Agency for Research on Cancer (IARC), a division of the World Health Organization (WHO)[15,16]. Subsequently, it is believed that H. pylori is the major risk factor of gastric cancer based on animal studies[17,18], as well as clinical observational and human interventional studies[10,19-21].
The clinical manifestations of H. pylori infection are as follows: (1) chronic gastritis, which almost all patients develop and most remain asymptomatic; (2) duodenal ulcer (DU) phenotype, which occurs in 10%-15% of infected individuals; (3) gastric ulcer/adenocarcinoma phenotype, which develops into gastric cancer in 1%-3% of infected individuals; and (4) gastric mucosa–associated lymphoid tissue lymphoma (MALToma), which develops in 0.1% of infected subjects[12,15,22,23]. The DU phenotype with antral colonization is associated with high gastrin and high output of gastric acid, and also related to a lowered risk for gastric cancer occurrence[15,20,24]. However, the gastric adenocarcinoma phenotype, which occurs more frequently when there is proximal colonization of the stomach (pangastritis), brings about damage to gastric glands, causing atrophic gastritis and associated hydrochlorhydria or achlorhydria, and it is characterized by low pepsinogen I and high gastrin levels and a low pepsinogen I/II ratio. This phenotype eventually progresses to a multistep process including intestinal metaplasia, dysplasia, and adenocarcinoma[20,23-25]. This series of histological changes may take as long as 7 or 8 decades[26] and is a well-known characteristic of intestinal-type adenocarcinoma, which is one of two distinct histological variants. It is also believed that H. pylori is associated with diffuse-type adenocarcinoma[11], which shows the paucity of glandular structure and comprises poorly cohesive cells that infiltrate the gastric wall[15]. However, pathological sequences of the diffuse-type are less characterized[26].
Gastric adenocarcinoma is also categorized into proximal tumors (esophagogastric junction and gastric cardia) and distal tumors (gastric antrum, body, and fundus)[15]. Proximal gastric cancers have different epidemiological and pathophysiological characteristics compared with distal cancer, and many studies support that this type of cancer is inversely associated with H. pylori infection[21,27,28] despite some debates[29,30]. Although the incidence of cancer of the proximal stomach has been increasing, the majority of gastric cancers worldwide arise from the distal stomach, and the significance of H. pylori in gastric carcinogenesis remains overwhelming.
As mentioned above, H. pylori-induced gastric carcinogenesis in humans rarely occurs among infected individuals. Many studies over the past three decades suggest that the combination of a bacterial virulent strain, a genetically susceptible host, and a predisposed gastric environment may be required for cancer to develop.
H. pylori yields various virulence factors that may dysregulate host intracellular signaling pathways and decrease the threshold for neoplastic transformation. Of all virulence factors, cagA (cytotoxin-associated gene A) and its pathogenicity island (cag PAI) and vacA (vacuolating cytotoxin A) are the major pathogenic factors.
Cag PAI and cagA: The most well-featured H. pylori virulence factor is the cag PAI, which is about 40 kb and contains 27-31 genes. The terminal gene of this island, cagA, is a highly immunogenic protein often used as a indicator for the entire cag PAI locus[26]. It is believed that cagA-positive (i.e., cag PAI-positive) strains are linked to more harsh inflammation, higher steps of atrophy, and a larger possibility of advancement to adenocarcinoma of stomach compared with cagA-negative (i.e., cag PAI-negative) strains[15,31-34]. The estimated relative risk (RR) ranges from 2 to as high as 28.4[23]. However, the same clinical diseases of these are also originated by infections with cagA-negative strains, compatible with the assumption that any other bacterial or host factor may contribute to increased risk of a significant clinical outcome[35].
The prevalence of cagA differs widely according to region. It varies dramatically, with the prevalence reaching almost 100% in East Asia, and less than 50% in some countries in the West[36]. It has been observed that people with cagA-positive strains of H. pylori are more susceptible to peptic ulcer disease or gastric adenocarcinoma than are those with cagA-negative strains in Western countries[37,38]. In East Asia, most H. pylori strains possess the cagA gene without regard to the disease; therefore, the pathogenic difference in East Asia is hard to explain concerning the existence of the cagA gene alone[39]. Thus, the combined circumstances that permit cagA to initiate carcinogenesis remain unclear.
The cag PAI encodes a type IV secretion system (T4SS; i.e., a molecular motors) that injects at least 18 proteins including cagA into host cells[14,40].
CagA and glutamate-proline-isoleucine-tyrosine-alanine motifs: The H. pylori cagA protein is a 120- to 140-kDa protein translocated into host cells by the T4SS after bacterial attachment. When the cagA enters the host cell, it can bind to the cell membrane inner surface and undergo tyrosine phosphorylation. This in turn results in morphological changes of the cell, and influences various intracellular signal transduction pathways. In addition, cagA exerts pathogenic effects without phosphorylation. Both the phosphorylation-dependent and -independent cagA signals interact with many host proteins to trigger downstream pathways, such as the ras/mitogen-activated protein kinase/extracellular signal-regulated kinase pathway[41,42], nuclear factor κB (NF-κB) pathway and B-catenin pathway[43].
Glutamate-proline-isoleucine-tyrosine-alanine (EPIYA) motifs are the sites of cagA phosphorylation. According to variations in the encompassing amino acid sequence, four distinct EPIYA-motifs are reported (-A, -B, -C, -D)[44,45]. The first repeat region comprises EPIYA-A/EPIYA-B segments and is present in strains throughout the world. However, the prevalence of the second repeat region varies by geographic area. The respective names of the second repeat region segments of the Western and East Asian strains are EPIYA-C and EPIYA-D[35,46]. In Western strains, an increased number of cagA EPIYA-C sites is an significant barometer of the risk of progressing to gastric adenocarcinoma[47]. East Asian strains are nearly the only strains to carry the EPIYA-D motif. These are strains from South Korea, Japan, and China. Many studies have concluded that when infections that occur in the same area are compared, infection with EPIYA-D strains have a higher risk of gastric cancer or peptic ulcer compared to infection with EPIYA-C strains[35,48-50]. However, the role of cagA remains unclear. In some reports, cagA can micro-evolve within an individual[51] or the EPIYA-B motif may be polymorphic[52]. Thus, further studies are required to explore the association between cagA EPIYA motifs and gastric carcinogenesis.
VacA: All strains of H. pylori possess and more than half express the vacA gene, which encodes a pore-forming protein that binds to epithelium via interaction with protein-tyrosine phosphatases[53]. vacA protein is a very potent inhibitor of T cell activation in vitro[54], and it has multiple activities, such as pore-formation in membranes, cytochrome C release from mitochondria progressing to apoptosis, and attaching to cell membrane receptors resulting in pro-inflammatory responses[36].
There are many studies showing that differences in vacA gene structure are associated with severities of clinical disease. According to variations in in vitro vacuolating capacity, studies have reported differences in the signal region (s1 and s2), the middle regions (m1 and m2), and recently the intermediate regions (i1 and i2)[47,55,56]. Investigators have suggested that when compared to s2 or m2 strains, individuals who have been infected with vacA s1 or m1 strains may have a heightened risk of gastric cancer and/or peptic ulcers in Africa, Latin America, and the Middle East[57,58]. More recently, i1 strains have been suggested to be correlated not only with inflammatory and dysplastic, but also malignant neoplastic tissue formation in Portugal, Belgium, and Iran[59-61]. However, unlike the above reports, in a reports of subjects from East and Southeast Asia, there was no correlation between the i-region and clinical disease[36]. In a recent long-term study (mean 12.8-year follow-up) based on Spanish populations, there was no correlation between the i-region and clinical outcome either[62]. In addition, there are relatively few studies that have controlled for variables associated with inflammation severity, such as the presence of cagA. In summary, despite numerous reports that the vacA s1/i1 genotypes are highly pathogenic, no clear association has been observed yet.
There is increasing evidence that the nature of the inflammatory response to H. pylori is in large part determined by polymorphisms in several host genes encoding cytokines and cytokine receptors.
IL-1 gene cluster polymorphism: El-Omar et al[63] first reported that pro-inflammatory IL-1 gene cluster polymorphisms (IL-1B gene encoding cytokine IL-1β and IL-IRN gene encoding its naturally occurring receptor antagonist, IL-1RA) were clearly related to an intense inflammatory response resulting in hypochlorhydria and high risk of cancer. Subjects with the IL-1B-31*C or -511*T and IL-1RN*2/*2 genotypes have a higher risk of gastric atrophy, gastric cancer, or hypochlorhydria as a result of H.pylori infection[23,63,64]. The heightened risk of cancer development with these genotypes was 2- to 3-fold compared with non-inflammatory genotypes[63-65]. These findings have been confirmed in other groups such as Caucasian, Hispanic, and Asian populations[65-71].
In addition, Figueiredo et al[66] analyzed the mutual effects of bacterial virulence factors of H. pylori (cagA-positive, vacA s1, and vacA m1) and proinflammatory IL-1 genotypes. They showed that pro-inflammatory polymorphisms of IL-1, together with carriage of H. pylori with the vacA s1 form, heightened the possibility of developing gastric cancer 87-fold compared with individuals who had neither of these risk factors yet were still colonized by H. pylori[66]. Crucial evidence, provided by a transgenic study, has confirmed the exclusive role of IL-1β in H. pylori-associated gastric carcinogenesis[72]. According to this study, in transgenic mice, human IL-1β stomach-specific expression resulted in gastric cancer and spontaneous gastric inflammation which were associated with early recruitment of myeloid-derived suppressor cells to the stomach.
Despite some conflicting results among Caucasian, Asian, and Hispanic populations, there is a consensus that IL-1B and IL-IRN are crucial cytokine receptors in the pathogenesis of H. pylori-induced gastric carcinogenesis[73-76].
Other cytokine gene polymorphism: Additional relations with gastric cancer risk for genetic polymorphisms in TNF-α and IL-10 have been reported[64]. Pro-inflammatory genotypes of TNF-α and IL-10 were each related to an approximately two-fold greater possibility of nocardia gastric cancer[64,65]. Additional reports have suggested that polymorphisms of the Toll-like receptor-4 (TLR-4) gene also heightens gastric cancer risk. An 11-fold increase in the odds ratio (OR) for hypochlorhydria was found in the TLR4 + 896G polymorphism carriers. Also, in Caucasian populations, these carriers had significantly more severe atrophic gastritis and inflammation[77].
Host genetics and gastric cancer in the era of Genome Wide Association Studies and future perspectives: In 2008, Sakamoto et al[78] first reported that an intronic single nucleotide polymorphism (SNP; rs2976392) in the prostate stem cell antigen (PSCA) was significantly associated with diffuse-type gastric cancer in Japan. Recent two meta-analyses also suggested that PSCA -rs2294008C>T and -rs2976392G>A were potential factors of gastric cancer development in East Asians[79,80]. In addition, it has been thought that the PSCA-rs2294008 polymorphism heightened risk of non-cardiac gastric cancer but protects against proximal cancer in Caucasian populations[81,82]. With recent advances in technology, we can increase our understanding of the genetic mechanisms of gastric carcinogenesis through SNP and next generation sequencing, which could be useful for screening and a necessary step for more effective treatment.
Environmental factors may also play a role in H. pylori-induced gastric carcinogenesis. Salt is a well-known dietary factor. In a Japanese prospective study in 2006[83], a significant correlation between salt consumption and gastric adenocarcinoma was reported in individuals who had both H. pylori infection and atrophic gastritis [age- and sex-adjusted hazard ratio, 2.87 (1.14-7.24)]. In addition, according to a recent animal study, high dietary salt intake potentiates the carcinogenic effects of cagA-positive H. pylori strains[84]. There are some suggestions on the mechanisms by which salt potentiates H. pylori-induced gastric carcinogenesis; however, they are not entirely understood. First, salt may destroy the gastric mucosa, thereby leading to inflammation and damage or permitting entry of carcinogens into stomach[14,85]. Second, upregulated production of proinflammatory enzymes and cytokines such as nitric oxide synthase and cyclooxygenase-2 (COX-2) in response to a high-salt consumption may be contributing[86]. Finally, recent reports suggest that high salt concentrations modulate virulence factors, including cagA, in H. pylori[87,88].
Smoking may be the most significant lifestyle-related risk factor. In a recent systemic review and meta-analysis of cohort studies, it was shown that smoking is correlated with an high relative risk for both gastric cardia [1.87 (1.31-2.67)] and non-cardia cancers [1.60 (1.41-1.80)] significantly[89].
H. pylori can be divided into seven global populations and subpopulations with distinct geographic distributions, genetically derived from ancestral populations such as those in Africa (Ancestral Africa 1 or 2; AA1 or 2), Europe (Ancestral Europe 1 or 2; AE1 or 2), and East Asia (Ancestral East Asia; AEA). While stomach cancer rates correlate with H. pylori prevalence in some areas, in other regions there is no correlation with H. pylori prevalence, such as some regions in Africa or South America[90]. In Columbia, the reported gastric cancer rate in the Andes Mountains (approximately 150 per 100000) is 25-fold higher than that in coastal regions (approximately 6 per 100000), in spite of similarly high (approximately 90%) prevalences of H. pylori in the two regions[2]. As recently reported[91] in those populations, the authors extracted both human ancestry, from the participants’ DNA, and H. pylori ancestry, from antral biopsies of the participants, and assessed how coevolution may have had on effect on gastric disease. Remarkably, they found that the interaction between Amerindian host ancestry and H. pylori ancestry AA1, which affects the severity of premalignant histopathology, was approximately five-fold larger than the effect of cagA (RR = 5.08 vs 0.98). This result suggested that ancestral coevolutionary relationships can be significant determinants of gastric cancer.
The most important primary prevention strategies for gastric cancer potentially include behavioral (dietary or lifestyle) modifications and a decline in the prevalence of H. pylori, the major causal factor of gastric cancer[92]. Although the pathogenesis remains unclear, prevention through dietary intervention would include increased fruit, allium, and non-starchy vegetable intake and reduced ingestion of salt or salt-preserved foods and N-nitroso compounds[93-98]. Lifestyle modifications such as maintaining normal weight, limiting alcohol consumption, and smoking cessation may also lower the risk of the disease[99].
H. pylori eradication may be the most efficient method to prevent gastric cancer, in that H. pylori infection can persist for decades and slowly progress from preneoplastic lesions to gastric cancer. It is believed that H. pylori eradication can suppress the recurrence of peptic ulcers, induce remission of MALToma of the stomach, and lower the rate of recurrence after endoscopic resection of early gastric cancer. However, demonstrating that H. pylori eradication directly decreases gastric cancer risk remains challenging.
Currently, a novel meta-analysis of six randomized trials performed in asymptomatic adults estimated a benefit from H. pylori eradication (RR = 0.66; 95%CI: 0.46-0.95)[100]. A Chinese randomized controlled trial performed in 2012 in the general adult population concluded that there was a significant decline of gastric cancer risk after 15 years of follow-up (4.6% in the control group, 3.0% in the treated group; OR = 0.61; 95%CI: 0.38-0.96)[101]. Despite some limitations of the study, such as examination of middle-aged groups only and a relative paucity of endpoint data, these well-designed studies have presented good results in terms of eradication therapy for prevention of gastric cancer. In addition, a recently published report from the WHO’s IARC, conducted in a working group population, concluded that H. pylori eradication can be efficient in gastric cancer prevention, and H. pylori screening and treatment strategies would be cost-effective. However, uncertainties regarding the generalizability of the results, cost-effectiveness, and possible adverse outcomes of programs applied in community settings need to be explored[92].
Peptic ulcer, MALToma, and endoscopic treatment of early gastric cancer are well-known indications for H. pylori eradication. Recently, it is generally acknowledged that iron deficiency anemia, idiopathic thrombocytopenic purpura, functional dyspepsia, and long-term use of non-steroidal anti-inflammatory drugs (NSAIDs) or proton pump inhibitors (PPIs) are considered highly evident indications[102-106]. In the context described above, Japanese guidelines revised in 2009 strongly recommend (Recommendation grade A) that all H. pylori infections should be eradicated regardless of the associated disease[106]. In addition, in the Kyoto Global Consensus Meeting on H. pylori Gastritis (From January 30, 2014 to February 1, 2014), it was suggested by 46 authorities that all H. pylori-infected individuals, including asymptomatic individuals, should be considered eradication subjects, especially in those with functional dyspepsia[107,108]. Thus, in other East Asian countries such as South Korea and China, where the prevalence of H. pylori infection and gastric cancer remains high, careful consideration is required for eradication therapy.
Although standard triple therapy (PPI + clarithromycin + amoxicillin or PPI + clarithromycin + metronidazole) is still recommended as a first-line regimen in recent Korean and Japanese guidelines[104-106], the increasing rate of eradication failure due to primary resistance to clarithromycin and metronidazole is a global concern[109-111]. Recently, high (≥ 20%) resistance rates of clarithromycin have been reported in the United States and developed countries in Europe and Asia, while relatively low (< 10%) rates have been reported in North Europe[112,113]. Especially, one Japanese multicenter study reported that clarithromycin-resistance rates have increased rapidly from 18.9% in 2002 to 27.2% in 2006[114]. In addition, over 80% of metronidazole-resistance rates have been observed in Africa, Iran, and South America, and 20%-40% of metronidazole-resistance rates have also been reported in United States, Europe, and East Asia[112,115]. Primary quinolone-resistance rates have also been increasing (> 10%) in developed countries in Europe and Asia[112,115,116]. Besides, amoxicillin resistance in Europe has been very low (0% to < 2%) but higher (6%-59%) in Asia, South America, and Africa, and tetracycline resistance has been low or absent (< 5%) in most countries while higher (9%-27%) in South America and Asia[112,115].
With regard to the high resistance to clarithromycin, recent European guidelines[102] recommend that first-line regimens should be tailored according to clarithromycin resistance. In low-resistance (< 20%) regions, standard triple therapy is recommended as a first-line regimen, while in high-resistance (> 20%) regions, bismuth quadruple therapy or sequential/concomitant therapy is recommended first. However, in East Asia, we could not evaluate the superiority of sequential/concomitant therapy over standard therapy[103,104,106,117].
Thus, to maximize the H. pylori eradication treatment effect, individually tailored treatment with consideration of a variety of demographic factors including genetic polymorphisms, antibiotic resistance, and age will be important in the future.
The role of aspirin, NSAIDs, and COX-2 inhibitors in gastric carcinogenesis should be considered, because H. pylori infection is thought to induce COX-2 overexpression[118,119], and higher levels of COX-2 expression have been observed in gastric carcinoma and premalignant lesions[120,121]. Therefore, it is believed that intervention with aspirin, NSAIDs, and COX-2 inhibitors inhibits or reverses the process of H. pylori-related carcinogenesis and prevents the development of gastric cancer[122].
Vitamin C and antioxidants are also considered protective against H. pylori-induced gastric carcinogenesis by strengthening the mucosal immune response, neutralizing free radicals, reducing the creation of gastric N-nitroso compounds, inhibiting cell proliferation, and directly influencing H. pylori growth[92]. According to a recent meta-analysis of randomized trials conducted in asymptomatic adults, H. pylori eradication in combination with antioxidants or vitamins showed a beneficial impact (RR = 0.52; 95%CI: 0.31-0.87)[100]. However, to date, there have been conflicting data in association with gastric cancer and NSAIDs or vitamin C; thus, further studies are required to support the roles of these agents in H. pylori-associated gastric carcinogenesis.
In addition, in a recent meta-analysis based on 45 randomized controlled trials, the additional use of probiotics with standard triple therapy was associated with an increased H. pylori eradication rate in the per-protocol set (OR = 1.13; 95%CI: 1.10-1.16), a reduction in adverse events (RR = 0.59; 95%CI: 0.48-0.71), and economic burden and a poor compliance rate[123].
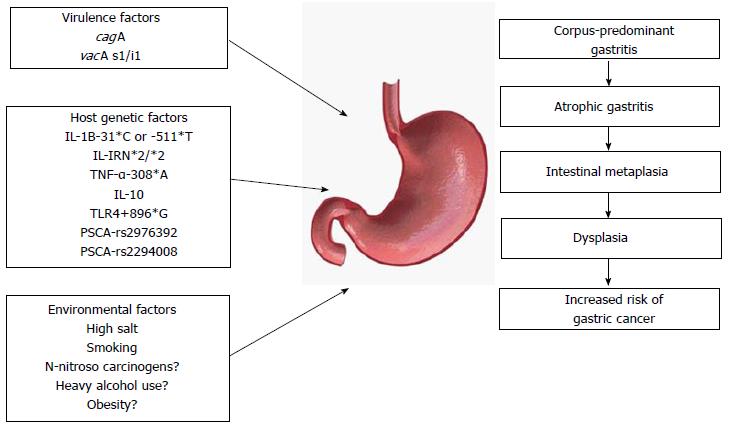
H. pylori infection is major factor for gastric carcinogenesis. During H. pylori infection and subsequent inflammation and carcinogenesis over a time span of decades, numerous factors including bacterial virulence, host genetic, and environmental factors interact and elicit variable clinical outcomes (Figure 1). Thus, understanding the complex mechanisms of a variety of factors is important and may provide future directions for novel therapy.
To date, prevention throughout behavioral management and H. pylori eradication may be an important strategy to reduce the occurrence of gastric cancer. A unique contrivance on potential dietary or other chemopreventive agents and related well-designed studies are required. In addition, it is important to take into account whom to eradicate, when to eradicate, and what regimen to use to eradicate H. pylori in the general population.
P- Reviewer: Manguso F, Tovey FI S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20108] [Cited by in RCA: 20515] [Article Influence: 2051.5] [Reference Citation Analysis (20)] |
| 2. | de Sablet T, Piazuelo MB, Shaffer CL, Schneider BG, Asim M, Chaturvedi R, Bravo LE, Sicinschi LA, Delgado AG, Mera RM. Phylogeographic origin of Helicobacter pylori is a determinant of gastric cancer risk. Gut. 2011;60:1189-1195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 112] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 3. | Cancer Reserch UK. Worldwide cancer incidence statistics, 2011 [accessed 2015 May 1]. Available from: http://www.cancerresearchuk.org/cancer-info/cancerstats/world/incidence/#By. |
| 4. | Schmidt N, Peitz U, Lippert H, Malfertheiner P. Missing gastric cancer in dyspepsia. Aliment Pharmacol Ther. 2005;21:813-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Figueiredo C, Garcia-Gonzalez MA, Machado JC. Molecular pathogenesis of gastric cancer. Helicobacter. 2013;18 Suppl 1:28-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 6. | McLean MH, El-Omar EM. Genetics of gastric cancer. Nat Rev Gastroenterol Hepatol. 2014;11:664-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 296] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 7. | Tan IB, Ng I, Tai WM, Tan P. Understanding the genetic basis of gastric cancer: recent advances. Expert Rev Gastroenterol Hepatol. 2012;6:335-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | de Martel C, Forman D, Plummer M. Gastric cancer: epidemiology and risk factors. Gastroenterol Clin North Am. 2013;42:219-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 277] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 9. | Kelley JR, Duggan JM. Gastric cancer epidemiology and risk factors. J Clin Epidemiol. 2003;56:1-9. [PubMed] |
| 10. | Suzuki H, Iwasaki E, Hibi T. Helicobacter pylori and gastric cancer. Gastric Cancer. 2009;12:79-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 92] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 11. | Polk DB, Peek RM. Helicobacter pylori: gastric cancer and beyond. Nat Rev Cancer. 2010;10:403-414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 880] [Cited by in RCA: 857] [Article Influence: 57.1] [Reference Citation Analysis (0)] |
| 12. | Noto JM, Peek RM. Helicobacter pylori: an overview. Methods Mol Biol. 2012;921:7-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 13. | Everhart JE. Recent developments in the epidemiology of Helicobacter pylori. Gastroenterol Clin North Am. 2000;29:559-578. [PubMed] |
| 14. | Wroblewski LE, Peek RM, Wilson KT. Helicobacter pylori and gastric cancer: factors that modulate disease risk. Clin Microbiol Rev. 2010;23:713-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 817] [Cited by in RCA: 1014] [Article Influence: 67.6] [Reference Citation Analysis (1)] |
| 15. | Abrams JA, Wang TC. Adenocarcinoma and other tumors of the stomach. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease Pathophysiology/Diagnosis/Management. 9th ed. Philadelphia, PA: Elsevier Saunders 2010; . [DOI] [Full Text] |
| 16. | Schistosomes , liver flukes and Helicobacter pylori. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 7-14 June 1994. IARC Monogr Eval Carcinog Risks Hum. 1994;61:1-241. [PubMed] |
| 17. | Honda S, Fujioka T, Tokieda M, Satoh R, Nishizono A, Nasu M. Development of Helicobacter pylori-induced gastric carcinoma in Mongolian gerbils. Cancer Res. 1998;58:4255-4259. [PubMed] |
| 18. | Watanabe T, Tada M, Nagai H, Sasaki S, Nakao M. Helicobacter pylori infection induces gastric cancer in mongolian gerbils. Gastroenterology. 1998;115:642-648. [PubMed] |
| 19. | Bornschein J, Malfertheiner P. Gastric carcinogenesis. Langenbecks Arch Surg. 2011;396:729-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3126] [Cited by in RCA: 3184] [Article Influence: 132.7] [Reference Citation Analysis (0)] |
| 21. | Helicobacter and Cancer Collaborative Group. Gastric cancer and Helicobacter pylori: a combined analysis of 12 case control studies nested within prospective cohorts. Gut. 2001;49:347-353. [PubMed] |
| 22. | Peek RM, Crabtree JE. Helicobacter infection and gastric neoplasia. J Pathol. 2006;208:233-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 446] [Article Influence: 22.3] [Reference Citation Analysis (2)] |
| 23. | Amieva MR, El-Omar EM. Host-bacterial interactions in Helicobacter pylori infection. Gastroenterology. 2008;134:306-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 392] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 24. | Datta De D, Roychoudhury S. To be or not to be: The host genetic factor and beyond in Helicobacter pylori mediated gastro-duodenal diseases. World J Gastroenterol. 2015;21:2883-2895. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (1)] |
| 25. | Ahmed A, Smoot D, Littleton G, Tackey R, Walters CS, Kashanchi F, Allen CR, Ashktorab H. Helicobacter pylori inhibits gastric cell cycle progression. Microbes Infect. 2000;2:1159-1169. [PubMed] |
| 26. | Lee DS, Moss SF. Targeting Helicobacter pylori in gastric carcinogenesis. Expert Opin Ther Targets. 2007;11:757-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Kamangar F, Dawsey SM, Blaser MJ, Perez-Perez GI, Pietinen P, Newschaffer CJ, Abnet CC, Albanes D, Virtamo J, Taylor PR. Opposing risks of gastric cardia and noncardia gastric adenocarcinomas associated with Helicobacter pylori seropositivity. J Natl Cancer Inst. 2006;98:1445-1452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 232] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 28. | da Costa DM, Dos Santos Pereira E, de Lima Silva-Fernandes IJ, Ferreira MV, Rabenhorst SH. Characterization of Gastric Cardia Tumors: Differences in Helicobacter pylori Strains and Genetic Polymorphisms. Dig Dis Sci. 2015;60:2712-2717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 29. | Kamangar F, Qiao YL, Blaser MJ, Sun XD, Katki H, Fan JH, Perez-Perez GI, Abnet CC, Zhao P, Mark SD. Helicobacter pylori and oesophageal and gastric cancers in a prospective study in China. Br J Cancer. 2007;96:172-176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 111] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 30. | Bornschein J, Selgrad M, Warnecke M, Kuester D, Wex T, Malfertheiner P. H. pylori infection is a key risk factor for proximal gastric cancer. Dig Dis Sci. 2010;55:3124-3131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 31. | Crabtree JE, Taylor JD, Wyatt JI, Heatley RV, Shallcross TM, Tompkins DS, Rathbone BJ. Mucosal IgA recognition of Helicobacter pylori 120 kDa protein, peptic ulceration, and gastric pathology. Lancet. 1991;338:332-335. [PubMed] |
| 32. | Kuipers EJ, Pérez-Pérez GI, Meuwissen SG, Blaser MJ. Helicobacter pylori and atrophic gastritis: importance of the cagA status. J Natl Cancer Inst. 1995;87:1777-1780. [PubMed] |
| 33. | Blaser MJ, Perez-Perez GI, Kleanthous H, Cover TL, Peek RM, Chyou PH, Stemmermann GN, Nomura A. Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. 1995;55:2111-2115. [PubMed] |
| 34. | Parsonnet J, Friedman GD, Orentreich N, Vogelman H. Risk for gastric cancer in people with CagA positive or CagA negative Helicobacter pylori infection. Gut. 1997;40:297-301. [PubMed] |
| 35. | Yamaoka Y, Graham DY. Helicobacter pylori virulence and cancer pathogenesis. Future Oncol. 2014;10:1487-1500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 101] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 36. | Yamaoka Y. Mechanisms of disease: Helicobacter pylori virulence factors. Nat Rev Gastroenterol Hepatol. 2010;7:629-641. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 500] [Cited by in RCA: 459] [Article Influence: 30.6] [Reference Citation Analysis (1)] |
| 37. | van Doorn LJ, Figueiredo C, Sanna R, Plaisier A, Schneeberger P, de Boer W, Quint W. Clinical relevance of the cagA, vacA, and iceA status of Helicobacter pylori. Gastroenterology. 1998;115:58-66. [PubMed] |
| 38. | Yamaoka Y, Kikuchi S, el-Zimaity HM, Gutierrez O, Osato MS, Graham DY. Importance of Helicobacter pylori oipA in clinical presentation, gastric inflammation, and mucosal interleukin 8 production. Gastroenterology. 2002;123:414-424. [PubMed] |
| 39. | Yamaoka Y, Kodama T, Gutierrez O, Kim JG, Kashima K, Graham DY. Relationship between Helicobacter pylori iceA, cagA, and vacA status and clinical outcome: studies in four different countries. J Clin Microbiol. 1999;37:2274-2279. [PubMed] |
| 40. | Tegtmeyer N, Wessler S, Backert S. Role of the cag-pathogenicity island encoded type IV secretion system in Helicobacter pylori pathogenesis. FEBS J. 2011;278:1190-1202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 202] [Cited by in RCA: 194] [Article Influence: 13.9] [Reference Citation Analysis (1)] |
| 41. | Xu X, Liu Z, Fang M, Yu H, Liang X, Li X, Liu X, Chen C, Jia J. Helicobacter pylori CagA induces ornithine decarboxylase upregulation via Src/MEK/ERK/c-Myc pathway: implication for progression of gastric diseases. Exp Biol Med (Maywood). 2012;237:435-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 42. | Mueller D, Tegtmeyer N, Brandt S, Yamaoka Y, De Poire E, Sgouras D, Wessler S, Torres J, Smolka A, Backert S. c-Src and c-Abl kinases control hierarchic phosphorylation and function of the CagA effector protein in Western and East Asian Helicobacter pylori strains. J Clin Invest. 2012;122:1553-1566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 202] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 43. | Wang F, Meng W, Wang B, Qiao L. Helicobacter pylori-induced gastric inflammation and gastric cancer. Cancer Lett. 2014;345:196-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 607] [Cited by in RCA: 571] [Article Influence: 51.9] [Reference Citation Analysis (1)] |
| 44. | Higashi H, Yokoyama K, Fujii Y, Ren S, Yuasa H, Saadat I, Murata-Kamiya N, Azuma T, Hatakeyama M. EPIYA motif is a membrane-targeting signal of Helicobacter pylori virulence factor CagA in mammalian cells. J Biol Chem. 2005;280:23130-23137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 107] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 45. | Naito M, Yamazaki T, Tsutsumi R, Higashi H, Onoe K, Yamazaki S, Azuma T, Hatakeyama M. Influence of EPIYA-repeat polymorphism on the phosphorylation-dependent biological activity of Helicobacter pylori CagA. Gastroenterology. 2006;130:1181-1190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 148] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 46. | Hatakeyama M. Anthropological and clinical implications for the structural diversity of the Helicobacter pylori CagA oncoprotein. Cancer Sci. 2011;102:36-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 47. | Basso D, Zambon CF, Letley DP, Stranges A, Marchet A, Rhead JL, Schiavon S, Guariso G, Ceroti M, Nitti D. Clinical relevance of Helicobacter pylori cagA and vacA gene polymorphisms. Gastroenterology. 2008;135:91-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 294] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 48. | Jones KR, Jang S, Chang JY, Kim J, Chung IS, Olsen CH, Merrell DS, Cha JH. Polymorphisms in the intermediate region of VacA impact Helicobacter pylori-induced disease development. J Clin Microbiol. 2011;49:101-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 49. | Vilaichone RK, Mahachai V, Tumwasorn S, Wu JY, Graham DY, Yamaoka Y. Molecular epidemiology and outcome of Helicobacter pylori infection in Thailand: a cultural cross roads. Helicobacter. 2004;9:453-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 74] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 50. | Hirai I, Sasaki T, Kimoto A, Yamamoto Y, Azuma T, Mahachai V, Hansomburana P, Lertkupinit C, Luangjaru S, Noophan P. Infection of less virulent Helicobacter pylori strains in asymptomatic healthy individuals in Thailand as a potential contributing factor to the Asian enigma. Microbes Infect. 2010;12:227-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 51. | Argent RH, Thomas RJ, Aviles-Jimenez F, Letley DP, Limb MC, El-Omar EM, Atherton JC. Toxigenic Helicobacter pylori infection precedes gastric hypochlorhydria in cancer relatives, and H. pylori virulence evolves in these families. Clin Cancer Res. 2008;14:2227-2235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 52. | Zhang XS, Tegtmeyer N, Traube L, Jindal S, Perez-Perez G, Sticht H, Backert S, Blaser MJ. A specific A/T polymorphism in Western tyrosine phosphorylation B-motifs regulates Helicobacter pylori CagA epithelial cell interactions. PLoS Pathog. 2015;11:e1004621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 78] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 53. | Yahiro K, Wada A, Nakayama M, Kimura T, Ogushi K, Niidome T, Aoyagi H, Yoshino K, Yonezawa K, Moss J. Protein-tyrosine phosphatase alpha, RPTP alpha, is a Helicobacter pylori VacA receptor. J Biol Chem. 2003;278:19183-19189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 89] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 54. | Gebert B, Fischer W, Weiss E, Hoffmann R, Haas R. Helicobacter pylori vacuolating cytotoxin inhibits T lymphocyte activation. Science. 2003;301:1099-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 409] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 55. | Atherton JC, Cao P, Peek RM, Tummuru MK, Blaser MJ, Cover TL. Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori. Association of specific vacA types with cytotoxin production and peptic ulceration. J Biol Chem. 1995;270:17771-17777. [PubMed] |
| 56. | Rhead JL, Letley DP, Mohammadi M, Hussein N, Mohagheghi MA, Eshagh Hosseini M, Atherton JC. A new Helicobacter pylori vacuolating cytotoxin determinant, the intermediate region, is associated with gastric cancer. Gastroenterology. 2007;133:926-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 312] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 57. | Sugimoto M, Zali MR, Yamaoka Y. The association of vacA genotypes and Helicobacter pylori-related gastroduodenal diseases in the Middle East. Eur J Clin Microbiol Infect Dis. 2009;28:1227-1236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 58. | Sugimoto M, Yamaoka Y. The association of vacA genotype and Helicobacter pylori-related disease in Latin American and African populations. Clin Microbiol Infect. 2009;15:835-842. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 81] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 59. | Ferreira RM, Machado JC, Letley D, Atherton JC, Pardo ML, Gonzalez CA, Carneiro F, Figueiredo C. A novel method for genotyping the Helicobacter pylori vacA intermediate region directly in gastric biopsy specimens. J Clin Microbiol. 2012;50:3983-3989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 60. | Douraghi M, Talebkhan Y, Zeraati H, Ebrahimzadeh F, Nahvijoo A, Morakabati A, Ghafarpour M, Esmaili M, Bababeik M, Oghalaie A. Multiple gene status in Helicobacter pylori strains and risk of gastric cancer development. Digestion. 2009;80:200-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 61. | Memon AA, Hussein NR, Miendje Deyi VY, Burette A, Atherton JC. Vacuolating cytotoxin genotypes are strong markers of gastric cancer and duodenal ulcer-associated Helicobacter pylori strains: a matched case-control study. J Clin Microbiol. 2014;52:2984-2989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 62. | Ferreira RM, Figueiredo C, Bonet C, Pardo ML, Liso JM, Alonso P, Sala N, Capella G, Sanz-Anquela JM, González CA. Helicobacter pylori vacA intermediate region genotyping and progression of gastric preneoplastic lesions. Am J Gastroenterol. 2012;107:145-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 63. | El-Omar EM, Carrington M, Chow WH, McColl KE, Bream JH, Young HA, Herrera J, Lissowska J, Yuan CC, Rothman N. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000;404:398-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1690] [Cited by in RCA: 1676] [Article Influence: 67.0] [Reference Citation Analysis (0)] |
| 64. | El-Omar EM, Rabkin CS, Gammon MD, Vaughan TL, Risch HA, Schoenberg JB, Stanford JL, Mayne ST, Goedert J, Blot WJ. Increased risk of noncardia gastric cancer associated with proinflammatory cytokine gene polymorphisms. Gastroenterology. 2003;124:1193-1201. [PubMed] |
| 65. | Machado JC, Figueiredo C, Canedo P, Pharoah P, Carvalho R, Nabais S, Castro Alves C, Campos ML, Van Doorn LJ, Caldas C. A proinflammatory genetic profile increases the risk for chronic atrophic gastritis and gastric carcinoma. Gastroenterology. 2003;125:364-371. [PubMed] |
| 66. | Figueiredo C, Machado JC, Pharoah P, Seruca R, Sousa S, Carvalho R, Capelinha AF, Quint W, Caldas C, van Doorn LJ. Helicobacter pylori and interleukin 1 genotyping: an opportunity to identify high-risk individuals for gastric carcinoma. J Natl Cancer Inst. 2002;94:1680-1687. [PubMed] |
| 67. | Furuta T, El-Omar EM, Xiao F, Shirai N, Takashima M, Sugimura H. Interleukin 1beta polymorphisms increase risk of hypochlorhydria and atrophic gastritis and reduce risk of duodenal ulcer recurrence in Japan. Gastroenterology. 2002;123:92-105. [PubMed] |
| 68. | Zeng ZR, Hu PJ, Hu S, Pang RP, Chen MH, Ng M, Sung JJ. Association of interleukin 1B gene polymorphism and gastric cancers in high and low prevalence regions in China. Gut. 2003;52:1684-1689. [PubMed] |
| 69. | Rad R, Prinz C, Neu B, Neuhofer M, Zeitner M, Voland P, Becker I, Schepp W, Gerhard M. Synergistic effect of Helicobacter pylori virulence factors and interleukin-1 polymorphisms for the development of severe histological changes in the gastric mucosa. J Infect Dis. 2003;188:272-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 138] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 70. | Palli D, Saieva C, Luzzi I, Masala G, Topa S, Sera F, Gemma S, Zanna I, D’Errico M, Zini E. Interleukin-1 gene polymorphisms and gastric cancer risk in a high-risk Italian population. Am J Gastroenterol. 2005;100:1941-1948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 71. | Garza-González E, Bosques-Padilla FJ, El-Omar E, Hold G, Tijerina-Menchaca R, Maldonado-Garza HJ, Pérez-Pérez GI. Role of the polymorphic IL-1B, IL-1RN and TNF-A genes in distal gastric cancer in Mexico. Int J Cancer. 2005;114:237-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 99] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 72. | Tu S, Bhagat G, Cui G, Takaishi S, Kurt-Jones EA, Rickman B, Betz KS, Penz-Oesterreicher M, Bjorkdahl O, Fox JG. Overexpression of interleukin-1beta induces gastric inflammation and cancer and mobilizes myeloid-derived suppressor cells in mice. Cancer Cell. 2008;14:408-419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 702] [Cited by in RCA: 681] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 73. | Camargo MC, Mera R, Correa P, Peek RM, Fontham ET, Goodman KJ, Piazuelo MB, Sicinschi L, Zabaleta J, Schneider BG. Interleukin-1beta and interleukin-1 receptor antagonist gene polymorphisms and gastric cancer: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:1674-1687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 181] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 74. | Kamangar F, Cheng C, Abnet CC, Rabkin CS. Interleukin-1B polymorphisms and gastric cancer risk--a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:1920-1928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 108] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 75. | Persson C, Canedo P, Machado JC, El-Omar EM, Forman D. Polymorphisms in inflammatory response genes and their association with gastric cancer: A HuGE systematic review and meta-analyses. Am J Epidemiol. 2011;173:259-270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 143] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 76. | Xue H, Lin B, Ni P, Xu H, Huang G. Interleukin-1B and interleukin-1 RN polymorphisms and gastric carcinoma risk: a meta-analysis. J Gastroenterol Hepatol. 2010;25:1604-1617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 97] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 77. | Hold GL, Rabkin CS, Chow WH, Smith MG, Gammon MD, Risch HA, Vaughan TL, McColl KE, Lissowska J, Zatonski W. A functional polymorphism of toll-like receptor 4 gene increases risk of gastric carcinoma and its precursors. Gastroenterology. 2007;132:905-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 207] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 78. | Sakamoto H, Yoshimura K, Saeki N, Katai H, Shimoda T, Matsuno Y, Saito D, Sugimura H, Tanioka F, Kato S. Genetic variation in PSCA is associated with susceptibility to diffuse-type gastric cancer. Nat Genet. 2008;40:730-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 328] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 79. | Shi D, Wang S, Gu D, Wu D, Wang M, Chu H, Tong N, Ma L, Zhong D, Zhang Z. The PSCA polymorphisms derived from genome-wide association study are associated with risk of gastric cancer: a meta-analysis. J Cancer Res Clin Oncol. 2012;138:1339-1345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 80. | Qiao L, Feng Y. Genetic variations of prostate stem cell antigen (PSCA) contribute to the risk of gastric cancer for Eastern Asians: a meta-analysis based on 16792 individuals. Gene. 2012;493:83-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 81. | Sala N, Muñoz X, Travier N, Agudo A, Duell EJ, Moreno V, Overvad K, Tjonneland A, Boutron-Ruault MC, Clavel-Chapelon F. Prostate stem-cell antigen gene is associated with diffuse and intestinal gastric cancer in Caucasians: results from the EPIC-EURGAST study. Int J Cancer. 2012;130:2417-2427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 82. | Lochhead P, Frank B, Hold GL, Rabkin CS, Ng MT, Vaughan TL, Risch HA, Gammon MD, Lissowska J, Weck MN. Genetic variation in the prostate stem cell antigen gene and upper gastrointestinal cancer in white individuals. Gastroenterology. 2011;140:435-441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 83. | Shikata K, Kiyohara Y, Kubo M, Yonemoto K, Ninomiya T, Shirota T, Tanizaki Y, Doi Y, Tanaka K, Oishi Y. A prospective study of dietary salt intake and gastric cancer incidence in a defined Japanese population: the Hisayama study. Int J Cancer. 2006;119:196-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 179] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 84. | Gaddy JA, Radin JN, Loh JT, Zhang F, Washington MK, Peek RM, Algood HM, Cover TL. High dietary salt intake exacerbates Helicobacter pylori-induced gastric carcinogenesis. Infect Immun. 2013;81:2258-2267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 152] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 85. | Liu C, Russell RM. Nutrition and gastric cancer risk: an update. Nutr Rev. 2008;66:237-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 88] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 86. | Toyoda T, Tsukamoto T, Hirano N, Mizoshita T, Kato S, Takasu S, Ban H, Tatematsu M. Synergistic upregulation of inducible nitric oxide synthase and cyclooxygenase-2 in gastric mucosa of Mongolian gerbils by a high-salt diet and Helicobacter pylori infection. Histol Histopathol. 2008;23:593-599. [PubMed] |
| 87. | Loh JT, Torres VJ, Cover TL. Regulation of Helicobacter pylori cagA expression in response to salt. Cancer Res. 2007;67:4709-4715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 113] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 88. | Gancz H, Jones KR, Merrell DS. Sodium chloride affects Helicobacter pylori growth and gene expression. J Bacteriol. 2008;190:4100-4105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 89. | Ladeiras-Lopes R, Pereira AK, Nogueira A, Pinheiro-Torres T, Pinto I, Santos-Pereira R, Lunet N. Smoking and gastric cancer: systematic review and meta-analysis of cohort studies. Cancer Causes Control. 2008;19:689-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 338] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 90. | Falush D, Wirth T, Linz B, Pritchard JK, Stephens M, Kidd M, Blaser MJ, Graham DY, Vacher S, Perez-Perez GI. Traces of human migrations in Helicobacter pylori populations. Science. 2003;299:1582-1585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 738] [Cited by in RCA: 708] [Article Influence: 32.2] [Reference Citation Analysis (1)] |
| 91. | Kodaman N, Pazos A, Schneider BG, Piazuelo MB, Mera R, Sobota RS, Sicinschi LA, Shaffer CL, Romero-Gallo J, de Sablet T. Human and Helicobacter pylori coevolution shapes the risk of gastric disease. Proc Natl Acad Sci USA. 2014;111:1455-1460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 180] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 92. | Park JY, von Karsa L, Herrero R. Prevention strategies for gastric cancer: a global perspective. Clin Endosc. 2014;47:478-489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 107] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 93. | Lee JK, Park BJ, Yoo KY, Ahn YO. Dietary factors and stomach cancer: a case-control study in Korea. Int J Epidemiol. 1995;24:33-41. [PubMed] |
| 94. | Hirayama T. Nutrition and cancer--a large scale cohort study. Prog Clin Biol Res. 1986;206:299-311. [PubMed] |
| 95. | Wang Q, Chen Y, Wang X, Gong G, Li G, Li C. Consumption of fruit, but not vegetables, may reduce risk of gastric cancer: results from a meta-analysis of cohort studies. Eur J Cancer. 2014;50:1498-1509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 96. | Guercio V, Galeone C, Turati F, La Vecchia C. Gastric cancer and allium vegetable intake: a critical review of the experimental and epidemiologic evidence. Nutr Cancer. 2014;66:757-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 97. | Loh YH, Jakszyn P, Luben RN, Mulligan AA, Mitrou PN, Khaw KT. N-Nitroso compounds and cancer incidence: the European Prospective Investigation into Cancer and Nutrition (EPIC)-Norfolk Study. Am J Clin Nutr. 2011;93:1053-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 143] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 98. | Jakszyn P, Bingham S, Pera G, Agudo A, Luben R, Welch A, Boeing H, Del Giudice G, Palli D, Saieva C. Endogenous versus exogenous exposure to N-nitroso compounds and gastric cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC-EURGAST) study. Carcinogenesis. 2006;27:1497-1501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 125] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 99. | Buckland G, Travier N, Huerta JM, Bueno-de-Mesquita HB, Siersema PD, Skeie G, Weiderpass E, Engeset D, Ericson U, Ohlsson B. Healthy lifestyle index and risk of gastric adenocarcinoma in the EPIC cohort study. Int J Cancer. 2015;137:598-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 91] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 100. | Ford AC, Forman D, Hunt RH, Yuan Y, Moayyedi P. Helicobacter pylori eradication therapy to prevent gastric cancer in healthy asymptomatic infected individuals: systematic review and meta-analysis of randomised controlled trials. BMJ. 2014;348:g3174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 456] [Cited by in RCA: 442] [Article Influence: 40.2] [Reference Citation Analysis (1)] |
| 101. | Ma JL, Zhang L, Brown LM, Li JY, Shen L, Pan KF, Liu WD, Hu Y, Han ZX, Crystal-Mansour S. Fifteen-year effects of Helicobacter pylori, garlic, and vitamin treatments on gastric cancer incidence and mortality. J Natl Cancer Inst. 2012;104:488-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 353] [Article Influence: 27.2] [Reference Citation Analysis (1)] |
| 102. | Malfertheiner P, Megraud F, O’Morain CA, Atherton J, Axon AT, Bazzoli F, Gensini GF, Gisbert JP, Graham DY, Rokkas T. Management of Helicobacter pylori infection--the Maastricht IV/ Florence Consensus Report. Gut. 2012;61:646-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1719] [Cited by in RCA: 1589] [Article Influence: 122.2] [Reference Citation Analysis (5)] |
| 103. | Liu WZ, Xie Y, Cheng H, Lu NH, Hu FL, Zhang WD, Zhou LY, Chen Y, Zeng ZR, Wang CW. Fourth Chinese National Consensus Report on the management of Helicobacter pylori infection. J Dig Dis. 2013;14:211-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 96] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 104. | Kim SG, Jung HK, Lee HL, Jang JY, Lee H, Kim CG, Shin WG, Shin ES, Lee YC. [Guidelines for the diagnosis and treatment of Helicobacter pylori infection in Korea, 2013 revised edition]. Korean J Gastroenterol. 2013;62:3-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 97] [Article Influence: 8.8] [Reference Citation Analysis (1)] |
| 105. | Fock KM, Katelaris P, Sugano K, Ang TL, Hunt R, Talley NJ, Lam SK, Xiao SD, Tan HJ, Wu CY. Second Asia-Pacific Consensus Guidelines for Helicobacter pylori infection. J Gastroenterol Hepatol. 2009;24:1587-1600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 420] [Cited by in RCA: 427] [Article Influence: 26.7] [Reference Citation Analysis (1)] |
| 106. | Asaka M, Kato M, Takahashi S, Fukuda Y, Sugiyama T, Ota H, Uemura N, Murakami K, Satoh K, Sugano K. Guidelines for the management of Helicobacter pylori infection in Japan: 2009 revised edition. Helicobacter. 2010;15:1-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 294] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 107. | Lee JY, Kim N. [Future trends of Helicobacter pylori eradication therapy in Korea]. Korean J Gastroenterol. 2014;63:158-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 108. | Sugano K, Tack J, Kuipers EJ, Graham DY, El-Omar EM, Miura S, Haruma K, Asaka M, Uemura N, Malfertheiner P. Kyoto global consensus report on Helicobacter pylori gastritis. Gut. 2015;64:1353-1367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1322] [Cited by in RCA: 1184] [Article Influence: 118.4] [Reference Citation Analysis (0)] |
| 109. | Oh HS, Lee DH, Seo JY, Cho YR, Kim N, Jeoung SH, Kim JW, Hwang JH, Park YS, Lee SH. Ten-day sequential therapy is more effective than proton pump inhibitor-based therapy in Korea: a prospective, randomized study. J Gastroenterol Hepatol. 2012;27:504-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 110. | Nishizawa T, Suzuki H, Suzuki M, Takahashi M, Hibi T. Proton pump inhibitor-amoxicillin-clarithromycin versus proton pump inhibitor-amoxicillin-metronidazole as first-line Helicobacter pylori eradication therapy. J Clin Biochem Nutr. 2012;51:114-116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 111. | Basu PP, Rayapudi K, Pacana T, Shah NJ, Krishnaswamy N, Flynn M. A randomized study comparing levofloxacin, omeprazole, nitazoxanide, and doxycycline versus triple therapy for the eradication of Helicobacter pylori. Am J Gastroenterol. 2011;106:1970-1975. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 112. | Boyanova L, Mitov I. Geographic map and evolution of primary Helicobacter pylori resistance to antibacterial agents. Expert Rev Anti Infect Ther. 2010;8:59-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 89] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 113. | De Francesco V, Giorgio F, Hassan C, Manes G, Vannella L, Panella C, Ierardi E, Zullo A. Worldwide H. pylori antibiotic resistance: a systematic review. J Gastrointestin Liver Dis. 2010;19:409-414. [PubMed] |
| 114. | Kobayashi I, Murakami K, Kato M, Kato S, Azuma T, Takahashi S, Uemura N, Katsuyama T, Fukuda Y, Haruma K. Changing antimicrobial susceptibility epidemiology of Helicobacter pylori strains in Japan between 2002 and 2005. J Clin Microbiol. 2007;45:4006-4010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 128] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 115. | Megraud F, Coenen S, Versporten A, Kist M, Lopez-Brea M, Hirschl AM, Andersen LP, Goossens H, Glupczynski Y. Helicobacter pylori resistance to antibiotics in Europe and its relationship to antibiotic consumption. Gut. 2013;62:34-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 653] [Cited by in RCA: 632] [Article Influence: 52.7] [Reference Citation Analysis (3)] |
| 116. | Miyachi H, Miki I, Aoyama N, Shirasaka D, Matsumoto Y, Toyoda M, Mitani T, Morita Y, Tamura T, Kinoshita S. Primary levofloxacin resistance and gyrA/B mutations among Helicobacter pylori in Japan. Helicobacter. 2006;11:243-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 112] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 117. | Kim KB, Kim YS. Recent Trends of Helicobacter pylori Eradication Therapy: Focusing on First Line Treatment. Korean J Helicobacter Up Gastrointest Res. 2014;14:237-241. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 118. | Yamac D, Ayyildiz T, Coşkun U, Akyürek N, Dursun A, Seckin S, Koybasioglu F. Cyclooxygenase-2 expression and its association with angiogenesis, Helicobacter pylori, and clinicopathologic characteristics of gastric carcinoma. Pathol Res Pract. 2008;204:527-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 119. | Shao Y, Sun K, Xu W, Li XL, Shen H, Sun WH. Helicobacter pylori infection, gastrin and cyclooxygenase-2 in gastric carcinogenesis. World J Gastroenterol. 2014;20:12860-12873. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 120. | He XP, Shao Y, Li XL, Xu W, Chen GS, Sun HH, Xu HC, Xu X, Tang D, Zheng XF. Downregulation of miR-101 in gastric cancer correlates with cyclooxygenase-2 overexpression and tumor growth. FEBS J. 2012;279:4201-4212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 121. | Tseng YC, Tsai YH, Tseng MJ, Hsu KW, Yang MC, Huang KH, Li AF, Chi CW, Hsieh RH, Ku HH. Notch2-induced COX-2 expression enhancing gastric cancer progression. Mol Carcinog. 2012;51:939-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 122. | Tan VP, Wong BC. Gastric cancer chemoprevention: the current evidence. Gastroenterol Clin North Am. 2013;42:299-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 123. | Zhang MM, Qian W, Qin YY, He J, Zhou YH. Probiotics in Helicobacter pylori eradication therapy: a systematic review and meta-analysis. World J Gastroenterol. 2015;21:4345-4357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 108] [Cited by in RCA: 114] [Article Influence: 11.4] [Reference Citation Analysis (1)] |