Published online Apr 15, 2025. doi: 10.4251/wjgo.v17.i4.104919
Revised: February 6, 2025
Accepted: February 25, 2025
Published online: April 15, 2025
Processing time: 78 Days and 14 Hours
Ileum adenocarcinoma (IA), a type of small bowel adenocarcinoma, is a rather uncommon factor associated with obstruction in small bowel. Owing to its location and indefinite clinical symptoms, the diagnosis of IA is difficult, and survival is usually poor. With respect to the rarity of this disease, very few studies have reported such cases to provide a reference for treatment.
In this manuscript, a case of a 48-year-old man presented with chronic right lower abdominal pain and distention, queasiness and emesis. A computed tomography scan revealed intestinal wall thickening and an intestinal obstruction in the terminal ileum. He was diagnosed with inflammatory bowel disease. However, his symptoms were not relieved after conservative treatment. The patient sub
IA should be considered as a differential diagnosis in cases of intestinal obstruction, and the recommended method for local disease treatment is surgery.
Core Tip: Adenocarcinoma in the ileum is an extremely rare pathological type. Its clinical presentation is indefinite, and its diagnosis is challenging. Here, the case of a 48-year-old man who was diagnosed with primary ileum adenocarcinoma was presented. Proper diagnosis and early intervention are important for the prognosis of patients. For local disease, surgical excision along with the removal of regional lymph nodes en bloc is recommended.
- Citation: Zhang XY, Li C, Lin J, Zhou Y, Shi RZ, Wang ZY, Jiang HB, Wang YY. Intestinal obstruction caused by early stage primary ileum adenocarcinoma: A case report and review of literature. World J Gastrointest Oncol 2025; 17(4): 104919
- URL: https://www.wjgnet.com/1948-5204/full/v17/i4/104919.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i4.104919
Compared with other gastrointestinal carcinomas, cancers in small bowel are rare, accounting for only 3% of the cancers that occur within this system[1]. The most frequent histological subtypes are neuroendocrine neoplasms, adenocarcinomas, lymphomas and gastrointestinal stromal neoplasms[2]. The proportion of small bowel adenocarcinomas (SBAs) is approximately 30%-40%. SBAs occur often in the duodenum but less in the jejunum or ileum[3]. Compared to those with jejunal tumours, patients with ileum adenocarcinoma (IA) are easier to develop gastrointestinal obstruction[4]. Among patients with small bowel cancers, jejunal tumours have a better overall survival (OS) than ileal and duodenal tumours do[5]. The rate of recurrence is higher among IA cases (77%) than among duodenal (54%) or jejunal adenocarcinoma cases (65%)[6]. Owing to its rarity and vague clinical manifestations, most patients with SBA have a poor prognosis because of advanced-stage presentations. It has been reported that 33% of patients have high-grade malignancies with lymph node involvement and that 56% of patients have distant metastasis at diagnosis[7]. The median OS and 5-year survival rates from cancer diagnosis data are 20 months and 26%, respectively[8]. Thus, early diagnosis and management are crucial, as this can significantly affect patient survival. In this manuscript, we present a case of the surgical treatment of a 48-year-old man with ileus of the small intestine resulting from early-stage primary adenocarcinoma in the ileum.
The patient was a 48-year-old man whose main symptoms were chronic right lower abdominal aches with distention, queasiness and emesis.
The patient came to our hospital after experiencing an abdominal ache and distention in the right lower abdomen for one month. He experienced nausea approximately one hour after eating and vomited stomach contents. These symptoms were alleviated by proton pump inhibitors. Five days prior to admission, the patient’s abdominal pain intensified with concomitant cessation of defecation and flatus. He reported weight loss of 5 kg over the last two months.
The patient presented with no history of previous abdominal surgery. He was diagnosed with hypertension two years prior, and this was effectively controlled with oral medication.
The patient presented no previous experience with SBA, and there was no history of cancer in his family.
A physical examination was performed on the patient. The body temperature was 36.8 °C, the pulse was normal at 88 beats/minute, and the respiratory rate was 18 breaths/minute. Abdominal examination indicated the presence of abdomen tenderness and muscle tension. The abdomen appeared distended, accompanied by a weak rumbling sound. No lump was palpated, and digital rectal examination was insignificant.
Routine blood tests revealed a white blood cell count of 6.0 × 109/L, a red blood cell count of 4.730 × 1012/L, a platelet count of 279 × 109/L, an alpha-fetoprotein level of 2.35 ng/mL (range: 0.00-9.00 ng/mL), a cancer antigen 199 (CA19-9) level of 4.8 U/mL (range: 0.0-35.0 U/mL), a carcinoembryonic antigen (CEA) level of 1.76 ng/mL (range: 0.00-5.00 ng/mL), and an abnormal triglyceride level of 8.84 mmol/L (range: 0.20-1.70 mmol/L).
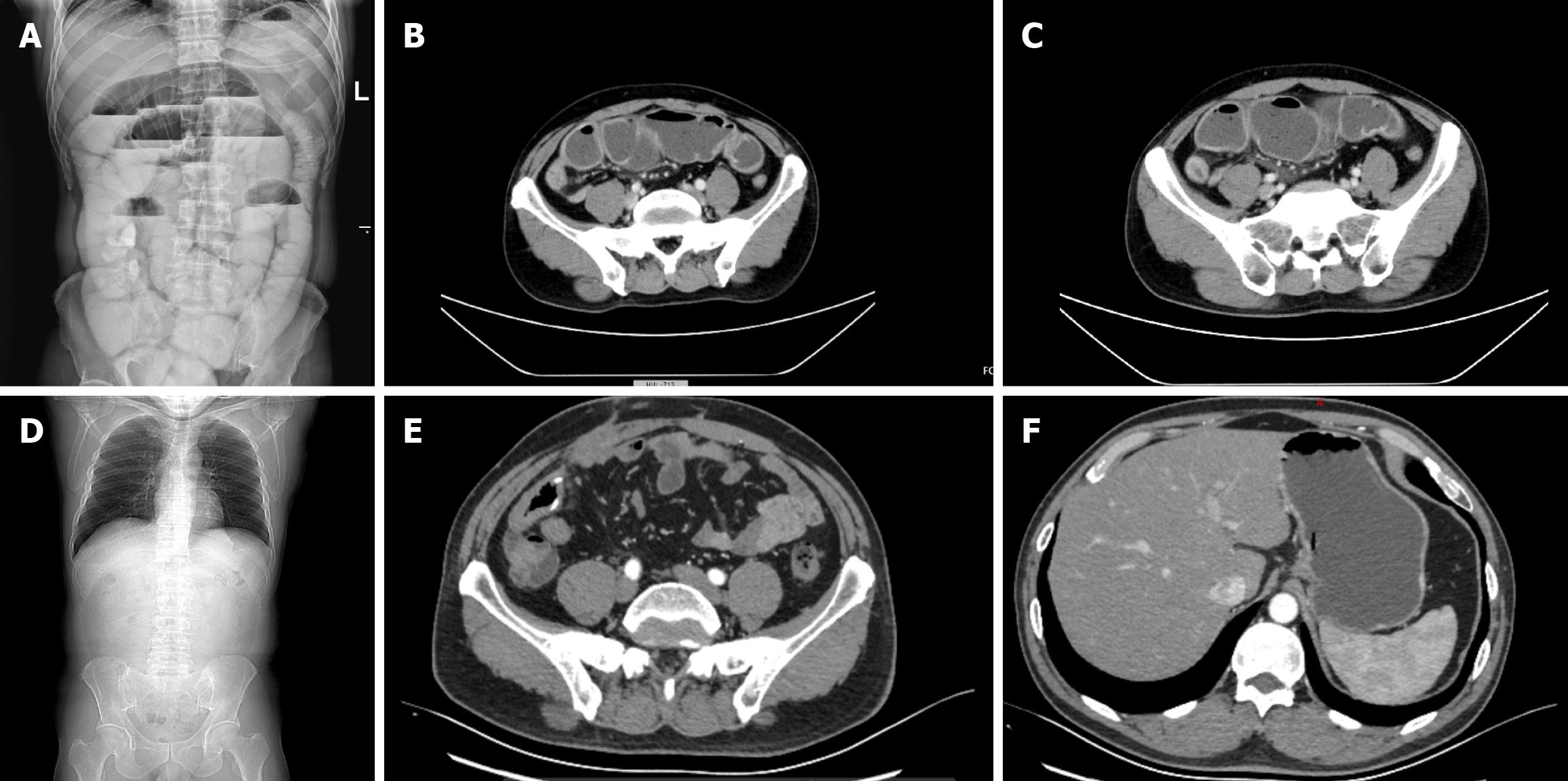
Abdominal radiography revealed dilation of the small bowel and the presence of gas-fluid interfaces (Figure 1A). Computed tomography (CT) scans revealed localized thickening of the ileum wall and small bowel obstruction in the terminal ileum, with no evident mass observed (Figure 1B and C).
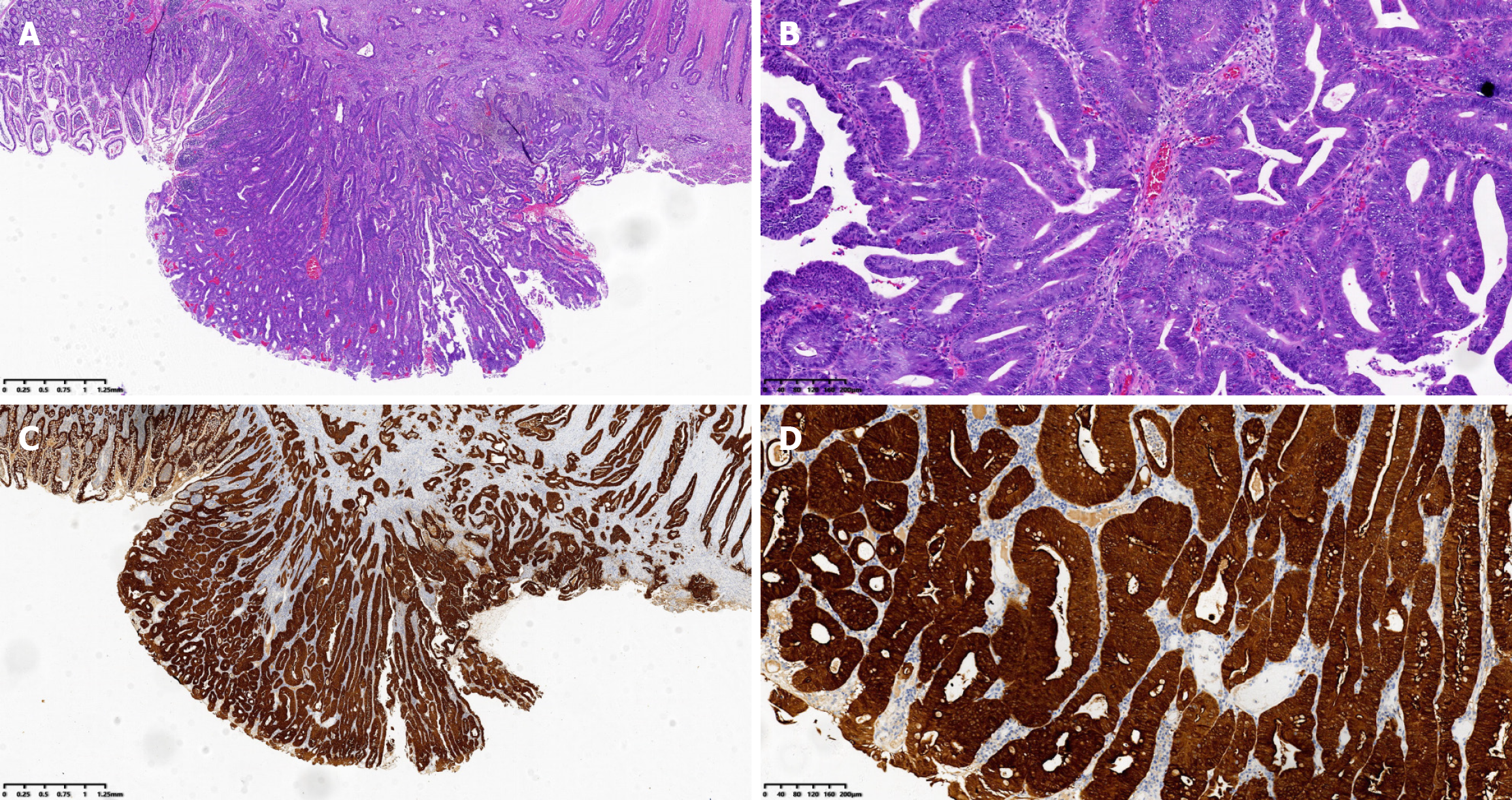
An ulcerative adenocarcinoma of the ileum measuring approximately 2.0 cm × 1.8 cm × 0.7 cm, that had invaded the full thickness of the ileal wall was confirmed by histopathology. The tumour cells manifested as moderately differentiated carcinoma. Tumour thrombus was found in the blood vessel, but perineural invasion was not observed. Furthermore, 12 lymph nodes were resected, and metastases were not discovered in any of them (Figure 2A and B). The immunohistochemical results indicated significant positivity for cytokeratin (Figure 2C and D). The patient was finally diagnosed with Stage IIA ulcerative IA (T3N0M0).
Owing to the lack of data regarding SBA, including in the patient’s history and in the physical examination, the normal CEA and CA19-9 values, vague results of abdominal CT, and limited digestive tract radiography, he was misdiagnosed with inflammatory bowel disease (IBD). A series of conservative treatments, including pharmacologic therapies and nasogastric tube decompression, were applied accordingly. However, the symptoms were not alleviated, so exploratory laparotomy was performed.
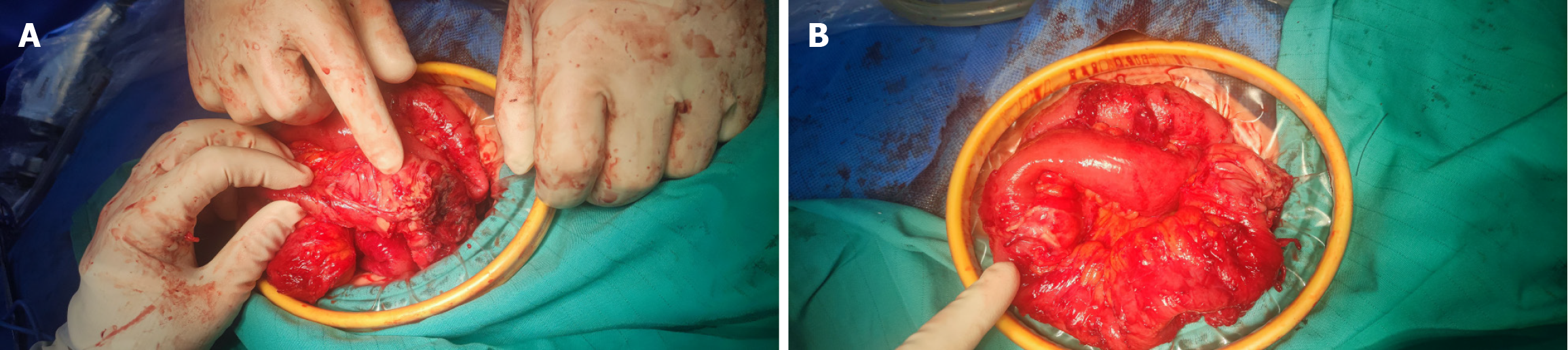
Laparotomy revealed constriction in the terminal ileum. Adhesions across the entire abdomen and approximately 100 mL of overt effusion were observed around the constriction. The small intestine was dilated on the proximal side of the constriction. A mass measuring 2.0 cm × 2.0 cm was located 20 cm from the ileocecal junction. No metastatic foci were found within the liver or peritoneal region (Figure 3). The constriction was resected en bloc, and end-to-end intestinal anastomosis was carried out. Approximately 1500 mL of physiological saline solution was injected to flush the abdominal cavity.
The operation was completed successfully, and the postoperative recovery of the patient was excellent. After two months of follow-up, a CT scan revealed that there was no reappearance of the disease (Figure 1D-F). Currently, his condition is stable and will be followed up further.
Although the small bowel accounts for 90% of the mucosal area in the gastrointestinal tract, small intestine cancers are relatively uncommon and comprise only approximately 3% of all cancers[9]. This rarity may be caused by the rapid turnover of small bowel cells or the brief transit period of the small bowel[10,11]. SBA comprises 30%-40% of all histological subtypes[3]. In most cases, the SBA is located in the duodenum (52%), much more than the jejunum (29%). IA is extremely rare and accounts for only 19% of cases[6]. There are no differences in altered genes between duodenal adenocarcinoma and IA[12]. The interactions of pancreaticobiliary secretions with carcinogens in the duodenum may explain these differences[13]. Risk factors for IA include smoking, alcohol consumption and IBD, especially Crohn’s disease, which has appeared to be the greatest risk factor in recent years[14]. Therefore, when a patient with chronic IBD has a change in clinical symptoms, a diagnosis of IA should be considered. However, these risk factors were not present in our patient. The clinical manifestations of IA are often nonspecific, and accurate differentiation from other diseases, such as appendicitis, pancreatitis or cholelithiasis, is a major challenge.
The incidence rate of SBA varies across geographic areas. It is more prevalent in Western Europe and North America but relatively less prevalent in Asian countries. On the basis of available reports, the incidence of SBA are more often among men. In one study, patients with a median age of 67 years were found to be more likely to be affected[1], whereas in another study, patients with a median age of 58 years were affected[6]. Our patient was 48 years old, which was younger than the previously reported trends. It is challenging to diagnose SBA accurately on the basis of only clinical presentations. In one study involving 241 patients with a diagnosis of SBA, that the presenting symptoms were shown to vary: 37.3% of patients presented with abdominal pain, 32.8% with weight reduction, 16.2% with jaundice, 14.6% with gastrointestinal bleeding, and 13.1% with no symptoms[15]. However, most patients with SBA occur with an urgent situation, commonly with cramping abdominal pain, ileus in the ileum or at the gastric outlet, and biliary obstruction in the duodenum[16]. Our patient had similar symptoms. With respect to this disease, both CEA and CA19-9 can assist in the diagnosis of SBA, with elevated levels of approximately 30% and 40%, respectively, in patients[17]. In our patient, the CEA and CA19-9 levels were both normal. Despite multiple efforts, there are no sensitive or specific tumour biomarkers for the identification of this disease. These biomarkers are usually employed for monitoring disease status.
Conventional CT or magnetic resonance (MR) imaging can be used to assess the degree of local tumour invasion or distant metastases at the initial workup. However, new imaging technologies, such as CT or MR enterography or MR enteroclysis (MRE), can improve the imaging effect of small intestine. An anticipatory investigation was conducted to identify the effect of CT and MR enterography in 150 patients suffering from small intestine disease. The results revealed that MR enterography had markedly higher sensitivity than CT enterography did, especially for neoplastic disease (P = 0.0412)[18]. In one study, two cases of Crohn’s disease-related IA were preoperatively diagnosed by MRE[19]. In a retrospective study involving 158 patients who received MRE and histological examination (HE) of surgical specimens, the consistency between MRE and HE was 100% for location and 62.2% for histological diagnosis[20]. On the basis of these data, MRE has an obvious impact on the localization of small bowel tumours and their staging, particularly if surgical planning is considered. Our patient underwent digestive tract radiography. The results revealed that there was a small bowel obstruction. Oesophago gastroduodenoscopy with endoscopic ultrasound is recommended for the detection and pathologic diagnosis of neoplasms in the proximal part of the duodenum and distal part of the ileum[21]. Other endoscopic methods, such as double-balloon endoscopy and capsule endoscopy, are recommended under certain conditions. The application of double-balloon endoscopy is especially beneficial for patients with small bowel constriction[22]. Even though capsule endoscopy is not recommended for initial workup, it is a preferred modality to detect the whole mucosa of the small intestine and reveal a primary lesion. However, it is contraindicated for situations involving small intestine obstruction or constriction, which may cause difficulty in capsule excretion[23].
SBA staging is based on American Joint Committee on Cancer Staging Manual, 8th edition. T1 neoplasms invade the submucosa or lamina; T2 neoplasms penetrate the submucosa to the muscularis propria; T3 neoplasms infiltrate the subserosa or invade through nonperitonealized perimuscular tissue; and T4 neoplasms penetrate the splanchnic peritoneum or infiltrate other organs. Lack of spread to regional lymph nodes is defined as N0; spread to 1-2 lymph nodes is defined as N1, and spread to 3 or more lymph nodes are referred to as N2. With respect to metastasis, SBA is considered M1. Stage I is defined as T1, T2, N0, M0; stage II is defined as T3, T4, N0, M0; stage III is defined as any T, N1, N2, M0; and stage IV is defined as any T, any N, M1. The IA in our patient was classified as stage IIA (T3N0M0)[24].
Surgical excision accompanied by resection of regional lymph nodes represents a main therapy for local SBA. The OS times of the patients with resected and unresected tumours are 94.4 and 30.1 months, respectively[25]. For patients with nonmetastatic disease, oncologic surgery results in the best outcomes, and negative margins predict longer survival[26]. The type of resection is contingent on the position of the primary neoplasm. In general, segmental resection is the standard surgical therapy[27]. Pancreaticoduodenectomy or segmental duodenal removal is needed for tumour located in the duodenum[28]. In cases of neoplasms in the ileum or jejunum, segmental resection along with lymph node removal and ileoileal or jejunojejunal anastomosis is the preferred approach[29]. The recommendation in multiple studies is to retrieve more than 9 regional lymph nodes during the process of SBA excision[30,31].
For unresectable and distant metastatic SBA, there is no truly effective standard adjuvant therapy. On the basis of limited studies, 5-fluorouracil plus platinum is referred to the first-line therapy for SBA. Irinotecan plus 5-fluorouracil is the most common second-line therapy[32]. Recently, programmed death-ligand 1-positive cell-targeting antibody drugs have been studied for the treatment of gastrointestinal malignancies. Several studies have reported the effectiveness of pembrolizumab in treating colorectal cancer (CRC) adenocarcinoma[33,34]. In another study, nivolumab, along with ipilimumab, thus inhibition of programmed death-1 and cytotoxic T-lymphocyte-associated protein 4, was reported to have clinical significance in terms of improvements with respect to metastatic CRC[35]. According to these positive results, the National Comprehensive Cancer Network guidelines suggest nivolumab or pembrolizumab, along with or without ipilimumab, as second-line therapies of SBA[36].
SBA patients have a dismal prognosis, with the 5-year OS rate varying from 15% to 35%[37-39]. The results of the multivariate analysis suggested that age, tumour stage, tumour location and lymph node invasion were significant prognostic factors for SBA. The prognosis among males aged over 55 years was predicted to be poor[40]. Additionally, the 5-year OS rates ranged from 50%-60% in patients of stage I, 30%-55% in patients of stage II, 10%-40% in patients of stage III and 3%-5% in patients of stage IV[41-43]. Compared with primary duodenal and ileal tumours, a jejunal tumour has a better prognosis[44]. Patients with fewer involved lymph nodes have longer survival following resection[45].
For our patient, IA led to complete small bowel obstruction and typical gastrointestinal symptoms. Symptomatic treatment was not provided until exploratory laparotomy was performed. A case of adenocarcinoma of the ileum was subsequently discovered unexpectedly. Here, we emphasize the importance of timely detection of tumours, which may strongly affect early staging and prognosis. If the symptoms and CT images are indefinite, surgery should be considered. Here, our aim was to describe our experience of diagnosis and treatment of IA, and we look forward to developing precision therapy for SBA soon.
Adenocarcinoma of the ileum poses diagnostic challenges because of vague clinical presentations. Imaging can be used only for auxiliary diagnosis. Although IA is insidious, attention should be provided to patients experiencing symptoms of small bowel obstruction. Laparotomy is recommended for the diagnosis and treatment of SBA in the initial stage. Currently, an accurate diagnosis is still lacking, and the long-term survival rate remains low (see Video 1). Consequently, new investigative strategies and treatments are needed for further research to achieve better outcomes.
| 1. | Raghav K, Overman MJ. Small bowel adenocarcinomas--existing evidence and evolving paradigms. Nat Rev Clin Oncol. 2013;10:534-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 126] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 2. | Vlachou E, Koffas A, Toumpanakis C, Keuchel M. Updates in the diagnosis and management of small-bowel tumors. Best Pract Res Clin Gastroenterol. 2023;64-65:101860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 3. | Pedersen KS, Raghav K, Overman MJ. Small Bowel Adenocarcinoma: Etiology, Presentation, and Molecular Alterations. J Natl Compr Canc Netw. 2019;17:1135-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 4. | Beltran MA, Cruces KS. Primary tumors of jejunum and ileum as a cause of intestinal obstruction: a case control study. Int J Surg. 2007;5:183-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Yu IS, Al-Hashami Z, Chapani P, Speers C, Davies JM, Lim HJ, Renouf DJ, Gill S, Stuart HC, Loree JM. Impact of Tumor Location on Patient Outcomes in Small Bowel Cancers. Clin Colorectal Cancer. 2022;21:107-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Colina A, Hwang H, Wang H, Katz MHG, Sun R, Lee JE, Thomas J, Tzeng CW, Wolff RA, Raghav K, Overman MJ. Natural history and prognostic factors for localised small bowel adenocarcinoma. ESMO Open. 2020;5:e000960. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 7. | Solem CA, Harmsen WS, Zinsmeister AR, Loftus EV Jr. Small intestinal adenocarcinoma in Crohn's disease: a case-control study. Inflamm Bowel Dis. 2004;10:32-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 74] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Shenoy S. Primary small-bowel malignancy: update in tumor biology, markers, and management strategies. J Gastrointest Cancer. 2014;45:421-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | de Latour RA, Kilaru SM, Gross SA. Management of small bowel polyps: A literature review. Best Pract Res Clin Gastroenterol. 2017;31:401-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Choi J, Rakhilin N, Gadamsetty P, Joe DJ, Tabrizian T, Lipkin SM, Huffman DM, Shen X, Nishimura N. Intestinal crypts recover rapidly from focal damage with coordinated motion of stem cells that is impaired by aging. Sci Rep. 2018;8:10989. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Albrecht H, Vetter M, Dauth W, Zoicas F, Neurath MF, Hagel AF. The impact of hospitalization on the performance of capsule endoscopy (CE). Dig Liver Dis. 2017;49:647-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Schrock AB, Devoe CE, McWilliams R, Sun J, Aparicio T, Stephens PJ, Ross JS, Wilson R, Miller VA, Ali SM, Overman MJ. Genomic Profiling of Small-Bowel Adenocarcinoma. JAMA Oncol. 2017;3:1546-1553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 165] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 13. | Lowenfels AB. Does bile promote extra-colonic cancer? Lancet. 1978;2:239-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Fields AC, Hu FY, Lu P, Irani J, Bleday R, Goldberg JE, Melnitchouk N. Small Bowel Adenocarcinoma: Is There a Difference in Survival for Crohn's Versus Sporadic Cases? J Crohns Colitis. 2020;14:303-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 15. | Zhang S, Zheng C, Chen Y, Xu Q, Ma J, Yuan W, Jiang Q, Zhao Y, Zhang J, Che X, Wang C, Huang X, Chen F, Wang N, Ma X, Lan Z. Clinicopathologic features, surgical treatments, and outcomes of small bowel tumors: A retrospective study in China. Int J Surg. 2017;43:145-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Sakae H, Kanzaki H, Nasu J, Akimoto Y, Matsueda K, Yoshioka M, Nakagawa M, Hori S, Inoue M, Inaba T, Imagawa A, Takatani M, Takenaka R, Suzuki S, Fujiwara T, Okada H. The characteristics and outcomes of small bowel adenocarcinoma: a multicentre retrospective observational study. Br J Cancer. 2017;117:1607-1613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 72] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 17. | Zaanan A, Costes L, Gauthier M, Malka D, Locher C, Mitry E, Tougeron D, Lecomte T, Gornet JM, Sobhani I, Moulin V, Afchain P, Taïeb J, Bonnetain F, Aparicio T. Chemotherapy of advanced small-bowel adenocarcinoma: a multicenter AGEO study. Ann Oncol. 2010;21:1786-1793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 102] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 18. | Masselli G, Di Tola M, Casciani E, Polettini E, Laghi F, Monti R, Bernieri MG, Gualdi G. Diagnosis of Small-Bowel Diseases: Prospective Comparison of Multi-Detector Row CT Enterography with MR Enterography. Radiology. 2016;279:420-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 19. | Placé V, Hristova L, Dray X, Lavergne-Slove A, Boudiaf M, Soyer P. Ileal adenocarcinoma in Crohn's disease: magnetic resonance enterography features. Clin Imaging. 2012;36:24-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Pappalardo G, Gualdi G, Nunziale A, Masselli G, Floriani I, Casciani E. Impact of magnetic resonance in the preoperative staging and the surgical planning for treating small bowel neoplasms. Surg Today. 2013;43:613-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | . Status evaluation: enteroscopy. Gastrointest Endosc. 1991;37:673-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Baars JE, Theyventhiran R, Aepli P, Saxena P, Kaffes AJ. Double-balloon enteroscopy-assisted dilatation avoids surgery for small bowel strictures: A systematic review. World J Gastroenterol. 2017;23:8073-8081. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Pennazio M, Rondonotti E, Despott EJ, Dray X, Keuchel M, Moreels T, Sanders DS, Spada C, Carretero C, Cortegoso Valdivia P, Elli L, Fuccio L, Gonzalez Suarez B, Koulaouzidis A, Kunovsky L, McNamara D, Neumann H, Perez-Cuadrado-Martinez E, Perez-Cuadrado-Robles E, Piccirelli S, Rosa B, Saurin JC, Sidhu R, Tacheci I, Vlachou E, Triantafyllou K. Small-bowel capsule endoscopy and device-assisted enteroscopy for diagnosis and treatment of small-bowel disorders: European Society of Gastrointestinal Endoscopy (ESGE) Guideline - Update 2022. Endoscopy. 2023;55:58-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 146] [Article Influence: 73.0] [Reference Citation Analysis (0)] |
| 24. | Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin. 2017;67:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2341] [Cited by in RCA: 4392] [Article Influence: 549.0] [Reference Citation Analysis (4)] |
| 25. | Alfagih A, Alrehaili M, Asmis T. Small Bowel Adenocarcinoma: 10-Year Experience in a Cancer Center-The Ottawa Hospital (TOH). Curr Oncol. 2022;29:7439-7449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Dabaja BS, Suki D, Pro B, Bonnen M, Ajani J. Adenocarcinoma of the small bowel: presentation, prognostic factors, and outcome of 217 patients. Cancer. 2004;101:518-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 349] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 27. | Agrawal S, McCarron EC, Gibbs JF, Nava HR, Wilding GE, Rajput A. Surgical management and outcome in primary adenocarcinoma of the small bowel. Ann Surg Oncol. 2007;14:2263-2269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 28. | De Pastena M, Zingaretti CC, Paiella S, Guerriero M, De Santis N, Luchini C, Bassi C, Malleo G, Salvia R. Impact of extra-ampullary duodenal adenocarcinoma subtypes on surgical and oncological outcomes following pancreaticoduodenectomy. Updates Surg. 2024;76:87-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 29. | Han SL, Cheng J, Zhou HZ, Guo SC, Jia ZR, Wang PF. Surgically treated primary malignant tumor of small bowel: a clinical analysis. World J Gastroenterol. 2010;16:1527-1532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 42] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (2)] |
| 30. | Tran TB, Qadan M, Dua MM, Norton JA, Poultsides GA, Visser BC. Prognostic relevance of lymph node ratio and total lymph node count for small bowel adenocarcinoma. Surgery. 2015;158:486-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 31. | Wilhelm A, Müller SA, Steffen T, Schmied BM, Beutner U, Warschkow R. Patients with Adenocarcinoma of the Small Intestine with 9 or More Regional Lymph Nodes Retrieved Have a Higher Rate of Positive Lymph Nodes and Improved Survival. J Gastrointest Surg. 2016;20:401-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 32. | Bhamidipati D, Colina A, Hwang H, Wang H, Katz M, Fournier K, Serpas V, Thomas J, Sun R, Wolff RA, Raghav K, Overman MJ. Metastatic small bowel adenocarcinoma: role of metastasectomy and systemic chemotherapy. ESMO Open. 2021;6:100132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 33. | Kim DW, Tan E, Zhou JM, Schell MJ, Martinez M, Yu J, Carballido E, Mehta R, Strosberg J, Imanirad I, Kim RD. A phase 1/2 trial of ibrutinib in combination with pembrolizumab in patients with mismatch repair proficient metastatic colorectal cancer. Br J Cancer. 2021;124:1803-1808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 34. | Wu X, Ye Y, Vega KJ, Yao J. Consensus Molecular Subtypes Efficiently Classify Gastric Adenocarcinomas and Predict the Response to Anti-PD-1 Immunotherapy. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 35. | Lenz HJ, Van Cutsem E, Luisa Limon M, Wong KYM, Hendlisz A, Aglietta M, García-Alfonso P, Neyns B, Luppi G, Cardin DB, Dragovich T, Shah U, Abdullaev S, Gricar J, Ledeine JM, Overman MJ, Lonardi S. First-Line Nivolumab Plus Low-Dose Ipilimumab for Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer: The Phase II CheckMate 142 Study. J Clin Oncol. 2022;40:161-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 412] [Article Influence: 137.3] [Reference Citation Analysis (0)] |
| 36. | Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen YJ, Ciombor KK, Cohen SA, Cooper HS, Deming DA, Garrido-Laguna I, Grem JL, Hoffe SE, Hubbard J, Hunt S, Kamel A, Kirilcuk N, Krishnamurthi S, Messersmith WA, Meyerhardt J, Miller ED, Mulcahy MF, Nurkin S, Overman MJ, Parikh A, Patel H, Pedersen KS, Saltz LB, Schneider C, Shibata D, Skibber JM, Sofocleous CT, Stoffel EM, Stotsky-Himelfarb E, Willett CG, Johnson-Chilla A, Gregory KM, Gurski LA. Small Bowel Adenocarcinoma, Version 1.2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2019;17:1109-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 113] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 37. | Teufel A, Meindl-Beinker NM, Hösel P, Gerken M, Roig A, Ebert MP, Herr W, Scheiter A, Pauer A, Schlitt HJ, Klinkhammer-Schalke M. Characteristics and outcome of patients with small bowel adenocarcinoma (SBA). J Cancer Res Clin Oncol. 2023;149:4579-4590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 38. | Makino S, Takahashi H, Haraguchi N, Nishimura J, Hata T, Matsuda C, Ikenaga M, Murata K, Yamamoto H, Doki Y, Mori M, Mizushima T. A Single Institutional Analysis of Systemic Therapy for Unresectable or Recurrent Small Bowel Adenocarcinoma. Anticancer Res. 2017;37:1495-1500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 39. | Eigenbrod T, Kullmann F, Klebl F. Resection of small bowel adenocarcinoma liver metastasis combined with neoadjuvant and adjuvant chemotherapy results in extended disease-free period--a case report. Int J Gastrointest Cancer. 2006;37:94-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 40. | Xu D, He Y, Liao C, Tan J. Development and validation of a nomogram for predicting cancer-specific survival in small-bowel adenocarcinoma patients using the SEER database. World J Surg Oncol. 2024;22:151. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 41. | Nicholl MB, Ahuja V, Conway WC, Vu VD, Sim MS, Singh G. Small bowel adenocarcinoma: understaged and undertreated? Ann Surg Oncol. 2010;17:2728-2732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (1)] |
| 42. | Tian J, Liu J, Guo C, Yang X, Yang Y, Gou H, Qiu M, Cao D. Prognostic factors and treatment outcomes in patients with non-ampullary small bowel adenocarcinoma: Long-term analysis. Medicine (Baltimore). 2019;98:e15381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 43. | Aparicio T, Svrcek M, Zaanan A, Beohou E, Laforest A, Afchain P, Mitry E, Taieb J, Di Fiore F, Gornet JM, Thirot-Bidault A, Sobhani I, Malka D, Lecomte T, Locher C, Bonnetain F, Laurent-Puig P. Small bowel adenocarcinoma phenotyping, a clinicobiological prognostic study. Br J Cancer. 2013;109:3057-3066. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 44. | Akce M, Jiang R, Zakka K, Wu C, Alese OB, Shaib WL, Behera M, El-Rayes BF. Clinical Outcomes of Small Bowel Adenocarcinoma. Clin Colorectal Cancer. 2019;18:257-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 45. | Overman MJ, Hu CY, Wolff RA, Chang GJ. Prognostic value of lymph node evaluation in small bowel adenocarcinoma: analysis of the surveillance, epidemiology, and end results database. Cancer. 2010;116:5374-5382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |