Published online Aug 15, 2024. doi: 10.4251/wjgo.v16.i8.3397
Revised: May 31, 2024
Accepted: June 21, 2024
Published online: August 15, 2024
Processing time: 210 Days and 14.7 Hours
Hepatocyte growth factor (HGF) and its receptor, c-Met, play important roles in the occurrence, development, and treatment of gastric cancer (GC). This review explored the function of the HGF/c-Met signaling pathway in GC and its potential targeted therapeutic mechanisms. As one of the most common malignant tumors worldwide, GC has a complex pathogenesis and limited therapeutic options. Therefore, a thorough understanding of the molecular mechanism of GC is very important for the development of new therapeutic methods. The HGF/c-Met signaling pathway plays an important role in the proliferation, migration, and invasion of GC cells and has become a new therapeutic target. This review summarizes the current research progress on the role of HGF/c-Met in GC and discusses targeted therapeutic strategies targeting this signaling pathway, providing new ideas and directions for the treatment of GC.
Core Tip: Hepatocyte growth factor (HGF) and its receptor, c-Met, play important roles in the occurrence, development, and treatment of gastric cancer (GC). This review explored the function of the HGF/c-Met signaling pathway in GC and its potential targeted therapeutic mechanisms. As one of the most common malignant tumors worldwide, GC has a complex pathogenesis and limited therapeutic options. Therefore, a thorough understanding of the molecular mechanism of GC is important for the development of new therapeutic methods. The HGF/c-Met signaling pathway plays an important role in the proliferation, migration, and invasion of GC cells and has become a novel therapeutic target.
- Citation: Wei WJ, Hong YL, Deng Y, Wang GL, Qiu JT, Pan F. Research progress on the development of hepatocyte growth factor/c-Met signaling pathway in gastric cancer: A review. World J Gastrointest Oncol 2024; 16(8): 3397-3409
- URL: https://www.wjgnet.com/1948-5204/full/v16/i8/3397.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i8.3397
Gastric cancer (GC) is the second most common cause of cancer death worldwide, with an annual incidence of approximately 1 million people and an annual death of approximately 700000[1-3]. Therefore, seeking effective treatment has become a research hotspot internationally. Current studies[4-6] have shown that hepatocyte growth factor (HGF) and its receptor (c-Met) signaling pathway are closely related to the genesis, development, metastasis, and prognosis of GC, and targeted drugs targeting this signaling pathway will become a new breakthrough point for the treatment of GC[7].
In the field of oncology, the incidence and mortality of stomach cancer remain high, making it a serious health problem worldwide[8]. Despite significant advances in the diagnosis and treatment of GC in recent years, the therapeutic effect on GC is still limited due to its complexity and heterogeneity[9-11]. Therefore, in-depth study of the molecular mechanism of GC and the search for new therapeutic targets and strategies are highly important for improving the therapeutic efficacy of treatments for GC[12]. The HGF/c-Met signaling pathway regulates cell proliferation, migration, and differentiation in normal physiological processes, but in tumors, it is closely related to tumor cell proliferation, survival, migration, invasion, and drug resistance[13-16].
Studies internationally have shown that the HGF/c-Met signaling pathway plays an important role in the occurrence and development of GC[17-20]. Some studies have shown that the expression level of c-Met in GC tissues is significantly greater than that in normal gastric tissues, and its overexpression is closely related to the poor prognosis of patients with GC[21]. In addition, activation of the HGF/c-Met signaling pathway not only promotes the proliferation and migration of GC cells but also enhances their resistance to chemotherapy drugs, increasing the difficulty of treatment[22].
In view of the important role of the HGF/c-Met signaling pathway in GC, this review further explored the specific mechanism of this signaling pathway in the occurrence and development of GC and evaluated targeted therapeutic strategies targeting the HGF/c-Met signaling pathway to provide new ideas for the clinical treatment of GC[23-30].
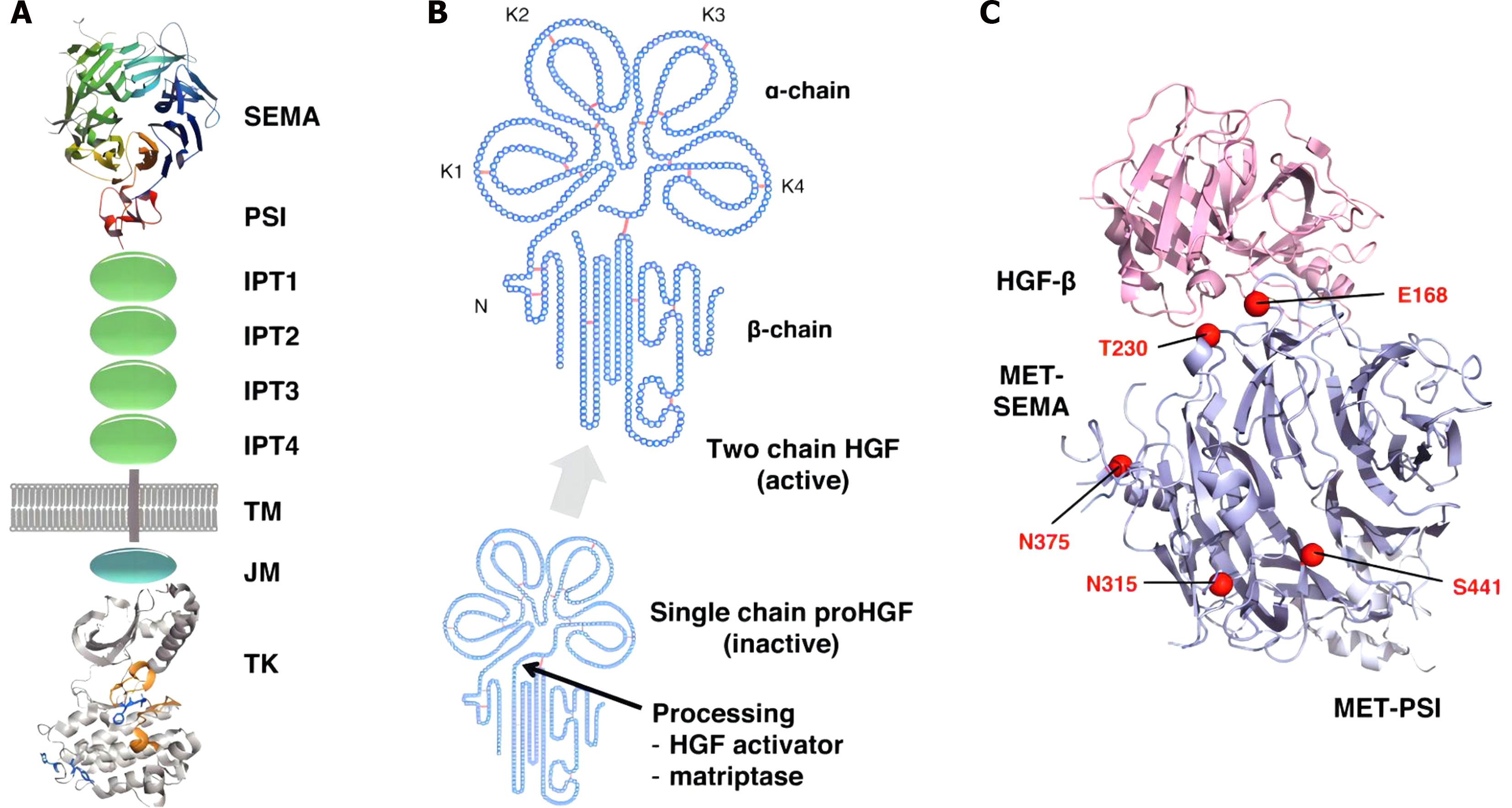
HGF is a multifunctional active factor that was first isolated and purified from the platelets and plasma of rats subjected to partial hepatectomy by the Japanese scholar Fushida et al[23] and is mainly produced by mesenchymal cells. The HGF gene is located at 7q21 in humans, and the protein is composed of 728 amino acids and is a heterodimer of 1 α chain and 1 β chain connected by a disulfide bond[31-33]. There is a hairpin structure at the N end of the α chain, which is the main component of the biological effect of HGF[34]. The β chain has a serine proteinase-like structure, which is the binding site of HGF to the interstitial epidermal transgene (or c-Met)[35]. HGF is a biological factor that can stimulate a variety of endothelial and epithelial cells to undergo mitosis and tissue formation and induce epithelial cell migration, invasion, vascular endothelial regeneration, and other functions.
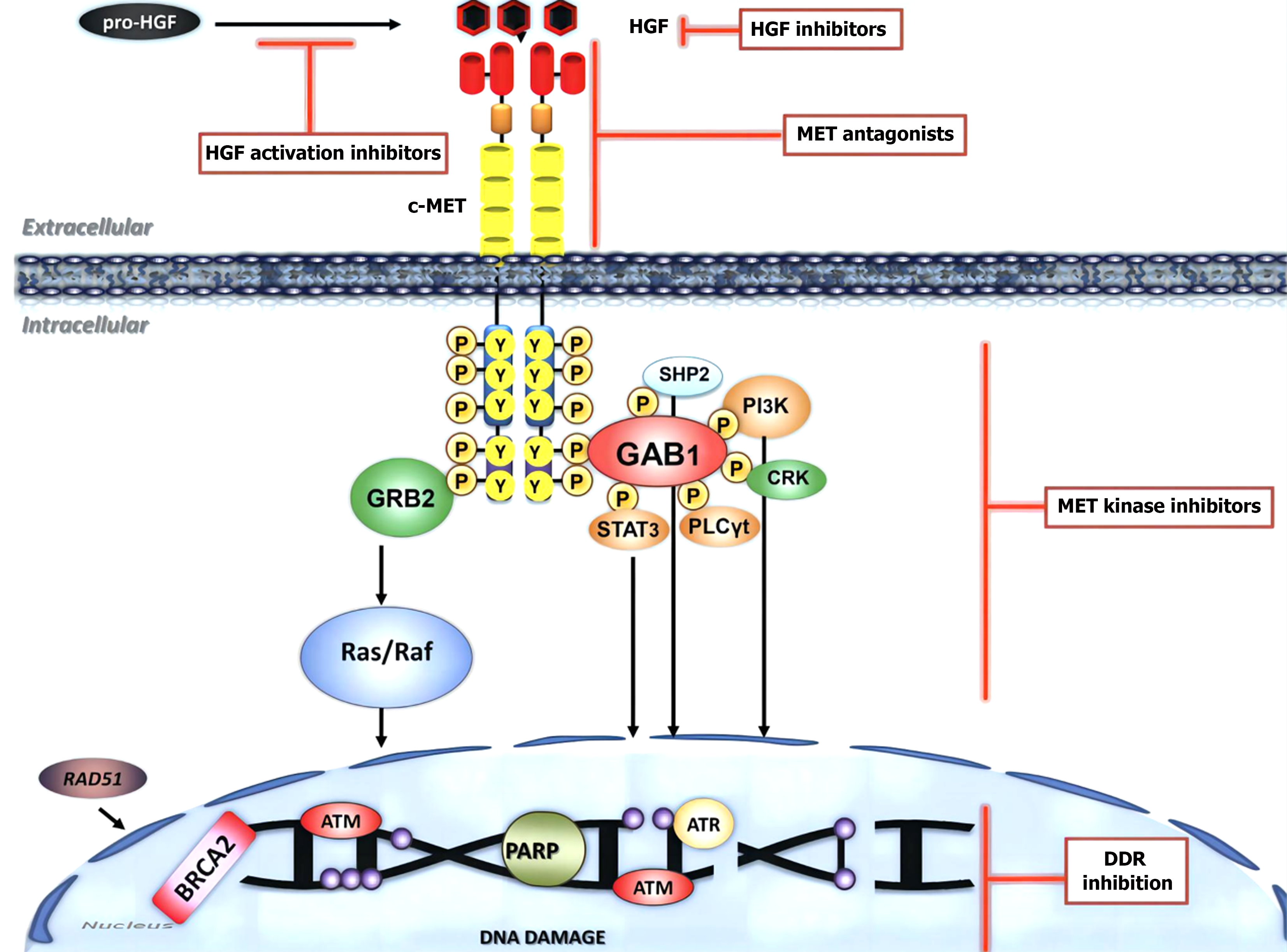
The c-Met protein, a member of the receptor tyrosine kinase (RTK) superfamily, is a transmembrane receptor with phosphorylated activity that is encoded by the MET proto-oncogene and is mainly produced by epithelial cells[36-38]. c-Met is a fragment with transformational activity that was first cloned. It fused with the translocation promoter region (TPR) in the osteosarcoma cell line to form a TPR-Met fusion body[39,40]. The MET gene is located on human chromosomes 7q21-q31, and the protein is connected by α and β subunits via disulfide bonds[41]. The α subunit contains only extracellular regions, while the β subunit has extracellular, transmembrane, and intracellular regions, with the former attached to the latter by disulfide bonds to form heterodimers. The extracellular region of the two subunits is the ligand recognition site, while intracellular division has tyrosine kinase activity[42]. c-Met is related to a variety of oncogene products and regulatory proteins and has a strong role in promoting cell proliferation, inducing the dispersal and motility of epithelial cells and fibroblasts[43-45]. It is involved in a variety of in vivo processes, such as signal transduction and cytoskeleton reconstruction, and is an important factor regulating cell proliferation, differentiation, and repair processes (Figure 1).
Under normal circumstances, HGF itself has no serine protease activity, and only after binding to the c-Met receptor can it exert its physiological effects[46-48]. Similar to other RTKs, the c-Met transmembrane region consists of a single alpha helix with two phosphorylation sites in most subdomains of the cytoplasmic near-membrane region: S985 and Y1003. After phosphorylation of S985, kinase activity is inhibited[49]. The phosphorylation of Y1003 can degrade internalized c-Met[50]. When c-Met binds to HGF, the tyrosine residue Tyr1234/Tyr1235 in the intracellular region is self-phosphorylated, and RTK in the protein kinase domain is activated. Activated RTK self-phosphorylates tyrosine Tyr1349/Tyr1356 at the carboxyl terminus of c-Met[51-54]. Finally, a variety of substrate proteins in the cytoplasm, such as phosphoinositide 3- kinase (PI3K) and phospholipase C, are recruited here and rapidly phosphorylated for use, thus activating a variety of intracellular signaling pathways such as the Ras/Raf, PI3K/AKT/mammalian target of rapamycin, Janus kinase/signal transducer and activator of transcription (STAT), and Crk/C3 glomerulopathy/Ras-associated protein-1 pathways. These pathways promote cell proliferation, regeneration, differentiation, angiogenesis, invasion, and metastasis; regulate cytoskeletal rearrangement; and promote cell movement. HGF and c-Met exist widely in a variety of cells and participate in a variety of physiological processes[55-57]. For example, it functions in repair, regeneration, and angiogenesis in damaged tissues and controls cell movement during embryonic development. However, when the HGF/c-Met signaling pathway is dysfunctional, the growth and movement of cells are disrupted, which may eventually lead to the occurrence, development, and metastasis of tumors (Figure 2).
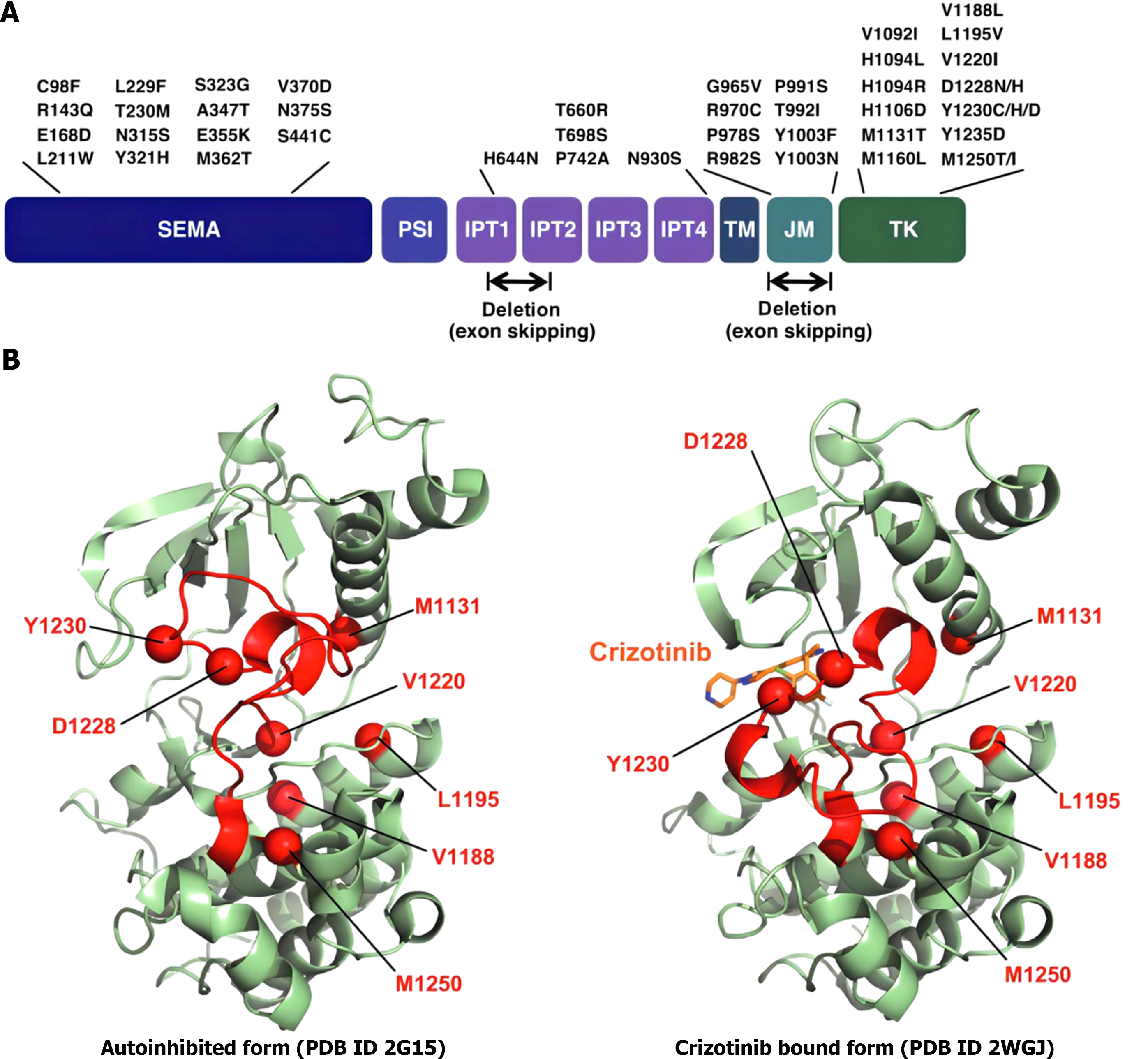
In a physiological state, the HGF/c-Met signaling pathway is strictly regulated by both positive and negative factors so that it is in a state of equilibrium[58-60]. Specific gene damage, transcriptional upregulation, and ligand-dependent autosecretory or paracrine mechanisms can cause abnormal activation of c-Met, resulting in cell proliferation, migration, invasion, and growth[61]. The c-Met gene mutation was originally reported to occur only in the tyrosine kinase region and is associated with inherited and sporadic renal cell carcinoma[62-64]. Since then, a large number of studies have reported that c-Met overexpression and amplification also occur in many tumors, such as lung, gastric, colorectal, liver, pancreatic, prostate, ovarian, lymphatic, and head and neck cancers (Figure 3).
The pathogenesis of GC is a multifactor, multistep process caused by the precursors of the disease, such as Helicobacter pylori infection, and genetic and epigenetic abnormalities that lead to cell proliferation and metastasis[65]. As early as 1995, researchers noted that the expansion or mutation of oncogenes encoding tyrosine kinase receptors plays an important role in the development of GC. c-Met is a member of the tyrosine kinase receptor family, which is also closely related to GC[66-68]. Under normal circumstances, the HGF/c-Met signaling pathway can be temporarily activated; for example, HGF and c-Met can be detected in the injured kidney, liver, and heart, indicating that the signaling pathway has a role in protecting the body from injury and promoting repair and regeneration under physiological conditions[69]. Abnormal regulation of this signaling pathway plays an important role in tumor survival, growth, angiogenesis, and metastasis. In recent years, domestic and foreign scholars have shown that high c-Met expression in GC tissues is related to GC invasion, metastasis, and poor prognosis but not to patient sex or age, tumor size, tumor location, or degree of differentiation. In this review, c-Met expression in 182 patients with GC, chronic atrophic gastritis, intestinal metaplasia, abnormal hyperplasia, and normal gastric mucosa was detected by immunohistochemistry. The results showed that the expression rate of c-Met in GC tissues was significantly greater than that in other tissues, and the expression rate in lymph node metastatic GC tissues was significantly greater than that in nonmetastatic GC tissues[70]. Another study used immunohistochemistry to detect c-Met expression in the tissues of 124 GC patients and found that the overexpression of c-Met was associated with lymph node metastasis and vascular invasion[71]. A retrospective immunohistochemistry study of tissue samples from 495 patients with GC suggested that the overall survival (OS) and disease-free survival of patients with c-Met overexpression were shorter than those of patients without c-Met overexpression (Figure 4).
HGF monoclonal antibodies prevent tumor growth mainly by interfering with the binding of HGF to c-Met. At present, many growth factor inhibitors are in the clinical trial stage, and the main HGF inhibitors under study are rilotumumab, ficlatuzumab, and TAK-701[72-74]. All three medicines are humanized immunoglobulin G monoclonal antibodies that bind only to HGF. They stop HGF from binding to c-Met and stop c-Met kinase from activating. Rilotumumab is currently in phase II clinical trials and is associated with fluorouracil, leucovorin, and oxaliplatin (FOLFOX). (fluorouracil + calcium folinate + oxaliplatin) and (epirubicin + cisplatin + xeloda, ECX) are combined for the treatment of advanced gastrointestinal tumors. A phase II randomized controlled trial with 121 people who had gastric or gastroesophageal junction cancer revealed that compared with ECX plus a placebo, ECX plus rilotumumab improved their chance of not having their cancer worse. Progression-free survival (PFS) increased from 4.2 mo to 5.6 mo (hazard ratio [HR] = 0.64), and OS increased from 5.7 mo to 11.1 mo (HR = 0.29)[75-78]. However, an independent data committee safety review revealed that patients treated with rilotumumab combined with chemotherapy had a greater mortality rate than patients treated with chemotherapy alone. Amgen announced the termination of the clinical study on November 26, 2014.
Onartuzumab/MetMAb is a c-Met monoclonal antibody that can inhibit dimerization of the c-Met receptor and further block the binding of HGF and c-Met, resulting in downstream signal transduction obstruction and tumor suppression[79]. More in-depth clinical research on non-small cell lung cancer (NSCLC) has focused on onartuzumab. The results of a phase II clinical trial revealed that after patients with NSCLC with mutations or amplifications of the c-Met gene were treated with the drug, PFS and OS were significantly longer than those in the placebo group. However, another phase III clinical trial ended early after failing to produce meaningful clinical results, and the trial ended in failure[80]. Researchers are still investigating the causes of the failure of phase III clinical trials. In a phase I clinical trial for GC, a chemotherapy-refractory patient with liver metastasis of GC with pathological stage PT3N1M1 achieved a sustained complete response (> 2 years) after treatment with onartuzumab[81-83]. The MetGastric study is a phase III randomized clinical trial of onartuzumab for GC that used onartuzumab combined with FOLFOX to treat human epidermal growth factor receptor (HER) (-)/c-Met (+) metastatic gastric and esophageal cancer patients (Figure 5).
Small molecule tyrosine kinase inhibitors inhibit downstream signal transduction by blocking c-Met kinase activation, thereby inhibiting tumor proliferation, invasion, and metastasis. According to the different binding sites of c-Met, small-molecule kinase inhibitors can be divided into class I (SU-11274) and class II (AM 7)[84-86]. In addition, there is a third class of small molecule kinases, namely, ARQ197 and tivantinib, which selectively bind to unphosphorylated kinases and cut off key catalytic sites, thereby inhibiting c-Met phosphorylation.
Tivantinib: Tivantinib (ARQ197) is a non-ATP-competitive small-molecule c-Met kinase inhibitor that inhibits ligand-dependent or ligand-independent c-MET phosphorylation, thereby preventing downstream signal transduction[87]. A phase II clinical trial of tivantinib and erlotinib in the treatment of non-small cell lung cancer showed that tivantinib is effective in inhibiting tumor invasion, metastasis, and proliferation and inducing apoptosis[88]. However, tivantinib monotherapy did not show significant efficacy in patients with advanced metastatic GC.
Crizotinib: Crizotinib, a dual inhibitor of c-Met and anaplastic lymphoma kinase, has been approved by the United States Food and Drug Administration for the treatment of NSCLC[89]. However, in patients with GC with c-Met amplification, overexpression, or mutation, there is no consensus on the efficacy of treatment[90]. Kawakami and Okamoto[7] generated a mouse model of GC with crizotinib, and found that mice with positive c-Met amplification showed tumor inhibition after being administered this drug. However, c-Met amplification did not have a significant curative effect on these mice. The clinical symptoms of the other 2 patients improved after medication, mainly manifesting as better gastric, pain relief, and mass reduction, and the curative effect was maintained for approximately 4 mo.
Foretinib: Foretinib is a multimolecule tyrosine kinase inhibitor with high affinity for c-Met, macrophage-stimulating protein receptor, angiogenin receptor, and vascular endothelial growth factor receptor (VEGFR)[91-93]. It changes its structure and inhibits kinase activity by binding to ATP. The results showed that 20.2% of patients had stable disease after taking foretinib, with a median time of 3.2 mo for stabilization, and none of the patients achieved complete or partial remission (Table 1).
| Drug (alternative name) | Study | Phase |
| Crizotinib (PF0234I066) | NCT00585I95 | I |
| Cabozantinib (XL184) | NCT0I8664I0 | II |
| NCT0I708954 | II | |
| NCT00940225 | II | |
| Foretinib (GSKI363089) | NCT0I068587 | I/II |
| Tepotinib (EMDI2I4063) | NCT02864992 | II |
| Rilotumumab (AMG102) | NCT02137343 | III |
| Ficlatuzumab | NCT03422536 | II |
| Tivantinib (ARQ197) | NCT01244191 | III |
| Capmatinib (INC280, INCB28060) | NCT0I324479 | I |
| NCT0I60336 | II |
In the past, although the potential of the HGF/c-Met signaling pathway for the treatment of GC has received much attention, the results of many clinical trials have been unsatisfactory. For example, a phase III clinical trial using the c-Met inhibitor rilotumumab showed no significant improvement in OS in patients with advanced GC, despite showing some antitumor activity in earlier trials. Similarly, another phase III trial using onartuzumab failed to achieve the expected efficacy, with no significant difference in PFS and OS compared with placebo. In addition, a phase II clinical trial of foretinib as a multitarget tyrosine kinase inhibitor in the treatment of GC showed limited efficacy of its monotherapy and failed to meet the expected clinical endpoint. These negative trial results suggest that the HGF/c-Met signaling pathway in the treatment of GC needs further research to explore its complex biological mechanisms and possible combination therapy strategies to improve patient outcomes.
This review focused on the expression and activation status of the HGF/c-Met signaling pathway in GC tissues and cells and revealed that this pathway plays a crucial role in the occurrence and development of GC. Studies have shown that abnormal activation of the HGF/c-Met signaling pathway is closely related to the proliferation, invasion, and metastasis of GC cells[94-96]. In particular, overexpression of the c-Met protein in GC tissues is negatively correlated with tumor malignancy and patient prognosis[97]. In addition, activation of the HGF/c-Met signaling pathway not only promotes the growth of tumor cells but may also affect the tumor microenvironment, thus participating in tumor immune escape. Therefore, targeted therapeutic strategies targeting the HGF/c-Met signaling pathway show broad application prospects for the treatment of GC[98]. Current research has also further explored the mechanism of action of this signaling pathway in the treatment of GC, hoping to provide more effective treatment options for patients with GC (Figure 6).
This review explored the downstream effects and biological functions of the HGF/c-Met signaling pathway in GC. When HGF binds to its receptor, c-Met, it initiates a chain of events that include the activation of signaling pathways such as the Ras/mitogen-activated protein kinase (MAPK), PI3K/AKT, and STAT pathways. The activation of these signaling pathways not only promotes the proliferation and survival of GC cells but also promotes tumor cell invasion and metastasis. The HGF/c-Met signaling pathway is a key part of the epithelial-mesenchymal transition of GC cells and is a necessary step for tumor cells to become more aggressive and able to move around. In addition, the activation of HGF/c-Met signaling is also associated with the stem cell properties of GC cells and may be involved in tumor chemotherapy resistance[99].
During the development of GC, abnormal activation of the HGF/c-Met signaling pathway is closely related to the biological behavior of the tumor. Clinical studies have shown that high expression of c-Met is closely associated with poor prognosis in patients with GC, suggesting its importance in tumor development[100-102]. The HGF/c-Met signaling pathway also affects different cells in the area around the tumor, such as tumor-associated macrophages and cancer-associated fibroblasts, which further impacts tumor growth and progression.
Based on these findings, the HGF/c-Met signaling pathway has become an important target for targeted therapy in GC. Several small molecule inhibitors and antibody drugs targeting c-Met are being evaluated in clinical trials, some of which have shown potential therapeutic efficacy in patients with GC. However, due to tumor heterogeneity and complex signaling networks, therapeutic strategies targeting the HGF/c-Met signaling pathway need to be more personalized when combined with other therapies. Future studies will focus on a more in-depth analysis of the mechanism of action of the HGF/c-Met signaling pathway in GC and search for more effective treatment combinations to improve treatment outcomes and quality of life for patients with GC.
Through their application in GC cell lines and animal models, these drugs have shown significant antitumor effects. The primary targeted drugs used in the study included small-molecule inhibitors of c-Met and monoclonal antibodies against HGF or c-Met. These drugs can effectively inhibit the proliferation, migration, and invasion of GC cells, indicating their potential in the treatment of GC[103].
In particular, these agents inhibit MAPK, PI3K/AKT, and STAT signaling, which are activated downstream of the HGF/c-Met pathway. They do this by stopping HGF from binding to c-Met or stopping c-Met from working as a kinase. This intervention reduces the proliferation and viability of tumor cells while inhibiting cell invasion and migration, which is directly related to the tumor's ability to metastasize. In addition, targeted drugs can also affect the tumor microenvironment, reducing the interaction between tumor cells and the surrounding microenvironment and thereby limiting the further development of tumors. The HGF/c-Met signaling pathway is thought to be one of the main reasons why chemotherapy and targeted therapy do not affect GC patients who are drug resistant. Therefore, drugs that target this signaling pathway show potential for overcoming drug resistance in GC. Studies have shown that the application of inhibitors of the HGF/c-Met signaling pathway can effectively reduce the drug resistance of tumor cells and improve the sensitivity of chemotherapy in GC cells and animal models treated with traditional chemotherapy drugs[104].
However, therapeutic strategies targeting the HGF/c-Met signaling pathway also face challenges. The heterogeneity and complex signaling network of tumors make it difficult for single-target therapy to completely inhibit the growth and spread of tumors. Therefore, future research needs to focus on how to combine these targeted drugs with other therapies, such as chemotherapy, radiation, and other types of targeted therapy, to improve treatment effectiveness[105]. At the same time, an in-depth understanding of the mechanism of the HGF/c-Met signaling pathway in different types of GC will help to more accurately select treatment strategies, develop personalized medicine, and improve the survival rate and quality of life of GC patients.
This study had some limitations. First, despite our best efforts to collect data from the latest and most comprehensive literature, there may still be recent advances that are not included in time due to the rapid pace of development in the field of research. Second, the complexity of the HGF/c-Met signaling pathway and heterogeneity of GC lead to different results in different studies, which increases the difficulty of summary and analysis.
In recent years, molecular targeted therapy has become a hot topic in the treatment of GC. Mutations in HER2, epidermal growth factor receptor, VEGFR, and other genes are all associated with GC, and c-Met is a potential target. Therefore, the use of HGF/c-Met signaling pathway inhibitors is a potential breakthrough in the treatment of GC. At present, some drugs targeting this signaling pathway are in clinical trials, including onartuzumab, crizotinib, and rilotumumab, have been shown to have fixed therapeutic effects in early-stage clinical trials for the treatment of late-stage GC. A phase III clinical trial of onartuzumab combined with chemotherapy, which is expected to become an effective means for the treatment of GC, is ongoing. However, the efficacy of most drugs has not yet been determined, so much research needs to be done by researchers. In addition, researchers can expand the research target to the downstream genes of the HGF/c-Met signaling pathway, increasing the scope of research to identify appropriate treatments.
| 1. | Anestis A, Zoi I, Karamouzis MV. Current advances of targeting HGF/c-Met pathway in gastric cancer. Ann Transl Med. 2018;6:247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 2. | Palle J, Hirsch L, Lapeyre-Prost A, Malka D, Bourhis M, Pernot S, Marcheteau E, Voron T, Castan F, Lacotte A, Benhamouda N, Tanchot C, François E, Ghiringhelli F, de la Fouchardière C, Zaanan A, Tartour E, Taieb J, Terme M. Targeting HGF/c-Met Axis Decreases Circulating Regulatory T Cells Accumulation in Gastric Cancer Patients. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Huang KH, Sung IC, Fang WL, Chi CW, Yeh TS, Lee HC, Yin PH, Li AF, Wu CW, Shyr YM, Yang MH. Correlation between HGF/c-Met and Notch1 signaling pathways in human gastric cancer cells. Oncol Rep. 2018;40:294-302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Yuan HH, Zhang XC, Wei XL, Zhang WJ, Du XX, Huang P, Chen H, Bai L, Zhang HF, Han Y. LncRNA UCA1 mediates Cetuximab resistance in Colorectal Cancer via the MiR-495 and HGF/c-MET Pathways. J Cancer. 2022;13:253-267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 5. | Wu L, Li X, Qian X, Wang S, Liu J, Yan J. Lipid Nanoparticle (LNP) Delivery Carrier-Assisted Targeted Controlled Release mRNA Vaccines in Tumor Immunity. Vaccines (Basel). 2024;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 59] [Reference Citation Analysis (0)] |
| 6. | Marano L, Chiari R, Fabozzi A, De Vita F, Boccardi V, Roviello G, Petrioli R, Marrelli D, Roviello F, Patriti A. c-Met targeting in advanced gastric cancer: An open challenge. Cancer Lett. 2015;365:30-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 7. | Kawakami H, Okamoto I. MET-targeted therapy for gastric cancer: the importance of a biomarker-based strategy. Gastric Cancer. 2016;19:687-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 8. | Wu L, Li H, Liu Y, Fan Z, Xu J, Li N, Qian X, Lin Z, Li X, Yan J. Research progress of 3D-bioprinted functional pancreas and in vitro tumor models. Int J Bioprinting. 2024;10:1256. [DOI] [Full Text] |
| 9. | Ding X, Ji J, Jiang J, Cai Q, Wang C, Shi M, Yu Y, Zhu Z, Zhang J. HGF-mediated crosstalk between cancer-associated fibroblasts and MET-unamplified gastric cancer cells activates coordinated tumorigenesis and metastasis. Cell Death Dis. 2018;9:867. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 82] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 10. | Ahn SY, Kim J, Kim MA, Choi J, Kim WH. Increased HGF Expression Induces Resistance to c-MET Tyrosine Kinase Inhibitors in Gastric Cancer. Anticancer Res. 2017;37:1127-1138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Wang C, Xi W, Ji J, Cai Q, Zhao Q, Jiang J, Zhou C, Shi M, Zhang H, Zhu Z, Zhang J. The prognostic value of HGF-c-MET signaling pathway in Gastric Cancer: a study based on TCGA and GEO databases. Int J Med Sci. 2020;17:1946-1955. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Mihailidou C, Karamouzis MV, Schizas D, Papavassiliou AG. Co-targeting c-Met and DNA double-strand breaks (DSBs): Therapeutic strategies in BRCA-mutated gastric carcinomas. Biochimie. 2017;142:135-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Wu L, Zheng Y, Liu J, Luo R, Wu D, Xu P, Wu D, Li X. Comprehensive evaluation of the efficacy and safety of LPV/r drugs in the treatment of SARS and MERS to provide potential treatment options for COVID-19. Aging (Albany NY). 2021;13:10833-10852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 64] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 14. | Hong SW, Jung KH, Park BH, Zheng HM, Lee HS, Choi MJ, Yun JI, Kang NS, Lee J, Hong SS. KRC-408, a novel c-Met inhibitor, suppresses cell proliferation and angiogenesis of gastric cancer. Cancer Lett. 2013;332:74-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Schmid E, Klotz M, Steiner-Hahn K, Konen T, Frisk AL, Schatz C, Krahn T, von Ahsen O. Detection of MET mRNA in gastric cancer in situ. Comparison with immunohistochemistry and sandwich immunoassays. Biotech Histochem. 2017;92:425-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Yonemura Y, Kaji M, Hirono Y, Fushida S, Tsugawa K, Fujimura T, Miyazaki I, Harada S, Yamamoto H. Correlation between overexpression of c-met gene and the progression of gastric cancer. Int J Oncol. 1996;8:555-560. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 17. | Zeng W, Xing ZT, Tan MY, Wu YW, Zhang CY. Lidocaine suppresses the malignant behavior of gastric cancer cells via the c-Met/c-Src pathway. Exp Ther Med. 2021;21:424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 18. | Zarei B, Javidan Z, Fatemi E, Rahimi Jamnani F, Khatami S, Khalaj V. Targeting c-Met on gastric cancer cells through a fully human fab antibody isolated from a large naive phage antibody library. Daru. 2020;28:221-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 19. | Lu J, Li X, Tu K, Guan Y, Fung KP, Liu F. Verticillin A suppresses HGF-induced migration and invasion via repression of the c-Met/FAK/Src pathway in human gastric and cervical cancer cells. Onco Targets Ther. 2019;12:5823-5833. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Wu Y, Yao X, Zhu M, Qian H, Jiang L, Lan T, Wu M, Pang J, Chen Y. PKG II reverses HGF-triggered cellular activities by phosphorylating serine 985 of c-Met in gastric cancer cells. Oncotarget. 2016;7:34190-34200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Lin X, Peng Z, Wang X, Zou J, Chen D, Chen Z, Li Z, Dong B, Gao J, Shen L. Targeting autophagy potentiates antitumor activity of Met-TKIs against Met-amplified gastric cancer. Cell Death Dis. 2019;10:139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Kasai S, Kuwayama N, Motoo Y, Kawashima A, Matsumoto K, Yano S, Matsushima K, Yasumoto K. Dual blockade of MET and VEGFR2 signaling pathways as a potential therapeutic maneuver for peritoneal carcinomatosis in scirrhous gastric cancer. Biochem Biophys Res Commun. 2022;600:80-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Fushida S, Yonemura Y, Urano T, Yamaguchi A, Miyazaki I, Nakamura T, Shiku H. Expression of hepatocyte growth factor(hgf) and C-met gene in human gastric-cancer cell-lines. Int J Oncol. 1993;3:1067-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 24. | Wu L, Zhong Y, Wu D, Xu P, Ruan X, Yan J, Liu J, Li X. Immunomodulatory Factor TIM3 of Cytolytic Active Genes Affected the Survival and Prognosis of Lung Adenocarcinoma Patients by Multi-Omics Analysis. Biomedicines. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 63] [Reference Citation Analysis (0)] |
| 25. | Kim HJ, Kang SK, Kwon WS, Kim TS, Jeong I, Jeung HC, Kragh M, Horak ID, Chung HC, Rha SY. Forty-nine gastric cancer cell lines with integrative genomic profiling for development of c-MET inhibitor. Int J Cancer. 2018;143:151-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 26. | Kim S, Ahn JM, Bae WJ, Han JH, Lee D. Quantitation of ligand is critical for ligand-dependent MET signalling activation and determines MET-targeted therapeutic response in gastric cancer. Gastric Cancer. 2021;24:577-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 27. | Xiang Y, Liang B, Jiang Y, Sun F, Zhao Y, Wu Q, Hu X, Liu Y, Huang Q, Liao W, Yao Z, Li S, Shi M. MET transcriptional regulator/serine peptidase inhibitor kunitz type 1 panel operating through HGF/c-MET axis as a prognostic signature in pan-cancer. Cancer Med. 2021;10:2442-2460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 28. | Wu L, Chen X, Zeng Q, Lai Z, Fan Z, Ruan X, Li X, Yan J. NR5A2 gene affects the overall survival of LUAD patients by regulating the activity of CSCs through SNP pathway by OCLR algorithm and immune score. Heliyon. 2024;10:e28282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 42] [Reference Citation Analysis (0)] |
| 29. | Oh HA, Lee G, Kang HJ, Kim YG, Bae SH, Lee JL, Lee KH, Hyun MS, Kim DS. Overexpression of c-met Protein in Gastric Cancer and Role of uPAR as a Therapeutic Target. Cancer Res Treat. 2003;35:9-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | Li S, Zhang H, Wang X, Qu Y, Duan J, Liu R, Deng T, Ning T, Zhang L, Bai M, Zhou L, Wang X, Ge S, Ying G, Ba Y. Direct targeting of HGF by miR-16 regulates proliferation and migration in gastric cancer. Tumour Biol. 2016;37:15175-15183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Omote R, Gion Y, Omote S, Tari A, Tanaka T, Nishikori A, Yoshino T, Sato Y. Clinicopathologic analysis of gastric mucosa-associated lymphoid tissue lymphoma with or without c-Met expression. Med Mol Morphol. 2020;53:149-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 32. | Amemiya H, Kono K, Takahashi A, Kamei S, Sugai H, Ichihara F, Fujii H, Matsumoto Y. c-Met expression in a gastric cancer cell line producing alpha-fetoprotein. Surg Today. 2004;34:115-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 33. | Amemiya H, Kono K, Itakura J, Tang RF, Takahashi A, An FQ, Kamei S, Iizuka H, Fujii H, Matsumoto Y. c-Met expression in gastric cancer with liver metastasis. Oncology. 2002;63:286-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 81] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 34. | Inoue T, Kataoka H, Goto K, Nagaike K, Igami K, Naka D, Kitamura N, Miyazawa K. Activation of c-Met (hepatocyte growth factor receptor) in human gastric cancer tissue. Cancer Sci. 2004;95:803-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 35. | Wu L, Li X, Yan J. Commentary: Machine learning developed an intratumor heterogeneity signature for predicting prognosis and immunotherapy benefits in cholangiocarcinoma. Transl Oncol. 2024;45:101995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 36. | Zhao L, Yasumoto K, Kawashima A, Nakagawa T, Takeuchi S, Yamada T, Matsumoto K, Yonekura K, Yoshie O, Yano S. Paracrine activation of MET promotes peritoneal carcinomatosis in scirrhous gastric cancer. Cancer Sci. 2013;104:1640-1646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 37. | Dong W, Wang L, Shen R. MYO5B is epigenetically silenced and associated with MET signaling in human gastric cancer. Dig Dis Sci. 2013;58:2038-2045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 38. | Catenacci DVT, Tebbutt NC, Davidenko I, Murad AM, Al-Batran SE, Ilson DH, Tjulandin S, Gotovkin E, Karaszewska B, Bondarenko I, Tejani MA, Udrea AA, Tehfe M, De Vita F, Turkington C, Tang R, Ang A, Zhang Y, Hoang T, Sidhu R, Cunningham D. Rilotumumab plus epirubicin, cisplatin, and capecitabine as first-line therapy in advanced MET-positive gastric or gastro-oesophageal junction cancer (RILOMET-1): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18:1467-1482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 284] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 39. | Wu L, Liu Q, Ruan X, Luan X, Zhong Y, Liu J, Yan J, Li X. Multiple Omics Analysis of the Role of RBM10 Gene Instability in Immune Regulation and Drug Sensitivity in Patients with Lung Adenocarcinoma (LUAD). Biomedicines. 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 56] [Reference Citation Analysis (0)] |
| 40. | Chen CT, Kim H, Liska D, Gao S, Christensen JG, Weiser MR. MET activation mediates resistance to lapatinib inhibition of HER2-amplified gastric cancer cells. Mol Cancer Ther. 2012;11:660-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 150] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 41. | Graziano F, Galluccio N, Lorenzini P, Ruzzo A, Canestrari E, D'Emidio S, Catalano V, Sisti V, Ligorio C, Andreoni F, Rulli E, Di Oto E, Fiorentini G, Zingaretti C, De Nictolis M, Cappuzzo F, Magnani M. Genetic activation of the MET pathway and prognosis of patients with high-risk, radically resected gastric cancer. J Clin Oncol. 2011;29:4789-4795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 151] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 42. | Liu SI, Lui WY, Mok KT, Wu CW, Chi CW. Effect of hepatocyte growth factor on cell cycle and c-met expression in human gastric cancer cells. Anticancer Res. 1997;17:3575-3580. [PubMed] |
| 43. | Li L, Jiang X, Zhang Q, Dong X, Gao Y, He Y, Qiao H, Xie F, Xie X, Sun X. Neuropilin-1 is associated with clinicopathology of gastric cancer and contributes to cell proliferation and migration as multifunctional co-receptors. J Exp Clin Cancer Res. 2016;35:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 44. | Graziano F, Catalano V, Lorenzini P, Giacomini E, Sarti D, Fiorentini G, De Nictolis M, Magnani M, Ruzzo A. Clinical impact of the HGF/MET pathway activation in patients with advanced gastric cancer treated with palliative chemotherapy. Pharmacogenomics J. 2014;14:418-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 45. | Amemiya H, Kono K, Mori Y, Takahashi A, Ichihara F, Iizuka H, Sekikawa T, Matsumoto Y. High frequency of c-Met expression in gastric cancers producing alpha- fetoprotein. Oncology. 2000;59:145-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 59] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 46. | Hao NB, Tang B, Wang GZ, Xie R, Hu CJ, Wang SM, Wu YY, Liu E, Xie X, Yang SM. Hepatocyte growth factor (HGF) upregulates heparanase expression via the PI3K/Akt/NF-κB signaling pathway for gastric cancer metastasis. Cancer Lett. 2015;361:57-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 88] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 47. | Kang YK, Muro K, Ryu MH, Yasui H, Nishina T, Ryoo BY, Kamiya Y, Akinaga S, Boku N. A phase II trial of a selective c-Met inhibitor tivantinib (ARQ 197) monotherapy as a second- or third-line therapy in the patients with metastatic gastric cancer. Invest New Drugs. 2014;32:355-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 98] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 48. | Lee JH, Han SU, Cho H, Jennings B, Gerrard B, Dean M, Schmidt L, Zbar B, Vande Woude GF. A novel germ line juxtamembrane Met mutation in human gastric cancer. Oncogene. 2000;19:4947-4953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 245] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 49. | Park WS, Oh RR, Kim YS, Park JY, Shin MS, Lee HK, Lee SH, Yoo NJ, Lee JY. Absence of mutations in the kinase domain of the Met gene and frequent expression of Met and HGF/SF protein in primary gastric carcinomas. APMIS. 2000;108:195-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 50. | Inoue T, Chung YS, Yashiro M, Nishimura S, Hasuma T, Otani S, Sowa M. Transforming growth factor-beta and hepatocyte growth factor produced by gastric fibroblasts stimulate the invasiveness of scirrhous gastric cancer cells. Jpn J Cancer Res. 1997;88:152-159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 51. | Amemiya H, Peña A, Chiurillo M, Moscoso J, Useche A, Baffi R. [Increased expression of the c-Met receptor mRNA in gastric cancer]. Invest Clin. 2013;54:284-298. [PubMed] |
| 52. | Feng Y, Ma PC. Anti-MET targeted therapy has come of age: the first durable complete response with MetMAb in metastatic gastric cancer. Cancer Discov. 2011;1:550-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 53. | Kermorgant S, Cadiot G, Lewin MJ, Lehy T. [Expression of hepatocyte growth factor and its receptor, C-Met in human digestive tissues and different gastric and colonic cancer cell lines]. Gastroenterol Clin Biol. 1996;20:438-445. [PubMed] |
| 54. | Lee KH, Choi EY, Hyun MS, Jang BI, Kim TN, Kim SW, Song SK, Kim JH, Kim JR. Hepatocyte growth factor/c-met signaling in regulating urokinase plasminogen activator in human stomach cancer: A potential therapeutic target for human stomach cancer. Korean J Intern Med. 2006;21:20-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 55. | Wang L, Lin L, Chen X, Sun L, Liao Y, Huang N, Liao W. Metastasis-associated in colon cancer-1 promotes vasculogenic mimicry in gastric cancer by upregulating TWIST1/2. Oncotarget. 2015;6:11492-11506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 56. | Kaji M, Yonemura Y, Harada S, Liu X, Terada I, Yamamoto H. Participation of c-met in the progression of human gastric cancers: anti-c-met oligonucleotides inhibit proliferation or invasiveness of gastric cancer cells. Cancer Gene Ther. 1996;3:393-404. [PubMed] |
| 57. | Catenacci DV, Henderson L, Xiao SY, Patel P, Yauch RL, Hegde P, Zha J, Pandita A, Peterson A, Salgia R. Durable complete response of metastatic gastric cancer with anti-Met therapy followed by resistance at recurrence. Cancer Discov. 2011;1:573-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 58. | Park M, Park H, Kim WH, Cho H, Lee JH. Presence of autocrine hepatocyte growth factor-Met signaling and its role in proliferation and migration of SNU-484 gastric cancer cell line. Exp Mol Med. 2005;37:213-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 59. | Ueda K, Iwahashi M, Matsuura I, Nakamori M, Nakamura M, Ojima T, Naka T, Ishida K, Matsumoto K, Nakamura T, Yamaue H. Adenoviral-mediated gene transduction of the hepatocyte growth factor (HGF) antagonist, NK4, suppresses peritoneal metastases of gastric cancer in nude mice. Eur J Cancer. 2004;40:2135-2142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 60. | Amemiya H, Menolascino F, Peña A. [Role of the expression of c-Met receptor in the progression of gastric cancer]. Invest Clin. 2010;51:369-380. [PubMed] |
| 61. | Tannapfel A, Wittekind C, Tahara E. Effect of hepatocyte growth factor (HGF)/scatter factor (SF) on cell adhesion in gastric cancer. Z Gastroenterol. 1994;32:91-93. [PubMed] |
| 62. | Yu S, Yu Y, Zhao N, Cui J, Li W, Liu T. C-Met as a prognostic marker in gastric cancer: a systematic review and meta-analysis. PLoS One. 2013;8:e79137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 63. | Liu J, Li S, Chen S, Chen S, Geng Q, Xu D. c-Met-dependent phosphorylation of RhoA plays a key role in gastric cancer tumorigenesis. J Pathol. 2019;249:126-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 64. | Zhang Q, Zhang H, Ning T, Liu D, Deng T, Liu R, Bai M, Zhu K, Li J, Fan Q, Ying G, Ba Y. Exosome-Delivered c-Met siRNA Could Reverse Chemoresistance to Cisplatin in Gastric Cancer. Int J Nanomedicine. 2020;15:2323-2335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 83] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 65. | Wu L, Zheng Y, Ruan X, Wu D, Xu P, Liu J, Wu D, Li X. Long-chain noncoding ribonucleic acids affect the survival and prognosis of patients with esophageal adenocarcinoma through the autophagy pathway: construction of a prognostic model. Anticancer Drugs. 2022;33:e590-e603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 71] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 66. | Lv WL, Hu YY, Li ZN, Zhang W, Pan Q. PAX3 silencing suppresses gastric cancer proliferation and angiogenesis via MET/PI3K signaling. Neoplasma. 2020;67:304-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 67. | Hou GX, Song BB. Gastric cancer patient with c-MET amplification treated with crizotinib after failed multi-line treatment: A case report and literature review. Math Biosci Eng. 2019;16:5923-5930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 68. | Pereira MA, Ramos MFKP, Dias AR, Cardili L, Ribeiro RRE, de Castria TB, Zilberstein B, Nahas SC, Ribeiro U Jr, de Mello ES. RhoA, Claudin 18, and c-MET in Gastric Cancer: Clinicopathological Characteristics and Prognostic Significance in Curative Resected Patients. Med Sci (Basel). 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 69. | Zhang Z, Miao L, Wang S, Zhao Y, Xie Y, Yun H, Ren Z, Wang G, Teng M, Li Y. Study on the expression of c-Met in gastric cancer and its correlation with preoperative serum tumor markers and prognosis. World J Surg Oncol. 2022;20:204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 70. | Peng Z, Zhu Y, Wang Q, Gao J, Li Y, Li Y, Ge S, Shen L. Prognostic significance of MET amplification and expression in gastric cancer: a systematic review with meta-analysis. PLoS One. 2014;9:e84502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 71. | Wang C, Li J, Qu L, Tang X, Song X, Yang F, Chen X, Lin Q, Lin W, Zhou Y, Tu Z, Chen Y, Zhang Z, Lu X. Discovery of D6808, a Highly Selective and Potent Macrocyclic c-Met Inhibitor for Gastric Cancer Harboring MET Gene Alteration Treatment. J Med Chem. 2022;65:15140-15164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 27] [Reference Citation Analysis (0)] |
| 72. | Yang Y, Wang C, Dai C, Liu X, Li W, Huang M, Zhao X, Ji D, Li J, Guo W. Amplification and expression of c-MET correlate with poor prognosis of patients with gastric cancer and upregulate the expression of PDL1. Acta Biochim Biophys Sin (Shanghai). 2021;53:547-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 73. | Zhu C, Xu J, Li M, Zhao G, Cao H. Heterogeneity of c-Met expression in Chinese gastric cancer patients. Hum Pathol. 2015;46:1901-1907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 74. | Koustas E, Karamouzis MV, Sarantis P, Schizas D, Papavassiliou AG. Inhibition of c-MET increases the antitumour activity of PARP inhibitors in gastric cancer models. J Cell Mol Med. 2020;24:10420-10431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 75. | Tong G, Cheng B, Li J, Wu X, Nong Q, He L, Li X, Li L, Wang S. MACC1 regulates PDL1 expression and tumor immunity through the c-Met/AKT/mTOR pathway in gastric cancer cells. Cancer Med. 2019;8:7044-7054. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 76. | Guo T, Yang J, Yao J, Zhang Y, Da M, Duan Y. Expression of MACC1 and c-Met in human gastric cancer and its clinical significance. Cancer Cell Int. 2013;13:121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 77. | Grojean M, Schwarz MA, Schwarz JR, Hassan S, von Holzen U, Zhang C, Schwarz RE, Awasthi N. Targeted dual inhibition of c-Met/VEGFR2 signalling by foretinib improves antitumour effects of nanoparticle paclitaxel in gastric cancer models. J Cell Mol Med. 2021;25:4950-4961. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 78. | Gu ML, Zhou XX, Ren MT, Shi KD, Yu MS, Jiao WR, Wang YM, Zhong WX, Ji F. Blockage of ETS homologous factor inhibits the proliferation and invasion of gastric cancer cells through the c-Met pathway. World J Gastroenterol. 2020;26:7497-7512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 79. | Kim BJ, Kim YJ, Sohn SH, Kim B, Sul HJ, Kim HS, Zang DY. Tivantinib inhibits the VEGF signaling pathway and induces apoptosis in gastric cancer cells with c-MET or VEGFA amplification. Invest New Drugs. 2020;38:1633-1640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 80. | Park CH, Cho SY, Ha JD, Jung H, Kim HR, Lee CO, Jang IY, Chae CH, Lee HK, Choi SU. Novel c-Met inhibitor suppresses the growth of c-Met-addicted gastric cancer cells. BMC Cancer. 2016;16:35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 81. | Zheng Z, Yan D, Chen X, Huang H, Chen K, Li G, Zhou L, Zheng D, Tu L, Dong XD. MicroRNA-206: Effective Inhibition of Gastric Cancer Progression through the c-Met Pathway. PLoS One. 2015;10:e0128751. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 82. | Nevisi F, Yaghmaie M, Pashaiefar H, Alimoghaddam K, Iravani M, Javadi G, Ghavamzadeh A. Correlation of HER2, MDM2, c-MYC, c-MET, and TP53 Copy Number Alterations in Circulating Tumor Cells with Tissue in Gastric Cancer Patients: A Pilot Study. Iran Biomed J. 2020;24:47-53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 83. | Sohn SH, Kim B, Sul HJ, Kim YJ, Kim HS, Kim H, Seo JB, Koh Y, Zang DY. INC280 inhibits Wnt/β-catenin and EMT signaling pathways and its induce apoptosis in diffuse gastric cancer positive for c-MET amplification. BMC Res Notes. 2019;12:125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 84. | Xu Z, Hu P, Fang D, Ni L, Xu J. Electrostatic explanation of D1228V/H/N-induced c-Met resistance and sensitivity to type I and type II kinase inhibitors in targeted gastric cancer therapy. J Mol Model. 2019;25:13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 85. | Wu L, Zhong Y, Yu X, Wu D, Xu P, Lv L, Ruan X, Liu Q, Feng Y, Liu J, Li X. Selective poly adenylation predicts the efficacy of immunotherapy in patients with lung adenocarcinoma by multiple omics research. Anticancer Drugs. 2022;33:943-959. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 67] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 86. | Zhao R, Zhang T, Xi W, Sun X, Zhou L, Guo Y, Zhao C, Bao Y. Human chorionic gonadotropin promotes cell proliferation through the activation of c-Met in gastric cancer cells. Oncol Lett. 2018;16:4271-4278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 87. | Erratum: Exosome-Delivered c-Met siRNA Could Reverse Chemoresistance to Cisplatin in Gastric Cancer [Corrigendum]. Int J Nanomedicine. 2022;17:1003-1004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 88. | Fuse N, Kuboki Y, Kuwata T, Nishina T, Kadowaki S, Shinozaki E, Machida N, Yuki S, Ooki A, Kajiura S, Kimura T, Yamanaka T, Shitara K, Nagatsuma AK, Yoshino T, Ochiai A, Ohtsu A. Prognostic impact of HER2, EGFR, and c-MET status on overall survival of advanced gastric cancer patients. Gastric Cancer. 2016;19:183-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 87] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 89. | Wei X, Juan ZX, Min FX, Nan C, Hua ZX, Qing FZ, Zheng L. Recombinant immunotoxin anti-c-Met/PE38KDEL inhibits proliferation and promotes apoptosis of gastric cancer cells. J Exp Clin Cancer Res. 2011;30:67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 90. | Liu M. Combination treatment with trastuzumab and crizotinib in metastatic gastric cancer harboring Her-2 amplification and c-MET amplification: A case report. Medicine (Baltimore). 2021;100:e27017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 91. | Metzger ML, Behrens HM, Böger C, Haag J, Krüger S, Röcken C. MET in gastric cancer--discarding a 10% cutoff rule. Histopathology. 2016;68:241-253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 92. | Seo S, Ryu MH, Ryoo BY, Park Y, Park YS, Na YS, Lee CW, Lee JK, Kang YK. Clinical significance of MET gene amplification in metastatic or locally advanced gastric cancer treated with first-line fluoropyrimidine and platinum combination chemotherapy. Chin J Cancer Res. 2019;31:620-631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 93. | Yang Y, Wu N, Shen J, Teixido C, Sun X, Lin Z, Qian X, Zou Z, Guan W, Yu L, Rosell R, Liu B, Wei J. MET overexpression and amplification define a distinct molecular subgroup for targeted therapies in gastric cancer. Gastric Cancer. 2016;19:778-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 94. | Ha SY, Lee J, Jang J, Hong JY, Do IG, Park SH, Park JO, Choi MG, Sohn TS, Bae JM, Kim S, Kim M, Kim S, Park CK, Kang WK, Kim KM. HER2-positive gastric cancer with concomitant MET and/or EGFR overexpression: a distinct subset of patients for dual inhibition therapy. Int J Cancer. 2015;136:1629-1635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 95. | Kim HS, Chon HJ, Kim H, Shin SJ, Wacheck V, Gruver AM, Kim JS, Rha SY, Chung HC. MET in gastric cancer with liver metastasis: The relationship between MET amplification and Met overexpression in primary stomach tumors and liver metastasis. J Surg Oncol. 2018;117:1679-1686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 96. | Yashiro M, Nishii T, Hasegawa T, Matsuzaki T, Morisaki T, Fukuoka T, Hirakawa K. A c-Met inhibitor increases the chemosensitivity of cancer stem cells to the irinotecan in gastric carcinoma. Br J Cancer. 2013;109:2619-2628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 97. | Ma JA, Hu C, Li W, Ren J, Zou F, Zhou D, Zou W, Wei Y, Zhou Y. Downregulation of c-Met expression does not enhance the sensitivity of gastric cancer cell line MKN-45 to gefitinib. Mol Med Rep. 2015;11:2269-2275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 98. | Asaoka Y, Tada M, Ikenoue T, Seto M, Imai M, Miyabayashi K, Yamamoto K, Yamamoto S, Kudo Y, Mohri D, Isomura Y, Ijichi H, Tateishi K, Kanai F, Ogawa S, Omata M, Koike K. Gastric cancer cell line Hs746T harbors a splice site mutation of c-Met causing juxtamembrane domain deletion. Biochem Biophys Res Commun. 2010;394:1042-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 99. | Shitara K, Kim TM, Yokota T, Goto M, Satoh T, Ahn JH, Kim HS, Assadourian S, Gomez C, Harnois M, Hamauchi S, Kudo T, Doi T, Bang YJ. Phase I dose-escalation study of the c-Met tyrosine kinase inhibitor SAR125844 in Asian patients with advanced solid tumors, including patients with MET-amplified gastric cancer. Oncotarget. 2017;8:79546-79555. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 100. | Wang XL, Chen XM, Fang JP, Yang CQ. Lentivirus-mediated RNA silencing of c-Met markedly suppresses peritoneal dissemination of gastric cancer in vitro and in vivo. Acta Pharmacol Sin. 2012;33:513-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 101. | Gavine PR, Ren Y, Han L, Lv J, Fan S, Zhang W, Xu W, Liu YJ, Zhang T, Fu H, Yu Y, Wang H, Xu S, Zhou F, Su X, Yin X, Xie L, Wang L, Qing W, Jiao L, Su W, Wang QM. Volitinib, a potent and highly selective c-Met inhibitor, effectively blocks c-Met signaling and growth in c-MET amplified gastric cancer patient-derived tumor xenograft models. Mol Oncol. 2015;9:323-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 103] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 102. | Sunakawa Y, Wakatsuki T, Yang D, Zhang W, Ning Y, Stintzing S, Stremitzer S, Yamauchi S, Sebio A, El-khoueiry R, Iqbal S, Barzi A, Gerger A, Stotz M, Azuma M, Watanabe M, Koizumi W, Lenz HJ. Prognostic impact of the c-MET polymorphism on the clinical outcome in locoregional gastric cancer patients. Pharmacogenet Genomics. 2014;24:588-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 103. | Wang H, Lu J, Tang J, Chen S, He K, Jiang X, Jiang W, Teng L. Establishment of patient-derived gastric cancer xenografts: a useful tool for preclinical evaluation of targeted therapies involving alterations in HER-2, MET and FGFR2 signaling pathways. BMC Cancer. 2017;17:191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 104. | Drebber U, Baldus SE, Nolden B, Grass G, Bollschweiler E, Dienes HP, Hölscher AH, Mönig SP. The overexpression of c-met as a prognostic indicator for gastric carcinoma compared to p53 and p21 nuclear accumulation. Oncol Rep. 2008;19:1477-1483. [PubMed] |
| 105. | Saisana M, Griffin SM, May FE. Importance of the type I insulin-like growth factor receptor in HER2, FGFR2 and MET-unamplified gastric cancer with and without Ras pathway activation. Oncotarget. 2016;7:54445-54462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |