Published online Mar 15, 2025. doi: 10.4251/wjgo.v17.i3.102083
Revised: December 16, 2024
Accepted: January 8, 2025
Published online: March 15, 2025
Processing time: 129 Days and 7.5 Hours
IL-22 plays a pivotal role in the processes of inflammation and tissue healing., but its role in cholangiocarcinoma (CCA) remains unclear. our study explored the IL-22/IL-22R1 pathway and its impact on CCA progression through the ERK1/2 signaling cascade.
To determine the mechanism of the IL-22/IL-22R1 pathway in CCA and provide new directions for its clinical treatment.
IL-22R1 expression was assessed in human and rat CCA tissues utilizing immunohistochemical techniques, Western blot analysis, and quantitative reverse transcription PCR. The impact of IL-22 on CCA cells was assessed in vitro via tests for proliferation, migration, invasion, and apoptosis assays. The rat models of thioacetamide-induced CCA and subcutaneous xenografts in nude mice were used to assess the in vivo effects. ERK1/2 inhibitors were applied to elucidate the mechanistic role of the pathway.
IL-22R1 was overexpressed in CCA cell lines and tissues. IL-22 treatment increased the phosphorylation of ERK1/2, promoting tumor cell proliferation, migration, invasion, and resistance to apoptosis. ERK1/2 inhibition considerably reversed these effects both in vitro and in vivo.
The IL-22/IL-22R1 axis promotes CCA progression by activating ERK1/2 signaling. Targeting this pathway with ERK1/2 inhibitors offers potential therapeutic strategies for CCA.
Core Tip: This research results show that IL-22R1 is highly expressed in cholangiocarcinoma (CCA) cell lines and tissues. The binding of IL-22 to IL-22R1 forms the IL-22/IL-22R1 axis, which can promote the progression of CCA both in vivo and in vitro. The application of ERK1/2 inhibitors can reverse the promoting effect of IL-22 on CCA, providing potential therapeutic strategies for CCA’s clinical treatment.
- Citation: Zhou J, Chen JR, Li JM, Han SQ, Deng XY, Li ZM, Tong W, Wang C, Bai Y, Zhang YM. IL-22/IL-22R1 pathway enhances cholangiocarcinoma progression via ERK1/2 activation. World J Gastrointest Oncol 2025; 17(3): 102083
- URL: https://www.wjgnet.com/1948-5204/full/v17/i3/102083.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i3.102083
Cholangiocarcinoma (CCA), the second most common type of liver cancer[1], is a type of malignant neoplasm that arises from the epithelial cells of the bile ducts. Despite the advances in diagnostic procedures and treatments, CCA is asso
Altogether, 27 CCA sufferers who underwent radical resection for CCA at the Hepatobiliary Surgery Department of Tianjin First Central Hospital from September 2021 to June 2024 were enrolled in this study. Cancer tissues were collected for immunohistochemical staining. Patient information, including sex, age, TNM staging, and tumor location, was also collected. No patients had undergone any anti-cancer therapy prior to surgery. The research was sanctioned by the Ethics Committee of Tianjin First Central Hospital. Comprehensive clinical data of the patients can be found in Supplementary Table 1.
The human intrahepatic bile duct epithelial cells (HIBEpiCs) as well as human CCA cell lines HuCCT1 and RBE were sourced from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China), The cells were maintained in RPMI-1640 medium (Gibco, Jiangsu, China), enriched with 10% fetal bovine serum (Gibco, Jiangsu, China) and 1% penicillin/streptomycin (Beyotime, Shanghai, China). Cultivation of all cell lines took place in an incubator (37 °C, 5% CO2). Thioacetamide (TAA) was purchased from Aike Reagent (Chengdu, China). SCH772984 was purchased from Selleck (Houston, United States). Details about the antibodies can be found in Supplementary Table 2.
The RNA from negative control (NC; n = 3) and IL-22 (n = 3) RBE CCA cells were extracted using the Trizol and Mjzol Animal RNA Kit (Majorivd), following the manufacturer’s protocols. Library preparations were done with the VAHTS Universal V6 RNA-seq Kit. The library quality was evaluated using an Agilent 4200 bioanalyzer before Illumina novaseq6000 sequencing. Seqtk-filtered raw reads were mapped to the genome with Hisat2 (v2.0.4), and gene expression was quantified with Stringtie (v1.3.3b), normalized with TMM. DEGs were identified using edgeR with FDR (Q < 0.05) and fold-change of > 2.
The 50 male SD rats required for this experiment (aged 6-8 weeks, weighing 250-300 g) were obtained from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). They were assigned at random to one of the following three groups: NC group (n = 10), experimental group A (n = 20), and experimental group B (n = 20). The rats were placed in an animal room with a constant indoor temperature (about 25 °C), humidity (60%), and a 12-hour light/dark cycle was implemented (light period from 08: 00 to 20: 00), allowing them unrestricted access to water and food. The animals are provided with humane care in accordance with the National Institutes of Health's Guidelines for the Care and Use of Laboratory Animals. The above experiments were approved by the Ethics Committee of the First Central Hospital of Tianjin. Both experimental groups A and B were given drinking water with a concentration of 300 mg TAA/L until the 18th week. Starting from the 19th week, both groups were switched from TAA water to regular drinking water. Experimental group A was given intraperitoneal injections of recombinant rat IL-22 (50 mg/kg) daily for 2 weeks (Sup
Cell counting kit-8 (CCK-8) proliferation assay was performed by seeding the treated cells into a 96-well plate. The CCK-8 reagent was introduced into each well at 0, 24, 48, and 72 hours, after which a 2-hour incubation in darkness was conducted to record the absorbance. the colony formation assay, 1000 treated cells were seeded into each well of a 6-well plate and cultured for 14 days, after which the cells were fixed and stained to count the number of colonies in each well.
For the assessment of cell proliferation capacity, we used the EdU (5-ethynyl-2'-deoxyuridine) detection kit (E-CK-A376, Elabscience, Wuhan, China). Cells were co-cultured with the EdU reagent, finally, EdU-positive cells were counted.
The Transwell migration assay, the counted cells were mixed with serum-free culture medium and added to the upper chamber, while the lower chamber was supplemented with complete culture medium to serve as a chemoattractant. Following a 24-hour incubation period, the insert was taken out, and the cells were fixed and stained. Non-migrated cells were carefully wiped away using a cotton swab. Imaging and counting were performed under an inverted microscope (× 20). The invasion assay, an extracellular matrix barrier was simulation by adding Matrigel to the upper chamber, thereby assessing the invasive capability of tumor cells. The scratch wound healing assay, the treated cells were grown in a 6-well plate until they achieved complete confluence. A vertical scratch was made on the cells, and after washing to remove floating cells, serum-free culture medium was added for further incubation. Images were taken at 0, 24, and 48 hours using an inverted microscope (× 10; Nikon, Japan). The migration rate: (0 hour scratch width and 48 hours scratch width)/0 hour scratch width × 100%.
The Annexin V-FITC/PI Apoptosis Detection Kit (Elabscience, Wuhan, China) was utilized for the assessment of apoptosis. Cells were digested and collected using trypsin that is free of 0.25% EDTA (Meilunbio, Dalian, China). After rinsing with phosphate buffered saline (PBS) and centrifugation, the cells were resuspended in 500 μL of binding buffer. Subsequently, FITC-Annexin V and PI working solution were added sequentially to the cell suspension. After incubating in the dark at room temperature, flow cytometry (BD Accuri C6 Plus, BD Biosciences, United States) was employed to detect apoptosis. Data analysis and processing were conducted using FlowJo V10.0 software.
The cell cycle detection kit (Elabscience, Wuhan, China) was utilized for cell cycle analysis. The processed cells were resuspended in 0.3 mL of PBS, followed by the gradual addition of 1.2 mL of -20 °C anhydrous ethanol. After mixing thoroughly, the sample was stored at -20 °C for 1 hour. Next, RNase A Reagent was added in a volume of 100 µL, and the mixture was incubated in a 37 °C incubator for 30 minutes. Then, 400 µL of PI reagent (50 µg/mL) was added and mixed well. The sample was incubated in the dark at 2 °C-8 °C for 30 minutes before immediate detection using the BD Accuri C6 Plus machine (BD Biosciences, United States), and the red fluorescence at a 488 nm excitation wavelength was recorded.
The collected tumor tissue samples were placed in a fixative solution (10% neutral formalin) for 48 hours, then dehydrated and embedded in paraffin. The paraffin-embedded tissues were cut into 4 μm sections by a microtome and then stained using HE. The human tissue specimens were procured from patients who underwent CCA resection at Tianjin First Central Hospital. The animal tissues were sourced from SD rats modeled with TAA. The experiments were approved by the ethics committee. Finally, the slices were evaluated by pathologists under a microscope (Nikon, Japan).
Deparaffinization and rehydration were performed on the tissue sections. The sections were placed in an EDTA solution for antigen retrieval. The activity of endogenous peroxidase was blocked by treating with a peroxidase-blocking agent at ambient temperature. Followed by blocking with 5% bovine serum albumin. Following incubation with the primary and secondary antibodies, the sections were treated with diaminobenzidine for staining, after which they were counterstained using hematoxylin. Finally, two pathologists independently examined and quantified the staining results. The antibody information is presented in Supplementary Table 2.
The RNA was isolated using the Trizol reagent (Vazyme Biotech, Nanjing, China). Reverse transcription was carried out with the ExScript RT-PCR kit (TaKaRa, Japan) as the manufacturer's instructions. The mRNA levels were quantified using SYBR Green Master Mix (Vazyme Biotech), each reaction was performed in quadruple, and the β-actin expression method was used for data normalization calibration. The relative expression levels of the target genes were calculation using the 2-ΔΔCt analysis approach. The primer sequences for PCR amplification are shown in Supplementary Table 3.
Proteins were extracted from the cells and tissues, and the protein concentration was measured. After separation by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (Bausde, Wuhan, China), The proteins were blotted onto a polyvinylidene fluoride membrane. The membrane was then sealed with 5% non-fat milk and incubated at room temperature for 90 minutes. Subsequent incubations with primary and secondary antibodies were conducted. Finally, the proteins were visualized using a chemiluminescence detection reagent in the dark. The protein band gray values analyzed utilizing ImageJ software.
The fluorescein TUNEL apoptosis assay kit (Servicebio, Wuhan, China) was employed to assess the apoptosis levels in tissues and cells. Cell climbing slices or tissue sections were prepared following the protocols provided by the manufacturer and treated with protease K, followed by staining with TUNEL-FITC (1:200). Using an anti-fluorescence quenching mounting medium containing DAPI to mount the slices, and images were captured using a fluorescence microscope (Nikon, Japan).
Blood samples were collected from SD rats at different stages in each group. After centrifugation, the serum was collected for biochemical analysis. The levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), and total bilirubin (T-Bil) were measured using an automatic animal biochemical analyzer (BS-240 VET, Mindray, Shenzhen). The ALT, AST, ALP, and T-Bil levels were then compared between the different groups.
Male BALB/C nude mice aged 4-5 weeks were provided by SPF (Beijing) Biotechnology Co., Ltd. and were randomly allocated into three groups, each group consisting of five mice. Each nude mouse was administered a subcutaneous injection of 6 × 106 RBE cells in the right flank. When the tumor diameter exceeded 100 mm3, the mice in the IL-22 group were administered a tail vein injection of 1 μg/100 μL recombinant human IL-22 protein (rIL-22) solution for 14 consecutive days. The total dosage of rIL-22 for each mouse during the experiment was 14 μg. The mice in the IL-22 + ERKi group received rIL-22 injections followed by intraperitoneal injections of SCH772984 (12.5 mg/kg) twice daily for 14 days. The mice in the control group received saline via tail vein injection for 14 consecutive days. After the experiment, the mice were euthanized, following which the tumors were excised, documented photographically, and their volume and weight were measured. The equation for determine tumor volume was as follows: Tumor volume = (length × width2)/2. The animal experiments were authorized by the Ethics Committee of Tianjin First Central Hospital.
Statistical analyses were conducted with GraphPad Prism 9.5 software (GraphPad Software, United States). For comparison between two groups, an unpaired t-test was used. For comparison among three or more groups, one-way analysis of variance (ANOVA) was applied. Statistical significance was defined as a P value < 0.05. Significance levels were indicated by asterisks as follows: aP < 0.05, bP < 0.01, cP < 0.001, dP < 0.0001.
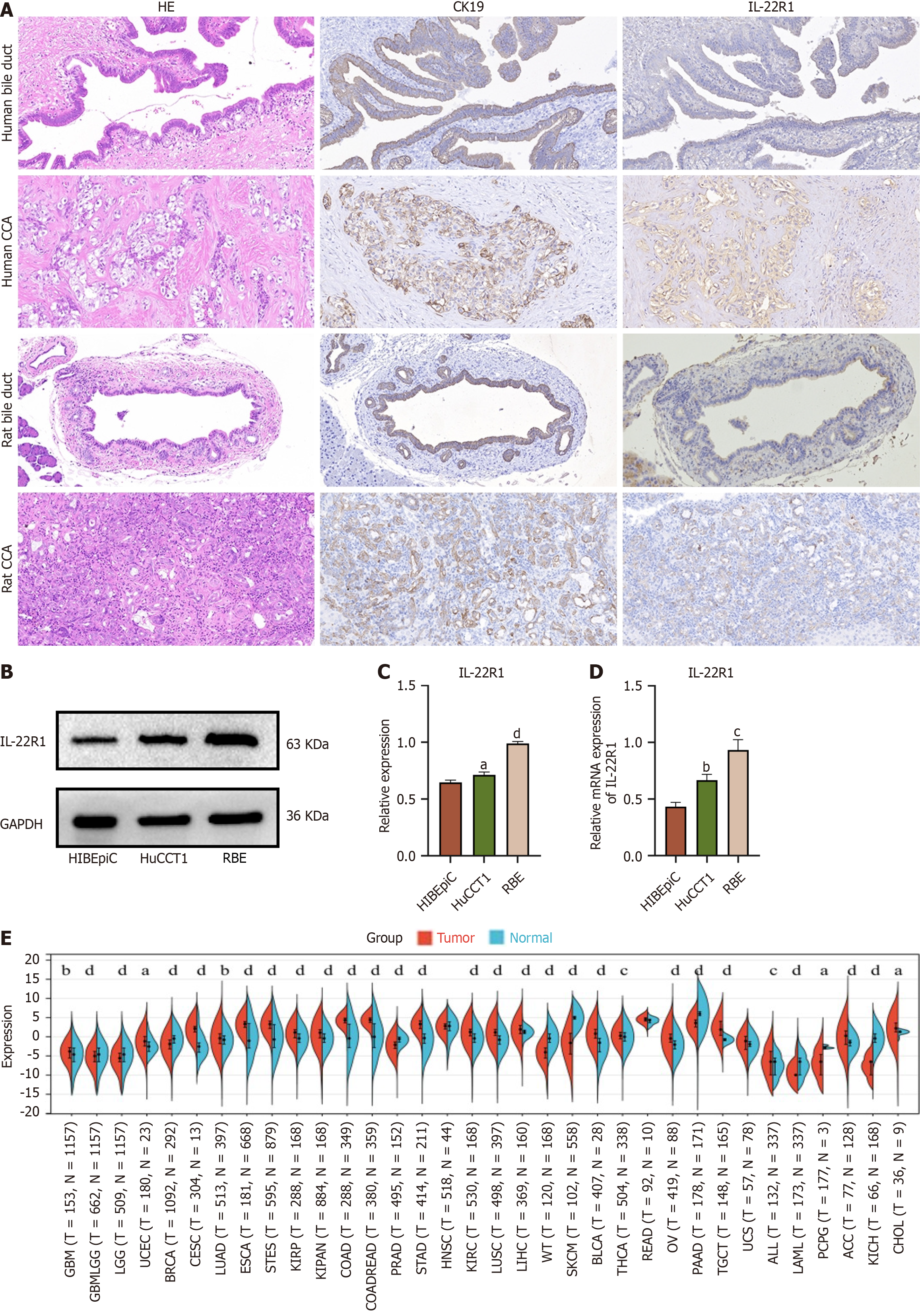
We collected human and rat CCA tissues (TAA) for immunohistochemistry, using CK-19 as a positive control. IL-22R1 was expressed in CCA tissues of humans and rats as shown in Figure 1A. Extract proteins and RNA from RBE, HuCCT1, and HIBEpiCs cells for Western blot analysis and qRT–PCR analysis. The expression level of IL-22R1 in RBE and HuCCT1 cells is higher than that in HIBEpiCs cells (Figure 1B-D). Using the UCSC database (https://xenabrowser.net/), we downloaded standardized pan-cancer datasets to analyze the IL-22R1 expressions across different cancer samples. We found that the IL-22R1 expression was elevated in CCA tissues compared to the adjacent tissues (Figure 1E).
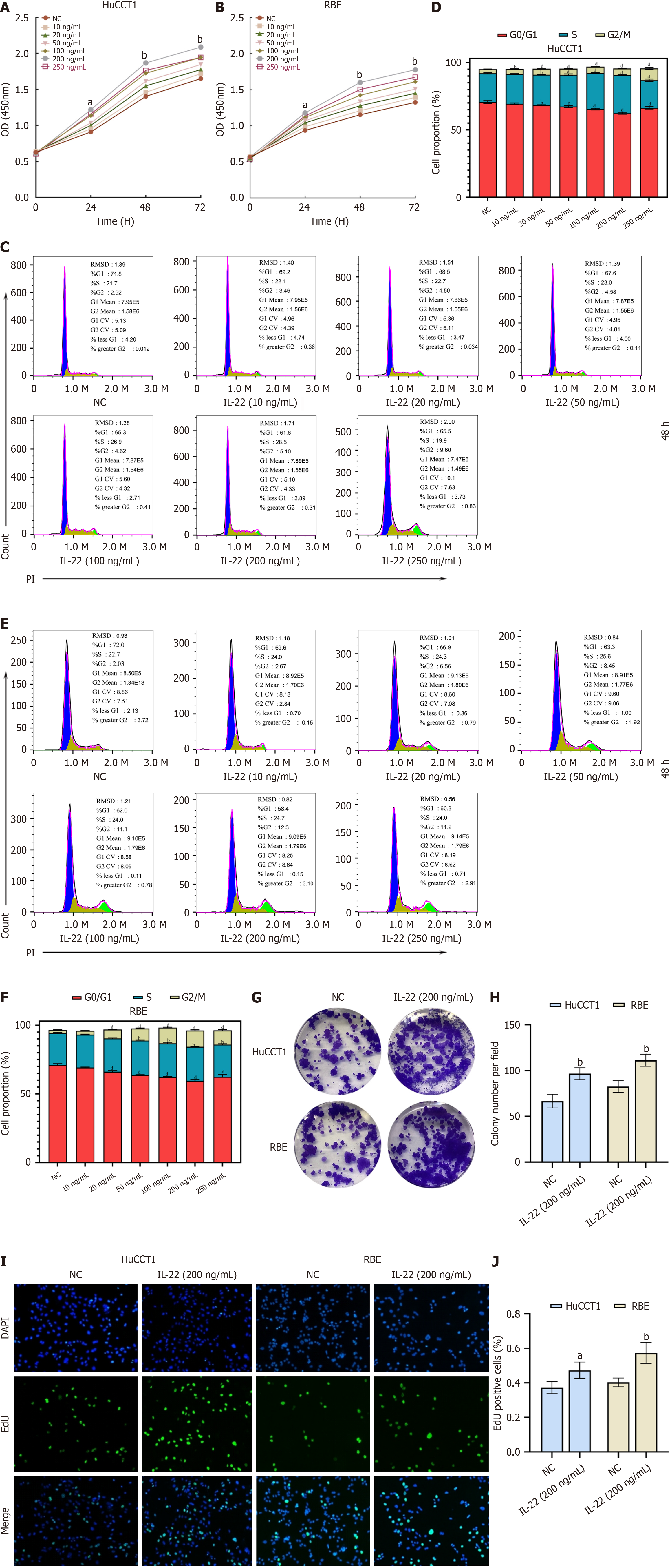
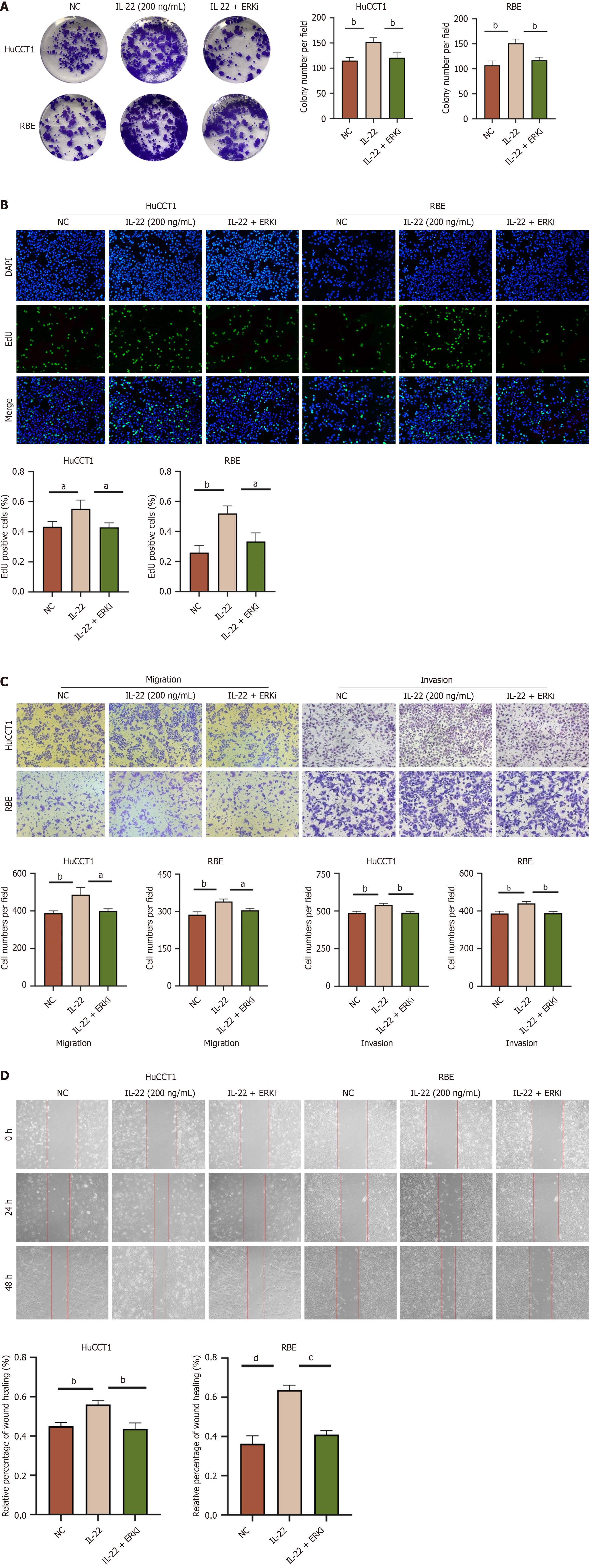
To explore the impact of IL-22 on the proliferation of CCA cell lines, a CCK-8 proliferation assay was performed. We set different concentration gradients of IL-22 (0, 10, 20, 50, 100, 200, and 250 ng/mL) and studied their effects on cell proliferation at different time points (0, 24, 48, and 72 hours). The optimal concentration and time were evaluated by measuring the absorbance at 450 nm (Figure 2A and B). The results indicated that the optimal concentration and duration for the proliferation of CCA cells was 200 ng/mL of IL-22 co-cultured with the cells for 48 hours. To confirm the results of the CCK-8 experiment, we treated the cells under the aforementioned conditions and conducted flow cytometry cell cycle experiments (Figure 2C-F), colony formation assays (Figure 2G and H), and EdU assays (Figure 2I and J). The results are consistent with those obtained from the CCK-8 assay.
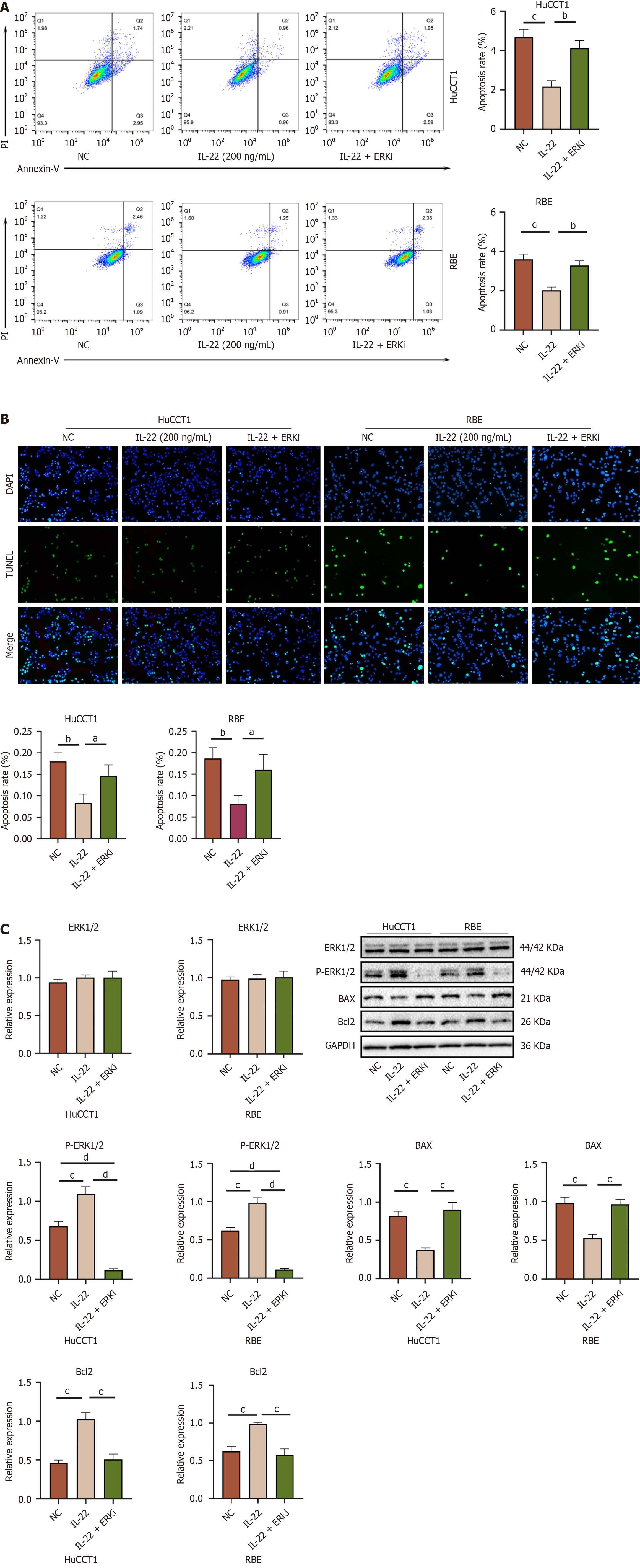
Tumor cells exhibit rapid proliferation and nutrient scavenging capabilities. We induced apoptosis in CCA cells through nutrient deprivation. Following the stimulation of CCA cells with 200 ng/mL of IL-22, apoptosis was evaluated using flow cytometry and the TUNEL assay as shown in Figure 3A and B. These assays showed that the CCA cells have significant resistance to apoptosis, following IL-22 stimulation.
Transwell migration and scratch assays revealed that after 48 h of stimulation with 200 ng/mL of IL-22, the cell migration rates notably exceeded those observed in the control group as shown in Figure 3C and D. Similarly, the Transwell invasion assays showed comparable results (Figure 3C). These findings demonstrate that IL-22 intensifies the migratory and invasive capabilities of CCA cells.
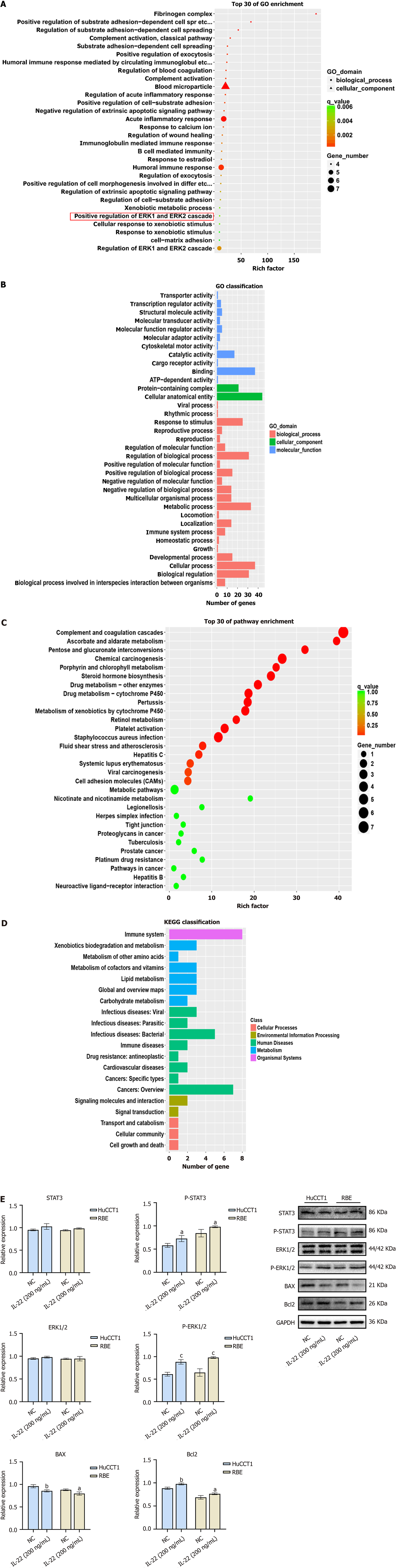
IL-22 binds to its receptor and activates multiple signaling pathways, including the JAK–STAT pathway, MAPK cascade, PI3K–AKT-mTOR, as well as the ERK1/2, p38, and JNK. IL-22 exerts its effects in various solid tumors by activating these different signaling pathways. We analyzed the RNA-seq data from the NC RBE (n = 3) and IL-22-treated cells at a concentration of 200 ng/mL (n = 3). The results of the KEGG and GO enrichment analyses (Figure 4A–D) suggested that IL-22 may promote a series of biological processes in CCA cells via the ERK cascade. To validate the RNA-seq results, we performed Western blot analysis on the proteins extracted from both groups. The findings showed that the IL-22 treatment enhanced the phosphorylated ERK1/2 (p-ERK1/2) and p-STAT3 expressions, with p-ERK1/2 expression being the most pronounced. Additionally, the levels of the anti-apoptotic proteins were markedly elevated in comparison to the control group (Figure 4E).
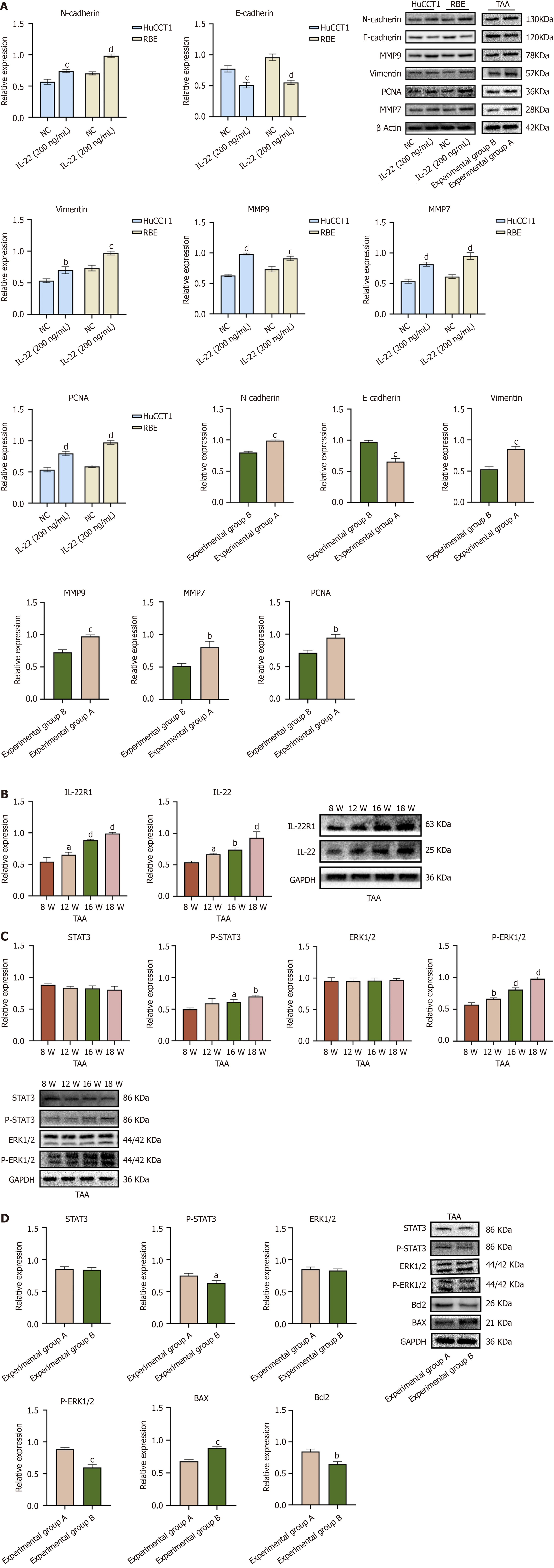
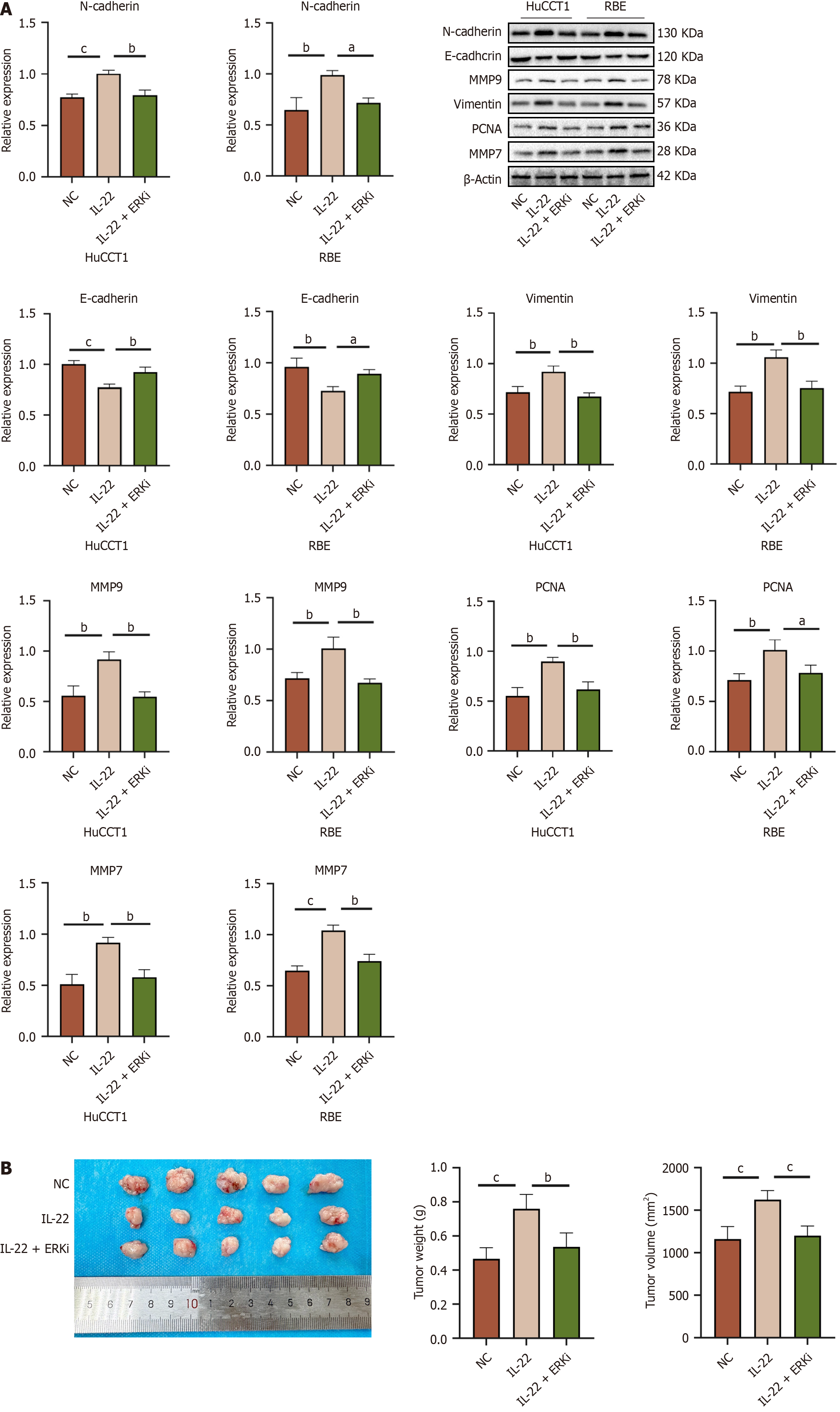
To examine the effects of IL-22 on the migration and invasion of CCA cells to investigate the impact of IL-22 on CCA cell migration and invasion, Western blot analyses were conducted. The results revealed that the protein expressions of neural cadherin (N-cadherin), Vimentin, PCNA, MMP9, and MMP7 were elevated in the IL-22-treated group compared to the control group, while the levels of epithelial cadherin (E-cadherin) were reduced in the IL-22-treated group. Statistically significant differences were noted between the two groups (Figure 5A). These results indicate that IL-22 promotes the epithelial-mesenchymal transition (EMT) and extracellular matrix (ECM) degradation, facilitating the breaching of the basement membrane by tumor cells and enhancing their migration and invasion.
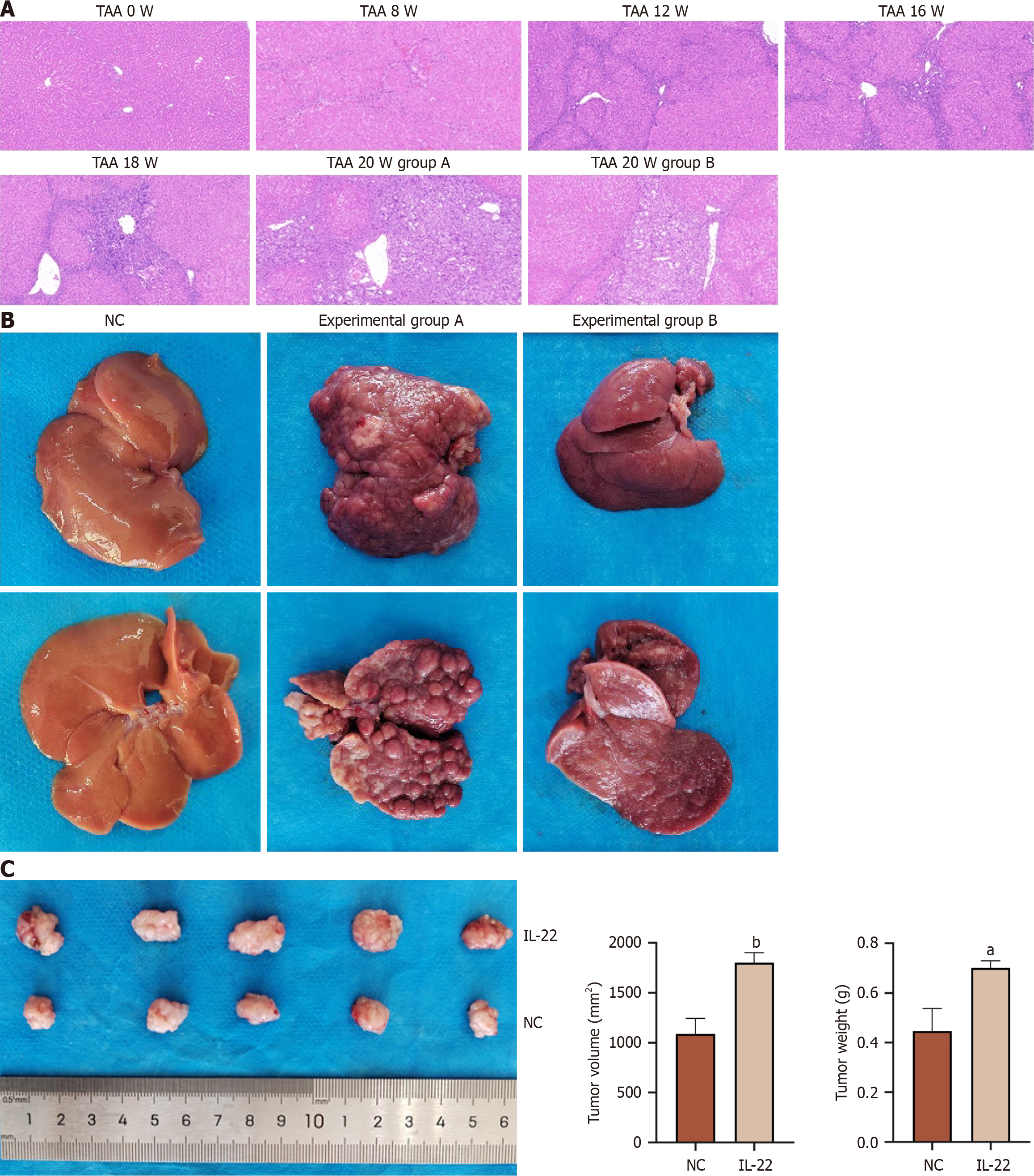
We induced a CCA model in the SD rats by using TAA. A previous study[8] has reported that approximately 50% of rats can exhibit the progression of biliary tissue dysplasia when consuming water containing 300 mg/L of TAA for 16 weeks. The present study confirmed this finding, and as the duration of administration was extended, the tumor formation rate gradually increased. We collected liver tumor tissues from rats at 8, 12, 16, and 18 weeks (n = 3) for subsequent analysis. Fresh tumor tissue proteins were extracted from SD rats consuming TAA-containing water at 8, 12, 16, and 18 weeks, as well as from experimental groups A and B. Subsequently, we prepared the tissue sections from these tumor samples for further analysis. Western blot analysis revealed that with prolonged TAA exposure, The levels of IL-22 and IL-22R1 protein expression in the tumor tissues progressively rose as shown in Figure 5B. Concurrently, the expression levels of p-ERK1/2 and p-STAT3 also increased, with p-ERK1/2 level showing the most significant elevation as shown in Figure 5C. When comparing experimental groups A and B, the protein expression levels of p-ERK1/2 and p-STAT3 were higher in group A, with p-ERK1/2 demonstrating the most notable increase (Figure 5D). The levels of pro-oncogenic and anti-apoptotic proteins were elevated in group A compared to group B (Figure 5 A and D), which is consistent with the results from the in vitro cell experiments. HE staining of the aforementioned tissue sections revealed a progressive worsening of lesions (Figure 6A), with experimental group A exhibiting more severe pathological changes compared to group B (Figure 6B). We prepared the liver tumor tissue sections from both groups for Ki67 immunohistochemical staining and TUNEL assays (Supplementary Figure 2). The results verified that IL-22 facilitates proliferation and has anti-apoptotic effects in TAA-induced CCA in vivo. Blood biochemical analyses (n = 3) indicated that the ALT, AST, ALP, and T-Bil levels were notably increased in both experimental groups A and B when compared to the control group, showing statistical significance. Furthermore, there were notable differences in the elevation of ALT, AST, ALP, and T-Bil levels between experimental groups A and B (Supplementary Table 4). In summary, these in vivo experimental findings demonstrate that IL-22 facilitates the development of TAA-induced CCA in rats.
To verify the impact of IL-22 on CCA cell proliferation in vivo, we established a subcutaneous xenograft mouse model. RBE cells were inoculated subcutaneously into the right shoulder of the nude mice. Following tumor development, the experimental group was treated with IL-22. Finally, the tumor weight and volume were measured. The results indicated that IL-22 promotes CCA cell proliferation in vivo as shown in Figure 6C.
After dissolving SCH772984 (ERK1/2 inhibitor) in DMSO, we co-treated the CCA cells with it at a concentration of 4 μM[9] along with IL-22 for 48 hours. The results indicated that this treatment inhibited the IL-22-induced proliferation (Figure 7A and B), migration (Figure 7C and D), invasion (Figure 7C), and anti-apoptotic (Figure 8A and B) effects in CCA cells. Furthermore, Western bloot analysis of the extracted proteins also revealed that the application of the ERK inhibitor can counteract the protein expression stimulated by IL-22 in these cells (Figures 8C and 9A). These in vitro findings inversely confirm that the IL-22/IL-22R1 axis exerts its influences on CCA cells through the ERK1/2 pathway.
We utilized the RBE cells to create a subcutaneous xenograft mouse model. After the tumors reached a diameter of 100 mm3, the mice were randomly allocated into the control, IL-22, and IL-22 + ERKi groups. Following the experimental period, all nude mice were euthanized, and the tumors were surgically removed, their weights and volumes were computed as shown in Figure 9B. The results indicated a significant difference in tumor volume and weight between the IL-22 group and both the control and IL-22 + ERKi groups, while no notable differences in tumor volume and weight were detected between the control and IL-22 + ERKi groups. This confirmed that the use of an ERK1/2 inhibitor can abrogate the tumor-promoting actions of IL-22 in vivo.
Multiple known risk factors are associated with the development of CCA, but they all share a common characteristic of being associated with chronic inflammation and bile stasis in the biliary epithelium[10]. Inflammation has become a marker of cancer, and the inflammatory environment can promote tumor progression. Understanding how to modulate the inflammatory response during the initial phases of tumor formation and inhibit tumor progression is a critical focus in cancer research. CCA exhibits greater heterogeneity as compared to other malignancies. In basic research, sample heterogeneity presents a considerable challenge; tumor tissues are typically obtained from transformed biliary tissues, whereas adjacent tissues are often sourced from neighboring liver tissues. CCA is characterized by its insidious onset, increased invasiveness and malignancy, a higher rate of postoperative recurrence, and low 5-year survival rate. Therefore, a deeper understanding of the underlying mechanisms of CCA occurrence and progression is crucial for its clinical prevention and treatment.
IL-22 is a cytokine secreted by immune cells, yet its particular receptor, IL-22R1, is exclusively expressed in non-immune cells. Initially, IL-22 was thought to primarily participate in inflammatory responses. However, recent researches have shown that IL-22 not only has a crucial role in inflammatory diseases but also correlates with the occurrence and exacerbation of various tumors. IL-22 is a double-edged sword; it can mitigate inflammation-induced tissue damage in the early stages, thereby preventing tumor formation, but, once cancerous transformation occurs, it may promote tumor cell proliferation and metastasis. Upon binding to its receptor IL-22R1, the signaling pathway of IL-22/IL-22R1 is triggered. This pathway enhances the proliferation, migration, and invasion of various gastrointestinal tumors[7]. However, its specific role in CCA remains unclear. The present research investigated the function and potential mechanisms of IL-22 in CCA through both in vivo and in vitro experiments. Our results confirm that IL-22 facilitates the proliferation, migration, and invasion of CCA cells through initiating the ERK1/2 signaling cascade.
The present research is the first to confirm the expression of IL-22R1 in human and rat CCA tissues through immunohistochemistry, establishing that IL-22 can function through its CCA IL-22 receptor. Additionally, IL-22R1 expression was observed in the RBE and HuCCT1 cells, with a greater expression level observed in these cells compared to HIBEpiC cells. Previous research has established that IL-22R1 is not expressed in immune cells and can also be used as a targeted receptor for treatment.
To elucidate the effect of IL-22 on CCA cell growth, we cultured RBE and HuCCT1 cells in vitro. By employing the CCK-8 assay, we established the optimal concentration and duration of IL-22 treatment for CCA cells. Flow cytometry was employed for the cell cycle analysis to validate these findings. Subsequent EdU proliferation and colony formation assays further confirmed that IL-22 promotes RBE and HuCCT1 cell proliferation. We also conducted wound healing assays, transwell migration and invasion assays to demonstrate that IL-22 acts as a promoter of migration and invasion in CCA cells. This suggests that IL-22 could act as a potential risk factor for CCA invasion and metastasis, warranting further investigation in future therapeutic studies. IL-22 can induce tumor cells to secrete various cytokines, including VEGF and TGF-β1[11]. IL-22 reportedly can increase the expression of MMP-9 in pancreatic and breast cancers and promote the expression of MMP-9, MMP-7, and MMP-13 in gastric cancer cells[7]. MMPs are a class of zinc-dependent enzymes that play crucial roles in physiological and pathological processes by degrading the ECM components. They have considerable implications for the occurrence, development, and metastasis of cancer. EMT refers to the process by which epithelial cells transdifferentiate into mesenchymal cells under specific pathophysiological conditions. EMT plays a critical role in the invasion and metastasis of various malignancies, including pancreatic ductal adenocarcinoma, melanoma, and breast, gastric, and liver cancers. Cadherin switching is a key mechanism of EMT, characterized by the transition from E-cadherin to N-cadherin. The co-expression of E-cadherin and N-cadherin is a hallmark of hepatocytes, cholangiocytes, and their derived tumors[12]. The present study found that IL-22 promotes EMT. The growth of tumor cells necessitates a continuous intake of nutrients from the surrounding environment, relying on nutrient scavenging to sustain their unrestricted proliferation. In our cell experiments, we investigated the effects of nutrient deprivation on CCA cells in response to IL-22 stimulation to determine any changes in apoptosis. Flow cytometry apoptosis and cell fluorescence TUNEL assays demonstrated that IL-22 stimulation resulted in a significant anti-apoptotic response in CCA cells.
To validate our in vitro results, we utilized a rat model of CCA induced by the oral administration of TAA. This model simulates the histological progression of human CCA at various disease stages. The advantages of this experimental approach include the continuity and integrity of the lesion process, as well as overcoming the heterogeneity associated with clinical sampling. By retaining the tissue and blood samples from different time points and conducting immunohistochemistry and Western blot experiments, we found that the IL-22 and IL-22R1 expression levels gradually increased with prolonged oral TAA administration. Furthermore, exogenous IL-22 administration promoted CCA progression in rats. We also established a subcutaneous xenograft mouse model by subcutaneously injecting RBE cells into nude mice to form subcutaneous tumors. After stimulation with exogenous IL-22, the subcutaneous tumors were enlarging. Collectively, the results of these in vivo experiments confirmed that IL-22 can promote CCA progression.
Following the binding of IL-22 to its receptor, the Janus kinases Jak1 and Tyk2 are activated, leading to the phosphorylation of tyrosine residues in the signal transducer and activator of the transcription proteins STAT3, STAT1, and STAT5, with STAT3 being identified as the primary target. Most current research focused specifically on the activation of STAT3 by IL-22. IL-22 functions through the phosphorylation of STAT3 in inflammatory bowel disease mucosal repair, liver injury repair, liver regeneration, as well as human colon, liver, and pancreatic cancer progression[5,13,14]. In addition, IL-22 can promote the progression of certain tumors through various signaling pathways, including the activation of the MAPK cascade, ERK1/2 cascade, JNK, and PI3K–AKT–mTOR[15]. To further investigate the mechanisms by which IL-22 affects CCA cells, we conducted the RNA-seq along with KEGG and GO enrichment analyses. The enrichment results suggest that the effects of IL-22 on CCA cells may be mediated through the REK1/2 cascade reaction. ERK1/2 is a widely expressed hydrophilic non-receptor protein that operates through a highly conserved signaling mechanism, playing an essential role in the Ras-Raf-MEK-ERK signaling cascade. This pathway governs a multitude of cellular function, including cell adhesion, cell cycle progression, migration, survival, differentiation, metabolism, proliferation, and transcription[16]. Under normal physiological conditions, the ERK cascade is finely regulated to ensure that its activation and deactivation align with the cellular growth requirements. However, ERK1/2 activation usually promotes cell proliferation, and its dysregulation is a sign of numerous cancers[17]. Previous research indicates that the ERK signaling pathway is over-activated in many malignancies, including breast, lung, colorectal, and pancreatic cancers, malignant melanoma, hepatocellular carcinoma, and CCA[18]. In hepatocellular carcinomas, ERK1/2 activation correlates with an aggressive tumor behavior and serves as an independent prognostic factor linked to overall survival. Elevated p-ERK1/2 expression has also been found in CCA[18]. Furthermore, elevated levels of p-ERK1/2 have been reported in CCA. The activation of ERK1/2 can be induced by various factors, including epidermal growth factor receptors, cytokine receptor-mediated inflammatory signals, oncogenic mutations in Ras and Raf, and bile acids[17]. Our cell and tissue protein blotting experiments demonstrated that IL-22 binding to its receptor may influence CCA cell mitosis, promote cell proliferation, and inhibit cell apoptosis through ERK1/2 phosphorylation. A previous study[19] has shown that, in liver schistosomiasis-related CCA, the IL-22 levels are significantly correlated with tumor progression. This research indicates that, in CCA caused by non-liver schistosomiasis infection, the IL-22/IL-22R1 axis continues to play a pro-carcinogenic role. SCH772984, a selective ERK1/2 inhibitor, potently inhibits the phosphorylation of REK1/2. To further clarify the function of the IL-22/IL22R1 axis in functioning through the ERK1/2 pathway, interventions with SCH772984 were conducted in vitro cellular experiments and in vivo subcutaneous xenograft mouse models. The conclusion demonstrated that SCH772984 can counteract the promotional effects of IL-22 on the CCA cells. Furthermore, ERK inhibitors play a positive role in cancer therapy, and their prospects are promising[9].
The importance of the IL-22/IL-22R1 pathway in cancer is gaining recognition. Although IL-22R1 is expressed on the surface of epithelial cells and does not directly affect the immune system, IL-22, as an immune modulator, inevitably influences the immune responses. First, IL-22 stimulates the epithelial or cancer cells to secrete cytokines that directly regulate the immune cells, promoting immune escape by inducing the production of immune-suppressive cytokines. Second, IL-22 influences adaptive immunity by interacting with receptors on lymphatic epithelial cells[20], subsequently affecting tumor progression in various ways. Although the results of the present study are commendable, it has certain limitations, including the relatively small number of clinical samples analyzed and the singularity of the animal model construction, which to some extent limit the persuasiveness of the findings. Nevertheless, despite these shortcomings, the research provides a valuable new perspective for the future clinical treatment of CCA, offering a theoretical foundation for clinical translational therapy and essential data for the further application of ERK inhibitors in clinical practice.
In summary, numerous studies have indicated that IL-22 can promote the progression of many gastrointestinal tumors. Balancing the role of IL-22 in tissue repair and tumor promotion may represent a potential therapeutic direction for cancer treatment in the future. Furthermore, elucidating the mechanisms by which IL-22 functions in different solid tumors and developing targeted interventions are expected to offer novel insights for developing clinical therapeutic strategies.
The IL-22/IL-22R1 axis promotes CCA progression by activating ERK1/2 signaling. Targeting this pathway with ERK1/2 inhibitors contributes to the development of potential therapeutic strategies for CCA.
| 1. | Cillo U, Fondevila C, Donadon M, Gringeri E, Mocchegiani F, Schlitt HJ, Ijzermans JNM, Vivarelli M, Zieniewicz K, Olde Damink SWM, Groot Koerkamp B. Surgery for cholangiocarcinoma. Liver Int. 2019;39 Suppl 1:143-155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 228] [Cited by in RCA: 225] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 2. | Banales JM, Marin JJG, Lamarca A, Rodrigues PM, Khan SA, Roberts LR, Cardinale V, Carpino G, Andersen JB, Braconi C, Calvisi DF, Perugorria MJ, Fabris L, Boulter L, Macias RIR, Gaudio E, Alvaro D, Gradilone SA, Strazzabosco M, Marzioni M, Coulouarn C, Fouassier L, Raggi C, Invernizzi P, Mertens JC, Moncsek A, Ilyas SI, Heimbach J, Koerkamp BG, Bruix J, Forner A, Bridgewater J, Valle JW, Gores GJ. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. 2020;17:557-588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1555] [Cited by in RCA: 1548] [Article Influence: 309.6] [Reference Citation Analysis (0)] |
| 3. | Cambridge WA, Fairfield C, Powell JJ, Harrison EM, Søreide K, Wigmore SJ, Guest RV. Meta-analysis and Meta-regression of Survival After Liver Transplantation for Unresectable Perihilar Cholangiocarcinoma. Ann Surg. 2021;273:240-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 86] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 4. | Ouyang W, O'Garra A. IL-10 Family Cytokines IL-10 and IL-22: from Basic Science to Clinical Translation. Immunity. 2019;50:871-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 536] [Cited by in RCA: 723] [Article Influence: 120.5] [Reference Citation Analysis (0)] |
| 5. | Wu Y, Min J, Ge C, Shu J, Tian D, Yuan Y, Zhou D. Interleukin 22 in Liver Injury, Inflammation and Cancer. Int J Biol Sci. 2020;16:2405-2413. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 6. | Mansur F, Arshad T, Liska V, Manzoor S. Interleukin-22 promotes the proliferation and migration of hepatocellular carcinoma cells via the phosphoinositide 3-kinase (PI3K/AKT) signaling pathway. Mol Biol Rep. 2023;50:5957-5967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 7. | Fukui H, Zhang X, Sun C, Hara K, Kikuchi S, Yamasaki T, Kondo T, Tomita T, Oshima T, Watari J, Imura J, Fujimori T, Sasako M, Miwa H. IL-22 produced by cancer-associated fibroblasts promotes gastric cancer cell invasion via STAT3 and ERK signaling. Br J Cancer. 2014;111:763-771. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 82] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 8. | Yeh CN, Maitra A, Lee KF, Jan YY, Chen MF. Thioacetamide-induced intestinal-type cholangiocarcinoma in rat: an animal model recapitulating the multi-stage progression of human cholangiocarcinoma. Carcinogenesis. 2004;25:631-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 113] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 9. | Gao Z, Chen JF, Li XG, Shi YH, Tang Z, Liu WR, Zhang X, Huang A, Luo XM, Gao Q, Shi GM, Ke AW, Zhou J, Fan J, Fu XT, Ding ZB. KRAS acting through ERK signaling stabilizes PD-L1 via inhibiting autophagy pathway in intrahepatic cholangiocarcinoma. Cancer Cell Int. 2022;22:128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 10. | Khan SA, Tavolari S, Brandi G. Cholangiocarcinoma: Epidemiology and risk factors. Liver Int. 2019;39 Suppl 1:19-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 498] [Article Influence: 83.0] [Reference Citation Analysis (0)] |
| 11. | Curd LM, Favors SE, Gregg RK. Pro-tumour activity of interleukin-22 in HPAFII human pancreatic cancer cells. Clin Exp Immunol. 2012;168:192-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 12. | Gerber TS, Goeppert B, Hausen A, Witzel HR, Bartsch F, Schindeldecker M, Gröger LK, Ridder DA, Cahyadi O, Esposito I, Gaida MM, Schirmacher P, Galle PR, Lang H, Roth W, Straub BK. N-Cadherin Distinguishes Intrahepatic Cholangiocarcinoma from Liver Metastases of Ductal Adenocarcinoma of the Pancreas. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Ren X, Hu B, Colletti LM. IL-22 is involved in liver regeneration after hepatectomy. Am J Physiol Gastrointest Liver Physiol. 2010;298:G74-G80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 98] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 14. | Jiang R, Wang H, Deng L, Hou J, Shi R, Yao M, Gao Y, Yao A, Wang X, Yu L, Sun B. IL-22 is related to development of human colon cancer by activation of STAT3. BMC Cancer. 2013;13:59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 163] [Cited by in RCA: 160] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 15. | Yao Y, Yang G, Lu G, Ye J, Cui L, Zeng Z, Chen J, Zhou J. Th22 Cells/IL-22 Serves as a Protumor Regulator to Drive Poor Prognosis through the JAK-STAT3/MAPK/AKT Signaling Pathway in Non-Small-Cell Lung Cancer. J Immunol Res. 2022;2022:8071234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 16. | Roskoski R Jr. ERK1/2 MAP kinases: structure, function, and regulation. Pharmacol Res. 2012;66:105-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 929] [Cited by in RCA: 1193] [Article Influence: 91.8] [Reference Citation Analysis (0)] |
| 17. | Ullah R, Yin Q, Snell AH, Wan L. RAF-MEK-ERK pathway in cancer evolution and treatment. Semin Cancer Biol. 2022;85:123-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 268] [Article Influence: 67.0] [Reference Citation Analysis (0)] |
| 18. | Jinawath A, Akiyama Y, Yuasa Y, Pairojkul C. Expression of phosphorylated ERK1/2 and homeodomain protein CDX2 in cholangiocarcinoma. J Cancer Res Clin Oncol. 2006;132:805-810. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Su SB, Zhang JF, Huang FF, Cen Y, Jiang HX. Large numbers of interleukins-22- and -17A-producing T helper cells in cholangiocarcinoma related to liver fluke infection. Microbiol Immunol. 2017;61:345-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Jiang R, Sun B. IL-22 Signaling in the Tumor Microenvironment. Adv Exp Med Biol. 2021;1290:81-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |