Published online Jul 15, 2024. doi: 10.4251/wjgo.v16.i7.3230
Revised: April 29, 2024
Accepted: May 17, 2024
Published online: July 15, 2024
Processing time: 140 Days and 6 Hours
Aldehyde (ALDH2) dysfunction has been verified to contribute to human cancers.
To investigate the molecular mechanism and biological function of ALDH2 in colorectal cancer (CRC) progression.
Human CRC cells with high expression of ALDH2 were screened. After shRNA ALDH2 (sh-ALDH2) transfection, phenotypes [proliferation, apoptosis, acetaldehyde (ACE) accumulation, DNA damage] of CRC cells were verified using cell counting kit-8, flow cytometry, ACE assay, and comet assays. Western blotting was used for evaluation of the apoptosis proteins (Bax and Bcl-2) and JNK/p38 MAPK pathway-associated proteins. We subjected CVT-10216 (a selective ALDH2 inhibitor) to nude mice for establishment of SK-CO-1 mouse xenograft model and observed the occurrence of CRC.
The inhibition of ALDH2 could promote the malignant structures of CRC cells, including apoptosis, ACE level, and DNA damage, and cell proliferation was decreased in the sh-ALDH2 group, whereas ALDH2 agonist Alda-1 reversed features. ALDH2 repression can cause ACE accumulation, whereas ACE enhanced CRC cell features related to increased DNA damage. Additionally, ALDH2 repression led to JNK/P38 MAPK activation, and apoptosis, ACE accumulation, and DNA damage were inhibited after p38 MAPK inhibitor SB203580 and JNK inhibitor SP600125 addition. ACE accumulation and raised DNA damage were recognized in CVT-10216 treated-mouse tumor tissues in vivo.
The repression of ALDH2 led to ACE accumulation, inducing cell apoptosis and DNA damage by the JNK/p38 MAPK signaling pathway activation in CRC.
Core Tip: This work demonstrated that a (ALDH2) repression caused the accumulation of acetaldehyde, inducing cell apoptosis and DNA damage by means of activating the JNK/p38 MAPK signaling pathway in colorectal cancer (CRC). ALDH2 is utilized as a therapeutic target for reversing patients with CRC.
- Citation: Yu M, Chen Q, Lu YP. Aldehyde dehydrogenase 2 family member repression promotes colorectal cancer progression by JNK/p38 MAPK pathways-mediated apoptosis and DNA damage. World J Gastrointest Oncol 2024; 16(7): 3230-3240
- URL: https://www.wjgnet.com/1948-5204/full/v16/i7/3230.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i7.3230
Colorectal cancer (CRC) is a common cancer, showing a high mortality throughout the world[1]. According to the Global Cancer Observatory report in 2020, over 1.9 million new CRC cases and 930000 deaths were estimated[2]. CRC has many risk factors, including environmental and inherited. And Fewer than 10% of patients have an indeed inherited predisposition to CRC[3]. Literatures show that many lifestyle-related factors associate with CRC, including obesity, physical activity, smoking, alcohol intake, and certain dietary variables. Other risk factors, such as being older, whether we have a history of adenomatous polyps (adenomas), personal history of inflammatory bowel disease, and family history of CRC or adenomas, are also risks that we cannot change[4]. Literature also shows that alcohol abuse is an essential risk factor for CRC[5]. Ethanol is mainly oxidized to acetaldehyde (ACE) through ethanol dehydrogenase, and ACE is a reagent that can trigger tumors, including CRC[6,7].
ACE is formed by the ethanol metabolism by ethanol dehydrogenase, catalase, and cytochrome P450 2E1 (CYP2E1). The previous study has shown that ACE can interfere with the antioxidant defense system and produce reactive oxygen species to inhibit DNA methylation and repair and form DNA and protein adducts[6]. The main mitochondrial enzyme that protects cells from ACE toxicity is Aldehyde (ALDH2)[8]. ALDH2 has 19 subtypes, and ALDH2 can detoxify ACE produced by ethanol metabolism in the liver[9]. In several tumor types, ALDH2 inhibition is related to cytotoxicity inhibition, DNA damage, and carcinogenic effects[10,11]. In addition, chromosomal instability helps cancer metastasis through cytoplasmic DNA produced by gDNA cleavage[12]. Furthermore, ALDH2 can inhibit cell migration and proliferation, help apoptosis, and change the epithelial-mesenchymal transition process[13].
We examined the ALDH2 function in CRC and identified that ALDH2 repression can cause raised malignant features. Proliferation capacity is measured by ACE accumulation. ALDH2 repression caused ACE accumulation, which induces DNA damage and cell apoptosis by the JNK/p38 MAPK signaling pathway activation in CRC.
Normal human colon mucosal epithelial cell line (NCM460), human CRC cell lines, NCM460, CL-40, SK-CO-1, SW-403, HT-29, COLO-678, and SW480 were purchased from American Type Culture Collection (Manassas, VA, United States). These cells were cultured in Iscove’s Modified Dulbecco’s Media and added with 10% fetal bovine serum (10099158, ThermoFisher, United States) and antibiotics (1%). The culture environment was 37 °C under 5% CO2. Cells were preserved with Alda-1 (1 μmol) or with a vehicle for 48 h at 37 °C. The plasmids (RiboBio, Beijing, China) of shRNA oligonucleotides targeting ALDH2 [shRNA ALDH2 (sh-ALDH2): 5’-ATGTCTCCGGTATTATGCC-3’), and NC (sh-NC: 5’-ACTACCGTTGTTATAGGTG-3’) were used. These above mentioned plasmids were transfected into CRC cells with Lipofectamine 3000 (L3000150, Invitrogen, United States) and cultured for 2 d.
cDNA synthesis from transfected cells was done using total RNA (500 ng) extracted by EcoDry Reverse Transcription Premix (639278, TaKaRa, Tokyo, Japan). Quantitative reverse transcriptase PCR (qRT-PCR) was done by means of SYBR-green (11784200, Invitrogen). The relative expression was calculated through the 2-ΔΔCT approach[14] with GAPDH serving as internal reference. Primers are listed in Table 1.
| Name | Sequence | |
| ALDH2 | Forward | 5′-CCTCGGCTACATCAACACG-3' |
| Reverse | 5’-CCCAACAACCTCCTCTATGG-3' | |
| GAPDH | Forward | 5′-GGACCTGACCTGCCGTCTAG-3′ |
| Reverse | 5′-GTAGCCCAGGATGCCCTTGA-3′ | |
The transfected CRC cells were dissolved. Then, the total protein (40 µg) was purified and quantified through PierceTM BCA protein assay kit (23227, ThermoFisher, United States). After that, we detached proteins by SDS-PAGE (10%) and then shifted them to PVDF membranes (IPVH00010, Millipore, United States). We blocked with 5% skimmed milk (232100, BD, United States) and cultured proteins with anti-ALDH2 (1:1000, ab227021, Abcam, United Kingdom), anti-Bax (1:1000, ab182733), anti-Bcl2 (1:1000, ab182858), anti-γH2AX (1:1000, ab243906), anti-p-JNK (phospho T183+Y185) (1:1000, ab307802), anti-JNK (1:1000, ab208035), anti-p-P38 MAPK (1:1000, ab39398), anti-P38 MAPK (1:1000, ab308333), and anti-β-actin (1:1000, ab8227) 24 h at 4 °C. Proteins continued to incubate with the anti-rabbit secondary antibody (1:5000; SA00001-2, SanYing, China) for one hour after washing the primary antibodies. We examined protein bands by the ECL chemiluminescent system (Thermo Fisher Scientific, United States). Image J was applied for the quantification of protein blots.
The proliferation capabilities of the transfected CRC cells were analyzed by cell counting kit-8 (CCK-8) assay. We seeded the sh-ALDH2-transfected cells (1 × 103/well) in a 96-well plate. After 1 d, we added CCK-8 reagent (10 μL, Catalog No. AD10, Dojindo Molecular Technologies, Inc., Kumamoto, Japan) to wells at room temperature. At 450 nm, we monitored absorbance at 0, 24, 48, 72, and 96 h for the evaluation of the cell viability.
The CRC cell apoptosis was detected using a flow cytometer (LSRII, BD Biosciences, United States). Briefly, cells were harvested by trypsinization and resuspended in 1 × buffer (Annexin V-FITC/PI apoptosis detection kit; SY0471, Beyotime Biotechnology, China). In total, 100 µL of this cell suspension (1 × 106 cells) was incubated with 5 µL Annexin V-FITC and propidium iodide at 4 ˚C in the dark for 15 min. The stained cells were analyzed using a BD FACSCalibur™ flow cytometer and FlowJo software (version 7.2.4; FlowJo LLC). Q2 (early apoptosis) and Q3 (late apoptosis) quadrants’ cells were considered as the apoptotic cells.
Methanol (80%, R40121, Thermo Fisher Scientific, United States) was used as an extraction reagent. For sample detection, 800 μL acetonitrile (80%, 4340863, Thermo Fisher Scientific) and dinitrophenylhydrazine (200 μL, D199303, SigmaAldrich, United States) were added. The samples underwent a triple homogenization step, employing the Bertin Precellys 24 Dual Multifunctional sample homogenizer (Bertin, France) at 5500 rpm for 20 s each. Following homogenization, the samples were subjected to a sequential temperature treatment, initially stored at -80 °C for 1 h and allowed to equilibrate at 25 °C for 4 h. Subsequent to these preparations, the sample homogenate underwent a derivatization process. Post-derivatization, the samples underwent centrifugation at 20000 g for 10 min. The supernatant was carefully collected. The collected supernatant was subjected to vacuum drying. To reconstitute the dried samples for subsequent LC-MS (AB SCIEX 4000) analysis, 200 μL of acetonitrile was added.
The assessment of DNA damage in CRC cells was performed using the comet assay, employing a Comet Assay kit (4250-050-K, TREVIGEN, United States). Cells were trypsinized and resuspended in ice-cold phosphate-buffered saline at 2 × 105 cells/mL, and a 50 μL cell suspension was combined with 500 μL preheated comet LMA garose. This mixture was deposited at the center of object slides and allowed to settle for 30 minutes at 4 °C until a distinct 0.5 mm clear ring emerged at the CometSlide™ area edge. Subsequently, slides were immersed in a 4 °C Lysis Solution overnight to enhance sensitivity. After a 30-min wash with neutral electrophoresis buffer (100 mmol/L tris base, 300 mmol/L sodium acetate, pH 9.0), samples underwent electrophoresis at 21 volts for 45 min at 4 °C. Neutral electrophoresis buffer was drained, and slides were submerged in DNA Precipitation Solution for 30 min, followed by a 30-min immersion in 70% ethanol at 25 °C. The dried slides were stained with SYBR green I (S7563, Invitrogen, United States), and images were captured using a Zeiss microscope (LSM 700, Carl Zeiss, Germany).
Mice (5-week-old of age; nude mice, BALB/c; males) were bought from Vital River Laboratories (Beijing, China). We raised mice routinely for one week to adapt to the environment. SK-CO-1 cells (3 × 106) were injected into the mice’s inguinal skin. The tumor growth was monitored for 7 d. All mice were randomized into two groups (n = 5 per group) and subjected to CVT-10216 treatment (experimental group) or Vehicle (control group) at 50 mg/kg daily after three weeks. After a period of 2 wk, we killed nude mice with an overdose of pentobarbital. All animal experiments were approved by the Animal Ethics Committee of Beijing Viewsolid Biotechnology Co. LTD (VS2126A00173). The authors have read the ARRIVE Guidelines, and the manuscript was prepared and revised according to the ARRIVE Guidelines.
GraphPad Prism 7.0 was utilized for analyzing data with an expression of ± SD. We performed single-group comparisons using a student’s t-test. We analyzed multiple group differences by means of an ANOVA test. P < 0.05 showed statistical significance.
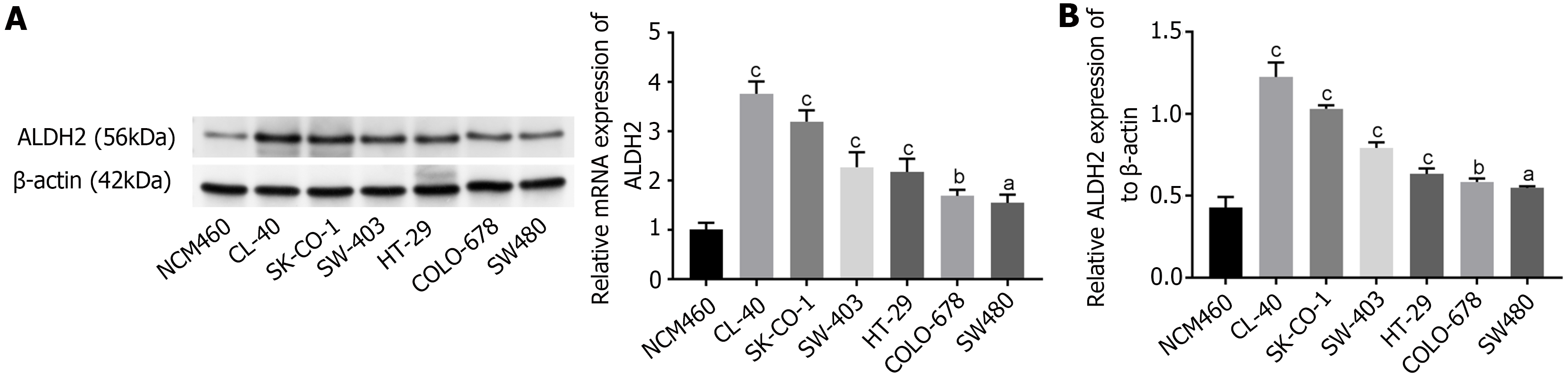
We analyzed expression levels of ALDH2 in NCM460, CL-40, SK-CO-1, SW-403, HT-29, COLO-678 and SW480 via western blot analysis. ALDH2 expression levels were up-regulated in CRC cell lines, including CL-40, SK-CO-1, SW-403, HT-29, COLO-678 and SW480, comparing to that in NCM460 (P < 0.05, P < 0.01, P < 0.001). Only two cell lines (CL-40, SK-CO-1) expressed relatively high levels of ALDH2 (Figure 1A). Moreover, the ALDH2 mRNA expression in CRC cell lines was higher than that in NCM460 (P < 0.001, P < 0.01, P < 0.05; Figure 1B).
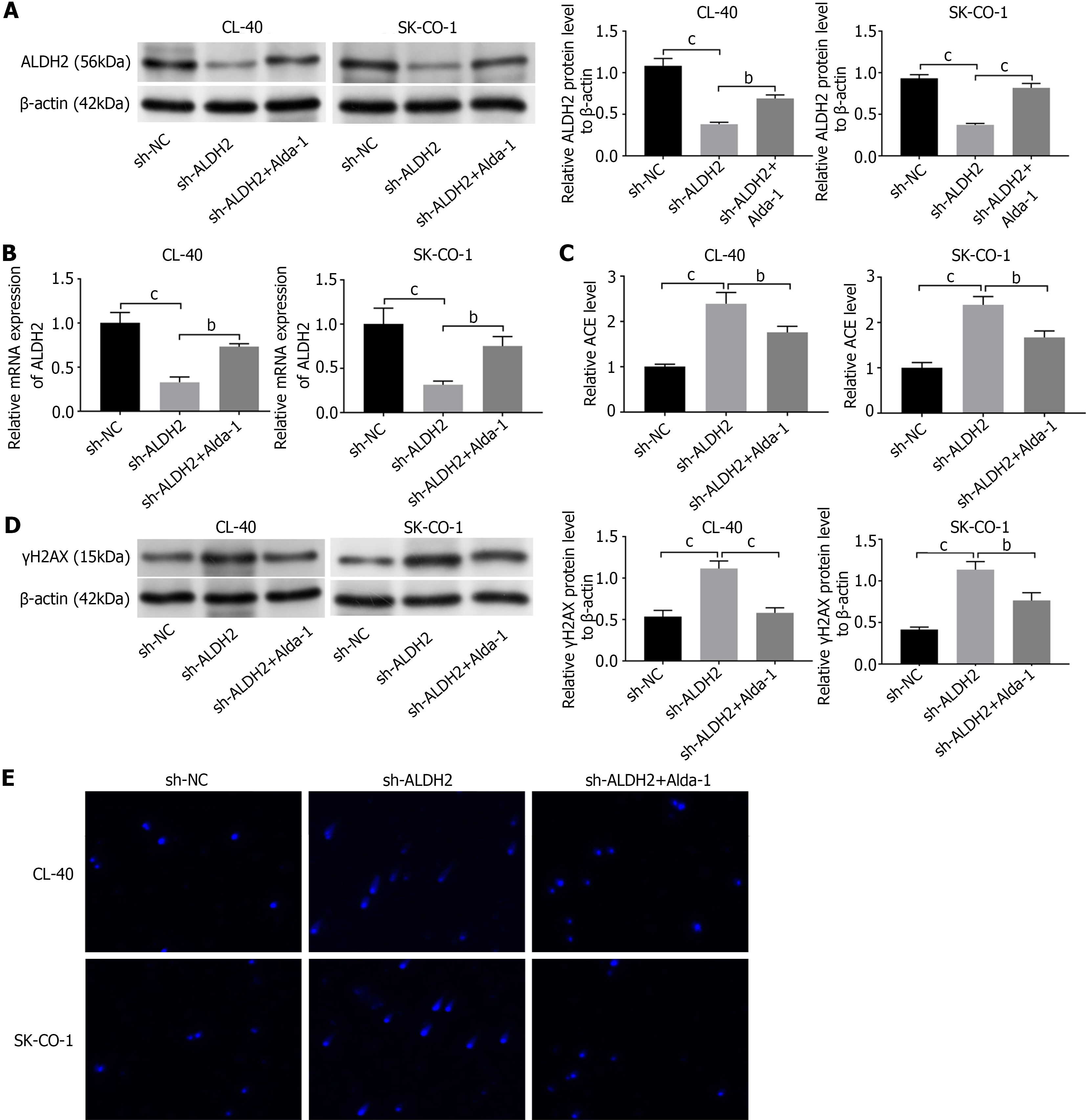
We transfected CL-40 and SK-CO-1 cells with a shRNA to knock down ALDH2 (sh-ALDH2). We examined the Alda-1’s effect (a selective agonist of ALDH2)[15]. Western blotting was utilized to detect cell transfection efficiency (Figure 2A). ALDH2 was lowly expressed in sh-IGF2-transfected cells (P < 0.001). while the sh-ALDH2 cells treated with Alda-1 (1 μM), could reverse the down-expressed ALDH2 when comparing with the sh-ALDH2 group (P < 0.01). qRT-PCR results revealed the same trend as Western blotting (P < 0.01, P < 0.001; Figure 2B). We measured the ACE amount in sh-ALDH2 CL-40/SK-CO-1 cells. Sh-ALDH2 indeed caused an increased ACE in CL-40 and SK-CO-1-shALDH2 cells when compared to that in sh-NC cells (P < 0.001); Alda-1 treatment could exhibit significantly reduced ACE level as compared to the shALDH2 group (P < 0.01; Figure 2C). We examined the γH2AX expression in the transfected cells with or without treatment of Alda-1. sh-ALDH2 exhibited increased levels of γH2AX in CL-40 and SK-CO-1 cells Without treatment (P < 0.001). However, under the Alda-1 treatment, sh-ALDH2+Alda-1 cells exhibited reduced γH2AX as compared to sh-ALDH2 group (P < 0.01, P < 0.001; Figure 2D). We investigated DNA damages in CL-40 and SK-CO-1 cells via comet assay. The induced intensive DNA damage in sh-ALDH2 cells was shown, addition of treatment inhibits the DNA damage in CL-40 and SK-CO-1 cells (Figure 2E). ALDH2 could remission exogenous ACE and DNA damage in CRC cells.
We did flow cytometric analysis and western blotting to evaluate the impact of ALDH2 deficiency on CL-40 and SK-CO-1 cell apoptosis. The cells transfected with sh-ALDH2 showed more apoptotic cells, indicating that the decreased expression of ALDH2 led to an increase in CL-40 and SK-CO-1 apoptosis, and Alda-1 treatment could reverse this trend (P < 0.001; Figure 3A). Likewise, we detected Bax and Bcl-2 expression levels by western blot assay. sh-AlDH2-transfected cells exhibited lower Bcl-2 expression level and higher Bax expression as comparing to the sh-NC group (P < 0.001). Alda-1 treatment reversed the outcome of sh-ALDH2 on Bax/Bcl-2 expression (P < 0.001; Figure 3B). Moreover, CCK-8 results showed that sh-ALDH2 transfection could inhibit the cell viability in CL-40 and SK-CO-1 cells, while cell viability was evidently increased after treatment of Alda-1 (P < 0.01, P < 0.001; Figure 3C).
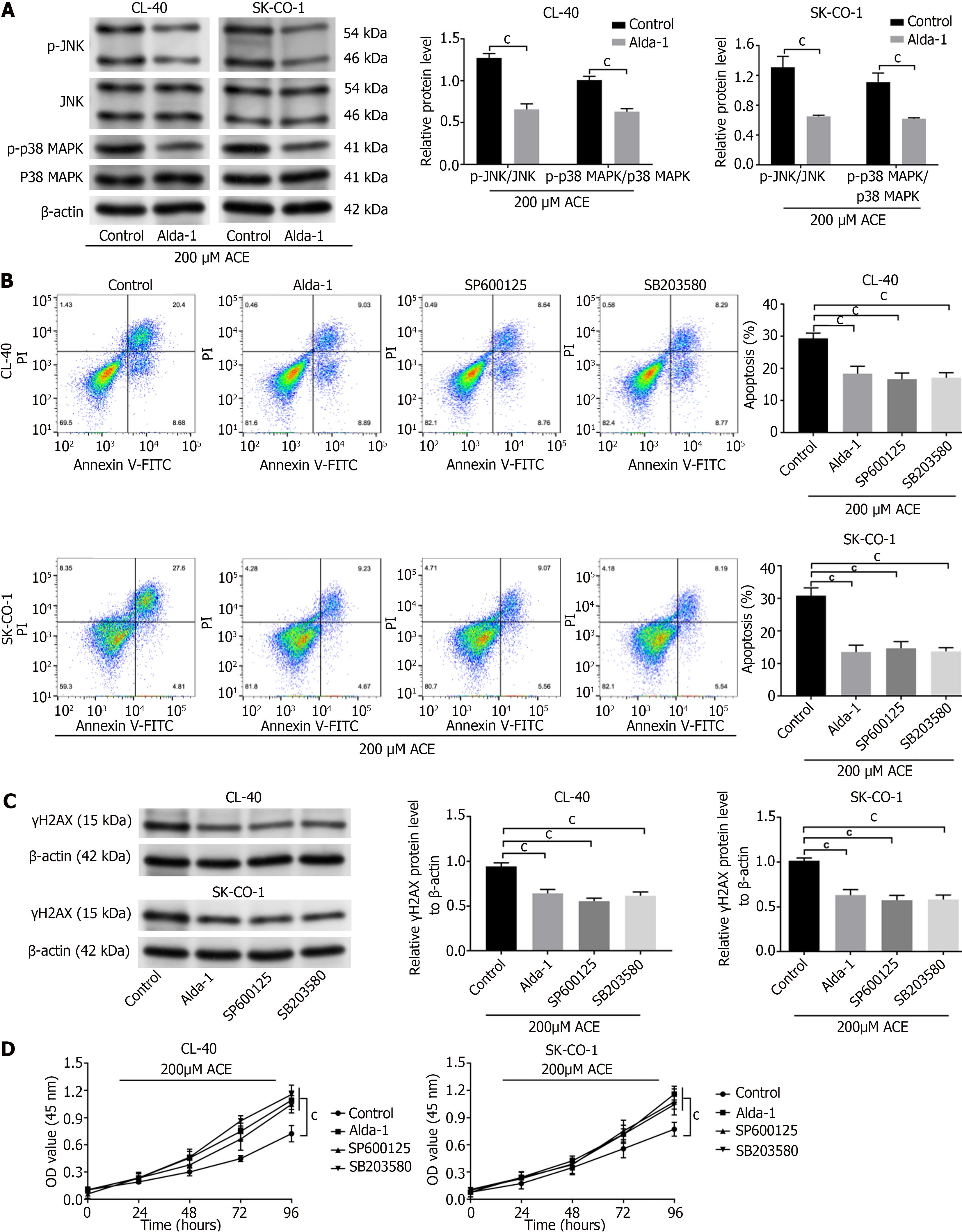
MAPK signal pathway was examined. SP600125 (20 μM), a highly efficient inhibitor of JNK, was added for the inhibition of p-JNK expression in sh-ALDH2 CL-40 and SK-CO-1 cells. SB203580 is a highly selective inhibitor of p38 MAPK[16]; SB203580 (5 μM) was added for the inhibition of the p-P38 MAPK expression in sh-ALDH2 CL-40 and SK-CO-1 cells. Western blot assay demonstrated p-P38 MAPK/P38 MAPK and p-JNK/JNK activation in sh-ALDH2 cell lines (P < 0.001), and Alda-1 treatment could reverse this trend (P < 0.01, P < 0.001). SP600125 treatment could inhibit p-JNK activation by sh-ALDH2 (P < 0.001) but did not affect the p-P38 MAPK. SB203580 treatment inhibited sh-ALDH2 activation of p-P38 MAPK (P < 0.01) and p-JNK expression (Figure 4A). Flow cytometry showed that sh-ALDH2 promoted apoptosis of CL-40 and SK-CO-1 cells, while Alda-1 treatment, JNK inhibitor (SP600125), and p38 MAPK inhibitor (SB203580) reversed this phenomenon (P < 0.001; Figure 4B). Then, expressions of Bax and Bcl-2 were identified to explore how the MAPK signal pathway influenced cell apoptosis. We found that sh-ALDH2 downregulated Bcl-2 expression and upregulated Bax expression (P < 0.001). Moreover, in sh-ALDH2 cells, Bcl-2 expression was obviously activated, while Bax level was decreased with Alda-1, SP600125, or SB203580 treatment (P < 0.05, P < 0.01, P < 0.001; Figure 4C).
We compared the activities of CRC cells in the presence of ACE. After ACE (200 μM) treatment, CL-40 and SK-CO-1 cells with Alda-1 had reduced p-P38 MAPK/P38 MAPK and p-JNK/JNK as compared to that of control cells (P < 0.001; Figure 5A). Importantly, cells in ACE presence, flow cytometric analysis showed that Alda-1, JNK inhibitor (SP600125), and p38 MAPK inhibitor (SB203580) treatment could inhibit the apoptosis of CL-40 and SK-CO-1 cells as compared to control cells (P < 0.001; Figure 5B). Similar results were obtained by DNA-damage response in γH2AX expression (P < 0.001; Figure 5C). CCK-8 assay also demonstrated that when exogenous ACE was added to CL-40 and SK-CO-1 cells, cells with Alda-1, SP600125, and SB203580 had increased proliferation as compared to that of the control group (P < 0.001; Figure 5D).
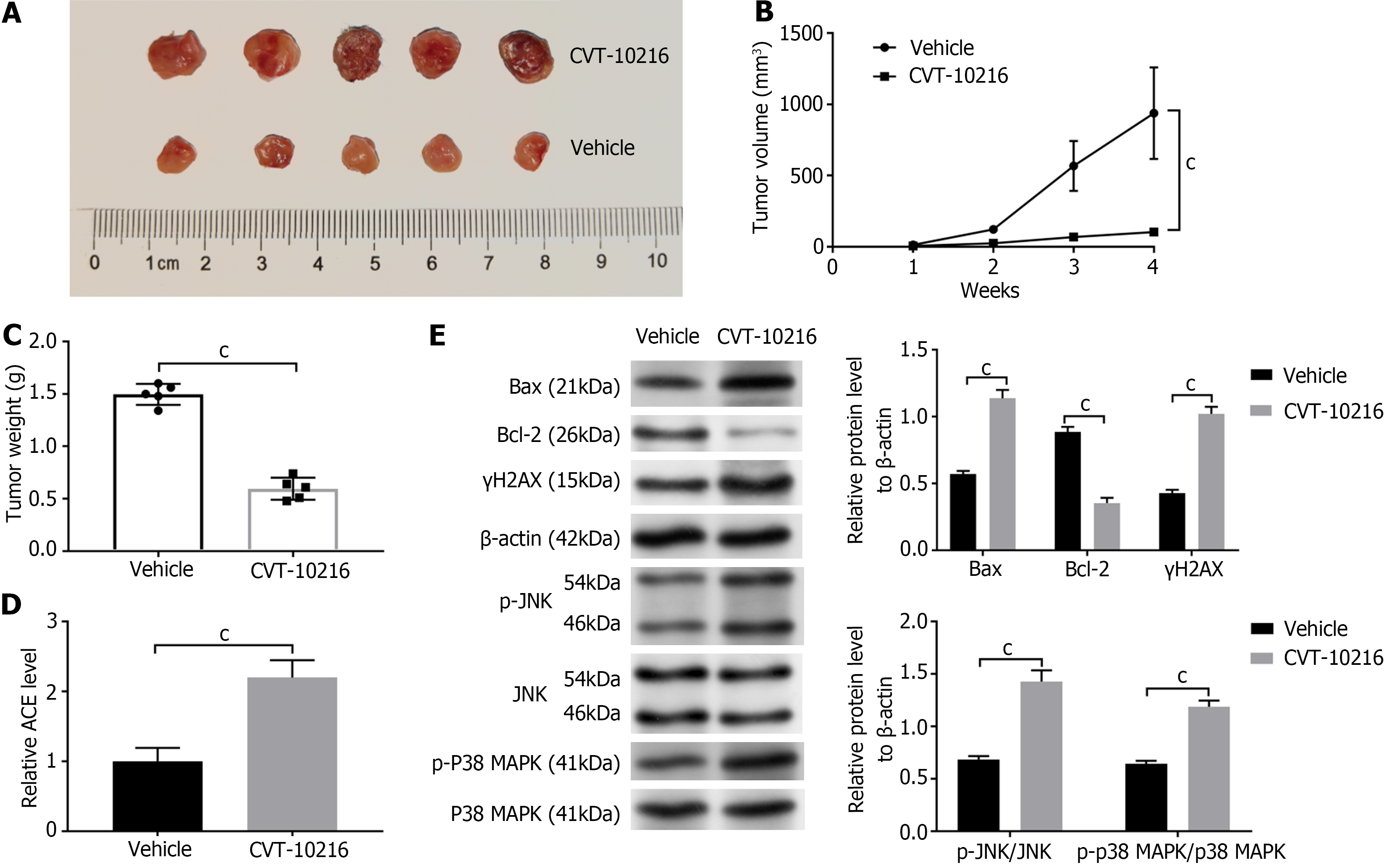
We constructed a xenograft model by inoculating SK-CO-1 cells into nude mice. 50 mg/kg CVT-10216 (a selective ALDH2 inhibitor) was used in mice once a day in the CVT-10216 group, and the control mice were treated with Vehicle once a day. Administration lasted for about two weeks. In the SK-CO-1 mouse xenograft models, tumor growth and volume were inhibited by CVT-10216 as compared to that of the Vehicle group (P < 0.001; Figure 6A-C). We measured ACE levels in the tumor tissues from CVT-10216 and Vehicle mice. The ACE level was significantly higher in the CVT-10216 mouse than in the Vehicle group (P < 0.001; Figure 6D). In addition, the western blot has submitted that after treatment with CVT-10216, the tumor of mice had decreased Bax/Bcl-2, p-P38 MAPK/P38 MAPK, γH2AX, p-JNK/JNK, and levels than that in Vehicle group (P < 0.001; Figure 6E).
CRC is an important cause of cancer-related deaths. CRC occurrence is closely associated with genetic factors, ulcerative colitis, intake of tobacco and alcohol, viral infections, environmental factors, etc[17]. Our study reported that inhibition of ALDH2 expression caused ACE accumulation and DNA damage in CRC cells and demonstrated that ALDH2 enhanced metastasis in CRC via suppression of accumulated ACE and DNA damage by activating the JNK/p38 MAPK pathways.
ALDH2 is expressed highly in patient tumor tissues consuming extreme alcohol[18]. ALDH2 is responsible for ACE metabolism to acetate[19]. ALDH2 reduction increased cell proliferation and stemness and enhanced DNA damage and migration through ACE accumulation in lung adenocarcinoma[11]. In addition, we observed highly expressed ALDH2 in tumor tissues from CRC patients with alcohol drinking history than non-drinkers[17]. The malignant features of CRC cells, including proliferation, apoptosis, ACE level, and DNA damage, were caused by ALDH2 silencing, which can then be reversed by the Alda-1. Alda-1 is a selective agonist of ALDH2[15].
ALDH2 plays a significant role in attenuating cell apoptosis. ALDH2 overexpression regulated autophagy, mitigating apoptosis of renal tubular epithelial cells and renal injury[20]. ALDH2 could decrease 4-HNE, inhibit the MAPK signaling pathway, and decreased apoptosis on liver injury[21]. Research has found that JNK and P38 MAPK pathways activation can induce cell apoptosis in hepatocellular carcinoma[22]. Moreover, ALDH2 represses the JNK/p38 MAPK activation to inhibit cell migration and proliferation in lung adenocarcinoma[13]. In our study, phosphorylated JNK and p38-MAPK expressions and cell apoptosis were observed by ALDH2 silencing. And ALDH2 repression induced apoptosis in both CL-40 and SK-CO-1 cells by decreasing the expression level of Bcl-2 and increasing the expression levels of Bax. On the other hand, JNK inhibitor SP600125 and p38-MAPK inhibitor SB203580 attenuated ALDH2-induced apoptosis. Likewise, P38/JNK MAPK signaling has participated in licochalcone B[23] and Echinatin[24] induced apoptosis in CRC cells by increased the protein level of Bax, and decreased the expression of Bcl-2. Thus, we concluded that ALDH2 repression could promote apoptosis through activating JNK/P38 MAPK pathways in CRC cells.
ACE can relate to DNA to form diverse types of adducts, which leads to carcinogenesis-related genetic mutations[25]. IARC has designated ACE to be a group I human carcinogen in 2009[26]. ACE is metabolized to acetate by ALDH2, and ALDH2's ability to repress cellular ACE levels is consistent in colon and pancreatic cancers. In heavy ethanol drinkers, it is supported by the connotation of ALDH2 Lack with a high occurrence of CRC and pancreatic cancer[6,27]. To verify whether ACE is involved in ALDH2 regulation in CRC cells by ALDH2, we treated cells with ACE and indicated that ALDH2 could inhibit the JNK/P38 MAPK-apoptosis and DNA damage by regulating ACE, thereby affecting the cell viability in CRC. Moreover, CVT-10216 is a highly selective, reversible inhibitor of ALDH-2 that reduces excessive alcohol drinking[28]. CVT-10216 significantly decrease migration and stemness properties of CRC cells[29]. In our in vivo study, CVT-10216 treatment also caused accumulated ACE, high DNA damage, and tumor growth in mice. Thereby, both in vivo and in vitro experiments have confirmed that ALDH2 repression caused the accumulation of ACE, and induced cell apoptosis and DNA damage in CRC.
In conclusion, our work demonstrated that ALDH2 repression caused the accumulation of ACE, inducing cell apoptosis and DNA damage by means of activating the JNK/p38 MAPK signaling pathway in CRC. ALDH2 is utilized as a therapeutic target for reversing patients with CRC.
| 1. | Siegel RL, Jakubowski CD, Fedewa SA, Davis A, Azad NS. Colorectal Cancer in the Young: Epidemiology, Prevention, Management. Am Soc Clin Oncol Educ Book. 2020;40:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 97] [Article Influence: 19.4] [Reference Citation Analysis (1)] |
| 2. | Morgan E, Arnold M, Gini A, Lorenzoni V, Cabasag CJ, Laversanne M, Vignat J, Ferlay J, Murphy N, Bray F. Global burden of colorectal cancer in 2020 and 2040: incidence and mortality estimates from GLOBOCAN. Gut. 2023;72:338-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 511] [Cited by in RCA: 901] [Article Influence: 450.5] [Reference Citation Analysis (1)] |
| 3. | Bogaert J, Prenen H. Molecular genetics of colorectal cancer. Ann Gastroenterol. 2014;27:9-14. [PubMed] |
| 4. | Aran V, Victorino AP, Thuler LC, Ferreira CG. Colorectal Cancer: Epidemiology, Disease Mechanisms and Interventions to Reduce Onset and Mortality. Clin Colorectal Cancer. 2016;15:195-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 231] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 5. | Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet. 2019;394:1467-1480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1570] [Cited by in RCA: 3006] [Article Influence: 501.0] [Reference Citation Analysis (3)] |
| 6. | Singh S, Arcaroli J, Thompson DC, Messersmith W, Vasiliou V. Acetaldehyde and retinaldehyde-metabolizing enzymes in colon and pancreatic cancers. Adv Exp Med Biol. 2015;815:281-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 7. | Johnson CH, Golla JP, Dioletis E, Singh S, Ishii M, Charkoftaki G, Thompson DC, Vasiliou V. Molecular Mechanisms of Alcohol-Induced Colorectal Carcinogenesis. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 8. | Zhang H, Fu L. The role of ALDH2 in tumorigenesis and tumor progression: Targeting ALDH2 as a potential cancer treatment. Acta Pharm Sin B. 2021;11:1400-1411. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 99] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 9. | Chen CH, Ferreira JC, Gross ER, Mochly-Rosen D. Targeting aldehyde dehydrogenase 2: new therapeutic opportunities. Physiol Rev. 2014;94:1-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 478] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 10. | Hou G, Chen L, Liu G, Li L, Yang Y, Yan HX, Zhang HL, Tang J, Yang YC, Lin X, Chen X, Luo GJ, Zhu Y, Tang S, Zhang J, Liu H, Gu Q, Zhao LH, Li Y, Liu L, Zhou W, Wang H. Aldehyde dehydrogenase-2 (ALDH2) opposes hepatocellular carcinoma progression by regulating AMP-activated protein kinase signaling in mice. Hepatology. 2017;65:1628-1644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 11. | Li K, Guo W, Li Z, Wang Y, Sun B, Xu D, Ling J, Song H, Liao Y, Wang T, Jing B, Hu M, Kuang Y, Wang Q, Yao F, Sun A, Zhu L, Wang L, Deng J. ALDH2 Repression Promotes Lung Tumor Progression via Accumulated Acetaldehyde and DNA Damage. Neoplasia. 2019;21:602-614. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 12. | Bakhoum SF, Ngo B, Laughney AM, Cavallo JA, Murphy CJ, Ly P, Shah P, Sriram RK, Watkins TBK, Taunk NK, Duran M, Pauli C, Shaw C, Chadalavada K, Rajasekhar VK, Genovese G, Venkatesan S, Birkbak NJ, McGranahan N, Lundquist M, LaPlant Q, Healey JH, Elemento O, Chung CH, Lee NY, Imielenski M, Nanjangud G, Pe'er D, Cleveland DW, Powell SN, Lammerding J, Swanton C, Cantley LC. Chromosomal instability drives metastasis through a cytosolic DNA response. Nature. 2018;553:467-472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 962] [Cited by in RCA: 1096] [Article Influence: 156.6] [Reference Citation Analysis (0)] |
| 13. | Yang M, Wang A, Li C, Sun J, Yi G, Cheng H, Liu X, Wang Z, Zhou Y, Yao G, Wang S, Liang R, Li B, Li D, Zhao H. Methylation-Induced Silencing of ALDH2 Facilitates Lung Adenocarcinoma Bone Metastasis by Activating the MAPK Pathway. Front Oncol. 2020;10:1141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 14. | Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149116] [Cited by in RCA: 133698] [Article Influence: 5570.8] [Reference Citation Analysis (1)] |
| 15. | Sun L, Ferreira JC, Mochly-Rosen D. ALDH2 activator inhibits increased myocardial infarction injury by nitroglycerin tolerance. Sci Transl Med. 2011;3:107ra111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 16. | Cuenda A, Rouse J, Doza YN, Meier R, Cohen P, Gallagher TF, Young PR, Lee JC. SB 203580 is a specific inhibitor of a MAP kinase homologue which is stimulated by cellular stresses and interleukin-1. FEBS Lett. 1995;364:229-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1651] [Cited by in RCA: 1736] [Article Influence: 57.9] [Reference Citation Analysis (0)] |
| 17. | Zhang H, Xia Y, Wang F, Luo M, Yang K, Liang S, An S, Wu S, Yang C, Chen D, Xu M, Cai M, To KKW, Fu L. Aldehyde Dehydrogenase 2 Mediates Alcohol-Induced Colorectal Cancer Immune Escape through Stabilizing PD-L1 Expression. Adv Sci (Weinh). 2021;8:2003404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 18. | Morita M, Oyama T, Kagawa N, Nakata S, Ono K, Sugaya M, Uramoto H, Yoshimatsu T, Hanagiri T, Sugio K, Kakeji Y, Yasumoto K. Expression of aldehyde dehydrogenase 2 in the normal esophageal epithelium and alcohol consumption in patients with esophageal cancer. Front Biosci. 2005;10:2319-2324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Rwere F, Yu X, Chen CH, Gross ER. Aldehydes, Aldehyde Metabolism, and the ALDH2 Consortium. Biomolecules. 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Xu T, Guo J, Wei M, Wang J, Yang K, Pan C, Pang J, Xue L, Yuan Q, Xue M, Zhang J, Sang W, Jiang T, Chen Y, Xu F. Aldehyde dehydrogenase 2 protects against acute kidney injury by regulating autophagy via the Beclin-1 pathway. JCI Insight. 2021;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 21. | Zhong Z, Ye S, Xiong Y, Wu L, Zhang M, Fan X, Li L, Fu Z, Wang H, Chen M, Yan X, Huang W, Ko DS, Wang Y, Ye Q. Decreased expression of mitochondrial aldehyde dehydrogenase-2 induces liver injury via activation of the mitogen-activated protein kinase pathway. Transpl Int. 2016;29:98-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Yang Z, Zhang H, Yin M, Cheng Z, Jiang P, Feng M, Liao B, Liu Z. Neurotrophin3 promotes hepatocellular carcinoma apoptosis through the JNK and P38 MAPK pathways. Int J Biol Sci. 2022;18:5963-5977. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 23. | Kwak AW, Kim WK, Lee SO, Yoon G, Cho SS, Kim KT, Lee MH, Choi YH, Lee JY, Park JW, Shim JH. Licochalcone B Induces ROS-Dependent Apoptosis in Oxaliplatin-Resistant Colorectal Cancer Cells via p38/JNK MAPK Signaling. Antioxidants (Basel). 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 24. | Kwak AW, Lee JY, Lee SO, Seo JH, Park JW, Choi YH, Cho SS, Yoon G, Lee MH, Shim JH. Echinatin induces reactive oxygen species-mediated apoptosis via JNK/p38 MAPK signaling pathway in colorectal cancer cells. Phytother Res. 2023;37:563-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 22] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 25. | Yu HS, Oyama T, Isse T, Kitagawa K, Pham TT, Tanaka M, Kawamoto T. Formation of acetaldehyde-derived DNA adducts due to alcohol exposure. Chem Biol Interact. 2010;188:367-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 111] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 26. | Secretan B, Straif K, Baan R, Grosse Y, El Ghissassi F, Bouvard V, Benbrahim-Tallaa L, Guha N, Freeman C, Galichet L, Cogliano V; WHO International Agency for Research on Cancer Monograph Working Group. A review of human carcinogens--Part E: tobacco, areca nut, alcohol, coal smoke, and salted fish. Lancet Oncol. 2009;10:1033-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 732] [Cited by in RCA: 779] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 27. | Kanda J, Matsuo K, Suzuki T, Kawase T, Hiraki A, Watanabe M, Mizuno N, Sawaki A, Yamao K, Tajima K, Tanaka H. Impact of alcohol consumption with polymorphisms in alcohol-metabolizing enzymes on pancreatic cancer risk in Japanese. Cancer Sci. 2009;100:296-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 28. | Overstreet DH, Knapp DJ, Breese GR, Diamond I. A selective ALDH-2 inhibitor reduces anxiety in rats. Pharmacol Biochem Behav. 2009;94:255-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Wei PL, Prince GMSH, Batzorig U, Huang CY, Chang YJ. ALDH2 promotes cancer stemness and metastasis in colorectal cancer through activating β-catenin signaling. J Cell Biochem. 2023;124:907-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |