Published online Mar 15, 2025. doi: 10.4251/wjgo.v17.i3.101734
Revised: November 12, 2024
Accepted: December 23, 2024
Published online: March 15, 2025
Processing time: 142 Days and 8.2 Hours
Metachronous gastric cancer usually refers to a tumor that occurs in the stomach more than half a year after esophageal cancer surgery, and metastasis of primary esophageal cancer should be excluded. There are few reports of metachronous gastric adenosquamous carcinoma with signet ring cell carcinoma combined with early tubular adenoma of the colon after esophageal cancer surgery, which has a high degree of malignancy. This is also the reason for the poor treatment results.
A 54-year-old male patient was admitted to the hospital with “dysphagia obstruc
He had a history of esophageal cancer resection. Gastroenteroscopy should be performed simultaneously to avoid missed diagnosis and misdiagnosis.
Core Tip: The common type of gastric cancer is adenocarcinoma, but the pathological type of gastric adenosquamous carcinoma is rare, so metachronous gastric adenosquamous carcinoma with signet ring cell carcinoma is even rarer. The metachronous tumors after esophageal cancer surgery are mostly found in the stomach, head and neck, but those occurring in the stomach and distal colon at the same time are rare.
- Citation: Liu GJ, Long XY, Zhang F, Ren T, Xia X. Heterochronic gastric adenosquamous carcinoma combined with colonic adenoma: A case report. World J Gastrointest Oncol 2025; 17(3): 101734
- URL: https://www.wjgnet.com/1948-5204/full/v17/i3/101734.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i3.101734
Metachronous gastric cancer is usually a malignant tumor that occurs in the stomach after esophageal squamous cell carcinoma resection[1,2]. The usual pathological type of gastric cancer is adenocarcinoma, while gastric adenosquamous carcinoma (GAC) accounts for < 1% of gastric cancers[3], accompanied by signet ring cell carcinoma (SRCC), and neoplastic lesions with early tubular adenoma of the colon (ETAC) are rarely reported. To date, the relationship between postoperative esophageal cancer and metachronous tumors is unclear. The occurrence of metachronous tumors is often closely related to normal physiological and structural changes in the digestive tract after esophageal cancer surgery, Helicobacter pylori infection, smoking, alcoholism, and other factors[2,4]. However, the patient had only a history of resection of esophageal cancer. Our case report aims to improve our understanding of metachronous tumors after esophageal cancer surgery.
A 54-year-old male patient was admitted to the hospital for swallowing obstruction half a month ago.
A 54-year-old male patient was admitted to the hospital for swallowing obstruction half a month ago and there was no abdominal pain or bloating. The patient did not receive medical treatment, his symptoms were not relieved, and he was admitted to our hospital for medical treatment.
The patient had a history of esophageal cancer resection.
The patient had a history of esophageal cancer resection and no adverse living habits. There was no family history of malignant tumors.
Physical examination showed that there were no abnormalities in the superficial lymph nodes of the whole body. The old surgical scar was approximately 15 cm in the chest and no abnormal signs, such as an abdominal mass, were observed.
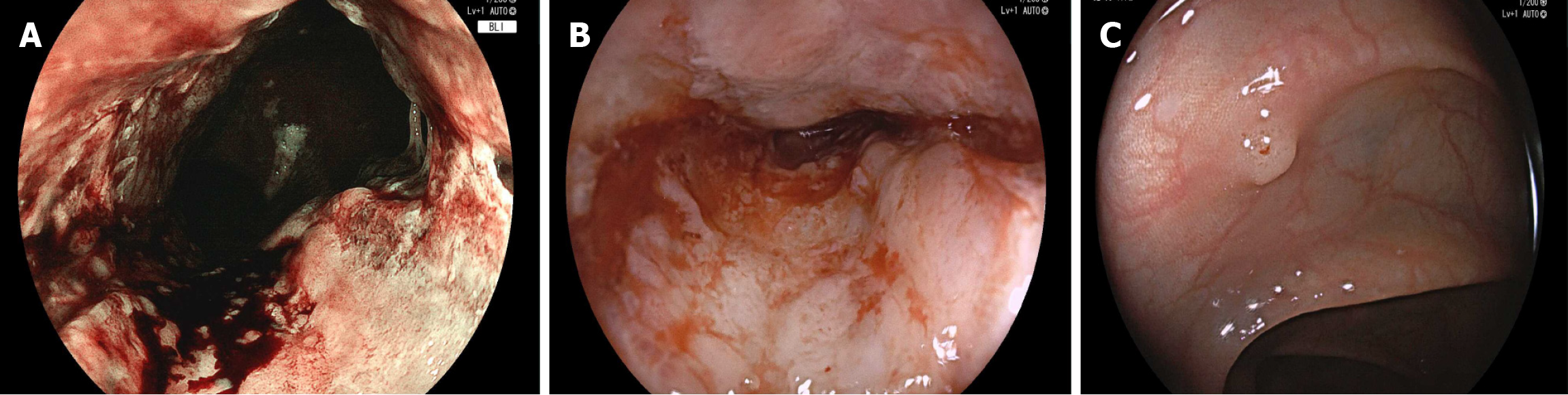
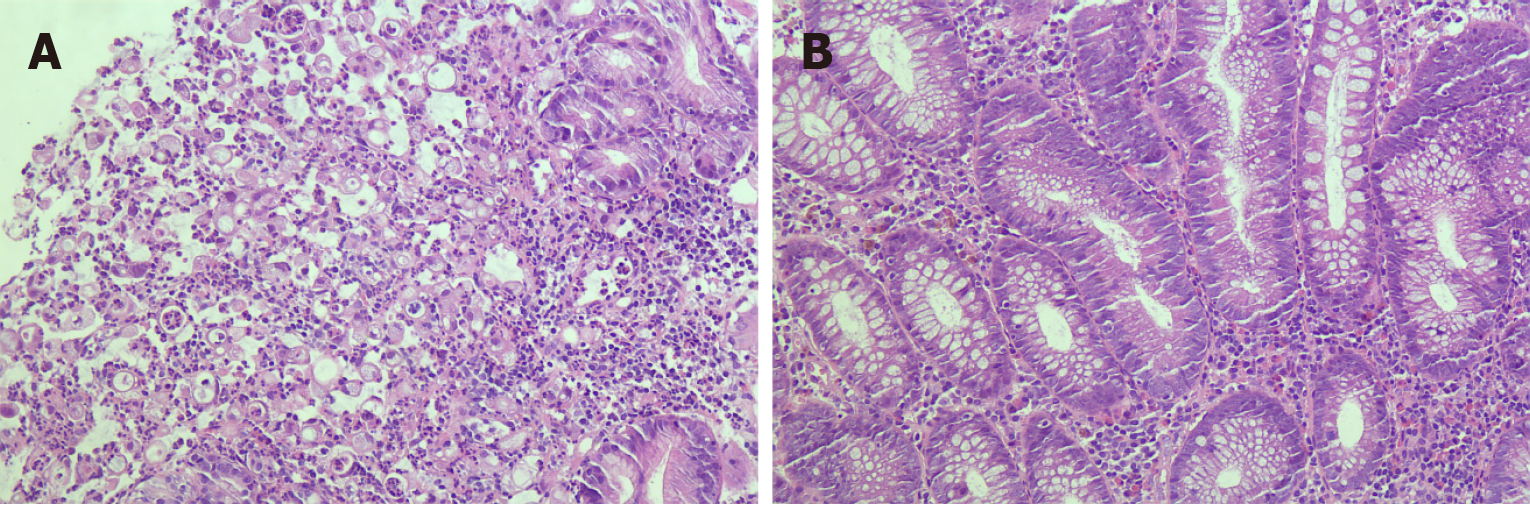
Blood cells, electrolytes, and liver and kidney functions were normal at admission. After admission, gastroscopy showed that the gastric mucosa was rough, erosive, brittle, and easily bled. The structure of the gland duct stained by blue light imaging disappeared and the lumen was narrow. Colonoscopy showed that the mucosa was orange-red, blood vessels were clearly visible and polyps with a diameter of approximately 0.4 cm were observed, with a smooth surface and a regular glandular duct structure (Figure 1). Hematoxylin-eosin staining of the gastric and colon lesions showed a poorly differentiated carcinoma, Helicobacter pylori (-), and ETAC in the stomach (Figure 2). Immunohistochemistry of the gastric tumor cells showed cytokeratin 7 (+), E-cadherin (+), human epidermal growth factor receptor 2 (+), Ki-67 (+), about (60%-70%) and S-100 (-). Caudal-type homeobox protein 2, cytokeratin 20, P40, CA5/6, and mucin 5AC (+) were detected in some tumor cells. The pathological diagnosis was a poorly differentiated GAC from SRCC. As there are many immu
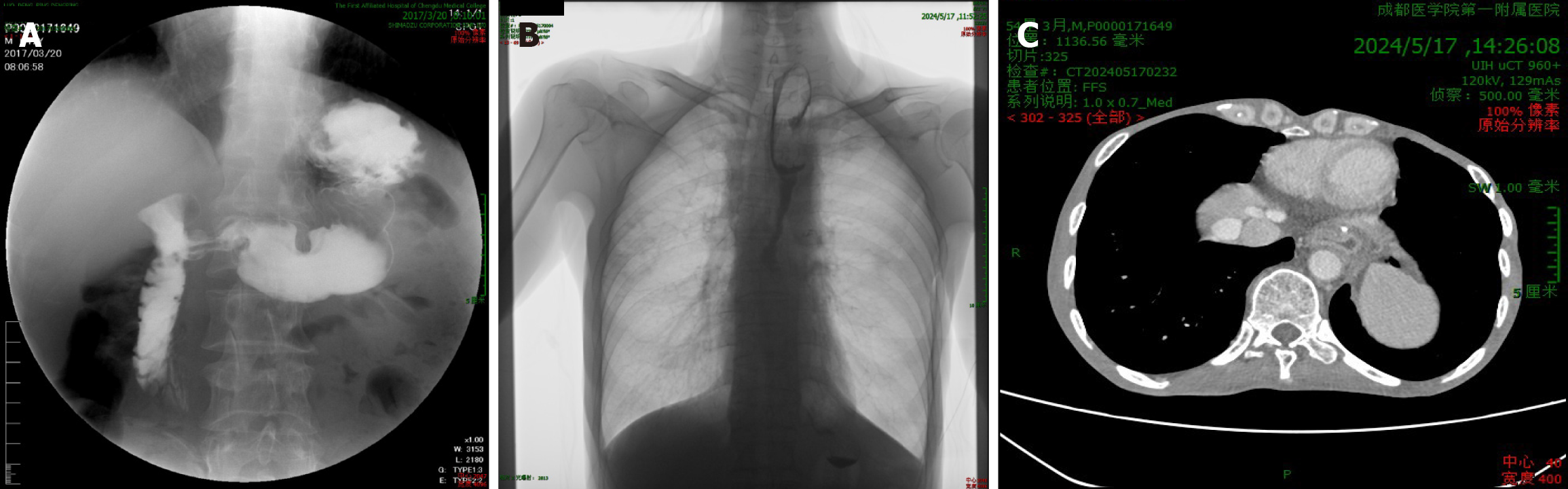
Seven years prior, the patient’s gastrointestinal radiography revealed a hooked stomach with a smooth and soft stomach wall and slightly thick mucosal folds. No filling defects or niches were found. After admission, gastrointestinal radio
Eventually, he was diagnosed with metachronous GAC with SRCC and metachronous ETAC.
The diagnosis was confirmed by gastroscopy after admission. Considering the complexity and high risk of a second surgery, the patient chose chemotherapy. The patient received 160 mg of intravenous oxaliplatin on day 1. At the same time, capecitabine 1.5 g was taken orally three times a day for 1-14 days. Treatment was administered every three weeks. On the first day of chemotherapy, the patient vomited, and the electrolyte levels decreased. Intravenous potassium supplementation and ondansetron hydrochloride injection (8 mg intravenous drip) were administered for symptomatic treatment. On day 5, blood tests showed that electrolytes and liver and kidney functions were normal, and the patient felt that symptoms were relieved, refused to continue treatment, and was discharged automatically.
The patient was discharged after symptom relief from initial chemotherapy. After discharge, the patient did not return to our hospital for further treatment or outpatient follow-up. We called 5 months later and were told that the patient had died.
Although current research shows that metachronous gastric cancer is increasing, the total incidence of gastric cancer for esophageal replacement is between 1.5% and 8.6%[1,5], and gastric cancer is common as a pathological type of adenocarcinoma. However, this pathological type of GAC is rare[1,3], so it is even rarer for metachronous GAC with SRCC combined with ETAC. Generally, the necessary condition for diagnosing adenosquamous carcinoma is that the pro
Imaging revealed that the contrast agent slowly passed through the digestive tract when our patient underwent gastrointestinal radiography. Chest-enhanced CT showed that the stomach wall was thickened, and the thickened stomach wall showed uneven enhancement. Combined with the literature[10], it is indicated that we should pay attention to the occurrence of metachronous gastric cancer when these imaging features appear because metachronous gastric cancer is asymptomatic in the early stage and is mostly found in the middle and late stages. Complications such as esophageal stricture, tumor recurrence, and metastasis can occur after esophageal cancer surgery, and the recurrence time of the tumor is different[11,12]. Esophageal cancer is asymptomatic or atypical in the early stages[11], and most patients do not know enough about the tumor. Therefore, follow-up is helpful in determining the patient’s condition in time, providing useful suggestions in time, and identifying and treating early. At the same time, postoperative follow-up can help to understand the quality of life of patients, which is beneficial for evaluating surgical effect[13]. Therefore, a gastroscopy must be performed every year after esophageal cancer surgery, which lasts more than 10 years[1]. Meta
After esophageal cancer surgery, the tumor can recur, which may also cause metachronous GAC and SRCC, and at the same time, it can be complicated by intestinal adenoma. Our literature search reveals that the latter is uncommon. Chest CT, gastrointestinal radiography, and other imaging examinations are often used to examine typical lesions that are not sufficient to diagnose early metachronous tumors of the digestive tract. Therefore, it is best to use a gastroscope as a reexamination method after esophageal cancer surgery and, at the same time, colonoscopy should be reexamined to avoid missed diagnosis. Combined with previous case reports, factors such as Helicobacter pylori infection and drinking can promote the occurrence of postoperative metachronous tumors. However, this patient only had factors of change in digestive tract structure. Therefore, we speculate that the latter may promote the occurrence of metachronous gas
We thank all authors who helped with this research.
| 1. | Lee GD, Kim YH, Choi SH, Kim HR, Kim DK, Park SI. Gastric conduit cancer after oesophagectomy for oesophageal cancer: incidence and clinical implications. Eur J Cardiothorac Surg. 2014;45:899-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 2. | Hirao M, Katada C, Yokoyama T, Yano T, Suzuki H, Furue Y, Yamamoto K, Doyama H, Koike T, Tamaoki M, Kawata N, Kawahara Y, Katagiri A, Ogata T, Yamanouchi T, Kiyokawa H, Kawakubo H, Konno M, Ishikawa H, Yokoyama A, Muto M. Metachronous primary gastric cancer after endoscopic resection in patients with esophageal squamous cell carcinoma. Gastric Cancer. 2023;26:988-1001. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 3. | Du Y, Tian H, Chen Z, Mao G, Shen Q, Jiang Q, Yin Y, Tao K, Zeng X, Zhang P. Analysis of clinicopathological characteristics and prognosis on primary gastric adenosquamous carcinoma. Sci Rep. 2024;14:16198. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 4. | Choi IJ, Kook MC, Kim YI, Cho SJ, Lee JY, Kim CG, Park B, Nam BH. Helicobacter pylori Therapy for the Prevention of Metachronous Gastric Cancer. N Engl J Med. 2018;378:1085-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 495] [Article Influence: 70.7] [Reference Citation Analysis (0)] |
| 5. | Bamba T, Kosugi S, Takeuchi M, Kobayashi M, Kanda T, Matsuki A, Hatakeyama K. Surveillance and treatment for second primary cancer in the gastric tube after radical esophagectomy. Surg Endosc. 2010;24:1310-1317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Li B, Liang H. [Research progress in primary gastric adenosquamous carcinoma]. Fubu WaiKe. 2022;35:77-80. |
| 7. | Sonnenberg A, Turner KO, Genta RM. Associations between gastric histopathology and the occurrence of colonic polyps. Colorectal Dis. 2020;22:814-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Harewood R, Wooldrage K, Robbins EC, Kinross J, von Wagner C, Cross AJ. Adenoma characteristics associated with post-polypectomy proximal colon cancer incidence: a retrospective cohort study. Br J Cancer. 2022;126:1744-1754. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Arai T. Where does signet-ring cell carcinoma come from and where does it go? Gastric Cancer. 2019;22:651-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Imaging Cooperative Group of Gastric Cancer Professional Committee of China Anti-Cancer Association; Abdomen Group of Chinese Society of Radiology. [Expert consensus on standardized process of imaging examination and diagnosis of gastric cancer (2022 edition)]. Zhonghua Wei Chang Wai Ke Za Zhi. 2022;25:859-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 11. | Nobel TB, Dave N, Eljalby M, Xing X, Barbetta A, Hsu M, Tan KS, Janjigian Y, Bains MS, Sihag S, Jones DR, Molena D. Incidence and Risk Factors for Isolated Esophageal Cancer Recurrence to the Brain. Ann Thorac Surg. 2020;109:329-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 12. | Kwon H, Popoff AM. Stenosis in Esophageal Cancer: A Poor Prognostic Indicator. Ann Surg Oncol. 2024;31:716-717. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 13. | Cheng Z, Johar A, Nilsson M, Lagergren P. Cancer-Related Fatigue After Esophageal Cancer Surgery: Impact of Postoperative Complications. Ann Surg Oncol. 2022;29:2842-2851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |