INTRODUCTION
Neuroendocrine tumors of the pancreas are rare malignancies, accounting for 1%-2% of pancreatic neoplasms. Also known as islet cell tumors, neoplasms in this heterogeneous group have distinct histological and biological behavior and are now believed to arise from multipotent stem cells located in the ductal epithelium[1]. From a clinical point of view, these tumors are classified into functioning and non-functioning. Functioning tumors are neoplasms that secrete inappropriate amounts of hormones causing clinical endocrinopathy. The most common secreting types are insulinoma and gastrinoma[2].
Glucagonomas are neuroendocrine tumors that arise from α cells of the pancreatic islets[3]. They present as encapsulated firm nodules that reach 25 cm in diameter and usually occur in the tail of the pancreas. Histologically, glucagonoma consist of cords and nests of well-differentiated islet cells. Nevertheless, despite their benign appearance, most glucagonomas are malignant and the disease is usually metastatic at diagnosis[3,4]. Metastatic disease usually involves the liver and lymph nodes and rarely extends to the bones. Therefore, only a few cases of glucagonomas with bone metastases have been reported in the literature. These bone metastases are mostly spinal[3-6]. We present the case of a male patient with a history of recurrent nonfunctioning glucagonoma who was found to have blastic bone lesions.
CASE REPORT
A 53-year-old male presented with left upper extremity numbness and weakness. His medical history revealed that he had been diagnosed with glucagonoma at age 36. More specifically, in April 1993, the patient experienced sudden epigastric pain radiating to the left upper quadrant. An abdominal ultrasound revealed a 5-6 cm mass located between the body of the pancreas and the anterior wall of the stomach and the patient underwent exploratory laparatomy converted into a distal pancreatectomy. Histology showed an islet cell tumor positive for glucagons, chromogranin and synaptophysin and negative for somatostatin, gastrin, insulin and pancreatic polypeptide, leading to the diagnosis of a non-functioning glucagonoma. His condition remained stable until February 1998 when an abdominal computed tomography (CT) scan revealed a recurrent mass in the mid-portion of the pancreas (6 cm × 8 cm × 6 cm). The tumor was surgically resected and histology confirmed the recurrence of the neuroendocrine tumor. The patient was on regular follow-up thereafter with serial CT scans of the abdomen and pelvis.
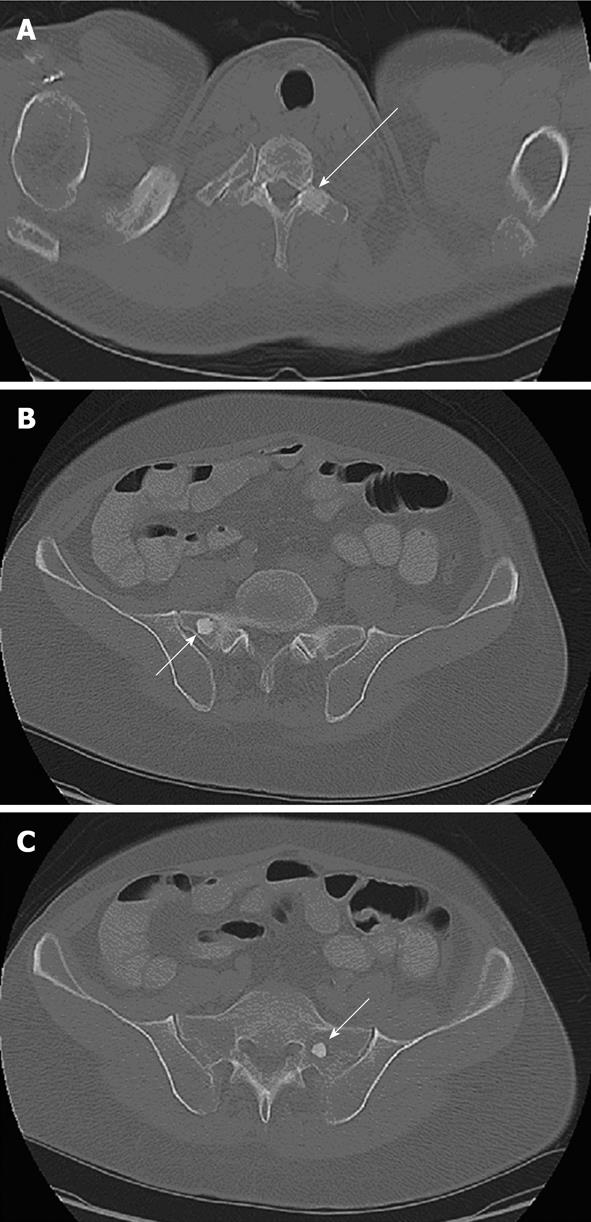
Seventeen years after the initial diagnosis, the patient presented to our clinic with left hand numbness and weakness radiating under the left arm and left axilla. The symptoms had started 2 mo before. He also noticed weakness in his left upper extremity but denied any pain. The patient experienced no other symptoms and review of the systems revealed weight gain, good appetite and performance status. Complete physical examination was unremarkable with no evidence of skin lesions, rashes, lymphadenopathy or focal neurological deficits. He underwent a chest CT scan that revealed a 20 mm blastic lesion suspicious of metastasis in the left transverse process of the T1 vertebra (Figure 1A). Additional blastic lesions were found on the posterior aspect of the right fifth rib and in both transverse processes of the S1 vertebra (measuring 3 mm, 15 mm and 15 mm in diameter, respectively) (Figure 1B and C). The magnetic resonance imaging (MRI) scan of the spine showed non-enhancing foci of low signal intensity in the bilateral sacrum, in accordance with the sclerotic lesions seen on prior CT scan. Bone scan showed no foci of abnormal increased activity. Octreotide scan did not show increased uptake in the area of bone lesions but showed focal activity in the surgical bed, raising the suspicion of recurrent disease. Although the diagnosis of osteosclerosis was part of the differential, the above mentioned lesions were not noticed in a similar study conducted 2 years before, suggesting that these lesions were metastatic. Chromogranin A (ChA) was found mildly elevated (48 ng/mL-normal values < 36.4 ng/mL) whereas glucagon, serotonin and gastrin levels were within the normal range. The patient moved to a different state and continued treatment there. The bone biopsy that had been scheduled was never performed.
Figure 1 Axial computed tomography scan of the patient.
A: Computed tomography (CT) through the upper chest displayed using the bone window settings. There is a 2-cm well-defined sclerotic lesion within the left transverse process of the T1 vertebra (arrow); B: CT through the pelvis displayed using the bone window settings. There is a 1.5-cm, well defined, sclerotic lesion (arrow) in the right sacrum adjacent to the sacroiliac joint; C: CT through the pelvis displayed using the bone window settings. There is a 1.5-cm, well defined, sclerotic lesion (arrow) in the left sacrum.
DISCUSSION
The diagnosis of glucagonoma includes: characteristic clinical features, elevated hormone levels, imaging findings and histological confirmation. The presentation of glucagonomas has been associated with necrolytic migratory erythema, diabetes mellitus, anemia, weight loss, diarrhea, deep venous thrombosis, neuropsychiatric symptoms and hypoaminoacidemia[4,6,7]. The main features of glucagonoma syndrome include hyperglycemia, increased muscle catabolism with wasting and cutaneous manifestations associated with necrolytic migratory erythema. Glucagon secretion is responsible for most of the observed signs and symptoms[6,8]. The endocrine manifestations are more common in advanced stages and may be related directly to tumor size, but lack of clinically important secretory activity can be observed in non-functioning tumors even in widely metastatic disease[9]. This was also the case in our patient who had no clinical manifestations of glucagonoma syndrome at initial presentation or later.
Laboratory abnormalities in functioning neoplasms include hyperglucagonemia and hyperglycemia. Glucagon levels are usually above 1000 pg/mL although in some glucagonomas, levels do not exceed the upper normal range[10]. Hormones not directly involved in the clinical syndrome may also be elevated: ChA and pancreatic peptide (PP) levels are raised in 50%-80% of cases, including nonsecretory tumors. A combined assessment of PP and ChA is particulary useful for the diagnosis of nonfunctioning cases and their increased levels also seem to correlate with overall disease burden[10]. Besides their role as tumor markers, they can also be utilized to monitor the therapeutic response[11]. In our case, glucagon levels were reported normal with only a mild increase in ChA levels.
The natural history of this malignancy reveals that the prevalence of metastatic disease at time of diagnosis varies from 50% to 100%[12]. Common metastatic sites are the liver and regional lymph nodes. Other reported sites for metastatic glucagonoma are adrenal glands, kidneys and lungs[6]. Bone metastases are rare events with only seven cases reported in the literature to date[3,13,14]. In most of these cases, vertebral metastases were described. In one of these cases, bone metastases were the initial finding of glucagonoma[13] and spinal cord compression was observed in another patient[3]. There has also been a case of misdiagnosis where the bone scan showed abnormality in the proximal femur, initially considered avascular necrosis[14]. Bone metastases from pancreatic islet carcinoma have also been reported in dogs[15]. Our patient had blastic lesions involving the vertebrae and the sacrum. Unfortunately, the patient moved to another state before bone biopsy was performed. However, the radiological findings deserve attention and clinicians should be aware of this rare site of glucagonoma metastases.
Octreotide scan has become one of the most important tools in the initial diagnosis and staging of these tumors. CT, MRI scans, endoscopic or perioperative ultrasonography are used for diagnosis and also for evaluation of response to treatment[16]. Arterial stimulation and venous sampling with calcium loading also seems to be an effective, although more invasive, method of detecting glucagonomas[17].
Somatostatin receptor scintigraphy using radiolabeled octreotide is an important diagnostic tool as glucagonomas express somatostatin receptors in more than 80% of cases. Due to the rarity of these neoplasms, the sensitivity and specificity of this imaging technique has not been clearly established. Possible causes of false-negative results are high levels of endogenous somatostatin competing for receptors with the radiolabeled octreotide or causing receptors downregulation. Absence or minimal expression of one of the somatostatin receptor subtypes (type 2) can also lead to poor visualization since this receptor holds the highest affinity for octreotide[5]. The octreoscan in our patient failed to show increased bone uptake but did show some uptake in the surgical bed, suggesting possible recurrent disease. As shown above, this study cannot rule out metastatic glucagonoma. Unfortunately, our patient did not undergo biopsy. However, the radiological findings deserve attention to make clinicians aware of this rare site of metastases of glucagonoma.
Regarding prognosis, glucagonomas are slowly growing tumors usually advanced by the time of diagnosis. When the primary tumor can be controlled, aggressive radical surgery and complete tumor resection offer long-term survival[18,19]. Once glucagonoma is metastatic, cure is rarely achieved.
Treatment of the glucagonomas with metastatic involvement other than the liver alone consists of targeting excessive hormonal secretion and tumor growth. Somatostatin analogues such as octreotide are highly effective in controlling symptoms related to glucagon hypersecretion[20]. No benefit in non-secreating tumors has been shown, as there is no documented antitumoral activity of octreotide. Interferon-alfa also improves symptoms in up to 50% of patients with pancreatic endocrine tumors. Multiple cytotoxic drugs have been used, mostly combinations of streptozocin with doxorubicin or fluorouracil. Cisplatin and etoposide have been used in rapidly progressive tumors[21]. Other studies have examined the role of topotecan, oxaliplatin, gemcitabine, capecitabine and temozolomide-based regimens in the treatment of neuroendocrine tumors of the gastrointestinal tract[22,23]. Unfortunately, the benefit of current regimens remains modest considering the poor tumor response and increased toxicity[6]. New molecularly targeted therapeutic options are under investigation. VEGF pathway inhibitors, such as sunitinib and bevacizumab have shown promise in delaying progression of metastatic pancreatic endocrine neoplasms. Inhibition of mTOR, using temsirolimus and everolimus, has also been studied[23]. Radioembolization with selective internal radiation microspheres in cases of liver metastases can achieve relatively long-term response[24]. Finally, external beam radiotherapy is used as palliative care in bone metastases or bulky disease[23].
In conclusion, glucagonomas are rare pancreatic endocrine tumors. By the time of diagnosis, more then half of these tumors are already metastatic. Bone metastases are rare in glucagonomas with only 7 other cases reported in the literature. Laboratory and imaging findings can be inconclusive, especially in case of non-secretory types. Even octreotide scan may lack sensitivity, depending on the somatostatin receptor profile and/or somatostatin endogenous secretion. In confirmed cases of bone metastases, therapy should include a systemic approach using chemotherapy combinations along with molecularly targeted therapy. Glucagonomas expressing somatostatin receptors 2 and 5 may benefit from radiolabelled somatostatin therapy. External beam radiation can be used palliatively.