Published online Jul 15, 2024. doi: 10.4251/wjgo.v16.i7.3241
Revised: May 7, 2024
Accepted: May 24, 2024
Published online: July 15, 2024
Processing time: 134 Days and 13.3 Hours
RNA binding motif 5 (RBM5) has emerged as crucial regulators in many cancers.
To explore more functional and mechanistic exploration of RBM5 since the lack of research on RBM5 in colorectal cancer (CRC) dictates that is essential.
Through Gene Expression Profiling Interactive Analysis, we analyzed RBM5 expression in colon adenocarcinoma and rectum adenocarcinoma tissues. For detecting the mRNA expression of RBM5, quantitative real time-polymerase chain reaction was performed. Protein expression levels of RBM5, hexokinase 2, lactate dehydrogenase A, phosphatase and tensin homolog (PTEN), phosphoinositide 3-kinase (PI3K), phosphorylated-protein kinase B (p-AKT), and AKT were determined via Western blot. Functionally, cell counting kit-8 and 5-ethynyl-2’-deoxyuridine (EDU) assay were performed to evaluate proliferation of CRC cells. Invasiveness and migration of CRC cells were evaluated through conducting transwell assays. Glucose consumption, lactate production and adenosine-triphosphate (ATP) production were measured through a glucose assay kit, a lactate assay kit and an ATP production assay kit, respectively. Besides, RNA immunoprecipitation assay, half-life RT-PCR and dual-luciferase reporter assay were applied to detect interaction between RBM5 and PTEN. To establish a xenotypic tumor mice, CRC cells were subcutaneously injected into the right flank of each mouse. Protein expression of RBM5, Ki67, and PTEN in tumor tissues was examined using immunohistochemistry staining. Haematoxylin and eosin staining was used to evaluate tumor liver metastasis in mice.
We discovered down-regulation of RBM5 expression in CRC tissues and cells. RBM5 overexpression repressed proliferation, migration and invasion of CRC cells. Meantime, RBM5 impaired glycolysis in CRC cells, presenting as decreased glucose consumption, decreased lactate production and decreased ATP production. Besides, RBM5 bound to PTEN mRNA to stabilize its expression. PTEN expression was positively regulated by RBM5 in CRC cells. The protein levels of PI3K and p-AKT were significantly decreased after RBM5 overexpression. The suppressive influences of RBM5 on glycolysis, proliferation and metastasis of CRC cells were partially counteracted by PTEN knockdown. RBM5 suppressed tumor growth and liver metastasis in vivo.
This investigation provided new evidence that RBM5 was involved in CRC by binding to PTEN, expanding the importance of RBM5 in the treatment of CRC.
Core Tip: RNA binding motif 5 (RBM5) exhibited low expression in colorectal cancer (CRC) tissues and cells. RBM5 retarded tumorigenesis and metastasis of CRC in vitro and in vivo. In terms of mechanism, RBM5 overexpression-induced tumor inhibition of CRC was partially mediated by the phosphatase and tensin homolog/phosphoinositide 3-kinase/anti-protein kinase pathway. These findings extend our knowledge of the link between RBM5 and CRC, shedding light on the mechanisms how RBM5 impair CRC tumorigenesis.
- Citation: Wang CX, Liu F, Wang Y. RBM5 suppresses proliferation, metastasis and glycolysis of colorectal cancer cells via stabilizing phosphatase and tensin homolog mRNA. World J Gastrointest Oncol 2024; 16(7): 3241-3255
- URL: https://www.wjgnet.com/1948-5204/full/v16/i7/3241.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i7.3241
Colorectal cancer (CRC) ranks the third most common malignancies, which has risen to the second major cause of tumor-associated death around the world[1-3]. Main manifestations of CRC include blood in the stool, iron deficiency, abdominal pain, anemia, loss of appetite and weight loss[4]. According to the statistics in 2018, approximately two million people are diagnosed with CRC and about 50% of them died annually[5,6]. Early diagnosis has a better prognosis, with 5-year survival rates of more than 90% in early stage CRC patients and less than 10% in late stage CRC patients[7]. Despite of much progress in treatment tactics, including surgery therapy, adjuvant therapy and targeted therapy, the prognosis of CRC patients at advanced stage remains challenging[8-10]. Therefore, exploring underlying mechanisms of CRC and seeking new therapeutic targets are indispensable.
RNA-binding proteins (RBPs) are recognized as a type of proteins containing the mRNA-binding domain[11,12]. Through the binding domain, RBPs can interact with intracellular mRNA to modulate mRNA maturation, translation and localization[13]. As a member of the RBPs family, RNA binding motif 5 (RBM5) is demonstrated to implicate in pathological processes of many cancers[14,15]. For instance, RBM5 up-regulation impairs proliferation of lung cancer cells[16,17]. RBM5 up-regulation suppresses invasion and growth of prostate cancer cells[18]. Long noncoding RNA AFAP1-AS1 facilitates cell proliferation and metastasis by decreasing RBM5 expression in prostate cancer[19]. Notably, Gene Expression Profilling Interactive Analysis (GEPIA) of The Cancer Genome Atlas database shows low expression of RBM5 in CRC tissues. However, few studies have clarified the function of RBM5 in CRC.
Phosphatase and tensin homolog (PTEN) is identified as a tumor suppressor gene that participates in development of diverse tumors[20]. PTEN is demonstrated to repress invasion and metastasis of breast cancer cells[21]. PTEN is demonstrated to prevent oncogenesis in liver cancer, prostate cancer and brain cancer[22,23]. Notably, existing evidence has indicated that several members of RBPs play important roles in cancers via interacting with PTEN. For example, RBM24 functions as a tumor suppressor via stabilizing PTEN mRNA[24]. RBM38 retards tumor development in breast cancer and CRC, which binds to PTEN and maintains PTEN stability[25,26]. However, whether RBM5 can bind to PTEN in CRC deserves further exploration.
Hereon, we explored the expression and role of RBM5 in CRC. At the same time, we probed the underlying mechanism of the RBM5/PTEN link in CRC. This study implicates RBM5 as a promising target for management of CRC and enriches investigation of CRC regulation mechanisms.
Human five CRC cell lines (HT29, HCT116, SW480, LOVO, and SW620) and human normal colon epithelial cell line [fetal human cell (FHC)] were bought by the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). Dulbecco’s Modification of Eagle’s Medium (DMEM; KeyGen Biotechn, Nanjing, Jiangsu Province, China) containing 10% fetal bovine serum (FBS) and 1% (v/v) penicillin-streptomycin was used to culture these cells. All cells were incubated in a humidified atmosphere (37 °C) with 5% CO2.
The pcDNA3.1-RBM5, short hairpin (sh)-RBM5, sh-PTEN, sh-negative control (NC) and pcDNA3.1-NC were bought from RiboBio (Guangzhou, Guangdong Province, China). HCT116 and SW480 cells were seeded into 6-well plates and cultured for 24 hours until they reached a confluency of 60%-70%. Then Lipofectamine 3000 (Invitrogen, Carlsbad, CA, United States) was used to transfect above plasmids (2 µg) into HCT116 and SW480 cells for 48 hours.
Total RNAs were extracted following the guidance of a GeneJET RNA Purification Kit (Thermo Fisher Scientific, Waltham, MA, United States). The RNA concentration and purity were measured using NanoDrop 2000 (Thermo Fisher Scientific). Based on instructions of EasyScript® First-Strand complementary DNA (cDNA) Synthesis SuperMix bought from Beijing TransGen (Beijing, China), cDNA was synthesized. The reaction conditions were as follows: 37 °C for 15 minutes and 85 °C for 5 seconds. The cDNA product was immediately used as a template for the PCR reaction. The ransScript® One-Step RT-PCR SuperMix (Beijing TransGen Biotech) was used to conduct quantitative real time-polymerase chain reaction (qRT-PCR). Amplification procedures were exhibited as follows: 5 minutes at 95 °C, 30 cycles of 20 seconds at 95 °C, 30 seconds at 55 °C and 2 minutes at 72 °C. Primer sequences from Yilaibo (Shanghai, China) were exhibited as follows: RBM5-F, 5’-GCACGACTATAGGCATGACAT-3’; RBM5-R, 5’-AGTCAAACTTGTCTGCTCCA-3’; PTEN-F, 5’-CCAGGACCAGAGGAAACCT-3’; PTEN-R, 5’-GCTAGCCTCTGGATTTGA-3’; glyceraldehyde-3-phosphate dehydrogenase (GAPDH)-F, 5’-CCATCTTCCAGGAGCGAGAT-3’; GAPDH-R, 5’-TGCTGATGATCTTGAGGCTG-3’. Relative mRNA expression of RBM5 and PTEN was calculated through the 2-ΔΔct, which was normalized to GAPDH.
The cell viability was evaluated via a cell counting kit-8 (CCK-8) kit (Dojindo Molecular Technologies, Shanghai, China). In detail, SW480 and HCT116 cells (5 × 103 cells/well) were seeded into 96-well plates. These cells were incubated for 0 hour, 24 hours, 48 hours, 72 hours, and 96 hours, after which CCK-8 reagent (10 μL/well) was added to incubate at 37 °C for 3 hours. At last, the optical density at a wavelength of 450 nm was measured through a microplate reader (MG LABTECH, Durham, NC, United States).
The cell proliferation was assessed via a 5-ethynyl-2’-deoxyuridine (EDU) assay kit (KeyGen Biotech., Nanjing, China). In brief, SW480 and HCT116 cells were put into 96-well plates, incubating two days. Then 50 μM EDU was added to treat these cells for 2 hours. After fixation through 4% polyformaldehyde, 4,6-diamino-2-phenyl indole (DAPI) was added to label cell nuclei. A fluorescence microscopy (Ribobio) was utilized for visualizing EDU-positive cells.
Transwell chambers (Millipore, Billerica, MA, United States) without Matrigel or with Matrigel (diluted to a final concentration of 5 μg/μL) were used to evaluate migration and invasion of SW480 and HCT116 cells, respectively. Firstly, SW480 and HCT116 cells (5 × 104 cells/well), which were re-suspended in serum-free medium, were put on upper transwell chambers (pore size, 8 μm). And 600 µL complete medium containing 10% FBS was added to lower transwell chambers and small bubbles were avoided in the lower chamber. Following incubation for 48 hours at 37 °C in a 5% humidified atmosphere, the chambers were taken out and residual cells were removed. Cells were unable to migrate or invade to the surface of the lower chamber were removed with cotton swabs. Meantime, migrating or invading cells were fixed via paraformaldehyde (4.0%) for 20 minutes and stained via crystal violet (0.1%) for 5 min. An optical microscope from Olympus (Tokyo, Japan) was utilized for observing stained cells.
Protein extraction was carried out through radio immunoprecipitation assay (RIPA) buffer (CWBio, Beijing, China). The lysates were centrifuged at 12000 r/minute for 10 minutes at 4 °C. The supernatant was mixed with 5 × loading buffer and denatured at 100 °C for 5 minutes. A BCA Kit (Beyotime, Shanghai, China) was applied to detect protein concentration. A sodium dodecyl sulphate-polyacrylamide gel with 10% separation gel and 5% stacking gel were prepared. The protein samples (30 μg) were separated by electrophoresis in 1 × buffer (1.51 g Tris-base, 9.4 g glycine, 0.5 g SDS, 500 mL ddH2O) at 80 V for 30 minutes and at 120 V for 1 hour, followed by moving onto polyvinylidene difluoride membranes (Cytiva, Shanghai, China). Following being blocked with 5% skim milk for 2 hours, the primary antibodies including anti-RBM5 (1:2000, ab245646, Abcam, Cambridge, CA, United States), anti-PTEN (1:1000, ab267787, Abcam), anti-hexokinase 2 (HK2; 1:1000, ab209847, Abcam), anti-lactate dehydrogenase A (LDHA; 1:5000, ab52488, Abcam), anti-protein kinase B (AKT; 1:2000, ab185633, Abcam), anti-phosphorylated-protein kinase B (p-AKT; 1:500, ab38449, Abcam), anti-phosphoinositide 3-kinase (PI3K; 1:1000, ab302958, Abcam) and anti-β-actin (1:200, ab115777, Abcam) were appended to membranes. Next day, the secondary antibody (1:2000, ab6721, Abcam) was appended. At last, protein blots were visualized through the ECL chemiluminescent system, which were quantified using Image J software (NIH, United States).
RNA immunoprecipitation (RIP) assay was conducted using a Thermo Fisher RIP kit from Thermo Fisher Scientific. HCT116 and SW480 cells were dissolved with RIP lysis buffer (25 mmol/L Tris-HCl, pH = 7.5, 150 mmol/L KCl, 2 mmol/L EDTA, 0.5% NP-40, 1 mmol/L DTT, 100 U/mL RNasin). Afterwards, RIP buffer containing magnetic beads with anti-RBM5 or anti-IgG was incubated with cell lysates. Following incubation at 4 °C for 2 hours, magnetic beads were washed and 0.5 mg/mL Proteinase K was used to digest the proteins. Then immunoprecipitated RNAs were eluted from the beads, after which purified RNAs were utilized for detecting PTEN.
Firstly, actinomycin D (Sigma, MO, United States) was appended to incubate transfected HCT116 and SW480 cells. After being treated with actinomycin D for 0 hour, 2 hours, 4 hours, and 6 hours, RNAs in HCT116 and SW480 cells were collected via TRIzol RNA Purification (Life Technologies, CA, United States) and then used for qRT-PCR. The RNA half-life was calculated using the time when percent remaining of PTEN reduced to 50%.
After collecting the culture medium of HCT116 and SW480 cells, glucose consumption, lactate production and adenosine-triphosphate (ATP) production were measured through a glucose assay kit (Sigma-Aldrich, St. Louis, MO, United States), a lactate assay kit (Sigma-Aldrich) and an ATP production assay kit (Solarbio), respectively.
Dual-luciferase reporter (DLR) assay was performed to confirm the binding of RBM5 to 3’UTR of PTEN. SW480 and HCT116 cells transfected with pcDNA3.1-RBM5/pcDNA3.1-NC were plated in a 24-well plate (1 × 105 cells/well). Next day, a pGL3 reporter (Promega, Madison, WI, United States) containing several regions of PTEN 3’UTR was co-transfected into HCT116 and SW480 cells using Lipofectamine 3000 (Invitrogen). After 48 hours of transfection, cells were lysed by Lysis Buffer. The lysate was incubated with Luciferase Assay Regent, and the fireny luciferase was measured by the dual-Luciferase reporter assay system (Promega). Stop Reagent was then added, and renilla luciferase was detected. The ratio of Firefly luciferase and Renilla luciferase indicated relative luciferase activity.
BALB/c nude mice (6-week-old, female, weighing 20-30 g) were bought from Shanghai Model Organisms Center (Shanghai, China). Animal experiments were executed in accordance with the Animal Care and Use Committee of Beijing Viewsolid Biotechnology Co. LTD (VS2126A00176). For establishing xenograft tumor models, 2 × 106 HCT116 cells (suspended in 0.2 mL PBS) transfected with pcDNA3.1-NC/pcDNA3.1-RBM5 were subcutaneously injected into the right flank of each mouse (n = 5/group). The short diameter (W) and long diameter (L) of tumors were monitored every week and calculated via the formula: Volume = 0.5 × L × W2. Following injection for four weeks, mice anesthetized through pentobarbital sodium were sacrificed. Isolated tumors were weighed and subjected to further experiments.
For establishing a mouse model of liver metastasis, the spleens of anaesthetized mice were exposed by making an incision through the skin and peritoneum. Then 1 × 106 HCT116 cells transfected with pcDNA3.1-NC/pcDNA3.1-RBM5 were introduced into the spleen of each mouse (n = 5/group). These mice were housed until the end of this investigation (6 weeks). To evaluate the metastatic lesions, mouse livers were dissected for haematoxylin and eosin (H&E) staining.
Protein expression levels of RBM5, Ki67 and PTEN were examined via immunohistochemistry (IHC) staining. In brief, mouse tumor tissues were fixed through formalin, embedded through paraffin, cut into sections (4 μm), dewaxed via xylene and rehydrated by gradient alcohol. After washing with PBS, tissue sections were repaired with an antigen retrieval solution at 100 °C for 3 minutes and then cooled to room temperature for 30 minutes. Endogenous peroxidases were then blocked with 3 % H2O2 for 15 minutes at room temperature. Afterwards, these tissue sections were incubated with primary antibodies including the RBM5 antibody (1:500, ab69770, Abcam), Ki67 antibody (1:200, ab16667, Abcam) and PTEN antibody (1:2000, ab267787, Abcam) at 4 °C. Next day, the anti-rabbit secondary antibody bought from Abcam (1:500, ab6112) was appended to incubate for 30 minutes at room temperature, after which 3,3’-diaminobenzidine substrate solution was appended. Eventually, hematoxylin was applied to stain tissue sections and a microscope (Olympus) was employed to observe staining areas.
Tumor tissues were fixed with 4% paraformaldehydeat 4 °C overnight. After dehydrated by ethanol, these tissues were paraffin-embedded, cut into 5 μm slices and deparaffinized. Then a H&E staining kit (Beyotime) was used to stain these sections, followed by dehydration via xylene and graded ethanol. Finally, stained sections were observed under an Eclipse Ti-S microscope (Nikon, Tokyo, Japan).
GraphPad Prism 7.0 (GraphPad Software Inc., La Jolla, CA, United States) was used to conduct statistical analysis. Data from at least three separate experiments were exhibited as mean ± SD. Comparisons among multiple groups were assessed through one-way analysis of variance and Tukey’s post hoc analysis. For comparisons between two groups, Student’s t-test was used. Any difference with P < 0.05 was considered as statistical significance.
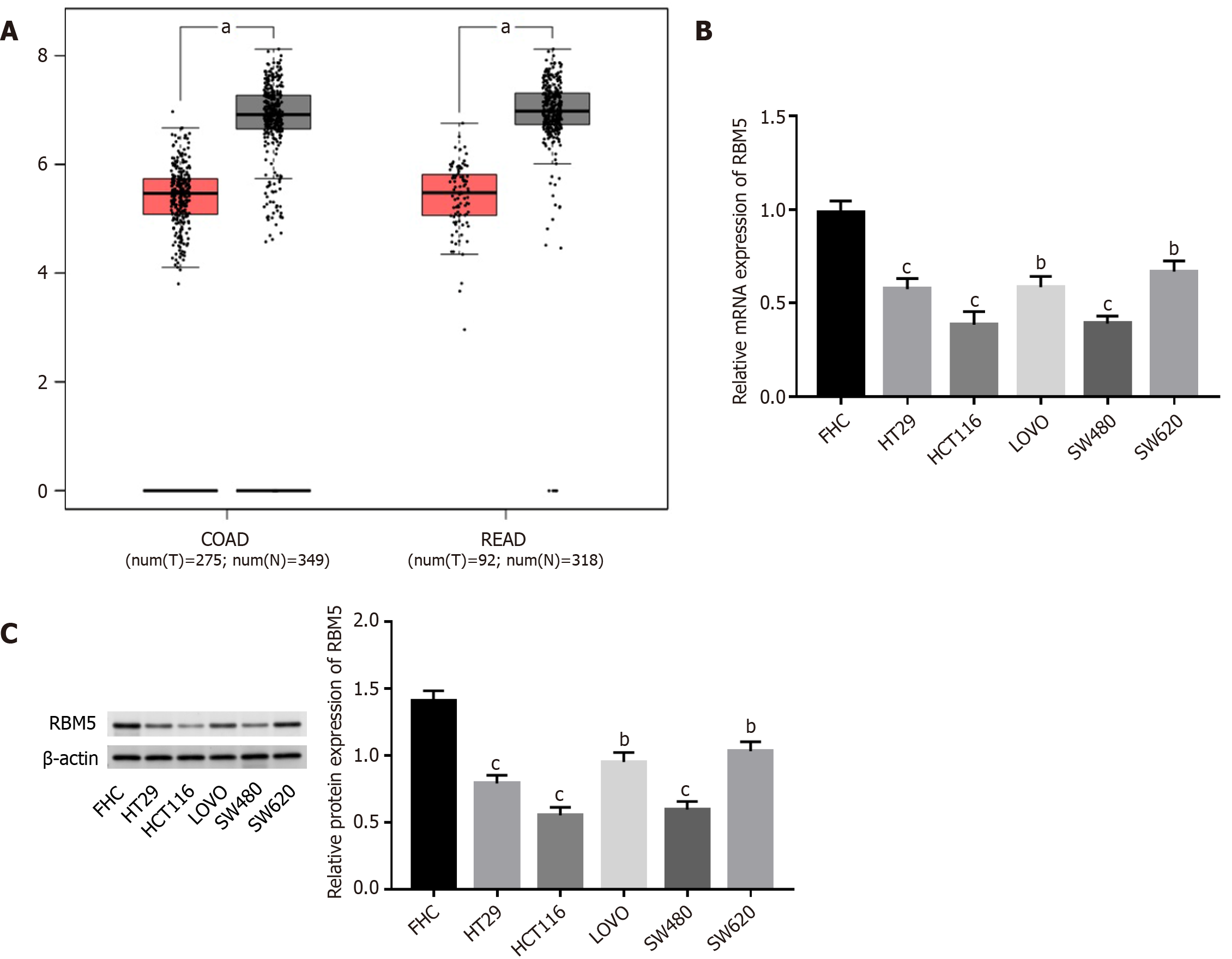
Through GEPIA, we discovered that RBM5 expression was dramatically down-regulated in colon adenocarcinoma (COAD) and rectum adenocarcinoma (READ) tissues as opposed to corresponding normal tissues (Figure 1A, P < 0.05). Then qRT-PCR and Western blot analysis were carried out for detecting expression of RBM5. Compared to FHC cells, mRNA and protein expression levels of RBM5 in CRC cells (SW620, SW480, HT29, LOVO, and HCT116) were distinctly decreased (Figure 1B and C, P < 0.01), especially SW480 and HCT116 cells.
For unraveling the influence of RBM5 on CRC cells, we overexpressed RBM5 through transfection of pcDNA3.1-RBM5. As expected, RBM5 displayed higher protein expression in the pcDNA3.1-RBM5 group than the pcDNA3.1-NC group (Figure 2A, P < 0.01). Subsequently, we performed functional experiments on RBM5. It was found that overexpression of RBM5 evidently attenuated the proliferation ability of SW480 and HCT116 cells, reflected by decreasing cell viability (Figure 2B, P < 0.001) and EDU positive cells (Figure 2C). Overexpression of RBM5 dampened migration and invasiveness of SW480 and HCT116 cells (Figure 2D-E, P < 0.01).
Furthermore, the effect of RBM5 on glycolysis was explored via measuring parameters of glycolysis. As exhibited in Figure 2F, glucose consumption, lactate production and ATP production were evidently reduced after RBM5 up-regulation in SW480 and HCT116 cells (P < 0.01). Inhibited glycolysis was also affirmed by detecting glycolysis-related proteins. It came out that protein levels of LDHA and HK2 in SW480 and HCT116 cells were all diminished in response to RBM5 overexpression (Figure 2G, P < 0.01).
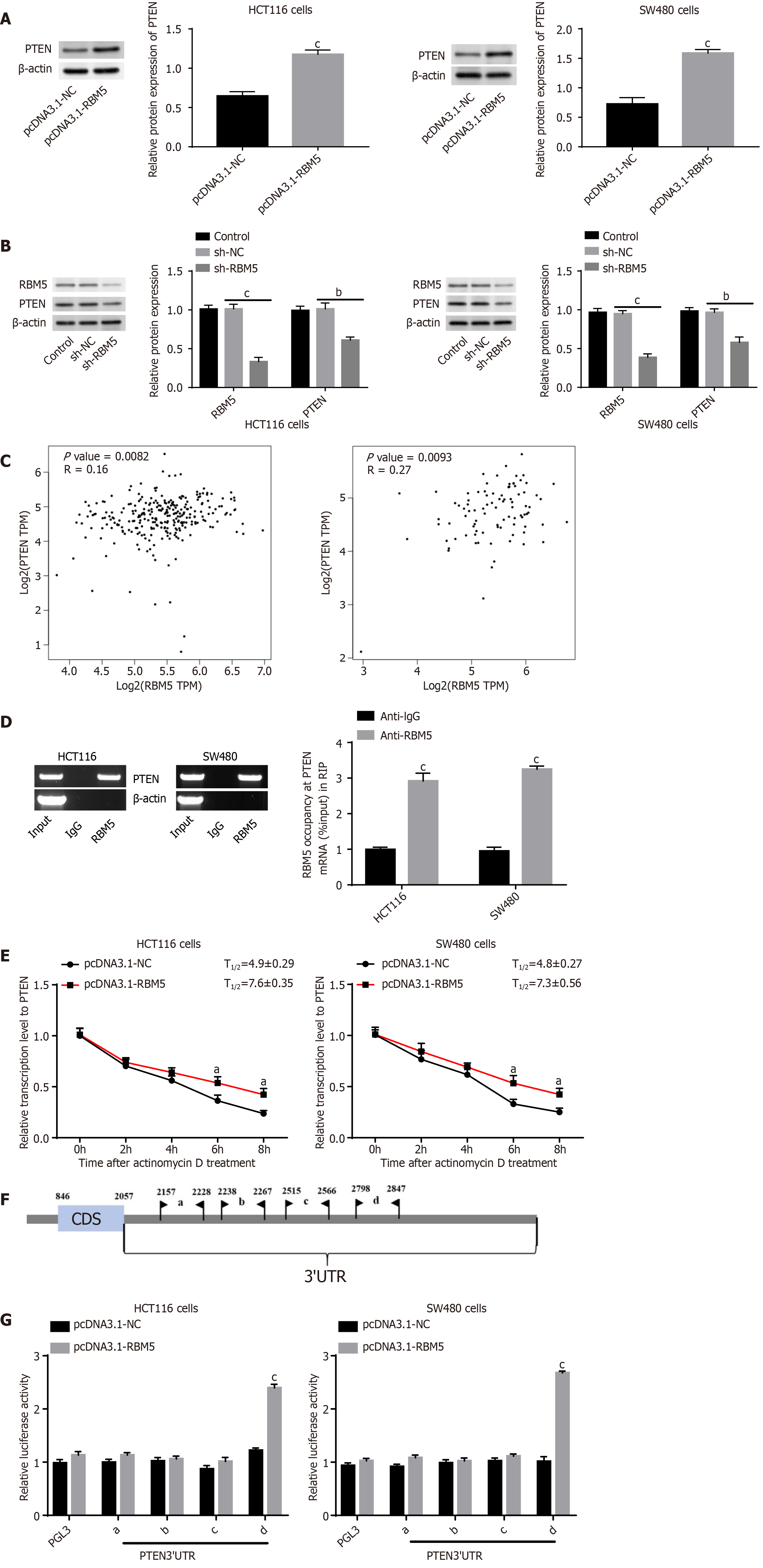
We next investigated whether RBM5 could regulate PTEN expression in CRC. Firstly, we discovered that PTEN protein expression in HCT116 and SW480 cells was boosted after RBM5 overexpression and diminished after RBM5 knockdown (Figure 3A and B, P < 0.01), suggesting that PTEN expression was positively regulated by RBM5 in CRC cells. Then correlation analysis (Figure 3C) exhibited an evident positive correlation between RBM5 and PTEN in COAD (P = 0.0082) and READ (P = 0.0093).
Afterwards, whether RBM5 could directly bind to PTEN and stabilize its expression in CRC cells was confirmed. Data from RIP assay displayed that the PTEN mRNA was dramatically enriched in RBM5 precipitate, but not in control IgG precipitate (Figure 3D, P < 0.001). Besides, data from qRT-PCR demonstrated that RBM5 overexpression led to an increase of PTEN half-life in HCT116 and SW480 cells (Figure 3E, P < 0.05). Then catRAPID (http://service.tartaglialab.com/page/catrapid_group) predicted a series of binding regions between RBM5 protein and PTEN 3’UTR (Figure 3F). DLR assay indicated that RBM5 overexpression markedly increased relative luciferase activity for reporters carrying 3’-UTR-d of PTEN, whereas failed to change 3’-UTR-a, b, and c of PTEN (Figure 3G, P < 0.001). Above data implied that 3’-UTR-d of PTEN was responsible for RBM5 to facilitate expression of PTEN.
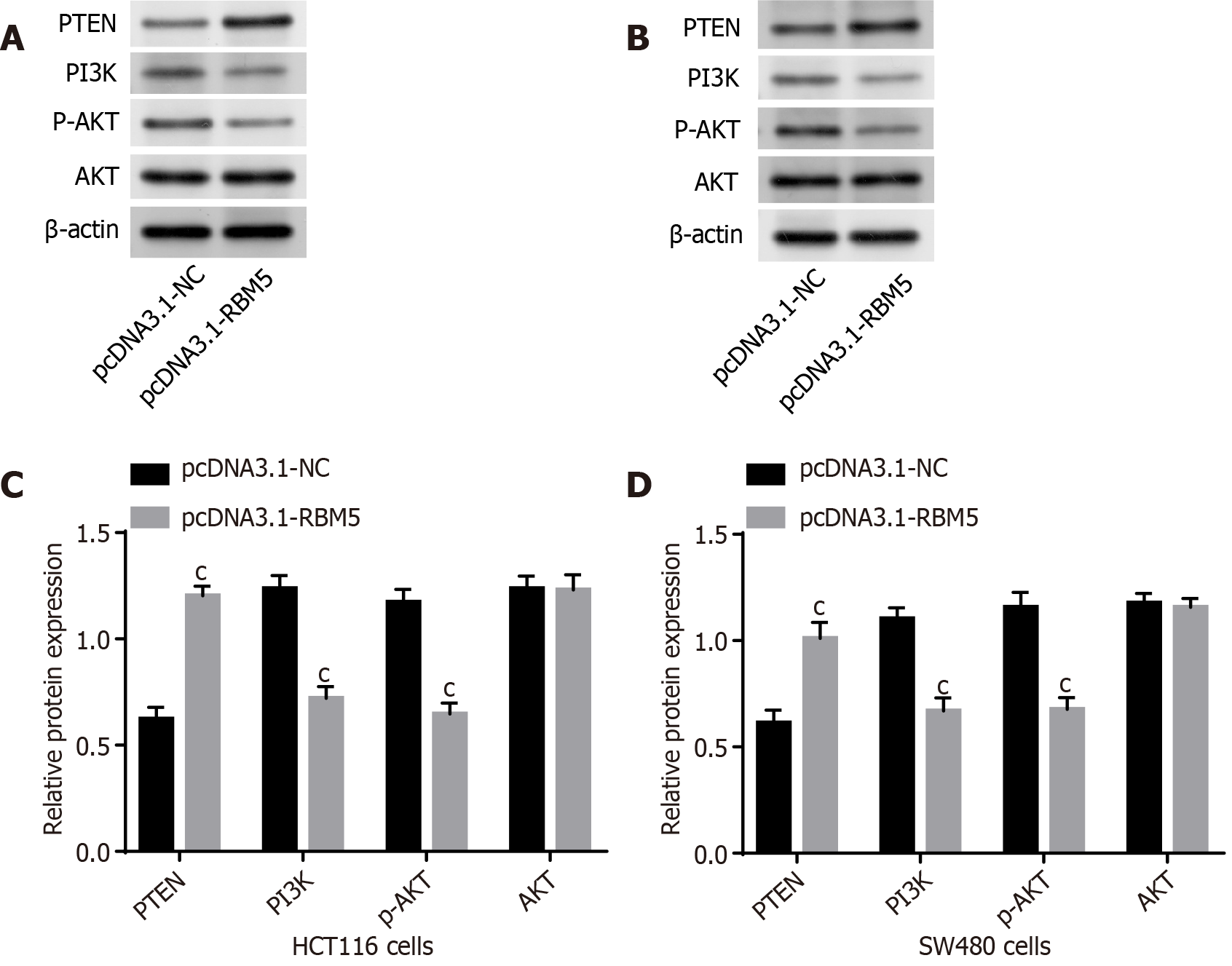
Previously, PTEN is proved to negatively modulate the PI3K/AKT signal pathway that participates in progression of diverse cancers[27,28]. We next delved into whether RBM5 made impact on the PTEN/PI3K/AKT pathway. We observed decreasing protein levels of PI3K and p-AKT as well as increasing PTEN after RBM5 overexpression in HCT116 and SW480 cells (Figure 4, P < 0.001). These findings suggested that RBM5 could regulate the PTEN/PI3K/AKT pathway in CRC cells.
For investigating whether PTEN overexpression participated in RBM5-mediated regulation in proliferation, migration, invasion and glycolysis, following experiments were conducted. Western blot analysis showed that PTEN expression was dramatically reduced after transfection of sh-PTEN in HCT116 and SW480 cells, suggesting successful PTEN silencing (Figure 5A, P < 0.001). PTEN expression was raised by RBM5 overexpression, which was reversed by PTEN silencing (Figure 5B, P < 0.01). Then rescue experiments were performed in HCT116 and SW480 cells. It was demonstrated that RBM5 overexpression-mediated inhibition of cell proliferation, migration and invasion was reversed by PTEN silencing (Figure 5C-F, P < 0.01). Besides, RBM5 overexpression-mediated suppression of glycolysis was abrogated by PTEN silencing in HCT116 and SW480 cells (Figure 5G, P < 0.05). Collectively, these data indicated that RBM5 may impair cell malignant behaviors in CRC by up-regulating PTEN expression.
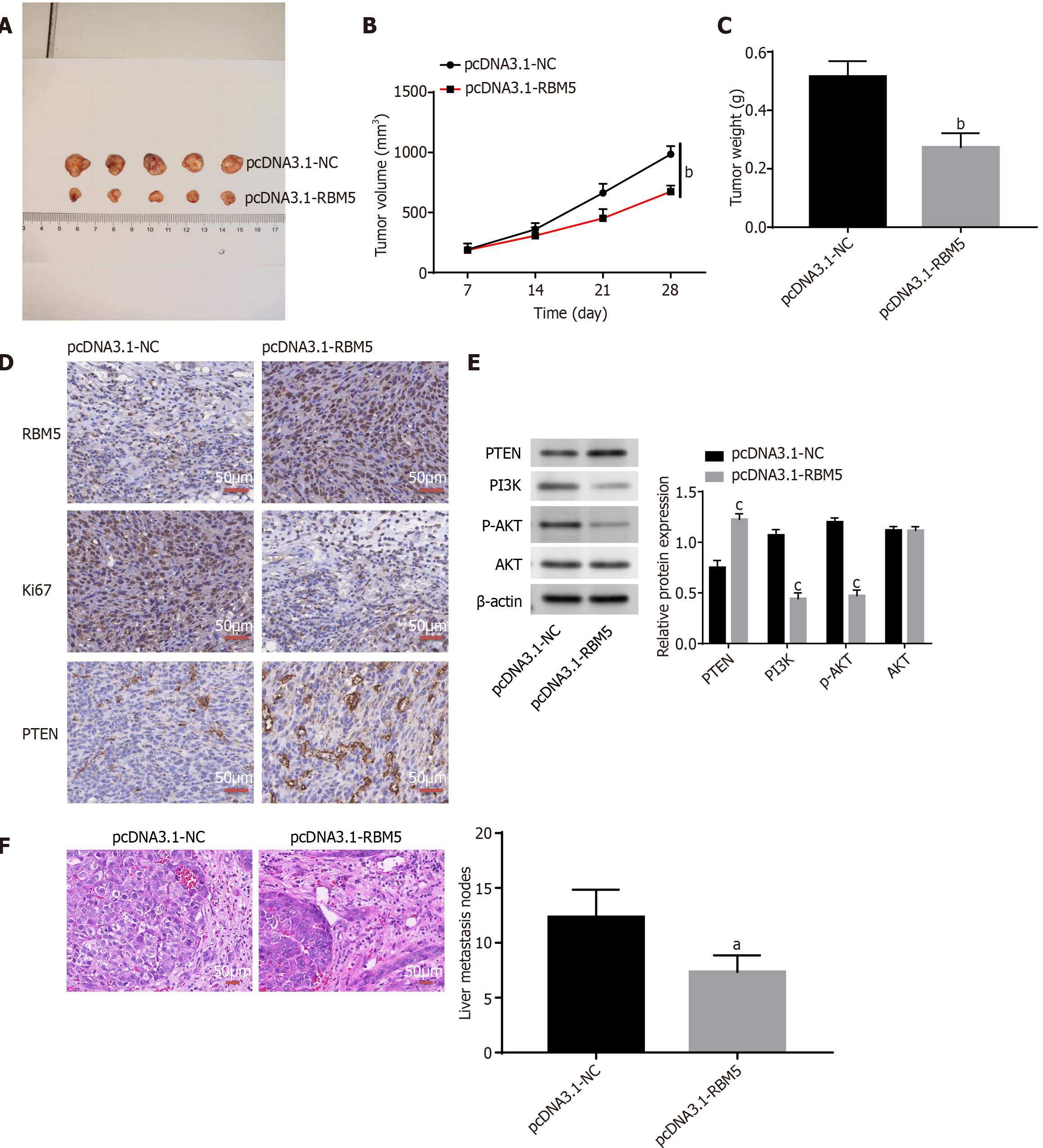
Finally, we explored the role of RBM5 in CRC in vivo. As displayed in Figure 6A-C, the mouse tumor volume (P < 0.01) and tumor weight (P < 0.01) were significantly reduced after RBM5 overexpression. Increasing PTEN expression and decreasing Ki-67 expression was found after RBM5 overexpression in xenograft mouse tumors according to IHC staining data (Figure 6D). Decreasing levels of PI3K and p-AKT as well as increasing PTEN expression were observed after RBM5 overexpression in xenograft mouse tumors (Figure 6E, P < 0.001). In addition, we discovered that less liver metastatic nodules were observed in the pcDNA3.1-RBM5 group than the pcDNA3.1-NC group (Figure 6F, P < 0.05).
Even if the CRC incidence among the elderly has declined, the incidence among younger patients is rising on account of unhealthy lifestyle[29,30]. As a result, CRC continues to be a serious health problem around the world[31,32]. In recent years, growing evidence has connected RBM5 to diverse cancers, which demonstrates that RBM5 expression is down-regulated in bladder cancer[33], non-small cell lung cancer[34], breast cancer[35] and vestibular schwannoma[36]. In our study, we also observed the decrease of RBM5 expression in CRC tissues and cells, which was in line with above mentioned studies. These results implied that RBM5 was closely related to CRC.
With the deepening research of RBM5, many studies have proved that RBM5 exerts crucial roles in occurrence and development of varied cancers. For instance, up-regulated RBM5 represses cell proliferation in lung cancer[16]. Up-regulated RBM5 suppresses cell growth and invasion in prostate cancer[18]. Down-regulated RBM5 prevents cell apoptosis in bladder cancer[33]. RBM5 overexpression inhibits cell proliferation and migration in medulloblastoma[37]. Here, we discovered that RBM5 overexpression distinctly attenuated CRC cell proliferative, migratory and invasive abilities in vitro, suggesting that RBM5 acted as a protective factor in CRC. We subsequently validated the ability of RBM5 to inhibit tumorigenesis and liver metastasis in vivo. Our findings were similar to previous studies which show that RBM5 functions as a tumor suppressor in CRC[16,18,33], and we inferred that RBM5 may be a potential target against CRC. It is uncovered that dependence on glycolysis is a sub-optimal way for cancer cells, which affects tumor microenvironment in favor of cancer malignancy and development[38-40]. Many genes are reported to inhibit glycolysis in CRC. For instance, STK25 overexpression represses aerobic glycolysis via reducing expression levels of LDHA, HK2 and GLUT1[42]. Similarly, we found that RBM5 repressed glycolysis of CRC cells, presenting as decreased glucose consumption, lactate production and ATP production as well as decreased expression levels of LDHA and HK2. Collectively, we deduced that targeting RBM5 may be a feasible method to treat CRC.
Given the fact that RBPs such as RBM24 and RBM38 play anti-tumor role in CRC through stabilizing PTEN[24,25], it is reasonable for us to assume that RBM5 may affect malignant behaviors of CRC cells via binding to PTEN. We then validated our assumption, and found that RBM5 stabilized transcript of PTEN and up-regulated PTEN via binding to 3’UTR of PTEN. Moreover, we performed rescue assays to confirm whether PTEN was involved in RBM5-mediated inhibition of cell proliferation, invasion, glycolysis and migration in CRC. We discovered that the suppressive influences of RBM5 on proliferation, invasiveness, glycolysis and migration of CRC cells were reversed by silencing of PTEN. Taken together, our findings offered evidence that RBM5 served as an anti-tumor gene via up-regulating PTEN expression in CRC, identifing a novel mechanism in which RBM5 enhanced the PTEN transcript by directly binding the 3’-UTR of PTEN mRNA. Moreover, PTEN is considered to an important negative regulator of the PI3K/Akt signal pathway that can facilitate cell proliferation, growth and migration[43,44]. This led us to focus on whether RBM5 regulated the PTEN/PI3K/Akt axis via interacting with PTEN in CRC cells. It turned out that RBM5 overexpression resulted in increasing PTEN expression as well as decreasing protein levels of PI3K and p-AKT in CRC cells and mice. This evidence strongly suggested that RBM5 overexpression regulated PTEN mRNA abundancy and leads to the inactivation of the PTEN/PI3K/Akt pathway, thereby inhibiting tumorigenesis in CRC.
In summary, RBM5 was lowly expressed in CRC tissues and cells. RBM5 overexpression-induced tumor inhibition was partially mediated by the PTEN/PI3K/Akt pathway. These findings extend our knowledge of RBM5 function in CRC, providing a potential target for treatment of CRC.
| 1. | Midgley R, Kerr D. Colorectal cancer. Lancet. 1999;353:391-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 277] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 2. | Testa U, Pelosi E, Castelli G. Colorectal cancer: genetic abnormalities, tumor progression, tumor heterogeneity, clonal evolution and tumor-initiating cells. Med Sci (Basel). 2018;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 177] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 3. | Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70:145-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2268] [Cited by in RCA: 3265] [Article Influence: 653.0] [Reference Citation Analysis (2)] |
| 4. | Morabito A, De Maio E, Di Maio M, Normanno N, Perrone F. Tyrosine kinase inhibitors of vascular endothelial growth factor receptors in clinical trials: current status and future directions. Oncologist. 2006;11:753-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 204] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 5. | Araghi M, Soerjomataram I, Jenkins M, Brierley J, Morris E, Bray F, Arnold M. Global trends in colorectal cancer mortality: projections to the year 2035. Int J Cancer. 2019;144:2992-3000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 346] [Article Influence: 57.7] [Reference Citation Analysis (0)] |
| 6. | Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin. 2023;73:233-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1548] [Reference Citation Analysis (3)] |
| 7. | Zhang X, Zhang H, Shen B, Sun XF. Chromogranin-A Expression as a Novel Biomarker for Early Diagnosis of Colon Cancer Patients. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 8. | Vassos N, Piso P. Metastatic Colorectal Cancer to the Peritoneum: Current Treatment Options. Curr Treat Options Oncol. 2018;19:49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 9. | Roth AD, Tejpar S, Delorenzi M, Yan P, Fiocca R, Klingbiel D, Dietrich D, Biesmans B, Bodoky G, Barone C, Aranda E, Nordlinger B, Cisar L, Labianca R, Cunningham D, Van Cutsem E, Bosman F. Prognostic role of KRAS and BRAF in stage II and III resected colon cancer: results of the translational study on the PETACC-3, EORTC 40993, SAKK 60-00 trial. J Clin Oncol. 2010;28:466-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 801] [Cited by in RCA: 917] [Article Influence: 57.3] [Reference Citation Analysis (0)] |
| 10. | Mohammad N, Singh SV, Malvi P, Chaube B, Athavale D, Vanuopadath M, Nair SS, Nair B, Bhat MK. Strategy to enhance efficacy of doxorubicin in solid tumor cells by methyl-β-cyclodextrin: Involvement of p53 and Fas receptor ligand complex. Sci Rep. 2015;5:11853. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 85] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 11. | Hentze MW, Castello A, Schwarzl T, Preiss T. A brave new world of RNA-binding proteins. Nat Rev Mol Cell Biol. 2018;19:327-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 764] [Cited by in RCA: 1167] [Article Influence: 166.7] [Reference Citation Analysis (0)] |
| 12. | Gerstberger S, Hafner M, Tuschl T. A census of human RNA-binding proteins. Nat Rev Genet. 2014;15:829-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1526] [Cited by in RCA: 1502] [Article Influence: 136.5] [Reference Citation Analysis (0)] |
| 13. | Brinegar AE, Cooper TA. Roles for RNA-binding proteins in development and disease. Brain Res. 2016;1647:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 124] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 14. | Kashuba VI, Szeles A, Allikmets R, Nilsson AS, Bergerheim US, Modi W, Grafodatsky A, Dean M, Stanbridge EJ, Winberg G. A group of NotI jumping and linking clones cover 2.5 Mb in the 3p21-p22 region suspected to contain a tumor suppressor gene. Cancer Genet Cytogenet. 1995;81:144-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Zhang L, Zhang Q, Yang Y, Wu C. The RNA recognition motif domains of RBM5 are required for RNA binding and cancer cell proliferation inhibition. Biochem Biophys Res Commun. 2014;444:445-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Oh JJ, Razfar A, Delgado I, Reed RA, Malkina A, Boctor B, Slamon DJ. 3p21.3 tumor suppressor gene H37/Luca15/RBM5 inhibits growth of human lung cancer cells through cell cycle arrest and apoptosis. Cancer Res. 2006;66:3419-3427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 68] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | Shao C, Zhao L, Wang K, Xu W, Zhang J, Yang B. The tumor suppressor gene RBM5 inhibits lung adenocarcinoma cell growth and induces apoptosis. World J Surg Oncol. 2012;10:160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Yang ZG, Ma XD, He ZH, Guo YX. miR-483-5p promotes prostate cancer cell proliferation and invasion by targeting RBM5. Int Braz J Urol. 2017;43:1060-1067. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 19. | Yang ZT, An F, Hu JD, Zhao WH. Long noncoding RNA AFAP1-AS1 accelerates the proliferation and metastasis of prostate cancer via inhibiting RBM5 expression. Eur Rev Med Pharmacol Sci. 2019;23:3284-3290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 20. | Nussinov R, Zhang M, Tsai CJ, Jang H. Phosphorylation and Driver Mutations in PI3Kα and PTEN Autoinhibition. Mol Cancer Res. 2021;19:543-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 21. | Qi Y, Liu J, Chao J, Scheuerman MP, Rahimi SA, Lee LY, Li S. PTEN suppresses epithelial-mesenchymal transition and cancer stem cell activity by downregulating Abi1. Sci Rep. 2020;10:12685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 22. | Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, Bigner SH, Giovanella BC, Ittmann M, Tycko B, Hibshoosh H, Wigler MH, Parsons R. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943-1947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3525] [Cited by in RCA: 3583] [Article Influence: 128.0] [Reference Citation Analysis (0)] |
| 23. | Li MF, Guan H, Zhang DD. Effect of overexpression of PTEN on apoptosis of liver cancer cells. Genet Mol Res. 2016;15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Xia RM, Liu T, Li WG, Xu XQ. RNA-binding protein RBM24 represses colorectal tumourigenesis by stabilising PTEN mRNA. Clin Transl Med. 2021;11:e383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 25. | Zhou XJ, Wu J, Shi L, Li XX, Zhu L, Sun X, Qian JY, Wang Y, Wei JF, Ding Q. PTEN expression is upregulated by a RNA-binding protein RBM38 via enhancing its mRNA stability in breast cancer. J Exp Clin Cancer Res. 2017;36:149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 26. | Guan B, Li G, Wan B, Guo X, Huang D, Ma J, Gong P, Guo J, Bu Y. RNA-binding protein RBM38 inhibits colorectal cancer progression by partly and competitively binding to PTEN 3'UTR with miR-92a-3p. Environ Toxicol. 2021;36:2436-2447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 27. | Carnero A, Blanco-Aparicio C, Renner O, Link W, Leal JF. The PTEN/PI3K/AKT signalling pathway in cancer, therapeutic implications. Curr Cancer Drug Targets. 2008;8:187-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 532] [Cited by in RCA: 659] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 28. | Worby CA, Dixon JE. PTEN. Annu Rev Biochem. 2014;83:641-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 431] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 29. | Thanikachalam K, Khan G. Colorectal Cancer and Nutrition. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 231] [Cited by in RCA: 474] [Article Influence: 79.0] [Reference Citation Analysis (0)] |
| 30. | Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66:683-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3058] [Cited by in RCA: 3299] [Article Influence: 412.4] [Reference Citation Analysis (3)] |
| 31. | Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, Brenner H, Dicker DJ, Chimed-Orchir O, Dandona R, Dandona L, Fleming T, Forouzanfar MH, Hancock J, Hay RJ, Hunter-Merrill R, Huynh C, Hosgood HD, Johnson CO, Jonas JB, Khubchandani J, Kumar GA, Kutz M, Lan Q, Larson HJ, Liang X, Lim SS, Lopez AD, MacIntyre MF, Marczak L, Marquez N, Mokdad AH, Pinho C, Pourmalek F, Salomon JA, Sanabria JR, Sandar L, Sartorius B, Schwartz SM, Shackelford KA, Shibuya K, Stanaway J, Steiner C, Sun J, Takahashi K, Vollset SE, Vos T, Wagner JA, Wang H, Westerman R, Zeeb H, Zoeckler L, Abd-Allah F, Ahmed MB, Alabed S, Alam NK, Aldhahri SF, Alem G, Alemayohu MA, Ali R, Al-Raddadi R, Amare A, Amoako Y, Artaman A, Asayesh H, Atnafu N, Awasthi A, Saleem HB, Barac A, Bedi N, Bensenor I, Berhane A, Bernabé E, Betsu B, Binagwaho A, Boneya D, Campos-Nonato I, Castañeda-Orjuela C, Catalá-López F, Chiang P, Chibueze C, Chitheer A, Choi JY, Cowie B, Damtew S, das Neves J, Dey S, Dharmaratne S, Dhillon P, Ding E, Driscoll T, Ekwueme D, Endries AY, Farvid M, Farzadfar F, Fernandes J, Fischer F, G/Hiwot TT, Gebru A, Gopalani S, Hailu A, Horino M, Horita N, Husseini A, Huybrechts I, Inoue M, Islami F, Jakovljevic M, James S, Javanbakht M, Jee SH, Kasaeian A, Kedir MS, Khader YS, Khang YH, Kim D, Leigh J, Linn S, Lunevicius R, El Razek HMA, Malekzadeh R, Malta DC, Marcenes W, Markos D, Melaku YA, Meles KG, Mendoza W, Mengiste DT, Meretoja TJ, Miller TR, Mohammad KA, Mohammadi A, Mohammed S, Moradi-Lakeh M, Nagel G, Nand D, Le Nguyen Q, Nolte S, Ogbo FA, Oladimeji KE, Oren E, Pa M, Park EK, Pereira DM, Plass D, Qorbani M, Radfar A, Rafay A, Rahman M, Rana SM, Søreide K, Satpathy M, Sawhney M, Sepanlou SG, Shaikh MA, She J, Shiue I, Shore HR, Shrime MG, So S, Soneji S, Stathopoulou V, Stroumpoulis K, Sufiyan MB, Sykes BL, Tabarés-Seisdedos R, Tadese F, Tedla BA, Tessema GA, Thakur JS, Tran BX, Ukwaja KN, Uzochukwu BSC, Vlassov VV, Weiderpass E, Wubshet Terefe M, Yebyo HG, Yimam HH, Yonemoto N, Younis MZ, Yu C, Zaidi Z, Zaki MES, Zenebe ZM, Murray CJL, Naghavi M. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2017;3:524-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2838] [Cited by in RCA: 2956] [Article Influence: 369.5] [Reference Citation Analysis (0)] |
| 32. | Zhang H, Ding C, Li Y, Xing C, Wang S, Yu Z, Chen L, Li P, Dai M. Data mining-based study of collagen type III alpha 1 (COL3A1) prognostic value and immune exploration in pan-cancer. Bioengineered. 2021;12:3634-3646. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 33. | Zhang YP, Liu KL, Wang YX, Yang Z, Han ZW, Lu BS, Qi JC, Yin YW, Teng ZH, Chang XL, Li JD, Xin H, Li W. Down-regulated RBM5 inhibits bladder cancer cell apoptosis by initiating an miR-432-5p/β-catenin feedback loop. FASEB J. 2019;33:10973-10985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 34. | Liang H, Zhang J, Shao C, Zhao L, Xu W, Sutherland LC, Wang K. Differential expression of RBM5, EGFR and KRAS mRNA and protein in non-small cell lung cancer tissues. J Exp Clin Cancer Res. 2012;31:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 35. | Rintala-Maki ND, Goard CA, Langdon CE, Wall VE, Traulsen KE, Morin CD, Bonin M, Sutherland LC. Expression of RBM5-related factors in primary breast tissue. J Cell Biochem. 2007;100:1440-1458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 36. | Welling DB, Lasak JM, Akhmametyeva E, Ghaheri B, Chang LS. cDNA microarray analysis of vestibular schwannomas. Otol Neurotol. 2002;23:736-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 80] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 37. | Yu J, Ji G, Shi W, Zhao R, Shen W, Zheng J, Li H, Jiang F. RBM5 Acts as Tumor Suppressor in Medulloblastoma through Regulating Wnt/β-Catenin Signaling. Eur Neurol. 2020;83:242-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 38. | Sun L, Suo C, Li ST, Zhang H, Gao P. Metabolic reprogramming for cancer cells and their microenvironment: Beyond the Warburg Effect. Biochim Biophys Acta Rev Cancer. 2018;1870:51-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 246] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 39. | Boedtkjer E, Pedersen SF. The Acidic Tumor Microenvironment as a Driver of Cancer. Annu Rev Physiol. 2020;82:103-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 236] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 40. | Lu H, Xu J, Yang J, Wang Z, Xu P, Hao Q, Luo W, Li S, Li Z, Xue X, Zheng H, Zhou Z, Wu H, Ma X, Li Y. On-demand targeting nanotheranostics with stimuli-responsive releasing property to improve delivery efficiency to cancer. Biomaterials. 2022;290:121852. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 41. | Wu F, Gao P, Wu W, Wang Z, Yang J, Di J, Jiang B, Su X. STK25-induced inhibition of aerobic glycolysis via GOLPH3-mTOR pathway suppresses cell proliferation in colorectal cancer. J Exp Clin Cancer Res. 2018;37:144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 42. | Zhang W, Tong D, Liu F, Li D, Li J, Cheng X, Wang Z. RPS7 inhibits colorectal cancer growth via decreasing HIF-1α-mediated glycolysis. Oncotarget. 2016;7:5800-5814. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 43. | Khalid A, Hussain T, Manzoor S, Saalim M, Khaliq S. PTEN: A potential prognostic marker in virus-induced hepatocellular carcinoma. Tumour Biol. 2017;39:1010428317705754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 44. | Chow LM, Baker SJ. PTEN function in normal and neoplastic growth. Cancer Lett. 2006;241:184-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 206] [Article Influence: 10.8] [Reference Citation Analysis (0)] |