Published online Jun 15, 2025. doi: 10.4251/wjgo.v17.i6.106603
Revised: March 23, 2025
Accepted: April 21, 2025
Published online: June 15, 2025
Processing time: 103 Days and 16.9 Hours
The hemoglobin-to-red cell distribution width ratio (HRR) is a recently intro
To investigate the prognostic significance of the HRR and other inflammation-based hematological markers in patients with metastatic CRC. Additionally, the study evaluated the impact of surgical interventions, particularly metastasectomy, and multiple clinical and laboratory parameters on overall survival. By iden
In this retrospective study, patients diagnosed with CRC between January 2020 and December 2024 were analyzed. The impact of HRR in conjunction with inflammatory markers and a total of 22 different clinical and laboratory para
A total of 155 patients with CRC were included in the study. The median age was 60 years, and 61.9% presented with de novo metastasis. In the receiver operating characteristic curve and area under the curve analysis performed to determine the optimal cutoff, the values were found to be 6.10 for carcinoembryonic antigen (CEA) (P = 0.036), 18.85 for platelet-to-red cell distribution width ratio (P = 0.028), and 10.87 for platelet distribution width-to-lymphocyte ratio (P = 0.028). For neutrophil-to-lymphocyte ratio, systemic immune-inflammation index (SII), platelet-to-lymphocyte ratio (PLR), monocyte-to-lymphocyte ratio, HRR, and carbohydrate antigen 19-9, an optimal cutoff could not be determined using the receiver operating characteristic-area under the curve analysis. Therefore, the median values were adopted as the cutoffs (3.09, 835.96, 177.50, 0.380, 0.824, and 21.6, respectively). Univariate analysis identified male gender (P = 0.045), being under 65 years of age (P = 0.001), history of metastasectomy (P = 0.001), low serum CEA level (P = 0.010), low PLR (P = 0.024), low SII (P = 0.010), and high HRR (P = 0.025) as favorable prognostic factors for overall survival. In the multivariate model, being under 65 years of age [hazard ratio (HR) = 1.59, 95% confidence interval (CI): 1.06-2.39, P = 0.025], metastasectomy (HR = 0.49, 95%CI: 0.29-0.85, P = 0.011), CEA (HR = 1.51, 95%CI: 1.0-2.28, P = 0.048), and PLR (HR = 1.63, 95%CI: 1.09-2.44, P = 0.018) emerged as independent prognostic factors for overall survival, whereas gender, SII, and HRR did not retain statistical significance.
In conclusion, low HRR alone was a prognostic indicator. However, when modelled with other inflammatory and clinical parameters, it did not provide a sufficiently strong marker feature.
Core Tip: Colorectal cancer is a leading cause of cancer-related deaths. While survival can be estimated with special tests, using inexpensive and validated methods such as hemogram parameters may be beneficial for clinicians. Blood parameter scales are currently used for some cancers, but there is not enough data on colorectal cancer. Hemoglobin/red cell distribution width ratio, which combines many parameters such as nutrition and inflammation in the same ratio, may be useful in predicting overall survival.
- Citation: Zeynelgil E, Duzkopru Y, Kocanoglu A, Karakaya S. Prognostic value of hemoglobin-to-red cell distribution width ratio and inflammation markers in colorectal cancer. World J Gastrointest Oncol 2025; 17(6): 106603
- URL: https://www.wjgnet.com/1948-5204/full/v17/i6/106603.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i6.106603
An estimated 2000000 new cancer cases and approximately 618000 cancer-related deaths are expected in the United States by 2025. Colorectal cancer (CRC) is the third most common type of cancer in males and females and is the leading cause of cancer-related deaths[1,2]. Stage 4 CRC was historically treated with surgery, chemotherapy, and currently targeted therapy methods. However, it still has a poor prognosis[3,4]. Determining prognostic indicators in advance guides clinicians in choosing intensive triplet treatment regimens for select patients[5,6].
Tumor-associated macrophages are known to be regulated by the cancer microenvironment and play a significant role in treatment failures[7]. Factors such as transforming growth factor-β and interleukin-10 have the potential to regulate monocytes and polarize them into M2 macrophages, which subsequently promote tumor cell proliferation, invasion, metastasis, angiogenesis, and matrix deposition and remodeling[7]. Chronic inflammation is thought to influence both cancer development and prognosis through DNA damage-induced mutations and abnormal DNA methylation[8]. Complete blood count is a routine assessment performed by clinicians in individuals with cancer.
Recently, various complete blood count parameters, such as the neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), systemic immune-inflammation index (SII), and monocyte-to-lymphocyte ratio (MLR), have been identified as potential prognostic biomarkers in CRC[9-11]. These markers, which reflect both the tumor microenvironment and systemic inflammation, stand out as accessible and cost-effective prognostic tools. Additionally, carcinoembryonic antigen (CEA) levels at the time of diagnosis and Eastern Cooperative Oncology Group have been shown to be prognostic markers[12,13].
Low hemoglobin levels in cancer patients may be associated with malnutrition, decreased bone marrow reserves due to inflammation, and tumor activity[14,15]. Red cell distribution width (RDW) is another important routine hemogram parameter. Traditionally, RDW has been used for the diagnosis and classification of anemia subtypes[16]. Recent studies suggest that RDW, similar to other inflammatory markers, may serve as an indicator of inflammation[17,18]. Moreover, changes in RDW have been shown to play a role in tumor etiology[19]. Based on this premise, RDW has been identified as a prognostic marker in various cancers, including breast cancer and non-small cell lung cancer[20,21]. Hemoglobin-to-red cell distribution width ratio (HRR) has been investigated as a prognostic marker in different types of cancer[22-24]. However, the relationship between HRR and prognosis in CRC remains insufficiently elucidated.
This study aimed to investigate the effects of surgical procedures, such as metastasectomy, on the prognosis of patients with metastatic CRC along with the impact of HRR and other pathological and hemogram parameters on survival.
Study design and patient selection: This retrospective study included a total of 155 outpatients diagnosed with metastatic CRC between January 2020 and December 2024. The inclusion criteria were as follows: (1) Patients aged ≥ 18 years; (2) Those who had received and completed at least 2 months of treatment (chemotherapy with or without biological agents) following diagnosis; (3) Patients with organ metastases confirmed through CT, magnetic resonance imaging, or other imaging modalities; and (4) Those without accompanying renal dysfunction. Patients with a history of malignancy, active smokers, had concomitant rheumatologic diseases, known hereditary anemia, multiple synchronous cancers, active infectious disease, or immunosuppressive drug use were excluded from the study.
Data collection: The demographic data, clinicopathological characteristics, and serum laboratory parameters of the patients before the first chemotherapy were recorded from the electronic medical records system. The pre-treatment laboratory parameters included NLR, PLR, MLR, HRR, platelet-to-red cell distribution width ratio (PRR), platelet distribution width-to-lymphocyte ratio (PDWLR), SII, CEA, and carbohydrate antigen 19-9. The SII was calculated as platelet × neutrophil/lymphocyte and recorded.
Statistical analyses were performed using SPSS Statistics software version 24. Categorical variables were presented as counts and percentages. To determine the optimal cutoff values for the analyses, a receiver operating characteristic (ROC) curve and area under the curve (AUC) analysis were initially performed. ROC-AUC analysis was preferred because it gave accurate results in similar biomarker studies[25]. Sensitivity and specificity analyses were conducted using the ROC curve. For laboratory parameters where a cutoff could not be determined using the ROC-AUC analysis, the median cutoff value was used. This cutoff value was utilized to classify the patients into “low” and “high” groups. Survival analysis was conducted using the Kaplan-Meier method, and group comparisons were performed using the log-rank test. Factors influencing survival were assessed using univariate and multivariate analyses based on the Cox regression model. The “Forward: LR” method was applied for multivariate analyses. Hazard ratios (HRs) were reported with corresponding 95% confidence intervals (95%CI). Overall survival (OS) was calculated from the date of metastasis diagnosis to the date of death or last follow-up. A P value of < 0.05 was considered statistically significant.
The values for NLR, PLR, SII, MLR, HRR, PRR, and PDWLR were calculated using the following formulas: NLR = neutrophil-to-lymphocyte ratio; SII = (platelet × neutrophil)/lymphocyte; PLR = platelet-to-lymphocyte ratio; MLR = monocyte-to-lymphocyte ratio; HRR = hemoglobin-to-RDW ratio; PRR = platelet-to-RDW ratio; and PDWLR = platelet distribution width-to-lymphocyte ratio.
OS was defined as the time from the date of metastasis diagnosis to either the date of death or the last follow-up. Survival analyses were conducted using the Kaplan-Meier method and the Cox regression model. Variables found to be significant in the univariate Cox regression analysis were included in the multivariate analysis. The “Forward: LR” method was applied for multivariate analysis. A P value of < 0.05 was considered statistically significant for all analyses.
The study was completed with 155 patients who met the inclusion criteria. The median age was 60 years (range: 24-83 years), and 99 patients (63.9%) were female. A total of 96 patients (61.9%) belonged to the de novo metastatic group, with the liver being the most common site of metastasis (69.7%). By the end of the study, 104 patients (67.1%) had died due to cancer-related causes. The median OS for all patients was 25.1 months (95%CI: 19.1-31.2). The general characteristics and laboratory data of the patients are presented in Table 1.
| Patients’ characteristics | Number | % |
| Age | ||
| < 65 | 94 | 60.6 |
| ≥ 65 | 61 | 39.4 |
| Sex | ||
| Male | 99 | 63.9 |
| Female | 56 | 36.1 |
| ECOG PS | ||
| < 2 | 55 | 64.5 |
| ≥ 2 | 100 | 35.5 |
| Tumor location | ||
| Right | 31 | 20.0 |
| Left | 124 | 80.0 |
| Tumor grade | ||
| < 2 | 22 | 14.2 |
| ≥ 2 | 133 | 85.8 |
| Body mass index | ||
| < 25 | 68 | 43.9 |
| ≥ 25 | 87 | 56.1 |
| Metastasis status | ||
| De novo | 96 | 61.9 |
| Recurrence | 59 | 38.1 |
| Liver metastasis | ||
| No | 47 | 30.3 |
| Yes | 108 | 69.7 |
| Lung metastasis | ||
| No | 93 | 60.0 |
| Yes | 62 | 40.0 |
| Peritoneal metastasis | ||
| No | 119 | 76.8 |
| Yes | 36 | 23.2 |
| Metastasectomy | ||
| No | 123 | 79.4 |
| Yes | 32 | 20.6 |
| Treatment lines | ||
| < 3 | 112 | 72.3 |
| ≥ 3 | 43 | 27.7 |
| Laboratory parameters | Low1 | High1 |
| CEA (ng/dL) | 71 (45.8) | 84 (54.2) |
| CA19-9 (U/mL) | 78 (50.3) | 77 (49.7) |
| NLR | 78 (50.3) | 77 (49.7) |
| PLR | 78 (50.3) | 77 (49.7) |
| SII | 78 (50.3) | 77 (49.7) |
| MLR | 77 (49.7) | 78 (50.3) |
| HRR | 77 (49.7) | 78 (50.3) |
| PRR | 81 (52.3) | 74 (47.7) |
| PDWLR | 75 (48.4) | 80 (51.6) |
The ROC-AUC analysis was conducted to determine the following optimal cutoff values: 6.10 for CEA (AUC = 0.604, 95%CI: 0.51-0.70, P = 0.036); 18.85 for PRR (AUC = 0.609, 95%CI: 0.52-0.70, P = 0.028); and 10.87 for PDWLR (AUC = 0.609, 95%CI: 0.52-0.70, P = 0.028). Since an optimal cutoff could not be determined using the ROC-AUC analysis for NLR, SII, PLR, MLR, HRR, and carbohydrate antigen 19-9, the median values were used as the cutoff points (3.09, 835.96, 177.50, 0.380, 0.824, and 21.6, respectively).
To identify factors associated with OS, univariate analysis revealed that male gender (P = 0.045), under 65 years old (P = 0.001), history of metastasectomy (P = 0.001), low serum CEA levels (P = 0.010), low PLR (P = 0.024), low SII (P = 0.010), and high HRR (P = 0.025) were favorable prognostic factors (Table 2). Using the parameters that were significant in the univariate analysis, a multivariate model was constructed to accurately evaluate prognostic factors for OS. In this model, being under 65 years of age (HR = 1.59, 95%CI: 1.06-2.39, P = 0.025), metastasectomy (HR = 0.49, 95%CI: 0.29-0.85, P = 0.011), CEA (HR = 1.51, 95%CI: 1.0-2.28, P = 0.048), and PLR (HR = 1.63, 95%CI: 1.09-2.44, P = 0.018) emerged as independent prognostic factors for OS. In the multivariate analysis, gender, SII, and HRR did not retain statistical significance (Table 2).
| Variable | Category | Univariate analysis | Multivariate analysis | ||
| HR (95%CI) | P value | HR (95%CI) | P value | ||
| Patients’ characteristics | |||||
| Sex | Male/female | 0.66 (0.44-0.99) | 0.0451 | ||
| ECOG PS | < 2/≥ 2 | 1.25 (0.84-1.87) | 0.270 | ||
| Body mass index | < 25/≥ 25 | 1.42 (0.95-2.13) | 0.084 | ||
| Age | < 65/≥ 65 | 1.51 (1.02-2.25) | 0.0401 | 1.59 (1.06-2.39) | 0.0251 |
| Tumor grade | < 2/≥ 2 | 0.97 (0.56-1.65) | 0.897 | ||
| Tumor location | Right/left | 1.51 (0.87-2.63) | 0.142 | ||
| Metastasis status | De novo/recurrence | 1.09 (0.72-1.66) | 0.693 | ||
| Liver metastasis | No/yes | 0.99 (0.64-1.54) | 0.976 | ||
| Peritoneal metastasis | No/yes | 1.32 (0.81-2.15) | 0.273 | ||
| Lung metastasis | No/yes | 0.90 (0.60-1.35) | 0.607 | ||
| Metastasectomy | No/yes | 0.42 (0.24-0.71) | 0.0011 | 0.49 (0.29-0.85) | 0.0111 |
| Treatment lines | < 3/≥ 3 | 0.86 (0.70-1.07) | 0.168 | ||
| Laboratory parameters | |||||
| CEA (ng/dL) | < 6.1/≥ 6.1 | 1.70 (1.14-2.55) | 0.0101 | 1.51 (1.00-2.28) | 0.0481 |
| CA19-9 (U/mL) | < 21.6/≥ 21.6 | 1.34 (0.91-1.98) | 0.138 | ||
| NLR | < 3.09/≥ 3.09 | 1.38 (0.93-2.04) | 0.108 | ||
| PLR | < 177.5/≥ 177.5 | 1.57 (1.06-2.33) | 0.0241 | 1.63 (1.09-2.44) | 0.0181 |
| SII | < 835.96/≥ 835.96 | 1.67 (1.13-2.46) | 0.0101 | ||
| MLR | < 0.38/≥ 0.38 | 1.00 (0.67-1.48) | 0.988 | ||
| HRR | < 0.83/≥ 0.83 | 0.64 (0.43-0.95) | 0.0251 | ||
| PRR | < 18.85/≥ 18.85 | 1.19 (0.80-1.77) | 0.384 | ||
| PDWLR | < 10.87/≥ 10.87 | 1.05 (0.71-1.57) | 0.794 | ||
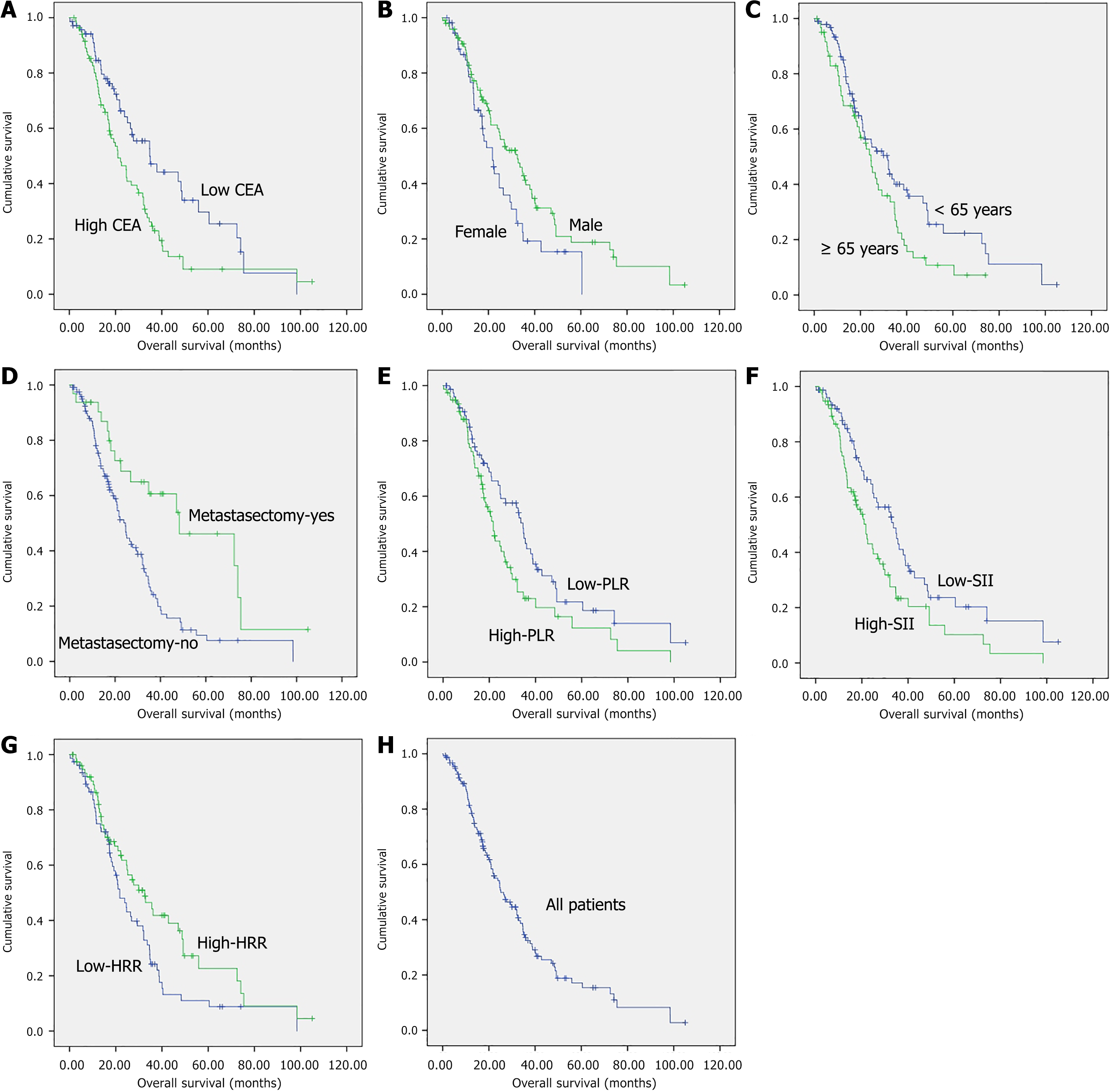
Kaplan-Meier survival curves were generated for the prognostic factors. The corresponding median OS values according to sex, age, metastasectomy, CEA levels, PLR, SII, and HRR were 21.7 months (95%CI: 16.3-27.1) vs 32.5 months (95%CI: 23.6-41.4) (log-rank P = 0.043), 31.8 months (95%CI: 22.1-41.5) vs 24.6 months (95%CI: 18.9-30.2) (log-rank P = 0.039), 24.6 months (95%CI: 20.9-28.3) vs 48.3 months (95%CI: 15.7-81.0) (log-rank P = 0.001), 34.8 months (95%CI: 22.0-47.6) vs 21.0 months (95%CI: 15.7-26.3) (log-rank P = 0.009), 34.6 months (95%CI: 31.0-38.2) vs 21.7 months (95%CI: 17.8-25.6) (log-rank P = 0.022), 33.7 months (95%CI: 25.0-42.4) vs 21.7 months (95%CI: 17.0-26.4) (log-rank P = 0.010), and 21.7 months (95%CI: 16.8-26.6) vs 32.5 months (95%CI: 21.8-43.2) (log-rank P = 0.024), respectively, with each comparison showing a significant difference (Figure 1).
In this study, which included 155 patients with metastatic CRC, being under 65 years of age and female and having a CEA level of ≥ 6.1, high PLR, high SII, and low HRR were found to be associated with poor prognosis. Additionally, patients eligible for liver metastasectomy at diagnosis and follow-up were identified as having a favorable prognostic indicator. Age, metastasis, CEA, and PLR collectively demonstrated the potential to serve as a low-cost prognostic marker model that could be utilized by clinicians.
The role of inflammation in cancer progression has been widely studied, with evidence linking immune dysregulation to tumor development and survival outcomes. The uncontrolled increase in the immune system leads to a decrease in immunity and results in tumoral progression[26]. The identification of inflammation using inexpensive and easily accessible markers plays a crucial role in survival assessment and consequently in clinicians’ decision-making. Kim et al[27] concluded that high PLR and high NLR are indicators of poor survival outcomes in patients with CRC. Similarly, Erstad et al[28], in a study involving 151 patients with resectable colorectal liver metastases, found that patients with PLR ≥ 220 and NLR ≥ 5 at diagnosis could have poor long-term survival outcomes. Tan et al[29] demonstrated through their analysis that SII, one of the inflammation markers, is a predictor for OS, progression-free survival, and distant metastasis-free survival. In our study, we concluded that patients with high NLR, high PLR, and high SII had poor prognoses, and these results were consistent with the literature.
RDW is a parameter that reflects the variation in the size and volume of erythrocytes in the circulatory system, indicating erythrocyte heterogeneity. Along with the type of anemia, it serves as a marker of patients’ nutritional statuses. RDW has been reported as an indicator of inflammation and has been associated with cardiovascular mortality, the clinical course of necrotizing pancreatitis, hepatitis B, and other inflammatory diseases[30-33]. Low hemoglobin levels, indicating anemia, have been correlated with poor prognosis in various tumors. This association in cancer patients may be attributed to cancer-related hypoxia[34]. Hypoxia can lead to increased angiogenesis in tumor tissues by upregulating molecules such as vascular endothelial growth factor and epidermal growth factor, which are involved in tumor angiogenesis and metabolism. Increased angiogenesis, in turn, can result in reduced treatment tolerance and poorer survival outcomes[35].
A decreased HRR has been identified as a poor prognostic marker in different cancer types due to factors such as poor nutritional status, increased inflammation, and enhanced angiogenesis[23,36]. In a study conducted by Tuncel et al[37], an HRR value below 0.89 was associated with poor prognosis in patients with rectal cancer[37]. In the study by Wu et al[38], low HRR was shown to be a poor prognostic indicator for small cell lung cancer. In our study, we determined the HRR cutoff value as 0.83 using ROC curve analysis and demonstrated that a low HRR was associated with poor prognosis, consistent with the literature.
Our study had certain limitations. One of the important limitations was the retrospective design of the study. Another limitation was the intra-patient variability of hemogram parameters due to factors such as bleeding and acute infections. However, a key strength of our study was the analysis of 22 different clinical and laboratory parameters using homogeneous survival data from a single center.
Our study was among the few that have demonstrated the prognostic significance of HRR as a marker reflecting both anemia and inflammation in patients with metastatic CRC. HRR, in combination with CEA, gender, metastasectomy, and other inflammation markers (PLR, SII), was found to be a prognostic indicator. Future prospective studies are needed to validate our findings and develop stronger predictive models by incorporating additional inflammation markers and using more robust analyses, including innovative machine learning-based risk models[39]. Furthermore, it is recommended that the external validation of these results be conducted using independent datasets.
| 1. | Siegel RL, Kratzer TB, Giaquinto AN, Sung H, Jemal A. Cancer statistics, 2025. CA Cancer J Clin. 2025;75:10-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 446] [Article Influence: 446.0] [Reference Citation Analysis (0)] |
| 2. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64594] [Article Influence: 16148.5] [Reference Citation Analysis (176)] |
| 3. | Robinson TP, Kaiser K, Lark M, Ruedinger B, Robb BW, Morgan T, Park S, Schleyer TKL, Haggstrom DA, Mohanty S. NCCN guideline concordance in colon and rectal cancer patients within a comprehensive health system. Am J Surg. 2025;240:116114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Sonkin D, Thomas A, Teicher BA. Cancer treatments: Past, present, and future. Cancer Genet. 2024;286-287:18-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 197] [Cited by in RCA: 184] [Article Influence: 184.0] [Reference Citation Analysis (0)] |
| 5. | Ychou M, Rivoire M, Thezenas S, Guimbaud R, Ghiringhelli F, Mercier-Blas A, Mineur L, Francois E, Khemissa F, Chauvenet M, Kianmanesh R, Fonck M, Houyau P, Aparicio T, Galais MP, Audemar F, Assenat E, Lopez-Crapez E, Jouffroy C, Adenis A, Adam R, Bouché O. Chemotherapy (doublet or triplet) plus targeted therapy by RAS status as conversion therapy in colorectal cancer patients with initially unresectable liver-only metastases. The UNICANCER PRODIGE-14 randomised clinical trial. Br J Cancer. 2022;126:1264-1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 6. | Parisi A, Porzio G, Cannita K, Venditti O, Avallone A, Filippi R, Salvatore L, Tortora G, Ribelli M, Nigro O, Gelsomino F, Spallanzani A, Zurlo V, Leo S, Dell'Aquila E, Claudia F, Lombardi P, Keränen SR, Aimar G, Depetris I, Giampieri R, Morelli C, De Tursi M, Tinari N, Di Pietro FR, De Galitiis F, Zanaletti N, Troiani T, Vitale P, Garajova I, Ghidini M, Spinelli GP, Zoratto F, Roberto M, Ierino D, Petrillo A, D'Orazio C, Ficorella C, Cortellini A. Clinicians' Attitude to Doublet Plus Anti-EGFR Versus Triplet Plus Bevacizumab as First-line Treatment in Left-Sided RAS and BRAF Wild-Type Metastatic Colorectal Cancer Patients: A Multicenter, "Real-Life", Case-Control Study. Clin Colorectal Cancer. 2021;20:318-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Si H, Yang Q, Hu H, Ding C, Wang H, Lin X. Colorectal cancer occurrence and treatment based on changes in intestinal flora. Semin Cancer Biol. 2021;70:3-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 93] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 8. | Hattori N, Niwa T, Ishida T, Kobayashi K, Imai T, Mori A, Kimura K, Mori T, Asami Y, Ushijima T. Antibiotics suppress colon tumorigenesis through inhibition of aberrant DNA methylation in an azoxymethane and dextran sulfate sodium colitis model. Cancer Sci. 2019;110:147-156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 9. | Naszai M, Kurjan A, Maughan TS. The prognostic utility of pre-treatment neutrophil-to-lymphocyte-ratio (NLR) in colorectal cancer: A systematic review and meta-analysis. Cancer Med. 2021;10:5983-5997. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 71] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 10. | Dong M, Shi Y, Yang J, Zhou Q, Lian Y, Wang D, Ma T, Zhang Y, Mi Y, Gu X, Fan R. Prognostic and clinicopathological significance of systemic immune-inflammation index in colorectal cancer: a meta-analysis. Ther Adv Med Oncol. 2020;12:1758835920937425. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 11. | Kang Y, Zhu X, Lin Z, Zeng M, Shi P, Cao Y, Chen F. Compare the Diagnostic and Prognostic Value of MLR, NLR and PLR in CRC Patients. Clin Lab. 2021;67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 12. | Yılmaz F, Karabulut CC, Özdemir F, Aydın F. Prognostic factors for survival in patients with metastatic colon carcinoma. UHOD. 2024;34:148-154. [DOI] [Full Text] |
| 13. | Roussille P, Auvray M, Vansteene D, Lecomte T, Rigault E, Maillet M, Locher C, Dior M, Hautefeuille V, Artru P, Mabro M, Touchefeu Y, Marthey L, Moulin V, Louafi S, Lecaille C, Chautard R, Lièvre A, Zaanan A, Bennouna J, Berger A, Emambux S, Randrian V, Tougeron D. Prognostic factors of colorectal cancer patients with brain metastases. Radiother Oncol. 2021;158:67-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Wilson MJ, van Haaren M, Harlaar JJ, Park HC, Bonjer HJ, Jeekel J, Zwaginga JJ, Schipperus M. Long-term prognostic value of preoperative anemia in patients with colorectal cancer: A systematic review and meta-analysis. Surg Oncol. 2017;26:96-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 15. | Xu J, Roy A, Palli SR. CREB-binding protein plays key roles in juvenile hormone action in the red flour beetle, Tribolium Castaneum. Sci Rep. 2018;8:1426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 16. | Tong L, Kauer J, Wachsmann-Hogiu S, Chu K, Dou H, Smith ZJ. A new red cell index and portable RBC analyzer for screening of iron deficiency and Thalassemia minor in a Chinese population. Sci Rep. 2017;7:10510. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Förhécz Z, Gombos T, Borgulya G, Pozsonyi Z, Prohászka Z, Jánoskuti L. Red cell distribution width in heart failure: prediction of clinical events and relationship with markers of ineffective erythropoiesis, inflammation, renal function, and nutritional state. Am Heart J. 2009;158:659-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 480] [Article Influence: 30.0] [Reference Citation Analysis (1)] |
| 18. | Lee WS, Kim TY. Relation between red blood cell distribution width and inflammatory biomarkers in rheumatoid arthritis. Arch Pathol Lab Med. 2010;134:505-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51728] [Cited by in RCA: 47121] [Article Influence: 3365.8] [Reference Citation Analysis (5)] |
| 20. | Seretis C, Seretis F, Lagoudianakis E, Gemenetzis G, Salemis NS. Is red cell distribution width a novel biomarker of breast cancer activity? Data from a pilot study. J Clin Med Res. 2013;5:121-126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 21. | Chen GP, Huang Y, Yang X, Feng JF. A Nomogram to Predict Prognostic Value of Red Cell Distribution Width in Patients with Esophageal Cancer. Mediators Inflamm. 2015;2015:854670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | Sun P, Zhang F, Chen C, Bi X, Yang H, An X, Wang F, Jiang W. The ratio of hemoglobin to red cell distribution width as a novel prognostic parameter in esophageal squamous cell carcinoma: a retrospective study from southern China. Oncotarget. 2016;7:42650-42660. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 91] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 23. | Tham T, Olson C, Wotman M, Teegala S, Khaymovich J, Coury J, Costantino P. Evaluation of the prognostic utility of the hemoglobin-to-red cell distribution width ratio in head and neck cancer. Eur Arch Otorhinolaryngol. 2018;275:2869-2878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 24. | Bozkaya Y, Kurt B, Gürler F. A prognostic parameter in advanced non-small cell lung cancer: the ratio of hemoglobin-to-red cell distribution width. Int J Clin Oncol. 2019;24:798-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 25. | Liu H, Weng J. A Pan-Cancer Bioinformatic Analysis of RAD51 Regarding the Values for Diagnosis, Prognosis, and Therapeutic Prediction. Front Oncol. 2022;12:858756. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 26. | Bardelčíková A, Šoltys J, Mojžiš J. Oxidative Stress, Inflammation and Colorectal Cancer: An Overview. Antioxidants (Basel). 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 100] [Reference Citation Analysis (0)] |
| 27. | Kim JH, Lee JY, Kim HK, Lee JW, Jung SG, Jung K, Kim SE, Moon W, Park MI, Park SJ. Prognostic significance of the neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in patients with stage III and IV colorectal cancer. World J Gastroenterol. 2017;23:505-515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 61] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 28. | Erstad DJ, Taylor MS, Qadan M, Axtell AL, Fuchs BC, Berger DL, Clancy TE, Tanabe KK, Chang DC, Ferrone CR. Platelet and neutrophil to lymphocyte ratios predict survival in patients with resectable colorectal liver metastases. Am J Surg. 2020;220:1579-1585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 29. | Tan Y, Hu B, Li Q, Cao W. Prognostic value and clinicopathological significance of pre-and post-treatment systemic immune-inflammation index in colorectal cancer patients: a meta-analysis. World J Surg Oncol. 2025;23:11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 30. | Cetinkaya E, Senol K, Saylam B, Tez M. Red cell distribution width to platelet ratio: new and promising prognostic marker in acute pancreatitis. World J Gastroenterol. 2014;20:14450-14454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 69] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 31. | Yeşil A, Senateş E, Bayoğlu IV, Erdem ED, Demirtunç R, Kurdaş Övünç AO. Red cell distribution width: a novel marker of activity in inflammatory bowel disease. Gut Liver. 2011;5:460-467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 32. | Xu WS, Qiu XM, Ou QS, Liu C, Lin JP, Chen HJ, Lin S, Wang WH, Lin SR, Chen J. Red blood cell distribution width levels correlate with liver fibrosis and inflammation: a noninvasive serum marker panel to predict the severity of fibrosis and inflammation in patients with hepatitis B. Medicine (Baltimore). 2015;94:e612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 33. | Agarwal S. Red cell distribution width, inflammatory markers and cardiorespiratory fitness: results from the National Health and Nutrition Examination Survey. Indian Heart J. 2012;64:380-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 34. | Larsson AM. Translational Aspects of Erythropoietin Receptor and Hypoxia-Inducible Factors in Breast Cancer. In: Lund University Faculty of Medicine Doctoral Dissertation Series. Center for Molecular Pathology, Faculty of Medicine. 2012. |
| 35. | Li S, Meng W, Guan Z, Guo Y, Han X. The hypoxia-related signaling pathways of vasculogenic mimicry in tumor treatment. Biomed Pharmacother. 2016;80:127-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 36. | Baysal M, Demirci H, Bas V, Gulsaran S, Umit E, Kırkızlar HO, Baysal S, Demir A. Could ratio of hemoglobin to red cell distribution width and ratio of absolute lymphocyte count to absolute monocyte count be a prognostic tool in newly diagnosed multiple myeloma patients? Acta Haematol Pol. 2020;51:81-87. [DOI] [Full Text] |
| 37. | Tuncel ET, Parvizi M, Kut E, Aydın M, Kasap E. Prognostic Significance of Hemoglobin/Prognostic Nutritional Index and Hemoglobin/Red Blood Cell Distribution in Rectal Cancer. Turk J Gastroenterol. 2023;34:128-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 38. | Wu F, Yang S, Tang X, Liu W, Chen H, Gao H. Prognostic value of baseline hemoglobin-to-red blood cell distribution width ratio in small cell lung cancer: A retrospective analysis. Thorac Cancer. 2020;11:888-897. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 39. | Liu H, Tang T. MAPK signaling pathway-based glioma subtypes, machine-learning risk model, and key hub proteins identification. Sci Rep. 2023;13:19055. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 17.5] [Reference Citation Analysis (0)] |