Published online Sep 15, 2024. doi: 10.4251/wjgo.v16.i9.3994
Revised: July 4, 2024
Accepted: August 1, 2024
Published online: September 15, 2024
Processing time: 110 Days and 18.8 Hours
Based on current knowledge, hepatocellular carcinoma (HCC) is a condition with numerous etiologies and risk factors. However, the pathogenesis of HCC remains unclear.
To investigate the roles of senegenin and O-GlcNAcylation in the growth and metastasis of HCC.
The levels of O-linked N-acetylglucosamine transferase (OGT) and O-GlcNA
Western blot analysis revealed that OGT and O-GlcNAcylation levels were significantly elevated in HCC tissues and cells. O-GlcNAcylation levels in HCC cells were significantly altered by drug treatment and lentiviral infection. An increase in the glycosylation level was linked to enhanced proliferation, inva
Senegenin lowers O-GlcNAcylation levels, decreases OGT expression, and inhibits cancer cell growth and metastasis by regulating proteins involved in NF-κB and JNK pathways.
Core Tip: This study investigated the effects of senegenin and O-GlcNAcylation on the growth and metastasis of hepatocellular carcinoma (HCC) cells. The findings indicated that O-GlcNAcylation levels are elevated in HCC tissues and cells, and senegenin can reduce these levels by targeting proteins involved in nuclear factor-kappa B and c-Jun N-terminal kinase signaling pathways. This modulation decreases the levels of O-linked N-acetylglucosamine transferase and inhibits the proliferation and metastasis of HCC cells, providing new therapeutic targets for liver cancer.
- Citation: Zhang X, Wang LQ, Liu ZY. Senegenin suppresses hepatocellular carcinoma by regulating O-GlcNAcylation. World J Gastrointest Oncol 2024; 16(9): 3994-4005
- URL: https://www.wjgnet.com/1948-5204/full/v16/i9/3994.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i9.3994
Hepatocellular carcinoma (HCC) arises from the malignant transformation of liver cells. It accounts for approximately 80% of all liver cancer cases[1]. Based on current knowledge, HCC is a condition with numerous etiologies and risk factors. The incidence and mortality of HCC in China are influenced by a number of known risk factors, such as hepatitis C virus (HCV) infection, hepatitis B virus (HBV) infection, aflatoxin exposure, alcohol use, and smoking[2]. A history of cirrhosis and chronic liver disease as well as risk factors such as chronic HBV infection, alcoholic liver disease, HCV infection, and nonalcoholic hepatitis are observed in 70%-90% of patients with HCC. Currently, the onset and progression of tumors as well as the acquisition of malignant biological phenotypes are believed to be caused by abnormal changes in the functions of specific proteins[3]. A protein’s function depends more on changes in its own activity than on changes in its expression. Post-translational modification (PTM) is the primary mechanism by which proteins regulate their functional activities[4]. Evidence indicates that the occurrence and growth of various types of tumors are accompanied with abnormal changes in glycosylation, including an imbalance of the constituent ratio of glycoproteins in tumors and changes in the glycosylation sites and glycan structure of proteins[5,6]. Numerous studies have demonstrated the importance of changes in protein glycosylation in the development of various malignant phenotypes.
Several studies have linked O-GlcNAcylation, a type of PTM, to the development of various cancers[7]. The enzyme O-linked N-acetylglucosamine transferase (OGT) catalyzes O-GlcNAcylation. The primary function of OGT is to transfer the N-acetylglucosamine group on uridine diphosphate-N-acetylglucosamine to the target protein. The reverse process is mediated by N-acetyl-β-glucosaminidase, which is encoded by MGEA5. Together, these two enzymes dynamically regulate O-GlcNAcylation and the function of corresponding proteins according to intracellular signals[8]. Based on the most recent findings, liver cancer caused by high glucose levels is considerably influenced by the O-GlcNAcylation of YAP protein in liver cells[9]. Previous research has demonstrated that cell proliferation is altered by the complete knockout of OGT in mouse embryonic fibroblasts[10]. O-GlcNAcylation can also alter a number of nuclear factor-kappa B (NF-κB) subunits, including p65 and c-Rel, which is an essential transcription factor that promotes the survival and proliferation of cancer cells[11]. In pancreatic adenocarcinoma cell lines, the p65 subunit of NF-κB and the upstream kinase IKKα/IKKβ have been demonstrated to be O-GlcNAcylated[12]. However, the specific regulatory mechanisms of O-GlcNAcylation in HCC remain unknown, necessitating further studies. Therefore, it is crucial to determine the role of O-GlcNAcylation and its regulatory mechanism in liver cancer.
Human HCC cell lines (MHCC97H, HEPG2, BEL-7402, HuH-7, SMMC-7721, and BEL-7404) and the human standard immortalized liver cell line THLE-3 were purchased from Cayman Chemical (Ann Arbor, MI, United States). The cells were cultured in DMEM (11995073, Gibco) containing 10% fetal bovine serum and 1% streptomycin double antibody solution, and the medium was replaced every 2-3 days. All cell culture reagents were purchased from Cayman Chemical. The cells were passaged upon reaching 80%-90% confluency.
The digested cells were seeded into a 6 cm dish, reaching 50%-60% confluency. TMG, PUG, and dimethyl sulfoxide (DMSO) were all purchased from Lonza (Walkersville, MD, United States) and used as control reagents. After 12 hours of drug treatment, total cellular protein was extracted.
OGT lentivirus was synthesized by Shanghai Jikai Company (Shanghai, China). In total, 3 × 105 cells were counted and seeded into 6-well plates after their digestion. The solution was changed after the cells were attached, and 1 mL of Opti-MEM (premixed 1:2000 with polybrene) was added to determine the amount of virus needed based on the MOI of the cells. The photoluminescence condition was observed after 3-5 days. The transfection efficiency was > 70%, and the stable transformant cells were screened using puromycin. After 8-12 hours, the cell status was observed, and the medium was replaced with DMEM. Puromycin (2 μg/mL) was applied to the culture medium for screening culture. Opti-MEM and puromycin were purchased from Cayman Chemical. The culture was then maintained for 1 week. The total protein was extracted, and western blot analysis was used to assess the infection efficiency.
After being digested, 3 × 103 cells were counted and seeded into 96-well plates with three duplicate wells for each condition. Starting on the second day after cell attachment, 20 μL of cell counting kit-8 (CCK8) staining solution (96992, Sigma-Aldrich) was added according to the instructions, and the cells were incubated for 4 hours. The absorbance at 450 nm was measured using a microplate reader. After 24 hours, the abovementioned incubation and measurement steps were repeated for 7 consecutive days.
The cells were digested with trypsin (25200056, Thermo Fisher Scientific). Overall, 1 × 103 cells were inoculated in each well of 6-well plates. After approximately 2 weeks, the formation of macroscopic clones was noted, the medium was discarded, and the cells were fixed by adding absolute ethanol. After removing the ethanol, the cells were washed with phosphate buffered saline (PBS) and stained with crystal violet. Photos were captured for observation, and the cells were counted for analysis.
MTT can be reduced by succinate dehydrogenase in living cells, forming blue-purple crystals. These crystals are soluble in DMSO and other organic solvents. The absorbance was determined using a spectrophotometer according to the color depth. The absorbance value reflects the number and activity of cells. Each well was treated with 10 μL of MTT (final concentration of 0.5 mg/mL; M5655, Sigma-Aldrich) for 4 hours at 37 °C. After removing the supernatant, 100 μL of DMSO (D2650, Sigma-Aldrich) was applied, and the mixture was shaken at a low speed for 10 minutes. After the crystals had completely dissolved, the absorbance was measured at 570 nm using a microplate reader. Each experiment was conducted in triplicate.
Cells in the logarithmic growth phase were harvested, trypsinized, and resuspended in serum-free DMEM. For counting, the cell density was adjusted to 1 × 105 cells/mL. Then, 500 μL of DMEM containing 20% serum was added to each well of 24-well plates. Transwell chambers were placed on the plates, and 200 μL of the cell suspension was seeded into each chamber with three replicates per group. The cells were incubated for 24 hours. The chambers were removed and fixed with absolute ethanol for 5 minutes. After washing with PBS, crystal violet stain (ab232855, Abcam) was applied for 5 minutes. After removing the top layer of the filter membrane with a cotton swab, the cells were washed again with PBS and counted under a microscope.
Using a reporter assay based on AP-1 activity, the effects of OGT overexpression and knockdown were examined. The cells were collected and tested 48 hours after plasmid transfection using a GloMax microplate photometer (Promega, Madison, WI, United States) and Assay System for Dual Luciferase Reporter (Promega).
The protein concentration in each group was adjusted to the same concentration after being extracted from the treated cells via lysis. Equal portions of 50 g of protein samples were subjected to 10% SDS-PAGE and transferred onto a polyvinylidene difluoride membrane using the wet rotation technique. The membrane was blocked with 5% skim milk and then incubated overnight at 4 °C with mouse monoclonal anti-OGT antibody (1:1000, Proteintech Group Inc., Chicago, MA, United States), phosphorylated c-Jun N-terminal kinase (p-SAPK/JNK, 1:1000), E-cadherin (1:1000), O-GlcNAc (CTD110.6, 1:1000), phospho-c-Jun (1:1000), c-Jun (1:1000), vimentin (D21H3), IKKβ (1:1000), IκBa (1:1000), phospho-IKKα/IKKβ (Ser176/180, 1:500), IKKα (1:500), glucose-regulated protein 78 (1:500), phospho-IκBα (Ser32, 1:500), inositol-requiring enzyme 1a (IRE1a), NAD-dependent protein deacetylase sirtuin-1, cleaved caspase 7 (1:1000), forkhead box M1 (1:500), cleaved caspase 3 (1:1000), caspase 7 (1:1000), NF-κB p50 (1:1000), cleaved poly (ADP-ribose) polymerase (1:500, Cell Signaling Technology, Boston, MA, United States), caspase 3 (1:1000), NF-κB p65 (1:1000), phospho-NF-κB p65 (1:1000), phospho-IKKα (1:1000), β-actin (1:500, Santa Cruz Biotechnology, Santa Cruz, CA, United States), and phospho-NF-κB p50 (1:1000). Subsequently, horseradish peroxidase-labeled secondary antibodies were applied at room temperature for 2 hours. Then, the membranes were washed three times with TBST for 10 minutes each. Images were captured using the BIO-RAD gel imager via the enhanced chemiluminescence method. β-actin was used as an internal reference. The experiment was conducted three times. ImageJ software was used to calculate the absorbance, and the relative absorbance (tau/-action) was used to represent the relative content of the target protein.
Statistical analysis was performed using SPSS 18.0. GraphPad Prism 9 was used for data visualization. All in vitro experiments included at least three replicates per group. Parametric data between groups were compared using the two-tailed Student’s t-test. When comparing multiple groups, data were compared via analysis of variance with Bonferroni’s post-hoc test. P values of < 0.05 were considered to indicate statistical significance.
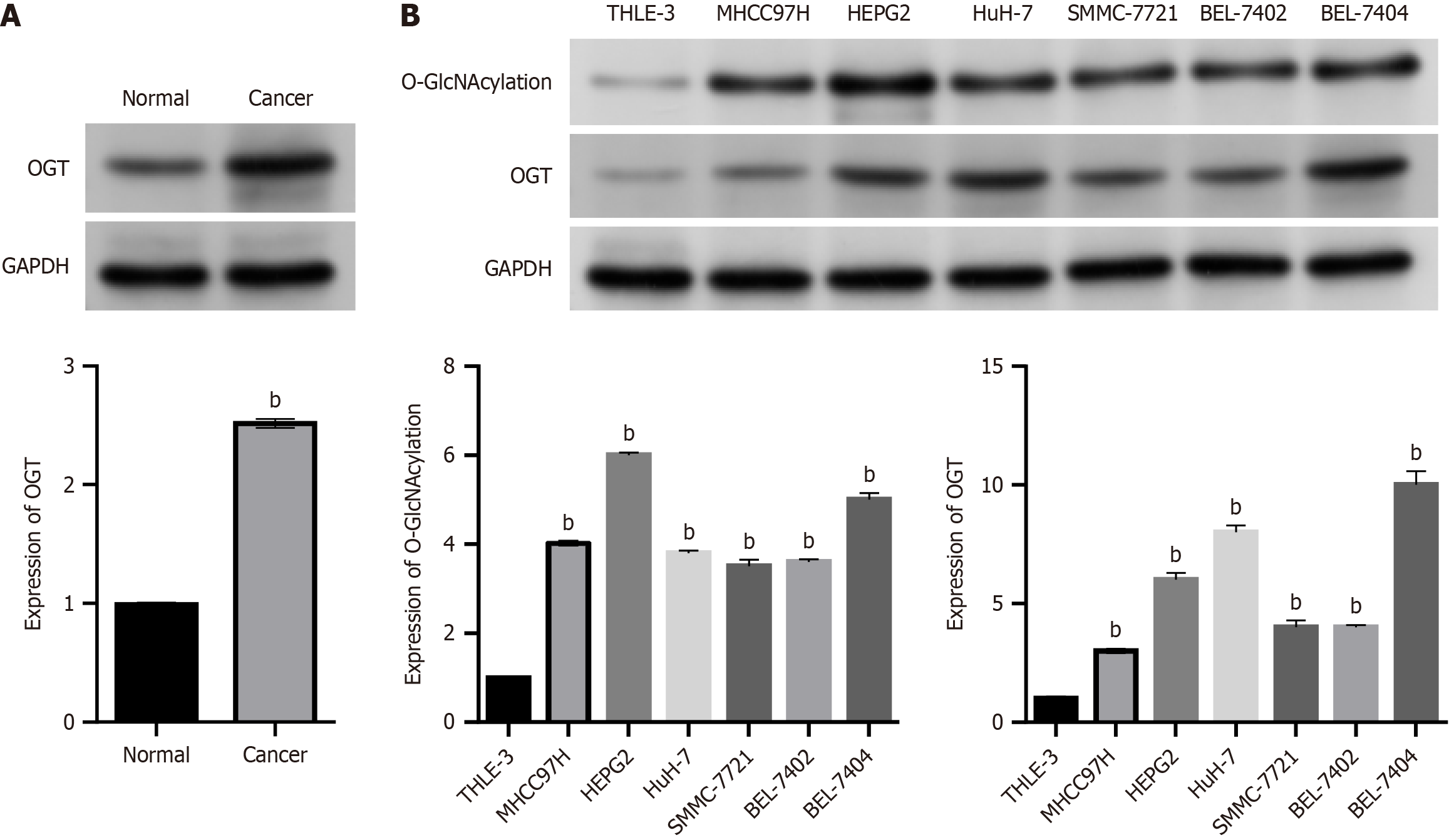
Western blot analysis was used to measure OGT levels in six pairs of adjacent normal tissues and HCC tissues to assess O-GlcNAcylation in HCC. The results are presented in Figure 1A. Compared with adjacent normal tissues, OGT level was significantly higher in each of the six pairs of HCC tissues. O-GlcNAcylation and OGT levels were investigated at the cellular level. Six different HCC cell lines (MHCC97H, HEPG2, HuH-7, BEL-7404, BEL-7402, and SMMC-7721) were used in this study, and the immortalized liver cell line THLE-3 was used as a control. The findings demonstrated that compared with THLE-3 cells, O-GlcNAcylation and OGT levels were consistently higher in HCC cell lines (all P < 0.05; Figure 1B).
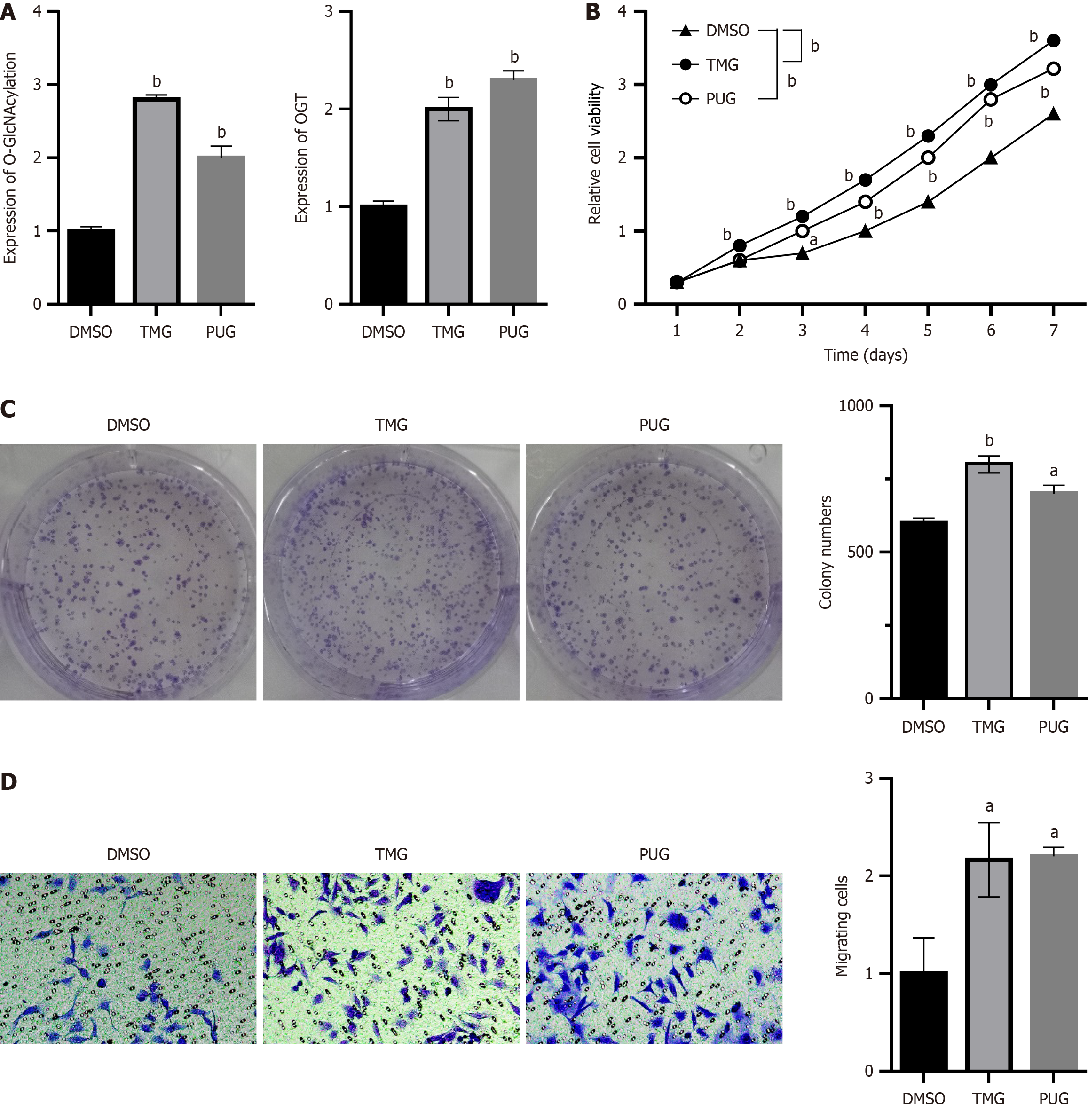
Two cell lines, namely BEL-7402 and BEL-7404, were chosen to examine the effect of O-GlcNAcylation on the proliferation of HCC cells based on the findings of the prior experiment. Because BEL-7402 cells exhibited low O-GlcNAcylation levels, we used an O-GlcNAcase inhibitor (TMG or PUG) to increase the O-GlcNAcylation level in BEL-7402 cells. BEL-7402 cells were treated with TMG (10 μmol/L), PUG (100 μmol/L), or DMSO (100 μmol/L), after which the proteins were extracted to observe the effects of treatment. Contrary to the effects of DMSO, the O-GlcNAcylation level was substantially increased by TMG and PUG treatment (P < 0.05; Figure 2A). As demonstrated by the CCK8 assay, increasing the level of O-GlcNAcylation via drug treatment significantly improved the proliferation of BEL-7402 cells (P < 0.05; Figure 2B). The proliferation of cells is significantly influenced by their capacity for colony formation. To further confirm the effects of O-GlcNAcylation on the proliferation of HCC cells, the effect of O-GlcNAcylation on cell colony formation was observed using the clonogenic assay. As shown in Figure 2C, after increasing O-GlcNAcylation levels by drug treatment, the ability of HCC cells to form colonies was enhanced (P < 0.05). As another important feature of tumor development, a crucial indicator of tumor malignancy is the capacity of tumor cells to metastasize and invade. The ability of BEL7402 cells to migrate was enhanced by TMG and PUG treatment based on the transwell migration assay (P < 0.05; Figure 2D).
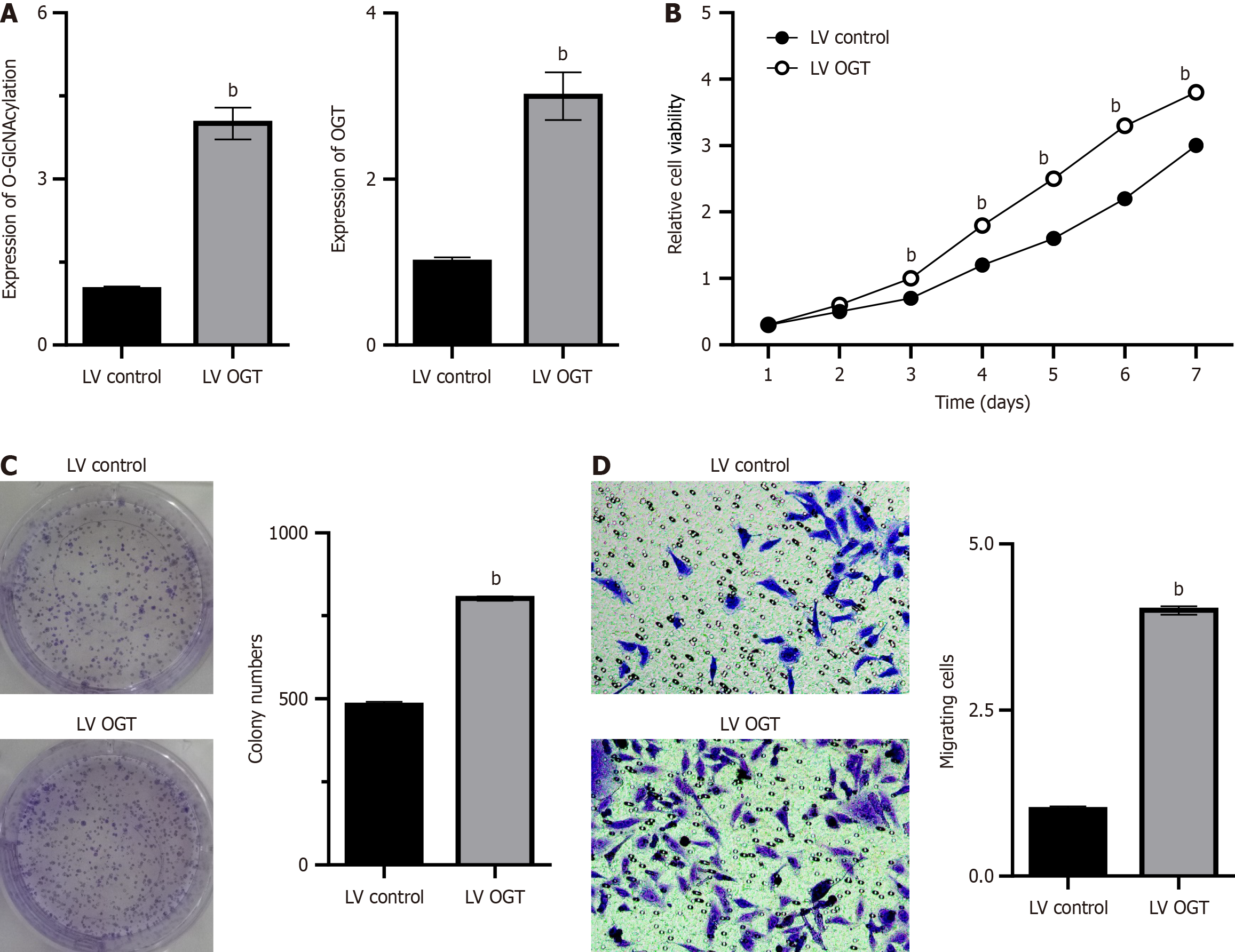
Overexpression of OGT in BEL-7402 cells via lentivirus infection increased the level of glycosylation, which was also verified via western blot analysis (P < 0.05; Figure 3A). Similarly, the CCK8 assay revealed that the proliferation of BEL-7402 cells was significantly improved after lentivirus infection (P < 0.05; Figure 3B). The clonogenic assay revealed that OGT overexpression increased O-GlcNAcylation levels, which enhanced the ability of HCC cells to form colonies (P < 0.05; Figure 3C). In addition, the migration of BEL7402 cells was improved to a certain extent after OGT overexpression (P < 0.05; Figure 3D).
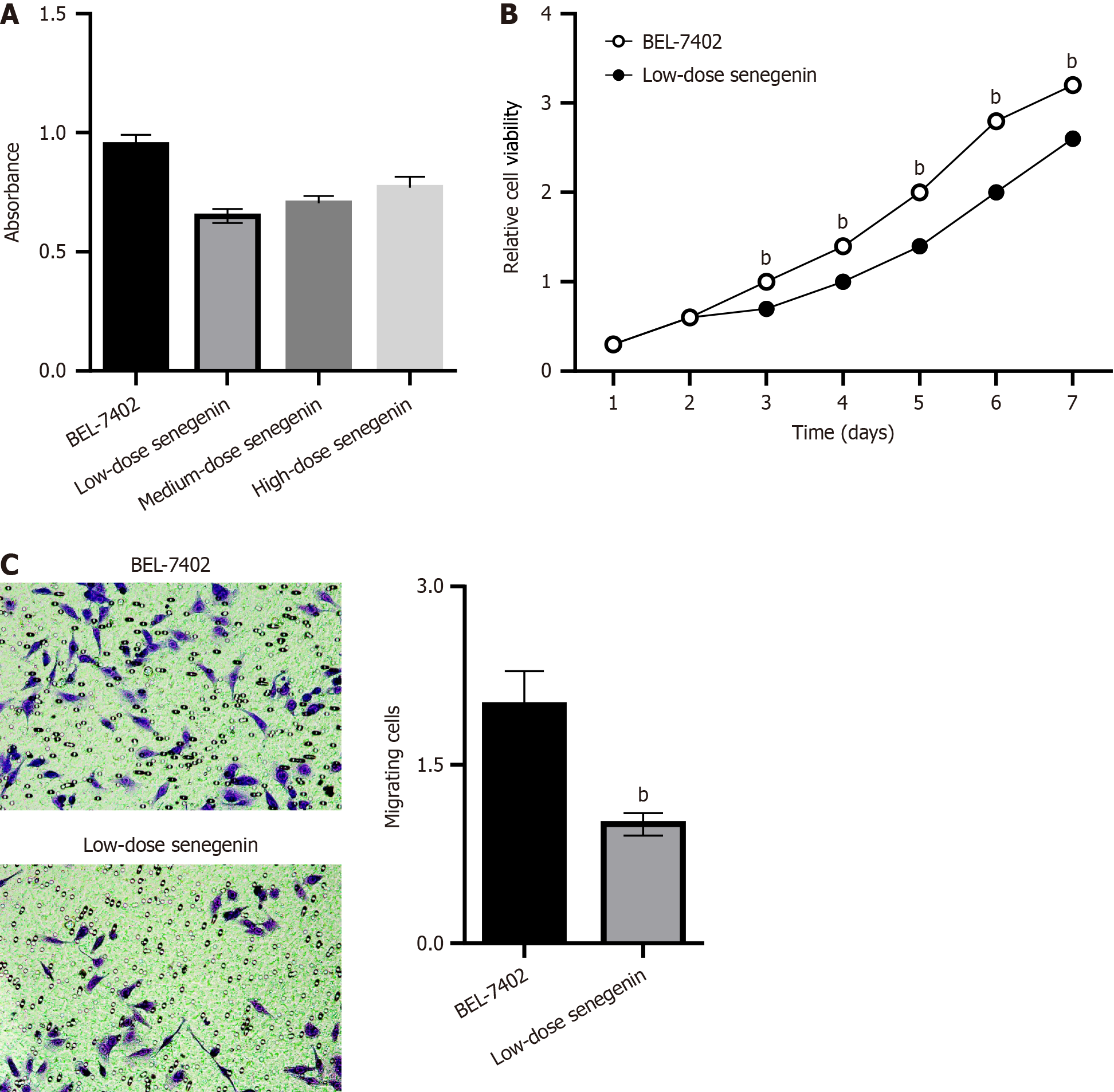
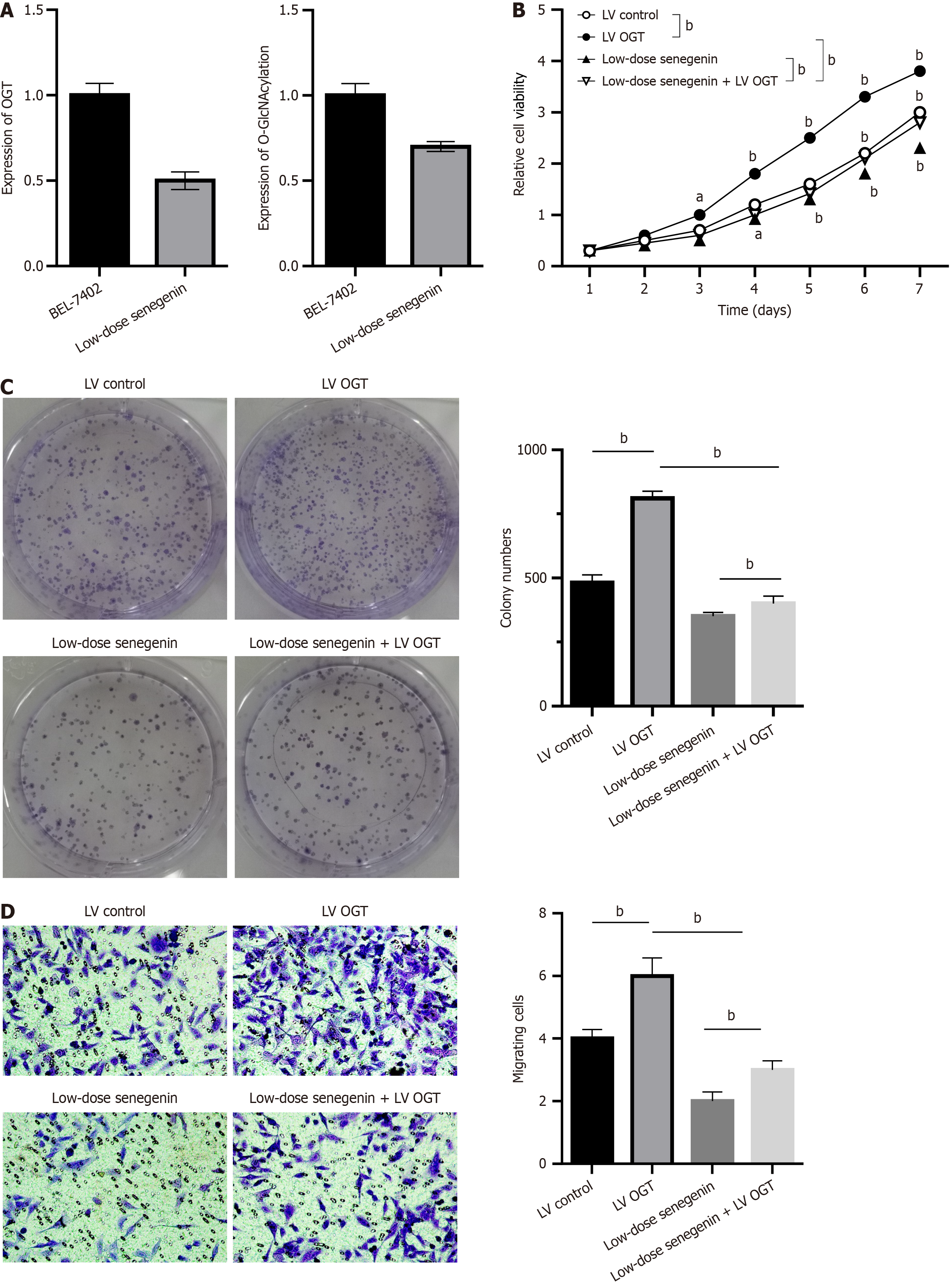
Based on the MTT assay, compared with the control group, the proliferation rate of BEL7402 cells decreased in various treatment groups of senegenin, with no considerable difference in the proliferation rate among the treatment groups (P < 0.05; Figure 4A). An attempt was made to demonstrate the effect of senegenin on the proliferative phenotype of HCC cells. The results of the CCK8 assay illustrated the ability of senegenin to inhibit the proliferation of BEL-7402 cells at low concentrations (P < 0.05; Figure 4B). The transwell migration assay revealed that the migration of BEL7402 cells was suppressed by senegenin (P < 0.05; Figure 4C).
The effects of senegenin on the levels of O-GlcNAcylation and proliferation and migration of HCC cells were examined. Western blot analysis indicated that senegenin can reduce O-GlcNAcylation and OGT levels in BEL-7402 cells (P < 0.05; Figure 5A). The CCK8 assay demonstrated that compared with the control group, overexpression of OGT improved the proliferation of BEL-7402 cells, which was then slowed down by low concentrations of senegenin (P < 0.05; Figure 5B). These findings demonstrated that senegenin administration decreased the ability of cancer cells to form colonies, whereas OGT overexpression led to increased colony formation by HCC cells (P < 0.05; Figure 5C). Based on transwell migration assay, senegenin inhibited the migration of BEL7402 cells, which was previously enhanced by OGT overexpression (P < 0.05; Figure 5D).
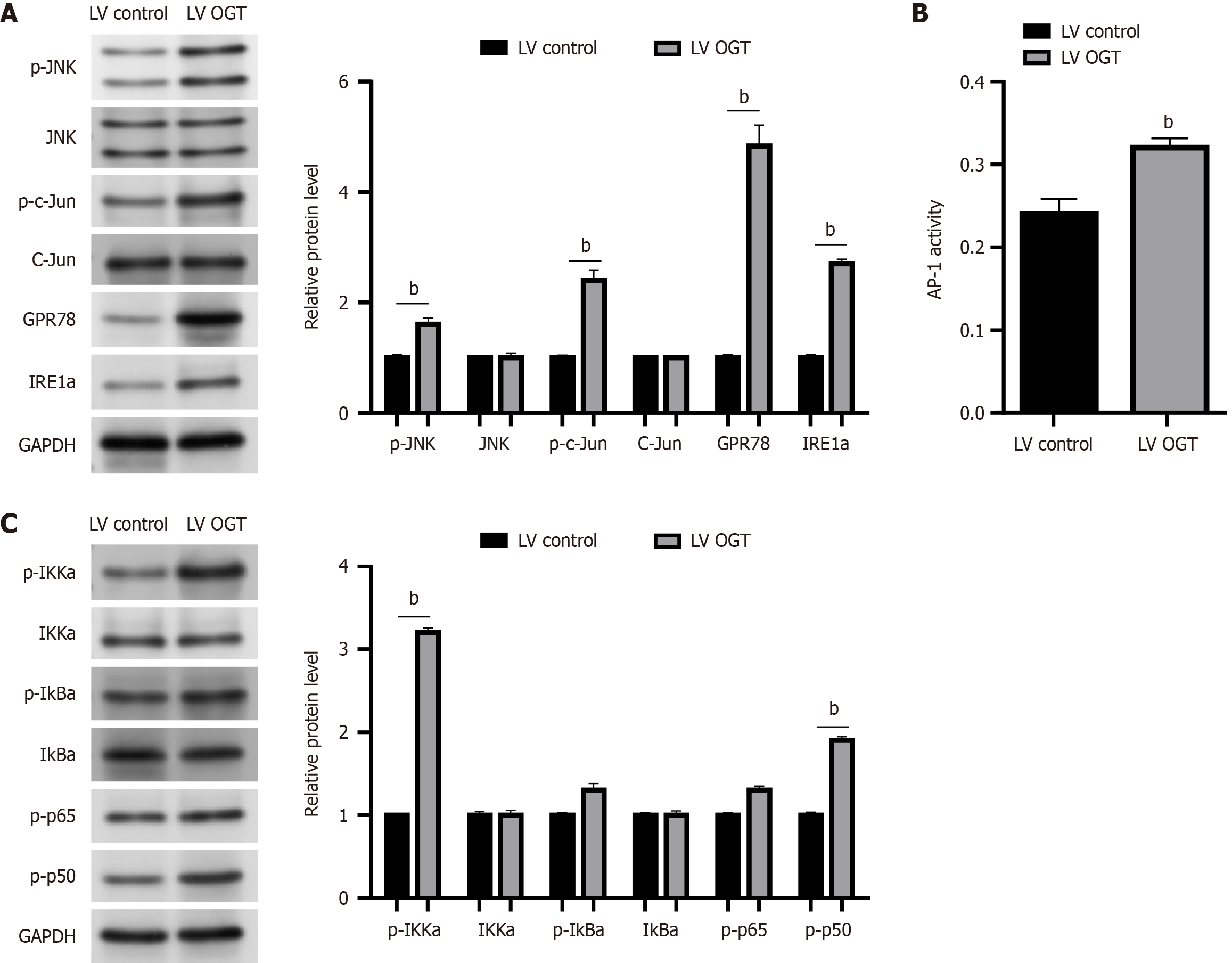
We investigated the relationship between endoplasmic reticulum (ER) stress and OGT-mediated HCC to understand the mechanism by which OGT influences carcinogenesis. We examined the effects of OGT on the activity of NF-κB and JNK/c-Jun/AP-1 cascades because these are the two main oncogenic signaling pathways linked to ER stress and involved in the development of NASH and HCC. As shown in Figure 6A, OGT overexpression in BEL-7402 cells resulted in an increase in the protein levels of p-JNK, p-c-Jun, GPR78, and IRE1a (P < 0.05). In line with this, OGT increased AP-1’s reporter activity (P < 0.05; Figure 6B). OGT, similar to JNK, increased the levels of p-p50, p-p65, p-IκBα, and p-IKKα, indicating the activation of the NF-κB cascade (P < 0.05; Figure 6C). Collectively, our findings imply that ER stress in HCC is at least partially responsible for the oncogenic effects of OGT.
HCC, a malignant tumor of the digestive system, is a serious threat to human health and is associated with high morbidity and mortality rates. HCC is a complex heterogeneous tumor, and its pathogenesis is extremely complicated, involving gene mutations, epigenetic changes, and abnormal molecular signal transduction pathways. The current research on HCC treatment is mainly focused on molecular targeted therapy[13].
Unlike conventional decoration of complex glycans on the surface of cells, O-GlcNAc is a simple monosaccharide modification that mostly occurs inside cells, specifically in the nucleus or cytoplasm. With the development of technologies related to identification, site mapping, quantitation, and site-specific function determination of O-GlcNAc proteins[14], studies have identified > 16000 O-GlcNAcylated proteins in 42 species[15]. A number of malignant phenotypes, including tumor occurrence and development, are influenced by protein glycosylation. O-GlcNAcylation is a unique intracellular glycosylation modification that is comparable to phosphorylation, and alternations in O-GlcNAcylation levels are considered crucial in tumorigenesis[16]. However, the O-GlcNAcylation level in HCC has not been systematically studied. Some previous studies have determined O-GlcNAcylation level in liver cancer, revealing that its level was higher in liver cancer tissues than in healthy tissues, which increasingly elevated in recurrent liver cancer tissues[17,18].
Evidence regarding O-GlcNAcylation levels in HCC and adjacent normal tissues is lacking. It is known that O-GlcNAcylation level is elevated in liver cancer tissues and cell lines[19], indicating that O-GlcNAcylation is involved in the emergence and development of malignant phenotypes of liver cancer, which was also demonstrated in the current study. O-GlcNAcylation plays a role in many diseases, including diabetes, diabetic nephropathy, and neurodegenerative diseases such as Alzheimer’s disease[20,21]. In recent years, several studies have addressed the role of protein O-GlcNAcylation in various types of cancer, including the effect of O-GlcNAcylation on the proliferation, angiogenesis, and metastasis of cancer cells[22,23]. The results of these studies suggested that suppressing O-GlcNAcylation levels is a universally applicable anticancer strategy that can effectively inhibit the growth and metastasis of many types of cancer. By developing specific inhibitors of O-GlcNAcylation and OGT, new directions and approaches for cancer treatment might be proposed.
Based on the findings of previous studies, BEL-7402 and BEL-7404 cells were selected for further examination. The existing O-GlcNAcase inhibitors (TMG and PUG) have been confirmed to enhance O-GlcNAcylation levels[24,25], and this effect was also replicated in our study. By concentrating on proliferation and metastasis, we investigated the effects of O-GlcNAcylation on HCC. In terms of proliferation, we first investigated its effects at the cellular level. Using the CCK8 assay, we revealed that O-GlcNAcylation promoted the proliferation of HCC cells in vitro. We also assessed the colony-forming ability of HCC cells, another important indicator of cell proliferation, using the clonogenic assay and showed that O-GlcNAcylation enhanced the colony-forming ability of HCC cells. Based on the transwell assay, O-GlcNAcylation might help regulate the ability of HCC cells to migrate and invade in culture.
The primary bioactive substance isolated from dry root of Changgung is senegenin. A number of pharmacological activities of senegenin have been demonstrated in recent years, including antioxidation, anti-inflammation, antiapoptosis, and cognitive enhancement[19]. It has a promising future for the treatment of ischemia-reperfusion injury, depression, osteoporosis, neurodegenerative diseases, and other illnesses[26]. However, no comprehensive study has fully established its role in tumor treatment. According to the study’s findings, senegenin can lower the proliferation rate of BEL7402 cells and slow their migration.
O-GlcNAcylation as a PTM requires the binding of corresponding target proteins[27]. Therefore, further research is being conducted to determine the precise mechanism by which O-GlcNAcylation promotes the malignant phenotype of liver cancer. The key is to identify the primary target proteins of O-GlcNAcylation, which remains to be further explored. The main oncogenic signaling pathways associated with ER stress and the emergence of NASH and HCC are NF-κB and JNK signaling pathways[28,29]. Numerous studies have suggested that NF-κB is closely related to tumorigenesis, development, invasion, and metastasis[30]. NF-κB is a nuclear protein factor that plays important roles in cellular physiological and pathological processes, such as inflammation, cell proliferation, and oxidative stress[31]. If NF-κB is activated to regulate the transcription of apoptotic genes and apoptosis, it can aggravate the occurrence and development of cell death by activating the DNA chain in the nucleus, destroying the effective caspase-3 protein, and disintegrating the cellular structure[32]. OGT plays an oncogenic role in NAFLD-associated HCC by regulating palmitic acid and inducing ER stress, consequently activating the oncogenic JNK/c-Jun/AP-1 and NF-κB cascades[33,34]. Therefore, we examined the effects of OGT on JNK/c-Jun/AP-1 and NF-κB cascades. In BEL-7402 cells, OGT overexpression resulted in increases in the protein levels of p-c-Jun, p-JNK, IRE1a, and GPR78. In line with this, OGT increased the reporter activity of AP-1. OGT, similar to JNK, increased the levels of the NF-κB subunits p-p50, p-p65, p-IκKa, and p-IκBa, demonstrating that OGT activated the NF-κB cascade. Overall, our findings imply that the oncogenic effects of OGT are at least partially mediated by the induction of ER stress by HCC. Nonetheless, this study had several limitations. Notably, because of a lack of sufficient serum samples from patients with HCC, all validation experiments were based on in vitro experiments. We plan to analyze some clinical trial results for further investigation.
Senegenin can suppress the levels of OGT and O-GlcNAcylation, which can prevent cancer cells from spreading and growing. HCC can potentially be treated by targeting the proteins involved in NK-κB and JNK pathways.
| 1. | Chidambaranathan-Reghupaty S, Fisher PB, Sarkar D. Hepatocellular carcinoma (HCC): Epidemiology, etiology and molecular classification. Adv Cancer Res. 2021;149:1-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 551] [Article Influence: 110.2] [Reference Citation Analysis (0)] |
| 2. | Kulik L, El-Serag HB. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology. 2019;156:477-491.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 754] [Cited by in RCA: 1215] [Article Influence: 202.5] [Reference Citation Analysis (1)] |
| 3. | Fernández-Barrena MG, Arechederra M, Colyn L, Berasain C, Avila MA. Epigenetics in hepatocellular carcinoma development and therapy: The tip of the iceberg. JHEP Rep. 2020;2:100167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 76] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 4. | Mak LY, Wong DK, Pollicino T, Raimondo G, Hollinger FB, Yuen MF. Occult hepatitis B infection and hepatocellular carcinoma: Epidemiology, virology, hepatocarcinogenesis and clinical significance. J Hepatol. 2020;73:952-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 134] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 5. | Duan F, Wu H, Jia D, Wu W, Ren S, Wang L, Song S, Guo X, Liu F, Ruan Y, Gu J. O-GlcNAcylation of RACK1 promotes hepatocellular carcinogenesis. J Hepatol. 2018;68:1191-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 84] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 6. | Zhao T, Jia L, Li J, Ma C, Wu J, Shen J, Dang L, Zhu B, Li P, Zhi Y, Lan R, Xu Y, Hao Z, Chai Y, Li Q, Hu L, Sun S. Heterogeneities of Site-Specific N-Glycosylation in HCC Tumors With Low and High AFP Concentrations. Front Oncol. 2020;10:496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 7. | Nie H, Ju H, Fan J, Shi X, Cheng Y, Cang X, Zheng Z, Duan X, Yi W. O-GlcNAcylation of PGK1 coordinates glycolysis and TCA cycle to promote tumor growth. Nat Commun. 2020;11:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 225] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 8. | Chang YH, Weng CL, Lin KI. O-GlcNAcylation and its role in the immune system. J Biomed Sci. 2020;27:57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 119] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 9. | Zhu G, Murshed A, Li H, Ma J, Zhen N, Ding M, Zhu J, Mao S, Tang X, Liu L, Sun F, Jin L, Pan Q. O-GlcNAcylation enhances sensitivity to RSL3-induced ferroptosis via the YAP/TFRC pathway in liver cancer. Cell Death Discov. 2021;7:83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 92] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 10. | Lee JS, Zhang Z. O-linked N-acetylglucosamine transferase (OGT) interacts with the histone chaperone HIRA complex and regulates nucleosome assembly and cellular senescence. Proc Natl Acad Sci U S A. 2016;113:E3213-E3220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 11. | Jiang M, Wu N, Xu B, Chu Y, Li X, Su S, Chen D, Li W, Shi Y, Gao X, Zhang H, Zhang Z, Du W, Nie Y, Liang J, Fan D. Fatty acid-induced CD36 expression via O-GlcNAcylation drives gastric cancer metastasis. Theranostics. 2019;9:5359-5373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 112] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 12. | Zhang D, Cai Y, Chen M, Gao L, Shen Y, Huang Z. OGT-mediated O-GlcNAcylation promotes NF-κB activation and inflammation in acute pancreatitis. Inflamm Res. 2015;64:943-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 13. | de Oliveira S, Houseright RA, Graves AL, Golenberg N, Korte BG, Miskolci V, Huttenlocher A. Metformin modulates innate immune-mediated inflammation and early progression of NAFLD-associated hepatocellular carcinoma in zebrafish. J Hepatol. 2019;70:710-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 141] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 14. | Nakashima M, Suga N, Ikeda Y, Yoshikawa S, Matsuda S. Relevant MicroRNAs of MMPs and TIMPs with Certain Gut Microbiota Could Be Involved in the Invasiveness and Metastasis of Malignant Tumors. Innov Discov. 2024;1:10. [DOI] [Full Text] |
| 15. | Ma J, Hart GW. O-GlcNAc profiling: from proteins to proteomes. Clin Proteomics. 2014;11:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 190] [Cited by in RCA: 212] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 16. | Wu J, Tan Z, Li H, Lin M, Jiang Y, Liang L, Ma Q, Gou J, Ning L, Li X, Guan F. Melatonin reduces proliferation and promotes apoptosis of bladder cancer cells by suppressing O-GlcNAcylation of cyclin-dependent-like kinase 5. J Pineal Res. 2021;71:e12765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 17. | Lee JB, Pyo KH, Kim HR. Role and Function of O-GlcNAcylation in Cancer. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 18. | Xiang J, Chen C, Liu R, Gou D, Chang L, Deng H, Gao Q, Zhang W, Tuo L, Pan X, Liang L, Xia J, Huang L, Yao K, Wang B, Hu Z, Huang A, Wang K, Tang N. Gluconeogenic enzyme PCK1 deficiency promotes CHK2 O-GlcNAcylation and hepatocellular carcinoma growth upon glucose deprivation. J Clin Invest. 2021;131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 74] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 19. | Chen Y, Bei J, Liu M, Huang J, Xie L, Huang W, Cai M, Guo Y, Lin L, Zhu K. Sublethal heat stress-induced O-GlcNAcylation coordinates the Warburg effect to promote hepatocellular carcinoma recurrence and metastasis after thermal ablation. Cancer Lett. 2021;518:23-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 20. | Zhu Q, Zhou L, Yang Z, Lai M, Xie H, Wu L, Xing C, Zhang F, Zheng S. O-GlcNAcylation plays a role in tumor recurrence of hepatocellular carcinoma following liver transplantation. Med Oncol. 2012;29:985-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 130] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 21. | Yi W, Clark PM, Mason DE, Keenan MC, Hill C, Goddard WA 3rd, Peters EC, Driggers EM, Hsieh-Wilson LC. Phosphofructokinase 1 glycosylation regulates cell growth and metabolism. Science. 2012;337:975-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 413] [Cited by in RCA: 510] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 22. | Bond MR, Hanover JA. O-GlcNAc cycling: a link between metabolism and chronic disease. Annu Rev Nutr. 2013;33:205-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 249] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 23. | Ferrer CM, Sodi VL, Reginato MJ. O-GlcNAcylation in Cancer Biology: Linking Metabolism and Signaling. J Mol Biol. 2016;428:3282-3294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 217] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 24. | Wu D, Jin J, Qiu Z, Liu D, Luo H. Functional Analysis of O-GlcNAcylation in Cancer Metastasis. Front Oncol. 2020;10:585288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 25. | Ruane PT, Tan CMJ, Adlam DJ, Kimber SJ, Brison DR, Aplin JD, Westwood M. Protein O-GlcNAcylation Promotes Trophoblast Differentiation at Implantation. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 26. | Zhang Z, Parker MP, Graw S, Novikova LV, Fedosyuk H, Fontes JD, Koestler DC, Peterson KR, Slawson C. O-GlcNAc homeostasis contributes to cell fate decisions during hematopoiesis. J Biol Chem. 2019;294:1363-1379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 27. | Chen Z, Yang Y, Han Y, Wang X. Neuroprotective Effects and Mechanisms of Senegenin, an Effective Compound Originated From the Roots of Polygala Tenuifolia. Front Pharmacol. 2022;13:937333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 28. | Zhu Y, Hart GW. Dual-specificity RNA aptamers enable manipulation of target-specific O-GlcNAcylation and unveil functions of O-GlcNAc on β-catenin. Cell. 2023;186:428-445.e27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 55] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 29. | Wu Q, Wu W, Fu B, Shi L, Wang X, Kuca K. JNK signaling in cancer cell survival. Med Res Rev. 2019;39:2082-2104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 233] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 30. | Jain MR, Giri SR, Bhoi B, Trivedi C, Rath A, Rathod R, Ranvir R, Kadam S, Patel H, Swain P, Roy SS, Das N, Karmakar E, Wahli W, Patel PR. Dual PPARα/γ agonist saroglitazar improves liver histopathology and biochemistry in experimental NASH models. Liver Int. 2018;38:1084-1094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 169] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 31. | Yan F, Liu L, Wang Q. Combinatorial dynamics of protein synthesis time delay and negative feedback loop in NF-κB signalling pathway. IET Syst Biol. 2020;14:284-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 32. | Thevkar-Nagesh P, Habault J, Voisin M, Ruff SE, Ha S, Ruoff R, Chen X, Rawal S, Zahr T, Szabo G, Rogatsky I, Fisher EA, Garabedian MJ. Transcriptional regulation of Acsl1 by CHREBP and NF-kappa B in macrophages during hyperglycemia and inflammation. PLoS One. 2022;17:e0272986. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 33. | Castro-Oropeza R, Vazquez-Santillan K, Díaz-Gastelum C, Melendez-Zajgla J, Zampedri C, Ferat-Osorio E, Rodríguez-González A, Arriaga-Pizano L, Maldonado V. Adipose-derived mesenchymal stem cells promote the malignant phenotype of cervical cancer. Sci Rep. 2020;10:14205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 34. | Xu W, Zhang X, Wu JL, Fu L, Liu K, Liu D, Chen GG, Lai PB, Wong N, Yu J. O-GlcNAc transferase promotes fatty liver-associated liver cancer through inducing palmitic acid and activating endoplasmic reticulum stress. J Hepatol. 2017;67:310-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 105] [Article Influence: 13.1] [Reference Citation Analysis (0)] |