Published online Oct 15, 2014. doi: 10.4251/wjgo.v6.i10.413
Revised: August 20, 2014
Accepted: September 16, 2014
Published online: October 15, 2014
Processing time: 173 Days and 2 Hours
AIM: To investigate plasma Monocyte Chemotactic Protein-1 levels preoperatively in colorectal cancer (CRC) and benign patients and postoperatively after CRC resection.
METHODS: A plasma bank was screened for minimally invasive colorectal cancer resection (MICR) for CRC and benign disease (BEN) patients for whom preoperative, early postoperative, and 1 or more late postoperative samples (postoperative day 7-27) were available. Monocyte chemotactic protein-1 (MCP-1) levels (pg/mL) were determined via enzyme linked immuno-absorbent assay.
RESULTS: One hundred and two CRC and 86 BEN patients were studied. The CRC patient’s median preoperative MCP-1 level (283.1, CI: 256.0, 294.3) was higher than the BEN group level (227.5, CI: 200.2, 245.2; P = 0.0004). Vs CRC preoperative levels, elevated MCP-1 plasma levels were found on postoperative day 1 (446.3, CI: 418.0, 520.1), postoperative day 3 (342.7, CI: 320.4, 377.4), postoperative day 7-13 (326.5, CI: 299.4, 354.1), postoperative day 14-20 (361.6, CI: 287.8, 407.9), and postoperative day 21-27 (318.1, CI: 287.2, 371.6; P < 0.001 for all).
CONCLUSION: Preoperative MCP-1 levels were higher in CRC patients (vs BEN). After MICR for CRC, MCP-1 levels were elevated for 1 mo and may promote angiogenesis, cancer recurrence and metastasis.
Core tip: In our past published studied we have shown that plasma levels of the pro-angiogenic proteins, vascular endothelial growth factor, angiopoietin-2, placental growth factor, and soluble vascular adhesion molecule-1, are significantly elevated for 2-4 wk following minimally invasive colorectal resection for colorectal cancer (CRC). Additionally, we also showed that postoperative plasma from cancer patients stimulates in vitro endothelial cell proliferation, migration, and invasion, all of which are critical steps in angiogenesis. In this manuscript we are presenting data to show that plasma Monocyte chemotactic protein-1 (MCP-1), a pro-angiogenic protein, in CRC patients remain elevated for month after MICR. Furthermore, we are also showing that the median preoperative plasma level of MCP-1 is significantly higher in the CRC patients than in the BEN group.
- Citation: Kumara HS, Myers EA, Herath SA, Jang JH, Njoh L, Yan X, Kirchoff D, Cekic V, Luchtefeld M, Whelan RL. Plasma monocyte chemotactic protein-1 remains elevated after minimally invasive colorectal cancer resection. World J Gastrointest Oncol 2014; 6(10): 413-419
- URL: https://www.wjgnet.com/1948-5204/full/v6/i10/413.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v6.i10.413
Surgery remains the mainstay of treatment for colorectal cancer (CRC), however, a significant number of patients develop disease recurrence following a “curative resection”, presumably from unrecognized tumor microfoci or from viable tumor cells that persist in the circulation[1]. There is growing evidence that tumor resection may indirectly stimulate the growth of residual cancer via surgery-related immunosuppression and elevated blood levels of proangiogenic proteins during the early postoperative period. Thus, the early postoperative period may be a dangerous time window for cancer patients who potentially harbor residual disease.
Plasma levels of the proangiogenic proteins, vascular endothelial growth factor (VEGF), angiopoietin-2 (Ang-2), placental growth factor (PlGF), and soluble vascular adhesion molecule-1 (sVCAM-1), have been noted to be significantly elevated for 2-4 wk following minimally invasive colorectal resection for CRC[2-5]. Additionally, prior studies have shown that postoperative plasma from cancer patients stimulates in vitro endothelial cell (EC) proliferation, migration, and invasion, all of which are critical steps in angiogenesis[6].
Monocyte chemotactic protein-1 (MCP-1), a member of the C-C chemokine family, is a protein that has several proangiogenic effects. Evidence shows that MCP-1 is produced by certain tumor cells as well as stromal cells such as fibroblasts, endothelial cells (EC’s), and monocytes[7]. It is a known chemo attractant for monocytes, macrophages, eosinophils, and lymphocytes, and is also a ligand for CC chemokine receptor 2 (CCR2)[7,8]. MCP-1 is thought to mediate angiogenesis via recruitment of proangiogenic protein producing monocytes and macrophages and endothelial cells into wounds and tumors. The chemotaxis of EC’ss is inhibited by MCP-1 antibodies in vitro and in vivo[9]. MCP-1, by binding to CCR2 on the surface of EC’s, has also been shown to promote EC migration, which is a critical early step in angiogenesis[9,10]. There also appears to be an intimate relationship between MCP-1 and VEGF. Interestingly, VEGF increases MCP-1 mRNA expression in EC in vitro cultures[11,12]. Also, there is evidence that MCP-1 modulates VEGF’s effects; MCP-1 antibody diminishes VEGF mediated tubule formation in angiogenesis assays[12].
Angiogenesis is fundamental to both wound healing and tumor growth. MCP-1 is found abundantly during the initial inflammatory stage of wound healing[9], where it plays a role in recruiting monocytes and macrophages[11,12]. Weber et al[10] showed that the presence of a MCP-1 receptor antagonist or neutralizing MCP-1 antibody impaired the ability of ECs to migrate and close wounds, whereas the addition of MCP-1 facilitated repair. Thus, MCP-1 appears to induce EC migration during wound repair[10]. Additionally, endothelial MCP-1 secretion is increased in the setting of multiple wounds[10]. Finally, wound re-epithelialization is significantly delayed in MCP-1 knockout mice[13].
There is also experiment evidence suggesting that MCP-1 plays a role in tumor growth. Nakashima et al[14] demonstrated that transfection of MCP-1 into a murine CRC cell line promoted lung metastases by augmenting neovascularization. Further, Salcedo et al[9] showed that treatment of immunodeficient mice, in whom metastases had been established via inoculation with human breast carcinoma cells, with administration of a neutralizing antibody to MCP-1 resulted in significant longer survival and decreased growth of lung micrometastases[9]. MCP-1 has also been associated with multiple human cancers. A study of breast cancer patients revealed high levels of MCP-1 expression in primary breast cancers by enzyme linked immuno-absorbent assay (ELISA) and immuno-histochemical analysis; this expression correlated significantly with macrophage accumulation in the tumors[15]. In another study, patients with primary and recurrent ovarian cancer were shown to have significantly higher MCP-1 serum levels compared to patients with benign ovarian pathology[16]. Furthermore, MCP-1 serum levels have been shown to correlate with histological grade in ovarian cancer patients[16].
The impact of CRC on plasma levels of MCP-1 is unknown. Further, the effect of minimally invasive colorectal resection (MICR) on postoperative (PostOp) plasma MCP-1 levels is unknown. MCP-1 may contribute to the overall proangiogenic state of plasma noted following surgery. The purpose of this study was twofold: (1) to assess plasma levels of MCP-1 before surgery in CRC and BEN disease patients; and (2) to determine levels after MICR for cancer.
Consenting patients with CRC or benign colorectal disease (BEN) that underwent elective MICR during the period of 2003-2011 were identified from a larger population of patients who had been enrolled in an IRB-approved multicenter prospective data and blood banking protocol. The broadly stated purpose of this effort is to study the physiologic, immunologic, and oncologic ramifications of major abdominal surgery. Enrolled patients underwent surgery alone and did not receive a novel drug or other therapy. The indications and type of surgery as well as the demographic, operative, and short term recovery data was prospectively collected for all patients. Recently transfused patients, immunosuppressed patients (medication-related, HIV+, etc.), and those who received radio- or chemotherapy within 6 wk of surgery were excluded. Patients undergoing urgent or emergent surgery were, likewise, excluded.
To be eligible for entry into this study plasma samples for the following time points needed to be available for CRC patients who underwent MICR: preoperative (PreOp), postoperative day (POD) 1, POD 3, and at least 1 later postoperative specimen from POD 7-28. Of note, blood samples after POD 7 were obtained at follow up office appointments but were not scheduled on a specific POD. Many patients refused late blood draws. Because the number of specimens on any given late postoperative day was small it was necessary to “bundle” the specimens from 7 d time blocks (POD 7-13, 14-20, 21-27) and consider these as single time points. PreOp blood samples were obtained prior to surgery and processed in an identical manner for comparison of MCP-1 levels in CRC patients and the BEN group. Samples were collected in heparin-containing tubes, were processed within 5-6 h of collection. After centrifugation, the plasma was frozen and stored at -80 ˚C until the assays were performed.
Plasma levels of MCP-1 were determined in duplicate using a commercially available enzyme linked immuno-absorbent assay (R and D Systems, Minneapolis, MN) according to the manufacturer’s instructions. MCP-1 concentrations (pg/mL) were calculated using a standard curve made in every assay and were reported as median and 95%CIs for the PreOp vs PostOp MCP-1 comparisons, the preoperative CRC vs BEN group comparison, and for the Stage 1-3 CRC sub group comparisons.
Demographic and clinical data are expressed as the mean and SD for continuous variables. Preoperative MCP-1 values in the cancer and Benign populations were not normally distributed and, thus, the median values for each group were calculated and compared using the Mann and Whitney U test. In regards to the CRC Pre vs Postoperative MCP-1 comparisons, the results are reported as the median and 95%CIs and the Wilcoxon paired test was used to analyze the data. Significance was set at P < 0.01 (Bonferroni adjustment was applied). In regards to the sub group comparisons of preoperative MCP-1 values vs the stage 1-3 CRC subgroups, the results are reported as the median and 95%CIs and the Mann and Whitney U test was utilized for the analysis. Correlation between postoperative MCP-1 plasma levels vs incision size and length of surgery was evaluated by the Spearman’s rank correlation coefficient (rs) and the correlation between complication rate and PostOp MCP-1 levels was calculated via logistic regression analysis. All data analysis was performed using SPSS version 15.0 (SPSS, Inc., Chicago, IL).
Overall, a total of 102 CRC patients (59 males, 43 females with a mean age of 67.1 ± 12.3 years) were included in the study. Seventy patients (69%) had colon cancers while 32 (31%) had rectal lesions. The final cancer stage breakdown is as follows: Stage I, 30.5%; Stage II, 30.5%; Stage III, 37%; and Stage IV, 2%. The majority of patients (66%) underwent laparoscopic-assisted (LA) resections, whereas 34% had a hand-assisted or hybrid laparoscopic (HAL) procedure. The types of resection performed, as well as other operative data are provided in Table 1. The overall complication rate for the CRC patients was 21% and there were no anastomotic leaks, intra-abdominal abscesses, or perioperative deaths. The complications noted included the following: wound infections (2 patients); cardiac (2); pulmonary (3); ileus (6); urinary retention (5); SBO (3); and C. difficile colitis (1).
| Cancer (n = 102) | |
| Age, yr (mean ± SD) | 67.1 ± 12.3 |
| Sex (n) | |
| Male | 59 |
| Female | 43 |
| Incision length, cm (mean ± SD) | 7.1 ± 2.8 |
| Operative time, min (mean ± SD) | 266.5 ± 113 |
| Length of stay, d (mean ± SD) | 5.9 ± 2.3 |
| Type of resection | |
| Right | 39 (38%) |
| Transverse | 4 (4%) |
| Left | 8 (8%) |
| Sigmoid/Rectosigmoid | 14/4 (18%) |
| LAR/AR | 24/2 (25%) |
| APR | 3 (3%) |
| Subtotal/total | 2/2 (4%) |
| Surgical method | |
| Laparoscopic-assisted | 67 (66%) |
| Hand-assisted/hybrid laparoscopic | 35 (34%) |
A group of 86 benign colorectal disease patients (BEN) who underwent MICR served as the control group for the preoperative MCP-1 levels comparison. The indications for MICR in the BEN group were diverticulitis (n = 30) and benign neoplasms (n = 56). The CRC group was significantly older than the BEN group (67.1 ± 12.3 vs 59.3 ± 13.4 years, P < 0.0001; Table 1) but with similar male to female ratios.
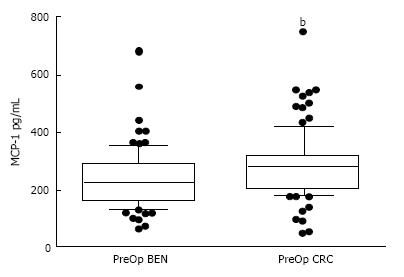
The median PreOp MCP-1 plasma level in the CRC patients (283.1, CI: 256.0, 294.4) was modestly but significantly higher (24%) than the level noted in the BEN patient group (227.5, CI: 200.2, 245.2; P = 0.0004; Figure 1).
In regards to final cancer stage, the median PreOp values for the Stage 1 to 3 CRC groups were as follows: Stage I, 296.5 (CI: 231.2, 343.7); Stage II, 274.2 (CI: 217.3, 292.2); and Stage III, 285.9 (CI: 251.1, 296.9). Although the results for each Stage group (1-3) were significantly higher than the BEN group’s median value, there was no significant difference amongst the Stage 1, 2, and 3 groups [Note: There were too few Stage 4 patients (n = 2) in the CRC population to permit statistical analysis].
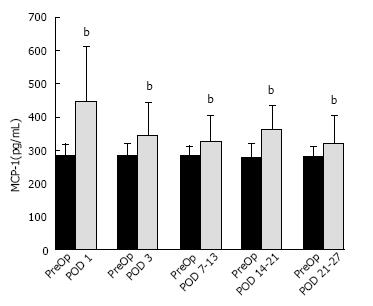
The median PreOp MCP-1 level in CRC patients was 283.1 (CI: 256.1, 294.4) pg/mL (n = 102). When compared to PreOp levels, significantly elevated mean MCP-1 plasma levels (pg/mL) were observed on POD 1 (446.3, CI: 418.0, 520.1; n = 102, P < 0.001), POD 3 (342.7, CI: 320.4, 377.4; n = 100, P < 0.001), POD 7-13 (326.5, CI: 299.4, 354.1; P < 0.001), POD 14-20 (361.6, CI: 287.8, 407.9; n = 27; P < 0.001), and POD 21-27 (318.1, CI: 287.2, 371.6; n = 28; P≤ 0.001). Because the “n” for the POD 3 and later time points was less than 102 and unique for each time point, the PreOp baseline level for each of these time points was somewhat different. This is reflected in Figure 2, which provides in bar graph form the mean PreOp baseline for each postoperative time point.
The percent increase over the PreOp baseline for each postoperative time point is as follows: POD 1 (73.5%); POD 3 (37.2%); POD 7-13 (24.6%); POD 14-20 (39.7%); and POD 21-27 (25%).The percentage of CRC patients that had plasma levels increased from the median PreOp baseline levels of each subgroup were: POD 1 (79%); POD 3 (81%); POD 7-13 (73.8%); POD 14-20 (89%); and POD 21-27 (89.3%).
There was a weak correlation between plasma MCP-1 levels on POD1 and the incision length (rs =0.217, P = 0.006) as well as the length of surgery (rs = 0.268, P = 0.007). There was no such correlation noted for the 4 other postoperative time points in regards to incision or operation length. Also, there was no correlation found between the presence of complication(s) and the degree of the postoperative plasma MCP-1 elevation at any of the postoperative time points.
The median preoperative plasma level of MCP-1 was significantly higher in the CRC patients than in the BEN disease group. This suggests that, in some patients, the tumor is generating MCP-1, either directly or indirectly. Unfortunately, this study did not include tumor analysis (microarray or RT-PCR) and thus, we can only speculate as to the origin of the additional MCP-1. Of note, no correlation was identified between PreOp levels and tumor stage.
In regards to the comparison of PreOp and Postop MCP-1 levels in the CRC patients, plasma levels were significantly elevated for the first month after MICR. The greatest increase was observed during the first week after surgery. MCP-1, therefore, joins the list of proteins whose blood levels are altered after MICR. The vast majority of surgery-related blood protein alterations (CRP, IL-6, IL-2, FGF, HGF, angiostatin, endostatin, etc.) are short lived and resolve within the first 3 to 5 d after surgery. Of note, the percent change from base line in regards to MCP-1 is amongst the highest when compared to the previously mentioned proteins. Many of the short duration blood compositional changes are related to the acute phase inflammatory response to surgical trauma as well as to the anesthesia. Because blood levels were increased during the entire first month after surgery, MCP-1 joins the small list of proteins (VEGF, Ang-2, PlGF, and sVCAM) with long duration plasma elevations; interestingly, all of these proteins play a role in angiogenesis[2-5]. MCP-1 facilitates angiogenesis through several mechanisms, including its intimate relationship with VEGF[17,18].
Interestingly, VEGF increases MCP-1 mRNA expression in endothelial cell in vitro cultures[17,18]. Also, there is evidence that MCP-1 modulates VEGF’s effects; MCP-1 antibody diminishes VEGF mediated tubule formation in angiogenesis assays[18]. Collectively; the above mentioned group of proteins play a role in the early stages of neovascularization and most modulate VEGF’s effects. What is the source of the plasma MCP-1 increases after MICR?
The authors believe that the tumor produced MCP-1 is not responsible for the postoperative increases in plasma MCP-1 levels. Logically, the blood levels should decrease after resection if the source of the added MCP-1 was the tumor. The significant correlation observed between POD1 MCP-1 levels and incision length and length of surgery suggests that the MCP-1 levels on POD 1 could be attributed, in part, to the surgical stress and the initial inflammatory response which takes place early after surgery. Of note, no such correlation was found from POD 3 onward. It is the authors’ opinion that the sustained plasma MCP-1 elevation is related to wound healing. MCP-1, in wounds, accelerates macrophage trafficking into inflammatory foci and also plays a role in angiogenesis. Angiogenesis is critical to wound healing which is a lengthy process that lasts, at least, 6 to 8 wk. There is evidence that VEGF levels in wounds are very high; it is assumed that some of the wound VEGF finds its way into the blood, raising plasma concentrations[19-21]. Although unproven, the authors believe it is likely that wound levels of the other proangiogenic proteins, including MCP-1, whose blood levels are persistently increased after surgery are also notably increased.
Interestingly, as mentioned earlier, it has been demonstrated via EC cultures that plasma from the second and third weeks after MICR stimulates EC proliferation (specifically, branch point formation which is the culture equivalent of microtubule formation), migration, and invasion when compared to culture results obtained with preoperative plasma. These EC functions are critical early steps in the process of neovascularization, critical to both wound healing and solid tumor growth beyond 2 mm[22]. Similar EC culture results were noted when plasma from open CRC resection patients was similarly assessed. What are the possible ramifications, if any, of the proangiogenic postoperative plasma?
In the proportion of patients that harbor residual micrometastases the proangiogenic postoperative plasma changes may promote tumor growth. Persistently elevated levels of MCP-1 after MICR for CRC may promote recurrence in patients who harbor tumor micro foci. The complex process of residual tumor growth and metastasis may be supported by other angiogenic proteins whose blood levels remained elevated after MICR for CRC such as VEGF, PLGF, sVCAM-1 ANG2 and MMP3. There are case reports of rapid tumor growth and the development of metastases in cancer patients who undergo major surgery[23,24]. Of note, there is also experimental evidence that laparotomy and bowel resection, in the murine setting, in general, are associated with increased rates of systemic tumor establishment and growth postoperatively[25-27]. Furthermore, human postoperative serum from POD 1 has been shown to stimulate in vitro growth of human colon cancer cells when compared to culture results obtained with preoperative plasma[28]. It is also well documented that surgery induces transient postoperative cell-mediated immune suppression. In addition, surgery also impairs lymphocyte and neutrophil chemotaxis, macrophage function, and delayed type hypersensitivity responses[29-31]. These changes might impact early postoperative tumor growth as well.
Thus, the first month after surgery may be a dangerous time for cancer patients. Standard adjuvant chemotherapy is most often started 4 to 8 wk after surgery because of fears that earlier administration may inhibit wound and anastomotic healing. Perhaps, the logical next step is to search for anti-cancer drugs that could be safely given during the first month following surgery to serve as a bridge between “curative” resection and the start of adjuvant chemotherapy. The ideal agent would effectively target tumor cells that remain after surgery without interfering with wound or anastomotic healing. The authors have done one human and numerous murine studies that have assessed the anti-cancer impact of perioperative administration of a number of immunomodulatory and anti-cancer agents[32-34].
One weakness of the present study is the limited number of blood samples obtained beyond the first postoperative week. The majority of these samples were obtained during office follow up visits, which were scheduled at the discretion of the patient. Additionally, many patients refused to have late samples drawn. Therefore, it was impossible to obtain blood samples on a set postoperative timeline. To permit statistical analysis, late samples were bundled into 7-d blocks and considered as single time points. Given the limited number of postoperative samples obtained after the first postoperative month, we were also not able to determine when MCP-1 levels return to baseline.
At baseline, plasma MCP-1 levels are significantly elevated in CRC patients. Also, for at least 1 mo after minimally invasive tumor resection, plasma MCP-1 levels are significantly elevated from the preoperative baseline. The early postoperative elevations (1st week) may be related to the acute inflammatory response associated with surgical trauma and anesthesia. Although unproven, it is believed that the elevations observed during weeks 2 through 4 are related to wound healing. MCP-1 joins the growing list of pro-angiogenic proteins whose blood levels are persistently elevated after colorectal resection (VEGF, PlGF, sVCAM, ANG-2, MMP-3, etc.). These surgery-related plasma compositional changes may stimulate the growth of residual micrometastases early after resection. Further investigations are needed to determine the clinical ramifications, if any, of these transient yet significant changes. The search for and administration of anti-cancer agents that do not inhibit wound healing may be indicated.
Blood levels of proangiogenic proteins are increased after minimally invasive colorectal cancer resection. Postoperative plasma enriched in proangiogenic proteins promotes angiogenesis in vitro. The angiogenic proteins in question [vascular endothelial growth factor (VEGF), angiopoetin-2 (Ang-2), placental growth factor (PlGF), soluble vascular adhesion molecule-1 (sVCAM-1) and Matrix metalloproteinase 3 (MMP-2)] have been noted to be significantly elevated for 2-4 wk following minimally invasive colorectal resection for CRC. Monocyte chemotactic protein-1 (MCP-1) has documented proangiogenic effects, however, little is known about plasma MCP-1 levels preoperatively in CRC and benign disease patients or in CRC patients after MICR.
MCP-1, a member of the C-C chemokine family, is expressed by some cancers and has been shown to support tumor angiogenesis and development. MCP-1 is thought to mediate angiogenesis via recruitment of proangiogenic protein producing monocytes and macrophages and endothelial cells into wounds and tumors. The authors evaluated preoperative and post-MICR MCP-1 levels in CRC patients. The concern is that significantly elevated blood levels of MCP-1 perioperatively may enhance the plasma’s proangiogenic properties during the first month after surgery which, in turn, may promote tumor angiogenesis in residual lesions.
Previous studies have established that significant elevations in plasma levels of VEGF, Ang-2, PlGF, sVCAM-1 and MMp-3 occur for 2-4 wk following MICR for CRC. Additionally, prior studies have shown that postoperative plasma from cancer patients stimulates in vitro endothelial cell (EC) proliferation, migration, and invasion, all of which are critical steps in angiogenesis and tumor development. This study found elevated levels of plasma MCP-1, a protein with proangiogenic effects, before and for 1 mo after surgery. Collectively, the sustained elevations in blood levels of the above mentioned group of proangiogenic proteins may support metastasis formation and the growth of residual tumors.
This study further supports the concept that surgery-related stress and post-surgery wound healing related plasma compositional changes may stimulate the growth of residual micrometastases early after resection. The search for and administration of anti-cancer agents during the perioperative period appears warranted; agents used in this time from must not inhibit wound healing.
It has earlier been shown that both MICR and open colorectal resection are associated with sustained (2-4 wk after surgery) plasma protein changes that collectively enhance the angiogenic properties of plasma. These changes, thought to be related to wound healing, may support tumor angiogenesis early after surgery. This study shows that plasma levels of MCP-1, another proangiogenic protein, are elevated after MICR for a month. Thus, another proangiogenic protein is added to the list. Collectively, these prolonged blood elevations may support the growth of residual cancer and initiation of cancer by circulating tumor cells.
This study is interesting and I would like to give my suggestions to impact the authors understanding of the tumor tissue in the elucidation of aberrant molecular aspect changes in the tumor microenvironment and surgical margins to impact the paper.
P- Reviewer: M’Koma A, Parsak C S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Jagoditsch M, Lisborg PH, Jatzko GR, Wette V, Kropfitsch G, Denk H, Klimpfinger M, Stettner HM. Long-term prognosis for colon cancer related to consistent radical surgery: multivariate analysis of clinical, surgical, and pathologic variables. World J Surg. 2000;24:1264-1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 2. | Belizon A, Balik E, Horst P, Feingold D, Arnell T, Azarani T, Cekic V, Skitt R, Kumara S, Whelan RL. Persistent elevation of plasma vascular endothelial growth factor levels during the first month after minimally invasive colorectal resection. Surg Endosc. 2008;22:287-297. [PubMed] |
| 3. | Kumara HM, Feingold D, Kalady M, Dujovny N, Senagore A, Hyman N, Cekic V, Whelan RL. Colorectal resection is associated with persistent proangiogenic plasma protein changes: postoperative plasma stimulates in vitro endothelial cell growth, migration, and invasion. Ann Surg. 2009;249:973-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | Shantha Kumara HM, Cabot JC, Yan X, Herath SA, Luchtefeld M, Kalady MF, Feingold DL, Baxter R, Whelan RL. Minimally invasive colon resection is associated with a persistent increase in plasma PlGF levels following cancer resection. Surg Endosc. 2011;25:2153-2158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Shantha Kumara HM, Tohme ST, Herath SA, Yan X, Senagore AJ, Nasar A, Kalady MF, Baxter R, Whelan RL. Plasma soluble vascular adhesion molecule-1 levels are persistently elevated during the first month after colorectal cancer resection. Surg Endosc. 2012;26:1759-1764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Shantha Kumara HM, Kirchoff D, Naffouje S, Grieco M, Herath SA, Dujovny N, Kalady MF, Hyman N, Njoh L, Whelan RL. Plasma from the second and third weeks after open colorectal resection for cancer stimulates in vitro endothelial cell growth, migration, and invasion. Surg Endosc. 2012;26:790-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Mackay CR. Chemokines: immunology’s high impact factors. Nat Immunol. 2001;2:95-101. [PubMed] |
| 8. | Murdoch C, Finn A. Chemokine receptors and their role in inflammation and infectious diseases. Blood. 2000;95:3032-3043. [PubMed] |
| 9. | Salcedo R, Ponce ML, Young HA, Wasserman K, Ward JM, Kleinman HK, Oppenheim JJ, Murphy WJ. Human endothelial cells express CCR2 and respond to MCP-1: direct role of MCP-1 in angiogenesis and tumor progression. Blood. 2000;96:34-40. [PubMed] |
| 10. | Weber KS, Nelson PJ, Gröne HJ, Weber C. Expression of CCR2 by endothelial cells: implications for MCP-1 mediated wound injury repair and In vivo inflammatory activation of endothelium. Arterioscler Thromb Vasc Biol. 1999;19:2085-2093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 217] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 11. | Grimm MC, Elsbury SK, Pavli P, Doe WF. Enhanced expression and production of monocyte chemoattractant protein-1 in inflammatory bowel disease mucosa. J Leukoc Biol. 1996;59:804-812. [PubMed] |
| 12. | Mazzucchelli L, Loetscher P, Kappeler A, Uguccioni M, Baggiolini M, Laissue JA, Mueller C. Monocyte chemoattractant protein-1 gene expression in prostatic hyperplasia and prostate adenocarcinoma. Am J Pathol. 1996;149:501-509. [PubMed] |
| 13. | Low QE, Drugea IA, Duffner LA, Quinn DG, Cook DN, Rollins BJ, Kovacs EJ, DiPietro LA. Wound healing in MIP-1alpha(-/-) and MCP-1(-/-) mice. Am J Pathol. 2001;159:457-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 248] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 14. | Nakashima E, Mukaida N, Kubota Y, Kuno K, Yasumoto K, Ichimura F, Nakanishi I, Miyasaka M, Matsushima K. Human MCAF gene transfer enhances the metastatic capacity of a mouse cachectic adenocarcinoma cell line in vivo. Pharm Res. 1995;12:1598-1604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Ueno T, Toi M, Saji H, Muta M, Bando H, Kuroi K, Koike M, Inadera H, Matsushima K. Significance of macrophage chemoattractant protein-1 in macrophage recruitment, angiogenesis, and survival in human breast cancer. Clin Cancer Res. 2000;6:3282-3289. [PubMed] |
| 16. | Hefler L, Tempfer C, Heinze G, Mayerhofer K, Breitenecker G, Leodolter S, Reinthaller A, Kainz C. Monocyte chemoattractant protein-1 serum levels in ovarian cancer patients. Br J Cancer. 1999;81:855-859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 85] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Marumo T, Schini-Kerth VB, Busse R. Vascular endothelial growth factor activates nuclear factor-kappaB and induces monocyte chemoattractant protein-1 in bovine retinal endothelial cells. Diabetes. 1999;48:1131-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 205] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 18. | Yamada M, Kim S, Egashira K, Takeya M, Ikeda T, Mimura O, Iwao H. Molecular mechanism and role of endothelial monocyte chemoattractant protein-1 induction by vascular endothelial growth factor. Arterioscler Thromb Vasc Biol. 2003;23:1996-2001. [PubMed] |
| 19. | Chen WY, Rogers AA, Lydon MJ. Characterization of biologic properties of wound fluid collected during early stages of wound healing. J Invest Dermatol. 1992;99:559-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 105] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 20. | Nissen NN, Polverini PJ, Koch AE, Volin MV, Gamelli RL, DiPietro LA. Vascular endothelial growth factor mediates angiogenic activity during the proliferative phase of wound healing. Am J Pathol. 1998;152:1445-1452. [PubMed] |
| 21. | Karayiannakis AJ, Zbar A, Polychronidis A, Simopoulos C. Serum and drainage fluid vascular endothelial growth factor levels in early surgical wounds. Eur Surg Res. 2003;35:492-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Takeda A, Shimada H, Imaseki H, Okazumi S, Natsume T, Suzuki T, Ochiai T. Clinical significance of serum vascular endothelial growth factor in colorectal cancer patients: correlation with clinicopathological factors and tumor markers. Oncol Rep. 2000;7:333-338. [PubMed] |
| 23. | Lee JW, Shahzad MM, Lin YG, Armaiz-Pena G, Mangala LS, Han HD, Kim HS, Nam EJ, Jennings NB, Halder J. Surgical stress promotes tumor growth in ovarian carcinoma. Clin Cancer Res. 2009;15:2695-2702. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 165] [Cited by in RCA: 173] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 24. | Coffey JC, Wang JH, Smith MJ, Bouchier-Hayes D, Cotter TG, Redmond HP. Excisional surgery for cancer cure: therapy at a cost. Lancet Oncol. 2003;4:760-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 278] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 25. | Allendorf JD, Bessler M, Kayton ML, Oesterling SD, Treat MR, Nowygrod R, Whelan RL. Increased tumor establishment and growth after laparotomy vs laparoscopy in a murine model. Arch Surg. 1995;130:649-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 140] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 26. | Allendorf JD, Bessler M, Horvath KD, Marvin MR, Laird DA, Whelan RL. Increased tumor establishment and growth after open vs laparoscopic bowel resection in mice. Surg Endosc. 1998;12:1035-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 49] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Gutt CN, Riemer V, Kim ZG, Jacobi CA, Paolucci V, Lorenz M. Impact of laparoscopic colonic resection on tumour growth and spread in an experimental model. Br J Surg. 1999;86:1180-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 73] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Kirman I, Cekic V, Poltaratskaia N, Asi Z, Bessler M, Huang EH, Forde KA, Whelan RL. Plasma from patients undergoing major open surgery stimulates in vitro tumor growth: Lower insulin-like growth factor binding protein 3 levels may, in part, account for this change. Surgery. 2002;132:186-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 29. | Allendorf JD, Bessler M, Whelan RL, Trokel M, Laird DA, Terry MB, Treat MR. Postoperative immune function varies inversely with the degree of surgical trauma in a murine model. Surg Endosc. 1997;11:427-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 100] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 30. | Lennard TW, Shenton BK, Borzotta A, Donnelly PK, White M, Gerrie LM, Proud G, Taylor RM. The influence of surgical operations on components of the human immune system. Br J Surg. 1985;72:771-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 258] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 31. | Nielsen HJ, Moesgaard F, Kehlet H. Ranitidine for prevention of postoperative suppression of delayed hypersensitivity. Am J Surg. 1989;157:291-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 32. | Wildbrett P, Oh A, Carter JJ, Schuster H, Bessler M, Jaboci CA, Whelan RL. Increased rates of pulmonary metastases following sham laparotomy compared to CO2 pneumoperitoneum and the inhibition of metastases utilizing perioperative immunomodulation and a tumor vaccine. Surg Endosc. 2002;16:1162-1169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 33. | Carter JJ, Feingold DL, Wildbrett P, Oh A, Kirman I, Asi Z, Stapleton G, Huang E, Fine RL, Whelan RL. Significant reduction of laparotomy-associated lung metastases and subcutaneous tumors after perioperative immunomodulation with flt3 ligand in mice. Surg Innov. 2005;12:319-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 34. | Shantha Kumara HM, Kirman I, Feingold D, Cekic V, Nasar A, Arnell T, Balik E, Hoffman A, Baxter R, Conte S. Perioperative GMCSF limits the proangiogenic plasma protein changes associated with colorectal cancer resection. Eur J Surg Oncol. 2009;35:295-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |