Published online Feb 15, 2025. doi: 10.4251/wjgo.v17.i2.97298
Revised: October 1, 2024
Accepted: November 1, 2024
Published online: February 15, 2025
Processing time: 235 Days and 16.4 Hours
Data on clinical characteristics, treatment outcomes, and prognosis of pancreatic primitive neuroectodermal tumors (PNETs) are limited.
To analyze the clinical data of 30 patients with pancreatic PNETs to identify their clinical characteristics and factors associated with prognosis.
We used the keywords "primary neuroectodermal tumor," "digestive tract," "pancreas," "pancreatic," and "gastrointestinal," individually or in combination, to collect data from a global database for all patients with pancreatic PNET to date. Univariate and Cox regression analyses were performed to identify prognostic factors for patient survival.
A total of 30 cases of pancreatic PNET were included in this study: 15 males and 15 females with a mean age of 24 years. The main symptom was abdominal pain (73.3%), and the median tumor size was 7.85 cm. Twenty-four patients (80.0%) underwent surgery and nineteen patients received adjuvant therapy. Local metastasis was observed in 13 patients (43.3%), lymph node metastasis in 10 patients (33.3%), and distant metastasis in 6 patients (20.0%). Local recurrence was observed in 13 patients (43.3%). The median survival time of all patients was 29.4 months, and the overall estimated 1-year and 3-year survival rates were approximately 66.0% and 36.4%, respectively. Univariate analysis showed that chemotherapy (P = 0.036), local metastasis (P = 0.041), lymph node metastasis (P = 0.003), distant metastasis (P = 0.049), and surgical margins (P = 0.048) were the prognostic factors affecting survival. Multivariate analysis revealed only lymph node metastasis (P = 0.012) as a prognostic factor.
Pancreatic PNET is extremely rare, occurs in young adults, has no apparent sex predisposition, has a high rate of metastasis and early recurrence, and has a very poor prognosis. The diagnosis of pancreatic PNET requires a combination of clinical symptoms, pathologic features, immunohistochemistry, and cytogenetic analysis. Univariate analysis suggested that chemotherapy, metastasis, and surgical margins were prognostic factors affecting survival, and multivariate analysis suggested that lymph node metastasis is an important prognostic factor. Therefore, early diagnosis, early and extensive resection, and adjuvant chemoradiotherapy may help improve prognosis.
Core Tip: Pancreatic primitive neuroectodermal tumor (PNET) is a rare disease, which lacks sound treatment strategies and prognostic data. We identified 30 cases of pancreatic PNET from global databases and retrospectively analyzed their demographic data, clinical characteristics, and treatment factors. Univariate analysis showed that surgical margins, chemotherapy, local metastasis, lymph node metastasis, and distant metastasis were the prognostic factors affecting survival. Multivariate analysis revealed that only lymph node metastasis was a significant prognostic factor. The results of this study suggest that early diagnosis of pancreatic PNET is critical, and early wide resection and adjuvant radiotherapy may help to improve prognosis.
- Citation: He YF, Wang HZ, Hu XD, Liu JQ, Li HM, Wang J, Lu SF. Pancreatic primitive neuroectodermal tumors: Clinical features, treatment, and influencing factors. World J Gastrointest Oncol 2025; 17(2): 97298
- URL: https://www.wjgnet.com/1948-5204/full/v17/i2/97298.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i2.97298
Primitive neuroectodermal tumors (PNETs) are a group of embryonal malignancies characterized by primitive undifferentiated small round cells[1]. They were first described by Stout[2] in 1918, and are common in the skeletal tissues of the limbs, central nervous system, and deep soft tissues of children and young adults, with pancreatic involvement being extremely rare, accounting for only 0.3% of primary tumors[3]. Its etiology and pathogenesis remain unclear. Because of the vague symptoms of pancreatic PNET, the lack of specific imaging features, and the extreme similarity of its morphologic and immunohistochemical features to those of some other tumors[4], the diagnosis of pancreatic PNET remains extremely challenging, although the accuracy of diagnosis of PNET has improved in recent years through a combination of pathology, immunohistochemistry, and genetic testing[5]. Data on the clinical features and prognosis of pancreatic PNET are scarce, with only a few case reports and small retrospective studies[6,7]. There are no therapeutic guidelines for pancreatic PNET, and no reliable clinical or histological parameters to predict its tumor behavior. Therefore, it is necessary to expand the amount of data to analyze; summarize the data; and provide a comprehensive understanding of its clinical features, pathomechanisms, and biological behaviors to improve its diagnosis and treatment and improve the quality of patient survival.
Herein, we searched global databases to accumulate a sufficient number of cases for analysis to identify prognostic factors associated with survival for the diagnosis and treatment of pancreatic PNET.
A comprehensive search of PubMed, MEDLINE, China National Knowledge Infrastructure, Wanfang, EMBASE, Google Scholar, Scopus, Wiley, Cochrane, and ScienceDirect online databases was conducted by an experienced information specialist; to maximize the scope of the search, we did not set time and literature type restrictions, and the last update date was May 2, 2024. We excluded studies other than those published in Chinese and English. We used the keywords "primitive neuroectodermal tumor," "pancreas," "pancreatic," "digestive tract," and "gastrointestinal tract" for a comprehensive literature search. We also searched the gray literature on the preprint servers bioRxiv and medRxiv. In addition, we manually searched the references of the original articles included in the studies to avoid missing important literature that was not identified in the initial search. Because Ewing's sarcoma and PNET share the same chromosomal translocation, t (11; 22) (q24; q12), which was classified as the same tumor type by the World Health Organization in 2002, some authors considered Ewing's sarcoma and neuroectodermal tumors to be the same tumor, and some of them were included in the present study after careful review of the full-text content. This systematic review has been registered on the PROSPERO platform (ID: 595567).
Demographic and clinical factors were analyzed using descriptive statistics, and correlations between categorical variables were calculated using the χ2 test. The area under the curve was used to determine the optimal tumor size threshold. Kaplan-Meier curves were constructed to assess disease-specific survival, and differences between groups were compared using log-rank analysis. Data extracted from global databases included age, sex, year of study, main symptoms at diagnosis, tumor size, tumor location, type of treatment, surgical margins, presence of metastasis and recurrence, and outcome. Cox regression analysis was performed for factors that were statistically significant in the univariate analysis. Descriptive statistics were performed with Statistical Package for the Social Sciences software version 25 (IBM, Armonk, NY, United States). The Kaplan-Meier method was used to estimate 1-year and 3-year overall survival. P < 0.05 indicated statistical significance.
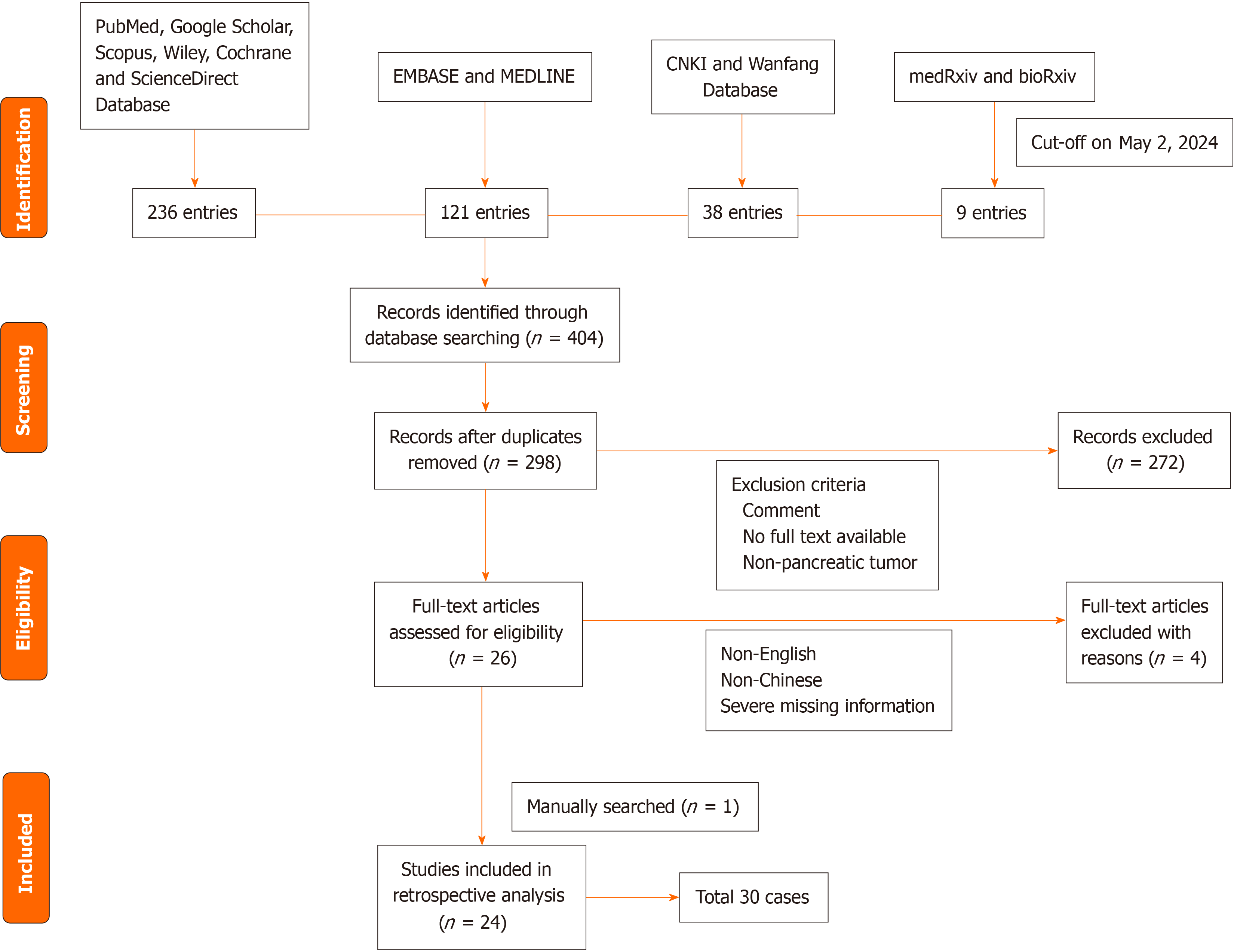
We retrieved a total of 27 papers, and after excluding 3 papers published in non-English and non-Chinese languages[3,8,9], as well as cases with unclear presentation, ambiguous data, and severe missing information[10], a total of 30 pancreatic PNET cases were finally included from 24 studies[6,7,11-32], the earliest of which was reported in 1989[30] and the latest in 2021[21]. Figure 1 shows a visual representation of the inclusion workflow.
There were 15 males and 15 females among 30 patients. The age range of the patients was 2-61 years, with a mean age of 24 years and a median age at diagnosis of 41 years. Sixteen patients (53.3%) were aged between 19 and 40 years, and 93% of the patients were younger than 60 years. Demographic data and tumor characteristics of the 30 patients with pancreatic PNET are shown in Table 1. Twenty-two of the thirty patients (73.3%) presented with abdominal pain and eight of the patients presented with jaundice. Two cases presented with rare precocious puberty[22,29]. The median tumor size was 7.85 cm and the maximum was 18 cm × 18 cm × 16 cm[27]. The most common site was the head of the pancreas (46.7%).
| Ref. | Age | Sex | Site | Size | Symp | Treat | Chemo | Surgi | Local meta | Regio | Distant meta | Follow up in months | Cytogenetics and molecular diagnostics | Patho | Necro | Recur | Site of recur | Out | ||
| Chro | Karyo | Gene fusion trans | ||||||||||||||||||
| Mao et al[6], 2013 | 13 | F | Pancrea | 3.5 × 2.5 × 1 | Abdo | Resec | VAC | (-) | (+) | (-) | (-) | 41 | t (11; 22) (q24; q12) | NR | NR | CD99, NSE, small round cells | Yes | (+) | Liver, kidney, colon | Died of disease after 41 months |
| Jayant et al[11], 2013 | 20 | F | Pancrea | 11 × 9 | Abdo | Distal pancrea | VAC | (-) | (-) | (-) | (-) | 24 | NR | NR | NR | CD99, NSE, small round cells | Yes | (+) | Bone, liver, lungs | Died of disease after 24 months |
| Shi et al[12], 2013 | 19 | M | Pancreas | 3.0 × 4.0 | Abdo | Whipple resection | No chemo | (-) | (-) | (-) | (-) | 48 | NR | NR | NR | CD99, NSE, vimen | Nr | (+) | Pancreas | No evidence of disease after 48 months |
| Changal et al[13], 2014 | 60 | NA | Pancrea | 4 × 3.7 × 2.5 | Abdo | No surgical resection | VIDE | (+) | (+) | (+) | (-) | 3 | t (11; 22) (q24; q12) | FISH | EWS-FLI1 | CD99, NSE, small round cells | Yes | NR | NR | Alive with disease |
| Nishi | 22 | M | Pancrea | 8.5 × 5.2 × 6.2 | Abdo | Whipple resection | No chemo | NA | (+) | (+) | (-) | 2 | t (11; 22) (q24; q12) | FISH | NR | CD99, vimen | Nr | NR | NR | Alive with disease |
| Amaro et al[15], 2015 | 8 | F | Pancrea | 4.5 × 4.0 | Abdo | Cholecy | NA | NA | NA | NA | NA | NA | NA | NA | NA | CD99, vimen | Yes | NR | NR | NA |
| Teixeira et al[16], 2015 | 28 | F | Pancrea | 12.8 × 12.1 × 10.9 | Abdo | Gastro | No chemo | (-) | (-) | (-) | (-) | 36 | NR | NR | NR | CD99, vimen | Yes | (-) | (-) | No evidence of disease after 36 months |
| Liu et al[17], 2018 | 36 | M | Pancrea | 6.3 × 3.6 × 4.8 | Abdo | RouxenY choledo | No chemo | (+) | (+) | (+) | NA | 2 | NR | NR | NR | CD99, vimen | Nr | (+) | Liver | Died of disease after 2 months |
| Achufusi et al[18], 2020 | 61 | M | Pancrea | NA | Abdo | cholecys | No chemo | (+) | (+) | (+) | (+) | 0.33 | NR | NR | NR | NA | Nr | (+) | Liver | Died 10 days after dis |
| Bülch | 6 | F | Pancrea | 6 × 5 × 5 | Paleness, dizzi | Modified Whipple-Opera | No chemo | (-) | (+) | (+) | (-) | 6 | t (11; 22) (q24; q12) | FISH | EWSR1 | CD99, small round cells | Nr | (+) | NA | Died of disease after 6 months |
| Saif et al[20], 2017 | 38 | F | Pancrea | 8 × 10 | Abdo | Distal pancrea | IE, VAC | NA | NA | (-) | (-) | 6 | t (11; 22) (q24; q12) | FISH | NR | CD99, small round cells | Yes | (-) | (-) | No evidence of disease after 6 months |
| Patil et al[21], 2021 | 51 | F | Pancrea | 6.2 × 5.5 × 4 | Asymp | Distal pancrea | IE, VAC | (+) | (-) | (-) | (-) | 2 | NR | NR | NR | CD99, small round cells, FLI-1 | Nr | (-) | (-) | No evidence of disease after 2 months |
| Schutte and Knight[22], 2006 | 2 | F | Pancrea | 6 × 4 | Preco | Distal pancrea | VAC, cisplati | NA | (-) | (-) | (-) | 12 | NR | NR | NR | CD99, vimen | Nr | (-) | (-) | No evidence of disease after 12 months |
| Maxwell et al[23], 2011 | 11 | M | Pancrea | 9.8 × 7.8 × 6.4 | Abdo | Whipple resec | IE, VAC | NA | (+) | (+) | (-) | 7 | t (21; 22) (q22; q12) | RT-PCR | EWS-ERG | CD99, vimentin | Nr | (-) | (-) | NA |
| O'Sulli | 20 | M | Pancrea | 3.5 | NA | Whipple resec | NA | (-) | (+) | (+) | (+) | 30 | t (11; 22) (q24; q12) | RT-PCR | EWS-FLI1 | CD99, vimen | Nr | (+) | Lung | Alive with disease |
| Mova | 17 | M | Pancreas | 9 | Abdo | Whipple resec | VAC | (-) | NA | NA | NA | 33 | t (11; 22) (q24; q12) | FISH | NR | CD99, NSE, small round cells | Yes | NR | NR | No evidence of disease after 33 months |
| 20 | M | Pancreas | 3.6 | Abdo | Whipple resection | NA | (-) | NA | NA | NA | 27 | t (11; 22) (q24; q12) | FISH | NR | CD99, NSE, small round cells | Yes | NR | NR | Alive with disease | |
| 25 | F | Pancreas | NA | Abdo | Biopsy | NA | NA | NA | NA | NA | NA | NA | NA | NR | CD99, small round cells | Nr | NR | NR | NA | |
| 21 | F | Pancrea | NA | Abdo | Whipple resection | NA | (-) | (+) | NA | NA | NA | t (11; 22) (q24; q12) | RT-PCR | EWS-FLI1 | CD99, NSE, small round cells | Nr | NR | NR | Died of disease | |
| 25 | F | Pancreas | 8 | Abdo | Biopsy | NA | NA | NA | NA | NA | NA | (-) | RT-PCR | NR | CD99, NSE, small round cells | Nr | NR | NR | NA | |
| 13 | M | Pancreas | 6 | Abdo | Biopsy, chemo | VAC | NA | NA | NA | NA | 43 | NA | NA | NR | CD99, NSE, small round cells | Nr | NR | NR | No evidence of disease after 43 months | |
| 6 | M | Pancreas | 3.5 | Abdo | Whipple resec | VAC | (-) | (+) | NA | NA | 48 | t (11; 22) (q24; q12) | RT-PCR | EWS-FLI1 | CD99, NSE, small round cells | Nr | NR | NR | Died of disease | |
| Perek et al[26], 2003 | 31 | M | Pancrea | 10 × 12 | Fever of un | Whipple resec | Doxoru | (-) | (-) | (-) | (+) | 50 | NR | NR | NR | CD99, vimen | Yes | (+) | Pancreas, lung, bone | Died of disease after 50 months |
| Welsch et al[27], 2006 | 33 | M | Pancrea | 18 × 18 × 16 | Abdo | Whipple resecti | VIDE, VAI | NA | NA | (+) | (+) | 12 | t (21; 22) (q22; q12) | FISH | EWS-ERG | CD99 | Yes | (+) | Liver | No evidence of disease after 12 months |
| Sang et al[7], 2006 | 13 | F | Pancrea | 9 × 11 × 17 | Abdo | Resec | VAC | (-) | (-) | (-) | (-) | 6 | t (11; 22) (q24; q12) | RT-PCR | EWS-FLI1 | CD99, NSE, small round cells | Yes | (-) | (-) | No evidence of disease after 6 months |
| Wu et al[28], 2006 | 19 | F | Pancreas | 10 × 8 × 7 | Abdo | Pancrea | NA | (-) | (-) | (-) | (-) | 10 | NR | NR | NR | CD99, NSE, small round cells | Yes | (+) | NA | Died of disease after 10 months |
| Menon et al[29], 2009 | 8 | F | Pancreas | 10 × 6 × 10 | Abdo | Biopsy, radio | NA | NA | (-) | (-) | (-) | 19 | NA | NA | NA | CD99 | Nr | (-) | (-) | Died of disease after 19 months |
| Pappo et al[30], 1989 | 13 | M | Pancrea | NA | Anemia, head | Bone marrow aspira | Vincri | (+) | (+) | (+) | (+) | 3.5 | t (2: 13) , iso-17 | Genomic DNA analysis | NA | NSE, vimen | Nr | (+) | Bones, liver, spleen, pan | Died of disease after 3.5 months |
| Doi et al[31], 2009 | 37 | M | Pancreas | 4.2 | Jaundice | Whipple resecti | VAC, IE | NA | (+) | (+) | (+) | 12 | t (21; 22) (q22; q12) | FISH | EWS-R1 | CD99, NSE, vimen | Nr | (+) | Liver, lungs | No evidence of disease after 12 months |
| Jing et al[32], 2011 | 24 | F | Pancrea | 10 × 10 × 8 | Asymp | Resec | NA | NA | (+) | NA | (-) | 18 | NR | NR | NR | NR | Yes | (+) | Pancreas | No evidence of disease after 18 months |
The resected tumor has a grossly pale, soft appearance and may have central necrosis. Microscopically, small round or ovoid cells of uniform size with large, deeply stained nuclei are visible. The cells were densely arranged and distributed in nests and sheets. Small round cells were seen in 23 patients (76.7%), cluster of differentiation 99 (CD99) was positive in 27 patients (90%), neuron-specific enolase (NSE) in 14 patients (46.7%), and vimentin in 11 patients (36.7%). Chromosomal translocations were reported in 15 of 30 patients (50%), of which 11 (73.3%) were t (11; 22) (q24; q12) translocations, and the gene fusion transcript Ewing’s sarcoma and friend leukemia integration 1 transcription factor (EWS-FLI1) was detected in 5 cases.
Abdominal computed tomography (CT) and magnetic resonance imaging (MRI) are the most commonly used imaging modalities. Unenhanced CT shows mainly a soft tissue mass with indistinct borders, irregular shape, and uneven density, and in some cases, cystic necrosis, calcification, and hemorrhage may be seen. Enhanced CT showed flocculent, grid-like, and apparently uneven enhancement, and MRI showed irregular shape, low signal on a T1 weighted image (T1WI), uneven high signal on T2WI, high signal on diffusion-weighted imaging, low signal on apparent diffusion coefficient, and high signal on fluid attenuated inversion recovery. Signs of cellular necrosis were reported in 13 cases (43.3%) in this study.
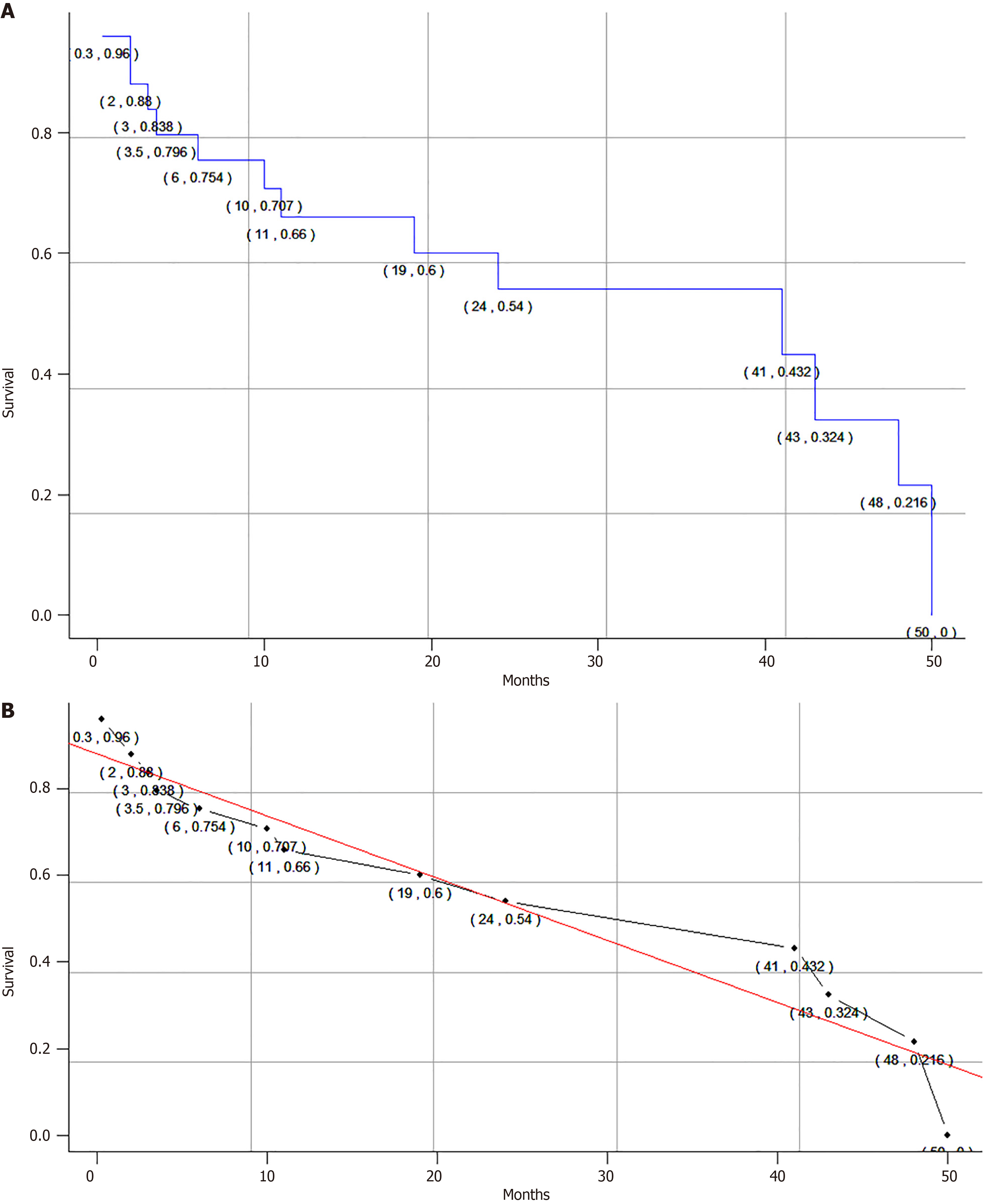
A total of 24 patients (80.0%) underwent surgical treatment, of whom 12 patients (50.0%) underwent complete surgical resection with negative margins and 5 patients (20.8%) had positive surgical margins. Nineteen patients received adjuvant therapy, including chemotherapy and/or radiotherapy, and were treated with a variety of chemotherapy regimens, including a vincristine, adriamycin, and cyclophosphamide (VAC) regimen in 11 patients (57.9%). Thirteen patients (43.3%) had local metastases, ten patients (33.3%) had lymph node metastases, and six patients (20.0%) had distant metastases. Thirteen patients (43.3%) had local recurrence, of whom seven (53.8%) had liver recurrence. Length of follow-up ranged from 10 days[18] to 50 months[26]. The mean survival time of all patients was 29.4 months, and the median survival time was 41.0 months. The overall estimated 1-year and 3-year survival rates were approximately 66.0% and 36.4%, respectively (Figure 2A), which we also calibrated with a linear function fit (Figure 2B). Ten patients died of disseminated metastatic disease.
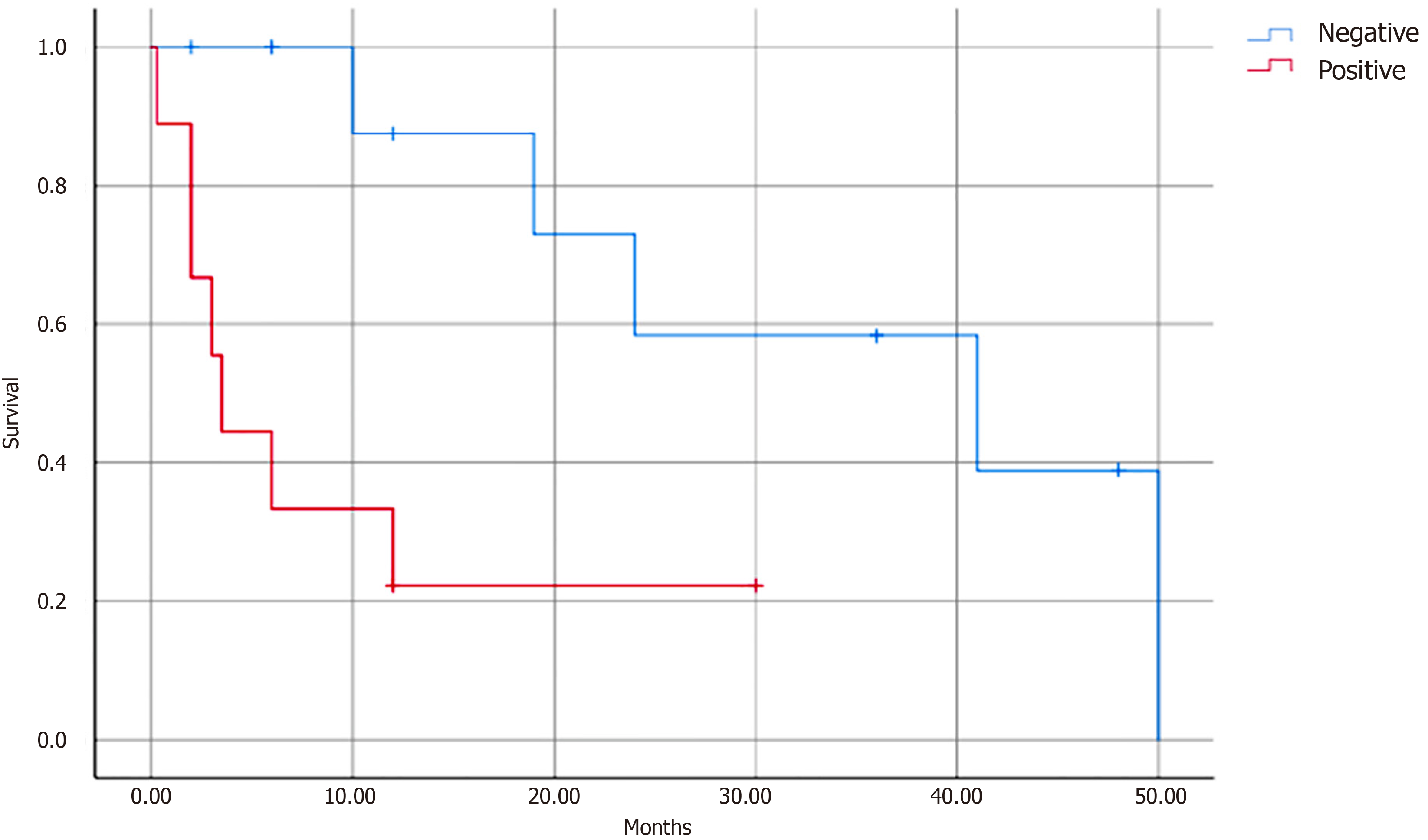
Data on age, sex, tumor size, tumor location, surgery, chemotherapy and radiotherapy, local metastasis, lymph node metastasis, distant metastasis, and the presence of recurrence were included in univariate analyses. We found that chemotherapy (P = 0.036), local metastasis (P = 0.041), lymph node metastasis (P = 0.003), distant metastasis (P = 0.049), and surgical margins (P = 0.048) were the prognostic factors affecting survival. Multivariate analysis revealed that only lymph node metastasis (P = 0.012) was a significant prognostic factor (Table 2, Figure 3).
| Variable | 3-year survival, n | Univariate P value | Cox P value | RR | 95%CI |
| Sex | 0.573 | ||||
| Men | 2 | ||||
| Women | 3 | ||||
| Age in years | 0.723 | ||||
| < 24 | 3 | ||||
| ≥ 24 | 3 | ||||
| Size | 0.795 | ||||
| < 8 | 4 | ||||
| ≥ 8 | 3 | ||||
| Surgical resection | 0.100 | ||||
| Yes | 4 | ||||
| No | 3 | ||||
| Chemotherapy | 0.036 | ||||
| Yes | 4 | ||||
| No | 1 | ||||
| Local metastasis | 0.041 | ||||
| Yes | 1 | ||||
| No | 2 | ||||
| Lymph node metastasis | 0.003 | 0.012 | 3.74 | 2.536-17.198 | |
| Yes | 0 | ||||
| No | 3 | ||||
| Distant metastasis | 0.049 | ||||
| Yes | 0 | ||||
| No | 1 | ||||
| Microscopic margin | 0.048 | ||||
| Positive | 0 | ||||
| Negative | 1 | ||||
| Recurrence | 0.088 | ||||
| Yes | 2 | ||||
| No | 3 |
PNET is a rare malignant soft tissue tumor whose pathogenesis and etiology are unclear, and it has been suggested that it may be related to the inhibition of apoptosis[33]. The cellular origin and histogenesis of PNET are not fully understood, and a previous study suggested that primitive neuroepithelial stem cells are the most likely source[34].
Pancreatic PNET disease has an insidious onset with no specific symptoms; the most common symptom is abdominal pain, which is usually non-specific and therefore often leads to a serious delay in diagnosis. In the present study, 22 patients (73.3%) presented with abdominal pain and 8 with jaundice. Anemia or loss of appetite may also be the first symptom, and in rare cases, precocious puberty[22,29] and elevated blood glucose[6] may occur at extreme ages due to endocrine dysfunction as a result of damage to the pancreas, possibly because of the role of neurons produced by neuroepithelial stem cells in regulating pancreatic islet function. The ages of the two girls with precocious puberty in this study were 2 years and 8 years. Abdominal CT and MRI are the most effective methods for detecting tumors, and the imaging manifestations of pancreatic PNET show diversity. The CT plain scan shows a solid mass of mixed density, when the mass is large, it is prone to cystic degeneration and necrosis leading to uneven density[35], and on enhancement, the mass shows heterogeneous enhancement, with mild-moderate enhancement of the solid portion and no enhancement of cystic degeneration and necrosis areas[36]. In advanced stages of the tumor, invasion, and metastasis to surrounding organs may occur[37].
Since there are no specific clinical symptoms or imaging features of pancreatic PNET, the definitive diagnosis of pancreatic PNET requires a combination of clinical, pathologic, immunohistochemical, and cytogenetic features. The most typical pathologic features of pancreatic PNET are small round tumor cells on light microscopy, immunohistochemical positivity for CD99 and NSE, and detectable waveform protein in most cases. In this study, small round cells were seen in 23 cases (76.7%) and CD99 was expressed in 27 patients (90%). CD99 is a cell surface glycoprotein abundantly expressed in peripheral PNETs and is a gene product of microneme protein 2[38]. Notably, CD99 positivity is helpful but not specific for the diagnosis of PNET. Detection of chromosomal translocations and gene fusion transcripts by molecular genetic analysis is the gold standard for the diagnosis of pancreatic PNET. A previous study showed that in approximately 85% of cases there is a t (11; 22) (q24; q12) translocation leading to the fusion product EWS-FLI1, i.e. the Ewing’s sarcoma breakpoint region 1 (EWSR1) gene on chromosome 22q12 is fused in reading frame with the FLI1 gene on chromosome 11q24; and in about 10%-15% of cases there is a t (21; 22) (q22; q12) translocation, i.e. the EWSR1 gene on chromosome 22q12 is fused to the ETS-related gene on chromosome 21q22[38].
Because pancreatic PNET is morphologically very similar to some tumors, while the cellular origin, biological behavior, and prognosis are very different, it is important to emphasize the differential diagnosis with these tumors.
Clear cell sarcoma of soft tissue: Morphologically, there are melanin deposits, immunophenotypically positive melanin markers, and electron microscopic observation of melanin granules support the diagnosis of clear cell sarcoma of soft tissue.
Melanoma: Pathologically, the tumor cells had different morphology and obvious heterogeneity, large nuclei were seen, and melanin granules were seen in the cytoplasm and interstitium of the cells. Immunohistochemical markers of tumor cells were positive for S-100 protein, SRY-box transcription factor 10, human melanoma black, and Melan A. Genetic testing of the tumor could have mutations in BRAF and c-KIT genes, with no rearrangement of EWSR1 gene.
Gastrointestinal mesenchymal tumors: Morphologically, spindle cells are common, and a few are epithelioid or mixed. Immunohistochemically, tumor cells are positive for CD117, discovered on gastrointestinal stromal tumors, and CD34; genetically, tumors usually have c-KIT or platelet-derived growth factor receptor alpha gene mutations and no EWSR1 gene rearrangement. In addition, we suggest differentiation from undifferentiated small cell carcinoma of the pancreas, pancreatoblastoma, and neuroendocrine carcinoma.
Age and sex: Our study showed that pancreatic PNET is mainly diagnosed in young adults, with the majority of patients between the ages of 19 years and 40 years, and in a few rare cases, it can be diagnosed at extreme ages. Different studies have come to different conclusions about whether sex affects prognosis. The results of a randomized controlled trial[39] showed no significant difference in 5-year progression-free survival between males and females, while the results of a study by Liu et al[40] showed that female patients had a significantly better prognosis than males. Our results suggest that males and females are equally affected and that neither age nor sex is an independent factor influencing the prognosis of pancreatic PNET.
Tumor size: In our study, the mean size of 30 pancreatic PNETs was 7.8 cm (range 3.5-18 cm), with 13 tumors measuring ≥ 8 cm. Tumor location was more common in the head of the pancreas (48.9%). Stratified by tumor size, the 1-year survival rates were 66.6% (tumor size < 8 cm) and 66.6% (tumor size ≥ 8 cm), and the 3-year survival rates were 50.0% (tumor size < 8 cm) and 44.4% (tumor size ≥ 8 cm). Theoretically, larger tumors would take longer to develop and invade surrounding tissues to a greater extent, with a higher risk of metastasis and recurrence, resulting in a lower 3-year survival rate. However, in our study, tumor size did not have a significant effect on the survival rate of patients with pancreatic PNET. Analysis of the reasons for this may be related to whether or not the patients were treated with chemotherapy postoperatively and the location of the tumor, e.g., patients with larger tumors may be more likely to opt for chemotherapy, and tumors occurring in the head of the pancreas, the smaller size may produce a greater compressive effect leading to a more severe impact. Further prospective randomized controlled trials are needed to determine the relationship between tumor size and prognosis in pancreatic PNET.
Surgical resection: Pancreatic PNET is a highly lethal and biologically relatively new malignancy. Clinical experience with this tumor is limited to a few case reports describing inconsistent treatment approaches, and there is no consensus on the optimal treatment strategy. Due to the high rate of early metastasis and recurrence of pancreatic PNET, early surgical resection combined with chemoradiotherapy is the first-line treatment modality, and aggressive reoperation is not recommended because of the poor prognosis. Moschovi et al[41] reported that adequate resection and effective systemic therapy are the keys to improving prognosis. Ozaki et al[42] concluded that surgical resection helps to improve disease control and survival in patients with pancreatic PNET. Unfortunately, our study showed that surgical treatment was not an important prognostic factor, and part of the reason for this result may be that patients who underwent surgical treatment had more lymphatic metastases and localized metastases. Despite this result, the importance of surgical resection cannot be excluded, because wide local excision is essential for negative margins, while early surgical resection reduces the rate of tumor metastasis.
Chemotherapy and/or radiotherapy: Our cases came from different countries and hospitals and they used different chemotherapy regimens, but the most commonly used was the VAC regimen. Among all chemotherapy regimens, the most commonly used drug was vincristine. According to Grier[43], a five-drug regimen (vincristine, doxorubicin, cyclophosphamide, ifosfamide, and etoposide) is the gold standard for the treatment of PNET. In a large randomized controlled clinical trial of bone PNETs by Grier et al[39], the results showed that a five-drug regimen significantly improved the prognosis of patients without metastases compared to a four-drug regimen (vincristine + doxorubicin + actinomycin D + cyclophosphamide), whereas it did not significantly improve the prognosis of patients with metastases. The mechanism may be that etoposide has a potentiating effect on alkylating agent-induced DNA damage by inhibiting DNA repair through inhibition of DNA topoisomerase II[39]. Sable et al[44] suggested that good induction chemotherapy improves the rate of negative surgical margins. Moschovi et al[41] also showed that intensive multi-agent chemotherapy prior to surgery further improves patient prognosis. Several other studies have investigated whether intensive standard regimens can improve outcomes. However, the results have been largely unsatisfactory. Womer et al[45] showed that increasing the dose of chemotherapeutic agents did not improve outcomes in patients with non-metastatic Ewing's sarcoma, while Granowetter et al[46] showed that shortening the intervals between cycles of chemotherapy did not improve survival in patients with tumors of the Ewing’s sarcoma family. In our study, although it was not possible to statistically analyze the therapeutic efficacy of the different regimens because the treatment options were too diverse, we observed that patients who received adjuvant chemotherapy appeared to have a better outcome than those who did not receive chemotherapy (P = 0.036), and thus our study suggests that pancreatic PNET has a certain degree of chemosensitivity. Local radiotherapy alone for pancreatic PNET should be used mainly in patients who are inoperable and have positive surgical margins, and despite all efforts, none of the efficacy is currently satisfactory[47].
Metastasis and recurrence: Pancreatic PNET is extremely aggressive, with a high rate of metastasis and a high rate of recurrence after surgical resection. Previous studies have shown that 25%-30% of patients have metastases at the time of diagnosis[38,43]. In our study, 13 patients (43.3%) developed local metastasis, 10 patients (33.3%) developed lymph node metastasis, and 6 patients (20.0%) developed distant metastasis. During follow-up, 13 patients (43.3%) developed local recurrence, and the minimum interval between diagnosis and recurrence was 2 months[21]. Our results demonstrate this property of pancreatic PNET, which indicates that subclinical metastatic spread is likely to be present at the time of PNET diagnosis, and therefore early diagnosis and systemic chemotherapy play an important role in the management of this type of tumor.
In our study, we found that patients with pancreatic PNET who developed metastasis had worse survival than those diagnosed at the localized stage. Univariate analysis showed that chemotherapy (P = 0.036), local metastasis (P = 0.041), lymph node metastasis (P = 0.003), distant metastasis (P = 0.049), and surgical margins (P = 0.048) had prognostic value for patients with pancreatic PNET. Multivariate analysis revealed that lymph node metastasis remained a significant variable associated with survival (P = 0.012), suggesting that tumor lymph node metastasis was negatively associated with survival in PNET. These results are consistent with the findings of Carvajal and Meyers[48], who concluded that the presence of metastases was the worst prognostic factor for pancreatic PNET. Therefore, stratification according to the presence or absence of metastases may be more helpful for patients with pancreatic PNET to benefit from adjuvant therapy. This finding is significant for the postoperative management of PNET and suggests the need for long-term follow-up of patients with pancreatic PNET to detect early tumor metastasis and recurrence in a timely manner.
To date, there have been no large-scale reports of survival in PNET, reflecting the rarity of this tumor. In our study, the longest follow-up was 50 months[26], and the median survival time of all patients was 41.0 months, with overall estimated 1-year and 3-year survival rates of approximately 66.0% and 36.4%, respectively. Our results suggest that the prognosis of pancreatic PNET is extremely poor. There are several reasons for this poor prognosis. First, given the rarity of pancreatic PNET, definitive diagnosis is challenging and the majority of patients are diagnosed late due to the lack of specific clinical manifestations. Second, pancreatic PNET has a high rate of early metastasis and recurrence and is therefore extremely lethal. In addition, the heterogeneity of treatment regimens may contribute to the ineffectiveness of treatment. Of course, our survival results may be lower than the actual survival rate of pancreatic PNET in the real world because the cases we included were case reports, and there may be selective publication bias such as delayed diagnosis, large tumor size, and patient-specific conditions.
To the best of our knowledge, this is the largest and most comprehensive retrospective study of pancreatic PNET to date. In contrast to the previously published literature on pancreatic PNET, which focused on describing the histopathologic nature of the disease, this is the first study to focus on describing the overall characteristics and survival data of pancreatic PNET. We believe that our study can make a new contribution to the understanding of pancreatic PNET because it is extremely rare. Second, this study included cases by searching global databases, and the greatest advantage is the high level of inclusion to minimize selection bias. At the same time, these data can truly reflect the demographic characteristics of patients and their management at that time, and the results of the study are reliable.
This study had some limitations. First, the small number of cases, the short follow-up period, the heterogeneity of the data (heterogeneity of population, heterogeneity of treatment habits, and heterogeneity of health care resources because the cases came from different countries), and the long time span limited the ability of the statistical analyses. Second, regarding the description of tumor size, we performed the statistical analyses in terms of the longest diameter of the tumor, which may be larger than the actual value of the tumor and may bias the interpretation of the results. Third, we only included studies published in English and Chinese, and there may have been very few missing cases. Also, the patient information extracted from these literatures may be incomplete, which may have affected the statistical power.
In conclusion, pancreatic PNET is extremely rare, usually has no specific clinical symptoms, is common in young adults aged 19-40 years, and has an equal sex distribution between men and women. A definitive diagnosis requires a combination of clinical symptoms, pathologic features, and cytogenetic analysis. Pancreatic PNET is inherently characterized by a high rate of metastatic spread, a high rate of early recurrence, and a very poor prognosis. Surgical resection followed by chemotherapy and/or radiotherapy is now a widely accepted treatment. Lymph node metastasis is an important prognostic factor; early diagnosis, early and extensive resection, and adjuvant radiotherapy may help improve prognosis, and long-term surveillance is necessary for effective detection of tumor recurrence. Accumulation of a larger number of cases, longer follow-up, and investigation of more variables are needed in the future to explore the biological behavior and optimal treatment strategy of this rare tumor.
Many thanks to Mr. Bo Feng for his great and selfless help in the statistical analyses of the data for this study. Many thanks to Mr. Bo-Ning Han for providing the audio for the core tip of the manuscript.
| 1. | Becker LE, Hinton D. Primitive neuroectodermal tumors of the central nervous system. Hum Pathol. 1983;14:538-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 147] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 2. | Stout AP. A tumor of the ulnar nerve. Proc NY Pathol Soc. 1918;12:2-12. |
| 3. | Lüttges J, Pierré E, Zamboni G, Weh G, Lietz H, Kussmann J, Klöppel G. [Malignant non-epithelial tumors of the pancreas]. Pathologe. 1997;18:233-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Kawai A, Nakayama R, Matsumine A, Matsumoto S, Ueda T, Tsuchiya H, Yabe H, Beppu Y. Clear cell sarcoma of tendons and aponeuroses: An analysis of 75 cases. J Clin Oncol. 2006;24:9572-9572. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 5. | Tsokos M. Peripheral primitive neuroectodermal tumors. Diagnosis, classification, and prognosis. Perspect Pediatr Pathol. 1992;16:27-98. [PubMed] |
| 6. | Mao Y, Sang X, Liang N, Yang H, Lu X, Yang Z, Du S, Xu Y, Zhao H, Zhong S, Huang J, Millis JM. Peripheral primitive neuroectodermal tumors arising in the pancreas: the first case report in Asia and a review of the 14 total reported cases in the world. Hepatobiliary Surg Nutr. 2013;2:51-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 7. | Sang XT, Liang NX, Mao YL, Lu X, Yang ZY, Zhong SX, Huang JF. [Diagnosis and treatment of primitive neuroectodermal tumors of pancreas]. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2006;28:191-195. [PubMed] |
| 8. | Dias AR, Arantes T, Sampaio RC, Jureidini R, Cunha JE, Cecconello I. [Pancreatic primitive neuroectodermal tumor: case report]. Arq Bras Cir Dig. 2013;26:159-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Pérez Marín JC, Apolinario Hidalgo RM, Mohamad Tubío AM, Santos Moyano Z, Peña Quintana P, Gómez Díaz J. [A 25-year-old man with pancreatic tumor, double vision and bone lesions]. Gastroenterol Hepatol. 2008;31:626-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Kim JY, Song JS, Park H, Byun JH, Song KB, Kim KP, Kim SC, Hong SM. Primary mesenchymal tumors of the pancreas: single-center experience over 16 years. Pancreas. 2014;43:959-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 11. | Jayant K, Agrawal S, Agarwal R, Khoiwal S. Pancreatic Ewings Sarcoma- A Dreadful Tumor. Am J Cancer Prev. 2013;1:24-26. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Shi L, Guo Z, Wu X. Primary pulmonary primitive neuroectodermal tumor metastasis to the pancreas: a rare case with seven-year follow-up. Diagn Pathol. 2013;8:51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Changal KH, Mir MH, Azaz SA, Qadri SK, Lone AR. Primitive neuroectodermal tumour of pancreas; second case from Asia. Malays J Med Sci. 2014;21:65-69. [PubMed] |
| 14. | Nishizawa N, Kumamoto Y, Igarashi K, Nishiyama R, Tajima H, Kawamata H, Kaizu T, Watanabe M. A peripheral primitive neuroectodermal tumor originating from the pancreas: a case report and review of the literature. Surg Case Rep. 2015;1:80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Amaro F, Colaiácovo R, Carbonari A, Saieg M, Baraldi AC, Rossini L. Pancreatic peripheral primitive neuroectodermal tumor diagnosed by endoscopic ultrasound. Endoscopy. 2015;47 Suppl 1:E11-E13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 16. | Teixeira U, Goldoni M, Unterleider M, Diedrich J, Balbinot D, Rodrigues P, Monteiro R, Gomes D, Sampaio J, Fontes P, Waechter F. Primitive Neuroectodermal Tumor of the Pancreas: A Case Report and Review of the Literature. Case Rep Surg. 2015;2015:276869. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Liu W, Xiao D, Yi X, Li W. Pancreatic primitive neuroectodermal tumor: Focus on radiological features and differential diagnosis - A case report and literature review. J Cancer Res Ther. 2018;14:S793-S795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Achufusi TG, Sohal R, Zamora E, Harne P, Russo R. Primitive neuroectodermal tumor of the pancreas. Proc (Bayl Univ Med Cent). 2020;34:144-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 19. | Bülchmann G, Schuster T, Haas RJ, Joppich I. Primitive neuroectodermal tumor of the pancreas. An extremely rare tumor. Case report and review of the literature. Klin Padiatr. 2000;212:185-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Saif MW, Kaley K. Extraosseous Ewing's Sarcoma of the Pancreas: An Uncommon but Treatable Disease. Cureus. 2017;9:e1882. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Patil R, Gandhe S, Ramesh YV, Ramesh YV. Pancreatic Ewing’s Sarcoma Synchronously Diagnosed in a Patient of Carcinoma Cervix: A Case Report and Literature Review. Cyprus J Med Sci. 2021;7:271-275. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 22. | Schutte WP, Knight PJ. Precocious puberty because of a pancreatic neuroectodermal tumor. J Pediatr Surg. 2006;41:1916-1918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Maxwell L, Hederman A, Jackson C, Sawaya D, Giles H, Nowicki MJ. Uncommon presentation of rare disorder-duodenal ulcer secondary to invasive pancreatic primitive neuroectodermal tumor: case report and review of the literature. J Pediatr Hematol Oncol. 2011;33:543-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | O'Sullivan MJ, Perlman EJ, Furman J, Humphrey PA, Dehner LP, Pfeifer JD. Visceral primitive peripheral neuroectodermal tumors: a clinicopathologic and molecular study. Hum Pathol. 2001;32:1109-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 73] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Movahedi-Lankarani S, Hruban RH, Westra WH, Klimstra DS. Primitive neuroectodermal tumors of the pancreas: a report of seven cases of a rare neoplasm. Am J Surg Pathol. 2002;26:1040-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 46] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Perek S, Perek A, Sarman K, Tuzun H, Buyukunal E. Primitive neuroectodermal tumor of the pancreas. A case report of an extremely rare tumor. Pancreatology. 2003;3:352-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Welsch T, Mechtersheimer G, Aulmann S, Mueller SA, Buechler MW, Schmidt J, Kienle P. Huge primitive neuroectodermal tumor of the pancreas: report of a case and review of the literature. World J Gastroenterol. 2006;12:6070-6073. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Wu JP, Chen HX, Wu WH. [A case of primary primitive neuroectodermal tumour in the pancreas]. Zhonghua Binglixue Zazhi. 2006;35:442. [DOI] [Full Text] |
| 29. | Menon BS, Juraida E, Mohamed M, Manaf Z, Zahari Z, Yusuf S, Ting TH, Muda Z, Ibrahim H. Pancreatic primitive neuroectodermal tumour associated with precocious puberty. Pediatr Blood Cancer. 2009;53:518-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Pappo AS, Cheah MS, Saldivar VA, Britton HA, Parmley RT. Disseminated primitive neuroectodermal tumor: diagnosis using immunocytochemistry, electron microscopy, and molecular probes. Cancer. 1989;63:2515-2521. [PubMed] [DOI] [Full Text] |
| 31. | Doi H, Ichikawa S, Hiraoka A, Ichiryu M, Nakahara H, Ochi H, Tanabe A, Kodama A, Hasebe A, Miyamoto Y, Ninomiya T, Horiike N, Takamura K, Kawasaki H, Kameoka C, Kan M, Doi S, Soga Y, Tamura H, Maeda T, Asaki A, Seno S, Iguchi H, Hasegawa T. Primitive neuroectodermal tumor of the pancreas. Intern Med. 2009;48:329-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 32. | Jing H, Li F, Chen L, Zhang T, Zhao Y. Detection of recurrent pancreatic primitive neuroectodermal tumor by tc-99m hydrazinonicotinyl-tyr3-octreotide scan. Clin Nucl Med. 2011;36:54-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 33. | Bhoopathi P, Chetty C, Gujrati M, Dinh DH, Rao JS, Lakka S. Cathepsin B facilitates autophagy-mediated apoptosis in SPARC overexpressed primitive neuroectodermal tumor cells. Cell Death Differ. 2010;17:1529-1539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 34. | Rubinstein LJ. Embryonal central neuroepithelial tumors and their differentiating potential. A cytogenetic view of a complex neuro-oncological problem. J Neurosurg. 1985;62:795-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 108] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 35. | Liu J, Zhao YL, Song SQ, Li ZH, Li PL. Primitive neuroectodermal tumors: a clinical and radiological analysis of six cases. Quant Imaging Med Surg. 2019;9:722-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 36. | Ba L, Tan H, Xiao H, Guan Y, Gao J, Gao X. Radiologic and clinicopathologic findings of peripheral primitive neuroectodermal tumors. Acta Radiol. 2015;56:820-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 37. | Obuz F, Kovanlikaya A, Olgun N, Sarialioğlu F, Ozer E. MR imaging of pancreatic metastasis from extraosseous Ewing's sarcoma. Pancreas. 2000;20:102-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 38. | de Alava E, Gerald WL. Molecular biology of the Ewing's sarcoma/primitive neuroectodermal tumor family. J Clin Oncol. 2000;18:204-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 217] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 39. | Grier HE, Krailo MD, Tarbell NJ, Link MP, Fryer CJ, Pritchard DJ, Gebhardt MC, Dickman PS, Perlman EJ, Meyers PA, Donaldson SS, Moore S, Rausen AR, Vietti TJ, Miser JS. Addition of ifosfamide and etoposide to standard chemotherapy for Ewing's sarcoma and primitive neuroectodermal tumor of bone. N Engl J Med. 2003;348:694-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 942] [Cited by in RCA: 912] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 40. | Liu Y, Yuan Y, Zhang F, Hu K, Qiu J, Hou X, Yan J, Lian X, Sun S, Liu Z, Shen J. Outcome of multidisciplinary treatment of peripheral primitive neuroectodermal tumor. Sci Rep. 2020;10:15656. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 41. | Moschovi M, Trimis G, Stefanaki K, Anastasopoulos J, Syriopoulou V, Koultouki E, Tzortzatou-Stathopoulou F. Favorable outcome of Ewing sarcoma family tumors to multiagent intensive preoperative chemotherapy: a single institution experience. J Surg Oncol. 2005;89:239-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 42. | Ozaki T, Hillmann A, Hoffmann C, Rübe C, Blasius S, Dunst J, Jürgens H, Winkelmann W. Significance of surgical margin on the prognosis of patients with Ewing's sarcoma. A report from the Cooperative Ewing's Sarcoma Study. Cancer. 1996;78:892-900. [PubMed] [DOI] [Full Text] |
| 43. | Grier HE. The Ewing family of tumors. Ewing's sarcoma and primitive neuroectodermal tumors. Pediatr Clin North Am. 1997;44:991-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 208] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 44. | Sable S, Gandhi V, Nagral A, Nagral S. Management of a large retroperitoneal primitive neuroectodermal tumour: 'a multimodal approach'. BMJ Case Rep. 2012;2012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 45. | Womer RB, West DC, Krailo MD, Dickman PS, Pawel BR, Grier HE, Marcus K, Sailer S, Healey JH, Dormans JP, Weiss AR. Randomized controlled trial of interval-compressed chemotherapy for the treatment of localized Ewing sarcoma: a report from the Children's Oncology Group. J Clin Oncol. 2012;30:4148-4154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 419] [Cited by in RCA: 519] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 46. | Granowetter L, Womer R, Devidas M, Krailo M, Wang C, Bernstein M, Marina N, Leavey P, Gebhardt M, Healey J, Shamberger RC, Goorin A, Miser J, Meyer J, Arndt CA, Sailer S, Marcus K, Perlman E, Dickman P, Grier HE. Dose-intensified compared with standard chemotherapy for nonmetastatic Ewing sarcoma family of tumors: a Children's Oncology Group Study. J Clin Oncol. 2009;27:2536-2541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 225] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 47. | Gaspar N, Hawkins DS, Dirksen U, Lewis IJ, Ferrari S, Le Deley MC, Kovar H, Grimer R, Whelan J, Claude L, Delattre O, Paulussen M, Picci P, Sundby Hall K, van den Berg H, Ladenstein R, Michon J, Hjorth L, Judson I, Luksch R, Bernstein ML, Marec-Bérard P, Brennan B, Craft AW, Womer RB, Juergens H, Oberlin O. Ewing Sarcoma: Current Management and Future Approaches Through Collaboration. J Clin Oncol. 2015;33:3036-3046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 371] [Cited by in RCA: 477] [Article Influence: 47.7] [Reference Citation Analysis (0)] |
| 48. | Carvajal R, Meyers P. Ewing's sarcoma and primitive neuroectodermal family of tumors. Hematol Oncol Clin North Am. 2005;19:501-525, vi. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 105] [Article Influence: 5.3] [Reference Citation Analysis (0)] |