Published online Jun 15, 2025. doi: 10.4251/wjgo.v17.i6.105016
Revised: March 10, 2025
Accepted: April 24, 2025
Published online: June 15, 2025
Processing time: 126 Days and 4.5 Hours
Sarcosine dehydrogenase (SARDH) and C-X-C motif chemokine ligand 1 (CXCL1) have been identified as potential tumor regulators, with growing evidence linking them to cancer progression. However, their specific roles, regulatory mechanisms, and influence on key signaling pathways remain unclear.
To investigate the regulatory mechanisms of SARDH and CXCL1 in cancer cells and their impact on key signaling pathways.
Real-time quantitative polymerase chain reaction and western blot analyses were used to assess the expression levels of SARDH and CXCL1 and their effects on protein kinase B (Akt) and extracellular signal-regulated kinase (ERK) signaling pathways. Gene overexpression was induced using an expression vector, while gene silencing was achieved using short hairpin RNA and small interfering RNA. CCK-8, migration, and invasion assays were used to evaluate the impact of gene suppression and overexpression on cancer cell proliferation, migration, and invasion.
SARDH silencing significantly enhanced cancer cell proliferation, whereas its overexpression suppressed proliferation in the early stages of the experiment. CXCL1 silencing reduced cancer cell migration and invasion. SARDH overexpression inhibited cell migration, invasion, and adhesion while increasing apoptosis. Conversely, SARDH silencing reversed these effects. Furthermore, simultaneous silencing of SARDH and CXCL1 strongly activated the Akt and ERK signaling pathways, indicating the potential role of these pathways in regulating cellular functions influenced by these genes.
This study revealed that SARDH and CXCL1 regulate cancer cell growth, migration, and invasion through Akt and ERK signaling pathways, highlighting their potential as therapeutic targets for cancer treatment.
Core Tip: In this study, we examined the roles of sarcosine dehydrogenase (SARDH) and C-X-C motif chemokine ligand 1
- Citation: Gao Z, Zhang X, He H. Role and mechanism of sarcosine dehydrogenase in the progression of gallbladder cancer through chemokine pathways. World J Gastrointest Oncol 2025; 17(6): 105016
- URL: https://www.wjgnet.com/1948-5204/full/v17/i6/105016.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i6.105016
Cancer is a complex and heterogeneous disease that continues to pose a significant global health challenge. Developing effective therapies requires a deeper understanding of the molecular mechanisms of the disease, particularly the intracellular signaling pathways that drive tumor formation and progression. Among the key regulators of cancer dynamics, sarcosine dehydrogenase (SARDH) and C-X-C motif chemokine ligand 1 (CXCL1) have garnered increasing attention for their critical roles in tumor proliferation, migration, and invasion. However, their precise roles and mechanisms in cancer progression remain unclear.
Uncontrolled tumor cell proliferation is a hallmark of cancer and is governed by various metabolic and signaling pathways[1,2]. Emerging research underscores metabolic dysregulation as a key driver of cancer development. SARDH, a vital metabolic enzyme, is significantly downregulated in certain cancers, and its reduced expression is linked to abnormal cell proliferation. This suggests that SARDH indirectly regulates tumor growth by modulating metabolic pathways[3,4]. However, despite growing evidence of its involvement in tumor metabolism, the exact mechanisms through which SARDH influences cancer progression remain unclear[5].
In addition to uncontrolled proliferation, tumor invasion and migration are key drivers of malignancy and metastasis. CXCL1, a small chemokine, plays a central role in various cancers by binding to its receptor, CXCR2, and activating key signaling pathways, such as protein kinase B (Akt) and extracellular signal-regulated kinase (ERK). These pathways promote tumor cell survival, proliferation, and migration[6,7], facilitating local and distant metastasis[8,9]. However, the regulatory mechanisms governing the function of CXCL1 in cancer remain poorly understood[10,11].
Notably, the dynamic interplay between metabolic enzymes and chemokines may be crucial in tumor progression. A potential link between SARDH and CXCL1 could offer new insights into the complex regulatory networks driving cancer. For instance, changes in SARDH activity may disrupt tumor cell energy metabolism, indirectly affecting their growth and proliferation. The interaction between these two molecules may work synergistically to accelerate tumor progression, enhance adaptation, and contribute to therapeutic resistance.
This study proposes that SARDH and CXCL1 functionally interact to regulate tumor progression through metabolic and inflammatory signaling pathways. By systematically exploring this relationship, our study aims to identify novel molecular targets for cancer therapy and establish a theoretical foundation for future therapeutic strategies[12,13].
Cells were cultured at 37 °C with 5% CO2 in DMEM (11995073, Gibco, United States) supplemented with 10% fetal bovine serum. Gene silencing and overexpression assays were performed by transfecting small interfering RNA (siRNA) or plasmids containing target gene expression vectors, such as pEF-Bos, 24 hours after cell inoculation.
Total RNA was extracted using Trizol reagent (Invitrogen) and reverse-transcribed into cDNA with Takara reverse transcriptase. Gene expression levels were quantified using SYBR Green dye (Applied Biosystems), with GAPDH as the reference gene. The PCR protocol included an initial denaturation at 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min.
Cells were collected and lysed, and total protein was extracted. Protein concentration was measured using the BCA Protein Assay reagent (Pierce). Samples were then separated through SDS-PAGE and transferred to PVDF membranes. After blocking the membranes with 5% skim milk, the membranes were incubated overnight with primary antibodies against p-Akt, Akt, p-ERK, ERK, and β-actin (1:1000, Cell Signaling Technology). Following a 1-h incubation with an HRP-conjugated secondary antibody (1:500, GE Healthcare), protein signals were detected using the ECL detection system (Millipore).
Cells (2000 per well) were seeded into 96-well plates. At 12, 36, 60, and 84 h, CCK-8 reagent (Dojindo) was added. After a 2-h incubation at 37 °C, absorbance at 450 nm was measured using a microplate reader (Bio-Rad), following the manufacturer’s instructions.
siRNAs targeting SARDH and CXCL1 were designed and synthesized using a commercial service (Sigma-Aldrich). When the cells reached 70% confluence, they were transfected using Lipofectamine 2000 (Invitrogen). A nonspecific control siRNA was used as the experimental control.
A pEF-Bos expression vector carrying full-length SARDH and CXCL1 cDNA was constructed and cloned through restriction enzyme digestion and ligation. Cells at 70% confluence were transfected using Lipofectamine 2000, and after 48 h, they were harvested for further analysis.
Cell migration and invasion were assessed using Transwell assays (Corning). For migration assays, cells were seeded onto uncoated 8-μm pore membranes, whereas for invasion assays, membranes were precoated with Matrigel. The lower chamber contained a medium supplemented with 10% fetal bovine serum as a chemoattractant. After 24 h, nonmigratory cells were removed with a cotton swab, whereas cells that had migrated or penetrated the bottom layer were fixed with formaldehyde and stained with crystal violet.
Matrigel was applied to a 96-well plate, and cells were seeded and incubated for 1 h. After incubation, nonadherent cells were washed off, and adhesion was assessed based on the remaining cells. Matrigel was dissolved in an ice bath at 4 °C, and the nozzle and 96-well plate were precooled to 4 °C. The Matrigel was diluted to 0.04 mg/mL in a serum-free culture medium. Matrigel (2 μL) was added to each well, and nonspecific binding sites were blocked by adding 20 μL of 3% BSA serum-free medium to each well. The plate was incubated at 37 °C for 1 h, washed twice with PBS, and prepared simultaneously for cell suspensions. The cells were resuspended in 0.1% BSA serum-free medium at a density of 5 × 105 cells/mL and incubated for 1 h in a 5% CO2 incubator with gentle shaking. Subsequently, 100 μL of the cell suspension was added to each well and incubated for 1 h at 37 °C. The cell suspension was discarded, and the wells were washed three times with PBS solution (shaking for 3 minutes each time to remove nonadherent cells). Finally, 100 μL of DMEM containing 1 μL of CCK-8 reagent was added to each well, and the plate was incubated for 4 h at 37 °C in a 5% CO2 incubator. Absorbance was measured at 450 nm using a microplate reader.
The cells were collected by aspirating the culture medium into a new sterile centrifuge tube and centrifuging at 1300 rpm for 3 min. The supernatant was discarded, and 500 μL of PBS was added and mixed. The cells were then digested with trypsin (without EDTA), centrifuged at 1300 rpm for 3 min, and collected. The cells were then combined and centrifuged at 1300 rpm for 10 min. Subsequently, 500 μL of binding buffer, 5 μL of Annexin V-FITC (Nanjing KGI), and 5 μL of propidium iodide (Nanjing KGI) were added, and flow cytometry was performed.
Data are expressed as mean ± SD. Group comparisons were performed using the t-test, with a statistically significant difference set at P < 0.05.
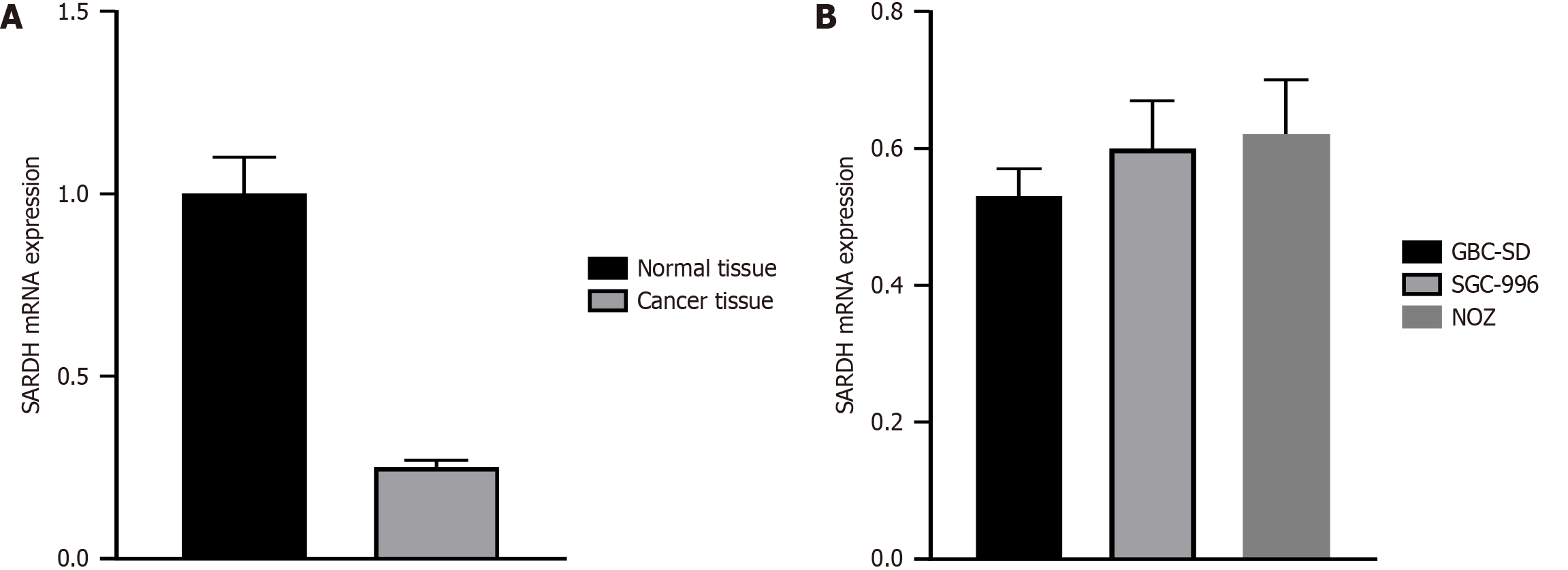
As shown in Figure 1A, real-time quantitative polymerase chain reaction (RT-PCR) analysis revealed significantly lower SARDH expression in gallbladder cancer tissues than in normal tissues, indicating its downregulation in tumor samples. Similarly, Figure 1B presents RT-PCR results for various gallbladder cancer cell lines, showing SARDH expression levels of 0.53 in GBC-SD, 0.60 in SGC-996, and 0.62 in NOZ cells. While the expression levels varied slightly among these cell lines, the overall trend indicated that SARDH expression was consistently downregulated, potentially playing a cell-specific role in the onset and progression of gallbladder cancer.
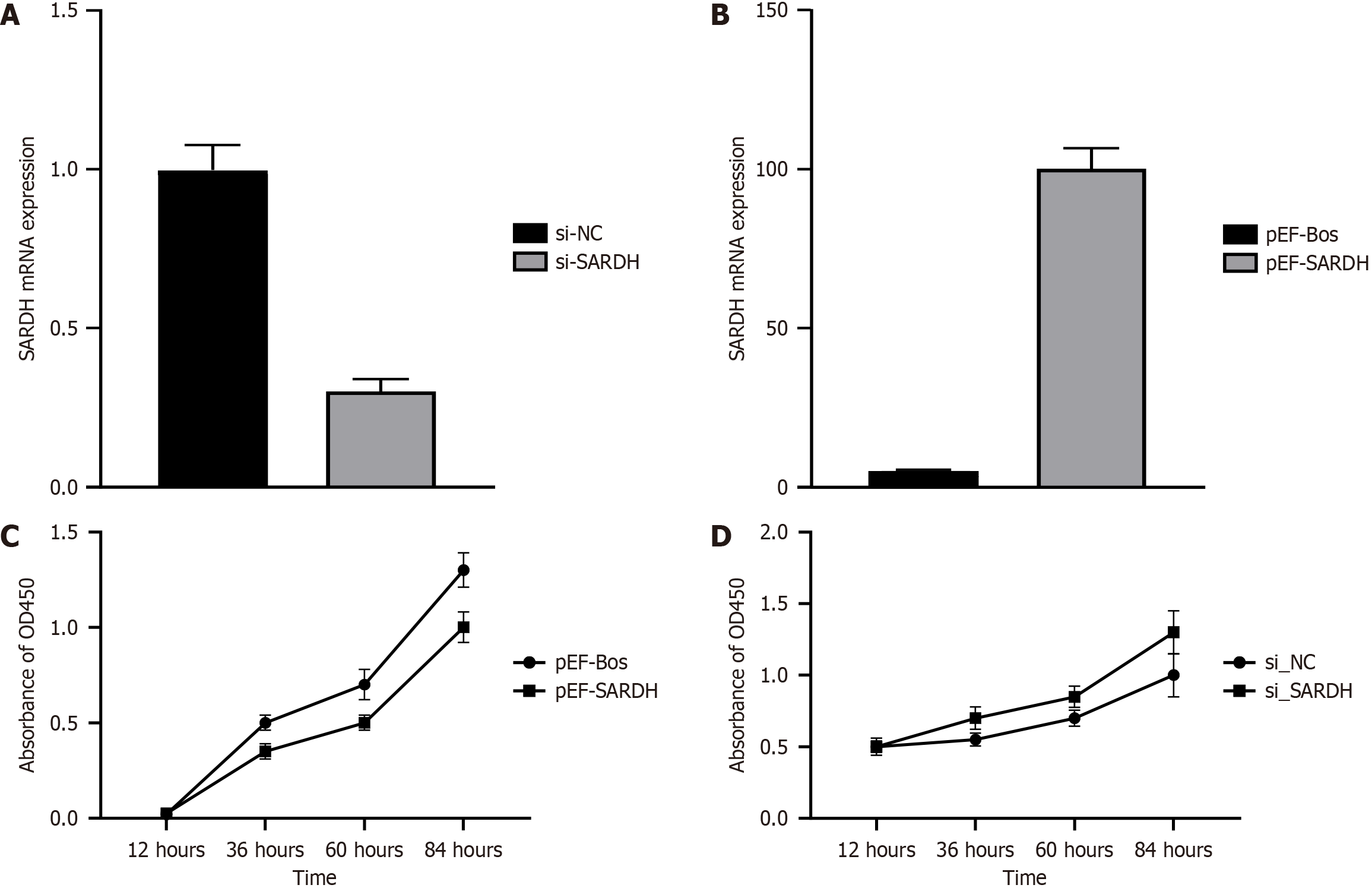
RT-PCR analysis confirmed successful SARDH silencing, with its expression levels decreasing from 1 to 0.3, indicating effective inhibition by the interfering sequence (Figure 2A). Conversely, SARDH overexpression led to a 100-fold increase in its expression, validating the efficacy of the overexpression strategy (Figure 2B). Initially, SARDH overexpression inhibited cell proliferation; however, this inhibitory effect diminished over time, with a proliferation rate lower than that of the control at 84 h (Figure 2C). Conversely, SARDH silencing gradually increased cell proliferation beyond that of the control group, indicating that SARDH silencing may promote cancer cell proliferation (Figure 2D).
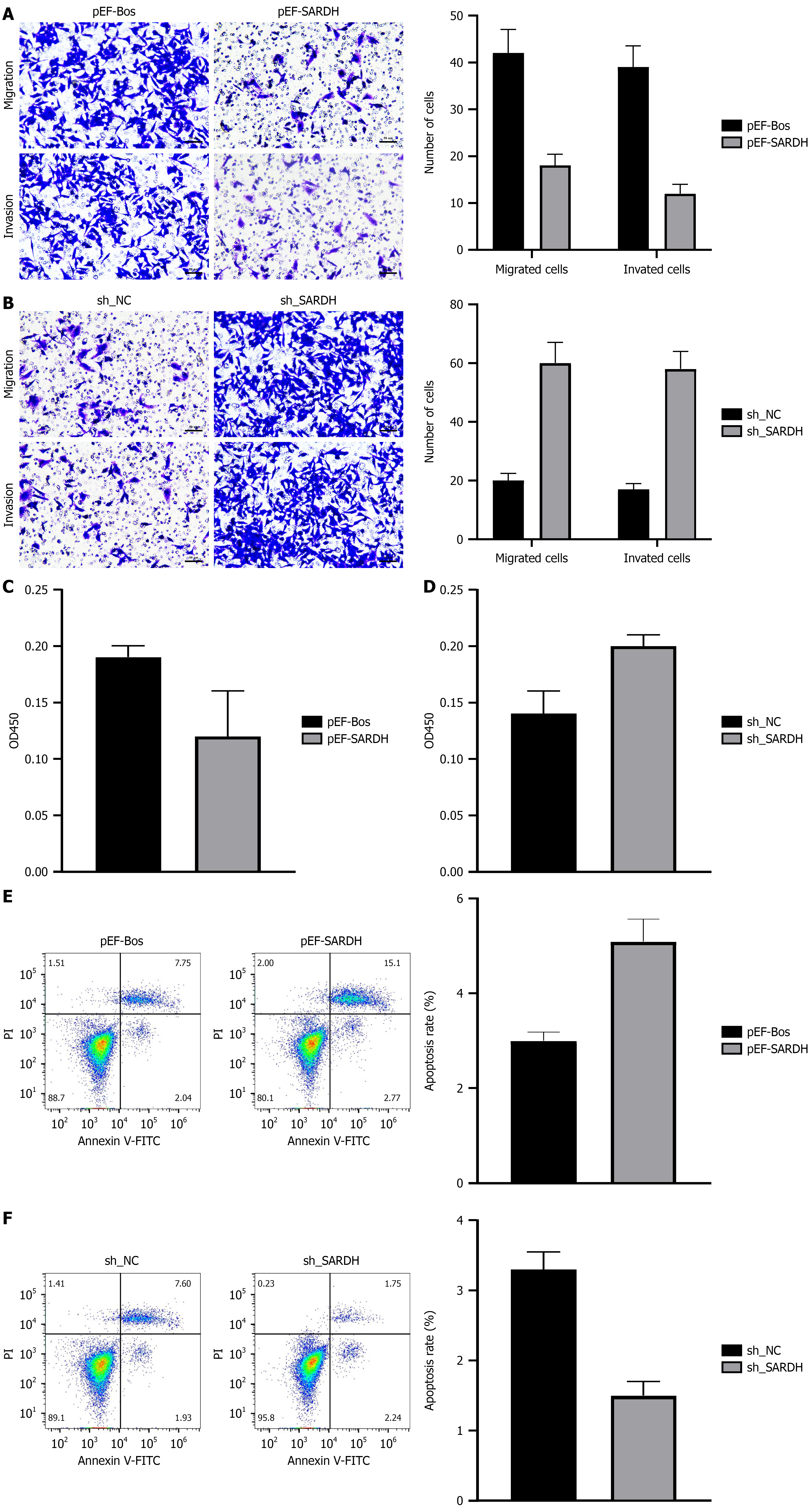
Figure 3A shows the comparisons of the migration and invasion abilities of SARDH-overexpressing cells and control cells (pEF-Bos). The results revealed that SARDH overexpression significantly reduced both cell migration and invasion, indicating that SARDH plays a crucial role in regulating tumor cell behavior. Figure 3B shows the effect of SARDH silencing on cell migration and invasion. Compared with the control group (sh_NC), the SARDH silencing group (sh_SARDH) showed a significant increase in the number of migrating and invasive cells. Figures 3C and 3D show the effect of SARDH on cell adhesion. SARDH overexpression significantly decreased adherent cell counts compared with the control (pEF-Bos), whereas SARDH silencing (sh_SARDH) resulted in a significant increase in adhesion compared with the control group (sh_NC). In addition, Figures 3E and 3F present flow cytometry analyses of apoptosis in gallbladder cancer cells. SARDH overexpression markedly increased the proportion of apoptotic cells compared to that in the control group (pEF-Bos), whereas SARDH silencing significantly reduced apoptosis rates. These findings indicate that SARDH is crucial in inhibiting tumor cell migration and invasion, as its silencing markedly increased cell migration and invasion.
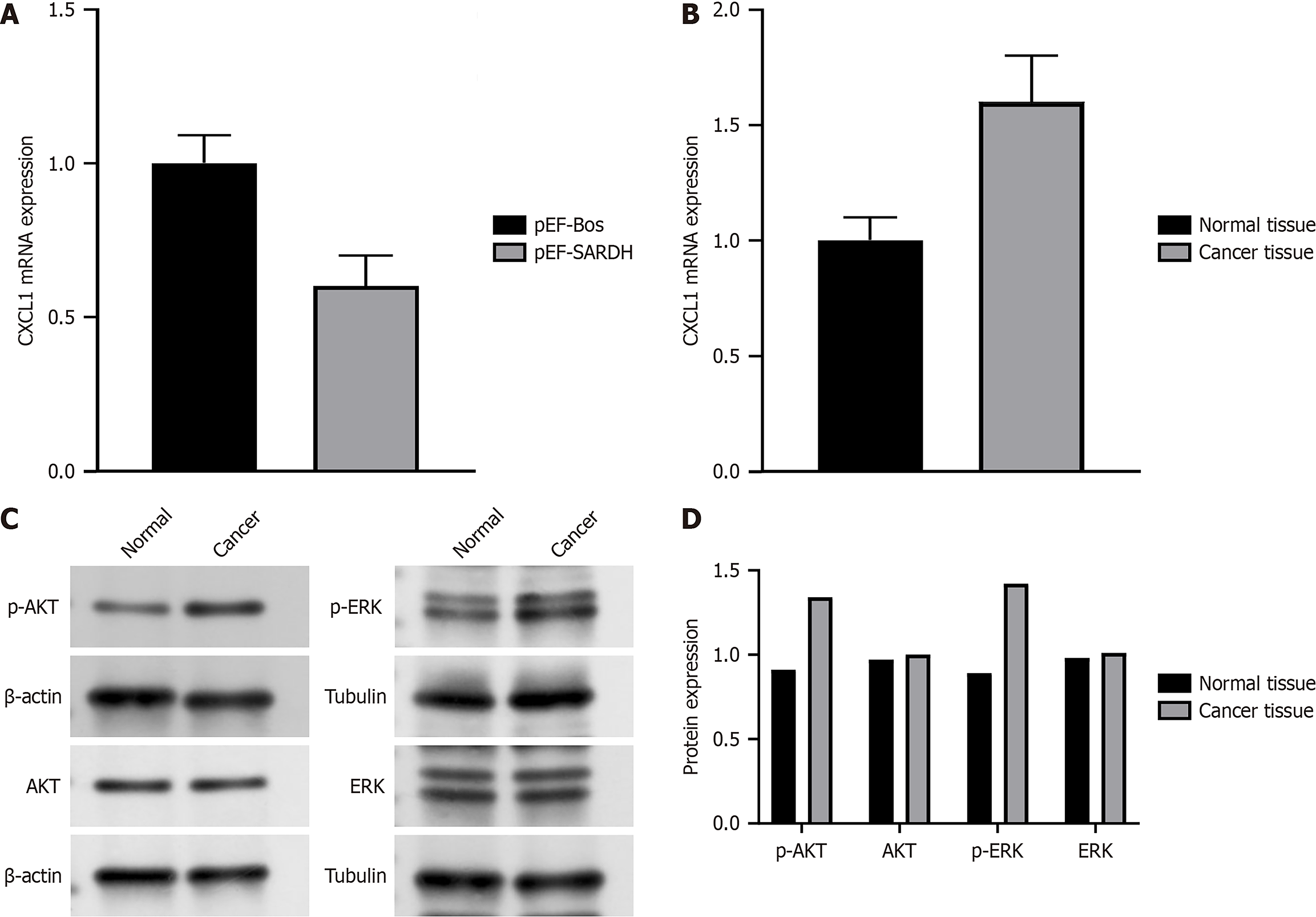
Figure 4A shows that SARDH overexpression downregulated CXCL1, suggesting potential reciprocal regulation between these two factors in cell signaling pathways. As shown in Figure 4B, CXCL1 expression was significantly elevated in cancerous tissues compared with that in healthy tissues, indicating its possible role in tumor progression. Correlation analysis revealed a negative association between SARDH and CXCL1 expression in cancer tissues (Figure 4C). In addition, Figure 4D shows that silencing SARDH and CXCL1 markedly increased the activation levels of p-Akt and p-ERK in cancer cells. This suggests that silencing these genes may promote cancer cell survival and proliferation through activating Akt and ERK signaling pathways.
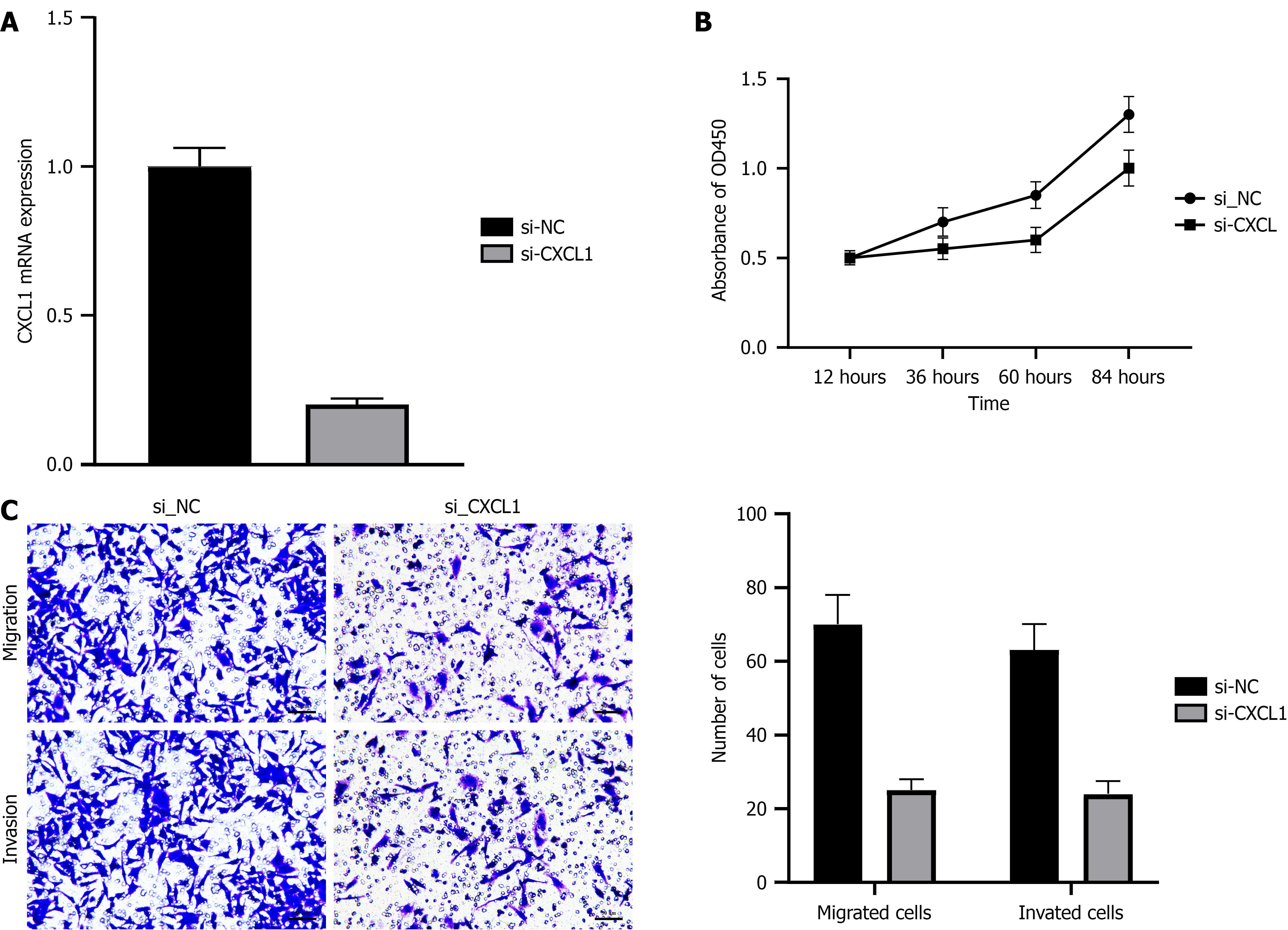
To further explore the role of CXCL1 in gallbladder cancer cell function, we conducted a series of experiments. Figure 5A presents RT-PCR results, showing that CXCL1 expression was significantly reduced in the silenced group (Si-CXCL1), indicating successful inhibition of CXCL1 by siRNA. Figure 5B shows the results of the CCK-8 assays, revealing that CXCL1 silencing reduced cell proliferation, with Si-CXCL1 exhibiting lower proliferation compared to the control group (Si-NC), particularly in the later stages of the experiment. Figure 5C shows that CXCL1 silencing significantly reduced both cell migration and invasion, highlighting the critical role of CXCL1 in regulating tumor cell migration and invasion.
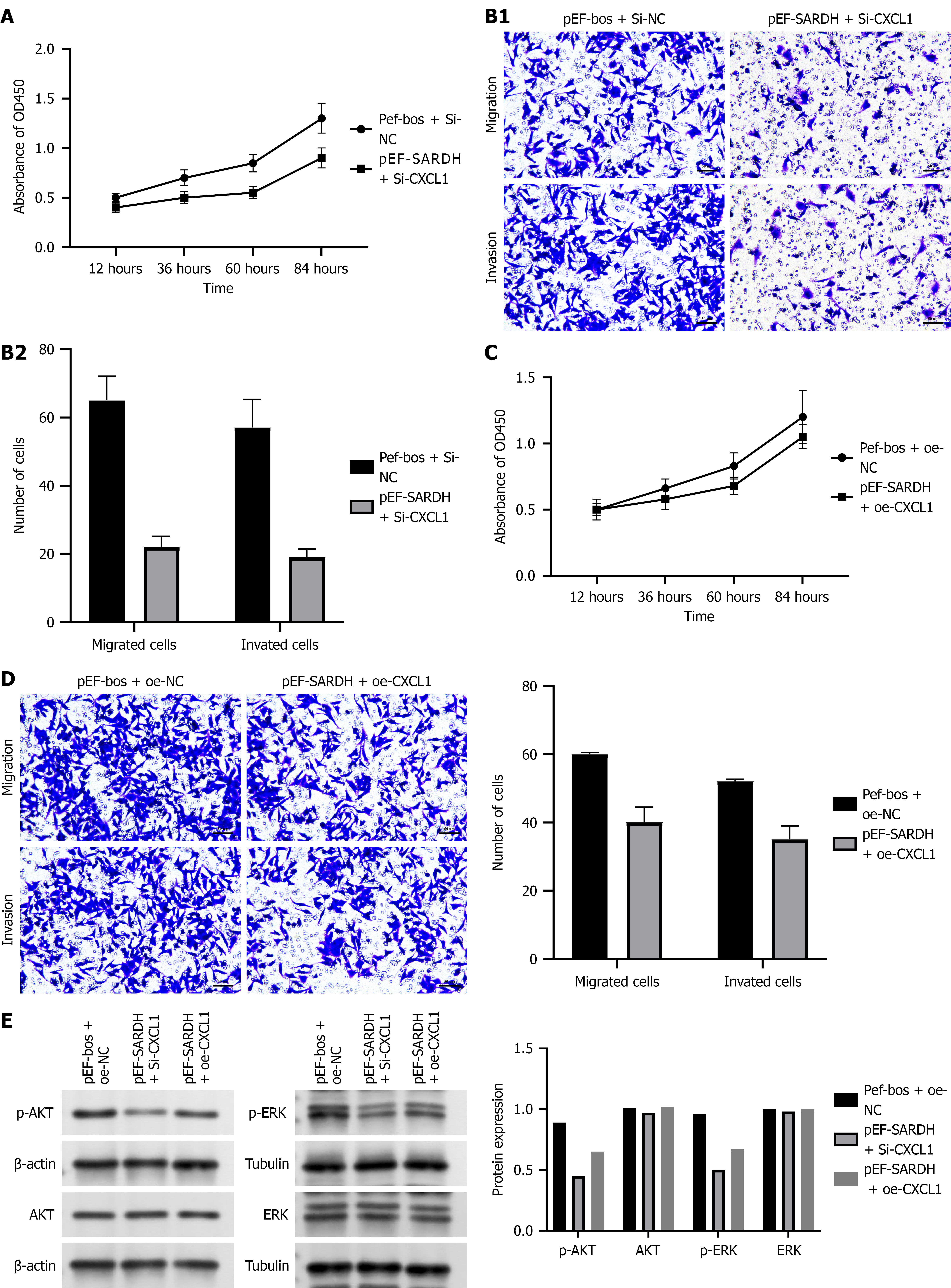
Figure 6 illustrates that the simultaneous silencing of SARDH and CXCL1 significantly inhibited cell proliferation, migration, and invasion, indicating their potential synergistic role in suppressing tumor cell function. Specifically, Figure 6A shows that the cell proliferation rate remained consistently lower in Si-CXCL1 than in Si-NC at all time points. Similarly, Figure 6B shows that migrating and invading cell counts significantly reduced after gene silencing. Conversely, Figures 6C and 6D show that the overexpression of SARDH and CXCL1 also inhibited cell proliferation, migration, and invasion, with the most pronounced effect observed in migration and invasion, where overexpression significantly reduced cell counts. These findings underscore the crucial role of SARDH and CXCL1 in regulating tumor cell activity.
In this study, we systematically investigated the effects of SARDH and CXCL1 overexpression and silencing on tumor cell function and explored their roles in tumor growth and progression, highlighting their potential as therapeutic targets for future cancer treatments. These findings enhance our understanding of the molecular mechanisms underlying cancer and provide a solid theoretical foundation for developing innovative treatment strategies.
SARDH, a key metabolic enzyme, has recently garnered attention in cancer research. In this study, SARDH silencing significantly accelerated tumor cell proliferation, aligning with previous studies and suggesting its tumor-suppressive role in various cancers[14,15]. For instance, in prostate and breast cancers, low SARDH expression is associated with increased malignancy and poorer prognosis[16]. Our findings indicate that SARDH may suppress tumor growth by regulating cell cycle-related proteins. Specifically, SARDH overexpression significantly suppressed early-stage cell proliferation, likely through the modulation of key regulatory proteins such as p53 and p21[17]. However, this inhibitory effect diminished over time, potentially owing to adaptive adjustments in intracellular signaling pathways or activation of negative feedback mechanisms[18]. For example, prolonged SARDH overexpression may trigger compensatory mechanisms that counteract its suppressive effects by modulating the expression of other regulatory genes.
Unlike SARDH, CXCL1 plays a more complex and multifaceted role in tumor progression. Our findings revealed that CXCL1 silencing significantly inhibited tumor cell migration and invasion, consistent with previous studies showing that CXCL1 interacts with its receptor CXCR2 to promote tumor metastasis[19,20]. The CXCL1–CXCR2 axis is recognized as a key driver of tumor invasion and metastasis in various cancers, including colorectal, lung, and breast cancers. Beyond its direct effects on tumor cell motility, CXCL1 modulates the tumor microenvironment by recruiting immunosuppressive cells, such as regulatory T cells and myeloid-derived suppressor cells, which facilitate immune evasion[21]. By suppressing antitumor immune responses, CXCL1 creates a permissive microenvironment for tumor progression and metastasis.
To further elucidate the tumor-promoting mechanisms of SARDH and CXCL1, we investigated their effects on the Akt and ERK signaling pathways, which play critical roles in cell survival, proliferation, and migration[22,23]. Our findings revealed that silencing both SARDH and CXCL1 activated these pathways, indicating that SARDH and CXCL1 may exert their antitumor effects, at least in part, by inhibiting these signaling pathways. The Akt signaling pathway is particularly essential for promoting cell survival, inhibiting apoptosis, and enhancing tumor cell proliferation and migration[24]. Thus, its inhibition by SARDH and CXCL1 may be a key mechanism through which they suppress tumor progression. Notably, despite their shared ability to modulate Akt and ERK signaling pathways, SARDH and CXCL1 silencing appears to function through distinct mechanisms. SARDH primarily regulates tumor proliferation by influencing metabolic and cell cycle pathways, whereas CXCL1 is involved in modulating the tumor microenvironment and enhancing metastatic potential.
This study identified SARDH and CXCL1 as promising therapeutic targets in cancer. Restoring SARDH expression may suppress tumor growth by regulating metabolism and the cell cycle, whereas inhibiting CXCL1 or its CXCR2 axis may reduce metastasis and modulate the immune microenvironment.
However, translating these findings into clinical applications presents challenges. The roles of SARDH and CXCL1 vary by cancer type and context; thus, further studies are required to identify patient subgroups that are most likely to benefit. In addition, CXCL1 inhibition may disrupt immune homeostasis, necessitating careful evaluation. A combination strategy involving SARDH activation and CXCL1 inhibition may enhance therapeutic efficacy, offering a more comprehensive approach to cancer treatment.
This study was limited to in vitro experiments, which may not fully reflect the complexity of tumor biology. The absence of in vivo models, such as xenograft or orthotopic mouse models, prevents a comprehensive assessment of SARDH and CXCL1 within a physiological context, particularly their interactions with the tumor microenvironment and immune system.
Future studies should incorporate in vivo models to validate the findings of this study and explore the therapeutic potential of targeting SARDH and CXCL1. In addition, integrating single-cell analysis or spatial transcriptomics could provide deeper insights into the roles of these genes in tumor heterogeneity and treatment resistance. Clinical validation using patient-derived tumor samples and correlation with disease outcomes are essential to establish their translational relevance.
In conclusion, our study shows that SARDH and CXCL1 play intricate and significant roles in tumor development. Targeting these proteins holds considerable therapeutic potential, given the possible tumor-suppressing function of SARDH and the involvement of CXCL1 in promoting tumor invasion and metastasis. However, future research is needed to fully understand the contributions of these genes to tumor progression. Investigating their interactions with other signaling molecules and their unique roles within the tumor microenvironment may pave the way for the development of novel treatment strategies.
| 1. | Chou J, Quigley DA, Robinson TM, Feng FY, Ashworth A. Transcription-Associated Cyclin-Dependent Kinases as Targets and Biomarkers for Cancer Therapy. Cancer Discov. 2020;10:351-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 209] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 2. | Loyer P, Trembley JH. Roles of CDK/Cyclin complexes in transcription and pre-mRNA splicing: Cyclins L and CDK11 at the cross-roads of cell cycle and regulation of gene expression. Semin Cell Dev Biol. 2020;107:36-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 3. | Xie H, Yang K, Qin C, Zhou X, Liu J, Nong J, Luo J, Wei Y, Hua H, Han C, Liao X, Yang C, Su H, Zhu G, Ye X, Peng T. Sarcosine dehydrogenase as an immune infiltration-associated biomarker for the prognosis of hepatocellular carcinoma. J Cancer. 2024;15:149-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 4. | Sun L, Yao X, Liu J, Zhang Y, Hu J. Curcumin Enhances the Efficacy of Docetaxel by Promoting Anti-Tumor Immune Response in Head and Neck Squamous Cell Carcinoma. Cancer Invest. 2023;41:524-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 5. | Cheng Y, Ma XL, Wei YQ, Wei XW. Potential roles and targeted therapy of the CXCLs/CXCR2 axis in cancer and inflammatory diseases. Biochim Biophys Acta Rev Cancer. 2019;1871:289-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 223] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 6. | Xu M, Wang Y, Xia R, Wei Y, Wei X. Role of the CCL2-CCR2 signalling axis in cancer: Mechanisms and therapeutic targeting. Cell Prolif. 2021;54:e13115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 204] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 7. | He H, Chen E, Lei L, Yan B, Zhao X, Zhu Z, Li Q, Zhang P, Zhang W, Xing J, Du L, Dong J, Yang J. Alteration of the tumor suppressor SARDH in sporadic colorectal cancer: A functional and transcriptome profiling-based study. Mol Carcinog. 2019;58:957-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Pan C, Li B, Simon MC. Moonlighting functions of metabolic enzymes and metabolites in cancer. Mol Cell. 2021;81:3760-3774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 103] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 9. | Zhuo C, Ruan Q, Zhao X, Shen Y, Lin R. CXCL1 promotes colon cancer progression through activation of NF-κB/P300 signaling pathway. Biol Direct. 2022;17:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 35] [Reference Citation Analysis (0)] |
| 10. | Korbecki J, Bosiacki M, Barczak K, Łagocka R, Chlubek D, Baranowska-Bosiacka I. The Clinical Significance and Role of CXCL1 Chemokine in Gastrointestinal Cancers. Cells. 2023;12:1406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 11. | Ullah A, Jiao W, Shen B. The role of proinflammatory cytokines and CXC chemokines (CXCL1-CXCL16) in the progression of prostate cancer: insights on their therapeutic management. Cell Mol Biol Lett. 2024;29:73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 12. | Qin Z, Xie B, Qian J, Ma X, Zhang L, Wei J, Wang Z, Fan L, Zhu Z, Qian Z, Yin H, Zhu F, Tan Y. Over-expression of RRM2 predicts adverse prognosis correlated with immune infiltrates: A potential biomarker for hepatocellular carcinoma. Front Oncol. 2023;13:1144269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 13. | Lee NH, Nikfarjam M, He H. Functions of the CXC ligand family in the pancreatic tumor microenvironment. Pancreatology. 2018;18:705-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 14. | Sainero-Alcolado L, Liaño-Pons J, Ruiz-Pérez MV, Arsenian-Henriksson M. Targeting mitochondrial metabolism for precision medicine in cancer. Cell Death Differ. 2022;29:1304-1317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 179] [Article Influence: 59.7] [Reference Citation Analysis (0)] |
| 15. | Łukaszewicz-Zając M, Pączek S, Mroczko P, Kulczyńska-Przybik A. The Significance of CXCL1 and CXCL8 as Well as Their Specific Receptors in Colorectal Cancer. Cancer Manag Res. 2020;12:8435-8443. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 16. | Yi M, Li T, Niu M, Zhang H, Wu Y, Wu K, Dai Z. Targeting cytokine and chemokine signaling pathways for cancer therapy. Signal Transduct Target Ther. 2024;9:176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 92] [Article Influence: 92.0] [Reference Citation Analysis (0)] |
| 17. | Zhou C, Gao Y, Ding P, Wu T, Ji G. The role of CXCL family members in different diseases. Cell Death Discov. 2023;9:212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 115] [Article Influence: 57.5] [Reference Citation Analysis (0)] |
| 18. | Yang K, Nong J, Xie H, Wan Z, Zhou X, Liu J, Qin C, Luo J, Zhu G, Peng T. DPF2 overexpression correlates with immune infiltration and dismal prognosis in hepatocellular carcinoma. J Cancer. 2024;15:4668-4685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | González-González M, Gutiérrez ML, Sayagués JM, Muñoz-Bellvís L, Orfao A. Genomic profiling of sporadic liver metastatic colorectal cancer. Semin Cancer Biol. 2021;71:98-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Yang B, Li G, Wang S, Zheng Y, Zhang J, Pan B, Wang N, Wang Z. Tumor-associated macrophages/C-X-C motif chemokine ligand 1 promotes breast cancer autophagy-mediated chemoresistance via IGF1R/STAT3/HMGB1 signaling. Cell Death Dis. 2024;15:743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 21. | Pandithar S, Galke D, Akume A, Belyakov A, Lomonaco D, Guerra AA, Park J, Reff O, Jin K. The role of CXCL1 in crosstalk between endocrine resistant breast cancer and fibroblast. Mol Biol Rep. 2024;51:331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 22. | Jing Y, Wang F, Zhang K, Chen Z. Comprehensive analysis of prognostic value and immune infiltration of CXC chemokines in pancreatic cancer. BMC Med Genomics. 2022;15:96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 23. | Ji HZ, Liu B, Ren M, Li S, Zheng JF, Liu TY, Yu HH, Sun Y. The CXCLs-CXCR2 axis modulates the cross-communication between tumor-associated neutrophils and tumor cells in cervical cancer. Expert Rev Clin Immunol. 2024;20:559-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Reference Citation Analysis (0)] |
| 24. | Hsu CC, Tseng LM, Lee HC. Role of mitochondrial dysfunction in cancer progression. Exp Biol Med (Maywood). 2016;241:1281-1295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 225] [Article Influence: 25.0] [Reference Citation Analysis (0)] |