Published online Jul 15, 2024. doi: 10.4251/wjgo.v16.i7.3284
Revised: May 14, 2024
Accepted: June 4, 2024
Published online: July 15, 2024
Processing time: 111 Days and 8.6 Hours
Colon adenocarcinoma (COAD) is a malignant tumor of the digestive system. The mechanisms underlying COAD development and progression are still largely unknown.
To identify the role of canopy FGF signaling regulator 3 (CNPY3) in the development and progression of COAD by using bioinformatic tools and functional experiments.
Bioinformatic data were downloaded from public databases. The associations of clinicopathological features, survival, and immune function with the expression of CNPY3 were analyzed. Gene Ontology and Kyoto Encyclopedia of Genes and Genomes analyses and Gene Set Enrichment Analysis were used to explore the related pathways. Then, quantitative real-time PCR and immunohistochemistry were used for validation of CNPY3 expression in clinical samples and tumor cell lines. Cell lines with CNPY3 knockdown were constructed to further analyze gene functions. The functional experiments included proliferation, invasion, migration and apoptosis assays.
In both the TCGA cohort and the merged dataset, elevated CNPY3 expression was observed in tumor tissues. High CNPY3 expression correlated with adverse survival and compromised immune functions. Functional enrichment analysis suggested that the pro-oncogenic properties of CNPY3 might be linked to the PI3K-AKT signaling pathway. CNPY3 expression was validated at both the RNA and protein levels. Functional assays indicated that cell proliferation, invasion, and migration were inhibited and cell apoptosis was promoted after CNPY3 knockdown. Additionally, Western blot results revealed the downregulation of key proteins in the PI3K/AKT pathway following CNPY3 knockdown. PI3K/AKT pathway activator reversed the decrease in proliferation, invasion, and migration and the increase in apoptosis. Notably, CNPY3 knockdown still affected the cells when the pathway was inhibited.
This study showed that CNPY3 is upregulated in COAD and might regulate COAD development and progression by the PI3K/AKT pathway. Thus, CNPY3 might be a promising therapeutic target.
Core Tip: First, this is the first study reporting that canopy FGF signaling regulator 3 (CNPY3) was up-regulated in colon adenocarcinoma and might regulate tumor development and progression. Second, CNPY3 expression was validated in clinical samples and tumor cell lines. Third, functional experiments indicated that cell proliferation, invasion, and migration were inhibited and cell apoptosis was promoted after CNPY3 knockdown.
- Citation: Gao XC, Zhou BH, Ji ZX, Li Q, Liu HN. Canopy FGF signaling regulator 3 affects prognosis, immune infiltration, and PI3K/AKT pathway in colon adenocarcinoma. World J Gastrointest Oncol 2024; 16(7): 3284-3298
- URL: https://www.wjgnet.com/1948-5204/full/v16/i7/3284.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i7.3284
Colon cancer is a malignant tumor affecting the digestive system[1,2]. In 2020, a total of 1.9 million cases of colon cancer and 0.9 million colon cancer-related deaths were reported worldwide[3]. This statistic highlights the pervasive nature of the disease and its major impact on global health. Furthermore, the prevalence and fatality rates of colon cancer continue to increase, presenting a major public health challenge[4]. Considerable progress has been made in increasing the survival rates, particularly in the field of immunotherapy. Immunotherapy is a promising treatment method that harnesses the body's immune functions and immune responses to effectively combat and eliminate cancer cells[5]. For example, the success of pembrolizumab and other programmed cell death 1 (PD-1) inhibitors in treating colon cancer underscores the importance of immunotherapy as a valuable addition to the treatment armamentarium for this disease[6]. However, the 5-year survival of this disease is low[7], and immune therapy does not work for some patients because of resistance to therapy and treatment intolerance[8]. Thus, exploring new treatment targets is necessary to expand colon cancer therapeutics.
The development of bioinformatic tools has shed new light on colon cancer research. Ding et al[9] reported that metabolism-related genes could augment the immunotherapeutic response and that CYP19A1 could promote vascular abnormalities and inhibit CD8+ T-cell functions. The combined use of CYP19A1 inhibition and PD-1 blockade exhibits promising therapeutic potential. A study conducted by Bao et al[10] revealed an important finding regarding YTHDF1 and its association with immune-related biomarkers in colorectal cancer (CRC). Specifically, the researchers reported that targeting YTHDF1 promotes anti-PD-1 efficacy and augments antitumor immunity. Sorrentino et al[11] analyzed the Cancer Genome Atlas (TCGA)-CRC data and reported the immune cell context in CRCs, which provided insight into overcoming immunotherapy resistance. However, our understanding of tumor immunity, biomarkers, and the underlying mechanisms is still limited[12,13]. Thus, further studies are still needed to identify more precise biomarkers that can increase the treatment efficacy of colon cancer treatment.
This study focused on colon adenocarcinoma (COAD), which is the most prevalent and malignant form of colon cancer. Through bioinformatic analysis, we found abnormal expression of canopy FGF signaling regulator 3 (CNPY3) in COAD. The function of CNPY3 has been implicated in various cellular processes, including tumor development. Notably, this protein is localized both within the endoplasmic reticulum and extracellular space, which underscores its versatility in modulating diverse signaling pathways[14]. The pro-oncogenic nature of this protein has been noted in hepatocellular carcinoma and gastric cancer[15]. Although the tumor-promoting role of CNPY3 has been implicated in various cancers, its specific involvement in COAD remains unknown. Hence, this study aimed to elucidate the function of CNPY3 in COAD using bioinformatic tools and functional experiments, in order to provide a reference for clinical treatment.
Bioinformatic analyses were performed based on the following databases: The Genotype Tissue Expression (GTEx), the Gene Expression Omnibus (GEO), and TCGA databases. The following datasets were used: GTEx-colon cohort (GTEx database, 308 normal samples), GSE17536 cohort (GEO database, 177 tumor samples), GSE17537 cohort (GEO database, 55 tumor samples), and TCGA-COAD cohort (TCGA database, 41 normal and 471 tumor samples). All datasets were annotated by gene symbols.
The expression data of CNPY3 among various cancers were acquired by accessing the TIMER database[16]. To expand the number of normal samples, the sequencing data from TCGA and GTEx cohorts were matched and batch-corrected. Then, CNPY3 gene expression was detected in two datasets: The merged dataset (349 normal and 471 tumor samples) and the TCGA-COAD dataset. Furthermore, the connections between clinical features (age, sex, and tumor-node-metastasis stage) and CNPY3 expression were analyzed using the nonparametric test.
Probabilities of survival, including overall survival (OS) and disease-specific survival (DSS), were evaluated. Receiver operating characteristic (ROC) curve analysis was performed to determine the prognostic value of CNPY3 expression. Prediction accuracy was determined by the area under curve (AUC). Possible prognostic factors were identified using univariate prognostic analysis. Then, multivariate analysis was performed to adjust confounding variables and identify independent prognostic factors.
Immune microenvironment-related indices were calculated with the help of the ESTIMATE algorithm[17]. The indices include stromal, immune, and ESTIMATE scores. The abundance of immune cells was calculated using the CIBERSORT algorithm[18] and the differences between groups were calculated. Correlations between CNPY3 and immune cells were calculated by Pearson correlation analysis and visualized in the Lollipop plot. Immune therapy-related factors, including 65 immune checkpoint-related factors, 43 immunostimulant-related factors, and 22 immunosuppressant-related factors, were acquired from previous studies. The relationship between CNPY3 and immune therapy-related factors was tested by Spearman's correlation analysis. Immunotherapy scores were used to assess the likelihood of a favorable therapeutic response to immunotherapy interventions. The scores were obtained from the Cancer Immunome Atlas database.
The genes co-expressed with CNPY3 were identified with the "limma" package. Functional annotation was performed based on the Gene Ontology (GO) terms. Pathway enrichment analysis was conducted using terms from the Kyoto Encyclopedia of Genes and Genomes (KEGG) and Reactome (obtained from Molecular Signatures Database)[19]. Gene Set Enrichment Analysis (GSEA) was carried out to identify pathways positively and negatively associated with CNPY3 expression.
The clinical sample collection process was performed after the approval of the local Ethics Committee. Eight normal people and thirty-two COAD patients were enrolled. All patients included in this study did not receive radiotherapy or chemotherapy before resection. The samples for quantitative real-time PCR (qRT-PCR) were immediately frozen at -80 °C, and the samples for immunohistochemistry were stored in 4% paraformaldehyde.
TRIzol was used for isolating RNA from samples. Briefly, the TRIzol method includes homogenization of the sample in TRIzol reagent to disrupt cells and release RNA, followed by chloroform extraction to separate RNA from DNA and proteins. On the 7900HT Sequence Detection System (ABI), qRT-PCR was performed. The procedure involved a denaturation program (95 °C, 10 min) and 45 amplification and quantification cycles, with each cycle lasting 15 s at 95 °C and 34 s at 60 °C. Primer sequences include: CNPY3: Forward, CGG AGC TGA GGA GAA CGA C and reverse, ATA GCC CGT GCC AAT CAC C; GAPDH: Forward, GGA CCT GAC CTG CCG TCT AG and reverse, GTA GCC CAG GAT GCC CTT GA. The CNPY3 expression level was determined using the 2-ΔΔCT method.
Immunohistochemistry was performed on a tissue microarray according to standard protocols. Antibodies used include rabbit anti-CNPY3 (Proteintech, 1:200) and HRP-conjugated rabbit secondary antibodies. The tissue section was dewaxed, repaired, blocked, and subsequently incubated with the primary antibody at 4 °C. The sections were then exposed to HRP-conjugated rabbit secondary antibody for 1 h and subsequently incubated with the DAB substrate solution to obtain brown immunoreactive signals. The immunohistochemistry results were evaluated by two pathologists independently and the immunohistochemical score (IHS) was calculated for the evaluation of staining intensity and quantity.
The following cell lines were used: Human colonic epithelial cell lines (HCoEpiC and NCM460) and colon cancer cell lines (Caco-2, HCT-116, HT-29, SW48, SW480, and SW620). Cell lines were sourced from the American Type Culture Collection or the Chinese Academy of Science Cell Bank. All cells were cultured in Dulbecco's modified Eagle's medium with 10% fetal calf serum. CNPY3 knockdown vectors were constructed, and lentivirus was packaged by GenePharma company (Shanghai, China). The cells were grouped into CNPY3-Con and CNPY3-knockdown groups based on treatment. Cell transfections were performed as described by the manufacturer[20]. Wherever mentioned, the cells were treated with 20 µmol/L 740Y-P (PI3K/AKT pathway activator) or 20 µmol/L LY294002 (PI3K/AKT pathway inhibitor) for 24 h.
The proliferation abilities of HT-29 and SW620 were evaluated by CCK-8 assay. A 96-well plate was used for cell culture and 2000 cells were seeded into each well. Following cell seeding, the plate was incubated under standard cell culture conditions. The cells were allowed to adhere to the culture plate and proliferate over the specified time intervals. The CCK-8 solution was added at predetermined time points (24, 48, 72, and 96 h post-incubation) and the absorbance values were measured.
Transwell device, which consists of two chambers separated by an 8 mm positron emission tomography semi-permeable membrane, was used. For the migration assay, medium without serum was added to the upper chamber, while the lower chamber was supplemented with medium with 20% serum. The cells were allowed to attach to the membrane and migrate to the lower chamber for 48 h. After the incubation period, cells migrating to the lower chamber were fixed (methyl) and stained (crystal violet). The Matrigel-coated membrane was used in the invasion assay to mimic the natural environment that cells encounter in tissues.
Apoptosis analysis was conducted by flow cytometry. The cells were centrifuged, rinsed with phosphate buffered saline for 3 times, and labeled with the FITC-labeled Annexin V and propidium iodide solution. The FACSCalibur flow cytometry system from BD Biosciences was used to identify apoptotic cells.
Antibodies used are as follows: Anti-CNPY3 (Proteintech, 1:2000), anti-PI3K (Affinity Biosciences, 1:1000), anti-p-PI3K (Affinity Biosciences, 1:1000), anti-AKT (Cell Signaling Technology, 1:1000), anti-p-AKT (Cell Signaling Technology, 1:000), anti-GAPDH (Cell Signaling Technology, 1:2000), and HRP-conjugated secondary antibodies (Cell Signaling Technology, 1:2000). The experiment was performed following routine experimental steps[21]. Briefly, proteins from tissue samples and cells were released using a lysis buffer. After centrifugation, the BCA method (KeyGEN BioTECH) was used to measure the protein concentration. Equal proteins were separated using 10% SDS-PAGE and a nitrocellulose membrane was used for protein transfer. Then, the membrane was blocked with milk and treated with primary and secondary antibodies. The immunoblots were developed using New Super ECL.
R (4.2.1) and SPSS (26.0) were used for all bioinformatic and statistical analyses. The Student’s t-test was used to evaluate differences in continuous data, and the χ2 test was used to test differences in count data. P-values less than 0.05 were considered significant.
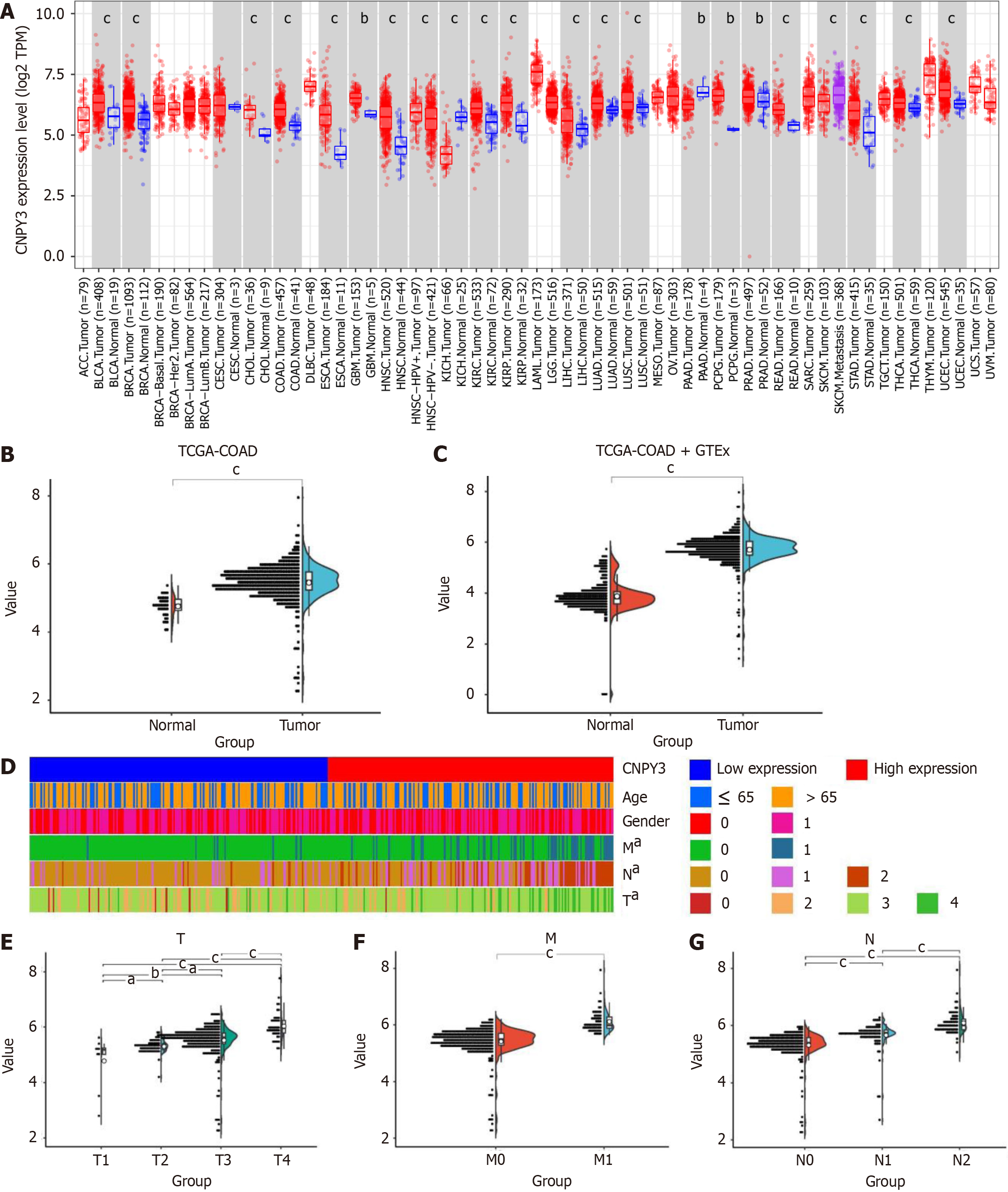
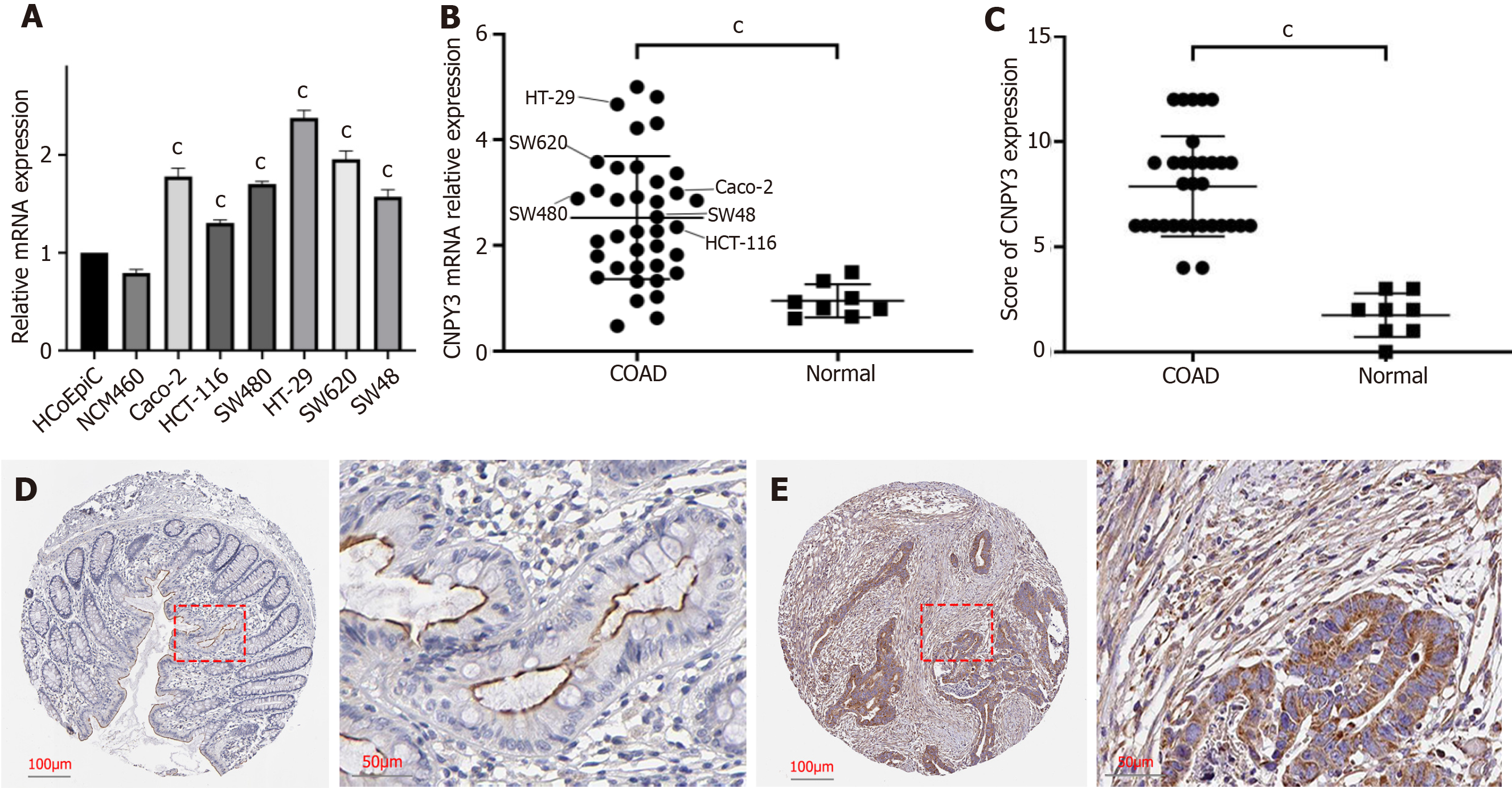
CNPY3 exhibited significant up-regulation in several digestive system cancers, including COAD, cholangiocarcinoma, esophageal carcinoma, liver hepatocellular carcinoma, rectum adenocarcinoma, and stomach adenocarcinoma (Figure 1A). The expression of CNPY3 in COAD is shown in Figure 1B and C. CNPY3 was highly expressed in COAD based on the TCGA and merged datasets. A heatmap of clinical relevance is shown in Figure 1D. There was no difference in CNPY3 expression regarding age (Supplementary Figure 1A) and gender (Supplementary Figure 1B). Furthermore, higher CNPY3 expression was correlated with increased T stage (Figure 1E), increased M stage (Figure 1F), and increased N stage (Figure 1G).
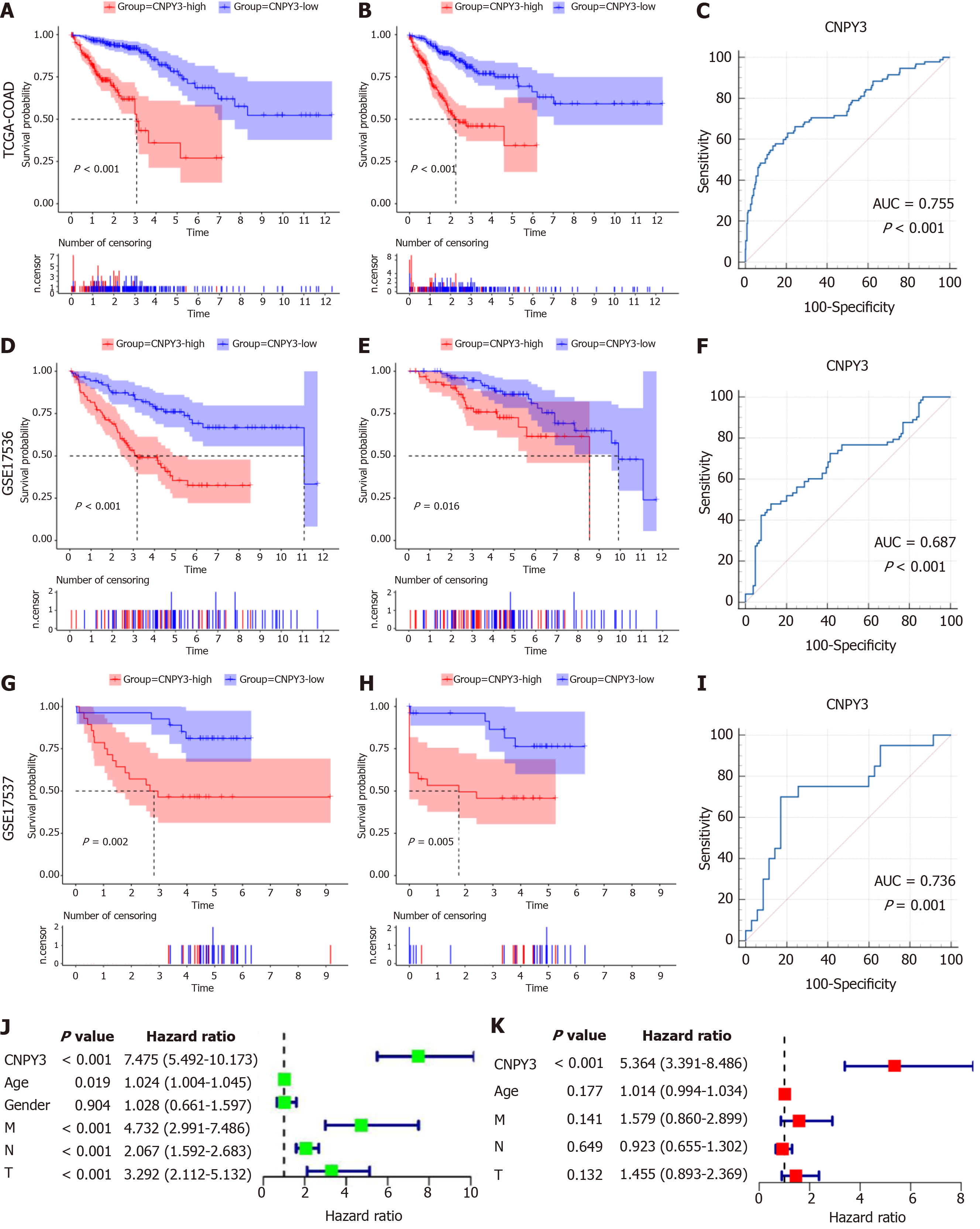
Kaplan-Meier curve analysis revealed that elevated CNPY3 expression correlated with decreased OS (Figure 2A) and DSS (Figure 2B). The AUC of CNPY3 expression for predicting the prognosis was 0.755 in TCGA-COAD (Figure 2C). Validation using the GSE17536 dataset yielded similar results (Figure 2D and E); the AUC was 0.687 (Figure 2F). Similar results were also acquired using GSE17537 (Figure 2G and H), and the AUC was 0.736 (Figure 2I), indicating the good predictive ability of CNPY3 expression for prognosis of COAD. Univariate regression analysis (Figure 2J) identified CNPY3, age, M stage, N stage, and T stage as significant prognostic factors for COAD and they were subsequently included in the multivariate regression analysis, which demonstrated that CNPY3 (P < 0.05) was an independent prognostic factor in COAD (Figure 2K).
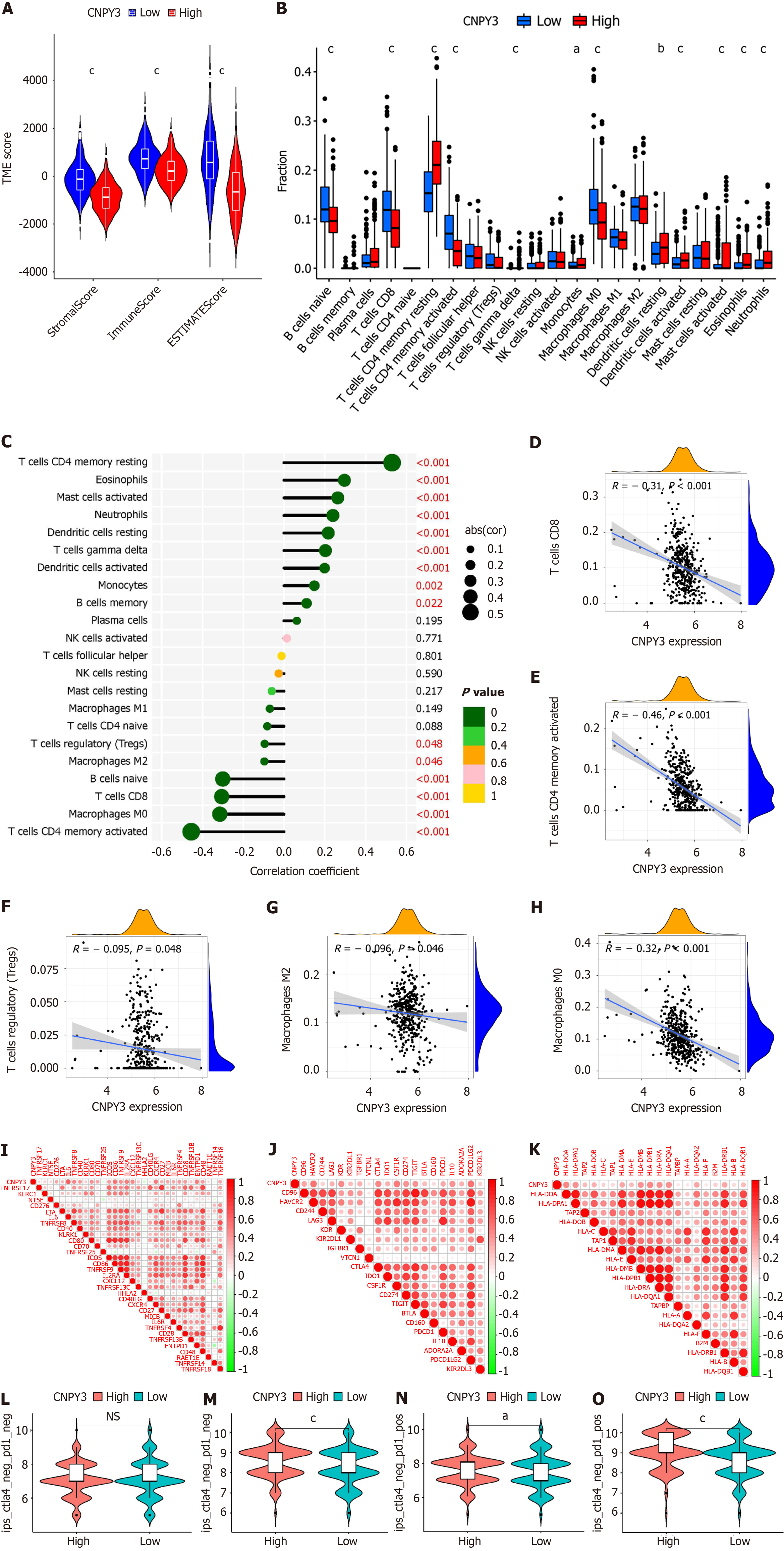
Immune microenvironment-related indexes were calculated using the EXTIMATE algorithm. Figure 3A indicates that the groups with high CNPY3 expression tended to have lower stomal, immune, and ESTIMATE scores. The difference in immune cells is shown in Figure 3B. Increased expression of CNPY3 decreased the proportion of naive B cells, activated CD4+ T cells, CD8+ T cells, and M0 macrophages. Figure 3C shows the results of the correlation analysis. Specifically, several immune therapy-related cell types were negatively related to CNPY3 expression, including CD8+ T cells (Figure 3D), CD4+ T cells (Figure 3E), regulatory T cells (Figure 3F), M2 macrophages (Figure 3G), and M0 macrophages (Figure 3H).
Next, the correlations between CNPY3 expression and immune checkpoints were analyzed. Within the TCGA-COAD cohort, CNPY3 expression was significantly correlated with 32 out of 43 immunostimulatory factors (Figure 3I), 20 out of 22 immunoinhibitors (Figure 3J), and 20 out of 21 MHC molecules (Figure 3K). Notably, CNPY3 was positively correlated with PD-1, CTLA4, and CD274, which are vital factors influencing immunotherapy efficacy. Then, the immunotherapy scores were compared. Patients in the high CNPY3 expression group might benefit more from immunotherapy when either programmed death ligand 1 (PD-L1) or CTLA4 was positively expressed (Figure 3L-O).
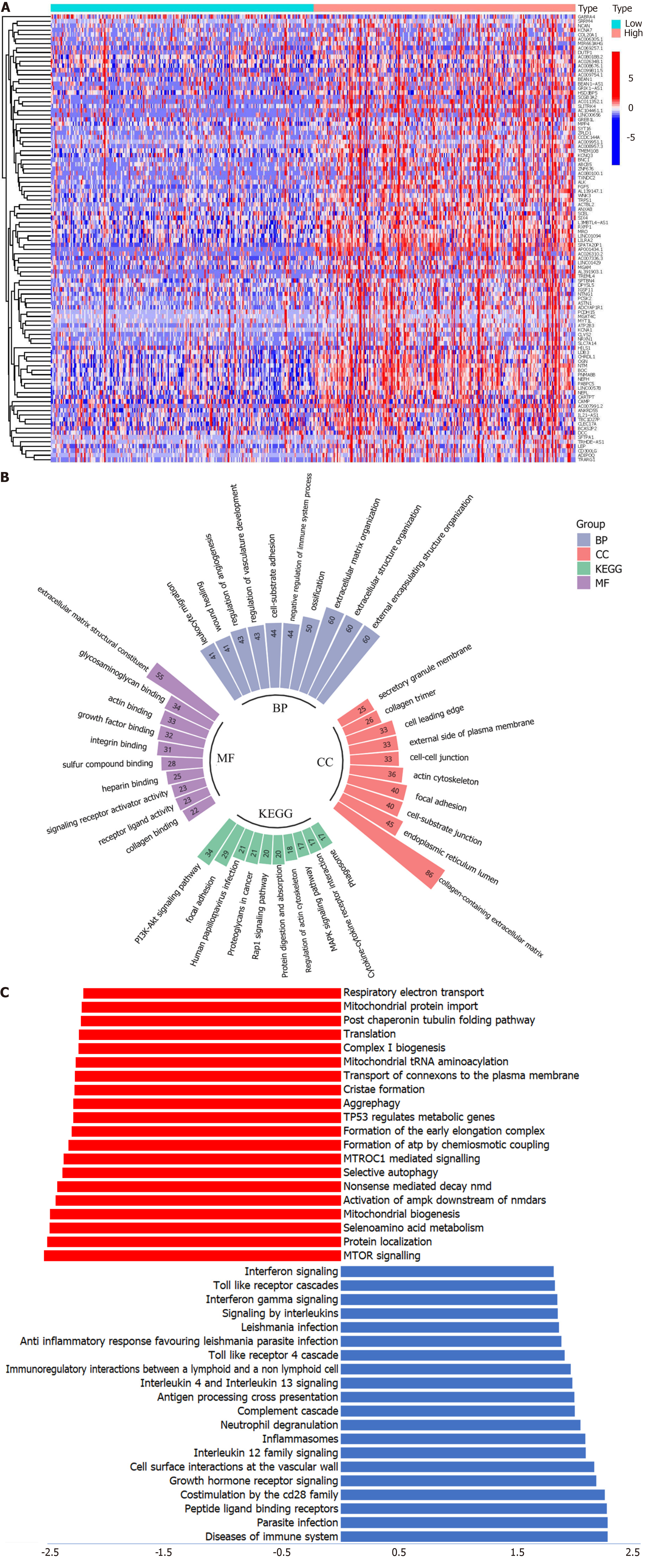
Based on the threshold (log |fold change| > 1 and P value < 0.05), 464 DEGs were identified. The top 50 DEGs are shown in Figure 4A. GO analysis (Figure 4B) showed that the top 3 biological process terms are external encapsulating structure, external structure, and extracellular matrix organization. The top 3 molecular function terms are extracellular matrix structural constituents, glycosaminoglycan binding, and actin binding. The top 3 cellular component terms are collagen-containing extracellular matrix, endoplasmic reticulum lumen, and cell-substrate junction. In the KEGG pathway analysis, the PI3K-AKT signaling pathway was the most enriched. Using the GSEA method, CNPY3 was found to be related to multiple cancer-related pathways, such as aggrephagy, TP53 regulating metabolic genes, and the mTOR signaling pathway (Figure 4C).
qRT-PCR was performed using colon cancer cell lines and normal tissue samples. As illustrated in Figure 5A, compared with that in normal cell lines, CNPY3 was more highly expressed in six cancer cell lines. CNPY3 was also more highly expressed in the tumor samples (Figure 5B). It is worth noticing that compared with normal tissues, six cancer cell lines expressed higher levels of CNPY3. However, no significant differences were found among the cancer cells and tumor samples. Then, immunohistochemistry was performed to evaluate the protein level, and the IHS was calculated. As illustrated in Figure 5C, the IHS of the COAD group (mean: 7.87 ± 2.38) was greater than that of the normal control (mean: 1.75 ± 1.03). Representative images of immunohistochemical staining are presented in Figure 5D and E. These findings collectively suggest that CNPY3 is highly expressed in COAD samples and cancer cell lines.
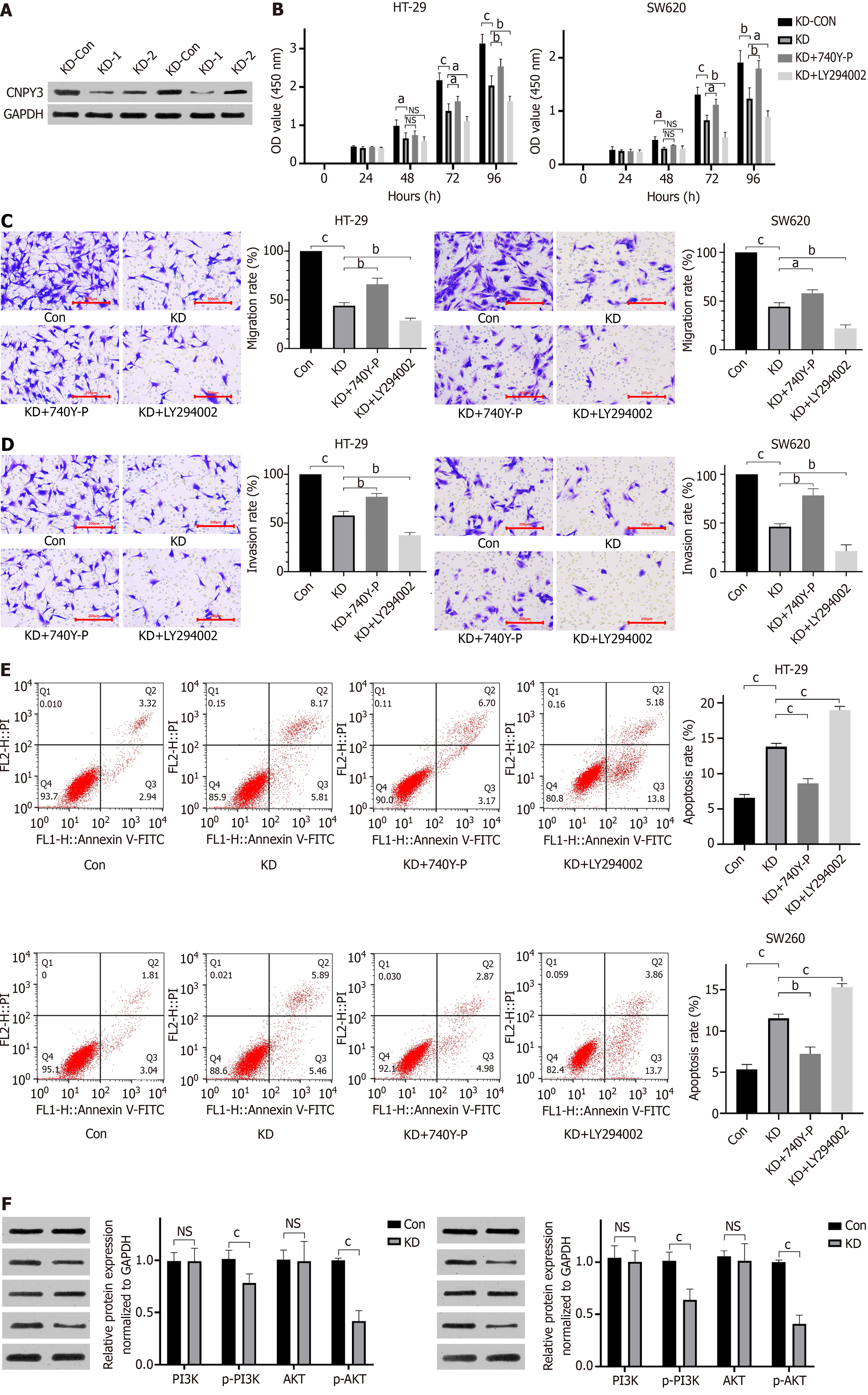
HT-29 and SW620 cell lines with CNPY3 knockdown were successfully established, as confirmed by PCR and Western blot experiments. Among the shRNA groups, shRNA1 exhibited superior silencing efficacy. Thus, it was selected for subsequent experiments (Figure 6A). CNPY3 knockdown hindered cell proliferation 24 h post-incubation (Figure 6B). Transwell assays demonstrated that CNPY3 knockdown suppressed HT-29 and SW620 cell migration (Figure 6C) and invasion (Figure 6D). In addition, flow cytometry revealed greater levels of apoptosis in CNPY3-KD cells than in control cells (Figure 6E).
As indicated by the bioinformatics analysis, CNPY3 might be correlated with the PI3K/AKT pathway. Thus, the changes of key proteins of this pathway were tested. It was found that downregulation of CNPY3 reduced the p-PI3K and p-AKT protein levels. However, the total PI3K and AKT levels were not significantly affected (Figure 6F). To further validate this finding, the pathway activator (740Y-P) and inhibitor (LY294002) were used to treat the CNPY3-KD cell line. The functional experiment was performed as described above. The results indicated that the pathway activator reversed the decreased cell proliferation (Figure 6A), migration (Figure 6C), and invasion (Figure 6D) and increased apoptosis (Figure 6E). Notably, CNPY3 knockdown still affected the cells when the pathway was inhibited. These results further prove that CNPY3 might regulate colon cancer progression through the PI3K/AKT pathway.
In this study, elevated CNPY3 expression was observed in COAD tissues. As indicated by the bioinformatic analysis, CNPY3 was correlated with tumor-node-metastasis stage, survival rate, tumor immune functions, and immunotherapeutic efficacy. The CNPY3’s pro-oncogenic nature appears to be linked to the PI3K/AKT signaling pathway, aggrephagy, and the mTOR signaling pathway. Furthermore, COAD tissues and cancer cell lines were used for CNPY3 expression validation. Functional experiments indicated that inhibition of CNPY3 inhibited COAD cell proliferation, invasion, and migration while inducing apoptosis-like cell behavior. Moreover, Western blot analysis revealed reduced levels of key proteins of the PI3K/AKT pathway. The pathway activator reversed the decreases in proliferation, invasion, and migration and the increase in apoptosis. Notably, CNPY3 knockdown still affected the cells when the PI3K/AKT pathway was inhibited. The findings of this study suggest that CNPY3 may function as a regulator of COAD development.
CNPY3 coordinates Toll-like receptor trafficking[22]. This protein is involved in endoplasmic reticulum functions via a signal peptide and a putative ER retention sequence[23,24]. Thus, CNPY3 is closely linked to immune responses, and its abnormal expression is associated with various diseases[25]. For instance, Zhou et al[15] reported that SLIT- and NTRK-like family member 4 could enhance the TrkB-related signaling pathway through interaction with CNPY3. In addition, CNPY3 could contribute to the formation of premetastatic niches, which could facilitate the cancer cell proliferation and metastasis. Liu et al[14] analyzed the internal reference of hepatocellular carcinoma cell lines and reported that CNPY3 was the stable reference gene of the cell line MHCC-97L. Zhang et al[26] identified CNPY3 as an important cellular target for the induction of pyroptosis, which may have therapeutic implications in prostate cancer. In addition to tumors, CNPY3 was also the hub gene of Alzheimer’s disease[27] and end-stage renal disease[28].
The immune microenvironment is crucial for COAD development and progression[29,30]. Ruan et al[8] noted that immunosurveillance gradually decreased during malignant transformation. This phase was characterized by decreased infiltration of cytotoxic T lymphocytes, Th1 CD4+ T lymphocytes, and natural killer cells and increased infiltration of immunosuppressive regulatory T cells[31]. Zhou et al[32] found that immune cell infiltration was an indicator for COAD prognosis. In this study, CNPY3 was found to be correlated with the proportion of immune cells and might regulate immune cell infiltration. Notably, CNPY3 expression was negatively correlated with the proportion of several types of T cells, especially CD8+ T cells. CD8+ T cells are capable of selectively detecting and eliminating cancer cells, and the exhaustion of CD8+ T cells has been found in multiple cancers[33]. In addition, tumor cells gradually become poor targets for immune attack due to the loss of tumor antigens and the increased production of immunosuppressive-related molecules, such as PD-L1, PD-L2, and CTLA4[34,35]. In this study, we found that CNPY3 was correlated with several immune regulators. The differences in immunotherapy scores also revealed that targeting CNPY3 might increase immunotherapeutic efficacy[36].
The functional enrichment and Western blot analyses revealed that CNPY3 might be related to the PI3K-AKT signaling pathway. The relationship between the PI3K/AKT pathway and COAD have been well illustrated in many studies. Narayanankutty[37] reported that PI3K/AKT/mTOR signaling plays an important role in COAD and concluded that inhibiting the pathway was effective in COAD treatment. Song et al[38] noted that TIMP1 was overexpressed in COAD and that knockdown of TIMP1 could reverse COAD tumorigenesis and metastasis through the PI3K/AKT pathway. Similar results were reported by Li et al[39], Lin et al[40], and Liu et al[41]. In our study, we utilized bioinformatic tools to explore the correlation of CNPY3 with the PI3K/AKT pathway, and these findings were subsequently validated through Western blot analysis and functional experiments. The connection was further validated by the finding that a PI3K/AKT pathway activator rescued the functional loss caused by CNPY3 knockdown. Another interesting finding was that CNPY3 knockdown still affected the cells when the pathway was inhibited. The results could be explained as follows. First, knockdown of CNPY3 affected the pathway to some extent, and the pathway was not fully blocked. Thus, treatment methods targeting CNPY3 might achieve better therapeutic results when combined with PI3K/AKT pathway inhibitors. Second, CNPY3 can affect tumorigenesis through other mechanisms and pathways. The underlying mechanisms are worthy of further exploration. Taken together, these findings support our conclusion regarding the association between CNPY3 knockdown and the suppression of PI3K/AKT pathway, underscoring the potential importance of CNPY3 as a player in COAD progression and offering insights into therapeutic avenues targeting this pathway.
This study has several limitations. First, the study was based on bioinformatic tools and functional experiments, and more high-quality studies are still needed for further validation of our findings. Notably, due to the limitations of bioinformatics data, a few cofounders were included in the univariate and multivariate analyses. Second, the underlying mechanism needs to be further explored by basic experiments. While this study has limitations, it is the first study reporting the prognostic value of CNPY3 and revealing its cancer-promoting role in COAD.
This study found that CNPY3 was a prognostic factor for COAD patients and higher CNPY3 was associated with poorer immune functions. The tumor-promoting role of CNPY3 might be correlated with the PI3K-AKT signaling pathway. Moreover, functional experiments revealed that knockdown of CNPY3 promoted HT-29 and SW620 apoptosis while suppressing cell invasion, migration, and proliferation. Finally, the expression of CNPY3 was validated in cancer cell lines and tumor samples, indicating that the findings of this study are reliable and worthy of further investigation.
| 1. | Fidelle M, Yonekura S, Picard M, Cogdill A, Hollebecque A, Roberti MP, Zitvogel L. Resolving the Paradox of Colon Cancer Through the Integration of Genetics, Immunology, and the Microbiota. Front Immunol. 2020;11:600886. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 2. | Verkuijl SJ, Jonker JE, Trzpis M, Burgerhof JGM, Broens PMA, Furnée EJB. Functional outcomes of surgery for colon cancer: A systematic review and meta-analysis. Eur J Surg Oncol. 2021;47:960-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 3. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64133] [Article Influence: 16033.3] [Reference Citation Analysis (174)] |
| 4. | Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Farkas L, Garrido-Laguna I, Grem JL, Gunn A, Hecht JR, Hoffe S, Hubbard J, Hunt S, Johung KL, Kirilcuk N, Krishnamurthi S, Messersmith WA, Meyerhardt J, Miller ED, Mulcahy MF, Nurkin S, Overman MJ, Parikh A, Patel H, Pedersen K, Saltz L, Schneider C, Shibata D, Skibber JM, Sofocleous CT, Stoffel EM, Stotsky-Himelfarb E, Willett CG, Gregory KM, Gurski LA. Colon Cancer, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19:329-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1054] [Cited by in RCA: 944] [Article Influence: 236.0] [Reference Citation Analysis (16)] |
| 5. | Almquist DR, Ahn DH, Bekaii-Saab TS. The Role of Immune Checkpoint Inhibitors in Colorectal Adenocarcinoma. BioDrugs. 2020;34:349-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 6. | Ganesh K, Stadler ZK, Cercek A, Mendelsohn RB, Shia J, Segal NH, Diaz LA Jr. Immunotherapy in colorectal cancer: rationale, challenges and potential. Nat Rev Gastroenterol Hepatol. 2019;16:361-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1114] [Cited by in RCA: 1214] [Article Influence: 202.3] [Reference Citation Analysis (0)] |
| 7. | Angell HK, Bruni D, Barrett JC, Herbst R, Galon J. The Immunoscore: Colon Cancer and Beyond. Clin Cancer Res. 2020;26:332-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 271] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 8. | Ruan H, Leibowitz BJ, Zhang L, Yu J. Immunogenic cell death in colon cancer prevention and therapy. Mol Carcinog. 2020;59:783-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 79] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 9. | Ding X, Liu H, Yuan Y, Zhong Q, Zhong X. Roles of GFPT2 Expression Levels on the Prognosis and Tumor Microenvironment of Colon Cancer. Front Oncol. 2022;12:811559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 10. | Bao Y, Zhai J, Chen H, Wong CC, Liang C, Ding Y, Huang D, Gou H, Chen D, Pan Y, Kang W, To KF, Yu J. Targeting m(6)A reader YTHDF1 augments antitumour immunity and boosts anti-PD-1 efficacy in colorectal cancer. Gut. 2023;72:1497-1509. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 124] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 11. | Sorrentino C, D'Antonio L, Fieni C, Ciummo SL, Di Carlo E. Colorectal Cancer-Associated Immune Exhaustion Involves T and B Lymphocytes and Conventional NK Cells and Correlates With a Shorter Overall Survival. Front Immunol. 2021;12:778329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 50] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 12. | Li X, Wen D, Li X, Yao C, Chong W, Chen H. Identification of an Immune Signature Predicting Prognosis Risk and Lymphocyte Infiltration in Colon Cancer. Front Immunol. 2020;11:1678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 13. | Tesniere A, Schlemmer F, Boige V, Kepp O, Martins I, Ghiringhelli F, Aymeric L, Michaud M, Apetoh L, Barault L, Mendiboure J, Pignon JP, Jooste V, van Endert P, Ducreux M, Zitvogel L, Piard F, Kroemer G. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene. 2010;29:482-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 692] [Cited by in RCA: 915] [Article Influence: 57.2] [Reference Citation Analysis (0)] |
| 14. | Liu Y, Qin Z, Cai L, Zou L, Zhao J, Zhong F. Selection of internal references for qRT-PCR assays of human hepatocellular carcinoma cell lines. Biosci Rep. 2017;37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Zhou YQ, Bao TS, Xie JX, Yao LL, Yu ST, Li Q, Huang PQ, Zhou WZ, Wang YY, Chen SY, Wang XQ, Zhang XL, Jiang SH, Yi SQ, Zhang ZG, Ma MZ, Hu LP, Xu J, Li J. The SLITRK4-CNPY3 axis promotes liver metastasis of gastric cancer by enhancing the endocytosis and recycling of TrkB in tumour cells. Cell Oncol (Dordr). 2023;46:1049-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 16. | Li T, Fan J, Wang B, Traugh N, Chen Q, Liu JS, Li B, Liu XS. TIMER: A Web Server for Comprehensive Analysis of Tumor-Infiltrating Immune Cells. Cancer Res. 2017;77:e108-e110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2728] [Cited by in RCA: 4047] [Article Influence: 505.9] [Reference Citation Analysis (0)] |
| 17. | Wang X, Zhang Y, Song N, Li K, Lei S, Wang J, Wang Z, Zhang W. CILP2: A prognostic biomarker associated with immune infiltration in colorectal cancer. Heliyon. 2023;9:e15535. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Chen B, Khodadoust MS, Liu CL, Newman AM, Alizadeh AA. Profiling Tumor Infiltrating Immune Cells with CIBERSORT. Methods Mol Biol. 2018;1711:243-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 993] [Cited by in RCA: 2427] [Article Influence: 346.7] [Reference Citation Analysis (0)] |
| 19. | Croft D, Mundo AF, Haw R, Milacic M, Weiser J, Wu G, Caudy M, Garapati P, Gillespie M, Kamdar MR, Jassal B, Jupe S, Matthews L, May B, Palatnik S, Rothfels K, Shamovsky V, Song H, Williams M, Birney E, Hermjakob H, Stein L, D'Eustachio P. The Reactome pathway knowledgebase. Nucleic Acids Res. 2014;42:D472-D477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1213] [Cited by in RCA: 1171] [Article Influence: 97.6] [Reference Citation Analysis (0)] |
| 20. | Kang T, Wei Y, Honaker Y, Yamaguchi H, Appella E, Hung MC, Piwnica-Worms H. GSK-3 beta targets Cdc25A for ubiquitin-mediated proteolysis, and GSK-3 beta inactivation correlates with Cdc25A overproduction in human cancers. Cancer Cell. 2008;13:36-47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 138] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 21. | Troschel FM, Palenta H, Borrmann K, Heshe K, Hua SH, Yip GW, Kiesel L, Eich HT, Götte M, Greve B. Knockdown of the prognostic cancer stem cell marker Musashi-1 decreases radio-resistance while enhancing apoptosis in hormone receptor-positive breast cancer cells via p21(WAF1/CIP1). J Cancer Res Clin Oncol. 2021;147:3299-3312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 22. | Tabeta K, Hoebe K, Janssen EM, Du X, Georgel P, Crozat K, Mudd S, Mann N, Sovath S, Goode J, Shamel L, Herskovits AA, Portnoy DA, Cooke M, Tarantino LM, Wiltshire T, Steinberg BE, Grinstein S, Beutler B. The Unc93b1 mutation 3d disrupts exogenous antigen presentation and signaling via Toll-like receptors 3, 7 and 9. Nat Immunol. 2006;7:156-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 529] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 23. | Morales C, Li Z. Drosophila canopy b is a cochaperone of glycoprotein 93. J Biol Chem. 2017;292:6657-6666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Hirate Y, Okamoto H. Canopy1, a novel regulator of FGF signaling around the midbrain-hindbrain boundary in zebrafish. Curr Biol. 2006;16:421-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 25. | Ghait M, Husain RA, Duduskar SN, Haack TB, Rooney M, Göhrig B, Bauer M, Rubio I, Deshmukh SD. The TLR-chaperone CNPY3 is a critical regulator of NLRP3-inflammasome activation. Eur J Immunol. 2022;52:907-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 26. | Zhang XW, Li L, Liao M, Liu D, Rehman A, Liu Y, Liu ZP, Tu PF, Zeng KW. Thermal Proteome Profiling Strategy Identifies CNPY3 as a Cellular Target of Gambogic Acid for Inducing Prostate Cancer Pyroptosis. J Med Chem. 2024;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 27. | Madar IH, Sultan G, Tayubi IA, Hasan AN, Pahi B, Rai A, Sivanandan PK, Loganathan T, Begum M, Rai S. Identification of marker genes in Alzheimer's disease using a machine-learning model. Bioinformation. 2021;17:348-355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 28. | Dai H, Zhou J, Zhu B. Gene co-expression network analysis identifies the hub genes associated with immune functions for nocturnal hemodialysis in patients with end-stage renal disease. Medicine (Baltimore). 2018;97:e12018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Chong W, Shang L, Liu J, Fang Z, Du F, Wu H, Liu Y, Wang Z, Chen Y, Jia S, Chen L, Li L, Chen H. m(6)A regulator-based methylation modification patterns characterized by distinct tumor microenvironment immune profiles in colon cancer. Theranostics. 2021;11:2201-2217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 167] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 30. | Wang Z, Song J, Azami NLB, Sun M. Identification of a Novel Immune Landscape Signature for Predicting Prognosis and Response of Colon Cancer to Immunotherapy. Front Immunol. 2022;13:802665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 31. | Finn OJ. Cancer immunology. N Engl J Med. 2008;358:2704-2715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 667] [Cited by in RCA: 644] [Article Influence: 37.9] [Reference Citation Analysis (0)] |
| 32. | Zhou R, Zhang J, Zeng D, Sun H, Rong X, Shi M, Bin J, Liao Y, Liao W. Immune cell infiltration as a biomarker for the diagnosis and prognosis of stage I-III colon cancer. Cancer Immunol Immunother. 2019;68:433-442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 194] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 33. | Philip M, Schietinger A. CD8(+) T cell differentiation and dysfunction in cancer. Nat Rev Immunol. 2022;22:209-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 590] [Article Influence: 196.7] [Reference Citation Analysis (0)] |
| 34. | Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348:56-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2870] [Cited by in RCA: 3565] [Article Influence: 356.5] [Reference Citation Analysis (0)] |
| 35. | Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27:450-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2655] [Cited by in RCA: 3198] [Article Influence: 319.8] [Reference Citation Analysis (0)] |
| 36. | Shi Y, Fu Y, Zhang X, Zhao G, Yao Y, Guo Y, Ma G, Bai S, Li H. Romidepsin (FK228) regulates the expression of the immune checkpoint ligand PD-L1 and suppresses cellular immune functions in colon cancer. Cancer Immunol Immunother. 2021;70:61-73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 37. | Narayanankutty A. PI3K/ Akt/ mTOR Pathway as a Therapeutic Target for Colorectal Cancer: A Review of Preclinical and Clinical Evidence. Curr Drug Targets. 2019;20:1217-1226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 194] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 38. | Song G, Xu S, Zhang H, Wang Y, Xiao C, Jiang T, Wu L, Zhang T, Sun X, Zhong L, Zhou C, Wang Z, Peng Z, Chen J, Wang X. TIMP1 is a prognostic marker for the progression and metastasis of colon cancer through FAK-PI3K/AKT and MAPK pathway. J Exp Clin Cancer Res. 2016;35:148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 223] [Cited by in RCA: 210] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 39. | Li G, Zhang C, Liang W, Zhang Y, Shen Y, Tian X. Berberine regulates the Notch1/PTEN/PI3K/AKT/mTOR pathway and acts synergistically with 17-AAG and SAHA in SW480 colon cancer cells. Pharm Biol. 2021;59:21-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 40. | Lin F, Zhang G, Yang X, Wang M, Wang R, Wan M, Wang J, Wu B, Yan T, Jia Y. A network pharmacology approach and experimental validation to investigate the anticancer mechanism and potential active targets of ethanol extract of Wei-Tong-Xin against colorectal cancer through induction of apoptosis via PI3K/AKT signaling pathway. J Ethnopharmacol. 2023;303:115933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 34] [Article Influence: 17.0] [Reference Citation Analysis (1)] |
| 41. | Liu F, Liang Y, Sun R, Yang W, Liang Z, Gu J, Zhao F, Tang D. Astragalus mongholicus Bunge and Curcuma aromatica Salisb. inhibits liver metastasis of colon cancer by regulating EMT via the CXCL8/CXCR2 axis and PI3K/AKT/mTOR signaling pathway. Chin Med. 2022;17:91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |