Published online Aug 15, 2024. doi: 10.4251/wjgo.v16.i8.3716
Revised: June 8, 2024
Accepted: July 2, 2024
Published online: August 15, 2024
Processing time: 147 Days and 21.2 Hours
Aggressive fibromatosis (AF), also known as desmoid tumor or desmoid-type fibromatosis, is a rare soft tissue neoplasm that can occur in almost any part of the body. Although it is a benign disease, AF is aggressive and infiltrative and has a high recurrence rate after surgery. Common sites for intra-abdominal AF are the small bowel mesentery, retroperitoneum, and pelvis. AF in the colon is extremely rare.
Here, we report the first case of sigmoid colon AF, which was accidentally discovered in a 27-year-old woman during laparoscopic myomectomy. Computed tomography confirmed a slightly enhanced mass in the sigmoid colon. Subse
AF should be considered in the differential diagnosis of subepithelial colon masses. Radical resection alone can achieve good outcomes.
Core Tip: Aggressive fibromatosis (AF) is a rare soft tissue neoplasm that can occur in almost any part of the body. Here, we report the first case of sigmoid colon AF, which was accidentally discovered in a 27-year-old woman during laparoscopic myomectomy. The patient underwent laparoscopic exploration, and sigmoidectomy with a negative margin was performed to excise the mass. Postoperative immunohistochemistry revealed that the mass was an AF. The patient recovered well and was recurrence-free at the 30-month follow-up without adjuvant therapy. AF should be considered in the differential diagnosis of subepithelial colon masses.
- Citation: Yu PP, Liu XC, Yin L, Yin G. Aggressive fibromatosis of the sigmoid colon: A case report. World J Gastrointest Oncol 2024; 16(8): 3716-3722
- URL: https://www.wjgnet.com/1948-5204/full/v16/i8/3716.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i8.3716
Aggressive fibromatosis (AF), also known as desmoid tumor or desmoid-type fibromatosis, is a rare soft tissue neoplasm with an estimated incidence rate of 5 to 6 cases per million per year[1]. AF occurs at a peak age of approximately 30 years and predominantly occurs in females[2,3]. Although rare, AF can occur in almost any part of the body and can be divided into 3 groups depending on the anatomical site: Extra-abdominal, abdominal wall and intra-abdominal[2]. For intra-abdominal AF, the common sites include the small bowel mesentery, retroperitoneum, and pelvis[4]. AF in the colon is extremely rare.
In this report, we describe a patient with sigmoid AF who was preoperatively misdiagnosed with having a gastrointestinal stromal tumor (GIST). This is the first case of AF located in the sigmoid colon in the literature. The following case is presented in accordance with the CARE reporting checklist[5].
A 27-year-old woman was referred to our hospital because of a sigmoid mass that was incidentally found during a laparoscopic myomectomy in a women’s hospital one week prior.
A mass in the sigmoid colon was incidentally found during laparoscopy for myomectomy. The impaired sigmoid colon was rigid with condensed adhesions to the left pelvic wall and enlarged lymph nodes around the sigmoid colon.
Before admission to the women’s hospital, the patient experienced a prolonged menstruation period for 20 days, while her normal menstrual cycle lasted 25 days, with a menstruation period of 7 days. An ultrasonography revealed a 4.9 cm × 4.0 cm × 3.0 cm mass in the uterine fundus. Physical examination revealed an enlarged uterus like at approximately 50-60 days gestation. The digital rectal examination was unremarkable. The patient also complained of slight pelvic discomfort and expressed a desire to bear a child. Therefore, laparoscopic myomectomy was recommended and scheduled. During laparoscopy for myomectomy, the impaired sigmoid colon was found to be rigid with condensed adhesions to the left pelvic wall and enlarged lymph nodes around the sigmoid colon. Early-onset colorectal cancer was suspected. After consulting with a general surgeon and discussing the options with the patient’s relatives, the gynecologist performed myomectomy with a frozen section that indicated a leiomyoma. The final pathological examination confirmed the diagnosis of uterine leiomyoma, with immunohistochemical staining positive for SMA and Desmin and negative for ALK and CD10. The Ki-67 index was less than 5%. The patient’s medical history was unremarkable except for a fibroadenoma of the left breast, which had been surgically removed five years prior.
The patient denied any family history of malignant tumors.
On admission to our hospital, the patient denied any abdominal symptoms, changes in bowel movement habitus or unintentional weight loss. The patient’s physical examination was unremarkable except for an enlarged uterus.
Serum tumor markers, including carcinoembryonic antigen, carbohydrate antigen 19-9, cancer antigen 125, cancer antigen 15-3 and alpha-fetoprotein, were all within the normal ranges. Other laboratory test results, including routine blood tests, blood biochemistry and coagulation function, were unremarkable, whereas the fecal occult blood test was positive.
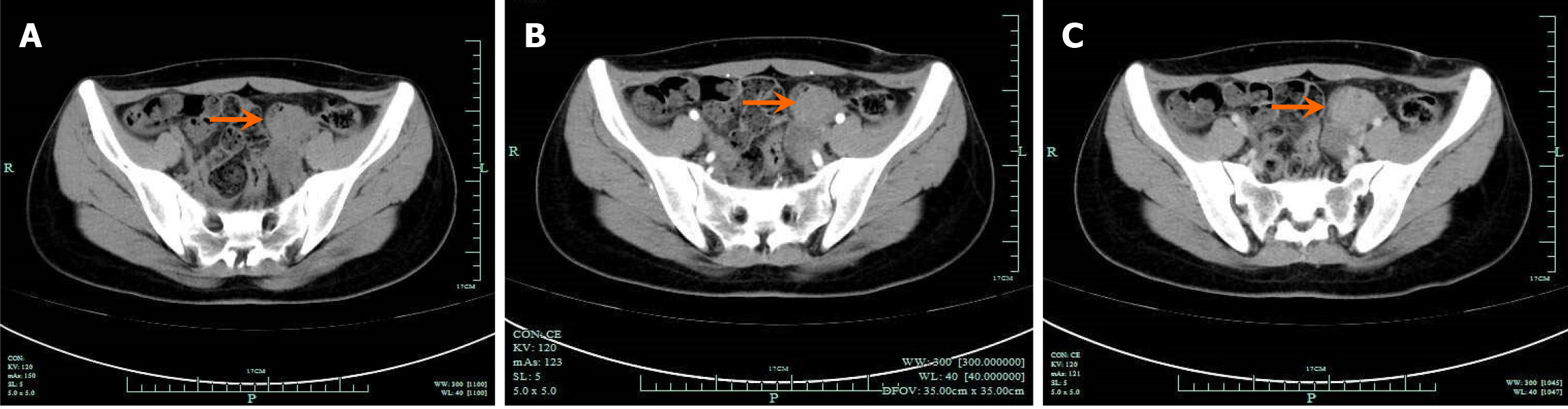
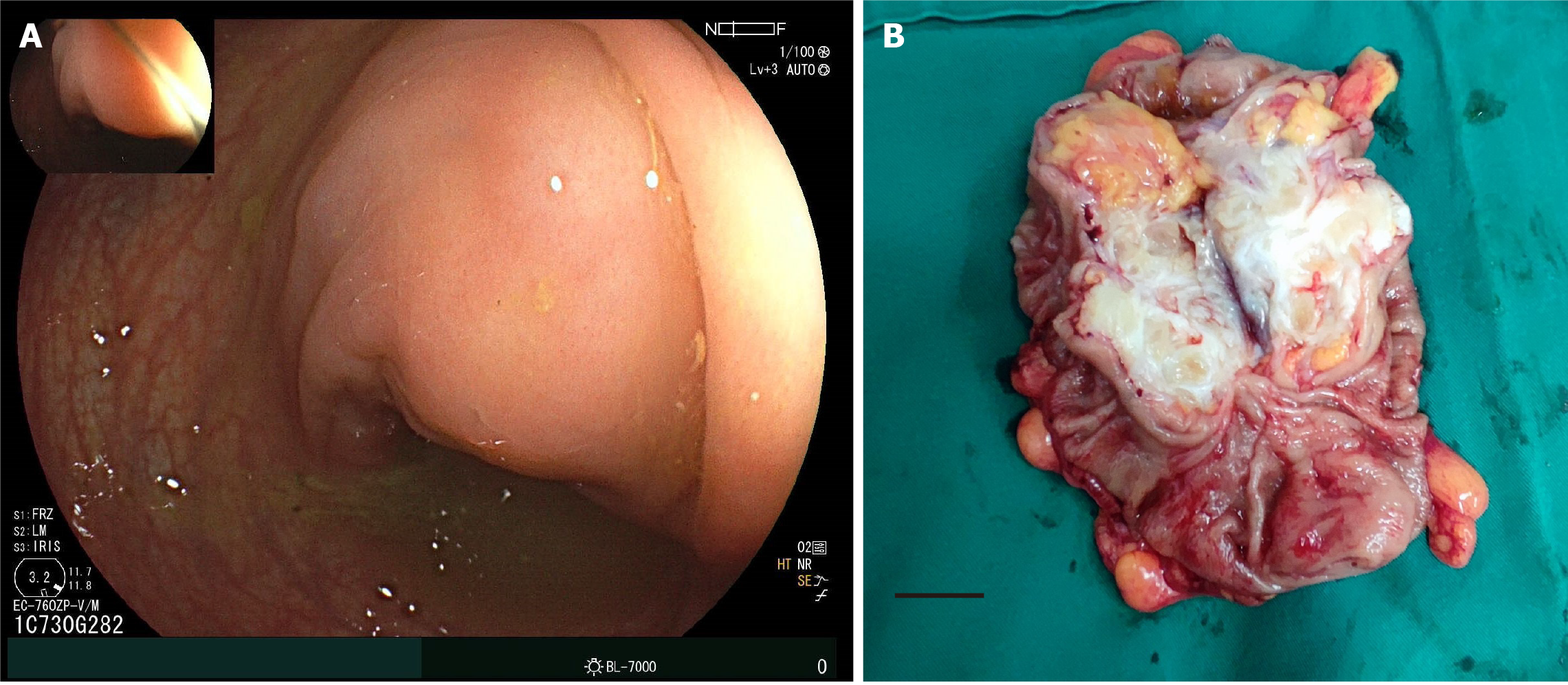
Contrast computed tomography revealed a mass measuring 3.9 cm × 3.5 cm in the sigmoid colon, which was slightly enhanced in the arterial phase (Figure 1). Colonoscopy was performed with the intention of biopsy. However, during the colonoscopy, no lesion was found in the lumen of the colon, but an external compress was found in the sigmoid colon (Figure 2A).
Based on the clinical presentation, laboratory findings and imaging findings, surgical disease was suspected, and a diagnosis of having a GIST was made. Endoscopic ultrasound fine-needle aspiration was suggested but refused by the patient due to the high cost and potential complications, such as bleeding. Therefore, laparoscopic exploration was performed.
During exploration, a lesion in the sigmoid colon was confirmed. Sigmoidectomy with D1 Lymphadenectomy was performed. The anastomosis was performed with a stapled side-to-end colocolostomy. The operation lasted 190 minutes, with an estimated blood loss of 30 mL. The tumor had a white-gray appearance (Figure 2B). Frozen resection indicated a spindle cell tumor of mesenchymal origin and a margin-negative resection.
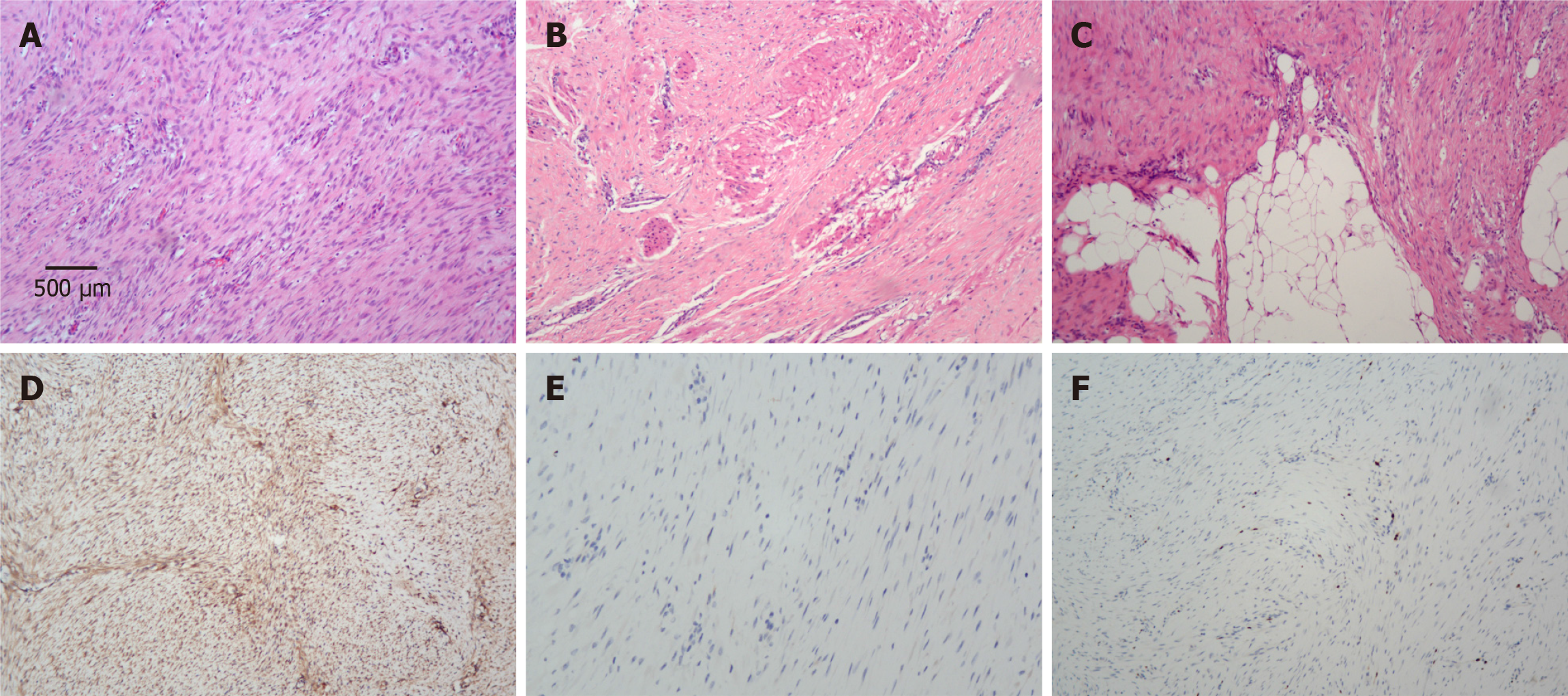
Pathological examination confirmed R0 resection and revealed that the size of the white-gray mass was 3.5 cm × 2.2 cm × 2.2 cm. There was no lymph node metastasis (0/12). Histology revealed that the tumor cells displayed a fascicular or interwoven pattern (Figure 3A) with infiltrative growth into the muscularis propria of the colon (Figure 3B) and the adipose tissue (Figure 3C). Immunohistochemical staining revealed diffuse nuclear staining for β-catenin in the tumor cells (Figure 3D), and the cells were also positive for CD34 but negative for CD117 (Figure 3E), Dog-1, S100, SMA and desmin. The Ki-67 index was less than 2% (Figure 3F). Taken together, these findings were consistent with AF.
Postoperatively, this patient recovered well. Despite the locally aggressive and infiltrative nature of the tumor, close follow-up by magnetic resonance imaging (MRI), instead of adjuvant therapy, such as radiotherapy, chemotherapy, and hormonal therapy, was suggested at the postoperative multidisciplinary conference.
During the follow-up, pelvic MRI was performed every three months for the first two years. The patient was free of recurrence 30 months after the operation. The patient was satisfied with the treatment and prognosis.
AF is a rare and locally aggressive monoclonal fibroblastic proliferation that does not metastasize but is characterized by infiltrative growth and a high rate of local recurrence[6]. Approximately 5%-10% of AFs are associated with familial adenomatous polyposis (FAP) or Gardner syndrome[7], and the remaining cases are sporadic, with trauma being the most common cause[8,9]. Since AF can occur at almost any anatomical site in the body, the presentations are variable. Diagnosis is based on pathological immunohistochemistry. Upfront surgery was the treatment of choice; however, a watch-and-wait approach has become the standard treatment since it has similar efficacy to upfront surgery in newly diagnosed patients[3]. Other treatment options include radiotherapy, cryoablation, chemotherapy, hormonal therapy, nonsteroidal anti-inflammatory drugs, and targeted therapy[10,11]. Sobczuk et al[12] reported that the 5-year event-free survival rates were 60% for surgical resection and 55% for the watch-and-wait approach with or without concomitant nonsteroid anti-inflammatory drugs as the first-line treatment, respectively[12]. By retrospectively analyzing 262 patients with AF treated at one single institution over a period of 30 years, Testa et al[13] reported that the 5-year progression-free survival rates were 50.6% after surgery, 64.9% after surgery followed by adjuvant radiotherapy, 57.1% after surgery followed by adjuvant systemic therapy, 24.9% after chemotherapy, 26.7% after hormonal therapy, 41.3% after treatments with tyrosine kinase inhibitors, 44.4% after cryoablation and high-intensity focused ultrasound, and 43.1% after active surveillance.
AF of the colon is extremely rare, and this extraluminal case is difficult to diagnose before surgery. Previously, one systematic review evaluated 11 different types of colon subepithelial tumors (SETs) and examined the endoscopic and EUS characteristics of colon SETs[14]. Recently, Kim et al[15] reported a total of 105 colon SETs, and a total of 25 entities were found. However, AF was not reported by these studies. Common entities include lipoma, parasitic infection, lymphangioma, and leiomyoma[15]. Previously, Mitrovic Jovanovic et al[16] and Linardoutsos et al[17] reported a case of AF located in the right colon that mimicked a GIST. Shah et al[18] reported an AF of the transverse colon that mimicked a perforated malignancy. Makis et al[19] reported an AF of the ascending colon that mimicked a colon malignancy. Abuji et al[20] reported a desmoid tumor in the medial wall of the cecum, which was suspected to be leiomyosarcoma. All of the abovementioned five patients underwent surgery without adjuvant therapy and were free of recurrence after 1 year of follow-up. In our case, we presented the first report in the literature of AF arising from the sigmoid colon. For intra-abdominal AF, a positive surgical margin is a validated risk factor for recurrence after surgery[21-23]. Since this patient had a negative margin, adjuvant therapy was not initiated. After resection with a negative margin, the patient was free of recurrence 30 months after surgery. However, this case was limited by the relatively short follow-up time. The patient should be under close follow-up for at least 5 years. In addition, although the above five cases in the literature and our case suggested that radical surgery with negative margins could achieve favorable outcomes, this approach did not preclude the use of an active surveillance strategy, which was found to have efficacy similar to that of upfront surgery[12,24]. Active surveillance with MRI has become the first-line approach for identifying AF[25]. In this situation, preoperative diagnosis is paramount. Therefore, computed tomography and MRI are commonly used imaging modalities[23]. However, neither of these two methods are diagnostic. Colonoscopy is commonly preferred for detecting lesions in the colon. However, for extraluminal masses without ulceration, it is difficult to diagnose using forceps biopsy during colonoscopy. Therefore, performing biopsy via endoscopic ultrasound fine-needle aspiration is essential for obtaining a pathological diagnosis of extraluminal masses. In this case, endoscopic ultrasound fine-needle aspiration was considered but was denied due to the costs and suspected surgical conditions of the GIST. Given the findings of this case, endoscopic ultrasound fine-needle aspiration should be emphasized to obtain full patient information and provide alternative treatment methods.
This case report describes the first known instance of AF originating from the sigmoid colon. Despite the favorable outcomes after surgery, a preoperative diagnosis was not achieved, and alternative treatment methods were not considered in the era of increasing modalities. Additionally, a potential link might exist among the uterine leiomyoma, fibroadenoma of the breast and AF of the sigmoid colon in this patient, which was not fundamentally investigated in this case. Nevertheless, colon polyps, which are characterized in 5%-10% of AF cases associated with FAP, were not found during the patient’s colonoscopy.
Although AF is a very rare entity, it should be considered in the differential diagnosis of subepithelial/extraluminal colon masses. Awareness of this condition will help surgeons perform thorough preoperative workups and determine the appropriate treatment strategies. For sporadic colonic AF, radical surgery alone could result in favorable outcomes.
The authors thank the patient for her kind willingness of consent for the anonymized publication of her case report findings and all accompanying images.
| 1. | Garcia-Ortega DY, Martín-Tellez KS, Cuellar-Hubbe M, Martínez-Said H, Álvarez-Cano A, Brener-Chaoul M, Alegría-Baños JA, Martínez-Tlahuel JL. Desmoid-Type Fibromatosis. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 2. | Zhang Z, Shi J, Yang T, Liu T, Zhang K. Management of aggressive fibromatosis. Oncol Lett. 2021;21:43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 3. | Pandrowala S, Jones RL, Gupta S, Gulia A. Desmoid fibromatosis: is the current picture changing? Future Oncol. 2021;17:3397-3408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Otero S, Moskovic EC, Strauss DC, Benson C, Miah AB, Thway K, Messiou C. Desmoid-type fibromatosis. Clin Radiol. 2015;70:1038-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 5. | Riley DS, Barber MS, Kienle GS, Aronson JK, von Schoen-Angerer T, Tugwell P, Kiene H, Helfand M, Altman DG, Sox H, Werthmann PG, Moher D, Rison RA, Shamseer L, Koch CA, Sun GH, Hanaway P, Sudak NL, Kaszkin-Bettag M, Carpenter JE, Gagnier JJ. CARE guidelines for case reports: explanation and elaboration document. J Clin Epidemiol. 2017;89:218-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 741] [Cited by in RCA: 1022] [Article Influence: 127.8] [Reference Citation Analysis (0)] |
| 6. | Kasper B, Baumgarten C, Garcia J, Bonvalot S, Haas R, Haller F, Hohenberger P, Penel N, Messiou C, van der Graaf WT, Gronchi A; Desmoid Working Group. An update on the management of sporadic desmoid-type fibromatosis: a European Consensus Initiative between Sarcoma PAtients EuroNet (SPAEN) and European Organization for Research and Treatment of Cancer (EORTC)/Soft Tissue and Bone Sarcoma Group (STBSG). Ann Oncol. 2017;28:2399-2408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 192] [Cited by in RCA: 273] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 7. | Nieuwenhuis MH, Casparie M, Mathus-Vliegen LM, Dekkers OM, Hogendoorn PC, Vasen HF. A nation-wide study comparing sporadic and familial adenomatous polyposis-related desmoid-type fibromatoses. Int J Cancer. 2011;129:256-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 153] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 8. | Escobar C, Munker R, Thomas JO, Li BD, Burton GV. Update on desmoid tumors. Ann Oncol. 2012;23:562-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 159] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 9. | DE Marchis ML, Tonelli F, Quaresmini D, Lovero D, Della-Morte D, Silvestris F, Guadagni F, Palmirotta R. Desmoid Tumors in Familial Adenomatous Polyposis. Anticancer Res. 2017;37:3357-3366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 10. | Desmoid Tumor Working Group. The management of desmoid tumours: A joint global consensus-based guideline approach for adult and paediatric patients. Eur J Cancer. 2020;127:96-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 286] [Article Influence: 57.2] [Reference Citation Analysis (0)] |
| 11. | Napolitano A, Mazzocca A, Spalato Ceruso M, Minelli A, Baldo F, Badalamenti G, Silletta M, Santini D, Tonini G, Incorvaia L, Vincenzi B. Recent Advances in Desmoid Tumor Therapy. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 12. | Sobczuk P, Agnieszczak IM, Grycuk W, Czarnecka AM, Świtaj T, Koseła-Paterczyk H, Morysiński T, Zdzienicki M, Rutkowski P. What is the best front-line approach in patients with desmoid fibromatosis? - A retrospective analysis from a reference center. Eur J Surg Oncol. 2021;47:2602-2608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 13. | Testa S, Bui NQ, Charville GW, Avedian RS, Steffner R, Ghanouni P, Mohler DG, Ganjoo KN. Management of Patients with Newly Diagnosed Desmoid Tumors in a First-Line Setting. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 14. | Kim TO. Colorectal Subepithelial Lesions. Clin Endosc. 2015;48:302-307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Kim A, Hong SN, Chang DK, Kim YH, Kim JE, Kim ER. Clinicopathologic and Endosonographic Characteristics of Colon Subepithelial Tumors Discovered Incidentally. Diagnostics (Basel). 2024;14. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 16. | Mitrovic Jovanovic M, Djuric-Stefanovic A, Velickovic D, Keramatollah E, Micev M, Jankovic A, Milosevic S, D Kovac J. Aggressive fibromatosis of the right colon mimicking a gastrointestinal stromal tumour: a case report. J Int Med Res. 2021;49:300060521994927. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Linardoutsos D, Patel N, Patel H. Colonic fibromatosis − a case report and review of the literature. J Coloproctol. 2018;38:346-350. [DOI] [Full Text] |
| 18. | Shah IA, Toor SA, Gerogiannis I. A rare case of desmoid fibromatosis of the transverse colon mimicking a perforated malignancy. Oxf Med Case Reports. 2021;2021:omab031. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 19. | Makis W, Ciarallo A, Abikhzer G, Stern J, Laufer J. Desmoid tumour (aggressive fibromatosis) of the colon mimics malignancy on dual time-point 18F-FDG PET/CT imaging. Br J Radiol. 2012;85:e37-e40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Abuji K, Naik A, Jain T, Dahiya D. Caecal desmoid tumour: a rare tumour at uncommon location and review of literature. BMJ Case Rep. 2021;14. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 21. | Huang PW, Tzen CY. Prognostic factors in desmoid-type fibromatosis: a clinicopathological and immunohistochemical analysis of 46 cases. Pathology. 2010;42:147-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Zheng Q, Liu B, Zhou Y, Liu D. Prognostic factors of abdominal desmoid tumor after surgery: A retrospective study of 52 patients. Asian J Surg. 2022;45:1770-1771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Xiao J, Mao J, Li B. Clinical Characteristics and Treatment of Intra-abdominal Aggressive Fibromatosis: A Retrospective Study of 16 Patients. Front Med (Lausanne). 2020;7:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Colombo C, Fiore M, Grignani G, Tolomeo F, Merlini A, Palassini E, Collini P, Stacchiotti S, Casali PG, Perrone F, Mariani L, Gronchi A. A Prospective Observational Study of Active Surveillance in Primary Desmoid Fibromatosis. Clin Cancer Res. 2022;28:4027-4032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 25. | Timbergen MJM, Schut AW, Grünhagen DJ, Sleijfer S, Verhoef C. Active surveillance in desmoid-type fibromatosis: A systematic literature review. Eur J Cancer. 2020;137:18-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |