Published online Mar 15, 2025. doi: 10.4251/wjgo.v17.i3.101859
Revised: November 27, 2024
Accepted: December 19, 2024
Published online: March 15, 2025
Processing time: 138 Days and 17.6 Hours
Solid pseudopapillary neoplasm (SPN) of the pancreas is a rare epithelial tumor that primarily affects young women. Since the condition is often asymptomatic or presents with non-specific symptoms, its diagnosis can be difficult.
This report details the case of a 15-year-old girl who presented with a 2-year history of abdominal pain, with no significant findings during physical exa
This case highlights the risk of SPN in adolescent girls and the necessity of early diagnosis and intervention for better outcomes.
Core Tip: Solid pseudopapillary neoplasm (SPN) of the pancreas is an uncommon pancreatic tumor that primarily impacts young women, especially those in their twenties and thirties. This report highlights the case of a 15-year-old female patient who experienced a two-year history of abdominal pain and was diagnosed with a solid pseudopapillary neoplasm localized to the pancreatic tail. The patient underwent an open distal pancreatectomy accompanied by splenectomy, and histopathological examination confirmed the diagnosis of SPN. This case underscores the possibility of SPN occurring in adolescent girls and points out the vital importance of early diagnosis and timely intervention, which can significantly enhance clinical outcomes.
- Citation: Sapkota A, Paudel R, Pandey S, Bhatt N. Solid pseudopapillary neoplasm of the pancreas in an adolescent: A case report and review of the literature. World J Gastrointest Oncol 2025; 17(3): 101859
- URL: https://www.wjgnet.com/1948-5204/full/v17/i3/101859.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i3.101859
Solid pseudopapillary neoplasm (SPN) of the pancreas is a rare epithelial tumor, representing less than 2% of pancreatic exocrine tumors and below 5% of cystic pancreatic tumors[1]. This neoplasm, first explained by Frantz[2], is distinguished for its slow growing behavior and favorable outcome, particularly affecting young women[3]. While often detected incidentally, SPNs may present with nonspecific symptoms such as abdominal pain or distension[4]. The diagnosis is primarily established through abdominal tomography or magnetic resonance imaging[5]. The primary treatment involves the surgical resection of the tumor, which yields a 5-year survival rate of ~95%[6]. Here, we present the condition of a 15-year-old female patient who presented with a 2-year history of abdominal pain and was diagnosed with a SPN localized to the pancreatic tail, for which she underwent an open distal pancreatectomy and splenectomy.
Left upper abdominal pain for 2 years.
A 15-year-old girl presented to the outpatient department with a complaint of upper abdominal pain, primarily in the left hypochondriac region for 2 years. The pain had an abrupt onset, was dull aching, and ranged from moderate to severe in intensity, significantly impacting her daily activities. The pain was non-radiating and had no known aggravating or relieving factors. She also reported occasional nausea and multiple episodes of vomiting, which were non-projectile, non-foul smelling, and not stained with blood. The patient reported a history of undocumented weight loss, noticeable by the loosening of her previously fitting clothes. She did not have a history of fever, abdominal distension, fat intolerance, shortness of breath, melena, constipation, jaundice, heartburn, or anorexia.
The patient had no previous medical or surgical history.
There was no history of alcohol or drug use, and the family history was unremarkable.
On clinical examination, the patient’s vital signs and general physical examination findings were within normal limits. The abdomen was flat, soft, and non-tender with no guarding or rigidity. There was no organomegaly or any palpable mass. Shifting dullness and fluid thrill were absent. Normal bowel sounds were heard on auscultation.
All preoperative investigations were within normal limits, including serum amylase and serum lipase levels.
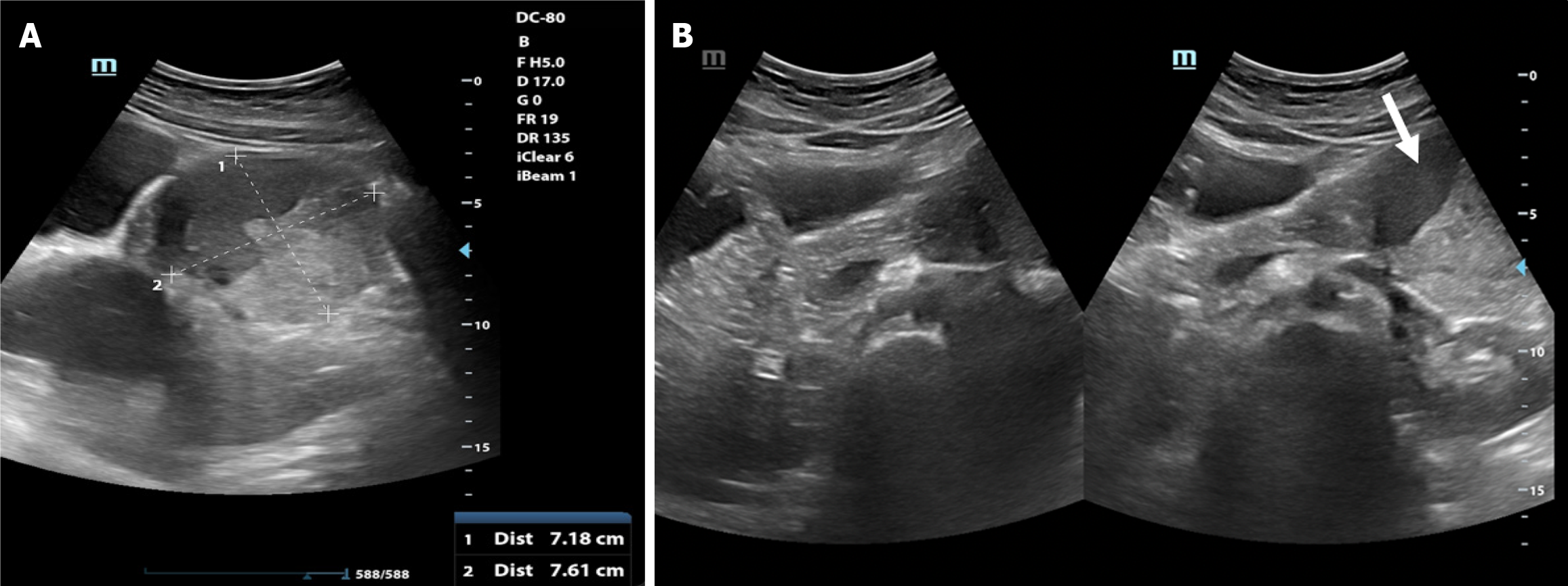
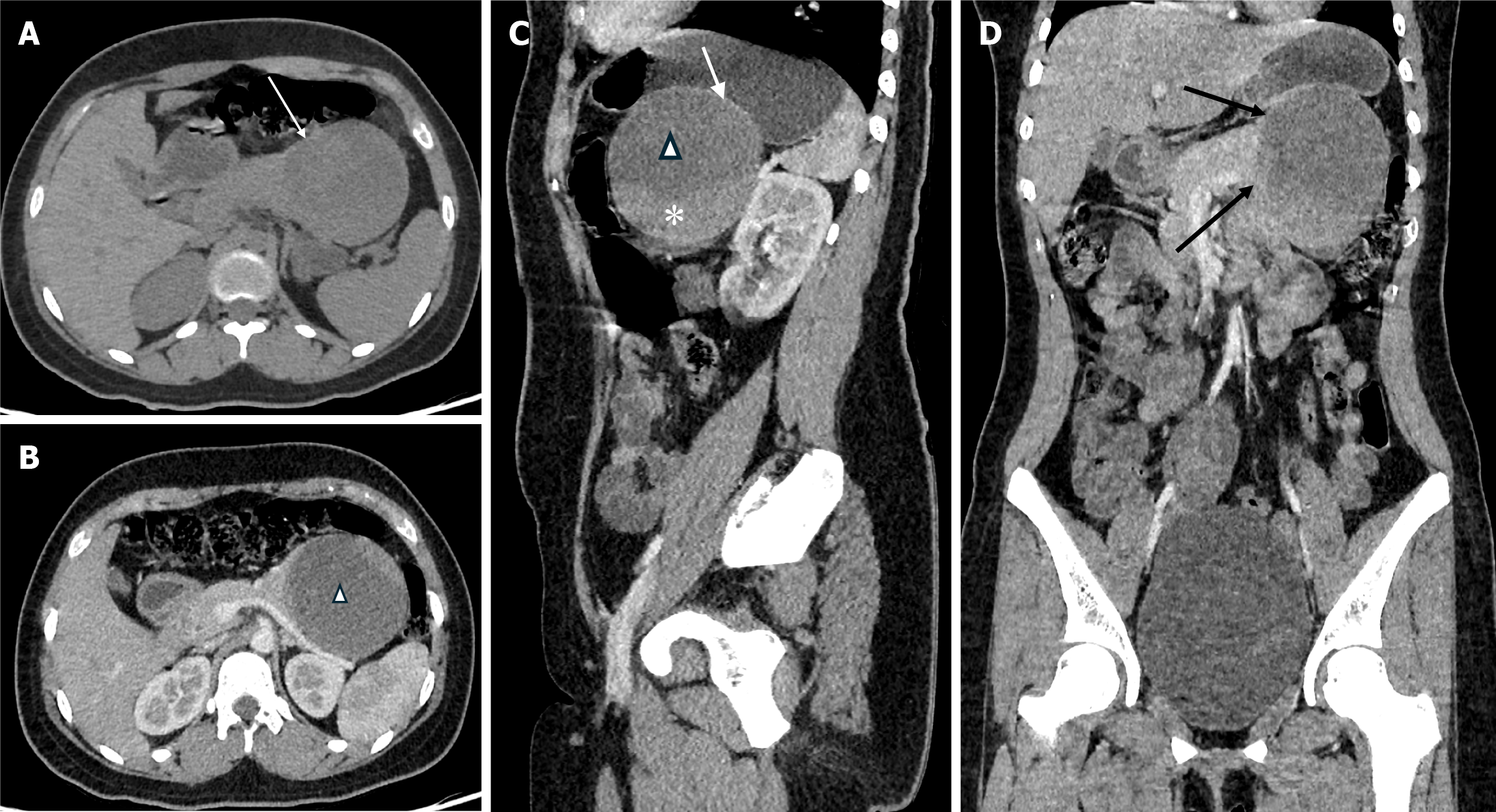
The patient underwent abdominal ultrasonography (Figure 1), which identified a well-defined heterogeneous solid-cystic mass measuring 7.6 cm × 7.2 cm in the epigastric region, seemingly originating from the tail of the pancreas. Notably, the solid component exhibited demonstrable vascularity raising suspicion for a pancreatic solid cystic tumor. A contrast-enhanced computed tomography scan of the abdomen and pelvis (Figure 2) was subsequently conducted, revealing a well-defined cystic lesion with an enhancing solid component and capsule, in the region of the tail of the pancreas. The lesion was giving a “claw sign[7] with the pancreas” confirming its intraparenchymal origin. The lesion was observed to be in close proximity to several structures. It abutted the posterior surface of the stomach superiorly, the left kidney, and the left splenic vessel posteriorly, and it displaced the bowel loops anteriorly. These findings were suggestive of a likely cystic neoplasm of the pancreas.
The final diagnosis of solid pseudopapillary epithelial neoplasm of the pancreas was confirmed by histopathological reports.
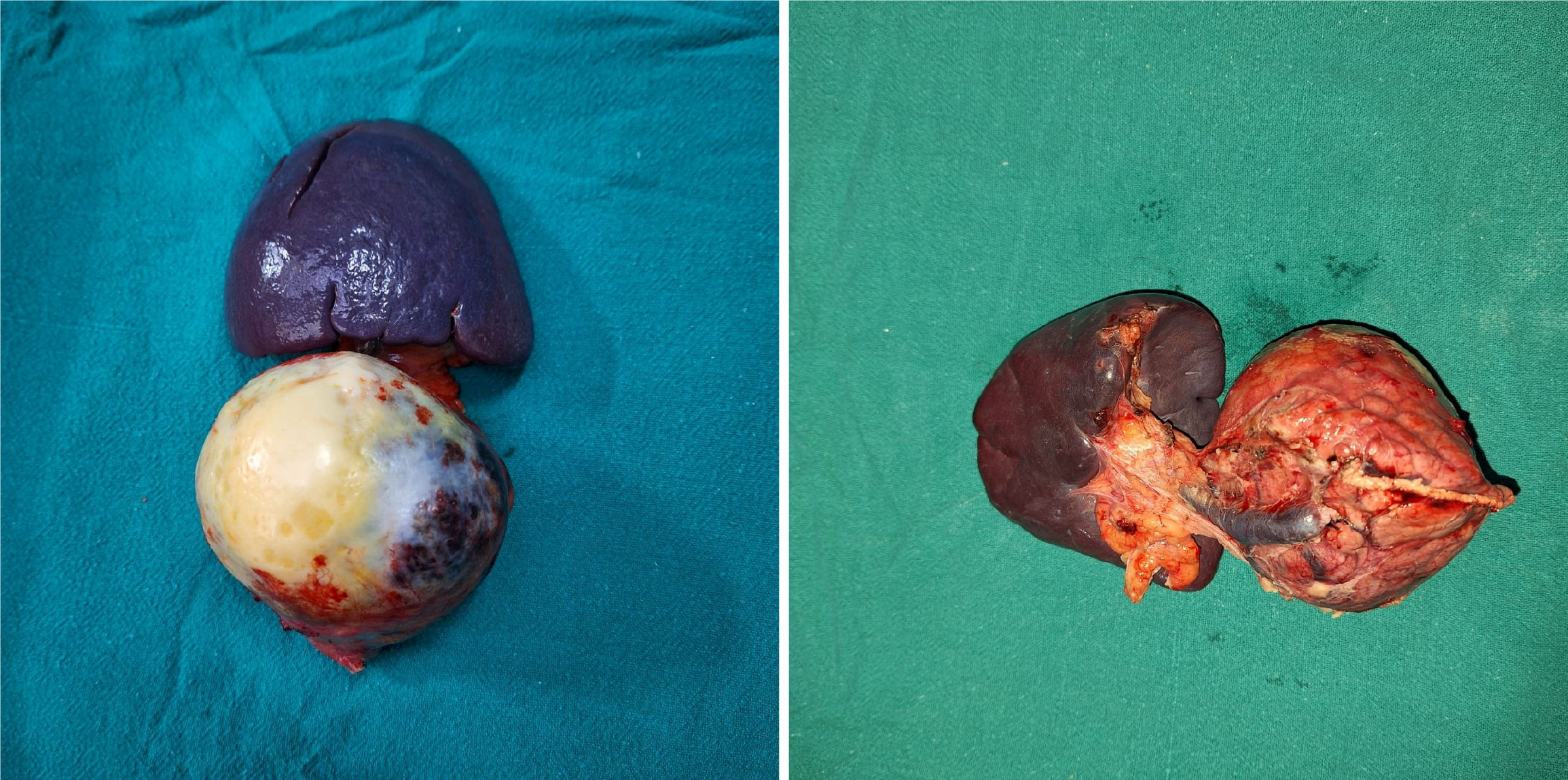
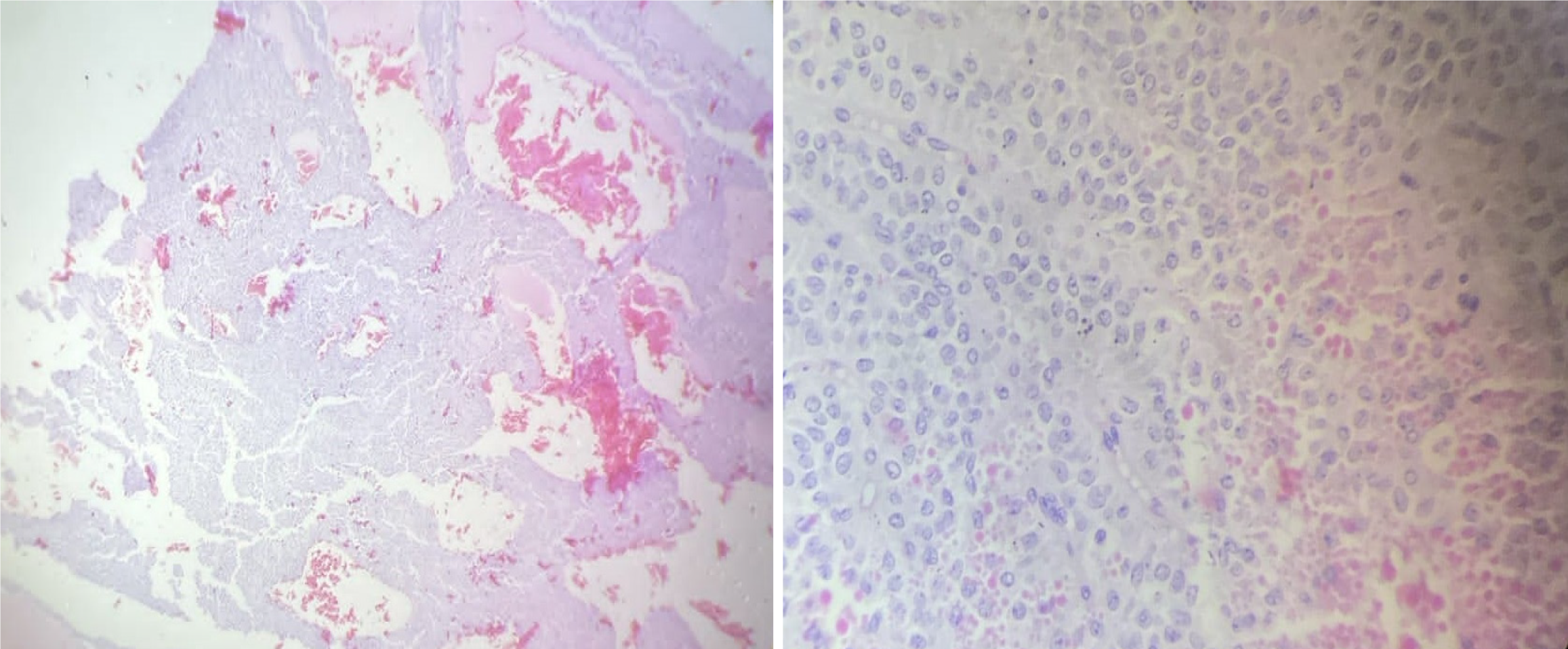
Surgical resection of the mass was planned, and an open distal pancreatectomy with splenectomy was carried out under general anesthesia. During the procedure, a well-encapsulated solid-cystic mass measuring 7 cm × 7 cm × 8 cm was identified, originating from the tail of the pancreas. An area of whitish patchy tissue was observed anteriorly over the lesion. There were no indications of omental, mesenteric, peritoneal, or hepatic deposits, and there was no regional lymphadenopathy or ascites. The entire tumor was successfully resected (Figure 3) and sent for histopathological examination (Figure 4). Postoperatively, the patient exhibited mild leukocytosis with a white blood cell count of 16780/mm³, predominantly composed of neutrophils (86%). Additionally, there was a slight elevation in prothrombin time at 17 seconds and an international normalized ratio of 1.21. On the third postoperative day, a significant increase in serum amylase level was noted, reaching 1742 U/L, suggestive of a postoperative pancreatic fistula, which gradually resolved spontaneously.
The patient successfully recovered from the surgery and was discharged from the hospital. She has been kept under regular follow-up to monitor for any potential recurrence of the tumor.
SPN of the pancreas is a very rare condition, primarily affecting young women in their second or third decades of life, though it can also occur in children[6]. The significant predominance of females has been suggested to result from the closeness of primitive pancreatic cells to the ovarian ridge during the early stage of development[8]. The clinical features of SPN of the pancreas are nonspecific and may include unusual abdominal pain, abdominal mass palpable during a clinical examination, or an incidental finding during imaging studies conducted for other purposes[9]. As the tumor is typically found in either the head or tail of the pancreas[6], its growth may lead to symptoms associated with the compression of nearby digestive structures, bile ducts, or blood vessels[10]. In some cases, the tumor may be detected following a spontaneous bleeding episode or as a result of bleeding due to abdominal trauma[11].
Despite the advancements in diagnostic techniques, accurately diagnosing SPNs preoperatively remains a clinical challenge[12]. This is due to their radiological and clinical similarities with a variety of differential diagnoses, such as pseudocysts, pancreatic mucinous neoplasms, well-differentiated ductal adenocarcinoma, pancreatic endocrine neoplasms, and acinic cell carcinoma[13]. In children, pancreatic tumors that arise from secondary origins, such as neuroblastoma, leukemia, lymphoma, and lymphoproliferative disorders, are more commonly observed[6].
A contrast-enhanced computed tomography scan of the abdomen is considered the most effective imaging modality for SPNs as it offers comprehensive information about tumors including their origin, size, and configuration, as well as details regarding the local invasion and the presence of metastasis[6]. The encapsulated lesions, characterized by a mixture of solid and cystic components, display both enhancing and non-enhancing areas and often contain intra-tumoral calcifications[6]. In addition, hemorrhage can arise from the growth of the tumor that results in internal degeneration[11]. Thus, a mass covered with a capsule, containing both cystic and solid components along with intra-tumoral hemorrhage, distinguishes SPNs from other malignant tumors[6,14,11]. Macroscopically, SPNs are typically large, well-circumscribed tumors with a diverse appearance, often exhibiting areas of necrosis, hemorrhage, and cystic degeneration, which can be attributed to vascular ischemia[15]. Pseudo-papillae are created when tumor cells detach from blood vessels and develop fibrovascular stalks or rosette-like structures[16].
Microscopically, SPNs are typically characterized by solid sheets of neoplastic cells, which are often interspersed with regions where the tumor cells are organized around the fibrovascular cores[17]. A distinctive feature of these tumors is the presence of periodic acid-Schiff-positive hyaline globules[18]. Other histological characteristics include the presence of pseudo-papillary architecture, microcystic changes, clear cells, nuclear grooves, eosinophilic cytoplasm, myxoid stroma, and hyaline globules. Additional features such as atypical cells, tumor giant cells, mitotic activity, calcification, cholesterol clefts, fibrosis, hemorrhage, infarction, and tumor necrosis might also be noted during histological evaluation[18].
To complement the histological findings and to differentiate SPNs from histological mimics, immunohistochemical staining is crucial. A panel of commonly used immunohistochemical markers includes CD10, CD56, beta-catenin, cyclin D1, CD99, cytokeratins, chromogranin A, synaptophysin, and progesterone receptor[17]. All patients with SPN possess activating somatic mutations in the β-catenin gene (catenin beta1, located on chromosome 3p)[19]. Increased expression of certain proteins involved in Wnt signaling, such as Dickkopf-related protein 4, along with other proteins like non-POU domain-containing octamer-binding and Fused in sarcoma, that interact directly with β-catenin, are upregulated in solid pseudopapillary tumor[20]. Moreover, nine metabolic proteins including SLC25A13, glycosylphosphatidylinositol, phosphoglycerate kinase 1, hexokinase 1, enzyme enolase 2, pyruvate dehydrogenase E1 component subunit beta, ALDH7A1, pyruvate kinase M2, and DLD are overexpressed[21]. But in our case, due to the limited resources available, the diagnosis was largely made based on the typical clinical presentation, imaging results, and histopathological features, which are often sufficient to confirm the diagnosis in such situations.
The mainstay of treatment of SPNs remains the surgical removal of the tumor[6]. Depending on the location, procedures such as distal pancreatectomy with or without splenectomy, pylorus-preserving pancreatoduodenectomy, Whipple procedure, or enucleation may be carried out[22]. Small tumors located away from the main pancreatic duct are enucleated, those located on the head or uncinate process of the pancreas are treated by pancreatoduodenectomy, while central pancreatectomy is carried out for the tumors of the neck or body of the pancreas, without vessel involvement[23]. The surgical resection must be performed carefully, as the spillage of the tumor contents can lead to the implantation of the tumor cells into the peritoneum[13,24].
Studies show that patients who had limited resection with microscopically positive margins showed outcomes similar to those who underwent extensive surgery with R0 resection[25]. Thus, longevity can be attained with minimally invasive procedures[26], even in patients with advanced or metastatic disease[27]. Moreover, chemotherapy (primarily 5-fluorouracil and gemcitabine) and radiotherapy have been reported to be effective treatment modalities in a limited number of patients[28]. Other modalities such as radiofrequency ablation[29], transcatheter arterial chemoembolization with pharmorubicin and iodized oil[30], and selective internal radiotherapy[31] have also been found to be suitable for inducing long-term remissions of the strongly vascularized liver metastases. In a study done by Dovigo et al[32], liver transplantation was found to result in recurrence-free survival of at least 1 year.
The reported recurrence rate after surgery lies between 3%-9%[14]. According to a study done by Serrano et al[33], recurrence commonly occurred 5 to 7 years after surgery, indicating that a follow-up period of more than 5 years is necessary by routine imaging, especially for patients with lymphatic and vascular invasion, metastases, and probable tumor capsule invasion. A meta-analysis reported a recurrence rate of 2% following resection, highlighting that male patients, those with positive lymph nodes, and individuals with lymphovascular invasion were at an increased risk for recurrence[34]. The patient in our case underwent surgery on April 24, 2024, and is under regular follow-up since then.
In conclusion, this case highlights the significant possibility of SPN occurring in adolescent girls, which is often overlooked in clinical practice. It underscores the importance of vigilance and thorough assessment in adolescent patients, ensuring that conditions like SPN are not missed and that appropriate care is provided promptly. Early intervention can significantly alter the course of the disease, leading to more favorable results and ultimately improving the quality of life for young patients.
We would like to extend our heartfelt gratitude to the patient and her family for their generous willingness to share this case with us for publication.
| 1. | Salvia R, Bassi C, Festa L, Falconi M, Crippa S, Butturini G, Brighenti A, Capelli P, Pederzoli P. Clinical and biological behavior of pancreatic solid pseudopapillary tumors: report on 31 consecutive patients. J Surg Oncol. 2007;95:304-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 73] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 2. | Botello-hernández Z, Fuentes-reyes R, Hernández-gonzález M, Mosqueira-mondragón C, Pérez-torres E, Chapa-azuela O. Frantz's tumour. Unusual presentation in two adolescents. Revista Médica del Hospital General de México. 2018;81:122-126. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 3. | Cai YQ, Xie SM, Ran X, Wang X, Mai G, Liu XB. Solid pseudopapillary tumor of the pancreas in male patients: report of 16 cases. World J Gastroenterol. 2014;20:6939-6945. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 4. | Branco C, Vilaça S, Falcão J. Solid pseudopapillary neoplasm-Case report of a rare pancreatic tumor. Int J Surg Case Rep. 2017;33:148-150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Eric D, Milosavljevic V, Gonzalez-Urquijo M, Tadic B, Veselinovic M, Grubor N, Jelic D, Bjelovic M. Laparoscopic enucleation of Frantz's tumor of the pancreas: Case report and literature review. Ann Med Surg (Lond). 2021;64:102221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Papavramidis T, Papavramidis S. Solid pseudopapillary tumors of the pancreas: review of 718 patients reported in English literature. J Am Coll Surg. 2005;200:965-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 533] [Article Influence: 26.7] [Reference Citation Analysis (1)] |
| 7. | Xiang H, Han J, Ridley WE, Ridley LJ. Claw sign: Origin of a mass. J Med Imaging Radiat Oncol. 2018;62 Suppl 1:66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Kosmahl M, Seada LS, Jänig U, Harms D, Klöppel G. Solid-pseudopapillary tumor of the pancreas: its origin revisited. Virchows Arch. 2000;436:473-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 226] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 9. | Limaiem F, Mestiri H, Mejri S, Lahmar A, Mzabi S. Solid pseudopapillary neoplasm of the pancreas in two male patients: gender does not matter. Pan Afr Med J. 2017;27:283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Maimaijiang A, Wang H, Li W, Wang Y. Diagnosis and treatment of solid pseudopapillary neoplasm of the pancreas in children: A report of 18 cases. Front Pediatr. 2022;10:899965. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 11. | Xu X, Chen D, Cao L, Feng X, Tong R, Zheng S, Wu J. Spontaneous rupture of solid pseudopapillary tumor of pancreas: A case report and review of literature. Medicine (Baltimore). 2019;98:e17554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Chon HK, Choi KH, Kim TH. An Unusual Presentation of a Solid Pseudopapillary Tumor of the Pancreas Mimicking Adenocarcinoma. Clin Endosc. 2020;53:615-619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Wu H, Huang YF, Liu XH, Xu MH. Extrapancreatic solid pseudopapillary neoplasm followed by multiple metastases: Case report. World J Gastrointest Oncol. 2017;9:497-501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Sharma PK, Mehrotra S, Gleisner AL, Schulick RD, McCarter MD. Recurrent Solid Pseudopapillary Neoplasm of Pancreas: Case Report and Review of Literature. J Pancreat Cancer. 2018;4:25-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Uppin SG, Hui M, Thumma V, Challa S, Uppin MS, Bheerappa N, Sastry RA, Paul TR, Prayaga AK. Solid-pseudopapillary neoplasm of the pancreas: a clinicopathological and immunohistochemical study of 33 cases from a single institution in Southern India. Indian J Pathol Microbiol. 2015;58:163-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | La Rosa S, Bongiovanni M. Pancreatic Solid Pseudopapillary Neoplasm: Key Pathologic and Genetic Features. Arch Pathol Lab Med. 2020;144:829-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 67] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 17. | Din NU, Rahim S, Abdul-Ghafar J, Ahmed A, Ahmad Z. Clinicopathological and immunohistochemical study of 29 cases of solid-pseudopapillary neoplasms of the pancreas in patients under 20 years of age along with detailed review of literature. Diagn Pathol. 2020;15:139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Zalatnai A, Kis-Orha V. Solid-pseudopapillary Neoplasms of the Pancreas is still an Enigma: a Clinicopathological Review. Pathol Oncol Res. 2020;26:641-649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 19. | Rodriguez-Matta E, Hemmerich A, Starr J, Mody K, Severson EA, Colon-Otero G. Molecular genetic changes in solid pseudopapillary neoplasms (SPN) of the pancreas. Acta Oncol. 2020;59:1024-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Guo M, Luo G, Jin K, Long J, Cheng H, Lu Y, Wang Z, Yang C, Xu J, Ni Q, Yu X, Liu C. Somatic Genetic Variation in Solid Pseudopapillary Tumor of the Pancreas by Whole Exome Sequencing. Int J Mol Sci. 2017;18:81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (1)] |
| 21. | Park M, Lim JS, Lee HJ, Na K, Lee MJ, Kang CM, Paik YK, Kim H. Distinct Protein Expression Profiles of Solid-Pseudopapillary Neoplasms of the Pancreas. J Proteome Res. 2015;14:3007-3014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Yagcı A, Yakan S, Coskun A, Erkan N, Yıldırım M, Yalcın E, Postacı H. Diagnosis and treatment of solid pseudopapillary tumor of the pancreas: experience of one single institution from Turkey. World J Surg Oncol. 2013;11:308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 23. | Coelho JCU, da Costa MAR, Ramos EJB, Torres AR, Savio MC, Claus CMP. Surgical Management of Solid Pseudopapillary Tumor of the Pancreas. JSLS. 2018;22:e2018.00032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 24. | Yamaguchi H, Morisaka H, Sano K, Nagata K, Ryozawa S, Okamoto K, Ichikawa T. Seeding of a Tumor in the Gastric Wall after Endoscopic Ultrasound-guided Fine-needle Aspiration of Solid Pseudopapillary Neoplasm of the Pancreas. Intern Med. 2020;59:779-782. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Estrella JS, Li L, Rashid A, Wang H, Katz MH, Fleming JB, Abbruzzese JL, Wang H. Solid pseudopapillary neoplasm of the pancreas: clinicopathologic and survival analyses of 64 cases from a single institution. Am J Surg Pathol. 2014;38:147-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 26. | Zhang H, Liang TB, Wang WL, Shen Y, Ren GP, Zheng SS. Diagnosis and treatment of solid-pseudopapillary tumor of the pancreas. Hepatobiliary Pancreat Dis Int. 2006;5:454-458. [PubMed] |
| 27. | You L, Yang F, Fu DL. Prediction of malignancy and adverse outcome of solid pseudopapillary tumor of the pancreas. World J Gastrointest Oncol. 2018;10:184-193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (1)] |
| 28. | Law JK, Ahmed A, Singh VK, Akshintala VS, Olson MT, Raman SP, Ali SZ, Fishman EK, Kamel I, Canto MI, Dal Molin M, Moran RA, Khashab MA, Ahuja N, Goggins M, Hruban RH, Wolfgang CL, Lennon AM. A systematic review of solid-pseudopapillary neoplasms: are these rare lesions? Pancreas. 2014;43:331-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 239] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 29. | Li JX, Wu H, Huang JW, Prasoon P, Zeng Y. Synchronous intraoperative radiofrequency ablation for multiple liver metastasis and resection of giant solid pseudopapillary tumors of the pancreas. Chin Med J (Engl). 2012;125:1661-1663. [PubMed] |
| 30. | Liu T, He J, Cao D, Huang Y. Successful treatment of liver metastasis from solid pseudopapillary tumor of the pancreas: a case report. Contemp Oncol (Pozn). 2013;17:400-403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 31. | Krug S, Bartsch DK, Schober M, Librizzi D, Pfestroff A, Burbelko M, Moll R, Michl P, Gress TM. Successful selective internal radiotherapy (SIRT) in a patient with a malignant solid pseudopapillary pancreatic neoplasm (SPN). Pancreatology. 2012;12:423-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Dovigo AG, Díaz MB, Gütierrez MG, Selles CF, Grobas JP, Valladares M, Suárez F, Marini M. Liver transplantation as treatment in a massive metastasis from Gruber-Frantz pancreatic tumor: a case report. Transplant Proc. 2011;43:2272-2273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 33. | Serrano PE, Serra S, Al-Ali H, Gallinger S, Greig PD, McGilvray ID, Moulton CA, Wei AC, Cleary SP. Risk factors associated with recurrence in patients with solid pseudopapillary tumors of the pancreas. JOP. 2014;15:561-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 34. | Yepuri N, Naous R, Meier AH, Cooney RN, Kittur D, Are C, Jain A, Dhir M. A systematic review and meta-analysis of predictors of recurrence in patients with Solid Pseudopapillary Tumors of the Pancreas. HPB (Oxford). 2020;22:12-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |