Published online Aug 15, 2024. doi: 10.4251/wjgo.v16.i8.3410
Revised: May 28, 2024
Accepted: July 4, 2024
Published online: August 15, 2024
Processing time: 105 Days and 12.3 Hours
Pyroptosis is a type of programmed cell death mediated by gasdermines (GSDMs). The N-terminal domain of GSDMs forms pores in the plasma mem
Core Tip: Pyroptosis plays an important role in inflammatory diseases and malignant tumors. With the further study of pyroptosis, an increasing number of studies have shown that the pyroptosis pathway can regulate the tumor microenvironment and antitumor immunity of colorectal cancer and is closely related to the occurrence, development, treatment and prognosis of colorectal cancer. This review aimed to explore the molecular mechanism of pyroptosis and the role of pyroptosis in the occurrence, development, treatment and prognosis of colorectal cancer (CRC) and to provide ideas for the clinical diagnosis and treatment of CRC.
- Citation: Wang X, Yin QH, Wan LL, Sun RL, Wang G, Gu JF, Tang DC. Research progress on the effect of pyroptosis on the occurrence, development, invasion and metastasis of colorectal cancer. World J Gastrointest Oncol 2024; 16(8): 3410-3427
- URL: https://www.wjgnet.com/1948-5204/full/v16/i8/3410.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i8.3410
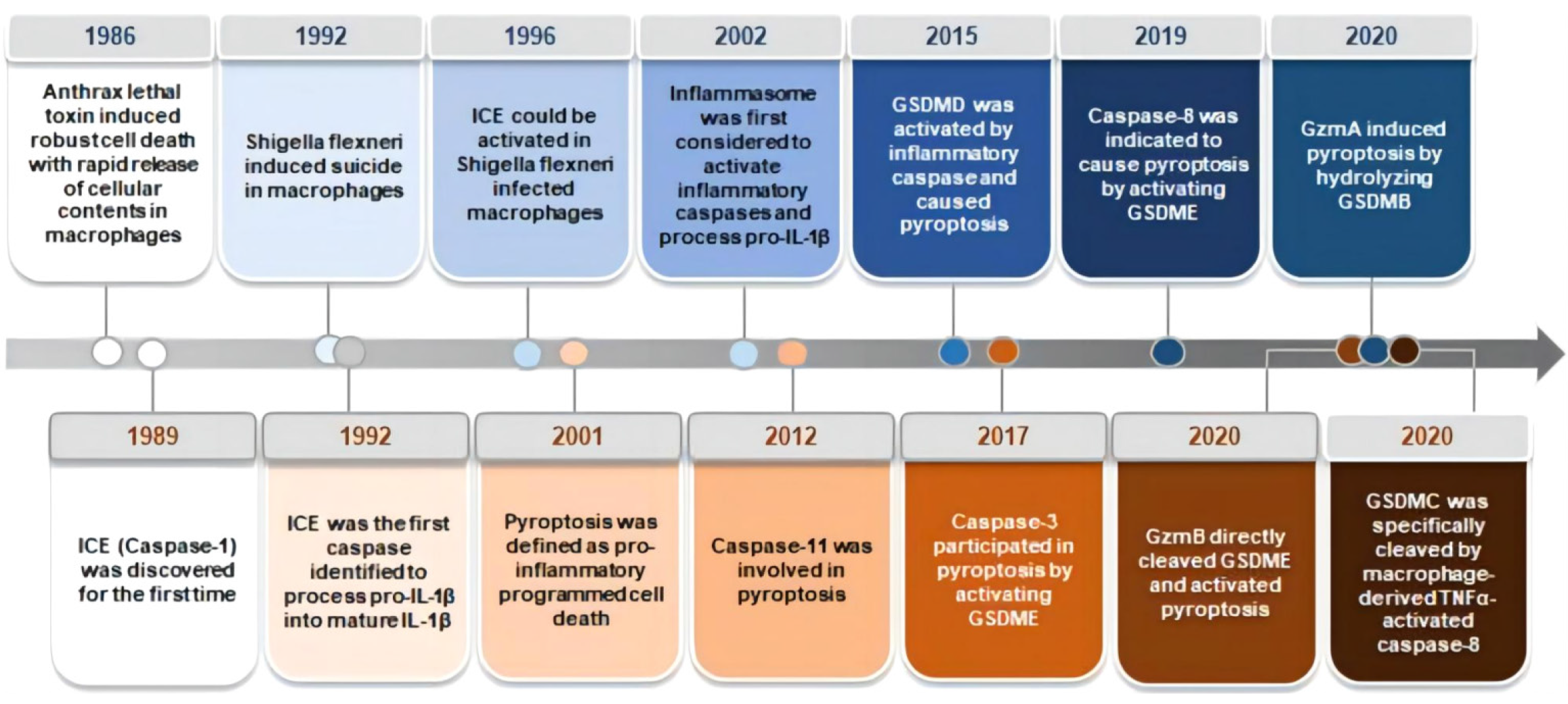
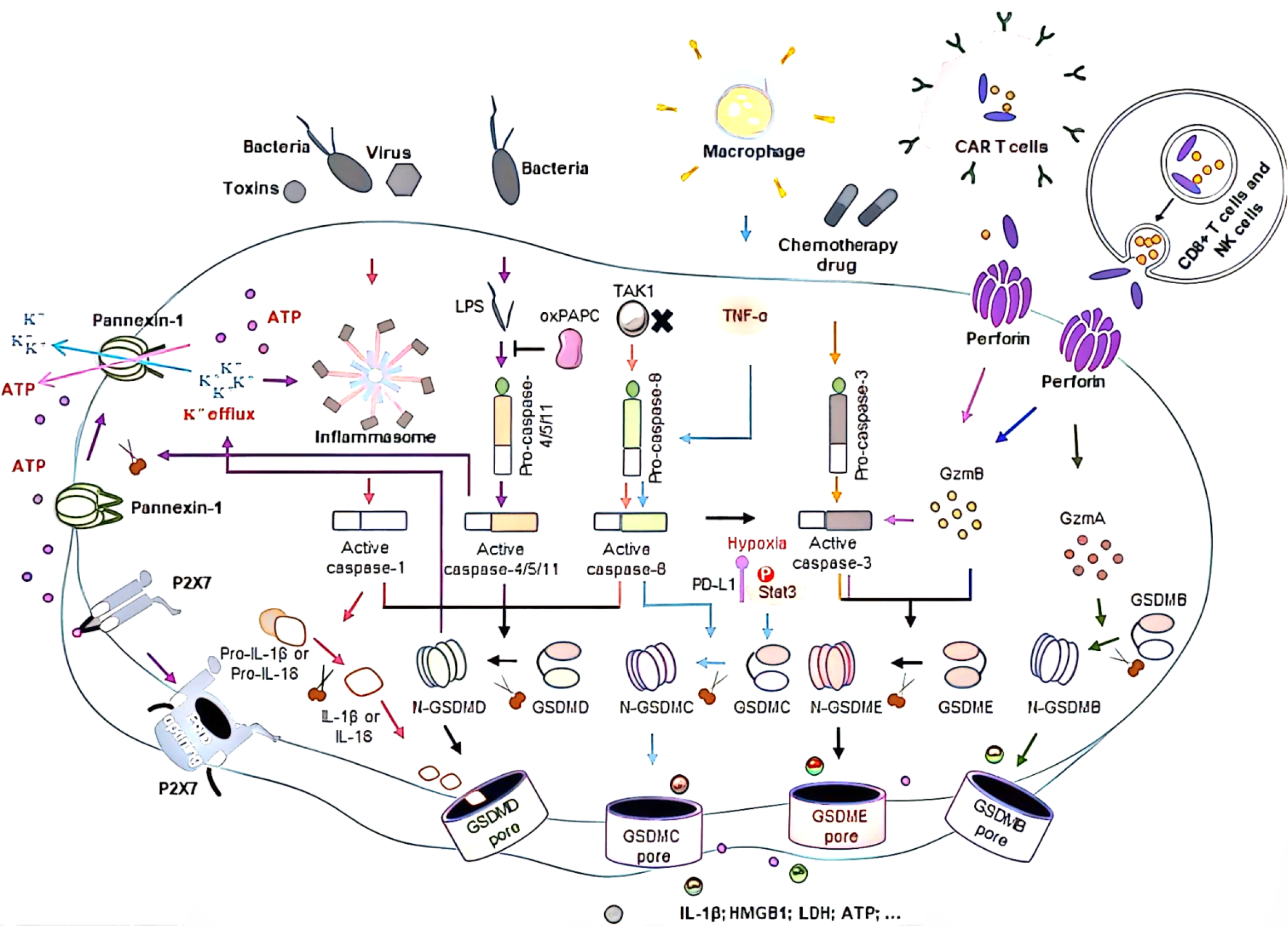
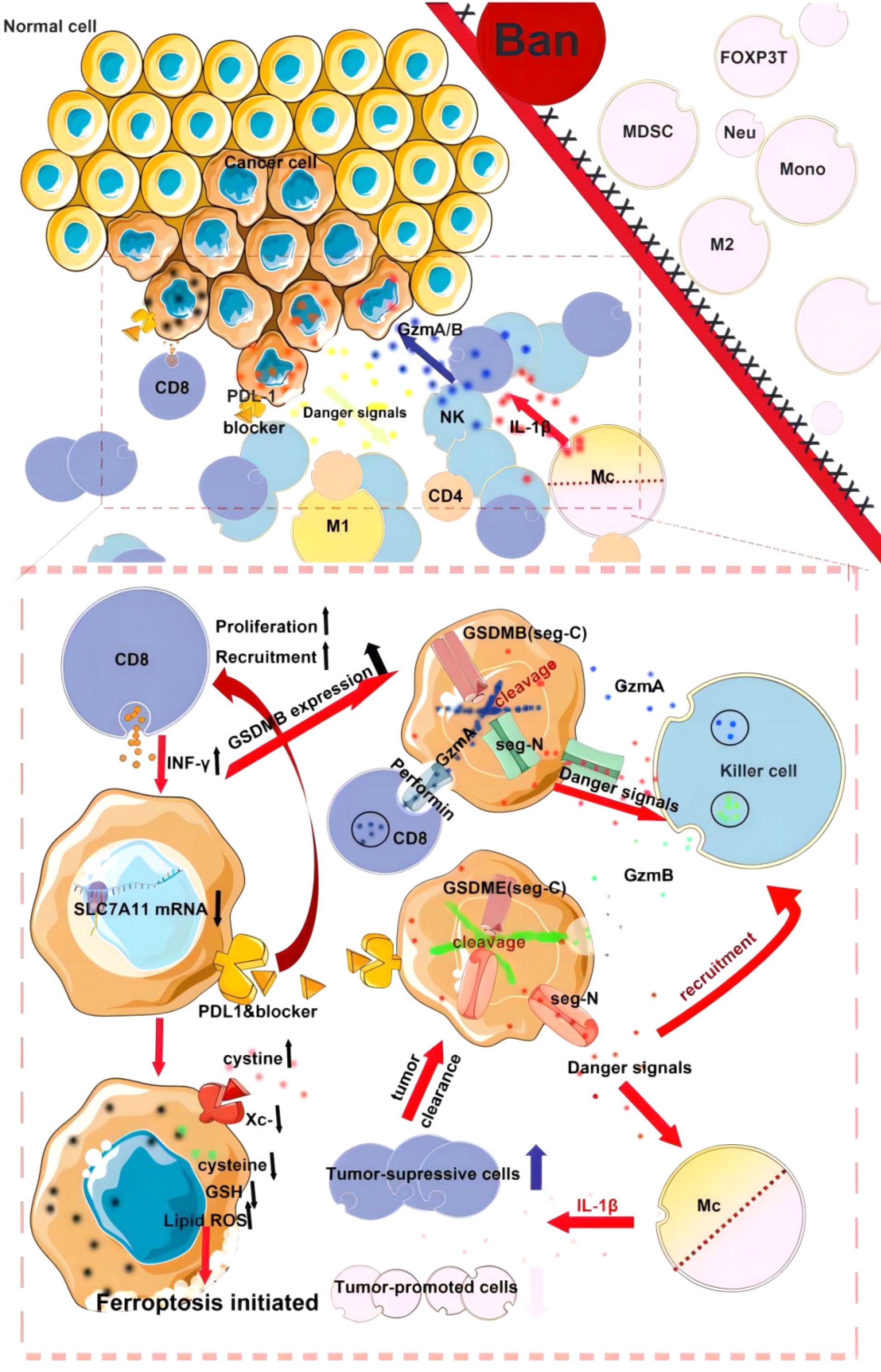
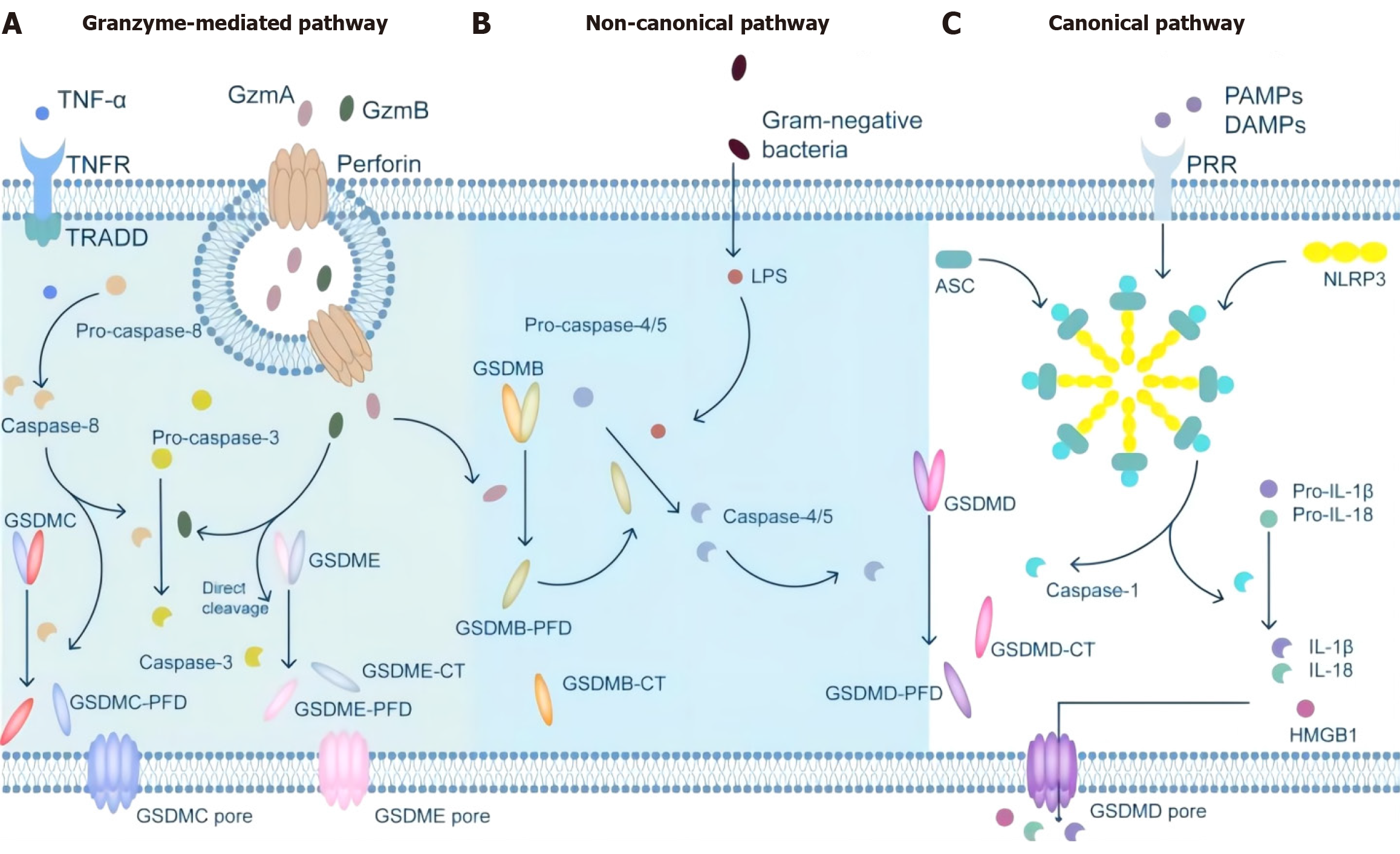
Colorectal cancer (CRC) is a gastrointestinal malignancy. According to China’s cancer statistics report in 2023, the incidence and mortality of colorectal cancer rank third and fifth, respectively, among all malignant tumors[1-5]. Pyroptosis is a type of programmed cell death that can be divided into typical and atypical pathways according to the pathways involved in inflammasome formation. The typical pathway is triggered by inflammasome assembly and promotes the activation of cysteinyl aspartate specific protein-1 (caspase-1), which then promotes the cleavage of the effector protein gasdermin D (GSDMD)[6-8]. In the nonclassical pathway, Caspase-4/5/11 directly cleaves GSDMD without assembling the inflammasome. Caspase-1 converts pro-interleukin (IL)-1β and pro-IL-18 to IL-1β and IL-18 through its proinflammatory activity[9]. By recruiting and activating exocellular inflammatory factors, immune cells induce the synthesis of inflammatory factors, adhesion factors and chemokines, thereby amplifying the inflammatory response. In addition to Caspase-1, Caspase-4/5/11, granzyme A (GZMA) and granzyme B (GZMB) may also cause pyroptosis. Recent studies[10-15] have shown that pyroptosis plays an important role in the occurrence, development and metastasis of cancer and affects therapeutic outcomes by affecting the infiltration of immune cells[16-18]. Therefore, extensive research is needed to elucidate the molecular mechanism underlying the relationship between pyrodeath and CRC (Figure 1).
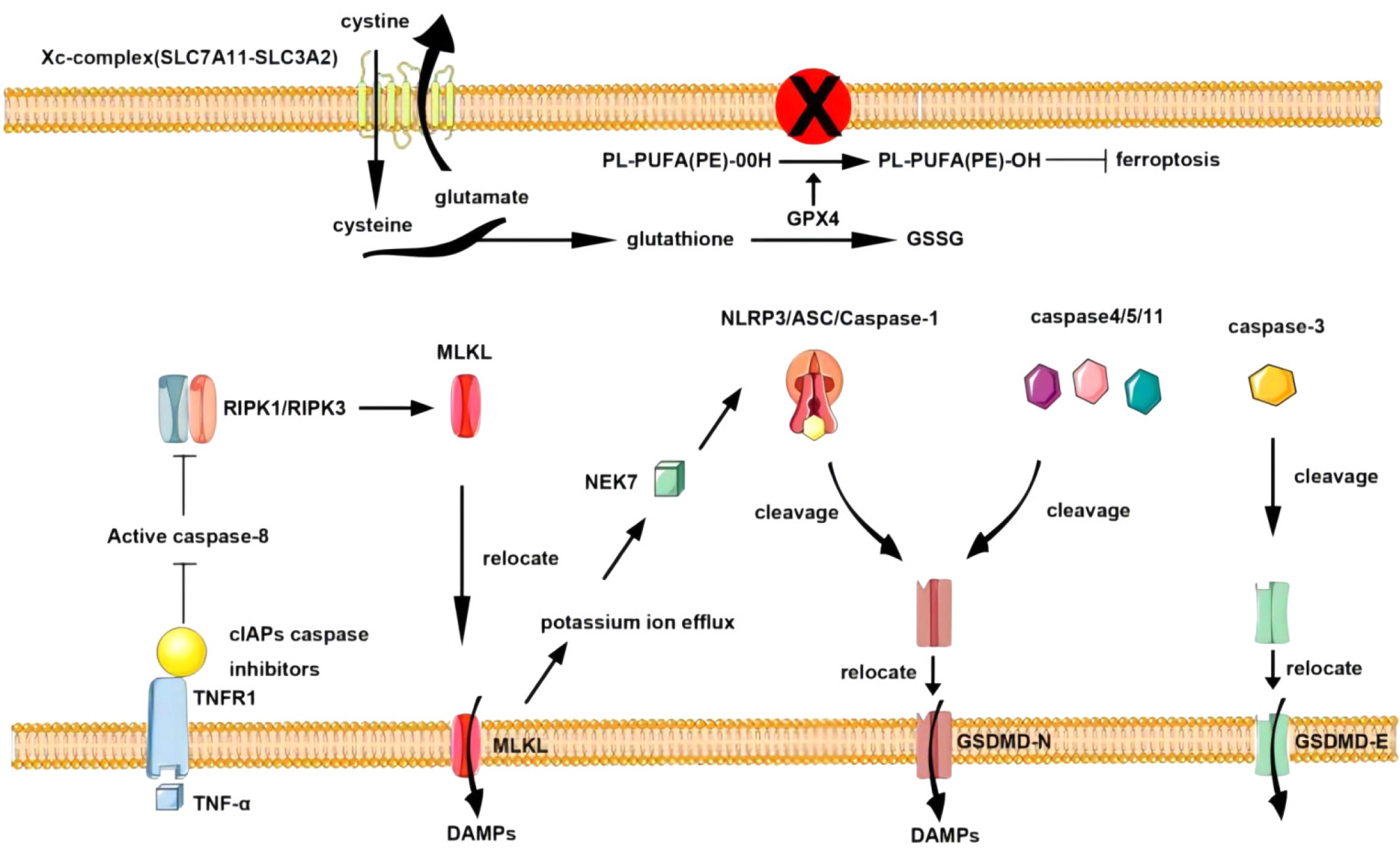
Typically, pyroptosis is mediated by Caspase-1, and the key step is the recruitment and activation of Caspase-1[19]. For example, in the NOD-like receptor thermal protein domain associated protein 3 (NLRP3) inflammasome, when the NLRP3 protein is exposed to pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs), the NLRP3 protein contains a caspase recruitment domain, an associated speck-like protein containing a caspase recruitment domain (ASC) and Caspase-1, and is assembled into the NLRP3 inflammasome[20]. NLRP3 inflammasome assembly activates precaspase-1, which not only mediates the maturation and secretion of IL-1β and IL-18 but also directly cleaves GSDMD to produce GSDMD-N[21-25]. Subsequently, GSDMD-N binds to phosphatidylinositol, phosphatidylic acid, and phosphatidylserine on the inner surface of the membrane through membrane-lipid interactions and forms oligopolymer pores (GSDMS pores) with inner diameters of 10 nm to 20 nm in the lipid bilayer, ultimately leading to cell pyrosis[26-30]. At the same time, small holes in the plasma membrane cause the connection between the inner and outer membranes to form nonselective membrane channels, resulting in the outflow of K + ions, causing the ion concentration on both sides of the plasma membrane to be unbalanced, and the solute to enter the cell, resulting in cell swelling and eventually cell death[31-34].
Like in the classical pyro pathway, the protein involved in the nonclassical pyro pathway is also GSDMD; the difference is that the nonclassical pyro pathway is directly recognized by caspase-11 (mouse) and caspase-4/5 (human) combined with lipopolysaccharide in the cell wall of gram-negative bacteria. Lipopolysaccharide (LPS) and activate their own protease activity[35-38]. After activation, Caspase-4/5/11 directly cleaves GSDMD into the C segment and N-terminal segment and forms cell membrane pores via the oligomerization of GSDMD-N[39-41]. This pyrodeath mediated by Caspase-4/5/11-GSDMD is called nonclassical pyroptosis and is a process independent of Caspase-1[42-44].
In addition to the classical and nonclassical pyroptosis pathways mentioned above, other signaling pathways can also induce pyroptosis[45]. When intracellular GSDME expression is high, Caspase-3 cleaves GSDME to induce pyroptosis, while when GSDME expression is low or absent, apoptosis occurs. Recent studies[46-50] have shown that the aminoprotease GZMA can enter target cells via perforin and induce pyrodeath in target cells through Lys229/Lys244 of gasdermin B (GSDMB), contradicting the conclusion that pyroptosis can be activated only by caspases.
DFNA5 and Pejvakin (PJVK, also known as DFNB59)[51-54]. With the exception of PJVK, all Gasdermin proteins have a dual-domain structure: A C-terminal effect domain and an N-terminal inhibition domain[55]. The N-terminal domain of GSDMD is lipophilic and can bind to phosphatidylinositol phosphate and phosphatidylinositol, which allows the cell membrane to form nonspecific pores that lead to pyrodeath by releasing cell contents[56-58].
GSDMD is widely expressed in a variety of cells and tissues and is a common substrate protein for mouse Caspase-11 and human Caspase-4/5. GSDMD has 487 amino acids with a relative molecular mass of 53 × 103 and consists of a 30 × 103 N-terminal domain and a 22 × 103 C-terminal domain. In general, the C-terminus of GSDMD is connected to the N-terminal domain by a long ring, leaving GSDMD in a self-inhibitory state, while GSDMD can be cleaved by Caspase-1/11 into two independent domains in a state of activation of the cell pyro pathway, where the N-terminus is inserted into the plasma membrane to induce pyroptosis (Figure 2). Therefore, GSDMD has become an important target for pyroptosis intervention.
GSDMC-induced pyroptosis plays a role in colorectal cancer, and GSDMC is significantly upregulated in CRC patients. GSDMD deficiency promotes the development of CRC, possibly through reduced pyroptosis caused by the downregulation of interferon-γ and transcriptional activator 1 signaling[59-62]. In addition, GSDMD expression is significantly downregulated in human CRC tissues, and patients with high GSDMD expression in CRC tissues have a decreased risk of distant metastasis[63-67]. GSDMD is involved in the proliferation, invasion, scorch death and metastasis of colorectal cancer cells and inhibits the occurrence and development of tumors[68-70]. Studies[71-75] have shown that GSDME plays a key role in the progression of colorectal cancer, and GSDME methylation can be used as a potential specific marker and a meaningful prognostic biomarker for patients with colorectal cancer. These findings suggest that colorectal cancer cells may inhibit their proliferation through Caspase-3/GSDME-mediated pyroptosis. In addition, GSDME-mediated pyroptosis promoted the development of colitis-associated colorectal cancer but not in xenograft models[76-78].
These findings show that CRC risk is associated with the NLR in CRC tissues and/or cell lines, providing preliminary evidence for the involvement of the NLR in CRC development. In addition, a study revealed that NLRP5, also known as MATER, is not expressed in normal colon tissue but is expressed in colon cancer tissue and cell lines[79]. The NLRP1, NLRP3, and NLRP12 inflammasomes are negative regulators of intestinal tumorigenesis[80]. Studies[81-84] have confirmed that human colorectal tumor tissues express lower levels of NALP1 than surrounding tumor tissues and are associated with patient survival and tumor metastasis[85]. Increasing the expression of NALP1 improved the survival rate of the mice. NLRP3 deficiency significantly increased mortality in mice with acute DSS-induced colitis, and the incidence of CRC tended to increase in NLRP3-deficient mice[86-90]. The NLRP3-/- receptor of immune cells that express NLRP3 can prevent tumor development[91-95]. Nlrp12-deficient mice are highly sensitive to colon inflammation and tumorigenesis and exhibit increased production of inflammatory cytokines, chemokines, and tumor necrosis factors. Further studies showed that NLRP12 deficiency in mice led to the activation of nuclear factor kappa-B (NF-κB) and extracellular signal-regulated kinase (ERK) in macrophages. This can promote the development of colitis or colon cancer[96-100].
The above evidence suggests that the NLRP inflammasome plays a key role in the prevention of colorectal cancer and can serve as a potential biomarker of the tumor microenvironment.
IL-18 can stimulate epithelial cell regeneration and induce antitumor responses mediated by T cells and natural killer cells. IL-18 administration reduces AOM/DSS-induced inflammation and tumorigenesis in CasP1-deficient mice[101-104]. Conversely, other studies have shown that IL-18 deletion is protective against AOM/DSS-induced colitis and CRC[105]. While IL-18 directly prevents inflammation-driven carcinogenesis, it also downregulates soluble IL-22-binding proteins that neutralize IL-22, thereby indirectly enhancing the carcinogenic effect of IL-22.
IL-1β has traditionally been considered a proinflammatory and procancer factor. However, recent studies[106-108] have shown that L-1 plays a dual role in CRC. In a mouse model of colitis-associated cancer, inhibition of the NLRP3 inflammasome reduced tumor expansion, which was associated with reduced levels of IL-1β and IL-18 at the tumor site[109]. The underlying mechanism of the dual role of IL-1β in CRC has recently been well understood. A study investigated the effect of IL-1 signaling on different cell types in the CRC microenvironment. Analysis of epithelial-specific IL-1R1 deletions showed that the proliferation of early CRC tumors was slowed and that NF-κB activation was reduced. T-cell-specific ablation of IL-1R1 similarly reduced tumor-induced inflammation in IL-17- and IL-22-dependent tumors, thereby reducing the progression of CRC[110]. However, related studies have shown that IL-1β plays the opposite role through ZEB1 activation, thereby promoting the dryness and invasion of colon cancer cells. Therefore, the dual role of IL-1β needs to be carefully considered when developing anticancer therapies that inhibit IL-1β (Figure 3).
Pyroptosis-related factors are important prognostic indicators of CRC. Recent studies[111-115] have shown that genes associated with pyroptosis play an important role in assessing the prognosis of patients with CRC. The mRNA expression of Caspase-9 is downregulated in colorectal cancer tissues, poorly differentiated tumors exhibit decreased Caspase-9 mRNA expression, and Caspase-9 mRNA expression can be used as an independent prognostic factor in stage II colorectal cancer patients[116]. Localization of GSDMD in the cytoplasm in vivo indicates a good clinical prognosis for CRC patients, while nuclear displacement of GSDMD is associated with a poor prognosis[117-120]. Nuclear GSDMD (but not cytoplasmic GSDMD) inhibits cell growth and promotes pyrodeath in cancer. Hypoxia in the TME causes mild or moderate nuclear displacement of GSDMD in vivo[121]. Pyroptosis-related genes in CRC patients included CAS P3, CHMP2A, CHMP2B, CHMP3, CHMP4C, CHMP6, CHMP7, GSDME, HMGB1, IL1A, IRF2, and TP63, and the above 12 pyrodeath-related genes were associated with immune cell infiltration[122-125]. These results indicate that pyroptosis plays an important role in the tumor immune microenvironment[126]. Similarly, 13 apoptosis-related genes, AIM2, CASP1, CASP5, CASP6, CASP8, CASP9, ELANE, GPX4, GSDMD, NLRP7, NOD2, PJVK, and PRKACA, have been shown to be associated with the prognosis of CRC patients[127-130].
Long noncoding RNAs (lncRNAs) are associated with CRC. Recent studies have shown that lncRNAs associated with pyroptosis play a role in the prognosis of CRC patients[131-133]. miR-21-5p also plays an important role in the prognosis of CRC patients. The overexpression of miR-21-5p resulted in the release of various inflammatory factors, including IL-1β and IL-18, and the mRNA associated with pyroptosis was significantly upregulated. In addition, overexpression of miR-21-5p, a downstream factor, leads to downregulation of growth factor beta-induced protein (TGFBI), which leads to pyroptosis[134]. Chen et al[132] reported that four lncRNAs associated with pyroptosis, namely, ELNF1-AS1, PCAT6, TNRC6C-AS1 and ZEB1-AS1, could be used as biomarkers to accurately predict the prognosis of CRC patients (Figure 4). Some studies showed that 8 lncRNAs associated with pyroptosis, Z99289.2, FENDRR, CCDC144NL-ASL, TEX41, MNX1-AS1, NKILA, LINC02798, and LINC02381, have potential roles in the response to treatment and prognosis in CRC patients[135-138].
Pyroptosis may be an important therapeutic target for cancer treatment. Studies have shown that arsenic trioxide (ATO) and ascorbic acid jointly upregulate the expression of Caspase-1 and promote inflammasome formation, thereby inducing pyroptosis in CRC cells[139]. Moreover, ATO inhibited the growth of CRC cells by inhibiting telomerase activation and inducing Caspase-3-dependent apoptosis[140]. The antitumor drug 5-aza-2’-deoxycytidine (DAC) is a DNA methylation inhibitor that treats CRC by upregulating the expression of NLRP1. After CRC cells were treated with DAC, the expression level of the inflammasome NLRP1 increased both in vivo and in vitro, suggesting that DAC inhibits the growth of colon cancer by inducing pyroptosis. In addition, DAC increased the expression of miR-133b and triggered the apoptosis of CRC cells[141]. Therefore, pyroptosis may be targeted for CRC treatment by ATO and DAC. LPS in the outer membrane of gram-negative bacteria increased the sensitivity of CRC to oxaliplatin and increased antitumor activity by inducing GSDMD-mediated pyrodeath in HT-29 cells. Loplatin induces pyroptosis by activating Caspase-3 and GSDME, thereby eliminating CRC cells. The camptothecin (CPT) analog FL118 inhibits CRC growth and metastasis by inducing NLRP3/caspase-1-mediated pyrodeath in SW48 and HT129 cells[142].
Two small molecule inhibitors, BI2536 and CPT, were further confirmed to induce GSDME-mediated pyrodeath via caspase-3-dependent apoptosis, demonstrating anticolorectal cancer activity in vitro and in vivo. Some studies[143-145] have shown that the forkhead box p2 gene (FOXP2) acts as a tumor inhibitor. FOXP2 can promote the activation of Caspase-1 to enhance cell pyrodeath. In a mouse model of colitis-associated tumors, FOXP2 was downregulated in colitis and tumor tissue, and CRC patients with low FOXP2 expression had lower survival rates. Further study indicated that knockdown of FOXP2 promoted the expression of proliferating cell nuclear antigen (PCNA) and cyclin D1 and downregulated the expression of Caspase family proteins and GSDMD. A recent study[146] showed that tumor cells are sensitive to apoptosis induced by alpha-ketoglutaric acid (αKG) in an acidic environment. αKG enhances reactive oxygen species levels and activates Caspase-8 to lyse GSDMC, thus providing a theoretical basis for the use of αKG as a new treatment strategy for CRC through the induction of GSDMC-mediated pyrodeath (Figure 5).
The GSDM family includes GSDMD, GSDME, GSDMC, and GSDMB in the gastrointestinal tract and is associated with a variety of gastrointestinal tumors[147-150]. Pyroptosis in CRC can be divided into three categories: (1) Different levels of GSDMs affect the occurrence and development of CRC; (2) GSDMs mediate pyroptosis to affect the TME; and (3) GSDMs can be used as target effectors for antitumor therapy.
Gsdms-mediated pyrogen death acts as a double-edged sword in the development of tumors and can either promote the occurrence of tumors or suppress tumors through tumor immunity, depending on the tumor environment in which the cells are located[151-154]. In nontumor cells, GSDMS-mediated pyroptosis may promote tumor development through chronic inflammatory stimulation, while in tumor cells, antitumor immune responses may predominate[155]. GSTM family proteins are downstream effector molecules of the inflammasome that play a role in the occurrence of CRC by mediating pyroptosis[156-158]. Changes in gut microbes and inflammasome activation can promote or inhibit the development of CRC. On the one hand, microbial stimulation can promote the proteolytic shear NT domain of GSDM, the formation of pores and the release of inflammatory factors to produce pyrodeath, and pyrodeath of tumor cells can inhibit the growth of CRC[159]. On the other hand, the inflammatory factors released by porous GSDM stimulate immune cells, which can increase the clearance of tumor cells, establish tolerance, and promote the tumor microenvironment.
GSDMD is the first member of the GSDM family to be extensively studied as an executor of pyroptosis. It is expressed in most tumors and affects tumor progression and prognosis[160-162]. Studies[163-165] have shown that the lncRNA RP1-85F18.6 can regulate the Np63 signaling pathway, induce the proliferation and invasion of CRC cells, disrupt the cell cycle, and inhibit the death and apoptosis of CRC cells. However, the lncRNA RP1-85F18.6 promoted the pyro-death of CRC cells after GSDMD knockdown[166]. This finding suggested that GSDMD may be a benign prognostic factor for CRC. Pinitrol diglucoside (SDG) induced pyroptosis in CRC cells by activating the BAX-Caspase-1-GSDMD pathway through the ROS/PI3K/AKT signaling pathway and significantly inhibited tumor growth in a HCT116 nude mouse model[167]. In addition, LPS enhances the antitumor activity of oxaliplatin in HT29 cells by activating GSDMD expression through a nonclassical pathway, thus inhibiting the growth of HT29 cells and promoting cell death. This study also analyzed the relationship between GSDMD expression and overall survival in 244 CRC patients, and immunohistochemical analysis revealed that the overall survival of patients with high GSDMD expression was significantly greater than that of patients with low GSDMD expression, indicating that compared with that of normal cells, the expression of GSDMD in CRC cells is reduced and is associated with poor prognosis in CRC patients. Studies[168-170] have shown that GSDMD mediates pyroptosis and the release of the inflammatory factors IL-1β and IL-18, which can inhibit the proliferation of tumor cells, to induce an immune response in colorectal adenocarcinoma. In conclusion, GSDMD may regulate the pyrodeath of CRC cells through different signaling pathways, inflammatory stimulation pathways and antitumor immunity pathways, which are closely related to the progression and prognosis of CRC, and high expression of GSDMD may be a prognostic factor for CRC[171].
GSDME usually acts as a cancer suppressor in CRC. Gene promoter hypermethylation is involved in the development and progression of cancer, and abnormal CpG island methylation of gene promoters is a common change in human CRC[172-174]. GSDME gene methylation was reported in 29 (34%) of 85 CRC patients, and methylation was significantly associated with lymphatic invasion and TNM stage, suggesting that GSDME gene methylation may play a role in the development of CRC. Recent studies[175-178] have shown that the expression of the lncRNA paraventular assembly transcript 1 (NEAT1) is upregulated in the ionizing radiation response and that the downregulated expression of miR-448 enhances the expression of GSDME and induces pyroptosis in CRC cells[179]. These results indicated that NEAT1/miR-448 could affect the pyrodeath and viability of CRC cells by regulating the expression of GSDME. Liu et al[175] used apopsin to activate Caspase-3 and Caspase-9 through the mitochondrial pathway to induce pyrodeath in GSDME cells, which significantly reduced tumor growth in an HCT-116 mouse tumor model. However, some studies have shown that GSDME mediates the release of intracellular HMGB1 through the ERK1/2 pathway, which promotes the progression of colitis-related colorectal cancer in mice. It is possible that pyroptosis causes the release of a large number of intracellular DAMPs (such as HMGB1, IL-1β, IL-18 and other inflammatory cytokines) that trigger chronic inflammation, thus promoting the development of chronic inflammation-related tumors[180]. Therefore, by inhibiting GSDME-mediated pyroptosis or reducing the release of intracellular HMGB1, the occurrence of colitis-associated CRC can be reduced. GSDME may regulate the pyroptosis of colorectal cancer cells according to pathological status, expression level and inflammatory factor stimulation[181-184].
GSDMC is mainly expressed in the gastrointestinal tract and skin. GSDMC converts apoptosis to necrosis via the action of tumor necrosis factor (TNF)[185]. In hypoxia, PD-L1 functions as a nonimmune checkpoint, generates nuclear translocation, and enhances the gene transcription of GSDMC. Caspase-8- and TNF-α-mediated cleavage of GSDMC causes pyroptosis, which promotes tumor development. Some studies[186-190] have shown that both the mRNA and protein levels of GSDMC are high in CRC tissues. Inhibition of GSDMC expression significantly reduced the proliferation and tumorigenesis of CRC cell lines in vivo and in vitro, while overexpression of GSDMC promoted the proliferation of CRC cells. These results suggest that GSDMC can act as an oncogene in the development of CRC. Therefore, the antitumor or protumor effects of GSDMC may depend on the degree to which pyroptosis is induced.
There are few studies on GSDMB in CRC, and its role is still unclear[191]. The GZMA-mediated cleavage of GSDMB enhances the expression of GSDMB in mouse CRC cells and thus promotes the clearance of mouse tumor cells[192]. These results suggest that GSDMB-mediated pyroptosis may promote the occurrence of CRC. Therefore, the occurrence and development of CRC cells can be affected by the pyrogenic pathway or the different expression levels of GSDMs, indicating that GSDMs may be potential tumor markers for CRC[193-195]. However, the specific mechanism regulating CRC progression is currently unclear and lacks clinical verification, and further research is still needed.
The TME includes immune cells, stromal cells, blood vessels, the extracellular matrix and extracellular vesicles[196]. The TME is crucial for the occurrence and development of CRC, can promote the formation and progression of CRC blood vessels, and can also predict the prognosis of CRC patients by calculating the infiltration of immune cells[197-200]. Pyroptosis, a highly immunogenic form of cell death, induces local inflammation accompanied by the recruitment of a large number of tumor-infiltrating lymphocytes (TILs) and macrophages, further amplifying the inflammatory response, thereby relieving immunosuppression in the TME and inducing a systemic immune response. Some studies[201,202] have shown that pyroptosis induced by GSDMs can regulate the antitumor immune response generated by the TME. Downregulation of GSDMD reduced the cytolytic ability of CD8 + T cells, indicating that GSDMD is necessary for the optimal CTL response against tumor cells and has antitumor activity. Pyroptosis mediated by GSDMs plays a dual role in regulating the TME. On the one hand, the inflammatory response associated with pyroptosis can induce normal cells to become cancerous and provide an appropriate TME for tumors to promote the growth of tumor cells. On the other hand, pyroptosis can be used as a cytotoxic lymphocyte killing mechanism. Increasing the number and function of TILs and the phagocytosis of macrophages can enhance the antitumor immune response and inhibit tumor development. GZMB can directly cleave GSDME by activating Caspase-3 to activate target cells to cause scorch death, which can increase the antitumor function of CD8 + T lymphocytes and NK cells in tumors, affect the tumor microenvironment and recruitment of immune cells, and inhibit the growth of tumor cells. A recent study analyzed the correlation between GSDMD expression and immune invasion in pancarcinoma tissues, and in rectal adenocarcinoma, GSDMD expression was positively correlated with NK cells and CD8 + T cells and negatively correlated with CD4 + T cells. In addition, a study analyzed the relationship between different sublocalization levels of GSDMD and the TME in 178 CRC patients, and the results showed that the cytoplasmic expression of GSDMD was correlated with the proliferation of CD3 + lymphocytes, while the membrane expression of GSDMD was positively correlated with CD68 + macrophages and CD8 + lymphocytes. The nuclear expression of GSDMD was negatively correlated with CD68 + macrophages and CD8 + lymphocytes. Recent research has shown that in a mouse model of colon cancer, oncolytic paraviruses stabilize GSDME by reducing the ubiquitination of GSDME, initiating pyrodeath, recruiting more cytotoxic lymphocytes, reshaping the TME, turning “cold” tumors into “hot” tumors, and activating antitumor immunity (Figure 6). At present, there are few studies on pyroptosis and the CRC microenvironment, but their relationship is worthy of further study.
Chemotherapy, radiotherapy and immunotherapy can induce the death of tumor cells, thereby enhancing local and systemic antitumor immune functions. In recent years, immunotherapy has attracted much attention for the treatment of CRC. Immunotherapy is a therapeutic method for restoring normal antitumor immunity by restarting and maintaining the tumor immune cycle to control and eliminate tumors. It can not only activate the immune system against tumors but also affect the tumor microenvironment. Pyroptosis is a highly inflammatory and lytic form of programmed cell death that involves the production of a large number of new antigens, stimulation of the systemic immune response, and inhibition of tumor progression. Some studies have shown that neither blocking the PD-1/PD-L1 pathway nor inducing transient pyroptosis can inhibit the growth of 4T-1 tumors alone, but the combination of the two has a strong inhibitory effect on tumor growth, indicating that immunotherapy agents and drugs that cause pyroptosis can synergistically and effectively clear tumors. In addition, in mouse colon cancer cells, upregulation of GSDMB expression did not affect tumor growth in immunoactive mice, but it could enhance the ability of anti-PD-1 antibodies to block immune checkpoints, thus playing a role in inhibiting tumor growth. In the future treatment of CRC patients, we may be able to achieve synergistic effects through GSDM-mediated pyrodeath and immune checkpoint inhibitors to improve the effectiveness of immunotherapy.
GSDMD and GSDME are the most common apoptosis-related proteins in cancer research and play important roles in the pathogenesis and treatment of cancer. In the treatment of CRC, many chemotherapy drugs can clear tumor cells by inducing apoptosis, and with the use of drugs, tumor cells exhibit apoptosis resistance and drug tolerance. An increasing number of studies have shown that many chemotherapy drugs induce GSDMD and GSDME to pyroath tumor cells through various cell signaling pathways. Therefore, the use of chemotherapeutic agents that induce the pyroptotic death of tumor cells may be a new antitumor treatment strategy for overcoming apoptosis resistance.
LPS-induced pyroptosis promoted the expression of GSDMD in CRC cells and increased the chemical sensitivity of HT29 cells to oxaliplatin, thus enhancing the antitumor effect of these cells. In addition, the relationship between the sublocalization of different GSDMD molecules and the prognosis of CRC patients and reported that high cytoplasmic GSDMD expression is an independent favorable indicator of prognosis and can improve the efficacy of chemotherapy in CRC patients. Studies have shown that multivalent CXCR4-targeted nanotoxins (T22-PE24-H6) induce GSDMD-mediated pyroptosis, thereby inhibiting the metastasis of CRC cells. Nanotoxins mediate the pyroptosis of CRC cells through the Caspase-1/GSDMD pathway, have strong antitumor effects and can effectively overcome the apoptotic resistance associated with chemotherapy resistance and metastatic CRC tumors. Therefore, the use of some drugs to regulate the expression level of GSDMD or induce the death of tumor cells through the Caspase-1/GSDMD pathway may provide a new strategy for the treatment of CRC.
In recent years, the Caspase-3/GSDME signaling pathway has been shown to be related to the chemotherapy and antitumor immunity of CRC. Activation of Caspase-3 by chemotherapeutic agents to inhibit GSDME can transform the mitochondrial apoptosis pathway into pyroptosis. In addition, GSDme-NT can also enhance the activation of Caspase-3 and play a positive feedback role in self-amplification. Further cutting of full-length GSDME can promote the transformation of Caspase-3-mediated apoptotic cells to pyroptotic cells through the Caspase-3/GSDME pathway, activate the body’s antitumor immune system, effectively inhibit the proliferation and metastasis of tumors, and improve the efficacy of chemotherapeutic drugs. TNF-α + CHX and ABT-263 (Navitoclax) chemotherapy drugs induced the apoptosis and pyrodeath of colon cancer cells through the BAK/BAX-Caspase-3-GSDME pathway and that GSDME-CT palmitoylation promoted chemotherapy-induced pyrodeath. The palmitoacylation inhibitor 2-bromopalmitate (2-BP) inhibited palmitoacylation and chemotherapy-induced pyrodeath in GSDME-CT, but total cell death did not change, indicating that 2-BP can transform pyrodeath into apoptosis, providing a new target for realizing the transformation between chemotherapy-induced pyrodeath and apoptosis. Chemotherapy drugs such as platinum can increase the level of phosphorylated ROS/JNK, activate the Bax mitochondrial apoptosis pathway to activate Caspase-3 and -9, and then cleave GSDME to mediate the pyroptosis of colon cancer cells, suggesting that GSDME pyroptosis may be a potential mechanism by which lobacplatin clears CRC tumor cells. Therefore, the selection of appropriate chemotherapy drugs for CRC treatment can activate the Caspase-3/GSDME pathway to induce pyroptosis, which can effectively clear tumor cells, increase sensitivity to chemotherapy drugs, and target tumor cell pyrodeath to overcome the apoptotic resistance of tumor cells and activate the body’s antitumor immune system, providing new insights for CRC antitumor therapy. However, the application of the activated pyroptosis pathway in the treatment of CRC patients’ needs further clinical validation.
Current research on CRC, a new type of programmed cell death, is still lacking, and there are still many unanswered questions, such as how to promote CRC tumor cell pyrodeath and avoid normal cell pyrodeath to inhibit the occurrence and development of CRC. How to reduce the death of CRC tumor cells to increase chemotherapy drug sensitivity and avoid adverse reactions and how to balance pyroptosis in CRC and antitumor immunity are important questions. Notably, pyroptosis can reshape the TME, transform “cold” tumors into “hot” tumors, and may improve the response to immune checkpoint inhibitors, which has great potential for antitumor therapy. However, most of the current studies on the correlation between pyroptosis regulation of CRC development and the TME have been conducted in cell and animal models and lack relevant clinical validation. Therefore, in-depth studies on the specific mechanism and biological role of pyroptosis regulation in CRC can identify new targets for CRC treatment and aid in the development of effective molecular targeted drugs for the clinical treatment of CRC.
| 1. | Zhou P, Zhang S, Wang M, Zhou J. The Induction Mechanism of Ferroptosis, Necroptosis, and Pyroptosis in Inflammatory Bowel Disease, Colorectal Cancer, and Intestinal Injury. Biomolecules. 2023;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 2. | Feng WQ, Zhang YC, Xu ZQ, Yu SY, Huo JT, Tuersun A, Zheng MH, Zhao JK, Zong YP, Lu AG. IL-17A-mediated mitochondrial dysfunction induces pyroptosis in colorectal cancer cells and promotes CD8 + T-cell tumour infiltration. J Transl Med. 2023;21:335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 34] [Reference Citation Analysis (1)] |
| 3. | Lin JF, Hu PS, Wang YY, Tan YT, Yu K, Liao K, Wu QN, Li T, Meng Q, Lin JZ, Liu ZX, Pu HY, Ju HQ, Xu RH, Qiu MZ. Phosphorylated NFS1 weakens oxaliplatin-based chemosensitivity of colorectal cancer by preventing PANoptosis. Signal Transduct Target Ther. 2022;7:54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 183] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 4. | Song W, Ren J, Xiang R, Kong C, Fu T. Identification of pyroptosis-related subtypes, the development of a prognosis model, and characterization of tumor microenvironment infiltration in colorectal cancer. Oncoimmunology. 2021;10:1987636. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 98] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 5. | Liu Z, Li Y, Zhu Y, Li N, Li W, Shang C, Song G, Li S, Cong J, Li T, Xiu Z, Lu J, Ge C, Yang X, Li Y, Sun L, Li X, Jin N. Apoptin induces pyroptosis of colorectal cancer cells via the GSDME-dependent pathway. Int J Biol Sci. 2022;18:717-730. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 58] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 6. | Xiao Y, Zhang T, Ma X, Yang QC, Yang LL, Yang SC, Liang M, Xu Z, Sun ZJ. Microenvironment-Responsive Prodrug-Induced Pyroptosis Boosts Cancer Immunotherapy. Adv Sci (Weinh). 2021;8:e2101840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 184] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 7. | Keshavarz Shahbaz S, Koushki K, Ayati SH, Bland AR, Bezsonov EE, Sahebkar A. Inflammasomes and Colorectal Cancer. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 8. | Zhao Q, Dai MY, Huang RY, Duan JY, Zhang T, Bao WM, Zhang JY, Gui SQ, Xia SM, Dai CT, Tang YM, Gonzalez FJ, Li F. Parabacteroides distasonis ameliorates hepatic fibrosis potentially via modulating intestinal bile acid metabolism and hepatocyte pyroptosis in male mice. Nat Commun. 2023;14:1829. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 88] [Reference Citation Analysis (0)] |
| 9. | Wu Y, Pi D, Zhou S, Yi Z, Dong Y, Wang W, Ye H, Chen Y, Zuo Q, Ouyang M. Ginsenoside Rh3 induces pyroptosis and ferroptosis through the Stat3/p53/NRF2 axis in colorectal cancer cells. Acta Biochim Biophys Sin (Shanghai). 2023;55:587-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 40] [Reference Citation Analysis (0)] |
| 10. | Huang Y, Yang W, Yang L, Wang T, Li C, Yu J, Zhang P, Yin Y, Li R, Tao K. Nrf2 inhibition increases sensitivity to chemotherapy of colorectal cancer by promoting ferroptosis and pyroptosis. Sci Rep. 2023;13:14359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 33] [Reference Citation Analysis (0)] |
| 11. | Jiang P, Fan J, Huang S, Liu L, Bai M, Sun Q, Shen J, Zhang N, Liu D, Zhou N, Feng Y, Jiang J, Zhu J. A pyroptosis-related signature in colorectal cancer: exploring its prognostic value and immunological characteristics. PeerJ. 2023;11:e16631. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 12. | Tan G, Huang C, Chen J, Zhi F. HMGB1 released from GSDME-mediated pyroptotic epithelial cells participates in the tumorigenesis of colitis-associated colorectal cancer through the ERK1/2 pathway. J Hematol Oncol. 2020;13:149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 197] [Cited by in RCA: 187] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 13. | Ai YL, Wang WJ, Liu FJ, Fang W, Chen HZ, Wu LZ, Hong X, Zhu Y, Zhang CX, Liu LY, Hong WB, Zhou B, Chen QT, Wu Q. Mannose antagonizes GSDME-mediated pyroptosis through AMPK activated by metabolite GlcNAc-6P. Cell Res. 2023;33:904-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 36] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 14. | Zhou Y, Zhang W, Wang B, Wang P, Li D, Cao T, Zhang D, Han H, Bai M, Wang X, Zhao X, Lu Y. Mitochondria-targeted photodynamic therapy triggers GSDME-mediated pyroptosis and sensitizes anti-PD-1 therapy in colorectal cancer. J Immunother Cancer. 2024;12. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 15. | Li R, Zhang S, Liu G. Identification and validation of a pyroptosis-related prognostic model for colorectal cancer. Funct Integr Genomics. 2022;23:21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 16. | Ding C, Yang X, Li S, Zhang E, Fan X, Huang L, He Z, Sun J, Ma J, Zang L, Zheng M. Exploring the role of pyroptosis in shaping the tumor microenvironment of colorectal cancer by bulk and single-cell RNA sequencing. Cancer Cell Int. 2023;23:95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 17. | Yu J, Li S, Qi J, Chen Z, Wu Y, Guo J, Wang K, Sun X, Zheng J. Cleavage of GSDME by caspase-3 determines lobaplatin-induced pyroptosis in colon cancer cells. Cell Death Dis. 2019;10:193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 164] [Cited by in RCA: 366] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 18. | Zhang Y, Li F, Wang L, Lou Y. A438079 affects colorectal cancer cell proliferation, migration, apoptosis, and pyroptosis by inhibiting the P2X7 receptor. Biochem Biophys Res Commun. 2021;558:147-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 19. | Zhang M, Dang P, Liu Y, Qiao B, Sun Z. Noncoding RNAs in pyroptosis and cancer progression: Effect, mechanism, and clinical application. Front Immunol. 2022;13:982040. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 20. | Ning Y, Lin K, Fang J, Chen X, Hu X, Liu L, Zhao Q, Wang H, Wang F. Pyroptosis-Related Signature Predicts the Progression of Ulcerative Colitis and Colitis-Associated Colorectal Cancer as well as the Anti-TNF Therapeutic Response. J Immunol Res. 2023;2023:7040113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 21. | Xu H, Zhang D, Wei R, Zhou Y, Dai G, Li J, Sun Y, Li F, Xi L. Gambogic Acid Induces Pyroptosis of Colorectal Cancer Cells through the GSDME-Dependent Pathway and Elicits an Antitumor Immune Response. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 33] [Reference Citation Analysis (0)] |
| 22. | Xie W, Peng M, Liu Y, Zhang B, Yi L, Long Y. Simvastatin induces pyroptosis via ROS/caspase-1/GSDMD pathway in colon cancer. Cell Commun Signal. 2023;21:329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 23. | Dal Z, Aru B. The role of curcumin on apoptosis and NLRP3 inflammasome-dependent pyroptosis on colorectal cancer in vitro. Turk J Med Sci. 2023;53:883-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 24. | Wittwer NL, Staudacher AH, Liapis V, Cardarelli P, Warren H, Brown MP. An anti-mesothelin targeting antibody drug conjugate induces pyroptosis and ignites antitumor immunity in mouse models of cancer. J Immunother Cancer. 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 25. | Liang H, He X, Tong Y, Bai N, Pu Y, Han K, Wang Y. Ferroptosis open a new door for colorectal cancer treatment. Front Oncol. 2023;13:1059520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 26. | Li Z, Liu Y, Lin B, Yan W, Yi H, Wang H, Wei Y. Pyroptosis-Related Signature as Potential Biomarkers for Predicting Prognosis and Therapy Response in Colorectal Cancer Patients. Front Genet. 2022;13:925338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 27. | Jiang R, Chen X, Ge S, Wang Q, Liu Y, Chen H, Xu J, Wu J. MiR-21-5p Induces Pyroptosis in Colorectal Cancer via TGFBI. Front Oncol. 2020;10:610545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 28. | Cai X, Liang X, Wang K, Liu Y, Hao M, Li H, Dai X, Ding L. Pyroptosis-related lncRNAs: A novel prognosis signature of colorectal cancer. Front Oncol. 2022;12:983895. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 29. | Tang Z, Ji L, Han M, Xie J, Zhong F, Zhang X, Su Q, Yang Z, Liu Z, Gao H, Jiang G. Pyroptosis is involved in the inhibitory effect of FL118 on growth and metastasis in colorectal cancer. Life Sci. 2020;257:118065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 30. | Chen M, Zhang J, Lin X, Zhu X, Xie T. A pyroptosis-related prognosis model to predict survival in colorectal cancer patients. Int J Clin Exp Pathol. 2022;15:168-182. [PubMed] |
| 31. | Wu H, Qian D, Bai X, Sun S. Targeted Pyroptosis Is a Potential Therapeutic Strategy for Cancer. J Oncol. 2022;2022:2515525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 32. | Zhou CB, Fang JY. The role of pyroptosis in gastrointestinal cancer and immune responses to intestinal microbial infection. Biochim Biophys Acta Rev Cancer. 2019;1872:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 117] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 33. | Yu YQ, Gamez-Belmonte R, Patankar JV, Liebing E, Becker C. The Role of Programmed Necrosis in Colorectal Cancer. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 34. | Liao P, Huang WH, Cao L, Wang T, Chen LM. Low expression of FOXP2 predicts poor survival and targets caspase-1 to inhibit cell pyroptosis in colorectal cancer. J Cancer. 2022;13:1181-1192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 35. | Hou X, Xia J, Feng Y, Cui L, Yang Y, Yang P, Xu X. USP47-Mediated Deubiquitination and Stabilization of TCEA3 Attenuates Pyroptosis and Apoptosis of Colorectal Cancer Cells Induced by Chemotherapeutic Doxorubicin. Front Pharmacol. 2021;12:713322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 36. | Zheng C, Tan Z. A novel identified pyroptosis-related prognostic signature of colorectal cancer. Math Biosci Eng. 2021;18:8783-8796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 37. | Rao J, Li W, Chen C. Pyroptosis-Mediated Molecular Subtypes and Tumor Microenvironment Infiltration Characterization in Colon Cancer. Front Cell Dev Biol. 2021;9:766503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 38. | Luo S, Cai S, Zhao R, Xu L, Zhang X, Gong X, Zhang Z, Liu Q. Comparison of left- and right-sided colorectal cancer to explore prognostic signatures related to pyroptosis. Heliyon. 2024;10:e28091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 39. | Wu Z, Wang B, Ye Y, Wang S, Jiang K. Development and verification of a prognostic model for colon cancer on pyroptosis-related genes. Front Genet. 2022;13:922055. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 40. | Guo J, Zheng J, Mu M, Chen Z, Xu Z, Zhao C, Yang K, Qin X, Sun X, Yu J. GW4064 enhances the chemosensitivity of colorectal cancer to oxaliplatin by inducing pyroptosis. Biochem Biophys Res Commun. 2021;548:60-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 41. | Chen J, Jin D, Shao L, Wang L, Zhou L, Cai J. Machine Learning-derived Multi-omics Prognostic Signature of Pyroptosis-related lncRNA with Regard to ZKSCAN2-DT and Tumor Immune Infiltration in Colorectal Cancer. Comb Chem High Throughput Screen. 2024;27:1161-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 42. | Zhou J, Guo H, Liu L, Feng M, Yang X, Hao S. Pyroptosis patterns of colon cancer could aid to estimate prognosis, microenvironment and immunotherapy: evidence from multi-omics analysis. Aging (Albany NY). 2022;14:7547-7567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 43. | Ren T, Guo X, Zhang J, Liu Z. Pyroptosis-Related Signatures for Predicting Prognosis in Breast Cancer. Front Surg. 2022;9:788437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 44. | Wu L, Shan L, Xu D, Lin D, Bai B. Pyroptosis in cancer treatment and prevention: the role of natural products and their bioactive compounds. Med Oncol. 2024;41:66. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 45. | Zhang Y, Xu Z, Feng W, Gao H, Xu Z, Miao Y, Li W, Chen F, Lv Z, Huo J, Tuersun A, Liu W, Zong Y, Shen X, Zhao J, Lu A. Small molecule inhibitors from organoid-based drug screen induce concurrent apoptosis and gasdermin E-dependent pyroptosis in colorectal cancer. Clin Transl Med. 2022;12:e812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 46. | Dai J, Chen S, Bai Y, Fu Y, Pan Y, Ye L. A Novel Pyroptosis-Associated Gene Signature to Predict Prognosis in Patients with Colorectal Cancer. Evid Based Complement Alternat Med. 2022;2022:6965308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 47. | Lin W, Zhang Y, Yang Y, Lin B, Zhu M, Xu J, Chen Y, Wu W, Chen B, Chen X, Liu J, Wang H, Teng F, Yu X, Wang H, Lu J, Zhou Q, Teng L. Anti-PD-1/Her2 Bispecific Antibody IBI315 Enhances the Treatment Effect of Her2-Positive Gastric Cancer through Gasdermin B-Cleavage Induced Pyroptosis. Adv Sci (Weinh). 2023;10:e2303908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 48. | Hu D, Cui L, Zhang S, He S, Zhuo Y, Li D, Zhang L, Wang Y, Yang L, Wang X. Antitumor effect of tubeimoside-I on murine colorectal cancers through PKM2-dependent pyroptosis and immunomodulation. Naunyn Schmiedebergs Arch Pharmacol. 2024;397:4069-4087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 49. | Zhu LH, Yang J, Zhang YF, Yan L, Lin WR, Liu WQ. Identification and validation of a pyroptosis-related prognostic model for colorectal cancer based on bulk and single-cell RNA sequencing data. World J Clin Oncol. 2024;15:329-355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (1)] |
| 50. | Peng X, Na R, Zhou W, Meng X, Yang Y, Amini S, Song L. Nuclear translocation of Gasdermin D sensitizes colorectal cancer to chemotherapy in a pyroptosis-independent manner. Oncogene. 2022;41:5092-5106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 51. | Chen T, Wang Z, Zhong J, Zhang L, Zhang H, Zhang D, Xu X, Zhong X, Wang J, Li H. Secoisolariciresinol diglucoside induces pyroptosis by activating caspase-1 to cleave GSDMD in colorectal cancer cells. Drug Dev Res. 2022;83:1152-1166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 52. | Man SM, Karki R, Kanneganti TD. AIM2 inflammasome in infection, cancer, and autoimmunity: Role in DNA sensing, inflammation, and innate immunity. Eur J Immunol. 2016;46:269-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 259] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 53. | Wei R, Li S, Yu G, Guan X, Liu H, Quan J, Jiang Z, Wang X. Deciphering the Pyroptosis-Related Prognostic Signature and Immune Cell Infiltration Characteristics of Colon Cancer. Front Genet. 2021;12:755384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 54. | Liu Q, Chen N, Liu L, Zheng Q, Liao W, Zhao M, Zeng J, Tang J. Construction and Validation of Pyroptosis-Related lncRNA Prediction Model for Colon Adenocarcinoma and Immune Infiltration Analysis. Dis Markers. 2022;2022:4492608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 55. | Wang N, Zhang L, Leng XX, Xie YL, Kang ZR, Zhao LC, Song LH, Zhou CB, Fang JY. Fusobacterium nucleatum induces chemoresistance in colorectal cancer by inhibiting pyroptosis via the Hippo pathway. Gut Microbes. 2024;16:2333790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 22] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 56. | Wu L, Zheng Y, Liu J, Luo R, Wu D, Xu P, Wu D, Li X. Comprehensive evaluation of the efficacy and safety of LPV/r drugs in the treatment of SARS and MERS to provide potential treatment options for COVID-19. Aging (Albany NY). 2021;13:10833-10852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 57. | Gong W, Liu P, Zhao F, Liu J, Hong Z, Ren H, Gu G, Wang G, Wu X, Zheng T, Zhao Y, Ren J. STING-mediated Syk Signaling Attenuates Tumorigenesis of Colitisassociated Colorectal Cancer Through Enhancing Intestinal Epithelium Pyroptosis. Inflamm Bowel Dis. 2022;28:572-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 58. | Hu H, Zhang F, Sheng Z, Tian S, Li G, Tang S, Niu Y, Yang J, Liu Y. Synthesis and mitochondria-localized iridium (III) complexes induce cell death through pyroptosis and ferroptosis pathways. Eur J Med Chem. 2024;268:116295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 59. | Hu J, Tian C, Zhao Y, Guo Y, Chen S. Prognostic prediction of systemic immune-inflammation status for patients with colorectal cancer: a novel pyroptosis-related model. World J Surg Oncol. 2022;20:234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 60. | Han W, Xing W, Wang K, Wang B, Bai K. Alisol A attenuates malignant phenotypes of colorectal cancer cells by inactivating PI3K/Akt signaling. Oncol Lett. 2022;24:249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 61. | Wang J, Kang Y, Li Y, Sun L, Zhang J, Qian S, Luo K, Jiang Y, Sun L, Xu F. Gasdermin D in Different Subcellular Locations Predicts Diverse Progression, Immune Microenvironment and Prognosis in Colorectal Cancer. J Inflamm Res. 2021;14:6223-6235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 62. | Chen H, Yin L, Yang J, Ren N, Chen J, Lu Q, Huang Y, Feng Y, Wang W, Wang S, Liu Y, Song Y, Li Y, Jin J, Tan W, Lin D. Genetic polymorphisms in genes regulating cell death and prognosis of patients with rectal cancer receiving postoperative chemoradiotherapy. Cancer Biol Med. 2023;20:297-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 63. | Chen S, Zhu J, Zhi X. A Novel Pyroptosis-Associated Long Noncoding RNA Signature to Predict the Prognosis of Patients with Colorectal Cancer. Int J Gen Med. 2021;14:6111-6123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 64. | Huo J, Shen Y, Zhang Y, Shen L. BI 2536 induces gasdermin E-dependent pyroptosis in ovarian cancer. Front Oncol. 2022;12:963928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 65. | Lu H, Sun Y, Zhu Z, Yao J, Xu H, Huang R, Huang B. Pyroptosis is related to immune infiltration and predictive for survival of colon adenocarcinoma patients. Sci Rep. 2022;12:9233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 66. | Su F, Duan J, Zhu J, Fu H, Zheng X, Ge C. Long noncoding RNA nuclear paraspeckle assembly transcript 1 regulates ionizing radiationinduced pyroptosis via microRNA448/gasdermin E in colorectal cancer cells. Int J Oncol. 2021;59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 67. | Yan Z, Da Q, Li Z, Lin Q, Yi J, Su Y, Yu G, Ren Q, Liu X, Lin Z, Qu J, Yin W, Liu J. Inhibition of NEK7 Suppressed Hepatocellular Carcinoma Progression by Mediating Cancer Cell Pyroptosis. Front Oncol. 2022;12:812655. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 68. | Mo W, Yu Q, Kuang X, He T, Lou J, Tang R, Zhang K, Li L, Zhao L. Focused ultrasound restrains the growth of orthotopic colon cancer via promoting pyroptosis. Folia Histochem Cytobiol. 2023;61:47-55. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 69. | Huntington KE, Louie AD, Srinivasan PR, Schorl C, Lu S, Silverberg D, Newhouse D, Wu Z, Zhou L, Borden BA, Giles FJ, Dooner M, Carneiro BA, El-Deiry WS. GSK-3 inhibitor elraglusib enhances tumor-infiltrating immune cell activation in tumor biopsies and synergizes with anti-PD-L1 in a murine model of colorectal cancer. bioRxiv. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 70. | Wu L, Zhong Y, Wu D, Xu P, Ruan X, Yan J, Liu J, Li X. Immunomodulatory Factor TIM3 of Cytolytic Active Genes Affected the Survival and Prognosis of Lung Adenocarcinoma Patients by Multi-Omics Analysis. Biomedicines. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 71. | Zhuang Z, Cai H, Lin H, Guan B, Wu Y, Zhang Y, Liu X, Zhuang J, Guan G. Development and Validation of a Robust Pyroptosis-Related Signature for Predicting Prognosis and Immune Status in Patients with Colon Cancer. J Oncol. 2021;2021:5818512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 72. | Tian W, Wang Z, Tang NN, Li JT, Liu Y, Chu WF, Yang BF. Ascorbic Acid Sensitizes Colorectal Carcinoma to the Cytotoxicity of Arsenic Trioxide via Promoting Reactive Oxygen Species-Dependent Apoptosis and Pyroptosis. Front Pharmacol. 2020;11:123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 73. | Chen Y, Tian Z, Hou H, Gai W. The noncoding RNAs regulating pyroptosis in colon adenocarcinoma were derived from the construction of a ceRNA network and used to develop a prognostic model. BMC Med Genomics. 2022;15:201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 74. | Tan Y, Lu L, Liang X, Chen Y. Identification of a pyroptosis-related lncRNA risk model for predicting prognosis and immune response in colon adenocarcinoma. World J Surg Oncol. 2022;20:118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 75. | Fan Y, Zhang X, Tong Y, Chen S, Liang J. Curcumin against gastrointestinal cancer: A review of the pharmacological mechanisms underlying its antitumor activity. Front Pharmacol. 2022;13:990475. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 76. | Pan XQ, Huang W, Jin LW, Lin HZ, Xu XY. A Novel Pyroptosis-Related Prognostic Signature for Risk Stratification and Clinical Prognosis in Clear Cell Renal Cell Carcinoma. Dis Markers. 2022;2022:8093837. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 77. | Ren CP, Zhang YN, Wu YL, DU XX, Cui XL. [Effects of resveratrol on inhibiting pyroptosis of intestinal cancer cells]. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2022;38:326-331. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 78. | Ling Y, Wang Y, Cao C, Feng L, Zhang B, Li S. Molecular subtypes identified by pyroptosis-related genes are associated with tumor microenvironment cell infiltration in colon cancer. Aging (Albany NY). 2022;14:9020-9036. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 79. | Ren Q, Yang B, Zhu G, Wang S, Fu C, Zhang H, Ross RP, Stanton C, Chen H, Chen W. Antiproliferation Activity and Mechanism of c9, t11, c15-CLNA and t9, t11, c15-CLNA from Lactobacillus plantarum ZS2058 on Colon Cancer Cells. Molecules. 2020;25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 80. | Wu L, Liu Q, Ruan X, Luan X, Zhong Y, Liu J, Yan J, Li X. Multiple Omics Analysis of the Role of RBM10 Gene Instability in Immune Regulation and Drug Sensitivity in Patients with Lung Adenocarcinoma (LUAD). Biomedicines. 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 81. | Derangère V, Chevriaux A, Courtaut F, Bruchard M, Berger H, Chalmin F, Causse SZ, Limagne E, Végran F, Ladoire S, Simon B, Boireau W, Hichami A, Apetoh L, Mignot G, Ghiringhelli F, Rébé C. Liver X receptor β activation induces pyroptosis of human and murine colon cancer cells. Cell Death Differ. 2014;21:1914-1924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 124] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 82. | Chen HX, Ren NX, Yang J, Chen JN, Lu QX, Feng YR, Huang Y, Yin LL, Lin DX, Li YX, Jin J, Tan W. [Associations of genetic variations in pyroptosis related genes with acute adverse events in postoperative rectal cancer patients receiving concurrent chemoradiotherapy]. Zhonghua Zhong Liu Za Zhi. 2023;45:146-152. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 83. | Wu LS, Liu Y, Wang XW, Xu B, Lin YL, Song Y, Dong Y, Liu JL, Wang XJ, Liu S, Kong P, Han M, Li BH. LPS Enhances the Chemosensitivity of Oxaliplatin in HT29 Cells via GSDMD-Mediated Pyroptosis. Cancer Manag Res. 2020;12:10397-10409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 84. | Jiang M, Wu Y, Qi L, Li L, Song D, Gan J, Li Y, Ling X, Song C. Dihydroartemisinin mediating PKM2-caspase-8/3-GSDME axis for pyroptosis in esophageal squamous cell carcinoma. Chem Biol Interact. 2021;350:109704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 85. | Yin H, Xie C, Zuo Z, Xie D, Wang Q. A CTL-Inspired Killing System Using Ultralow-Dose Chemical-Drugs to Induce a Pyroptosis-Mediated Antitumor Immune Function. Adv Mater. 2024;36:e2309839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 86. | Jiang M, Wu Y, Qi L, Li L, Song D, Gan J, Li Y, Ling X, Song C. Corrigendum to "Dihydroartemisinin mediating PKM2-caspase-8/3-GSDME axis for pyroptosis in esophageal squamous cell carcinoma" [Chem. Biol. Interact. 350 (2021) 109704]. Chem Biol Interact. 2022;352:109765. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 87. | Lei A, Maloy KJ. Colon Cancer in the Land of NOD: NLRX1 as an Intrinsic Tumor Suppressor. Trends Immunol. 2016;37:569-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 88. | Wu L, Zheng Y, Ruan X, Wu D, Xu P, Liu J, Wu D, Li X. Long-chain noncoding ribonucleic acids affect the survival and prognosis of patients with esophageal adenocarcinoma through the autophagy pathway: construction of a prognostic model. Anticancer Drugs. 2022;33:e590-e603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 89. | Tong X, Tang R, Xiao M, Xu J, Wang W, Zhang B, Liu J, Yu X, Shi S. Targeting cell death pathways for cancer therapy: recent developments in necroptosis, pyroptosis, ferroptosis, and cuproptosis research. J Hematol Oncol. 2022;15:174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 456] [Cited by in RCA: 449] [Article Influence: 149.7] [Reference Citation Analysis (0)] |
| 90. | Rao Z, Zhu Y, Yang P, Chen Z, Xia Y, Qiao C, Liu W, Deng H, Li J, Ning P, Wang Z. Pyroptosis in inflammatory diseases and cancer. Theranostics. 2022;12:4310-4329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 344] [Article Influence: 114.7] [Reference Citation Analysis (0)] |
| 91. | Wei X, Xie F, Zhou X, Wu Y, Yan H, Liu T, Huang J, Wang F, Zhou F, Zhang L. Role of pyroptosis in inflammation and cancer. Cell Mol Immunol. 2022;19:971-992. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 260] [Cited by in RCA: 371] [Article Influence: 123.7] [Reference Citation Analysis (0)] |
| 92. | Yang F, Bettadapura SN, Smeltzer MS, Zhu H, Wang S. Pyroptosis and pyroptosis-inducing cancer drugs. Acta Pharmacol Sin. 2022;43:2462-2473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 82] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 93. | Faria SS, Costantini S, de Lima VCC, de Andrade VP, Rialland M, Cedric R, Budillon A, Magalhães KG. NLRP3 inflammasome-mediated cytokine production and pyroptosis cell death in breast cancer. J Biomed Sci. 2021;28:26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 118] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 94. | Su L, Chen Y, Huang C, Wu S, Wang X, Zhao X, Xu Q, Sun R, Kong X, Jiang X, Qiu X, Huang X, Wang M, Wong PP. Targeting Src reactivates pyroptosis to reverse chemoresistance in lung and pancreatic cancer models. Sci Transl Med. 2023;15:eabl7895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 66] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 95. | Zhang C, Liu N. Ferroptosis, necroptosis, and pyroptosis in the occurrence and development of ovarian cancer. Front Immunol. 2022;13:920059. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 42] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 96. | Hsu SK, Li CY, Lin IL, Syue WJ, Chen YF, Cheng KC, Teng YN, Lin YH, Yen CH, Chiu CC. Inflammation-related pyroptosis, a novel programmed cell death pathway, and its crosstalk with immune therapy in cancer treatment. Theranostics. 2021;11:8813-8835. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 286] [Article Influence: 71.5] [Reference Citation Analysis (0)] |
| 97. | Khan M, Ai M, Du K, Song J, Wang B, Lin J, Ren A, Chen C, Huang Z, Qiu W, Zhang J, Tian Y, Yuan Y. Pyroptosis relates to tumor microenvironment remodeling and prognosis: A pan-cancer perspective. Front Immunol. 2022;13:1062225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 36] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 98. | Al Mamun A, Mimi AA, Aziz MA, Zaeem M, Ahmed T, Munir F, Xiao J. Role of pyroptosis in cancer and its therapeutic regulation. Eur J Pharmacol. 2021;910:174444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 99. | Ruan J, Wang S, Wang J. Mechanism and regulation of pyroptosis-mediated in cancer cell death. Chem Biol Interact. 2020;323:109052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 169] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 100. | Ju X, Yang Z, Zhang H, Wang Q. Role of pyroptosis in cancer cells and clinical applications. Biochimie. 2021;185:78-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 101. | Wu L, Zhong Y, Yu X, Wu D, Xu P, Lv L, Ruan X, Liu Q, Feng Y, Liu J, Li X. Selective poly adenylation predicts the efficacy of immunotherapy in patients with lung adenocarcinoma by multiple omics research. Anticancer Drugs. 2022;33:943-959. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 62] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 102. | Qi S, Wang Q, Zhang J, Liu Q, Li C. Pyroptosis and Its Role in the Modulation of Cancer Progression and Antitumor Immunity. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 103. | Wang Y, Wang Y, Pan J, Gan L, Xue J. Ferroptosis, necroptosis, and pyroptosis in cancer: Crucial cell death types in radiotherapy and post-radiotherapy immune activation. Radiother Oncol. 2023;184:109689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 104. | Xia J, Chu C, Li W, Chen H, Xie W, Cheng R, Hu K, Li X. Mitochondrial Protein UCP1 Inhibits the Malignant Behaviors of Triple-negative Breast Cancer through Activation of Mitophagy and Pyroptosis. Int J Biol Sci. 2022;18:2949-2961. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 105. | Long K, Gu L, Li L, Zhang Z, Li E, Zhang Y, He L, Pan F, Guo Z, Hu Z. Small-molecule inhibition of APE1 induces apoptosis, pyroptosis, and necroptosis in non-small cell lung cancer. Cell Death Dis. 2021;12:503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 84] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 106. | Zhang CC, Li CG, Wang YF, Xu LH, He XH, Zeng QZ, Zeng CY, Mai FY, Hu B, Ouyang DY. Chemotherapeutic paclitaxel and cisplatin differentially induce pyroptosis in A549 lung cancer cells via caspase-3/GSDME activation. Apoptosis. 2019;24:312-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 311] [Article Influence: 51.8] [Reference Citation Analysis (0)] |
| 107. | Wu L, Lu H, Pan Y, Liu C, Wang J, Chen B, Wang Y. The role of pyroptosis and its crosstalk with immune therapy in breast cancer. Front Immunol. 2022;13:973935. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Reference Citation Analysis (0)] |
| 108. | Ren Y, Feng M, Hao X, Liu X, Li J, Li P, Gao J, Qi Q, Du L, Wang C, Wang Q, Wang Y. USP48 Stabilizes Gasdermin E to Promote Pyroptosis in Cancer. Cancer Res. 2023;83:1074-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 41] [Reference Citation Analysis (0)] |
| 109. | Wang A, Wang Y, Du C, Yang H, Wang Z, Jin C, Hamblin MR. Pyroptosis and the tumor immune microenvironment: A new battlefield in ovarian cancer treatment. Biochim Biophys Acta Rev Cancer. 2024;1879:189058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Reference Citation Analysis (0)] |
| 110. | Wu L, Li H, Liu Y, Fan Z, Xu J, Li N, Qian X, Lin Z, Li X, Yan J. Research progress of 3D-bioprinted functional pancreas and in vitro tumor models. IJB. 2024;10:1256. [DOI] [Full Text] |
| 111. | Tan Y, Chen Q, Li X, Zeng Z, Xiong W, Li G, Li X, Yang J, Xiang B, Yi M. Pyroptosis: a new paradigm of cell death for fighting against cancer. J Exp Clin Cancer Res. 2021;40:153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 301] [Article Influence: 75.3] [Reference Citation Analysis (0)] |
| 112. | Wang Q, Qin W, Qiao L, Gao M, Zhou M, Zhang H, Sun Q, Yao W, Yang T, Ren X, Sun G, He X. Biomimetic Nanophotosensitizer Amplifies Immunogenic Pyroptosis and Triggers Synergistic Cancer Therapy. Adv Healthc Mater. 2023;12:e2301641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 113. | Hu Y, Liu Y, Zong L, Zhang W, Liu R, Xing Q, Liu Z, Yan Q, Li W, Lei H, Liu X. The multifaceted roles of GSDME-mediated pyroptosis in cancer: therapeutic strategies and persisting obstacles. Cell Death Dis. 2023;14:836. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 114. | Chao L, Zhang W, Feng Y, Gao P, Ma J. Pyroptosis: a new insight into intestinal inflammation and cancer. Front Immunol. 2024;15:1364911. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 115. | Liang X, Qin Y, Wu D, Wang Q, Wu H. Pyroptosis: a double-edged sword in lung cancer and other respiratory diseases. Cell Commun Signal. 2024;22:40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 116. | Tian F, Chen X, Yin K, Lin X, Song Y. The role of pyroptosis in lung cancer and compounds regulated pyroptosis of lung cancer cells. J Cancer Res Ther. 2021;17:1596-1602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 117. | Zhang Y, Yu W, Chen M, Zhang B, Zhang L, Li P. The applications of nanozymes in cancer therapy: based on regulating pyroptosis, ferroptosis and autophagy of tumor cells. Nanoscale. 2023;15:12137-12156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 29] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 118. | Chen Q, Sun Y, Wang S, Xu J. New prospects of cancer therapy based on pyroptosis and pyroptosis inducers. Apoptosis. 2024;29:66-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 119. | Ding B, Chen H, Tan J, Meng Q, Zheng P, Ma P, Lin J. ZIF-8 Nanoparticles Evoke Pyroptosis for High-Efficiency Cancer Immunotherapy. Angew Chem Int Ed Engl. 2023;62:e202215307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 141] [Reference Citation Analysis (0)] |
| 120. | Wu L, Li X, Qian X, Wang S, Liu J, Yan J. Lipid Nanoparticle (LNP) Delivery Carrier-Assisted Targeted Controlled Release mRNA Vaccines in Tumor Immunity. Vaccines (Basel). 2024;12. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 121. | Zhu M, Liu D, Liu G, Zhang M, Pan F. Caspase-Linked Programmed Cell Death in Prostate Cancer: From Apoptosis, Necroptosis, and Pyroptosis to PANoptosis. Biomolecules. 2023;13. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 122. | Wang M, Wu M, Liu X, Shao S, Huang J, Liu B, Liang T. Pyroptosis Remodeling Tumor Microenvironment to Enhance Pancreatic Cancer Immunotherapy Driven by Membrane Anchoring Photosensitizer. Adv Sci (Weinh). 2022;9:e2202914. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 72] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 123. | Xu X, Fan H, Yang Y, Yao S, Yu W, Guo Z, Tan W. Virus-Like Particle-Induced cGAS-STING Activation and AIM2 Inflammasome-Mediated Pyroptosis for Robust Cancer Immunotherapy. Angew Chem Int Ed Engl. 2023;62:e202303010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 124. | Zhang L, Song A, Yang QC, Li SJ, Wang S, Wan SC, Sun J, Kwok RTK, Lam JWY, Deng H, Tang BZ, Sun ZJ. Integration of AIEgens into covalent organic frameworks for pyroptosis and ferroptosis primed cancer immunotherapy. Nat Commun. 2023;14:5355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 76] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 125. | Wu L, Chen X, Zeng Q, Lai Z, Fan Z, Ruan X, Li X, Yan J. NR5A2 gene affects the overall survival of LUAD patients by regulating the activity of CSCs through SNP pathway by OCLR algorithm and immune score. Heliyon. 2024;10:e28282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 126. | Deng W, Shang H, Tong Y, Liu X, Huang Q, He Y, Wu J, Ba X, Chen Z, Chen Y, Tang K. The application of nanoparticles-based ferroptosis, pyroptosis and autophagy in cancer immunotherapy. J Nanobiotechnology. 2024;22:97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 127. | Chen H, Luo H, Wang J, Li J, Jiang Y. Identification of a pyroptosis-related prognostic signature in breast cancer. BMC Cancer. 2022;22:429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 128. | Yan H, Luo B, Wu X, Guan F, Yu X, Zhao L, Ke X, Wu J, Yuan J. Cisplatin Induces Pyroptosis via Activation of MEG3/NLRP3/caspase-1/GSDMD Pathway in Triple-Negative Breast Cancer. Int J Biol Sci. 2021;17:2606-2621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 185] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 129. | Zhang Q, Tan Y, Zhang J, Shi Y, Qi J, Zou D, Ci W. Pyroptosis-Related Signature Predicts Prognosis and Immunotherapy Efficacy in Muscle-Invasive Bladder Cancer. Front Immunol. 2022;13:782982. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 130. | Zhang Z, Zhang H, Li D, Zhou X, Qin Q, Zhang Q. Caspase-3-mediated GSDME induced Pyroptosis in breast cancer cells through the ROS/JNK signalling pathway. J Cell Mol Med. 2021;25:8159-8168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 120] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 131. | Li L, Tian H, Zhang Z, Ding N, He K, Lu S, Liu R, Wu P, Wang Y, He B, Luo M, Peng P, Yang M, Nice EC, Huang C, Xie N, Wang D, Gao W. Carrier-Free Nanoplatform via Evoking Pyroptosis and Immune Response against Breast Cancer. ACS Appl Mater Interfaces. 2023;15:452-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 39] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 132. | Chen L, Ma X, Liu W, Hu Q, Yang H. Targeting Pyroptosis through Lipopolysaccharide-Triggered Noncanonical Pathway for Safe and Efficient Cancer Immunotherapy. Nano Lett. 2023;23:8725-8733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 27] [Reference Citation Analysis (0)] |
| 133. | Lia T, Shao Y, Regmi P, Li X. Development and validation of pyroptosis-related lncRNAs prediction model for bladder cancer. Biosci Rep. 2022;42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 134. | Du X, Zhao X, Tang Y, Tang W. Construction of Pyroptosis-Related Prognostic and Immune Infiltration Signature in Bladder Cancer. Dis Markers. 2022;2022:6429993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 135. | Sang R, Fan R, Deng A, Gou J, Lin R, Zhao T, Hai Y, Song J, Liu Y, Qi B, Du G, Cheng M, Wei G. Degradation of Hexokinase 2 Blocks Glycolysis and Induces GSDME-Dependent Pyroptosis to Amplify Immunogenic Cell Death for Breast Cancer Therapy. J Med Chem. 2023;66:8464-8483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 136. | You HM, Wang L, Meng HW, Huang C, Fang GY, Li J. Pyroptosis: shedding light on the mechanisms and links with cancers. Front Immunol. 2023;14:1290885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 137. | Luo J, Lai J. Pyroptosis-related molecular classification and immune microenvironment infiltration in breast cancer: A novel therapeutic target. J Cell Mol Med. 2022;26:2259-2272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 138. | Wang W, Sun W, Xu H, Liu Y, Wei C, Wang S, Xian S, Yan P, Zhang J, Guo H, Qin H, Lian J, Han X, Zhang J, Guo R, Zhang J, Huang Z. Bibliometric analysis and mini-review of global research on pyroptosis in the field of cancer. Apoptosis. 2023;28:1076-1089. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 139. | Wang H, Zhou X, Li C, Yan S, Feng C, He J, Li Z, Tu C. The emerging role of pyroptosis in pediatric cancers: from mechanism to therapy. J Hematol Oncol. 2022;15:140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 39] [Reference Citation Analysis (0)] |
| 140. | Zhang B, Li Z, Wang K, Duan M, Yin Y, Zhan Q, Wang F, An R. Exploration of pyroptosis-associated prognostic gene signature and lncRNA regulatory network in ovarian cancer. Comput Biol Med. 2023;164:107343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 141. | Gao L, Ying F, Cai J, Peng M, Xiao M, Sun S, Zeng Y, Xiong Z, Cai L, Gao R, Wang Z. Identification and validation of pyroptosis-related gene landscape in prognosis and immunotherapy of ovarian cancer. J Ovarian Res. 2023;16:27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 142. | Liu A, Shen L, Li N, Shen L, Li Z. Pan-cancer analyses of pyroptosis with functional implications for prognosis and immunotherapy in cancer. J Transl Med. 2022;20:109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 143. | Luo L, Yao X, Xiang J. Pyroptosis-Related Gene Model Predicts Prognosis and Immune Microenvironment for Non-Small-Cell Lung Cancer. Oxid Med Cell Longev. 2022;2022:1749111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 144. | Jiang SC, Tao SH, Chen SY, Xie H, Feng YJ. Characterization of pyroptosis-related genes in esophageal cancer and construction of a prognostic model. Eur Rev Med Pharmacol Sci. 2023;27:6592-6604. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 145. | Ding B, Zheng P, Tan J, Chen H, Meng Q, Li J, Li X, Han D, Li Z, Ma X, Ma P, Lin J. Sodium Bicarbonate Nanoparticles for Amplified Cancer Immunotherapy by Inducing Pyroptosis and Regulating Lactic Acid Metabolism. Angew Chem Int Ed Engl. 2023;62:e202307706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 49] [Reference Citation Analysis (0)] |
| 146. | Xu K, Chang M, Wang Z, Yang H, Jia Y, Xu W, Zhao B, Chen Y, Yao F. Multienzyme-Mimicking LaCoO(3) Nanotrigger for Programming Cancer-Cell Pyroptosis. Adv Mater. 2023;35:e2302961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 35] [Reference Citation Analysis (0)] |
| 147. | Luo QW, Yao L, Li L, Yang Z, Zhao MM, Zheng YZ, Zhuo FF, Liu TT, Zhang XW, Liu D, Tu PF, Zeng KW. Inherent Capability of Self-Assembling Nanostructures in Specific Proteasome Activation for Cancer Cell Pyroptosis. Small. 2023;19:e2205531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 148. | Zhou L, Wong C, Liu Y, Jiang W, Yang Q. Development and validation of stable ferroptosis- and pyroptosis-related signatures in predicting prognosis and immune status in breast cancer. J Cell Mol Med. 2023;27:3827-3838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 149. | Huang G, Zhou J, Chen J, Liu G. Identification of pyroptosis related subtypes and tumor microenvironment infiltration characteristics in breast cancer. Sci Rep. 2022;12:10640. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 150. | Luo CG, Gui CP, Huang GW, Chen JL, Li JY, Li PJ, Xu QH, Wang YH, Zhu JQ, Liang H, Wang Z, Deng Q, Cao JZ, Luo JH, Lu J, Chen W. Identification of ZDHHC1 as a Pyroptosis Inducer and Potential Target in the Establishment of Pyroptosis-Related Signature in Localized Prostate Cancer. Oxid Med Cell Longev. 2022;2022:5925817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 151. | Li L, Liao A. Application of pyroptosis score in the treatment and prognosis evaluation of gastric cancer. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2023;48:1882-1889. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 152. | Liang M, Li JW, Luo H, Lulu S, Calbay O, Shenoy A, Tan M, Law BK, Huang S, Xiao TS, Chen H, Wu L, Chang J, Lu J. Epithelial-Mesenchymal Transition Suppresses AMPK and Sensitizes Cancer Cells to Pyroptosis under Energy Stress. Cells. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 153. | Yang J, Guo W, Lu M. Confusion about the article: Natural product triptolide induces GSDME-mediated pyroptosis in head and neck cancer through suppressing mitochondrial hexokinase-ΙΙ. Cancer Lett. 2022;534:115568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 154. | Zhu X, Li S. Ferroptosis, Necroptosis, and Pyroptosis in Gastrointestinal Cancers: The Chief Culprits of Tumor Progression and Drug Resistance. Adv Sci (Weinh). 2023;10:e2300824. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 155. | Yang J, Wang C, Zhang Y, Cheng S, Xu Y, Wang Y. A Novel pyroptosis-related signature for predicting prognosis and evaluating tumor immune microenvironment in ovarian cancer. J Ovarian Res. 2023;16:196. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 156. | Zeng T, Ye J, Wang H, Tian W. Identification of pyroptosis-related lncRNA subtype and signature predicts the prognosis in bladder cancer. Medicine (Baltimore). 2023;102:e35195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 157. | Zhao Q, Feng H, Yang Z, Liang J, Jin Z, Chen L, Zhan L, Xuan M, Yan J, Kuang J, Cheng X, Zhao R, Qiu W. The central role of a two-way positive feedback pathway in molecular targeted therapies-mediated pyroptosis in anaplastic thyroid cancer. Clin Transl Med. 2022;12:e727. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 158. | Wang H, He Z, Gao Y, Feng D, Wei X, Huang Y, Hou J, Li S, Zhang W. Dual-Pronged Attack: pH-Driven Membrane-Anchored NIR Dual-Type Nano-Photosensitizer Excites Immunogenic Pyroptosis and Sequester Immune Checkpoint for Enhanced Prostate Cancer Photo-Immunotherapy. Adv Sci (Weinh). 2023;10:e2302422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 159. | Qiu S, Hu Y, Dong S. Pan-cancer analysis reveals the expression, genetic alteration and prognosis of pyroptosis key gene GSDMD. Int Immunopharmacol. 2021;101:108270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 160. | Wu J, Zhu Y, Luo M, Li L. Comprehensive Analysis of Pyroptosis-Related Genes and Tumor Microenvironment Infiltration Characterization in Breast Cancer. Front Immunol. 2021;12:748221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 161. | Yu B, Wang Y, Bing T, Tang Y, Huang J, Xiao H, Liu C, Yu Y. Platinum Prodrug Nanoparticles with COX-2 Inhibition Amplify Pyroptosis for Enhanced Chemotherapy and Immune Activation of Pancreatic Cancer. Adv Mater. 2024;36:e2310456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 40] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 162. | Gong M, Liu X, Zhao X, Wang H. A pyroptosis-related gene signature predicting survival and tumor immune microenvironment in breast cancer and validation. BMC Cancer. 2022;22:1005. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 163. | Zeng Y, Cai Y, Chai P, Mao Y, Chen Y, Wang L, Zeng K, Zhan Z, Xie Y, Li C, Zhan H, Zhao L, Chen X, Zhu X, Liu Y, Chen M, Song Y, Zhou A. Optimization of cancer immunotherapy through pyroptosis: A pyroptosis-related signature predicts survival benefit and potential synergy for immunotherapy in glioma. Front Immunol. 2022;13:961933. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 164. | Guo H, Wang Z, Ma R, Chen X, Li H, Tang Y, Du G, Zhang Y, Yin D. A novel pharmacological mechanism of anti-cancer drugs that induce pyroptosis. Inflammopharmacology. 2023;31:745-754. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 165. | Luo L, Wei Q, Xu C, Dong M, Zhao W. Immune landscape and risk prediction based on pyroptosis-related molecular subtypes in triple-negative breast cancer. Front Immunol. 2022;13:933703. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 166. | Xu JZ, Xia QD, Sun JX, Liu CQ, Lu JL, Xu MY, An Y, Xun Y, Liu Z, Hu J, Li C, Wang SG. Establishment of a novel indicator of pyroptosis regulated gene transcription level and its application in pan-cancer. Sci Rep. 2023;13:17911. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 167. | Liu Z, Wang C, Lin C. Pyroptosis as a double-edged sword: The pathogenic and therapeutic roles in inflammatory diseases and cancers. Life Sci. 2023;318:121498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 168. | Hou J, Wang S, Miao R, Zhang X, Hung MC. Detection of Gasdermin C-Mediated Cancer Cell Pyroptosis. Methods Mol Biol. 2023;2641:135-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 169. | Ma L, Bian M, Gao H, Zhou Z, Yi W. A novel 3-acyl isoquinolin-1(2H)-one induces G2 phase arrest, apoptosis and GSDME-dependent pyroptosis in breast cancer. PLoS One. 2022;17:e0268060. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 170. | Li YT, Tan XY, Ma LX, Li HH, Zhang SH, Zeng CM, Huang LN, Xiong JX, Fu L. Targeting LGSN restores sensitivity to chemotherapy in gastric cancer stem cells by triggering pyroptosis. Cell Death Dis. 2023;14:545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 171. | Ling YY, Xia XY, Hao L, Wang WJ, Zhang H, Liu LY, Liu W, Li ZY, Tan CP, Mao ZW. Simultaneous Photoactivation of cGAS-STING Pathway and Pyroptosis by Platinum(II) Triphenylamine Complexes for Cancer Immunotherapy. Angew Chem Int Ed Engl. 2022;61:e202210988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 61] [Reference Citation Analysis (0)] |
| 172. | Du Q, Luo Y, Xu L, Du C, Zhang W, Xu J, Liu Y, Liu B, Chen S, Wang Y, Wang Z, Ran H, Wang J, Guo D. Smart responsive Fe/Mn nanovaccine triggers liver cancer immunotherapy via pyroptosis and pyroptosis-boosted cGAS-STING activation. J Nanobiotechnology. 2024;22:95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 173. | Wang Z, Cao L, Zhou S, Lyu J, Gao Y, Yang R. Construction and Validation of a Novel Pyroptosis-Related Four-lncRNA Prognostic Signature Related to Gastric Cancer and Immune Infiltration. Front Immunol. 2022;13:854785. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 174. | Zhang M, Wu P. Construction of prognosis model of pyroptosis gene and characterization of tumor microenvironment infiltration in cervical cancer. Medicine (Baltimore). 2022;101:e31599. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 175. | Liu Z, Kuang S, Chen Q. A review focusing on the role of pyroptosis in prostate cancer. Medicine (Baltimore). 2023;102:e36605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 176. | Bi K, Yang J, Wei X. Alternative splicing variants involved in pyroptosis and cuproptosis contribute to phenotypic remodeling of the tumor microenvironment in cervical cancer. Reprod Sci. 2023;30:3648-3660. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 177. | Fu J, Li G, Luo R, Lu Z, Wang Y. Classification of pyroptosis patterns and construction of a novel prognostic model for prostate cancer based on bulk and single-cell RNA sequencing. Front Endocrinol (Lausanne). 2022;13:1003594. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 178. | Zhang G, Luo Y, Dong W, Zhong W. Characterization of a Pyroptosis-Related Signature for Prognosis Prediction and Immune Microenvironment Infiltration in Prostate Cancer. Comput Math Methods Med. 2022;2022:8233840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 179. | Xue S, Shen K, Wang K, Ge W, Mao T, Li S, Zhang X, Xu H, Wang Y, Yao J, Yue M, Ma J, Wang Y, Shentu D, Cui J, Wang L. Prediction of Survival and Tumor Microenvironment Infiltration Based on Pyroptosis-Related lncRNAs in Pancreatic Cancer. Dis Markers. 2022;2022:5634887. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 180. | Zhang W, Liu Z, Zhu J, Liu Z, Zhang Y, Qin G, Ren J, Qu X. Bioorthogonal Disruption of Pyroptosis Checkpoint for High-Efficiency Pyroptosis Cancer Therapy. J Am Chem Soc. 2023;145:16658-16668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 46] [Reference Citation Analysis (0)] |
| 181. | Hu J, You Y, Zhu L, Zhang J, Song Y, Lu J, Xu X, Wu X, Huang X, Xu X, Du Y. Sialic Acid-Functionalized Pyroptosis Nanotuner for Epigenetic Regulation and Enhanced Cancer Immunotherapy. Small. 2024;20:e2306905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 182. | Li S, Chen P, Cheng B, Liu Y, Zhang X, Xu Q, Huang M, Dai X, Huang K, Zhang L, Cheng Y, Liu L. Pyroptosis predicts immunotherapy outcomes across multiple cancer types. Clin Immunol. 2022;245:109163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 183. | Zeng X, Huang X, Yin L, Yu H, Wang S, Li L. Molecular subtyping and immune score system by a novel pyroptosis-based gene signature precisely predict immune infiltrating, survival and response to immune-checkpoint blockade in breast cancer. Cancer Genet. 2023;276-277:60-69. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 184. | El-Kenawi A, Berglund A, Estrella V, Zhang Y, Liu M, Putney RM, Yoder SJ, Johnson J, Brown J, Gatenby R. Elevated Methionine Flux Drives Pyroptosis Evasion in Persister Cancer Cells. Cancer Res. 2023;83:720-734. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 185. | Wu Z, Zeng J, Wu M, Liang Q, Li B, Hou G, Lin Z, Xu W. Identification and validation of the pyroptosis-related long noncoding rna signature to predict the prognosis of patients with bladder cancer. Medicine (Baltimore). 2023;102:e33075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 186. | Chen J, Ge L, Shi X, Liu J, Ruan H, Heng D, Ye C. Lobaplatin Induces Pyroptosis in Cervical Cancer Cells via the Caspase-3/GSDME Pathway. Anticancer Agents Med Chem. 2022;22:2091-2097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 187. | Xiao L, Chen B, Wang W, Tian T, Qian H, Li X, Yu Y. Multifunctional Au@AgBiS(2) Nanoparticles as High-Efficiency Radiosensitizers to Induce Pyroptosis for Cancer Radioimmunotherapy. Adv Sci (Weinh). 2023;10:e2302141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 188. | Liu J, Dai Y, Lu Y, Liu X, Deng J, Lu W, Liu Q. Identification and validation of a new pyroptosis-associated lncRNA signature to predict survival outcomes, immunological responses and drug sensitivity in patients with gastric cancer. Math Biosci Eng. 2023;20:1856-1881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 189. | Liu Y, Zhang W, Zhou H, Chen J. Steroidal saponins PPI/CCRIS/PSV induce cell death in pancreatic cancer cell through GSDME-dependent pyroptosis. Biochem Biophys Res Commun. 2023;673:51-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 190. | Jun H, ZeXin Z. Screening of pyroptosis-related genes influencing the therapeutic effect of dehydroabietic acid in liver cancer and construction of a survival nomogram. Biochem Biophys Res Commun. 2021;585:103-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 191. | Xie B, Liu T, Chen S, Zhang Y, He D, Shao Q, Zhang Z, Wang C. Combination of DNA demethylation and chemotherapy to trigger cell pyroptosis for inhalation treatment of lung cancer. Nanoscale. 2021;13:18608-18615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 192. | Liu ZS, Jing CL. A novel risk prediction model of pyroptosis-related genes for the prognosis and immunotherapy response of endometrial cancer. Eur Rev Med Pharmacol Sci. 2022;26:2259-2278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 193. | Kobayashi T, Mitsuhashi A, Hongying P, Shioya M, Kojima K, Nishikimi K, Yahiro K, Shozu M. Bexarotene-induced cell death in ovarian cancer cells through Caspase-4-gasdermin E mediated pyroptosis. Sci Rep. 2022;12:11123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 194. | Lu H, Wu J, Liang L, Wang X, Cai H. Identifying a Novel Defined Pyroptosis-Associated Long Noncoding RNA Signature Contributes to Predicting Prognosis and Tumor Microenvironment of Bladder Cancer. Front Immunol. 2022;13:803355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 48] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 195. | Ding B, Sheng J, Zheng P, Li C, Li D, Cheng Z, Ma P, Lin J. Biodegradable Upconversion Nanoparticles Induce Pyroptosis for Cancer Immunotherapy. Nano Lett. 2021;21:8281-8289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 100] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 196. | Chang M, Wang Z, Dong C, Zhou R, Chen L, Huang H, Feng W, Wang Z, Wang Y, Chen Y. Ultrasound-Amplified Enzyodynamic Tumor Therapy by Perovskite Nanoenzyme-Enabled Cell Pyroptosis and Cascade Catalysis. Adv Mater. 2023;35:e2208817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 72] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 197. | Lv W, Tan Y, Zhao C, Wang Y, Wu M, Wu Y, Ren Y, Zhang Q. Identification of pyroptosis-related lncRNAs for constructing a prognostic model and their correlation with immune infiltration in breast cancer. J Cell Mol Med. 2021;25:10403-10417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 198. | Chen J, Peng R, Niu Z, Zhou H, Kang C. Betulinic acid enhanced the chemical sensitivity of esophageal cancer cells to cisplatin by inducing cell pyroptosis and reducing cell stemness. Ann Palliat Med. 2020;9:1912-1920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 199. | Huang K, Lin Y, Qiu G, Wang S, Feng L, Zheng Z, Gao Y, Fan X, Zheng W, Zhuang J, Luo F, Feng S. Comprehensive characterization of pyroptosis phenotypes with distinct tumor immune profiles in gastric cancer to aid immunotherapy. Aging (Albany NY). 2023;15:8113-8136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 200. | Chen Z, Ge L, Xu S, Li Q, Zhou L. A novel defined pyroptosis-related gene signature predicts prognosis and correlates with the tumour immune microenvironment in lung adenocarcinoma. Sci Rep. 2023;13:9921. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 201. | Tang J, Bei M, Zhu J, Xu G, Chen D, Jin X, Huang J, Dong J, Shi L, Xu L, Hu B. Acute cadmium exposure induces GSDME-mediated pyroptosis in triple-negative breast cancer cells through ROS generation and NLRP3 inflammasome pathway activation. Environ Toxicol Pharmacol. 2021;87:103686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 52] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 202. | Xu W, Song C, Wang X, Li Y, Bai X, Liang X, Wu J, Liu J. Downregulation of miR-155-5p enhances the anti-tumor effect of cetuximab on triple-negative breast cancer cells via inducing cell apoptosis and pyroptosis. Aging (Albany NY). 2021;13:228-240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 203. | BioRender. [cited 2 July 2024]. Available from: https://www.biorender.com/. |