Published online Mar 15, 2025. doi: 10.4251/wjgo.v17.i3.101991
Revised: November 21, 2024
Accepted: January 8, 2025
Published online: March 15, 2025
Processing time: 133 Days and 6.8 Hours
Irreversible electroporation (IRE) is a novel local tumor ablation approach with the potential to activate the host’s immune system. However, this approach is insufficient to prevent cancer progression, and complementary approaches are required for effective immunotherapy.
To assess the immunomodulatory effects and mechanism of IRE combined anti-programmed cell death protein 1 (PD-1) treatment in subcutaneous pancreatic cancer models.
C57BL-6 tumor-bearing mice were randomly divided into four groups: Control group; IRE group; anti-PD-1 group; and IRE + anti-PD-1 group. Tumor-infiltrating T, B, and natural killer cell levels and plasma concentrations of T helper type 1 cytokines (interleukin-2, interferon-γ, and tumor necrosis factor-α) were evaluated. Real-time PCR was used to determine the expression of CD8 (marker of CD8+ T cells) in tumor tissues of the mice of all groups at different points of time. The growth curves of tumors were drawn.
The results demonstrated that the IRE + anti-PD-1 group exhibited significantly higher percentages of T lymphocyte infiltration, including CD4+ and CD8+ T cells compared with the control group. Additionally, the IRE + anti-PD-1 group showed increased infiltration of natural killer and B cells, elevated cytokine levels, and higher CD8 mRNA expression. Tumor volume was significantly reduced in the IRE + anti-PD-1 group, indicating a more pronounced therapeutic effect.
The combination of IRE and anti-PD-1 therapy promotes CD8+ T cell immunity responses, leading to a more effective reduction in tumor volume and improved therapeutic outcomes, which provides a new direction for ablation and immunotherapy of pancreatic cancer.
Core Tip: This study highlighted the synergistic effect of combining irreversible electroporation with anti-programmed cell death protein 1 therapy in the treatment of subcutaneous pancreatic cancer. The combination significantly enhanced T lymphocyte infiltration, elevated key cytokine levels, and promoted CD8+ T cell-mediated immune responses, resulting in a marked reduction in tumor volume. These findings suggest a promising avenue for improving immunotherapy strategies in pancreatic cancer through enhanced local tumor ablation methods.
- Citation: Ma YY, Wang XH, Zeng JY, Chen JB, Niu LZ. Irreversible electroporation combined with anti-programmed cell death protein 1 therapy promotes tumor antigen-specific CD8+ T cell response. World J Gastrointest Oncol 2025; 17(3): 101991
- URL: https://www.wjgnet.com/1948-5204/full/v17/i3/101991.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i3.101991
Pancreatic cancer is recognized as a notably aggressive type of cancer and is characterized by an alarmingly unfavorable prognosis. The 5-year survival rate for individuals diagnosed with this disease is persistently below 8%[1]. Although sur
Irreversible electroporation (IRE) represents an innovative, non-thermal approach to localized tissue ablation. This technique employs high-voltage electrical pulses to induce disruption of cellular membranes, leading to the formation of irreversible nanoscale pores and ultimately precipitating apoptotic cell death[5,6]. As an effective local treatment, the safety and effectiveness of IRE have been explored in various types of cancer[7], with the most promising overall survival and recurrence results in pancreas[8], liver[9], and prostate cancer[10]. Boosting IRE-induced immune activation was also proposed[11,12]. Moreover, Babikr et al[13] and Scheffer et al[14] demonstrated that IRE for locally advanced pancreatic cancer temporarily reduced immune suppression, thereby establishing an opportunity for the activation of antitumor T cells. Meanwhile, recent studies have shown that an immune response can be elicited after IRE ablation, but this immune response is not sufficient to restrain tumor progression and recurrence[15-18].
Immune checkpoint inhibitors play a pivotal role in enhancing the ability of the immune system to target and combat tumors, thereby transforming the landscape of cancer treatment. Notably, therapies that target programmed cell death protein 1 (PD-1) have demonstrated significant effectiveness across a range of malignancies, such as melanoma, non-small cell lung carcinoma, and renal cell carcinoma[19-21]. PD-1 serves as an inhibitory receptor found on activated T cells. The interaction of PD-1 with its ligands, PD-L1 and PD-L2, which are often found in elevated levels in tumor cells, results in T cell exhaustion and a weakened immune response against tumors. By blocking this interaction, anti-PD-1 therapies reinvigorate T cells by enhancing their proliferation and cytotoxic activity against tumor cells. Moreover, T helper type 1 (Th1) cytokines, primarily produced by CD4+ Th1 cells, play a crucial role in driving robust immune responses[22]. Crucial cytokines, including interferon (IFN)-γ and tumor necrosis factor (TNF)-α, play a significant role in bolstering antitumor immunity. They achieve this by facilitating enhanced antigen presentation, activating macrophages, and supporting the maturation of cytotoxic CD8+ T cells[23,24]. Elevated levels of Th1 cytokines create a proinflammatory tumor microenvironment that can combat tumor growth. Despite the promising mechanisms of immune checkpoint inhibitors, their use in pancreatic cancer has been disappointing due to the immunosuppressive microenvironment and low tumor immunogenicity. Therefore, combining IRE with anti-PD-1 therapy could potentially augment the immunogenicity of pancreatic tumors and facilitate a more robust antitumor immune response, thus overcoming existing limi
In this study, we investigated the therapeutic efficacy and immune response of combining IRE with anti-PD-1 therapy in a C57BL-6 mouse model of pancreatic cancer using Panc02 cells. We hypothesized that the combination of IRE and anti-PD-1 would potentially augment the immunogenicity of pancreatic tumors and facilitate a more robust antitumor immune response compared to either treatment alone. To test this hypothesis, we established a pancreatic tumor model in C57BL-6 mice and evaluated tumor growth and immune cell populations following treatment.
Our findings provide critical insights into the potential synergistic effects of IRE and anti-PD-1 therapy in pancreatic cancer. By elucidating the mechanisms underlying the enhanced antitumor immune response, this study aimed to pave the way for novel combination therapies that could improve outcomes for patients with this devastating disease.
Panc02 cells were purchased from BeNa Culture Collection (BNCC, Beijing, China). Panc02 cells were cultured in 90% DMEM (Gibco) and 10% FBS (Hyclone). The cells were then maintained in an incubator at 37 °C and 5% CO2.
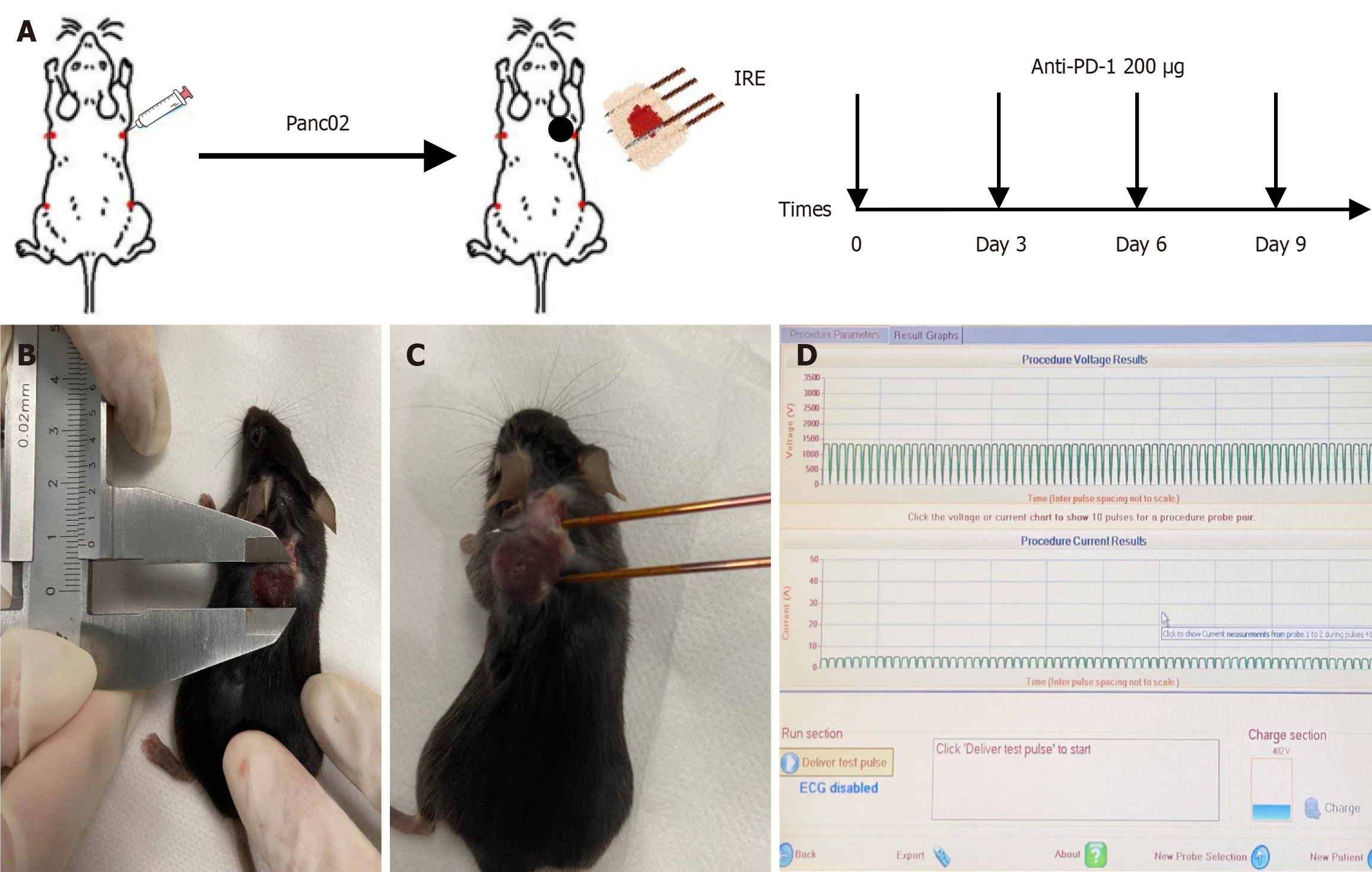
To establish a mouse model with a normal immune system, female C57BL/6 mice were subcutaneously inoculated with 1 × 106 Panc02 cells in 100 µL of Hanks balanced salt solution into the right flank of 6-week-old C57BL/6 mice. This animal experiment was examined and approved by the laboratory animal welfare ethics committee of the Jinan University.
Forty-eight female C57BL-6 mice were randomly assigned to four groups: Control group without any treatment (n = 12); IRE group (n = 12); anti-PD-1 group (n = 12); and IRE + anti-PD-1 group (n = 12). Panc02 cells (1 × 106/mL) were injected subcutaneously into the right flank subcutaneous area of mice, and the tumor growth was detected every 2 days. The maximum diameter (a) and shortest diameter (b) of the tumor were measured and recorded with vernier calipers, and the volume of the tumor was calculated according to the formula a × b2/2. When the maximum diameter of the tumor reached 10 mm, IRE was performed, an anti–PD-L1 antibody (200 μg/mouse) was administered intraperitoneally immediately after the IRE procedure, and tumor tissues and peripheral blood were taken from the mice at different time points (1, 3, 7, and 14 days) after the IRE procedure. The tumor size was recorded every 3 days by calculating the length and the width of the tumor. The flowchart for model establishment and IRE ablation parameters are shown in Figure 1.
Mice were fixed on the operating table, anesthetized with tribromoethanol intraperitoneally according to their body weight, and the surgical area was prepared, disinfected, and toweled. Two electrode needles were inserted along the long axis of the tumors (the parameters were set at 1200 V/cm, 50 A, 70 pulses, 70 μs wavelength, and 100 ms intervals, and the distance of the needles was adjusted according to the size of the tumors). The PD-1/PD-L1 inhibitor was purchased from Aladdi. Anti-PD-1 (200 μg) was injected intraperitoneally once every 3 days for a total of 4 times. The needle distance was adjusted according to the tumor size.
Mouse peripheral blood samples (1 mL) were drawn at different time points. Flow cytometry techniques (FACS caliber, four-color system, BD Bioscience, CA, United States) were used to analyze the absolute number of CD3+ T cells (CD3+), CD4+ T cells (CD3+ CD4+), CD8+ T cells (CD3+ CD8+), the ratio of CD4+ T cells (CD3+ CD4+) and CD8+ T cells, natural killer (NK) cells (CD16+ CD56+), and B cells (CD19). A similar method was used to measure serum cytokines, including interleukin-2 (IL-2), TNF-β, and IFN-γ. A record of the data was made.
The expression of CD8 in mouse tumor tissues was detected by SYBR Green real-time PCR (RT-PCR) with glyceraldehyde 3-phosphate dehydrogenase as the internal reference gene. Tumor tissues were collected and cut into pieces of 0.3 cm × 0.3 cm × 0.3 cm. Total RNA was extracted by adding TRIzol reagent, and cDNA was synthesized according to the instructions. The reaction system (20 μL) was prepared according to the instructions of the reagents and then subjected to RT-PCR amplification and detection. The RT-PCR primer sequences were: β-actin (forward) GGGAAATCGTGCGTGAC, (reverse) AGGCTGGAA AAG -AGCCT; CD8 (forward) CAAACACGCTTTCGG CTCCTG, (reverse) CGGATTGGACTTCGCCTGTGA. The results were analyzed using the 2-ΔΔCt method.
All statistical analyses were performed and compiled using GraphPad Prism (version 8.01, GraphPad Software, San Diego, CA, United States) and SPSS software (version 20.0, SPSS Inc., Chicago, IL, United States). The measured data were expressed as mean ± standard deviation. The t test was used to compare the data between two groups, and one-way analysis of variance was used to compare the data of multiple groups. P < 0.05 was considered statistically significant.
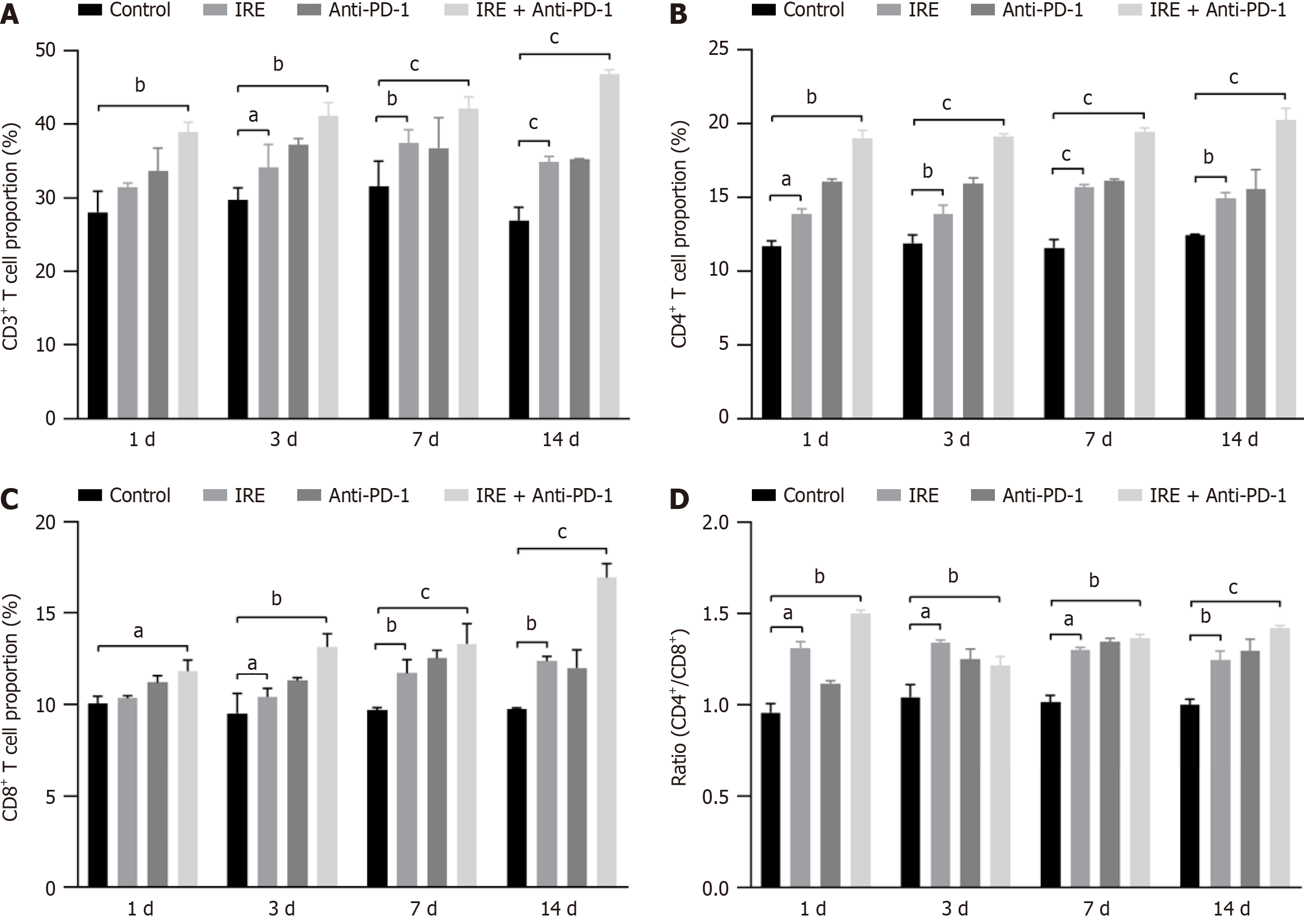
Flow cytometric evaluation revealed that the proportion of T lymphocytes was significantly elevated in the IRE group (P < 0.01), the anti-PD-1 group (P < 0.01), and the IRE + anti-PD-1 group (P < 0.001) compared with the control group, demonstrating a time-dependent effect. Additionally, the percentage of CD4+ T cells was markedly higher in all three treatment groups relative to the control group (P < 0.001). Notably, the IRE + anti-PD-1 group exhibited an increased proportion of infiltrating CD8+ T cells (P < 0.001, Figure 2). These findings indicated that the immune response induced by IRE ablation is primarily driven by the infiltration of CD8+ T cells, while the combination of IRE with anti-PD-1 therapy elicits a more robust CD8+ T cell response.
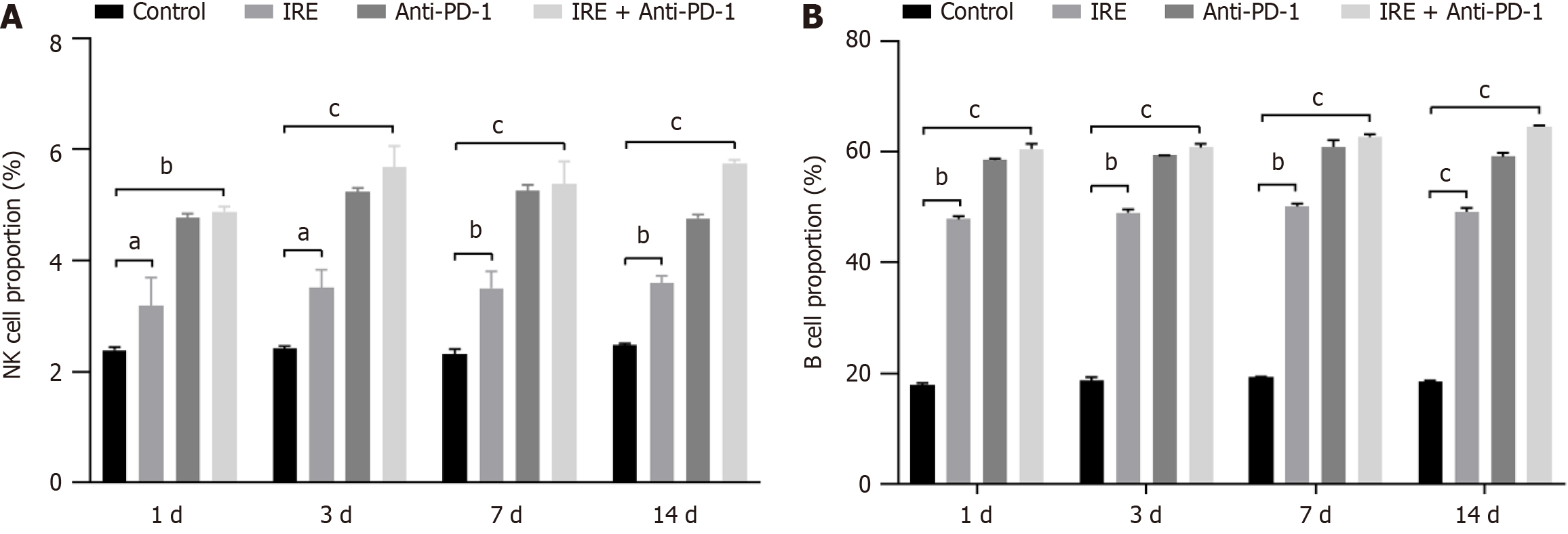
To explore whether humoral and innate immune responses were involved in the therapeutic response, we also analyzed the B cell and NK cell populations in the tumor tissues. The percentages of NK cells and B cells were significantly higher in the IRE group, the anti-PD-1 group, and the IRE + anti-PD-1 group compared to the control group. Notably, the extent of infiltration was significantly greater in the IRE + anti-PD-1 group relative to the anti-PD-1 group (P < 0.001, Figure 3). These findings indicated that all three therapeutic strategies bolstered both humoral and innate immune responses, with the combination of IRE and anti-PD-1 treatment demonstrating superior efficacy.
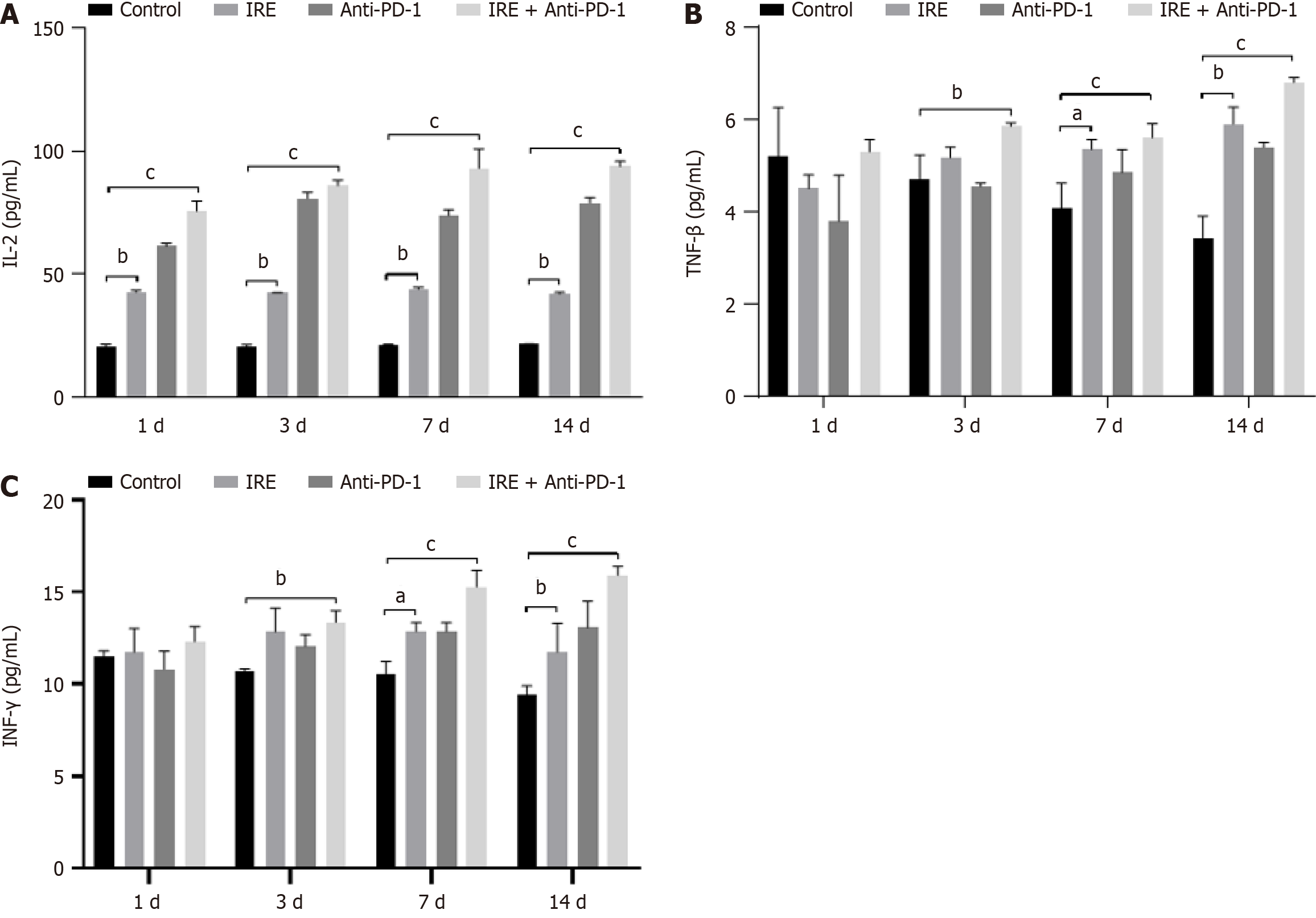
We conducted a quantitative analysis of cytokine levels, specifically IL-2, TNF-β, and IFN-γ, in the plasma of mice with tumors using flow cytometry techniques. Our findings revealed that the levels of IL-2, TNF-β, and IFN-γ were markedly elevated in the IRE, anti-PD-1, and IRE + anti-PD-1 treatment groups compared with the control group (P < 0.01 for both IRE and anti-PD-1 groups; P < 0.001 for the IRE + anti-PD-1 group). Notably, within the IRE + anti-PD-1 groups, the concentration of IL-2 exhibited a significant variation (P < 0.001, Figure 4). These observations indicated that all three therapeutic strategies successfully instigated a systemic immune response through the modulation of various cytokine levels; however, the combination of IRE ablation with anti-PD-1 therapy produced a more pronounced systemic immune response.
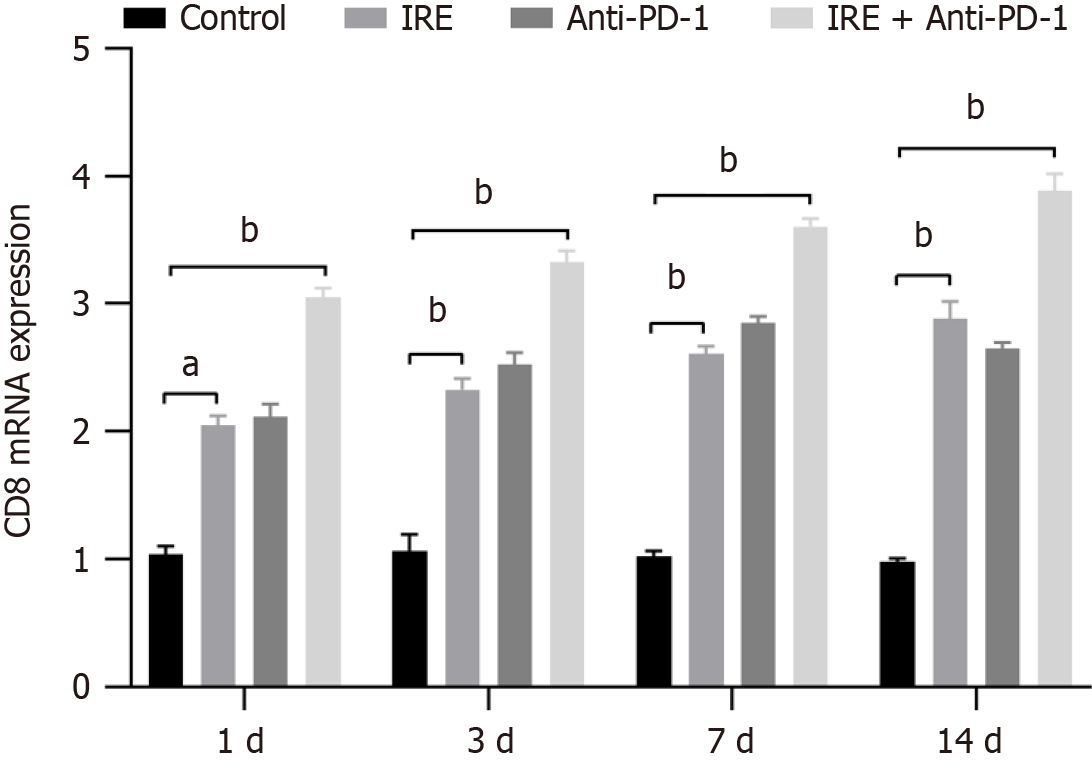
We examined the expression of CD8 mRNA by RT-PCR. It was found that the expression of CD8 mRNA in the IRE group, the anti-PD-1 group, and the IRE + anti-PD-1 group exhibited a markedly elevated response compared to the control group (P < 0.001). The expression of CD8 mRNA in the IRE + anti-PD-1 group was stronger in a time-dependent manner
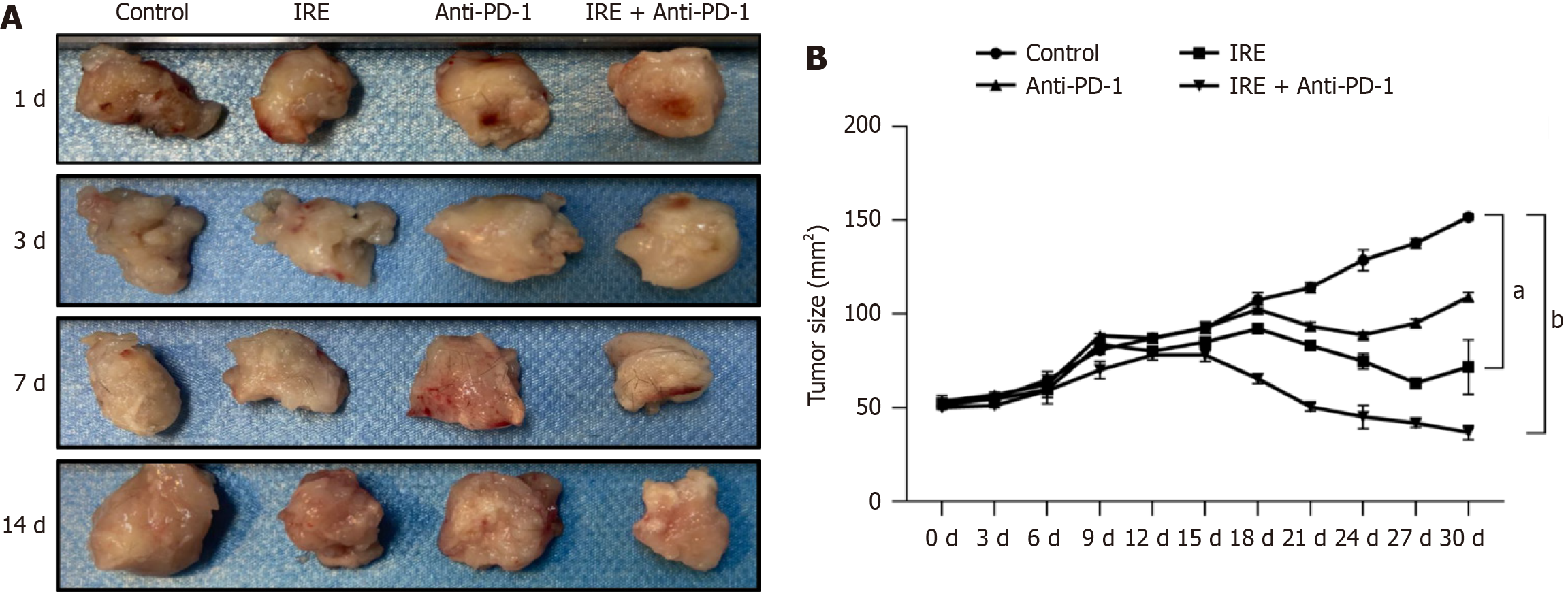
We detected the tumor volume every 3 days after IRE treatment. The results revealed that the tumor volumes in the IRE group, the anti-PD-1 group, and the IRE + anti-PD-1 group were markedly reduced compared with those in the control group (P < 0.01, P < 0.001). However, the change in tumor volume in the IRE + anti-PD-1 group was significantly greater in a time-dependent manner (Figure 6). These results suggested that all three interventions can reduce tumor volume. However, IRE ablation combined with anti-PD-1 treatment induced a more significant reduction in tumor volume.
In this study, we investigated the therapeutic effects and immune responses of IRE and anti-PD-1 therapy in a C57BL-6 mouse model of pancreatic cancer. Our findings indicated that the combination of IRE and anti-PD-1 therapy significantly inhibited tumor growth compared with the control group (P < 0.01). Additionally, flow cytometry analysis revealed an increase in the absolute number and proportion of CD4+ T cells, CD8+ T cells, NK cells, and B cells in the peripheral blood of treated mice. RT-PCR further demonstrated elevated CD8 expression in tumor tissues, suggesting enhanced cytotoxic T cell activity. These results support our hypothesis that the synergistic effect of IRE and anti-PD-1 therapy can potentiate antitumor immunity and improve therapeutic outcomes in pancreatic cancer.
The primary novelty of this study lies in its comprehensive evaluation of the combined effects of IRE + anti-PD-1 the
The main mechanism of IRE in treating pancreatic cancer is the induction of tumor cell apoptosis, which can lead to immune tolerance. Consequently, traditional theories suggest that combining IRE with immunotherapy may struggle to achieve synergistic effects. However, Shao et al[27] discovered that IRE is superior to thermal and cryoablation in inducing T cell immunity, indicating that IRE combined with immunotherapy may have synergistic effects. Zhao et al[15] utilized IRE in conjunction with PD-1 antibodies in a mouse model of orthotopic pancreatic cancer, and the results illu
The clinical implications of our study are significant, particularly in the context of pancreatic cancer, which remains one of the most lethal malignancies with limited treatment options. The combination of IRE and anti-PD-1 therapy de
Our study demonstrated that the combination of IRE and anti-PD-1 therapy significantly enhanced the therapeutic efficacy against pancreatic cancer mouse model. Furthermore, CD8+ T cell infiltration and activation, suggesting that the combination therapy not only directly targets tumor cells but also modulates the immune microenvironment to favor antitumor responses.
We wish to thank the staff from the Department of Surgery and Anesthesia of Guangzhou Fuda Cancer Hospital for their technical support.
| 1. | Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4235] [Cited by in RCA: 11430] [Article Influence: 3810.0] [Reference Citation Analysis (4)] |
| 2. | Rahib L, Wehner MR, Matrisian LM, Nead KT. Estimated Projection of US Cancer Incidence and Death to 2040. JAMA Netw Open. 2021;4:e214708. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 277] [Cited by in RCA: 787] [Article Influence: 196.8] [Reference Citation Analysis (0)] |
| 3. | Li W, Li T, Sun C, Du Y, Chen L, Du C, Shi J, Wang W. Identification and prognostic analysis of biomarkers to predict the progression of pancreatic cancer patients. Mol Med. 2022;28:43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 4. | Fernandes P, O'Donovan TR, McKenna SL, Forde PF. Electrochemotherapy Causes Caspase-Independent Necrotic-Like Death in Pancreatic Cancer Cells. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 5. | Rai ZL, Feakins R, Pallett LJ, Manas D, Davidson BR. Irreversible Electroporation (IRE) in Locally Advanced Pancreatic Cancer: A Review of Current Clinical Outcomes, Mechanism of Action and Opportunities for Synergistic Therapy. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 6. | O'Neill C, Hayat T, Hamm J, Healey M, Zheng Q, Li Y, Martin RCG 2nd. A phase 1b trial of concurrent immunotherapy and irreversible electroporation in the treatment of locally advanced pancreatic adenocarcinoma. Surgery. 2020;168:610-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 7. | Yun JH, Fang A, Khorshidi F, Habibollahi P, Kutsenko O, Etezadi V, Hunt S, Nezami N. New Developments in Image-Guided Percutaneous Irreversible Electroporation of Solid Tumors. Curr Oncol Rep. 2023;25:1213-1226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 8. | Ruarus AH, Vroomen LGPH, Geboers B, van Veldhuisen E, Puijk RS, Nieuwenhuizen S, Besselink MG, Zonderhuis BM, Kazemier G, de Gruijl TD, van Lienden KP, de Vries JJJ, Scheffer HJ, Meijerink MR. Percutaneous Irreversible Electroporation in Locally Advanced and Recurrent Pancreatic Cancer (PANFIRE-2): A Multicenter, Prospective, Single-Arm, Phase II Study. Radiology. 2020;294:212-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 92] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 9. | Meijerink MR, Ruarus AH, Vroomen LGPH, Puijk RS, Geboers B, Nieuwenhuizen S, van den Bemd BAT, Nielsen K, de Vries JJJ, van Lienden KP, Lissenberg-Witte BI, van den Tol MP, Scheffer HJ. Irreversible Electroporation to Treat Unresectable Colorectal Liver Metastases (COLDFIRE-2): A Phase II, Two-Center, Single-Arm Clinical Trial. Radiology. 2021;299:470-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 10. | Wang H, Xue W, Yan W, Yin L, Dong B, He B, Yu Y, Shi W, Zhou Z, Lin H, Zhou Y, Wang Y, Shi Z, Ren S, Gao X, Wang L, Xu C. Extended Focal Ablation of Localized Prostate Cancer With High-Frequency Irreversible Electroporation: A Nonrandomized Controlled Trial. JAMA Surg. 2022;157:693-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 11. | Li X, Xu K, Li W, Qiu X, Ma B, Fan Q, Li Z. Immunologic response to tumor ablation with irreversible electroporation. PLoS One. 2012;7:e48749. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 12. | Dai Z, Wang Z, Lei K, Liao J, Peng Z, Lin M, Liang P, Yu J, Peng S, Chen S, Kuang M. Irreversible electroporation induces CD8(+) T cell immune response against post-ablation hepatocellular carcinoma growth. Cancer Lett. 2021;503:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 13. | Babikr F, Wan J, Xu A, Wu Z, Ahmed S, Freywald A, Chibbar R, Wu Y, Moser M, Groot G, Zhang W, Zhang B, Xiang J. Distinct roles but cooperative effect of TLR3/9 agonists and PD-1 blockade in converting the immunotolerant microenvironment of irreversible electroporation-ablated tumors. Cell Mol Immunol. 2021;18:2632-2647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 14. | Scheffer HJ, Stam AGM, Geboers B, Vroomen LGPH, Ruarus A, de Bruijn B, van den Tol MP, Kazemier G, Meijerink MR, de Gruijl TD. Irreversible electroporation of locally advanced pancreatic cancer transiently alleviates immune suppression and creates a window for antitumor T cell activation. Oncoimmunology. 2019;8:1652532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 15. | Zhao J, Wen X, Tian L, Li T, Xu C, Wen X, Melancon MP, Gupta S, Shen B, Peng W, Li C. Irreversible electroporation reverses resistance to immune checkpoint blockade in pancreatic cancer. Nat Commun. 2019;10:899. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 189] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 16. | Imran KM, Nagai-Singer MA, Brock RM, Alinezhadbalalami N, Davalos RV, Allen IC. Exploration of Novel Pathways Underlying Irreversible Electroporation Induced Anti-Tumor Immunity in Pancreatic Cancer. Front Oncol. 2022;12:853779. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Ma Y, Xing Y, Li H, Yuan T, Liang B, Li R, Li J, Li Z, Li S, Niu L. Irreversible electroporation combined with chemotherapy and PD-1/PD-L1 blockade enhanced antitumor immunity for locally advanced pancreatic cancer. Front Immunol. 2023;14:1193040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 18. | Tian G, Guan J, Chu Y, Zhao Q, Jiang T. Immunomodulatory Effect of Irreversible Electroporation Alone and Its Cooperating With Immunotherapy in Pancreatic Cancer. Front Oncol. 2021;11:712042. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Bagchi S, Yuan R, Engleman EG. Immune Checkpoint Inhibitors for the Treatment of Cancer: Clinical Impact and Mechanisms of Response and Resistance. Annu Rev Pathol. 2021;16:223-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 1338] [Article Influence: 267.6] [Reference Citation Analysis (0)] |
| 20. | Carlino MS, Larkin J, Long GV. Immune checkpoint inhibitors in melanoma. Lancet. 2021;398:1002-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 702] [Article Influence: 175.5] [Reference Citation Analysis (0)] |
| 21. | Kubli SP, Berger T, Araujo DV, Siu LL, Mak TW. Beyond immune checkpoint blockade: emerging immunological strategies. Nat Rev Drug Discov. 2021;20:899-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 273] [Article Influence: 68.3] [Reference Citation Analysis (0)] |
| 22. | Dong C. Cytokine Regulation and Function in T Cells. Annu Rev Immunol. 2021;39:51-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 348] [Article Influence: 87.0] [Reference Citation Analysis (0)] |
| 23. | Kalbasi A, Ribas A. Tumour-intrinsic resistance to immune checkpoint blockade. Nat Rev Immunol. 2020;20:25-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 1017] [Article Influence: 169.5] [Reference Citation Analysis (0)] |
| 24. | Salomon BL, Leclerc M, Tosello J, Ronin E, Piaggio E, Cohen JL. Tumor Necrosis Factor α and Regulatory T Cells in Oncoimmunology. Front Immunol. 2018;9:444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 145] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 25. | Ringel-Scaia VM, Beitel-White N, Lorenzo MF, Brock RM, Huie KE, Coutermarsh-Ott S, Eden K, McDaniel DK, Verbridge SS, Rossmeisl JH Jr, Oestreich KJ, Davalos RV, Allen IC. High-frequency irreversible electroporation is an effective tumor ablation strategy that induces immunologic cell death and promotes systemic anti-tumor immunity. EBioMedicine. 2019;44:112-125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 118] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 26. | Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9936] [Cited by in RCA: 10350] [Article Influence: 796.2] [Reference Citation Analysis (34)] |
| 27. | Shao Q, O'Flanagan S, Lam T, Roy P, Pelaez F, Burbach BJ, Azarin SM, Shimizu Y, Bischof JC. Engineering T cell response to cancer antigens by choice of focal therapeutic conditions. Int J Hyperthermia. 2019;36:130-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 87] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 28. | Narayanan JSS, Ray P, Hayashi T, Whisenant TC, Vicente D, Carson DA, Miller AM, Schoenberger SP, White RR. Irreversible Electroporation Combined with Checkpoint Blockade and TLR7 Stimulation Induces Antitumor Immunity in a Murine Pancreatic Cancer Model. Cancer Immunol Res. 2019;7:1714-1726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 83] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 29. | Zhang N, Li Z, Han X, Zhu Z, Li Z, Zhao Y, Liu Z, Lv Y. Irreversible Electroporation: An Emerging Immunomodulatory Therapy on Solid Tumors. Front Immunol. 2021;12:811726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 30. | Peng H, Shen J, Long X, Zhou X, Zhang J, Xu X, Huang T, Xu H, Sun S, Li C, Lei P, Wu H, Zhao J. Local Release of TGF-β Inhibitor Modulates Tumor-Associated Neutrophils and Enhances Pancreatic Cancer Response to Combined Irreversible Electroporation and Immunotherapy. Adv Sci (Weinh). 2022;9:e2105240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 58] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 31. | Burbach BJ, O'Flanagan SD, Shao Q, Young KM, Slaughter JR, Rollins MR, Street TJL, Granger VE, Beura LK, Azarin SM, Ramadhyani S, Forsyth BR, Bischof JC, Shimizu Y. Irreversible electroporation augments checkpoint immunotherapy in prostate cancer and promotes tumor antigen-specific tissue-resident memory CD8+ T cells. Nat Commun. 2021;12:3862. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |