Published online Jul 15, 2024. doi: 10.4251/wjgo.v16.i7.3256
Revised: May 3, 2024
Accepted: May 17, 2024
Published online: July 15, 2024
Processing time: 127 Days and 19.4 Hours
The combination of transcatheter arterial chemoembolization (TACE) and tyrosine kinase inhibitors (TKIs) has shown broad prospects in prolonging the survival of patients with hepatocellular carcinoma (HCC). TACE and TKIs can affect the immune microenvironment in patients with HCC.
To determine the overall effects and differences between TACE and different TKIs combinations on the immune microenvironment.
Data and immune cell profile test results from 213 HCC patients treated with TACE combined with apatinib, lenvatinib, sorafenib, or donafenib before and after 3 wk of treatment were collected. Monocytes were co-cultured with LM3 liver cancer cells, and their ability to inhibit cancer cell growth was analyzed using the MTT method and a nude mouse subcutaneous tumorigenesis experiment. Simulated combined therapy was done using an in situ liver cancer C57BL/6 male mouse model, and the immune response of tumor tissues was analyzed using immunohistochemistry.
Compared to before combination therapy, the proportion of programmed cell death protein 1 (PD-1)+ mononuclear cells and the number of CD4+ T cells decreased in the TACE + apatinib group, while the number of absolute count of CD4+ and CD8+ T cells increased in the TACE + lenvatinib group. Furthermore, the number of regulatory cells decreased in the TACE + donafenib group, whereas the number of CD8+ T and natural killer cells increased. Additionally, monocytes in the TACE combined with donafenib or lenvatinib groups had a stronger ability to inhibit cancer cell growth than those in the other groups. Combining TACE with donafenib or lenvatinib increased CD8+ T cell infiltration into the tumor tissue. In addition, the proportion of PD-1+ in CD8+ cells, absolute CD8+ T lymphocyte count, and regulatory T cells proportion were independent prognostic factors affecting the survival time of patients with HCC.
TACE, in combination with different TKIs, produces different immune responses. Specifically, TACE combined with donafenib or lenvatinib may induce strong anti-tumor immune responses.
Core Tip: This study revealed that the combination of transcatheter arterial chemoembolization and donafinib or lenvatinib may be relatively beneficial in improving the immune suppression status of patients and inducing relatively robust anti-tumor immune responses. In addition, certain immune cells and protein molecules are significantly correlated with the prognosis of patients. The study results provide patients with reference for their selection of combination therapy strategies and help them predict their clinical prognosis.
- Citation: Guo Y, Li RC, Xia WL, Yang X, Zhu WB, Li FT, Hu HT, Li HL. Immune effect and prognosis of transcatheter arterial chemoembolization and tyrosine kinase inhibitors therapy in patients with hepatocellular carcinoma. World J Gastrointest Oncol 2024; 16(7): 3256-3269
- URL: https://www.wjgnet.com/1948-5204/full/v16/i7/3256.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i7.3256
Liver cancer is the sixth most common cancer worldwide and the third leading cause of cancer-related deaths. Hepatocellular carcinoma (HCC) accounts for the largest proportion of liver cancers[1]. Transcatheter arterial chemoembolization (TACE) is a widely used local treatment for advanced liver cancer[2]. The use of tyrosine kinase and immune checkpoint inhibitors has changed the systemic treatment of various cancers, including liver cancer. The widespread application of systemic therapy and its combination with local therapy shows prospects for prolonging patient survival[3].
TACE mainly kills cancer cells by blocking the arteries that supply blood to the tumor and injecting chemotherapeutic drugs into the tumor interior. Tumor cell necrosis can induce the release of inflammatory factors and new antigens, thus affecting the liver immune microenvironment in multiple ways and exerting a pleiotropic effect on the immune status of a patient[4]. After TACE, the levels of activated CD4+ and anti-tumor CD8+ T cells increase[5], and the infiltration of regulatory T cells (Tregs) decreases, thereby weakening the immunosuppressive state[6]. However, the increased ex
Anti-angiogenic drugs play a role in releasing antigens and regulating the tumor microenvironment, affecting the efficacy of immunotherapy[8]. Sorafenib and apatinib can improve the tumor microenvironment and induce an anti-HCC immune response by inactivating signal transduction and downregulating programmed cell death protein 1 (PD-1) expression through the transcription-activating factor 3 pathways[9,10]. Lenvatinib regulates tumor immunity and enhances anti-tumor activity by reducing the proportion of tumor-associated macrophages[11]. Donafenib combined with lipiodol embolization reportedly upregulated interleukin (IL)-6, tumor necrosis factor-α, and interferon-γ expression in the plasma of a liver cancer rat model[12].
Although local and systemic treatments have improved patient prognoses to a certain extent, they are not beneficial for all patients[13]. Exploring the overall effect of TACE combined with different tyrosine kinase inhibitors (TKIs) on the human immune microenvironment can guide the selection of combination therapy strategies, lay a foundation for combined TACE targeting and immunotherapy, and guide the development of new immunotherapy targets. We conducted subcutaneous tumorigenesis experiments in male nude mice and constructed an in situ liver cancer C57BL/6 male mouse model for simulated treatment, analyzing tumor growth and immune response. The aim of this study was to analyze the prognosis, overall impact, and immune microenvironment differences in patients with HCC receiving TACE combined with different TKIs.
In total, 213 patients with HCC (confirmed using imaging or histology) who underwent TACE combined with TKIs at Zhengzhou University Affiliated Cancer Hospital between June 2019 and December 2021 were retrospectively included in the study. Fifty-eight patients were in the TACE combined with apatinib group (T + A), 42 in the TACE combined with lenvatinib group (T + L), 64 in the TACE combined with sorafenib group (T + S), and 49 in the TACE combined with donafenib group (T + D). Patient clinical data, including age, sex, Barcelona Clinic Liver Cancer (BCLC) stage, Child-Pugh score, causative pathogen, tumor size and number, baseline alpha-fetoprotein, alanine aminotransferase, aspartate aminotransferase, and vascular endothelial growth factor levels, baseline and 3-wk post-treatment lymphoid immune cell test results, and metastasis status were collected.
The inclusion criteria were as follows: (1) Met the diagnostic and treatment criteria for HCC with at least one measurable liver target lesion; (2) Not suitable for surgical resection or refusal of surgical treatment; (3) Aged between 18 and 75 years; (4) Liver function was suitable for TACE treatment (Child-Pugh A or B, score ≤ 7); and (5) No history of liver cancer-related treatment.
The exclusion criteria were as follows: (1) BCLC phase A stage; (2) Received anti-tumor treatments, such as surgery, ablation, and radiotherapy; (3) Suffered from severe comorbidities, such as severe heart failure and respiratory system diseases; (4) Uncorrectable abnormalities in renal and coagulation functions; (5) Severe liver dysfunction (Child-Pugh C) or irreversible liver decompensation; (6) Eastern Cooperative Tumor Group score > 2 points; (7) Life expectancy of < 3 months; and (8) A history of other tumors.
HCC was staged according to the BCLC standards[14]. The Child-Pugh score was calculated based on patient clinical examination results, laboratory parameters, and imaging results. Treatment efficacy was evaluated using the modified response evaluation criteria in solid tumors based on enhanced computed tomography or magnetic resonance imaging[15]. The primary endpoint of this study was progression-free survival (PFS), defined as the time from the start of treatment to progression or death from any cause. The secondary endpoint was overall survival (OS), defined as the period from initial TACE to patient death or lack of follow-up.
The Seldinger technique was used as described previously[16] to puncture the femoral artery and evaluate hepatic artery blood flow and tumor blood supply via angiography. The dose of epirubicin (Haizheng Pharmaceutical, Hangzhou, China) was 50-75 mg/m2, and was adjusted based on tumor size, blood vessels, liver function, and body surface area. Epirubicin was mixed with 5-20 mL of lipiodol (Lipiodol Ultra-Fluid; Laboratoire Guerbet, Paris, France), the tumor supply artery was superselected through a microcatheter (Progreat; Terumo, Tokyo, Japan), and the mixture was injected at a rate of 1 mL/min until the blood flow stopped. Thereafter, gelatin sponge particles (500-700 μm; Caligel; Alicon Pharmaceutical, Hangzhou, China) were added to block the artery supplying the tumor.
The multidisciplinary team of the hospital determines the final combination treatment plan based on the BCLC guidelines or the China National Liver Cancer guidelines and the individual patient’s conditions. The included patients started taking TKIs daily, according to the instructions, 3 d after the first TACE[17]. The patients received 250 mg of oral apatinib daily. Patients with a body weight < 60 kg received an 8-mg daily oral dose of lenvatinib, while those with a body weight ≥ 60 kg received a 12-mg daily dose. In addition, 0.4 g of sorafenib and 0.2 g of donafenib were administered orally to all patients twice daily. We then determined whether TACE should be repeated based on a regular magnetic resonance imaging of tumor activity. If TACE treatment was repeated, the targeted drug was discontinued before TACE, and the medication was resumed 3 d after TACE. If intolerable toxicity or disease progression occurred, the treatment plan was changed according to each patient’s individual circumstances, medication dosage was reduced, or the combination therapy was terminated.
LM3 human liver cancer cells were pre-inoculated at a density of 2000 cells/well in a 96-well plate. Peripheral venous blood samples were collected from three patients in different combination therapy groups after 3 wk of treatment with no significant differences in their baseline characteristics; samples from three patients who received TACE alone served as controls (T group). All patients provided written informed consent before being included in the study. This study was approved by the Medical Ethics Committee of Henan Cancer Hospital (approval code: 2017002). Peripheral blood monocytes were separated from the obtained blood samples using Ficoll-Paque density gradient centrifugation, cultured in RPMI-1640 medium, and stimulated with IL-2 (50 ng/mL) for 16 h[18]. Subsequently, 2 × 104 monocytes (at a ratio of 10:1 relative to the LM3 cells) were added to each well of the 96-well plate for coculture. Next, 20 μL of 5 mg/mL MTT was added to each well at 24, 48, 72, and 96 h, and the reaction mixtures were incubated at 36 °C for another 2 h. Subsequently, 100 μL of dimethyl sulfoxide was used to dissolve the formazan crystals formed, and the absorbance of the samples was measured at 490 nm.
After coculturing with monocytes for 48 h, 5 × 106 LM3 cells were resuspended in 100 μL of phosphate-buffered saline and subcutaneously injected into the right back flank of 4-wk-old male nude mice weighing 18-20 g. LM3 cells were used as the control group, and a single animal was used as the experimental unit. Thirty mice were randomly divided into six groups, with 5 mice in each group for the experiment. Two data with high dispersion were removed for result analysis. Four weeks after tumor implantation, the mice were euthanized using the spinal dislocation method, and tumors were excised and weighed. The experimental operation and data analysis were conducted independently by two individuals.
C57BL/6 mice were placed inside an anesthesia induction box. Subsequently, we induced anesthesia with 5% isoflurane, and then switched to a breathing mask to maintain anesthesia with 2% isoflurane (gas flow maintained at 1 L/min). We shaved off abdominal hair, disinfected the area with iodine three times, exposed the liver at the center of the abdomen, injected 50 uL of H22 cells (including 5 × 106 cells) into the left lobe of the liver, sutured the wound layer by layer after surgery, disinfected the area again, and placed the mouse back inside a cage for normal feeding. Thirty mice were randomly divided into 6 groups, with 5 mice in each group. The two data with high dispersion were removed for result analysis. The solvent injection group is used as the control group, with a single animal as the experimental unit. All mice were four week old male mice weighing between 18-20 g. This model was approved by the Animal Experiment Ethics Committee of Hubei Provincial Center for Disease Control and Prevention (approval number: 202310157). As previously reported, one week after liver tumor cell implantation, 2 mg/kg epirubicin was intraperitoneally injected into the mice[19-22]; the control group was administered the same dose of physiological saline. Apatinib, lenvatinib, sorafenib, or donafenib was administered at a daily dose of 50, 5, 30, or 20 mg/kg, respectively, via oral gavage for 2 wk. After the treatment period, the mice were euthanized, and their livers were dissected. The tumor tissues were separated, weighed, and subjected to subsequent analyses. The experimental operation and data analysis were conducted independently by two individuals. Mice were housed in a standard animal laboratory with free activity and access to water and chow. They were kept under constant environment conditions with a 12 h light-dark cycle.
The collected tumors were stored in 4% paraformaldehyde for 24 h for fixation. After paraffin embedding, the sections were baked and dewaxed, and the antigens were retrieved using sodium citrate. The samples were blocked in 3% bovine serum albumin at room temperature for 30 min and then incubated overnight with primary antibodies at 4 °C. The samples were then incubated with the corresponding secondary antibodies at room temperature for 60 min. After the diaminobenzidine (DAB) color reaction, reverse staining with a hematoxylin staining solution, and dehydration, CD8+ T cells and the results of PD-L1 and Ki-67 staining of the tissues were observed under a microscope. DAB expression was considered positive when there was brownish-yellow staining. At least three independent areas from each section were observed under 20 × magnification. Images were captured and analyzed using the ImageJ software (National Institutes of Health, Bethesda, MD, United States).
SPSS software (version 22.0) was used for data analysis. To compare the differences between groups, two independent sample t-tests, paired t-tests, or Mann-Whitney U tests were used whenever appropriate. Multiple group comparisons were conducted using one-way analysis of variance and the Kruskal-Wallis H method. Qualitative data in a group of four grid tables that met Pearson’s χ2 test conditions were analyzed accordingly, whereas those that did not meet the conditions were analyzed using Pearson’s continuous correction χ2 test or Fisher’s exact probability method. The Kaplan-Meier method was used to analyze the PFS and OS, and the log-rank test was used for intergroup comparisons. Cox univariate analysis was conducted, and variables with statistical significance were included in a multivariate Cox proportional risk regression model to screen for independent factors that affect prognosis. Results with P < 0.05 were considered statistically significant.
This study retrospectively included 213 patients with HCC. Their average age was 57.5 ± 9.9 years (range: 35-73), with the majority being males (85.4%, 182/213). Hepatitis B viral infection was the main cause of liver disease in the recruited patients (94.4%, 201/213), and all patients had liver cirrhosis. The number of patients in the T + A, T + L, T + S, and T + D groups was 58 (27.2%), 42 (19.7%), 64 (30.1%), and 49 (23.0%), respectively. The detailed baseline patient demographic data are shown in Table 1.
| Variables | T + A (n = 58) | T + L (n = 42) | T + S (n = 64) | T + D (n = 49) | P value |
| Age (≥ 55/< 55) | 33/25 | 33/9 | 36/28 | 30/19 | 0.092 |
| Sex (female/male) | 10/48 | 6/36 | 7/57 | 8/41 | 0.768 |
| Etiology | 0.075 | ||||
| Hepatitis B virus | 54 | 40 | 60 | 47 | |
| Hepatitis C virus | 0 | 0 | 0 | 0 | |
| Others | 4 | 2 | 4 | 2 | |
| AFP level (ng/mL) | 0.084 | ||||
| ≤ 400 | 26 | 14 | 18 | 24 | |
| > 400 | 32 | 28 | 46 | 25 | |
| ALT (U/L) | 0.483 | ||||
| ≤ 40 | 12 | 8 | 19 | 14 | |
| > 40 | 46 | 34 | 45 | 35 | |
| AST (U/L) | 0.087 | ||||
| ≤ 40 | 5 | 2 | 12 | 9 | |
| > 40 | 53 | 40 | 52 | 40 | |
| VEGF (pg/mL), mean (SD) | 456.06 (216.38) | 412.81 (163.02) | 403.03 (168.45) | 417.99 (170.99) | 0.419 |
| Cirrhosis | |||||
| No | 0 | 0 | 0 | 0 | |
| Yes | 58 | 42 | 64 | 49 | |
| Child-Pugh class | 0.102 | ||||
| A | 41 | 24 | 49 | 29 | |
| B | 17 | 18 | 15 | 20 | |
| Tumor size (cm) | 0.103 | ||||
| ≤ 5 | 34 | 18 | 41 | 23 | |
| > 5 | 24 | 24 | 23 | 26 | |
| Tumor number | 0.338 | ||||
| Single | 21 | 12 | 15 | 11 | |
| Multiple | 37 | 30 | 49 | 38 | |
| Metastasis | 0.091 | ||||
| Yes | 18 | 20 | 16 | 19 | |
| No | 40 | 22 | 48 | 30 | |
| BCLC stage | 0.471 | ||||
| B | 38 | 22 | 35 | 26 | |
| C | 20 | 20 | 29 | 23 |
After 3 wk of treatment with TACE combined with apatinib, the proportion of PD-1+ mononuclear cells (P = 0.045) and the absolute count of CD4+ T lymphocytes significantly decreased (P = 0.027). In addition, after 3 wk of TACE combined with lenvatinib, the absolute count of total lymphocytes (P = 0.032), CD4+ T lymphocytes (P = 0.009), and CD8+ T lymphocytes (P = 0.023) significantly increased, whereas the percentage of helper/index cells significantly decreased (P = 0.003). After 3 wk of TACE combined with donafenib, the proportion of Tregs (P = 0.013) significantly decreased, whereas the absolute count of CD8+ T lymphocytes (P = 0.001) and natural killer (NK) cells (P = 0.039) significantly increased (Table 2). However, after 3 wk of TACE combined with sorafenib, no significant change in the patients’ lymphatic immune cell proportions was observed.
| Type | T + A | T + L | T + S | T + D | ||||||||
| Before treatment | After treatment | P value | Before treatment | After treatment | P value | Before treatment | After treatment | P value | Before treatment | After treatment | P value | |
| Total T lymphocytes (%) | 78.79 (9.22) | 76.50 (10.59) | 0.260 | 70.71 (10.78) | 71.55 (9.20) | 0.755 | 73.84 (11.88) | 74.44 (9.47) | 0.869 | 72.75 (13.79) | 71.26 (14.45) | 0.606 |
| Suppressor/cytotoxic cells (%) | 32.20 (13.03) | 31.82 (14.55) | 0.907 | 28.05 (12.29) | 25.73 (7.82) | 0.490 | 26.74 (12.65) | 26.06 (8.73) | 0.756 | 28.59 (14.91) | 27.07 (13.53) | 0.478 |
| Helper/inducible cells (%) | 39.04 (8.31) | 37.98 (7.35) | 0.608 | 40.89 (9.73) | 32.54 (9.74) | 0.003a | 42.05 (8.64) | 44.06 (7.17) | 0.434 | 34.70 (7.35) | 35.93 (5.64) | 0.446 |
| Natural killer cell (%) | 12.68 (8.13) | 11.56 (8.28) | 0.505 | 17.13 (8.63) | 16.86 (8.16) | 0.922 | 18.81 (11.28) | 17.56 (9.27) | 0.715 | 16.31 (11.20) | 18.61 (12.75) | 0.261 |
| B lymphocytes (%) | 7.20 (4.71) | 9.43 (6.42) | 0.093 | 10.68 (8.34) | 10.82 (5.38) | 0.952 | 5.58 (3.38) | 5.96 (3.23) | 0.707 | 10.30 (6.15) | 10.40 (4.44) | 0.933 |
| Helper T cells/suppressor T cells | 1.58 (1.20) | 1.67 (1.27) | 0.659 | 1.79 (1.25) | 1.64 (0.93) | 0.674 | 1.98 (0.99) | 1.86 (0.63) | 0.585 | 1.46 (0.54) | 1.59 (0.61) | 0.198 |
| Regulatory cells (%) | 11.65 (3.90) | 11.60 (3.63) | 0.918 | 10.47 (2.60) | 11.79 (2.88) | 0.110 | 10.04 (2.93) | 10.31 (3.11) | 0.789 | 12.23 (4.35) | 10.78 (3.27) | 0.013a |
| Proportion of PD-1+ in mononuclei (%) | 2.05 (4.13) | 1.21 (2.89) | 0.045a | 4.45 (6.24) | 2.37 (6.43) | 0.136 | 5.11 (8.10) | 2.66 (6.40) | 0.132 | 1.36 (4.86) | 2.15 (6.24) | 0.533 |
| Proportion of PD-1+ in CD3+ cells (%) | 2.43 (5.68) | 1.85 (5.18) | 0.563 | 6.93 (9.04) | 3.79 (8.64) | 0.205 | 6.91 (10.95) | 3.48 (8.89) | 0.071 | 1.69 (6.48) | 2.79 (8.01) | 0.330 |
| Proportion of PD-1+ in CD4+ cells (%) | 1.69 (4.39) | 1.72 (5.14) | 0.974 | 6.11 (8.56) | 3.16 (7.42) | 0.144 | 8.06 (12.81) | 3.91 (10.43) | 0.146 | 2.00 (8.42) | 2.62 (7.94) | 0.660 |
| Proportion of PD-1+ in CD8+ cells (%) | 3.32 (8.42) | 1.86 (5.36) | 0.262 | 7.92 (11.64) | 4.60 (10.61) | 0.262 | 3.36 (6.86) | 1.98 (7.22) | 0.375 | 1.13 (4.02) | 2.12 (6.05) | 0.292 |
| Absolute count of total lymphocytes (/uL) | 1537.33 (650.17) | 1349.96 (534.78) | 0.154 | 1248.86 (442.71) | 1624.48 (602.20) | 0.032a | 1555.82 (503.27) | 1319.00 (374.04) | 0.148 | 1891.05 (784.70) | 1721.86 (466.61) | 0.378 |
| Absolute T lymphocyte count (/uL) | 1194.63 (461.58) | 1058.63 (467.70) | 0.223 | 898.57 (347.46) | 1143.43 (436.88) | 0.058 | 1137.41 (484.47) | 976.18 (230.22) | 0.266 | 1251.73 (531.50) | 1270.27 (452.22) | 0.860 |
| CD4+T lymphocyte absolute count (/uL) | 625.46 (346.62) | 488.96 (184.78) | 0.027a | 421.43 (38.52) | 603.24 (62.16) | 0.009a | 628.12 (184.95) | 579.65 (173.29) | 0.425 | 627.45 (198.58) | 590.09 (464.05) | 0.700 |
| CD8+T lymphocyte absolute count (/uL) | 492.25 (266.83) | 457.58 (368.59) | 0.652 | 379.71 (130.84) | 502.71 (267.30) | 0.023a | 425.24 (327.35) | 340.24 (125.24) | 0.294 | 390.64 (183.19) | 530.18 (169.23) | 0.001a |
| Absolute NK cell count (/uL) | 214.25 (217.71) | 156.75 (143.46) | 0.112 | 299.10 (233.67) | 201.76 (113.38) | 0.114 | 333.12 (238.31) | 258.47 (191.34) | 0.323 | 288.41 (223.55) | 384.86 (229.53) | 0.039a |
| Absolute B cell count (/uL) | 109.21 (95.35) | 127.29 (89.20) | 0.333 | 178.76 (153.83) | 134.33 (77.23) | 0.285 | 80.18 (46.41) | 74.82 (46.18) | 0.720 | 179.60 (108.93) | 164.73 (93.09) | 0.650 |
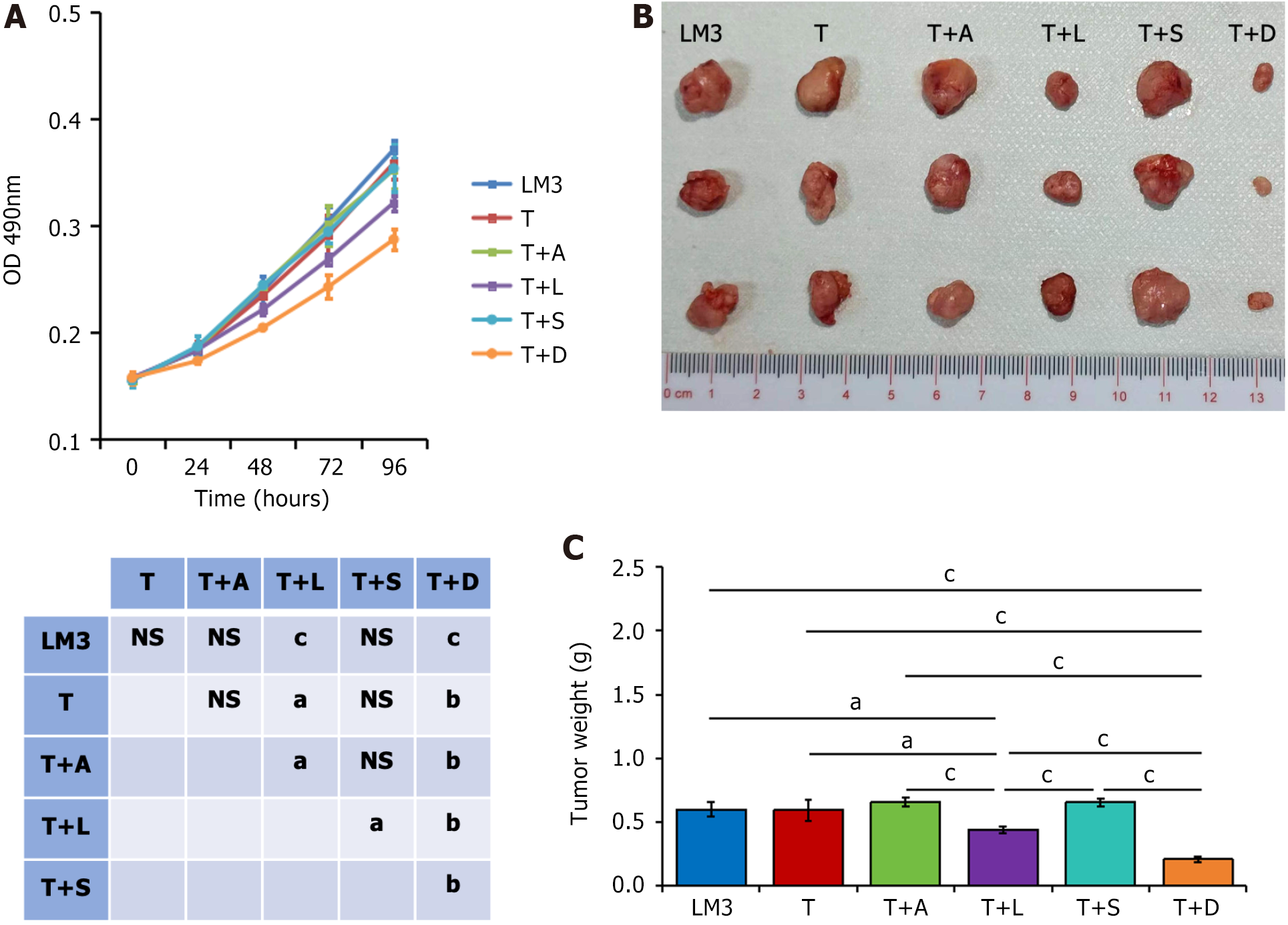
The growth and proliferation of LM3 liver cancer cells cocultured with monocytes from patients in the T + D group after 3 wk of treatment were the lowest, followed by those from the T + L group (Figure 1A). There was no significant difference in the growth and proliferation of cells among the other groups. Similar results were obtained in the subcutaneous tumor formation experiment with nude mice (Figure 1B and C). The tumor weight was the lowest after coculture with monocytes from the T + D group, followed by those from the T + L group; there was no significant difference among the other groups.
As shown in Figure 2A and B, the tumor weight of the solvent control group was significantly higher than that of the T group, and was higher than that of each combined TKI group. The tumor weight of the mice in the TACE group alone was significantly higher than that of each combined TKI group; however, there was no significant difference in the tumor weight among the four groups combined.
Figure 2C and D show that the number of infiltrating CD8+ T cells in the T group tumor tissues and the four combined groups was significantly higher than that in the solvent control group, with the T + D group having the highest level CD8+ T cell infiltration, followed by the T + L group. Figure 2E and F show that the expression of PD-L1 in the T group tumor tissues was significantly higher than that in the solvent control group, whereas PD-L1 expression in the tumor tissues of the four groups combined was significantly lower than that in the T group; there was no significant difference between them. Figure 2G and H show that the expression of Ki-67 in the tumor tissues of the solvent control group was significantly higher than that in the other groups, while its expression in the T group tumor tissues was significantly higher than in each combination group. Specifically, Ki-67 expression in the T + D group was significantly lower than that in the T + A, T + L, and T + S groups, whereas no significant difference was observed among the T + A, T + L, and T + S groups.
Based on the existing immune-related results, the survival prognosis of patients in different combination therapy groups was analyzed. As shown in Figure 3, although the median PFS of patients in the T + L and T + D groups was slightly longer than that of the T + A and T + S groups, there was no significant difference in PFS between the different combination therapy groups [T + A: median PFS, 9.8 months; 95% confidence interval (CI): 8.495-11.105; T + L: median PFS, 11.4 months; 95%CI: 8.535-14.265; T + S: median PFS, 10.3 months; 95%CI: 8.733-11.867; T + D: median PFS, 10.9 months; 95%CI: 9.528-12.272; P = 0.184]. Similarly, there was no significant difference in OS between the different combination therapy groups (T + A: median OS, 22.9 months; 95%CI: 19.515-26.285; T + L: median OS, 22.0 months; 95%CI: 17.731-26.269; T + S: median OS, 23.8 months; 95%CI: 20.348-27.252; T + D: median OS, 24.3 months; 95%CI: 23.390-25.210; P = 0.270).
As shown in Tables 3 and 4, tumor size (P = 0.023), the proportion of PD-1+ in CD8+ cells (P = 0.022), and absolute CD8+ T lymphocyte count (P = 0.001) were independent prognostic factors affecting PFS. Furthermore, tumor size (P = 0.032) and Tregs proportion (P = 0.046) were independent prognostic factors affecting OS.
| Variables | Univariate analysis | Multivariate analysis | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Age | 0.989 (0.968-1.010) | 0.302 | ||
| Sex | 0.964 (0.538-1.725) | 0.901 | ||
| Etiology | 1.242 (0.537-2.873) | 0.612 | ||
| AFP | 1.318 (0.845-2.057) | 0.224 | ||
| ALT | 1.002 (0.999-1.005) | 0.309 | ||
| AST | 1.001 (0.999-1.003) | 0.518 | ||
| VEGF | 1.000 (0.999-1.001) | 0.798 | ||
| Child-Pugh class | 1.135 (0.721-1.788) | 0.585 | ||
| ECOG score | ||||
| 0 | 0.925 (0.338-2.534) | 0.880 | ||
| 1 | 0.980 (0.562-1.708) | 0.943 | ||
| Tumor size | 1.011 (1.004-1.019) | 0.002a | 1.009 (1.001-1.017) | 0.023a |
| Tumor number | 0.974 (0.860-1.103) | 0.677 | ||
| Metastasis | 0.947 (0.578-1.554) | 0.831 | ||
| BCLC stage | 1.436 (0.914-2.254) | 0.116 | ||
| Treatment methods | ||||
| T + A | 0.722 (0.396-1.317) | 0.288 | ||
| T + L | 0.608 (0.321-1.152) | 0.608 | ||
| T + S | 0.586 (0.300-1.145) | 0.118 | ||
| Total T lymphocytes | 0.986 (0.966-1.008) | 0.211 | ||
| Suppressor/cytotoxic cells | 0.998 (0.981-1.016) | 0.859 | ||
| Helper/inducible cells | 0.976 (0.954-0.998) | 0.033a | 0.982 (0.959-1.006) | 0.133 |
| Natural killer cell | 1.016 (0.991-1.043) | 0.210 | ||
| B lymphocytes | 1.024 (0.984-1.065) | 0.252 | ||
| Helper T cells/suppressor T cells | 1.017 (0.823-1.257) | 0.873 | ||
| Regulatory cells | 1.100 (1.033-1.172) | 0.003a | 1.063 (0.989-1.144) | 0.098 |
| Proportion of PD-1+ in peripheral blood mononuclei | 1.014 (0.975-1.054) | 0.498 | ||
| Proportion of PD-1+ in CD3+ cells | 1.011 (0.984-1.039) | 0.410 | ||
| Proportion of PD-1+ in CD4+ cells | 1.010 (0.985-1.037) | 0.435 | ||
| Proportion of PD-1+ in CD8+ cells | 0.975 (0.946-0.999) | 0.040a | 0.966 (0.938-0.995) | 0.022a |
| Absolute count of total lymphocytes | 1.000 (1.000-1.000) | 0.782 | ||
| Absolute T lymphocyte count | 1.000 (0.999-1.000) | 0.451 | ||
| Absolute count of CD3+CD4+ lymphocytes | 1.000 (0.999-1.001) | 0.477 | ||
| Absolute count of CD3+CD8+ lymphocytes | 0.998 (0.997-0.999) | 0.002a | 0.998 (0.997-0.999) | 0.001a |
| CD4/CD8 ratio | 0.934 (0.741-1.177) | 0.564 | ||
| Absolute NK cell count | 1.001 (1.000-1.002) | 0.168 | ||
| Absolute B cell count | 1.002 (1.000-1.004) | 0.085 | ||
| Variables | Univariate analysis | Multivariate analysis | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Age | 0.994 (0.972-1.015) | 0.554 | ||
| Sex | 1.273 (0.710-2.282) | 0.418 | ||
| Etiology | 1.444 (0.619-3.368) | 0.396 | ||
| AFP | 1.215 (0.769-1.919) | 0.405 | ||
| ALT | 1.062 (0.617-1.827) | 0.828 | ||
| AST | 1.002 (1.000-1.004) | 0.064 | ||
| VEGF | 1.000 (0.999-1.001) | 0.780 | ||
| Child-Pugh class | 0.947 (0.594-1.512) | 0.821 | ||
| ECOG score | ||||
| 0 | 1.029 (0.393-2.690) | 0.954 | ||
| 1 | 0.884 (0.490-1.595) | 0.683 | ||
| Tumor size | 1.011 (1.003-1.020) | 0.005a | 1.010 (1.001-1.016) | 0.032a |
| Tumor number | 0.954 (0.842-1.081) | 0.461 | ||
| Metastasis | 0.928 (0.556-1.548) | 0.774 | ||
| BCLC stage | 1.209 (0.760-1.924) | 0.423 | ||
| Treatment methods | ||||
| T + A | 0.926 (0.499-1.720) | 0.809 | ||
| T + L | 0.781 (0.408-1.492) | 0.453 | ||
| T + S | 0.698 (0.358-1.360) | 0.291 | ||
| Total T lymphocytes | 0.990 (0.968-1.012) | 0.379 | ||
| Suppressor/cytotoxic cells | 0.992 (0.974-1.011) | 0.421 | ||
| Helper/inducible cells | 0.999 (0.976-1.021) | 0.903 | ||
| Natural killer cell | 1.007 (0.982-1.032) | 0.590 | ||
| B lymphocytes | 1.031 (0.988-1.076) | 0.164 | ||
| Helper T cells/suppressor T cells | 1.088 (0.885-1.337) | 0.426 | ||
| Regulatory cells | 1.082 (1.008-1.161) | 0.029a | 1.074 (1.001-1.152) | 0.046a |
| Proportion of PD-1+ in peripheral blood mononuclei | 1.012 (0.972-1.054) | 0.561 | ||
| Proportion of PD-1+ in CD3+ cells | 1.011 (0.983-1.041) | 0.432 | ||
| Proportion of PD-1+ in CD4+ cells | 1.007 (0.981-1.034) | 0.618 | ||
| Proportion of PD-1+ in CD8+ cells | 0.989 (0.963-1.015) | 0.402 | ||
| Absolute count of total lymphocytes | 1.000 (1.000-1.000) | 0.612 | ||
| Absolute T lymphocyte count | 1.000 (0.999-1.000) | 0.784 | ||
| Absolute count of CD3+CD4+ lymphocytes | 1.000 (0.999-1.001) | 0.926 | ||
| Absolute count of CD3+CD8+ lymphocytes | 0.999 (0.998-1.000) | 0.096 | ||
| CD4/CD8 ratio | 0.992 (0.795-1.239) | 0.945 | ||
| Absolute NK cell count | 1.000 (0.999-1.001) | 0.527 | ||
| Absolute B cell count | 1.003 (1.001-1.005) | 0.017a | 1.002 (1.000-1.004) | 0.108 |
In the current study, we found that the immune response of patients with HCC receiving TACE combined with different TKIs varies, and TACE combined with donafenib or lenvatinib was more likely to cause a strong anti-tumor immune response than the other TKIs. Unfortunately, patient survival analysis revealed that although patients with HCC who received TACE combined with donafenib or lenvatinib treatment had a longer survival time, it was not statistically significant. However, certain immune cells are independent prognostic factors affecting patient survival time.
The results of the mismatch between the immune response and survival prognosis in patients may be diverse. Generally, a more severe inhibitory tumor immune microenvironment predicts a poorer prognosis; however, the degree of response and changes produced after being stimulated by some treatment methods varies. Although some changes are beneficial for improving the immune microenvironment, the extent of their effects still needs to be further explored. Meanwhile, the components that make up the immune microenvironment and their relationships are complex. In addition, numerous factors affect the prognosis of patients, and stronger anti-tumor immune responses may be interfered with or offset by other factors.
In addition, the difference in immune response between TACE combined with TKIs and TACE alone is intriguing. TACE alone may increase the number of CD4+ T and NK cells in cancer tissues or circulating blood[23], decrease the number of CD8+ T cells and Tregs[24], and increase PD-1 and PD-L1 expression[25]. In our study, no significant change was observed in the lymphocyte profile in the circulating blood of patients after three weeks of TACE combined with sorafenib treatment compared with that before treatment; however, this finding does not indicate that the patients’ immune status remained unaffected. This phenomenon may be because of the counteracting effect of sorafenib binding, which also suggests that the positive and negative immune effects of TACE are offset simultaneously.
We also observed changes in the other combination treatment groups similar to those in the TACE-alone group, such as a significant decrease in the proportion of Tregs and an increase in the number of NK cells in the T + D group. Certain combination groups also showed changes opposite to those of the TACE-alone group, such as a decrease in the proportion of PD-1+ monocytes in the apatinib group and an increase in the number of CD8+ T cells in the lenvatinib and donafenib groups. In addition, some inconspicuous changes, such as the absolute counts of total lymphocytes and helper/index cells were observed, which require further research.
CD8+ T cells are considered the main effectors of the anti-tumor adaptive immune response as they can directly kill cancer cells[26]. However, a higher PD-1 and PD-L1 expression imply greater inhibition of immune cell activity and killing ability[27]. Therefore, in this study, the immunosuppressive status of patients treated with TACE combined with donafenib significantly improved. Subsequently, monocytes from each patient group were cocultured with LM3 liver cancer cells, and the growth of cancer cells in each group was inhibited to different levels. The TACE combined with donafenib group showed the strongest inhibition of cancer cell growth, followed by the TACE combined with lenvatinib group. The subcutaneous tumorigenesis experiment in nude mice showed the same results.
Although there was no significant difference in tumor weight among the combination therapy groups in the in situ liver cancer mouse model, PD-L1 expression increased in the group administered with TACE alone. However, the number of CD8+ T cells infiltrating the tumor in the TACE combined with donafenib and lenvatinib groups was significantly higher and Ki-67 expression was lower than those in the other groups. This finding indicates that these two groups combined can create a tumor immune microenvironment more conducive to killing cancer cells than the other groups. The lack of a significant difference in tumor weight could be attributed to other factors, such as other components of the immune microenvironment and specific phenotypic differences in immune cells, which also require further research. This study has certain limitations. A relatively simple method was used to simulate treatment on mice, and the number of immune indicators analyzed and detected was limited. A larger sample and more in-depth research are required to verify these findings.
Immune-related factors were associated with patient prognosis, which may aid in predicting patient prognosis. Moreover, the immune responses generated in different combination therapy groups varied. Collectively, our results suggest that TACE combined with donafenib or lenvatinib may aid in improving a patient’s immunosuppressive state and inducing stronger anti-tumor immune responses than the other TKIs investigated in this study. Further research will provide patients with reference for selecting suitable combination therapy options.
We thank the editors for their kind work and the reviewers for their constructive comments.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64390] [Article Influence: 16097.5] [Reference Citation Analysis (176)] |
| 2. | Reichert MC, Massmann A, Schulz A, Buecker A, Glanemann M, Lammert F, Malinowski M. Volume-Function Analysis (LiMAx Test) in Patients with HCC and Cirrhosis Undergoing TACE-A Feasibility Study. Dig Dis Sci. 2021;66:2452-2460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Leone P, Solimando AG, Fasano R, Argentiero A, Malerba E, Buonavoglia A, Lupo LG, De Re V, Silvestris N, Racanelli V. The Evolving Role of Immune Checkpoint Inhibitors in Hepatocellular Carcinoma Treatment. Vaccines (Basel). 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 70] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 4. | Piscaglia F, Ogasawara S. Patient Selection for Transarterial Chemoembolization in Hepatocellular Carcinoma: Importance of Benefit/Risk Assessment. Liver Cancer. 2018;7:104-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 104] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 5. | Ayaru L, Pereira SP, Alisa A, Pathan AA, Williams R, Davidson B, Burroughs AK, Meyer T, Behboudi S. Unmasking of alpha-fetoprotein-specific CD4(+) T cell responses in hepatocellular carcinoma patients undergoing embolization. J Immunol. 2007;178:1914-1922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 118] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 6. | Park H, Jung JH, Jung MK, Shin EC, Ro SW, Park JH, Kim DY, Park JY, Han KH. Effects of transarterial chemoembolization on regulatory T cell and its subpopulations in patients with hepatocellular carcinoma. Hepatol Int. 2020;14:249-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Montasser A, Beaufrère A, Cauchy F, Bouattour M, Soubrane O, Albuquerque M, Paradis V. Transarterial chemoembolisation enhances programmed death-1 and programmed death-ligand 1 expression in hepatocellular carcinoma. Histopathology. 2021;79:36-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 69] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 8. | Huang A, Yang XR, Chung WY, Dennison AR, Zhou J. Targeted therapy for hepatocellular carcinoma. Signal Transduct Target Ther. 2020;5:146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 473] [Cited by in RCA: 490] [Article Influence: 98.0] [Reference Citation Analysis (0)] |
| 9. | Wang X, Hu R, Song Z, Zhao H, Pan Z, Feng Y, Yu Y, Han Q, Zhang J. Sorafenib combined with STAT3 knockdown triggers ER stress-induced HCC apoptosis and cGAS-STING-mediated anti-tumor immunity. Cancer Lett. 2022;547:215880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 50] [Reference Citation Analysis (0)] |
| 10. | Liang LJ, Hu CX, Wen YX, Geng XW, Chen T, Gu GQ, Wang L, Xia YY, Liu Y, Fei JY, Dong J, Zhao FH, Ahongjiang Y, Hui KY, Jiang XD. Apatinib Combined with Local Irradiation Leads to Systemic Tumor Control via Reversal of Immunosuppressive Tumor Microenvironment in Lung Cancer. Cancer Res Treat. 2020;52:406-418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Kato Y, Tabata K, Kimura T, Yachie-Kinoshita A, Ozawa Y, Yamada K, Ito J, Tachino S, Hori Y, Matsuki M, Matsuoka Y, Ghosh S, Kitano H, Nomoto K, Matsui J, Funahashi Y. Lenvatinib plus anti-PD-1 antibody combination treatment activates CD8+ T cells through reduction of tumor-associated macrophage and activation of the interferon pathway. PLoS One. 2019;14:e0212513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 242] [Cited by in RCA: 355] [Article Influence: 59.2] [Reference Citation Analysis (0)] |
| 12. | Shi Q, Li T, Huang S, Bai Y, Wang Y, Liu J, Zhou C, Chen Y, Xiong B. Transcatheter Arterial Embolization Containing Donafenib Induces Anti-Angiogenesis and Tumoricidal CD8(+) T-Cell Infiltration in Rabbit VX2 Liver Tumor. Cancer Manag Res. 2021;13:6943-6952. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Guan YS, Liu Y, Zhou XP, Li X, He Q, Sun L. p53 gene (Gendicine) and embolisation overcame recurrent hepatocellular carcinoma. Gut. 2006;55:1684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 14. | Guo Y, Hu HT, Xu SJ, Xia WL, Zhao Y, Zhao XH, Zhu WB, Li FT, Li HL. Proteoglycan-4 predicts good prognosis in patients with hepatocellular carcinoma receiving transcatheter arterial chemoembolization and inhibits cancer cell migration in vitro. Front Oncol. 2022;12:1023801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Xia WL, Xu SJ, Guo Y, Zhao XH, Hu HT, Zhao Y, Yao QJ, Zheng L, Zhang DY, Guo CY, Fan WJ, Li HL. Plasma arginase-1 as a predictive marker for early transarterial chemoembolization refractoriness in unresectable hepatocellular carcinoma. Front Oncol. 2022;12:1014653. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 16. | Guo Y, Hu HT, Xu SJ, Xia WL, Li Y, Lu J, Zhao XH, Zhao Y, Li FT, Li HL. Correlation of Serum Chemokine Ligand 14 with Barcelona Clinic Liver Cancer Stage, Lymphocyte Profile, and Response to Transarterial Chemoembolization in Patients with Hepatocellular Carcinoma. J Vasc Interv Radiol. 2023;34:991-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Liu Z, Lin Y, Zhang J, Zhang Y, Li Y, Liu Z, Li Q, Luo M, Liang R, Ye J. Molecular targeted and immune checkpoint therapy for advanced hepatocellular carcinoma. J Exp Clin Cancer Res. 2019;38:447. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 166] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 18. | Santagata S, Rea G, Castaldo D, Napolitano M, Capiluongo A, D'Alterio C, Trotta AM, Ieranò C, Portella L, Di Maro S, Tatangelo F, Albino V, Guarino R, Cutolo C, Izzo F, Scala S. Hepatocellular carcinoma (HCC) tumor microenvironment is more suppressive than colorectal cancer liver metastasis (CRLM) tumor microenvironment. Hepatol Int. 2024;18:568-581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 19. | Li X, Xu A, Li H, Zhang B, Cao B, Huang J. Novel role of apatinib as a multi-target RTK inhibitor in the direct suppression of hepatocellular carcinoma cells. Biochim Biophys Acta Mol Basis Dis. 2018;1864:1693-1701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 20. | Shi T, Iwama H, Fujita K, Kobara H, Nishiyama N, Fujihara S, Goda Y, Yoneyama H, Morishita A, Tani J, Yamada M, Nakahara M, Takuma K, Masaki T. Evaluating the Effect of Lenvatinib on Sorafenib-Resistant Hepatocellular Carcinoma Cells. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 21. | Yuan S, Wei C, Liu G, Zhang L, Li J, Li L, Cai S, Fang L. Sorafenib attenuates liver fibrosis by triggering hepatic stellate cell ferroptosis via HIF-1α/SLC7A11 pathway. Cell Prolif. 2022;55:e13158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 252] [Article Influence: 84.0] [Reference Citation Analysis (0)] |
| 22. | Zhao Y, Wang J, Liu WN, Fong SY, Shuen TWH, Liu M, Harden S, Tan SY, Cheng JY, Tan WWS, Chan JKY, Chee CE, Lee GH, Toh HC, Lim SG, Wan Y, Chen Q. Analysis and Validation of Human Targets and Treatments Using a Hepatocellular Carcinoma-Immune Humanized Mouse Model. Hepatology. 2021;74:1395-1410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 23. | Huang M, Wang X, Bin H. Effect of Transcatheter Arterial Chemoembolization Combined with Argon-Helium Cryosurgery System on the Changes of NK Cells and T Cell Subsets in Peripheral Blood of Hepatocellular Carcinoma Patients. Cell Biochem Biophys. 2015;73:787-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Pinato DJ, Murray SM, Forner A, Kaneko T, Fessas P, Toniutto P, Mínguez B, Cacciato V, Avellini C, Diaz A, Boyton RJ, Altmann DM, Goldin RD, Akarca AU, Marafioti T, Mauri FA, Casagrande E, Grillo F, Giannini E, Bhoori S, Mazzaferro V. Trans-arterial chemoembolization as a loco-regional inducer of immunogenic cell death in hepatocellular carcinoma: implications for immunotherapy. J Immunother Cancer. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 126] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 25. | Guo J, Wang S, Han Y, Jia Z, Wang R. Effects of transarterial chemoembolization on the immunological function of patients with hepatocellular carcinoma. Oncol Lett. 2021;22:554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (1)] |
| 26. | Zhao G, Bi M, Liu S, Ma J, Xu F, Liu Y, Gao F, Yu Y, Zhou J, Feng Z, Wu J. Variation of NK, NKT, CD4(+) T, CD8(+) T cells, and IL-17A by CalliSpheres(®) microspheres-transarterial chemoembolization in refractory liver metastases patients. Scand J Clin Lab Invest. 2022;82:549-555. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 27. | El-Gebaly F, Abou-Saif S, Elkadeem M, Helmy A, Abd-Elsalam S, Yousef M, Elkhouly RA, Amer IF, El-Demerdash T. Study of Serum Soluble Programmed Death Ligand 1 as a Prognostic Factor in Hepatocellular Carcinoma in Egyptian Patients. Curr Cancer Drug Targets. 2019;19:896-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |