Published online Aug 15, 2024. doi: 10.4251/wjgo.v16.i8.3457
Revised: June 21, 2024
Accepted: June 27, 2024
Published online: August 15, 2024
Processing time: 88 Days and 23.7 Hours
Numerous studies have assessed surgical resection as a standard treatment option for patients with colorectal cancer (CRC) and resectable pulmonary metastases (PM). However, the role of perioperative chemotherapy after complete resection of isolated PM from patients with CRC patients remains controversial. We hy
To determine whether perioperative chemotherapy affects survival after radical resection of isolated PM from CRC.
We retrospectively collected demographic, clinical, and pathologic data on patients who underwent radical surgery for isolated PM from CRC. Cancer-specific survival (CSS) and disease-free survival were calculated using Kaplan-Meier analysis. Inter-group differences were compared using the log-rank test. For multivariate analysis, Cox regression was utilized when indicated.
This study included 120 patients with a median age of 61.6 years. The 5-year CSS rate was 78.2%, with 36.7% experiencing recurrence. Surgical resection for isolated PM resulted in a 5-year CSS rate of 50.0% for second metastases. Perioperative chemotherapy (P = 0.079) did not enhance survival post-resection. Factors associated with improved survival included fewer metastatic lesions [hazard ratio (HR): 2.51, P = 0.045], longer disease-free intervals (HR: 0.35, P = 0.016), and wedge lung resections (HR: 0.42, P = 0.035). Multiple PM predicted higher recurrence risk (HR: 2.22, P = 0.022). The log-rank test showed no significant difference in CSS between single and repeated metastasectomy (P = 0.92).
Perioperative chemotherapy shows no survival benefit post-PM resection in CRC. Disease-free intervals and fewer metastatic lesions predict better survival. Repeated metastasectomy is warranted for eligible patients.
Core Tip: Several studies have evaluated surgical resection of isolated pulmonary metastasis as a standard treatment option for colorectal cancer (CRC) patients with resectable pulmonary metastases (PM). However, the role of peri-operative chemotherapy after complete resection of isolated PM from CRC patients is still controversial. We sought to determine whether peri-operative chemotherapy affects survival after radical resection of isolated PM from CRC. As a single center, our study still has certain value. At the same time, due to the long survival period of patients, they will receive a lot of subsequent treatments and there are many factors that interfere with the prognosis. We believe that cancer-specific survival can better reflect the focus of research on these patients, but many studies do not give this. Due to the lack of randomized prospective trials and high-level evidence, our study may support valuable data support for individual participant data meta-analysis and help further research on this type of disease.
- Citation: Gao Z, Jin X, Wu YC, Zhang SJ, Wu SK, Wang X. Effect of perioperative chemotherapy on resection of isolated pulmonary metastases from colorectal cancer: A single center experience. World J Gastrointest Oncol 2024; 16(8): 3457-3470
- URL: https://www.wjgnet.com/1948-5204/full/v16/i8/3457.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i8.3457
Recently, with the increasing incidence of colorectal cancer (CRC), the incidence of metastatic CRC continues to rise[1]. Pulmonary metastases (PM) occur in 10%-20% of patients with metastatic CRC, making the lung the second most common site of metastases after the liver[2]. However, while the European Society for Medical Oncology recommends adjuvant chemotherapy (ACT) for patients with liver metastases (LM) after LM resection, the biology of LM and PM differs[3].
The management of metastatic patients with CRC and lung disease requires a multidisciplinary approach, considering various factors related to patient and tumor characteristics that might affect prognosis. While it is generally assumed that complete surgical removal of PM from CRC is beneficial for improving outcomes[4], it remains unclear which patients will benefit from surgical resection and which are at higher risk of recurrence. The role of perioperative chemotherapy after pulmonary metastasectomy is also a subject of controversy, with some studies supporting its use[5,6] and others having reported drawbacks[7,8]. It is possible that inconsistent data and significant bias in patient selection contributes to the differing conclusions.
A meta-analysis has shown that perioperative chemotherapy prolongs patient survival; however, the primary endpoint of the analysis was overall survival, and this study only included literature published before 2019[9]. Considering the advancements in diagnosis and treatment recently, it is necessary to update the literature on this topic. Therefore, our aim was to investigate whether patients could benefit from ACT after PM resection.
Patients have a long survival period and can receive multiple subsequent treatments, which can potentially interfere with their prognosis. We believe that cancer-specific survival (CSS) better reflects the research focus on these patients. Nevertheless, many studies fail to consider this factor. Due to the lack of randomized prospective trials and high-level evidence, our study can provide valuable data support for individual participant data meta-analysis and contribute to further research on this disease type.
Prognostic factors for isolated PM remain uncertain, and better stratification may be necessary to determine the most appropriate therapeutic approach. This study retrospectively analyzed the clinical data of patients with isolated PM from CRC who underwent surgery. The analysis aimed to identify prognostic factors that facilitates informed decision making for physicians regarding how to best manage these patients.
A hospital database was used to identify patients who had undergone pulmonary metastasectomy for a diagnosis of CRC at Peking University First Hospital between January 1, 2008, and June 31, 2023. Patients who had received their initial pulmonary metastasectomy at another facility were excluded. The inclusion criteria consisted of: (1) A confirmed diagnosis of CRC adenocarcinoma that had been completely resected without signs of local recurrence; (2) Preoperative imaging showing no metastases outside the lungs and multidisciplinary discussions confirming the feasibility of completely resecting all metastatic nodules with sufficient pulmonary function reserves; and (3) Confirmation through post-operative pathology that pulmonary metastasis originated from CRC. The exclusion criteria included the presence of metastases outside the lungs or multiple bilateral PM that could not be completely resected (R0).
Isolated pulmonary metastasis was defined as the diagnosis of pulmonary metastasis of CRC without metastases outside the lungs. Synchronous PM were defined as metastases detected during the initial CRC staging workup, while metachronous PM were defined as metastases detected after baseline workup.
Surgical methods for PM can be divided into lobectomy and sublobar resection (including wedge resection and segmentectomy). In most cases, perioperative chemotherapy after pulmonary metastasectomy comprises an oxaliplatin-based doublet (FOLFOX: Folinic acid, 5-fluorouracil, oxaliplatin or CAPEOX: Capecitabine and oxaliplatin) or a topoisomerase inhibitor (FOLFIRI: folinic acid, 5-fluorouracil, irinotecan) administered for about 6 months. This study was approved by the Ethics Committee of Peking University First Hospital.
Follow-up data were collected through hospital records, telephone calls, outpatient visits, and rehospitalization. Data included age, sex, diagnosis date, surgical strategy, TNM stage, tumor size, blood levels of carcinoembryonic antigen, postoperative chemotherapeutic regimen (including targeted therapy), time from CRC diagnosis to PM, pulmonary lesion size, type of surgical approach, and extent of pulmonary resection and nodal dissection.
The last follow-up date was in January 2024. Survival time was defined as the time from pulmonary metastasectomy to death from any cause. CSS was the primary outcome, calculated from the date of pulmonary surgery to the date of death attributed to CRC. For cases where no death occurred, the date of the last available follow-up was considered. Disease-free survival (DFS) was the secondary outcome and was calculated monthly, with the date of the operation as the starting point and end event being tumor progression or death from any cause.
Analyses were conducted in R statistical software, version 4.3.1. A significance level of P ≤ 0.05 was used. The t-test was used to test continuous variables, while the chi-square test was used for categorical variables. Survival analysis was conducted using the Kaplan-Meier method and log-rank test. Multivariate analyses were performed using multivariate Cox regression.
Prior to data analysis, missing values in the clinic cohorts were examined for each variable. The proportion of missing data among the predictors ranged from 0 to 28.3%. To include these data in the multivariate analyses, missing data were imputed using multiple imputations by chained equations. The mice package for R was used, which incorporates predictive mean matching with a default setting of k = 5. The study factors and confounding factors were analyzed using different data sets. For univariate analysis, the complete subject data were used before imputation. For multivariate analysis, the adjustment result of the factor should also use the complete data before imputation, while other confounding factors can be analyzed using the imputed data[10].
During the study period, 120 patients met the inclusion criteria, predominantly men (n = 73; 60.8%) with a median age of 62 years (interquartile range, 55-70 years; Table 1). Of which, 34 (28.3%) had a reported history of tobacco use. PM were detected in most cases (90%) during follow-up, with a median disease-free interval (DFI) of 29.4 months. The remaining cases were patients with synchronous PM from CRC. Among these patients, 96 had one metastatic lesion, 11 had two metastatic lesions, 12 had three metastatic lesions, and one had five metastatic lesions. In total, 45% of patients received perioperative chemotherapy during radical resection for isolated PM.
| Factors | Total |
| Sex | |
| Male | 73 (60.8) |
| Female | 47 (39.2) |
| Age at primary cancer | |
| Median (IQR) | 59.10 (51.75-67.25) |
| DFI | |
| Median (IQR) | 2.47 (1.22-3.20) |
| Age at time of pulmonary surgery | |
| Median (IQR) | 62.00 (55.00-70.00) |
| Pulmonary metastasis | |
| Synchronous | 12 (10.0) |
| Metachronous | 108 (90.0) |
| Access | |
| Open | 25 (20.8) |
| VATS | 95 (79.2) |
| Type of resection | |
| Sublobar resection | 70 (58.3) |
| Lobectomy | 50 (41.7) |
| Lymph node dissection | |
| No | 66 (55.0) |
| Yes | 54 (45.0) |
| Perioperative chemotherapy | |
| No | 66 (55.0) |
| Yes | 54 (45.0) |
| Primary tumor T stage | |
| T1 | 3 (3.5) |
| T2 | 8 (9.3) |
| T3 | 62 (72.1) |
| T4 | 13 (15.1) |
| Primary tumor N stage | |
| N0 | 37 (40.7) |
| N1 or N2 | 54 (59.3) |
| Primary tumor location | |
| Left colon | 35 (32.4) |
| Right colon | 19 (17.6) |
| Rectum | 54 (50.0) |
| Adjuvant chemotherapy for CRC | |
| No | 35 (29.2) |
| Yes | 85 (70.8) |
| CEA levels | |
| ≤ 5 ng/mL | 62 (61.4) |
| > 5 ng/mL | 39 (38.6) |
| Number of metastatic lesions | |
| 1 | 96 (80.0) |
| 2 | 11 (9.2) |
| 3 | 12 (10.0) |
| 5 | 1 (0.8) |
| Tumor size (cm) | |
| ≤ 2 cm | 63 (53.8) |
| > 2 cm | 54 (46.2) |
| CRC differentiation | |
| Well/well-to-moderate | 11 (12.2) |
| Moderate | 76 (84.4) |
| Moderate-to-poor/poor | 3 (3.3) |
| Smoking history | |
| No | 86 (71.7) |
| Yes | 34 (28.3) |
| RAS | |
| Wild type | 14 (53.8) |
| Mutant type | 12 (46.2) |
| Bilateral pulmonary nodules | |
| No | 113 (94.2) |
| Yes | 7 (5.8) |
| LN sampling at PM | |
| No | 66 (55.0) |
| Yes | 54 (45.0) |
| Positive LN at PM | |
| No | 112 (93.3) |
| Yes | 8 (6.7) |
| CRC LVI | |
| No | 58 (84.1) |
| Yes | 11 (15.9) |
| CRC PNI | |
| No | 50 (72.5) |
| Yes | 19 (27.5) |
Within the entire cohort, 66 patients did not receive perioperative chemotherapy, while 54 patients did. There were no differences in terms of sex, type of resection, number of metastatic lesions, tumor size, bilateral pulmonary nodules, lymph node (LN) sampling at pulmonary metastasis, or positive LN at pulmonary metastasis when stratified by perioperative chemotherapy. However, significant differences were found for age at the time of primary cancer diagnosis (P = 0.012), age at the time of pulmonary surgery (P = 0.002), and smoking history (P = 0.006) (Table 2).
| Factors | Levels | Surgery alone (n = 66) | Perioperative chemotherapy (n = 54) | P value |
| Sex | Male | 43 (65.2) | 30 (55.6) | 0.377 |
| Female | 23 (34.8) | 24 (44.4) | ||
| Age at CRC diagnosis | mean ± SD | 61.2 ± 9.7 | 56.6 ± 10.1 | 0.012 |
| Age at time of pulmonary surgery | mean ± SD | 64.1 ± 9.4 | 58.6 ± 9.9 | 0.002 |
| Smoking history | No | 40 (60.6) | 46 (85.2) | 0.006 |
| Yes | 26 (39.4) | 8 (14.8) | ||
| Adjuvant chemotherapy for CRC | No | 24 (36.4) | 11 (20.4) | 0.086 |
| Yes | 42 (63.6) | 43 (79.6) | ||
| CRC differentiation | Moderate | 36 (81.8) | 40 (87.0) | 0.744 |
| Moderate to poor/poor | 2 (4.5) | 1 (2.2) | ||
| Well/well to moderate | 6 (13.6) | 5 (10.9) | ||
| Primary tumor T stage | 0 | 1 (2.5) | 2 (4.3) | 0.969 |
| II | 4 (10.0) | 4 (8.7) | ||
| III | 29 (72.5) | 33 (71.7) | ||
| IV | 6 (15.0) | 7 (15.2) | ||
| Primary tumor N stage | 0 | 17 (39.5) | 20 (41.7) | 0.560 |
| 1 | 18 (41.9) | 17 (35.4) | ||
| 2 | 8 (18.6) | 11 (23.0) | ||
| MSS | pMMR | 26 (100.0) | 31(100.0) | |
| RAS | Wild type | 5 (71.4) | 9 (47.4) | 0.517 |
| Mutant type | 2 (28.6) | 10 (52.6) | ||
| Access | Open | 18 (27.3) | 7 (13.0) | 0.090 |
| VATS | 48 (72.7) | 47 (87.0) | ||
| Type of resection | Lobe | 33 (50.0) | 17 (31.5) | 0.063 |
| Segmental wedge | 33 (50.0) | 37 (68.5) | ||
| Number of metastatic lesions | 1 | 53 (80.3) | 43 (79.6) | 1.000 |
| > 1 | 13 (19.7) | 11 (20.4) | ||
| Tumor size (cm) | ≤ 2 cm | 32 (50.0) | 31 (58.5) | 0.465 |
| > 2 cm | 32 (50.0) | 22 (41.5) | ||
| Bilateral pulmonary nodules | No | 62 (93.9) | 51 (94.4) | 1.000 |
| Yes | 4 (6.1) | 3 (5.6) | ||
| LN sampling at PM | No | 32 (48.5) | 34 (63.0) | 0.161 |
| Yes | 34 (51.5) | 20 (37.0) | ||
| Positive LN at PM | No | 63 (95.5) | 49 (90.7) | 0.508 |
| Yes | 3 (4.5) | 5 (9.3) | ||
| CEA | ≤ 5 ng/mL | 33 (61.1) | 29 (61.7) | 1.000 |
| > 5 ng/mL | 21 (38.9) | 18 (38.3) | ||
| Pulmonary metastasis | Synchronous | 3 (4.5) | 9 (16.7) | 0.058 |
| Metachronous | 63 (95.5) | 45 (83.3) | ||
| DFI | mean ± SD | 1005.3 ± 774.6 | 771.9 ± 701.5 | 0.089 |
| Primary tumor location | Left colon | 18 (31.0) | 17 (34.0) | 0.363 |
| Rectum | 27 (46.6) | 27 (54.0) | ||
| Right colon | 13 (22.4) | 6 (12.0) |
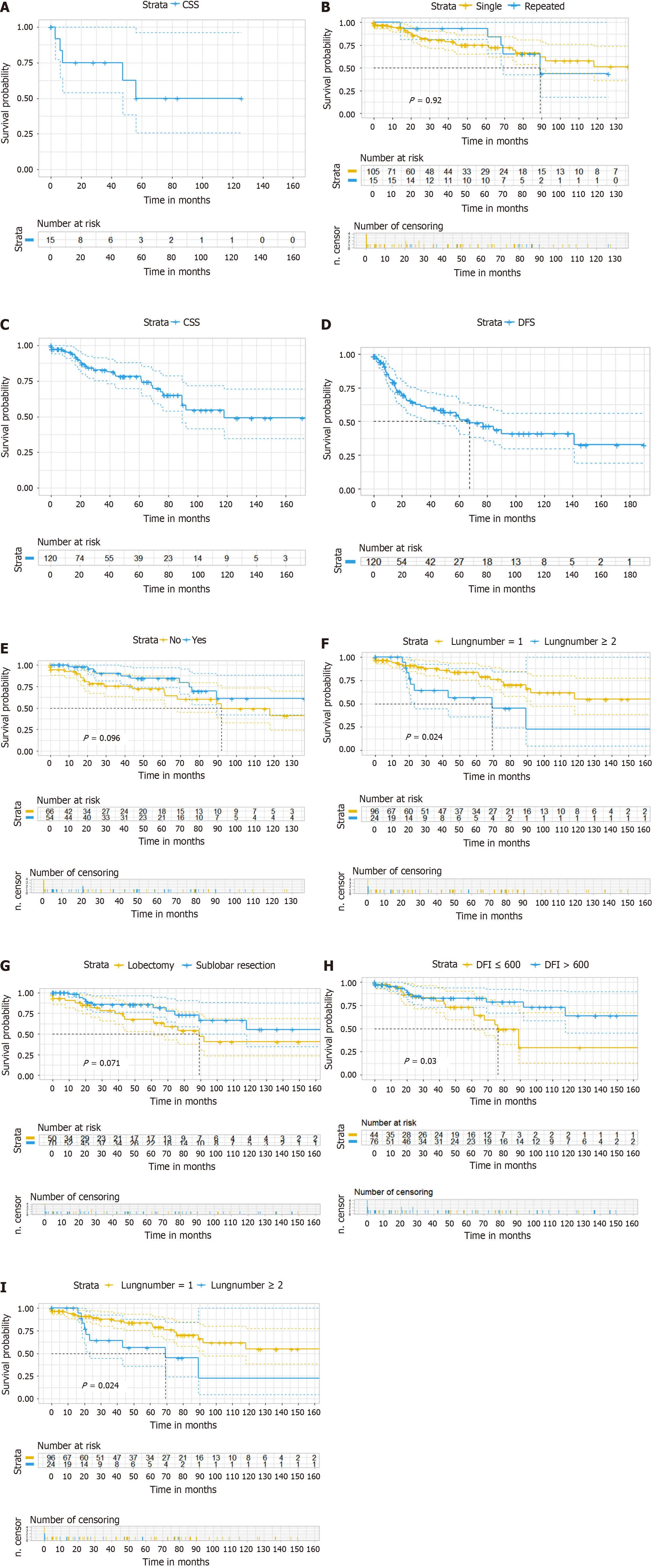
Overall, 17 patients developed second pulmonary metastasis after pulmonary metastasis resection, and 15 of them underwent surgical treatment. The 5-year CSS rate for second metastases treated with local therapy was 50.0% (Figure 1A). The median follow-up time since the first resection was 56.2 months for patients who received a second localized treatment, and the median DFI for the second resection was 22.6 months. Among patients who underwent repeated metastasectomy, three patients had a third metastasectomy and one had a fourth metastasectomy. The log-rank test comparing CSS between one-time metastasectomy and repeated metastasectomy did not show significant differences (P = 0.92) (Figure 1B).
The median follow-up time was 48.8 months. At the time of analysis, 30 patients had died, of which 28 deaths were due to cancer causes. The CSS rates at 1, 2, and 5 years were 95% [95% confidence interval (95%CI): 90.8%-99.4%], 85.4% (95%CI: 78.4%-93.2%), and 78.2% (95%CI: 69.6%-87.8%) (Figure 1C). Progressive disease occurred in 44 patients (36.7%) at a median interval of 67.4 months. The progression-free survival rates at 1, 2, and 5 years were 80.6% (95%CI: 72.9%-89.1%), 61.2% (95%CI: 51.5%-72.8%), and 52.7% (95%CI: 42.4%-65.5%), respectively (Figure 1D). Tables 3 and 4 provided an overview of the survival data based on risk factors.
| Factors | HR (univariable) | |
| Sex | Male | |
| Female | 1.01 (0.47-2.17, P = 0.980) | |
| Age at CRC diagnosis | ≤ 60 years | |
| > 60 years | 1.32 (0.63-2.78, P = 0.460) | |
| Age at time of pulmonary surgery | ≤ 60 years | |
| > 60 years | 0.86 (0.48-2.03, P = 0.970) | |
| Smoking history | No | |
| Yes | 1.37 (0.63-2.97, P = 0.420) | |
| Pulmonary metastasis | Synchronous | |
| Metachronous | 2.52 (0.34-18.7, P = 0.360) | |
| Surgical approach for PM | Open | |
| VATS | 0.56 (0.25-1.24, P = 0.150) | |
| Type of resection | Lobectomy | |
| Sublobar resection | 0.51 (0.24-1.08, P = 0.077) | |
| Lymph node dissection | No | |
| Yes | 1.24 (0.59-2.61, P = 0.560) | |
| Perioperative chemotherapy | No | |
| Yes | 0.52 (0.24-1.14, P = 0.100) | |
| Primary tumor T stage | T1-T3 | |
| T4 | 1.70 (0.57-5.08, P = 0.370) | |
| Primary tumor N stage | N0 | |
| N1 or N2 | 1.39 (0.57-3.33, P = 0.460) | |
| Primary tumor location | Left colon | |
| Right colon | 0.61 (0.26-1.42, P = 0.260) | |
| Rectum | 0.56 (0.17-1.78, P = 0.320) | |
| CEA levels | ≤ 5 ng/mL | |
| > 5 ng/mL | 1.38 (0.64-3.00, P = 0.420) | |
| Number of metastatic lesions | 1 | |
| > 1 | 2.47 (1.11-5.51, P = 0.027) | |
| Tumor size (cm) | ≤ 2 cm | |
| > 2 cm | 0.81 (0.37-1.77, P = 0.600) | |
| DFI | ≤ 600 | |
| > 600 | 0.44 (0.20-0.94, P = 0.030) | |
| Factors | HR (univariable) | |
| Sex | Male | |
| Female | 0.82 (0.44-1.52, P = 0.530) | |
| Age at CRC diagnosis | ≤ 60 years | |
| > 60 years | 0.86 (0.46-1.58, P = 0.630) | |
| Age at time of pulmonary surgery | ≤ 60 years | |
| > 60 years | 0.58 (0.32-1.06, P = 0.077) | |
| Smoking history | No | |
| Yes | 1.33 (0.70-2.50, P = 0.380) | |
| Pulmonary metastasis | Synchronous | |
| Metachronous | 1.62 (0.50-5.27, P = 0.420) | |
| Surgical approach for PM | Open | |
| VATS | 0.98 (0.48-1.99, P = 0.950) | |
| Type of resection | Lobectomy | |
| Sublobar resection | 0.82 (0.45-1.48, P = 0.510) | |
| Lymph node dissection | No | |
| Yes | 0.98 (0.54-1.78, P = 0.950) | |
| Perioperative chemotherapy | No | |
| Yes | 0.86 (0.48-1.57, P = 0.640) | |
| Primary tumor T stage | T1-T3 | |
| T4 | 2.63 (1.18-5.87, P = 0.017) | |
| Primary tumor N stage | N0 | |
| N1 or N2 | 1.94 (0.95-3.96, P = 0.069) | |
| Primary tumor location | Left colon | |
| Right colon | 0.71 (0.26-1.91, P = 0.500) | |
| Rectum | 0.96 (0.47-1.96, P = 0.920) | |
| CEA levels | ≤ 5 ng/mL | |
| > 5 ng/mL | 1.11 (0.58-2.14, P = 0.750) | |
| Number of metastatic lesions | 1 | |
| > 1 | 2.34 (1.21-4.53, P = 0.012) | |
| Tumor size (cm) | ≤ 2 cm | |
| > 2 cm | 0.82 (0.44-1.53, P = 0.540) | |
| DFI | ≤ 600 | |
| > 600 | 0.60 (0.33-1.09, P = 0.090) | |
Perioperative chemotherapy (P = 0.10) did not improve survival rates following PM resection in patients with CRC. However, DFI (P = 0.03) and the number of metastatic lesions (P = 0.027) were identified as key predictors for higher chances of survival. By the end of the study, 44 patients experienced relapse, with > 1 multiple PM (P = 0.012) and DFI < 600 days identified as risk factors for recurrence (Table 3).
In the multivariate analysis, perioperative chemotherapy (P = 0.079) did not confer survival benefits for patients following PM resection in CRC. Factors such as the number of metastatic lesions (HR: 2.51, P = 0.045), DFI (HR: 0.35, P = 0.016), and wedge resection of the lungs (HR: 0.42, P = 0.035) were found to be associated with the highest survival rates, while having more than one pulmonary metastasis (HR: 2.22, P = 0.022) was confirmed as a predictor of disease recurrence. Table 5 summarizes the results of the multivariate Cox regression analysis. Figure 1E-I display the survival curves for perioperative chemotherapy, the number of PM, type of resection, and DFI based on covariates.
| HR | 95%CI | P value | ||
| Recurrence | ||||
| Age at time of pulmonary surgery | ≤ 60 years | |||
| > 60 years | 0.59 | 0.32-1.10 | 0.099 | |
| Number of metastatic lesions | 1 | |||
| ≥ 2 | 2.22 | 1.12-4.38 | 0.022 | |
| Primary tumor N stage | N0 | |||
| N1 or N2 | 1.51 | 0.79-2.87 | 0.215 | |
| DFI | ≤ 600 | |||
| > 600 | 0.78 | 0.42-1.45 | 0.432 | |
| Survival | ||||
| Age at time of pulmonary surgery | ≤ 60 years | |||
| > 60 years | 1.57 | 0.69-3.58 | 0.286 | |
| Primary tumor T stage | T1 or T2 | |||
| T3 or T4 | 1.16 | 0.38-3.57 | 0.079 | |
| Primary tumor N stage | N0 | |||
| N1 or N2 | 1.35 | 0.58-3.13 | 0.485 | |
| Pulmonary metastasis | Synchronous | |||
| Metachronous | 2.35 | 0.29-18.85 | 0.423 | |
| Perioperative chemotherapy | No | |||
| Yes | 0.48 | 0.21-1.09 | 0.079 | |
| Number of metastatic lesions | 1 | |||
| ≥ 2 | 2.51 | 1.02-6.15 | 0.045 | |
| Type of resection | Lobectomy | |||
| Sublobar resection | 0.42 | 0.19-0.94 | 0.035 | |
| DFI | ≤ 600 | |||
| > 600 | 0.35 | 0.15-0.82 | 0.016 |
Supplementary Table 1 presents the data before and after imputation, demonstrating consistency in data distribution and indicating that imputation did not introduce significant bias. Additionally, a sensitivity analysis, which replaced the imputation of missing laboratory values with a complete case analysis, showed no substantial changes in the results (Supplementary Tables 2 and 3). Supplementary Table 4 shows the sensitivity analysis of recurrence-related factors, including the original T and N stages of colorectal cancer.
Currently, there is insufficient high-level evidence regarding the role of perioperative chemotherapy following complete resection of isolated PM in patients with CRC. While ACT is recommended post-surgery for CRC patients with LM[11], the situation is different for those with PM[12]. The expert Consensus on Multidisciplinary Comprehensive Treatment of Colorectal Cancer Pulmonary Metastases (2018 Edition)[13] noted that unlike distant metastases from other sites of mCRC, pulmonary metastatic lesions grew relatively slowly and have a better overall prognosis[14]. A “watch and wait” protocol is considered appropriate after pulmonary metastasectomy in patients with CRC for pulmonary involvement, as it is associated with a better outcome[9]. To verify the efficacy of perioperative chemotherapy for CRC PM, this study analyzed the effectiveness of perioperative chemotherapy for PM, and the results showed that perioperative chemotherapy had no significant effect on CSS (HR: 0.48; 95%CI: 0.21-1.09; P = 0.079) in patients after PM resection from CRC.
A meta-analysis of 18 cohort studies involving 3885 patients with colorectal PM evaluated the role of chemotherapy after radical pneumonectomy compared to surgery alone. The analysis found that postoperative treatment did not improve CSS (HR: 0.52; 95%CI: 0.24-1.14, P = 0.10) and DFS (HR: 0.86; 95%CI: 0.48-1.57, P = 0.64) compared to surgery alone. However, the limitations of this meta-analysis, including the retrospective nature of the studies analyzed and high degree of heterogeneity, may negatively affect the results[15]. Despite the lack of randomized prospective trials and limited evidence, perioperative or postoperative chemotherapy is commonly used in clinical practice for treating patients with resectable PM, especially in those with poor prognostic factors. These recommendations are largely based on data following the resection of metastatic CRC LM[16].
During postoperative chemotherapy for PM, the literature provides data on its potential use in patients undergoing surgery for isolated PM. Rapicetta et al[17] did not report any survival advantage with adjuvant treatment, whereas Guerrera et al[18] reported better outcomes with adjuvant chemotherapy in patients with multiple metastases, suggesting that there are no reliable data on isolated PM. The authors confirmed their theory in the recent Best Evidence Thread, which suggested that adjuvant chemotherapy may improve the prognosis of specific patients with advanced disease or specific molecular patterns[19]. Therefore, there is no clear evidence that patients undergoing PM resection benefit when treated with a single locoregional therapy, suggesting that this treatment is particularly applicable to patients with multiple metastases. However, further planned studies are needed to better clarify this issue.
Many studies have shown that patients with single metastases treated with PM have a higher survival rate. Nevertheless, treatment of multiple metastases remains controversial. According to a meta-analysis, resection of three to four metastases appears to be reasonable as long as complete resection is possible[20]. Herein, we found a highly significant correlation between survival and the number of PM as a linear continuous variable. This may be important for grading patients with a poorer prognosis who may receive chemotherapy pre- or post-metastasectomy.
The most common thoracic surgeries for colorectal PM are wedge resection and segmental pulmonary resection[21]. Surgical options for PM include wedge resection, segmental pulmonary resection, lobectomy, and total pneumonectomy[22]. However, sublobar resection may be a viable option for patients who are older or have chronic obstructive pul
Some researchers concluded that lobectomy results in a greater loss of pulmonary function and poorer prognosis compared to wedge or segmental resection. However, the prognostic impact of U-VATS sublobar resection of mCRC PM requires further investigation with larger sample sizes and a long-term perspective[26]. Furthermore, sublobar resection is more suitable when the number of metastases is low, suggesting that more pulmonary function may be preserved after sublobar resection in patients with appropriately located lesions.
While resections for recurrent PM from CRC with curative intent may lead to greater long-term survival, recurrence is common after the initial metastasectomy[27]. In the present study, the Kaplan-Meier analysis did not show differences in survival curves between first-time metastasectomy and repeated metastasectomy (P = 0.92). Similar outcomes were reported by Menna et al[28] and Lehtomäki et al[29]. These results are caused by the fact that repeat operations targeting locally confined recurrences in the lungs can effectively regain control within the thoracic cavity. Jaklitsch et al[30] showed that prognosis remains favorable (5-year survival: 33%-59%) when repeated resections achieve local control (R0). However, once local control has been lost, survival drops significantly to a median of 8 months, regardless of the number of previous procedures.
In the present study, patients were at advanced stages, so the impact of chemotherapy on overall survival time may not be significant, despite our correction in multivariate analysis for the influence of confounding variables, including stage. Furthermore, medical treatment strategies were diverse due to the availability of different multimodal treatment options during the study period, including follow-up measures. While a meta-analysis demonstrates that perioperative chemotherapy can indeed extend patient survival, its primary endpoint was overall survival, and all literature included predates 2019[9]. Considering the advancements in diagnosis and treatment, incorporating recent literature is imperative. Although our study was conducted at a single center, it still holds certain value. Additionally, due to the prolonged survival period of patients, they may undergo numerous subsequent treatments and encounter various factors that impact prognosis. Therefore, we believe that CSS better reflects the focus of research in these patients; however, many studies fail to consider this measure. Considering the absence of randomized prospective trials and high-level evidence, our study may provide valuable data support for individual participant data meta-analysis and facilitate further research on this particular disease.
Surgical resection of isolated PM from CRC prolongs patient survival. However, perioperative chemotherapy does not provide survival benefits for patients after PM resection from CRC. The presence of > 1 PM is an unfavorable predictor of death or disease recurrence, suggesting that chemotherapy could potentially be beneficial. Repeated pulmonary metastasectomy is justified and should be offered to patients who have sufficient cardiopulmonary reserve and technically resectable recurrent CRC PM.
| 1. | Cervantes A, Adam R, Roselló S, Arnold D, Normanno N, Taïeb J, Seligmann J, De Baere T, Osterlund P, Yoshino T, Martinelli E; ESMO Guidelines Committee. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023;34:10-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 756] [Article Influence: 378.0] [Reference Citation Analysis (33)] |
| 2. | Liu K, Cui Y, Li H, Mi J, Wang H, Zhuang Y, Tang L, Liu J, Tian C, Zhang Z, Zhou J, Shi H, Tian X, Liu P. The mechanism investigation of mutation genes in liver and lung metastasis of colorectal cancer by using NGS technique. Crit Rev Oncol Hematol. 2023;188:104057. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 3. | Van Cutsem E, Nordlinger B, Cervantes A; ESMO Guidelines Working Group. Advanced colorectal cancer: ESMO Clinical Practice Guidelines for treatment. Ann Oncol. 2010;21 Suppl 5:v93-v97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 350] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 4. | Handy JR, Bremner RM, Crocenzi TS, Detterbeck FC, Fernando HC, Fidias PM, Firestone S, Johnstone CA, Lanuti M, Litle VR, Kesler KA, Mitchell JD, Pass HI, Ross HJ, Varghese TK. Expert Consensus Document on Pulmonary Metastasectomy. Ann Thorac Surg. 2019;107:631-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 136] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 5. | Hao Z, Parasramka S, Chen Q, Jacob A, Huang B, Mullett T, Benson AB. Neoadjuvant Versus Adjuvant Chemotherapy for Resectable Metastatic Colon Cancer in Non-academic and Academic Programs. Oncologist. 2023;28:48-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 6. | Vidarsdottir H, Siesing C, Nodin B, Jönsson P, Eberhard J, Jirström K, Brunnström H. Clinical significance of RBM3 expression in surgically treated colorectal lung metastases and paired primary tumors. J Surg Oncol. 2021;123:1144-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Okumura T, Boku N, Hishida T, Ohde Y, Sakao Y, Yoshiya K, Higashiyama M, Hyodo I, Mori K, Kondo H. Surgical Outcome and Prognostic Stratification for Pulmonary Metastasis From Colorectal Cancer. Ann Thorac Surg. 2017;104:979-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 8. | Karim S, Nanji S, Brennan K, Pramesh CS, Booth CM. Chemotherapy for resected colorectal cancer pulmonary metastases: Utilization and outcomes in routine clinical practice. Eur J Surg Oncol. 2017;43:1481-1487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Li Y, Qin Y. Peri-operative chemotherapy for resectable colorectal lung metastasis: a systematic review and meta-analysis. J Cancer Res Clin Oncol. 2020;146:545-553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 10. | Omar R. Clinical Prediction Models: A Practical Approach to Development, Validation and Updating by STEYERBERG. E. W. Biometrics. 2010;66:661-662. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, Aranda Aguilar E, Bardelli A, Benson A, Bodoky G, Ciardiello F, D'Hoore A, Diaz-Rubio E, Douillard JY, Ducreux M, Falcone A, Grothey A, Gruenberger T, Haustermans K, Heinemann V, Hoff P, Köhne CH, Labianca R, Laurent-Puig P, Ma B, Maughan T, Muro K, Normanno N, Österlund P, Oyen WJ, Papamichael D, Pentheroudakis G, Pfeiffer P, Price TJ, Punt C, Ricke J, Roth A, Salazar R, Scheithauer W, Schmoll HJ, Tabernero J, Taïeb J, Tejpar S, Wasan H, Yoshino T, Zaanan A, Arnold D. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27:1386-1422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2286] [Cited by in RCA: 2403] [Article Influence: 267.0] [Reference Citation Analysis (31)] |
| 12. | Gkikas A, Kakos C, Lampridis S, Godolphin PJ, Patrini D. Preoperative prognostic factors for 5-year survival following pulmonary metastasectomy from colorectal cancer: a systematic review and meta-analysis. Eur J Cardiothorac Surg. 2023;63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 13. | Li J, Yuan Y, Yang F, Wang Y, Zhu X, Wang Z, Zheng S, Wan D, He J, Wang J, Ba Y, Bai C, Bai L, Bai W, Bi F, Cai K, Cai M, Cai S, Chen G, Chen K, Chen L, Chen P, Chi P, Dai G, Deng Y, Ding K, Fan Q, Fang W, Fang X, Feng F, Fu C, Fu Q, Gu Y, He Y, Jia B, Jiang K, Lai M, Lan P, Li E, Li D, Li J, Li L, Li M, Li S, Li Y, Li Y, Li Z, Liang X, Liang Z, Lin F, Lin G, Liu H, Liu J, Liu T, Liu Y, Pan H, Pan Z, Pei H, Qiu M, Qu X, Ren L, Shen Z, Sheng W, Song C, Song L, Sun J, Sun L, Sun Y, Tang Y, Tao M, Wang C, Wang H, Wang J, Wang S, Wang X, Wang X, Wang Z, Wu A, Wu N, Xia L, Xiao Y, Xing B, Xiong B, Xu J, Xu J, Xu N, Xu R, Xu Z, Yang Y, Yao H, Ye Y, Yu Y, Yu Y, Yue J, Zhang J, Zhang J, Zhang S, Zhang W, Zhang Y, Zhang Z, Zhang Z, Zhao L, Zhao R, Zhou F, Zhou J, Jin J, Gu J, Shen L. Expert consensus on multidisciplinary therapy of colorectal cancer with lung metastases (2019 edition). J Hematol Oncol. 2019;12:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 82] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 14. | Prasanna T, Karapetis CS, Roder D, Tie J, Padbury R, Price T, Wong R, Shapiro J, Nott L, Lee M, Chua YJ, Craft P, Piantadosi C, Sorich M, Gibbs P, Yip D. The survival outcome of patients with metastatic colorectal cancer based on the site of metastases and the impact of molecular markers and site of primary cancer on metastatic pattern. Acta Oncol. 2018;57:1438-1444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 15. | Zhang C, Tan Y, Xu H. Does adjuvant chemotherapy improve the prognosis of patients after resection of pulmonary metastasis from colorectal cancer? A systematic review and meta-analysis. Int J Colorectal Dis. 2019;34:1661-1671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, Bechstein WO, Primrose JN, Walpole ET, Finch-Jones M, Jaeck D, Mirza D, Parks RW, Mauer M, Tanis E, Van Cutsem E, Scheithauer W, Gruenberger T; EORTC Gastro-Intestinal Tract Cancer Group; Cancer Research UK; Arbeitsgruppe Lebermetastasen und-tumoren in der Chirurgischen Arbeitsgemeinschaft Onkologie (ALM-CAO); Australasian Gastro-Intestinal Trials Group (AGITG); Fédération Francophone de Cancérologie Digestive (FFCD). Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2013;14:1208-1215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 760] [Cited by in RCA: 910] [Article Influence: 75.8] [Reference Citation Analysis (0)] |
| 17. | Rapicetta C, Lococo F, Davini F, Carleo F, Kauppi J, Di Stefano TS, Ricciardi S, Di Martino M, Räsänen J, Paci M, Melfi F, Cardillo G. Is Adjuvant Chemotherapy Worthwhile After Radical Resection for Single Lung Metastasis From Colorectal Cancer? A Multicentric Analysis Evaluating the Risk of Recurrence. Front Oncol. 2019;9:763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Guerrera F, Mossetti C, Ceccarelli M, Bruna MC, Bora G, Olivetti S, Lausi PO, Solidoro P, Ciccone G, Ruffini E, Oliaro A, Filosso PL. Surgery of colorectal cancer lung metastases: analysis of survival, recurrence and re-surgery. J Thorac Dis. 2016;8:1764-1771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 19. | Guerrera F, Falcoz PE, Renaud S, Massard G. Does perioperative chemotherapy improve survival in patients with resectable lung metastases of colorectal cancer? Interact Cardiovasc Thorac Surg. 2017;24:789-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Gonzalez M, Poncet A, Combescure C, Robert J, Ris HB, Gervaz P. Risk factors for survival after lung metastasectomy in colorectal cancer patients: a systematic review and meta-analysis. Ann Surg Oncol. 2013;20:572-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 290] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 21. | Pfannschmidt J, Dienemann H, Hoffmann H. Surgical resection of pulmonary metastases from colorectal cancer: a systematic review of published series. Ann Thorac Surg. 2007;84:324-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 410] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 22. | Cheung F, Alam N, Wright G. Pulmonary metastasectomy: analysis of survival and prognostic factors in 243 patients. ANZ J Surg. 2018;88:1316-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Balata H, Blandin Knight S, Barber P, Colligan D, Crosbie EJ, Duerden R, Elton P, Evison M, Greaves M, Howells J, Irion K, Karunaratne D, Kirwan M, Macnab A, Mellor S, Miller C, Newton T, Novasio J, Sawyer R, Sharman A, Slevin K, Smith E, Taylor B, Taylor S, Tonge J, Walsham A, Waplington S, Whittaker J, Booton R, Crosbie PAJ. Targeted lung cancer screening selects individuals at high risk of cardiovascular disease. Lung Cancer. 2018;124:148-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 24. | Hennon M, Landreneau RJ. Role of Segmentectomy in Treatment of Early-Stage Non-Small Cell Lung Cancer. Ann Surg Oncol. 2018;25:59-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 25. | Zhang Y, Sun Y, Wang R, Ye T, Zhang Y, Chen H. Meta-analysis of lobectomy, segmentectomy, and wedge resection for stage I non-small cell lung cancer. J Surg Oncol. 2015;111:334-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 26. | Vogelsang H, Haas S, Hierholzer C, Berger U, Siewert JR, Präuer H. Factors influencing survival after resection of pulmonary metastases from colorectal cancer. Br J Surg. 2004;91:1066-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 90] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 27. | Yang KM, Park IJ, Lee JL, Kim CW, Yoon YS, Lim SB, Yu CS, Kim JC. Benefits of repeated resections for liver and lung metastases from colorectal cancer. Asian J Surg. 2020;43:102-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 28. | Menna C, Berardi G, Tierno SM, Andreetti C, Maurizi G, Ciccone AM, D'Andrilli A, Cassiano F, Poggi C, Diso D, Venuta F, Rendina EA, Ibrahim M. Do Repeated Operations for Recurrent Colorectal Lung Metastases Result in Improved Survival? Ann Thorac Surg. 2018;106:421-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 29. | Lehtomäki K, Soveri LM, Osterlund E, Lamminmäki A, Uutela A, Heervä E, Halonen P, Stedt H, Aho S, Muhonen T, Ålgars A, Salminen T, Kallio R, Nordin A, Aroviita L, Nyandoto P, Kononen J, Glimelius B, Ristamäki R, Isoniemi H, Osterlund P. Resectability, Resections, Survival Outcomes, and Quality of Life in Older Adult Patients with Metastatic Colorectal Cancer (the RAXO-Study). J Clin Med. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 30. | Jaklitsch MT, Mery CM, Lukanich JM, Richards WG, Bueno R, Swanson SJ, Mentzer SJ, Davis BD, Allred EN, Sugarbaker DJ. Sequential thoracic metastasectomy prolongs survival by re-establishing local control within the chest. J Thorac Cardiovasc Surg. 2001;121:657-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 65] [Article Influence: 2.7] [Reference Citation Analysis (0)] |