Published online Jul 15, 2024. doi: 10.4251/wjgo.v16.i7.3211
Revised: April 25, 2024
Accepted: May 11, 2024
Published online: July 15, 2024
Processing time: 148 Days and 11 Hours
Gastric intestinal metaplasia (IM) is a precancerous lesion that is associated with an elevated risk of gastric carcinogenesis. Weiwei Decoction (WWD) is a prom
To verify the regulation of the OLFM4/NOD1/CDX2 pathway in IM, specifically investigating WWD’s effectiveness on IM through this pathway.
Immunohistochemistry for OLFM4, NOD1, and CDX2 was conducted on tissue microarray. GES-1 cells treated with chenodeoxycholic acid were utilized as IM cell models. OLFM4 short hairpin RNA (shRNA), NOD1 shRNA, and OLFM4 pcDNA were transfected to clarify the pathway regulatory relationships. Protein interactions were validated by co-immunoprecipitation. To explore WWD’s pharmacological actions, IM rat models were induced using N-methyl-N’-nitro-N-nitrosoguanidine followed by WWD gavage. Gastric cells were treated with WWD-medicated serum. Cytokines and chemokines content were assessed by enzyme-linked immunosorbent assay and quantitative reverse transcription polymerase chain reaction.
The OLFM4/NOD1/CDX2 axis was a characteristic of IM. OLFM4 exhibited direct binding and subsequent down-regulation of NOD1, thereby sustaining the activation of CDX2 and promoting the progression of IM. WWD improved gastric mucosal histological lesions while suppressing intestinal markers KLF transcription factor 4, villin 1, and MUCIN 2 expression in IM rats. Regarding pharmacological actions, WWD suppressed OLFM4 and restored NOD1 expression, consequently reducing CDX2 at the mRNA and protein levels in IM rats. Parallel regulatory mechanisms were observed at the protein level in IM cells treated with WWD-medicated serum. Furthermore, WWD-medicated serum treatment strengthened OLFM4 and NOD1 interaction. In case of anti-inflammatory, WWD restrained interleukin (IL)-6, interferon-gamma, IL-17, macrophage chemoattractant protein-1, macrophage inflammatory protein 1 alpha content in IM rat serum. WWD-medicated serum inhibited tumor necrosis factor alpha, IL-6, IL-8 transcriptions in IM cells.
The OLFM4/NOD1/CDX2 pathway is involved in the regulation of IM. WWD exerts its therapeutic efficacy on IM through the pathway, additionally attenuating the inflammatory response.
Core Tip: Gastric intestinal metaplasia (IM) is a precancerous lesion that is associated with an elevated risk of gastric carcinogenesis. Weiwei Decoction (WWD) is a traditional Chinese herbal formula derived from Sijunzi Decoction that has been utilized to treat gastric disease for thousands of years. Nowadays, WWD is widely employed in clinical for treating IM. Previous studies suggested the potential involvement of the olfactomedin 4 (OLFM4)/nucleotide-binding oligomerization domain 1 (NOD1)/caudal-type homeobox gene 2 (CDX2) pathway in IM regulation. In this study, we preliminarily validated the pivotal role of the OLFM4/NOD1/CDX2 signaling pathway in regulating gastric IM. Focusing on this pathway, our investigation centered on the therapeutic effects of WWD against gastric IM.
- Citation: Zhou DS, Zhang WJ, Song SY, Hong XX, Yang WQ, Li JJ, Xu JQ, Kang JY, Cai TT, Xu YF, Guo SJ, Pan HF, Li HW. Weiwei Decoction alleviates gastric intestinal metaplasia through the olfactomedin 4/nucleotide-binding oligomerization domain 1/caudal-type homeobox gene 2 signaling pathway. World J Gastrointest Oncol 2024; 16(7): 3211-3229
- URL: https://www.wjgnet.com/1948-5204/full/v16/i7/3211.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i7.3211
Gastric intestinal metaplasia (IM) denotes the replacement of normal gastric epithelium with intestinal-type epithelium, often occurring concomitantly with chronic atrophic gastritis (CAG). Metaplasia is considered to serve as a protective and healing response to severe injury or inflammation. Histologically, IM is characterized by the loss of glandular lineages, particularly parietal and chief cells (atrophy), which are replaced by goblet cells, absorptive cells, and enteroendocrine cells (IM)[1]. Gastric IM is regarded as a typical precancerous lesion. Epidemiological studies have confirmed that IM accounts for 23.6% of patients with chronic gastritis[2]. The annual incidence of gastric cancer (GC) was 0.25% for patients with gastric IM[3]. As more studies have confirmed that IM increases the risk of carcinogenesis[4,5], preventing the malignant transformation of gastric IM is crucial for reducing GC incidence.
The pathogenesis of gastric IM remains elusive; however, it is commonly associated with repetitive damage caused by physical and chemical factors, bile reflux, infection by Helicobacter pylori (H. pylori), and inflammatory infiltration[6]. N-methyl-N’-nitro-N-nitrosoguanidine (MNNG) is a mutagenic carcinogenic reagent that induces the alkylation of bases on the DNA chain[7], which was administered to create animal models of gastric IM. Additionally, chenodeoxycholic acid (CDCA) was utilized to induce intestine-like phenotypes in GES-1 cells, representing a reliable IM cell modeling approach[8].
Traditional Chinese medicines (TCMs) have a unique potential in treating chronic gastric diseases[9]. Numerous researchers have reported TCM formulas and compounds exhibiting potential in treating IM[10,11]. According to TCM theory, IM and CAG are highly correlated with “Spleen Qi deficiency”[12]. Weiwei Decoction (WWD) is a traditional herbal formula approved for the preparation of medical institutions in Guangdong Province [Weiwei granule (Z20070548)]; its components and dosages are shown in Table 1. WWD is a derivative of the Sijunzi Decoction, which has been utilized to treat gastric disease and prevent GC for thousands of years in China. Based on the theory of TCM, WWD may alleviate gastric IM by strengthening the spleen, dispersing blood stasis, and detoxifying[13]. Previous clinical trials have indicated that WWD may ameliorate the symptoms of IM in combination with standard quadruple therapy, partially eradicate H. pylori, and improve the pathological manifestations of IM[14,15]. Experimentally, WWD effectively reduces spasmolytic polypeptide-expressing metaplasia (SPEM) markers expression in H. pylori-infected mice and
| Latin name | Local name | Material | Quantities (g) |
| Astragalus mongholicus Bunge | Huangqi | Root | 30 |
| Codonopsis pilosula Nannf. | Dangshen | Root | 30 |
| Sparganium stoloniferum (Buch.-Ham. ex Graebn.) Buch.-Ham. ex Juz | Sanleng | Tuber | 15 |
| Curcuma phaeocaulis Valeton | Ezhu | Rhizoma | 15 |
| Panax notoginseng (Burkill) F.H.Chen | Sanqi | Radix and rhizoma | 10 |
| Lycium barbarum L. | Gouqizi | Fruit | 20 |
| Ophiopogon japonicus (Thunb.) Ker Gawl. | Maidong | Tuberous root | 15 |
| Dendrobium nobile Lindl. | Shihu | Stem | 15 |
| Scleromitrion diffusum (Willd.) R.J.Wang | Baihuasheshecao | Herb | 20 |
| Scutellaria barbata D.Don | Banzhilian | Herb | 20 |
| Atractylodes macrocephala Koidz. | Baizhu | Rhizoma | 15 |
| Glycyrrhiza uralensis Fisch | Zhigancao | Radix and rhizoma | 5 |
Earlier, we conducted quality control of WWD and identified 9 representative chemical constituents[13]. Some ind
Olfactomedin 4 (OLFM4), also known as GW112 or HGC-1, is a secreted glycoprotein belonging to the olfactomedin family[23]. Notably, OLFM4 is significantly up-regulated in gastric IM and is considered a sensitive biomarker of early GC[24,25]. Caudal-type homeobox gene 2 (CDX2) is a pivotal transcription factor that directs the transformation of gastric IM by promoting the expression of intestinal markers, including KLF transcription factor 4 (KLF4), villin 1 (VIL1), MUCIN 2 (MUC2), and sucrase-isomaltase (SI)[8]. While the importance of CDX2 in regulating intestinal differentiation is widely accepted, the mechanism underlying CDX2 activation in IM remains incompletely explored. OLFM4 may mediate gastric IM preceding CDX2 and is involved in the regulation of CDX2 expression[25]. Specifically, OLFM4 expression is detected in gastric-type glands surrounding IM before CDX2 expression. Additionally, a positive correlation between OLFM4 and CDX2 has been identified in non-tumorous gastric tissue[26]. Furthermore, nucleotide-binding oligomerization domain 1 (NOD1) inhibits gastric IM progression by down-regulating CDX2. In cases of persistent H. pylori infection, CDX2 induction relies on the activation of the nuclear factor-kappaB (NF-κB) P65 subunit, whereas NF-κB is suppressed by NOD1-mediated activation of tumor necrosis factor (TNF) receptor associated factor 3[27]. In addition, OLFM4 may interact with NOD1 during IM transformation. OLFM4 is a target gene of the H. pylori-activated NF-κB signaling, which has a negative feedback effect on NF-κB in turn through direct interaction with NOD1[28]. In this study, we preliminarily validated the pivotal role of the OLFM4/NOD1/CDX2 signaling pathway in regulating gastric IM. Focusing on this pathway, our investigation centered on the therapeutic effects of WWD against gastric IM.
A total of 26 cases of normal tissue and 35 cases of IM tissue were saved from the gastroscopic biopsy remaining tissue at Shenzhen Traditional Chinese Medicine Hospital between June 2022 and April 2023, which were used for the paraffin-embedded slides of human gastric tissue microarrays. Pathology results were evaluated by two expert pathologists independently, and all the samples were shown to be correctly labeled clinically and histologically. Written informed consent was obtained from all patients. The studies involving clinical research conformed to the Ethics Committee at Shenzhen Traditional Chinese Medicine Hospital and were approved by the Ethics Committee of the Shenzhen Traditional Chinese Medicine Hospital, The Fourth Clinical Medical College of Guangzhou University of Chinese Medicine (No. K2022-011-02).
WWD herbal formula was prepared by the Pharmacy Department of Shenzhen Traditional Chinese Medicine Hospital. The components and dosages of WWD are described in Table 1. Folic acid (FA, Lot: 22012311) was purchased from Changzhou Pharmaceutical Co., Ltd (Changzhou, China). CDCA (purity 98%, CAS. No. 474-25-9) was purchased from Macklin Biochemical Technology Co., Ltd (Shanghai, China), dissolved in DMSO solution, and diluted to the corresponding concentration when disposed to GES-1. MNNG (purity 95%, CAS. No. 70-25-7) purchased from Rhawn Technology Development Co. Ltd (Shanghai, China) was utilized to establish IM rat models. Other unspecified chemicals were of analytical grade.
Antibodies against OLFM4 (ab105861), MUC2 (ab134119), VIL1 (ab97512), and GAPDH (ab8245) were purchased from Abcam (Cambridge, MA); antibody against CDX2 (D11D10) was obtained from Cell Signaling Technology (Beverly, MA); antibody against NOD1 (sc-398696) was gained from Santa Cruz (Dallas, TX) (For samples of human origin). Antibodies against CDX2 (GB121501) and MUC2 (GB11344) were purchased from Servicebio (Wuhan, China); antibodies against NOD1 (ab217798), VIL1 (ab97512), KLF4 (ab214666), and GAPDH (ab8245) were purchased from Abcam (Cambridge, MA); antibody against OLFM4 (PA5-115687) was obtained from Thermo Fisher Scientific (Waltham, MA) (for samples of rat origin). Normal IgG (#2729) was gained from Cell Signaling Technology (Beverly, MA). Secondary antibodies (ab205718, ab205719, ab131368, #5127) were obtained from Abcam (Cambridge, MA) and Cell Signaling Technology (Beverly, MA).
Human gastric mucosal epithelial cell-line GES-1 (Procell, Wuhan, China) and human gastric adenocarcinoma cell-line AGS (ATCC, Manassas, VA) were cultured in RPMI-1640 medium (Gibco, United States) supplemented with 10% (V/V) fetal bovine serum (Gibco, United States) in a humidified incubator at 37 °C and 50 mL/L CO2.
For CDCA treatment, GES-1 cells were seeded into culture dishes and then treated with 0, 50, 100, 150, 200, 400, and 600 μM of CDCA for 24 h. The control group was treated with an equal concentration of DMSO. Cell counting kit-8 (CCK-8) solution was added to each well and incubated for 1 h according to the instructions. Absorbance at 450 nm was recorded by a microplate reader (Thermo Fisher Scientific, Waltham, MA) to calculate the cell viability rate. As for WWD-medicated serum groups, CDCA-pre-treated GES-1 cells, and AGS cells were cultured for 0, 6, 12, 24, and 48 h in a medium supplemented with 10% (V/V) serum of rats that were treated with corresponding WWD dosages, and the cell viability rates were determined. Cell viability rate (%) = (OD of test sample/OD of control sample) × 100.
GES-1 cells were washed twice with cold phosphate buffered saline (PBS) and homogenized in IP lysis buffer (Beyotime, Shanghai, China) with 1% protease inhibitors. Co-immunoprecipitation (Co-IP) experiment was then performed using the protein A + G agarose beads (Beyotime, Shanghai, China) according to the manufacturer’s instructions. Normal IgG was used as a negative control antibody. OLFM4, NOD1, and CDX2 antibodies were used as the capture antibodies. 30 μL beads were incubated with 1 μL antibody at room temperature for 1 h. Then mix the lysate with the beads-antibody complex and incubate at 4 °C overnight. Beads were washed three times with lysis buffer. Inputs and the bound proteins eluted from the beads were detected by western blot.
Short hairpin RNAs (shRNAs) against OLFM4 and NOD1, OLFM4 (NM_006418.5) overexpression plasmid, and the corresponding negative control were designed and constructed by Hanbio (Wuhan, China), which are listed in Supplementary Table 1. Transfection reagent GO3000 HiTrans Reagent was obtained from ECOTOP SCIENTIFIC Biotechnology Co., Ltd (Guangzhou, China). For transfection, plasmids and transfection reagent hybrid were added to Opti-MEM to generate the transfection medium. Subsequently, cells were cultured in the transfection medium, which was replaced with a fresh medium after 6 h. Then, the cells were cultured for an additional 48 h before being collected. The transfection reagent was used following the manufacturer’s instructions.
The herbs were weighed, soaked, and then extracted in distilled water twice (first in water 8 times the amount of the herbs; second in water 5 times the amount of the herbs, extracted for 1 h respectively). The two extracts were merged, filtered, and then centrifuged before retaining the supernatant, 55 °C 140 rpm rotary evaporate until the concentration was about 4 g/mL. For in vivo management, volumes of drugs were calculated based on the body weight of the rats.
Male Sprague-Dawley rats (200-250 g) were obtained from the Laboratory Animal Centre of the Guangzhou University of Chinese Medicine [permission No. SCXK-(Yue) 2018-0034], the rats were housed in the animal facilities of the specific pathogen-free animal laboratory. Rats were raised commonly 2 wk before the experiments and maintained under stan
A total of 48 rats were randomly divided into six groups, including the control group, model group, WWD low-dose (9.45 g/kg) group (WWD-L), WWD medium-dose (18.9 g/kg) group (WWD-M), WWD high-dose (37.8 g/kg) group (WWD-H) and the FA (2.7 mg/kg) group. Rats in the control group were conventional feeding, whereas the rest were administered an MNNG solution (200 μg/mL) with free drinking water for 18 wk to establish the IM models. Next, all rats were treated with the corresponding drugs once daily for 9 wk. The control group was given an equal amount of distilled water. By the end of the experiment, 7 rats remained in the model group (1 rat was lost), and 8 rats in each group in the other groups. Blood samples were collected under isoflurane anesthesia. Subsequently, rats were euthanized by isoflurane overdose, and gastric tissues were collected for further experiments.
Rats’ stomach tissues were fixed in 40 g/L paraformaldehyde, dehydrated before being embedded in paraffin wax, then cut into 4 μm slices. Serial sections were stained with hematoxylin and eosin (H&E) and Alcian blue-Periodic acid-Schiff (AB-PAS) following the standard protocols for light microscopy. All the relevant chemicals and reagents were purchased from Servicebio Technology Co., Ltd (Wuhan, China). The histological alternations and IM manifestations were assessed.
Immunohistochemistry (IHC) staining was performed using the IHC Application Solutions Kit (cell signaling technology, Beverly, MA). Stomach tissue sections were deparaffinized, antigens retrieved, and then antigens blocked. Primary antibodies were incubated at 4 °C overnight, and HRP-conjugated secondary antibodies were incubated for 1 h. Dia
In case of tissue microarray, the percentage of positive cells was divided into four categories as follows: 0 (< 5%), 1 (5%-25%), 2 (25%-50%), 3 (50%-75%), and 4 (> 75%). The intensity of staining was divided into four categories as follows: 0 (negative), 1 (weakly positive), 2 (moderately positive) and 3 (strongly positive). Results were evaluated by two expert pathologists independently. The immunoreactivity score = the percentage score × the intensity score.
In case of rat gastric mucosa, the expression of OLFM4, NOD1, CDX2, and MUC2 was evaluated with integrated option density (IOD). 5 images were captured from each sample and analyzed using Image-Pro-Plus 7.0 (Rockville, MD), the IOD was measured for each sample of each group.
Total RNAs were extracted from samples using TRIzol Reagent, which were subsequently reverse transcripted to cDNAs. Quantitative reverse transcription polymerase chain reaction (RT-qPCR) was performed by SYBR Green Premix qPCR Kit (the reagents above were obtained from Accurate Biotech Co., Ltd., Hunan, China) and analyzed on CFX Connect™ Real-Time PCR Detection System (Bio-Rad, Hercules, CA). The primer sequences are shown in Supplementary Table 2. The procedures were conducted according to the instructions, and the relative mRNA expressions were calculated by the 2-∆∆Ct method.
A total of 12 rats were randomly divided into 4 groups (n = 3). Rats in WWD serum groups were treated with intra
Samples were washed twice with cold PBS and homogenized in RIPA Lysis Buffer (Beyotime, Shanghai, China) with 1% protease and phosphatase inhibitors. The samples were quantified by BCA Protein Assay reagent (Thermo Scientific, Waltham, MA). Equal amounts of proteins were resolved by sodium-dodecyl sulfate gel electrophoresis and transferred to polyvinylidene fluoride membranes (Millipore, Billerica, MA). Antigens were blocked and then incubated with primary antibodies overnight, further incubating HRP-conjugated secondary antibodies for 1 h. Signals were detected using enhanced chemiluminescence (Millipore, Billerica, MA). Moreover, data was analyzed by Image J (Bethesda, MD).
Serum cytokines and chemokines levels of rats were detected, including interferon-gamma (IFN-γ), interleukin (IL)-6, IL-17, macrophage chemoattractant protein-1 (MCP-1), and macrophage inflammatory protein 1 alpha (MIP-1α). The enzyme-linked immunosorbent assay kits were from Jianglai Biotechnology Co., Ltd (Shanghai, China). All mea
All data is presented as mean ± SE and analyzed with the SPSS 23 (Chicago, IL). Differences between the two groups were examined using the independent t-test or the Mann-Whitney U test. Variances among multiple groups were compared using the one-way analysis of variance followed by the least significant difference or Dunnett’s T3 tests. The correlation between OLFM4, NOD1, and CDX2 expression was examined using Spearman’s correlation analysis. P < 0.05 was co
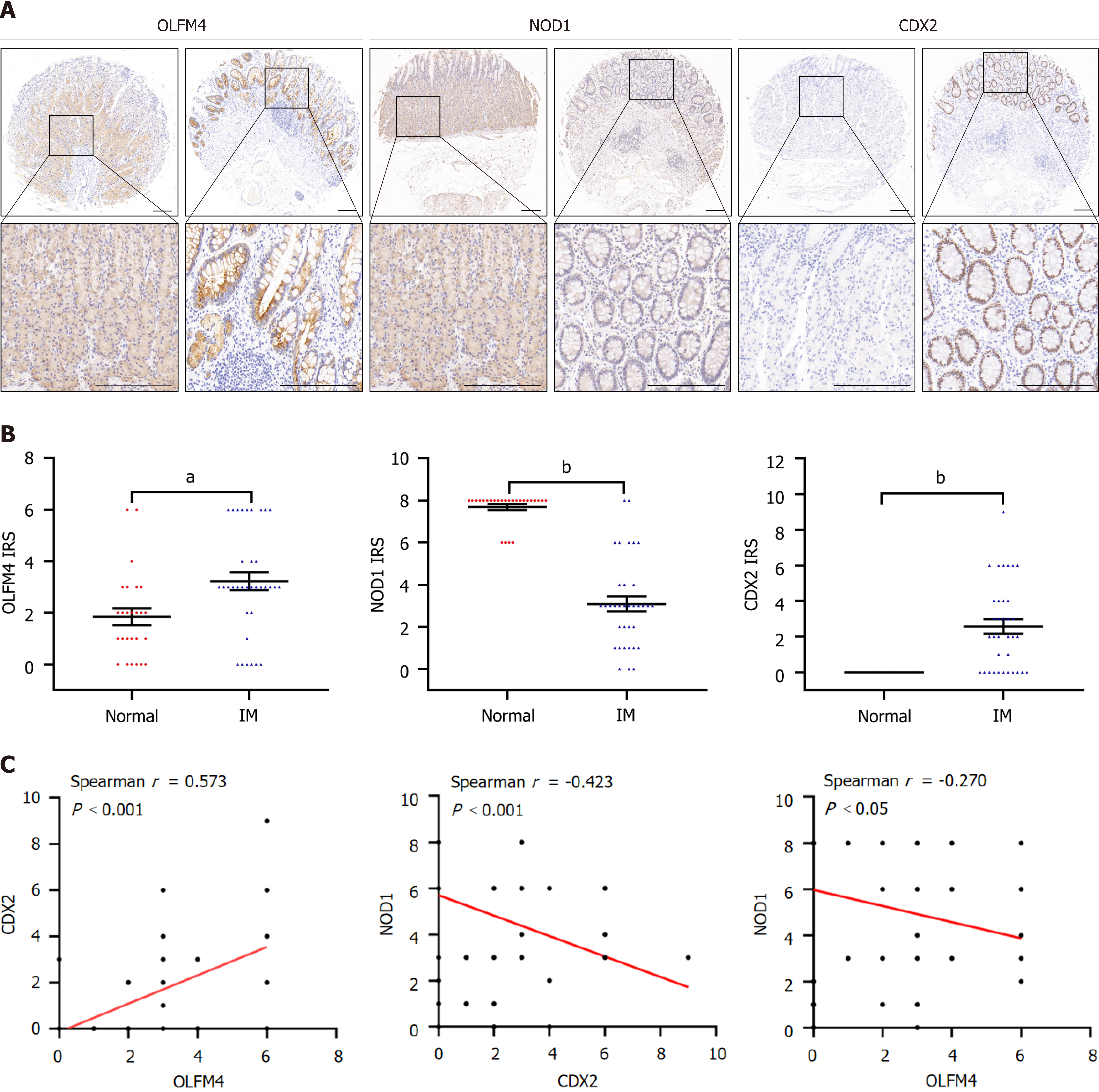
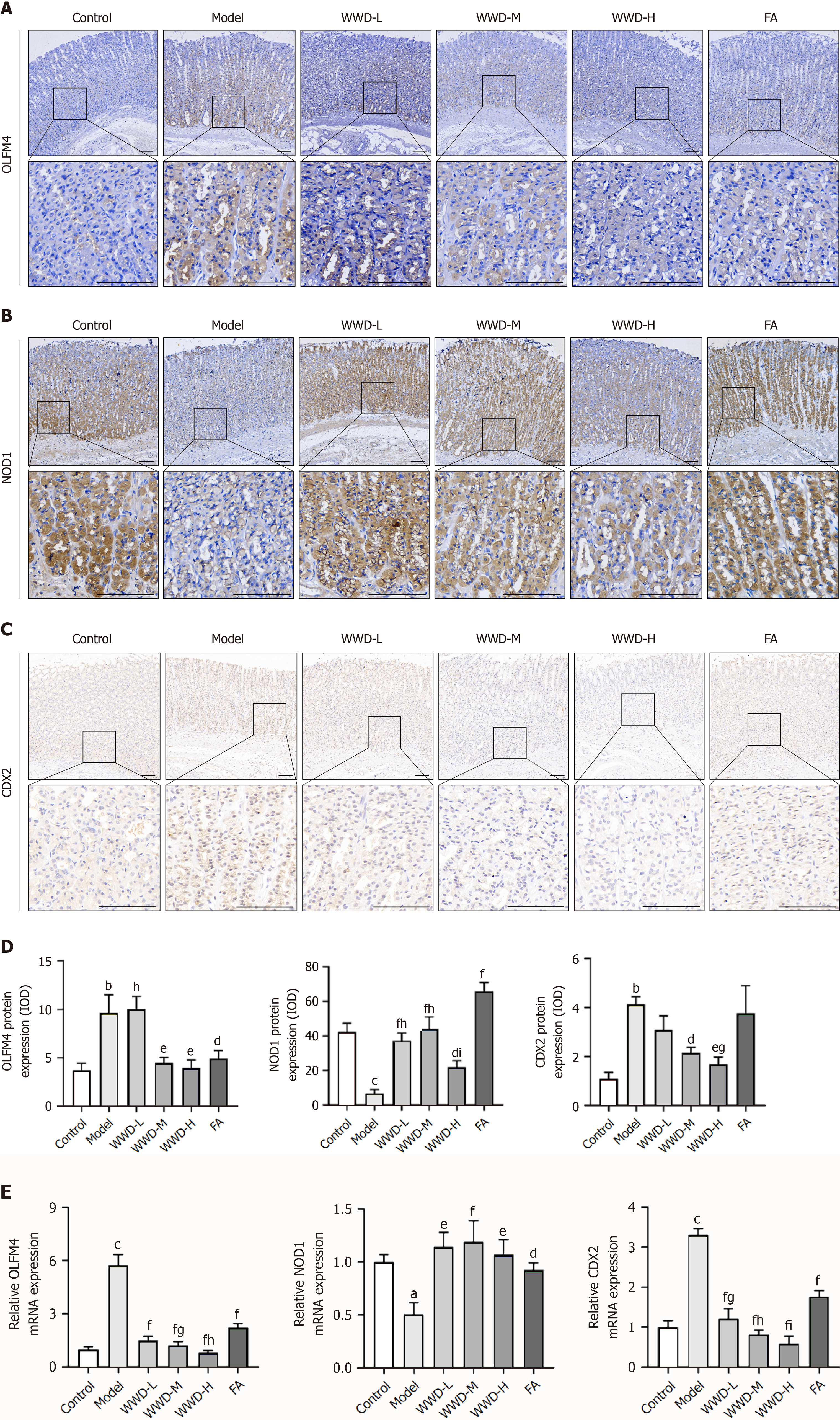
To ascertain the clinical relevance of the OLFM4/NOD1/CDX2 pathway regulation in gastric IM, immunohistochemical staining for OLFM4, NOD1, and CDX2 was conducted on the gastric tissue microarray. Comparative analysis revealed elevated OLFM4 levels (P < 0.01) and reduced NOD1 levels (P < 0.001) in IM tissues when compared to normal tissues. Notably, CDX2 expression was extremely low in normal gastric tissues, but its presence was significantly increased in IM tissues (P < 0.001) (Figure 1A and B). Further analysis demonstrated a positive correlation between CDX2 and OLFM4 expressions (P < 0.001), while a negative correlation was observed between CDX2 and NOD1 expressions (P < 0.001). Additionally, a negative correlation between OLFM4 and NOD1 expressions in gastric tissues was also identified (P < 0.05) (Figure 1C). These findings underscore the characteristics of the OLFM4/NOD1/CDX2 axis in human gastric IM.
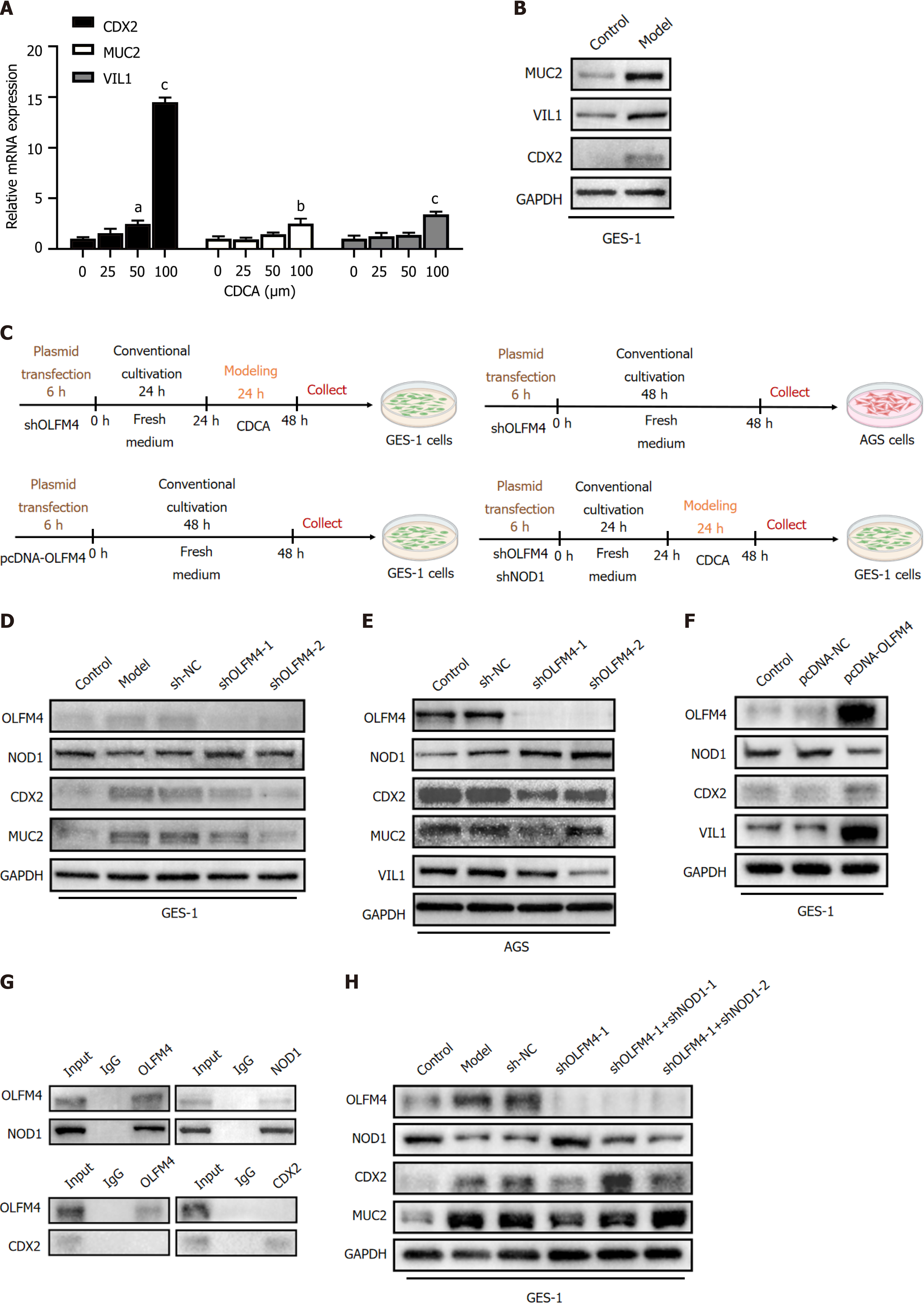
To validate the regulatory effect of the OLFM4/NOD1/CDX2 pathway on gastric IM, we first established IM cell models, GES-1 cells were exposed to 0, 50, 100, 150, 200, 400, and 600 μM of CDCA for 24 h. Cell viability rates were determined using CCK-8 assays. Substantial inhibition of cellular viability at CDCA doses exceeding 100 μM when compared to the control group (P < 0.001) (Supplementary Figure 1A). Consequently, GES-1 cells were subsequently treated with CDCA at a concentration ≤ 100 μM for 24 h. The mRNA expression of intestinal markers was examined using RT-qPCR after CDCA treatment. The results revealed that the mRNA level of MUC2, VIL1, and CDX2 significantly elevated in a dose-dependent manner. The transcription was mostly activated when CDCA was 100 μM (CDX2, VIL1, P < 0.001; MUC2, P < 0.01) (Figure 2A). Western blot results further corroborated these findings, demonstrating elevated expression of MUC2, VIL1, and CDX2 proteins (Figure 2B). Hence, we established the pattern for the gastric IM cell model in subsequent expe
Following the experimental procedures (Figure 2C), GES-1 cells were exposed to CDCA after being transfected with shRNAs targeting OLFM4. IM cell models displayed up-regulated OLFM4, down-regulated NOD1, and enhanced CDX2 and MUC2 protein levels. The introduction of OLFM4 shRNAs effectively reversed these alterations in downstream proteins (Figure 2D). In addition, transfection of AGS cells with OLFM4 shRNAs led to an increase in NOD1 expression, accompanied by the suppression of CDX2, MUC2, and VIL1 (Figure 2E). After GES-1 cells were transfected with OLFM4-pcDNAs, NOD1 protein level decreased as the CDX2 and VIL1 levels increased (Figure 2F). These findings indicated that OLFM4 may induce CDX2 through NOD1 and promote the progression of gastric IM. Further, we performed Co-IP assays using protein extracted from GES-1 cells and verified that OLFM4 directly interacted with NOD1. However, no interaction between OLFM4 and CDX2 was observed (Figure 2G). The results certified that OLFM4 induces CDX2 by interacting with NOD1, rather than activating CDX2 directly. Moreover, OLFM4 and NOD1 shRNAs were co-transfected to IM cell models, The introduction of NOD1 shRNAs restored the CDX2 and MUC2 down-regulation mediated by OLFM4 shRNA transfection (Figure 2H). These findings demonstrate that OLFM4 may activate CDX2 through the down-regulation of NOD1. Taken together, our findings suggest that OLFM4 exhibits direct binding and subsequent down-regulation of NOD1, thereby sustaining the activation of CDX2 at the protein level and promoting the progression of IM.
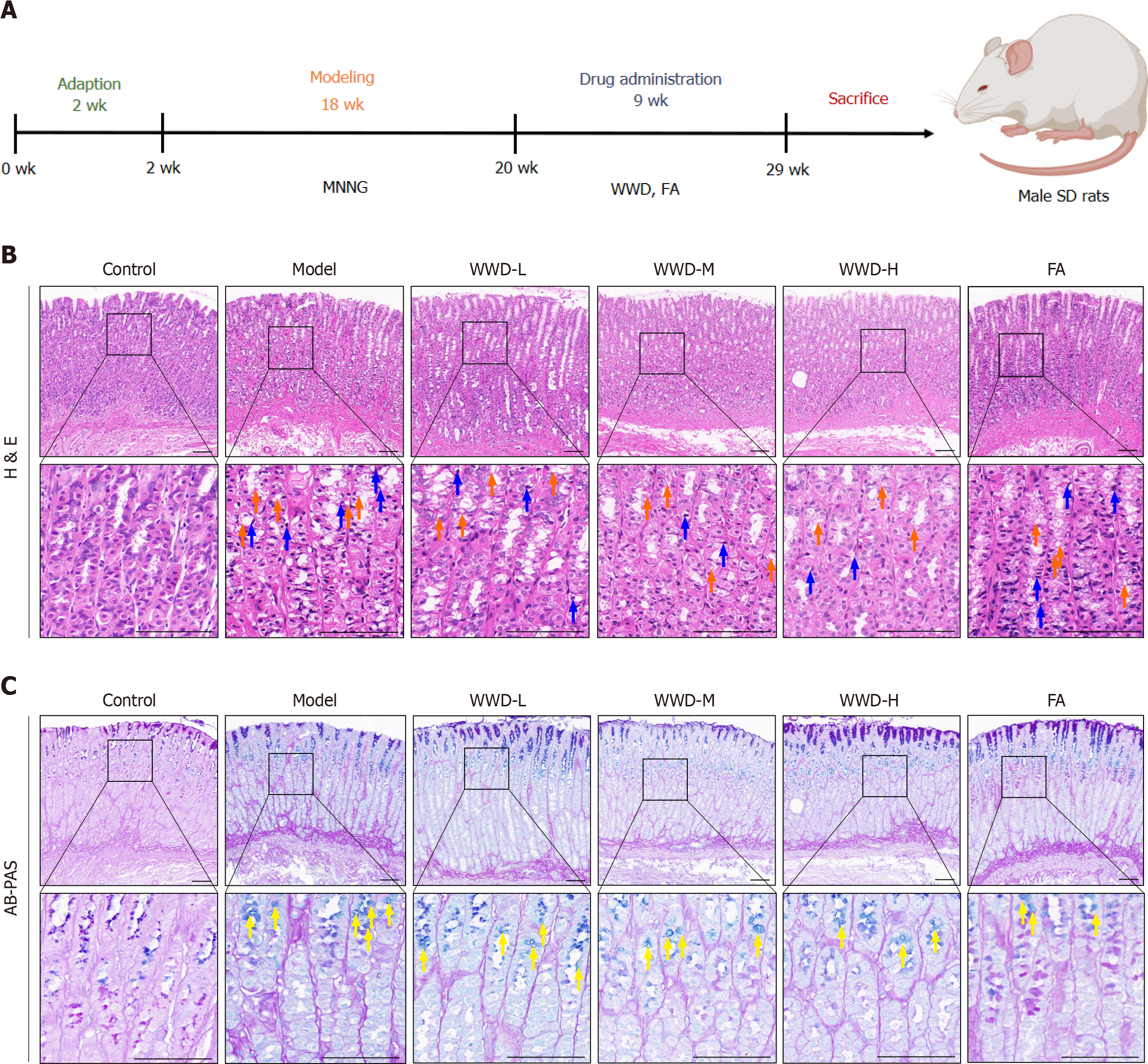
Following the experimental procedures (Figure 3A), the pharmacodynamic actions of WWD were evaluated in the MNNG-induced IM rats. According to the H&E staining, the epithelium of the gastric mucosa in the model group showed substantial vacuolation changes, with goblet cell formation (IM). Epithelial cells were observed with pyknotic and hyperchromatic nuclei in the lamina propria (dysplasia). Other observed features included glandular cavity dila
Furthermore, AB-PAS staining showed that the mucous vacuoles in the model group were stained blue, particularly noticeable at the neck of the gastric gland, suggesting acidic sialomucin secretion. The gastric mucous membranes of the WWD and FA groups appeared to have lighter bluish staining. Notably, the staining was visibly weaker in the WWD-M and WWD-H groups than in the WWD-L group, demonstrating the attenuation of IM in rats following WWD treatment (Figure 3C).
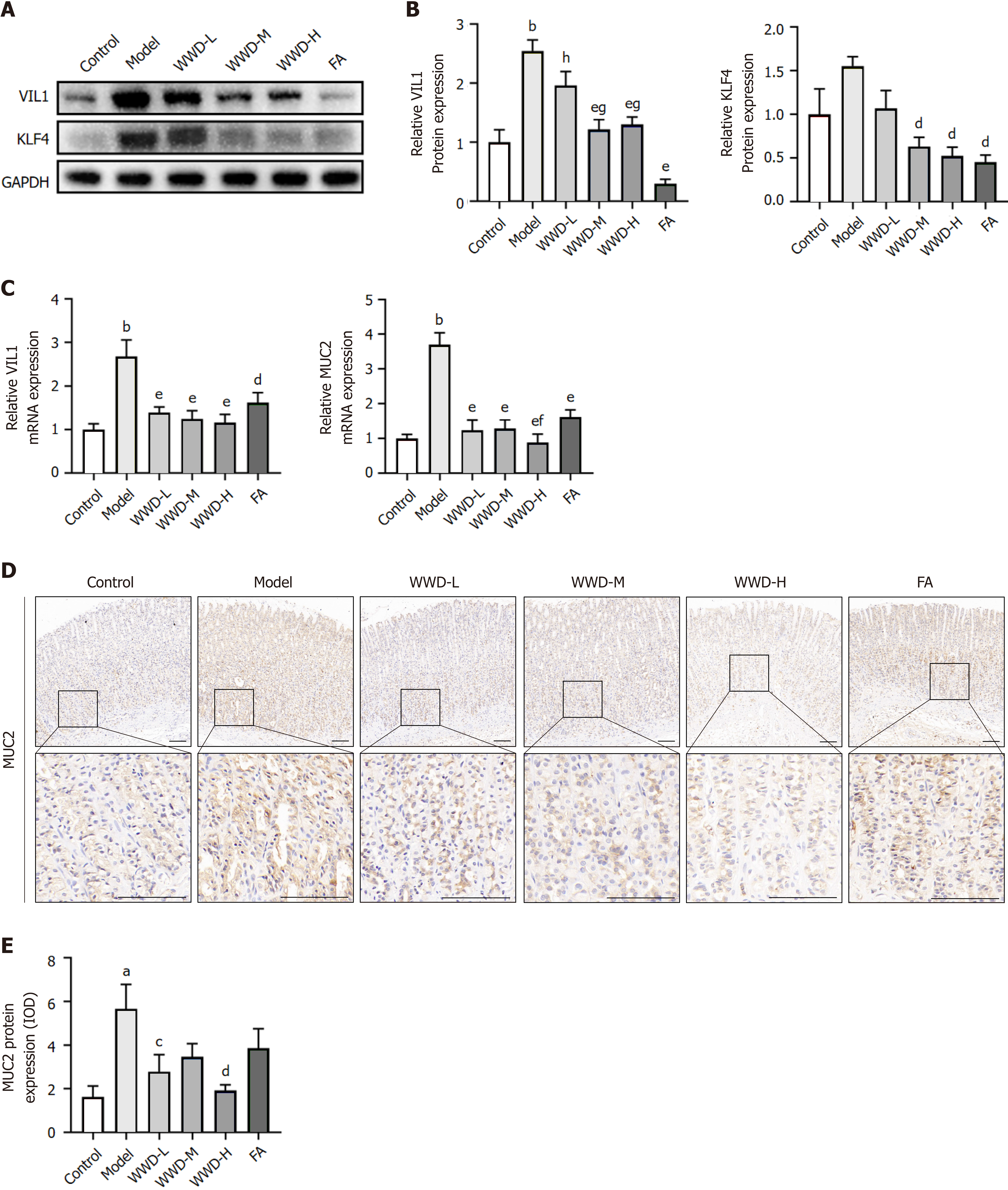
To further clarify the ameliorative effect of WWD on IM in rats, we measured intestinal markers regulated by CDX2. In the model group, the expression of VIL1 protein was significantly higher than that in the control group (P < 0.001). This elevation was effectively reversed by the administration of WWD-M, WWD-H, and FA (P < 0.001). Furthermore, exposure to MNNG enhanced KLF4 protein expressions; however, the difference was not statistically significant. WWD-M, WWD-H, and FA (P < 0.01) inhibited KLF4 protein levels compared to the model group (Figure 4A and B). Addi
To further visualize the effect of WWD on the regulation of the OLFM4/NOD1/CDX2 pathway in gastric IM, we determined the expression of each molecule in IM rats at the mRNA and protein levels. IHC revealed that OLFM4 and CDX2 expressions were extremely low in the control group samples, but the presences were significantly increased in the model group (P < 0.01). Correspondingly, WWD treatment suppressed OLFM4 (WWD-M, WWD-H, P < 0.01) and CDX2 (WWD-M, P < 0.05; WWD-H, P < 0.01) expressions. The most significant therapeutic effects were observed in the WWD-H group (Figure 5A, C, and D). Compared to the control group, significant NOD1 restraint was noted in MNNG-treated rats (P < 0.001). WWD (WWD-L, WWD-M, P < 0.001; WWD-H, P < 0.05) and FA (P < 0.001) interventions demonstrated up-regulatory effects on NOD1. The WWD-M group had remarkable potency among the WWD treatment groups (Figure 5B and D).
The transcription of OLFM4 and CDX2 was dramatically elevated in the model group (P < 0.001). Conversely, WWD and FA administrations decreased these trends (P < 0.001). Moreover, the inhibitory effects of WWD were dose-dependent. Additionally, a decrease in NOD1 transcription was observed in the model group compared to the control group (P < 0.05). WWD (WWD-L, WWD-H, P < 0.01; WWD-M, P < 0.001) and FA (P < 0.05) significantly stimulated NOD1 transcription (Figure 5E). These results illustrate that WWD suppresses OLFM4 expression and restores NOD1 level, consequently reducing CDX2 at both the mRNA and protein levels in IM rats.
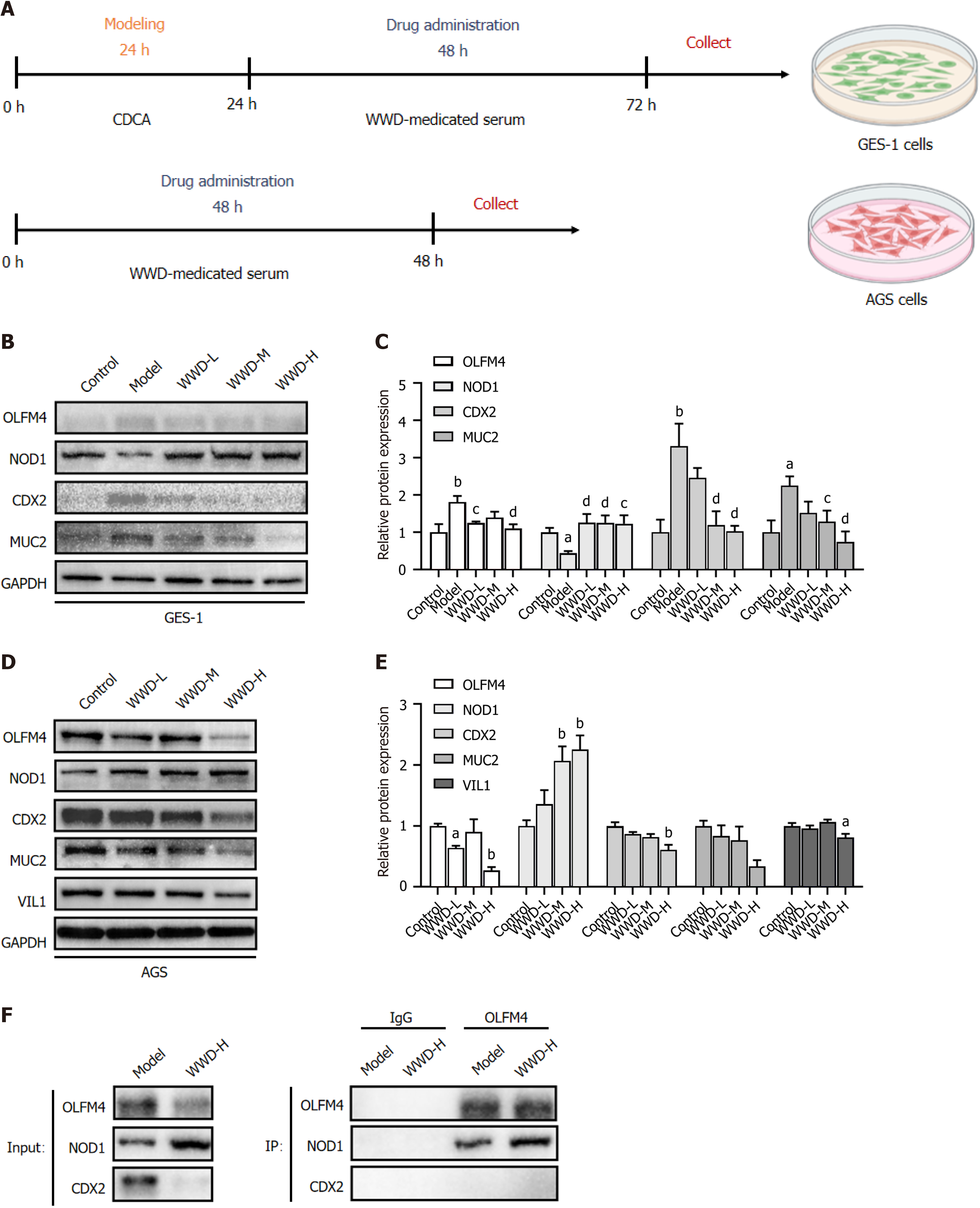
To explore the possible pharmacological actions of WWD-medicated serum in treating gastric IM, we examined the effects of WWD-medicated serum on the expression of relevant proteins of the OLFM4/NOD1/CDX2 pathway in gastric cells. To screen for the culture valid time of WWD-medicated serum, CDCA-pre-treated GES-1 cells, and AGS cells were incubated with the serum of rats treated with the corresponding WWD dosages. Light absorption values were measured at 0, 6, 12, 24, and 48 h, and cell viability rates were calculated. The results showed that WWD-medicated serum at each intragastric administration dose restrained GES-1 and AGS cells in a time-dependent manner respectively. Inhibition was the strongest at 48 h (P < 0.001), which was chosen as the culture-valid time for further investigation (Supplementary Figure 1B and C).
Following the experimental procedures (Figure 6A), the expression of OLFM4 (P < 0.01), CDX2 (P < 0.01), and MUC2 (P < 0.05) in GES-1 cells was dramatically elevated in the model group, while WWD-medicated serum was capable of reversing these upward trends. The WWD-L (P < 0.05) and WWD-H (P < 0.01) groups exerted OLFM4 attenuation, whereas the WWD-M (CDX2, P < 0.01; MUC2, P < 0.05) and WWD-H (P < 0.01) groups exhibited CDX2 and MUC2 inhibitions. Additionally, the WWD-H group demonstrated the most significant therapeutic effects. In contrast, there was a substantial decrease in NOD1 expression in the model group (P < 0.05). The WWD-medicated serum groups showed augmented NOD1 expression, and each WWD-medicated serum group exhibited similarly enhancing levels compared to the model group (WWD-L, WWD-M, P < 0.01; WWD-H, P < 0.05) (Figure 6B and C).
WWD-medicated serum markedly diminished the level of OLFM4 in AGS cells (WWD-L, P < 0.05; WWD-H, P < 0.01), while the expression of NOD1 was remarkably enhanced (WWD-M, WWD-H, P < 0.01). In addition, WWD-medicated serum mitigated the expression of CDX2 and VIL1, and the therapeutic effects in the WWD-H group were optimal (CDX2, P < 0.01; VIL1, P < 0.05). Notably, a decreasing trend for MUC2 was observed in the WWD groups compared to that in the control group; however, the differences were not statistically significant (Figure 6A, D, and E).
Subsequently, to validate the impact of WWD-medicated serum on intracellular OLFM4 and NOD1 interactions, we performed Co-IP assays using protein extracted from GES-1 cells. The results addressed that the WWD-H group strengthened OLFM4 and NOD1 interaction slightly compared to the model group. Whereas no interaction between OLFM4 and CDX2 was observed in either group (Figure 6F). Together, these results demonstrate that WWD-medicated serum attenuates OLFM4 level and simultaneously slightly strengthens OLFM4 and NOD1 binding, thereby enhancing NOD1 expression and consequently mitigating CDX2 and intestinal markers downstream at the protein level in gastric cells.
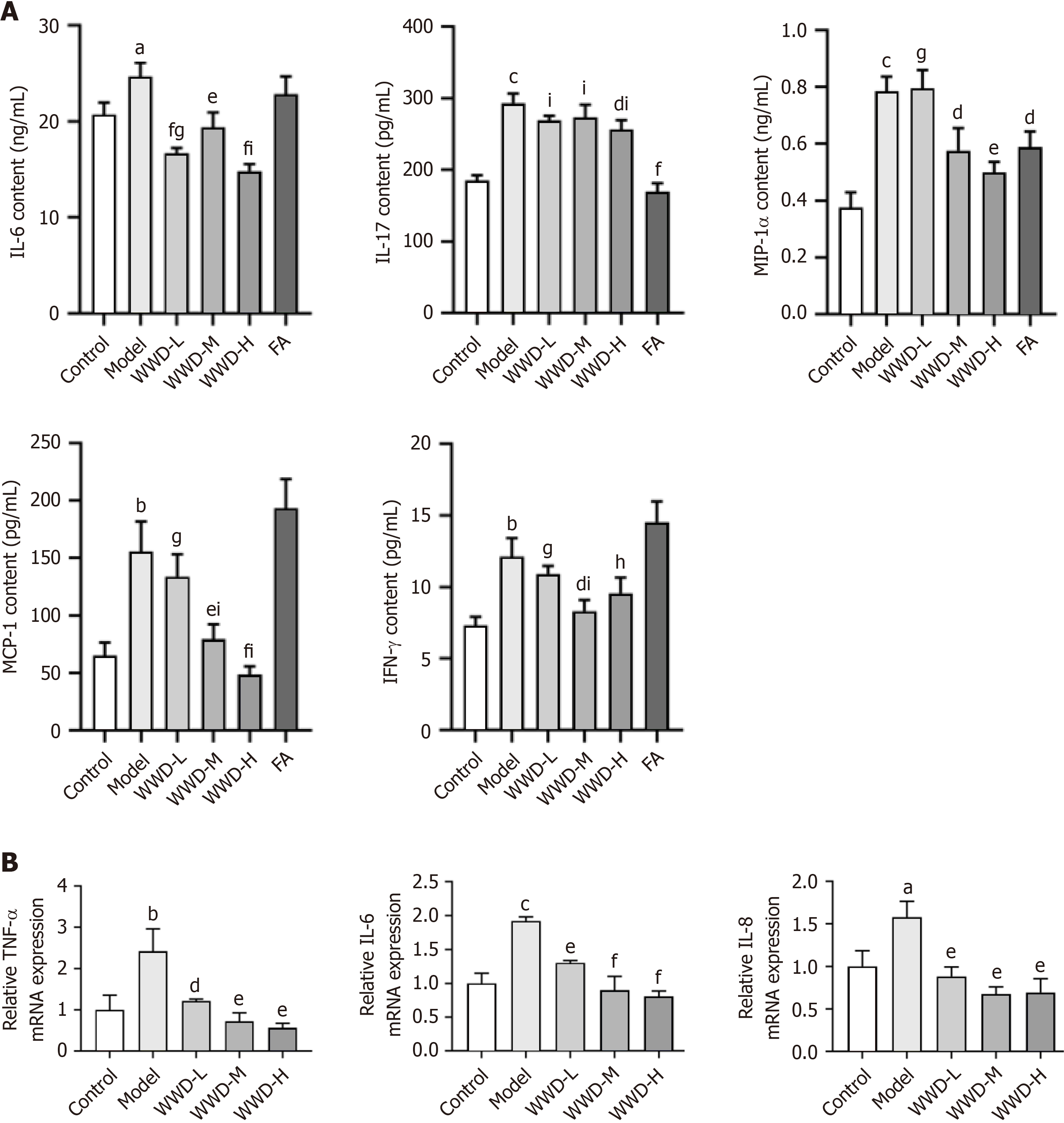
To determine the anti-inflammatory effect of WWD, we assessed cytokines and chemokines in rats and GES-1 cells, respectively. The content of cytokines such as IL-6, IFN-γ, IL-17, and chemokines such as MCP-1 and MIP-1α was tested in the serum of IM rats. Biomarker levels in the MNNG group were significantly higher than those in the control group (IL-6, P < 0.05; IL-17, MIP-1α, P < 0.001; MCP-1, IFN-γ, P < 0.01). Conversely, the WWD administration diminished these trends. The WWD-M (MIP-1α, P < 0.05; MCP-1, P < 0.01) and WWD-H (MIP-1α, P < 0.01; MCP-1, P < 0.001) groups dramatically decreased the content of MIP-1α and MCP-1, whereas the WWD-M group inhibited the content of IFN-γ (P < 0.05). Additionally, the WWD-H group showed reduced IL-17 levels (P < 0.05). WWD suppressed IL-6 levels in all dose groups (WWD-L, WWD-H, P < 0.001; WWD-M, P < 0.01). Likewise, markedly attenuation of IL-17 and MIP-1α levels upon FA intervention was also noted (IL-17, P < 0.001; MIP-1α, P < 0.05) (Figure 7A).
TNF-α, IL-6, and IL-8 are crucial biomarkers mediating gastric epithelial cell inflammatory injuries. In the present study, we investigated the transcription of these genes in GES-1 cells. TNF-α, IL-6, and IL-8 showed enhanced transcription levels in the model group (TNF-α, P < 0.01; IL-6, P < 0.001; IL-8, P < 0.05). In contrast, WWD-medicated serum intervention attenuated IL-8 transcription (P < 0.01). Moreover, WWD-medicated serum could effectively diminish the transcription of TNF-α (WWD-L, P < 0.05; WWD-M, WWD-H, P < 0.01) and IL-6 (WWD-L, P < 0.01; WWD-M, WWD-H, P < 0.001) in a dose-dependent manner (Figure 7B). In brief, WWD exerts anti-inflammatory effects during the treatment of gastric IM.
The incidence and mortality rate of GC have generally decreased in recent years globally, but it is still one of the major cancer types worldwide. Most cases are diagnosed in advanced stages with poor prognoses[29]. Intestinal-type adenocarcinoma is the most common type of GC, there is substantial evidence suggesting that it is preceded by precancerous lesions, including atrophy and IM[30]. Hence, improving the treatment of gastric precancerous lesions and thereby preventing GC is an essential public health issue that demands prompt solutions. Correspondingly, our study showed that WWD exerts a therapeutic effect on gastric IM. Clinically, it was found that WWD could attenuate IM symptoms in combination with standard quadruple therapy while improving IM pathological manifestations[14,15]. Experimentally, WWD was capable of improving histological alterations, simultaneously reducing the expression of the specific markers in advanced SPEM and IM[13]. Therefore, our research mainly investigated the pharmacological actions underlying the therapeutic effects of WWD on gastric IM.
CDX2, a crucial transcription factor that promotes intestinal differentiation, also considered a pivotal marker of gastric IM, is capable of inducing the expression of various intestinal markers downstream, including KLF4, VIL1, MUC2, SI, and cadherin 17 (CDH17). CDX2 is an intestinal-specific homeobox gene, which is one of the pivotal transcription factors in regulating the homeostasis of the adult gastrointestinal epithelium. Histological expression of CDX2 in proximal organs, such as the stomach and esophagus, causes metaplasia. Currently, the importance of CDX2 in regulating intestinal differentiation has been widely accepted. VIL1 and SI are the markers of intestinal columnar epithelial cells, whereas MUC2 is a marker of goblet cells. KLF4 is an important transcription factor involved in the development of intestinal mucosa similar to CDX2. KLF4 is up-regulated in metaplasia and induces the production of MUC2[31]. In gastric IM, CDX2 is elevated and directs the transformation by promoting the expression of intestinal markers, such as KLF4, VIL1, MUC2, and SI[8]. Furthermore, overexpression of CDX2 induces intestinal crypt-like structure differentiation in human gastric organoids while enhancing the expression of intestinal genes, namely MUC2 and CDH17[32].
In the present study, GES-1 cells treated with CDCA were utilized as cell models of IM. IM rat models were induced using MNNG. Pathogenic factors of gastric IM, such as H. pylori infection, bile reflux, and exposure to N-nitroso compounds, can promote the expression of CDX2[8,33]. Our assay confirmed that CDX2, MUC2, and VIL1 were up-regulated at the mRNA and protein levels in both in vivo and in vitro models of IM. Together with previous research, these findings indicate that CDX2 induces the transformation of IM and the expression of relevant intestinal markers.
In case of pharmacodynamic actions, it is noted that WWD could inhibit CDX2 elevation in IM and suppress the intestinal-like phenotype. Our study confirmed that WWD administration improved IM histological alternations in rat models. In addition, WWD reduced CDX2, VIL1, and MUC2 at the mRNA and protein levels while suppressing KLF4 protein level. Moreover, treatment of IM cell models with WWD-medicated serum reduced CDX2 and MUC2 proteins. Likewise, incubation of AGS cells in WWD-medicated serum reduced CDX2 and VIL1 proteins. These findings have corroborated this hypothesis in vitro. Beyond that, a clinical trial has indicated that Panax notoginseng (Burkill) F.H.Chen in this formula, together with Chinese herbal compounds, alleviates atrophy and IM in the treatment of CAG with erosion, which might help to explain the efficacious material basis of WWD against the intestinal-like phenotype[34].
Notably, current evidence suggests that OLFM4 is a biomarker of gastric IM, and has a strong connection to gastric IM that progresses to GC[35]. OLFM4 is remarkably increased in IM compared to that in chronic gastritis[25]. It is a robust marker of intestinal stem cells in both normal and metaplastic contexts[30]. Consistently, OLFM4 is co-expressed with intestinal stem cell markers, such as leucine rich repeat containing G protein-coupled receptor 5, EPH receptor B2, and achaete-scute family bHLH transcription factor 2. Thus, we presume that OLFM4 may be involved in the evolution and progression of metaplastic lineages in stomach[26]. OLFM4 is capable of mediating various cellular processes such as cell adhesion, migration, apoptosis, cell cycling, and proliferation. It is also regarded as a highly sensitive biomarker of early GC. The expression of OLFM4 is more frequently positive in stages I/II than in stages III/IV[24]. Here, we employed it as an entry point to study the pathogenic mechanisms of IM and the pharmacological actions of WWD.
NOD1 is a prominent pathogen-recognition molecule in epithelial cells. It may activate the immune reaction, prevent excessive inflammation, and protect against malignant transformation of gastric IM. The inflammation in response to H. pylori infection has been demonstrated to be more severe in NOD1-deficient mice of different genetic backgrounds[36]. Prolonged infection of NOD1-deficient mice with H. pylori leads to CDX2 up-regulation and aggravation of IM[27]. In INS-GAS FVB/N mouse stomach cancer models, NOD1 knockout increases the prevalence of dysplasia and accelerates gastric carcinogenesis[36]. Previous studies have also indicated that H. pylori activates NOD1 to promote the production of IL-33 in gastric epithelial cells, which functions as an anti-inflammatory cytokine that mediates tissue repair[37]. In contrast, analysis of NOD1 in human GC and normal tissues illustrated that the epithelial staining intensity of NOD1 is diminished in human malignant tissues[38].
In terms of pathogenic mechanisms, our study confirmed OLFM4 elevation and NOD1 reduction in IM progression, which is consistent with the dominant view of the current research that OLFM4 plays a promoting role in IM progression and carcinogenesis, whereas NOD1 effectively protects against IM malignant transformation.
Regarding WWD’s pharmacological actions in the treatment of gastric IM, our study demonstrated that WWD administration suppressed OLFM4 and enhanced NOD1 at the mRNA and protein levels in IM rat models. A parallel regulatory mechanism was demonstrated at the protein level in IM cell models and AGS cells treated with WWD-medicated serum, suggesting that WWD may exert a therapeutic effect by suppressing OLFM4 and enhancing NOD1 in gastric IM. TCM formula has the characteristics of multi-target, multi-pathway, and multi-mechanism. Our study has confirmed that OLFM4 is a vital target of WWD in the treatment of IM, revealing that TCM compounds could alleviate gastric IM by targeting OLFM4. On the other hand, functional components of WWD, such as naringin, ginsenoside Rb1, quercetin, and astragaloside IV, possess the potential capacity to modulate NOD-like receptors and coordinate immuno-inflammatory responses[39-42]. These findings provide evidence for the regulation of OLFM4 and NOD1 expressions by WWD in the process of treating gastric IM.
Another important issue is, what is the specific regulatory relationship between OLFM4, NOD1, and CDX2 in IM? Previous studies showed that OLFM4 may mediate gastric IM preceding CDX2, and is involved in the indirect regulation of CDX2 expression[25]. In addition, OLFM4 may interact with NOD1 during IM transformation. OLFM4 is a target gene of H. pylori-activated NF-κB signaling, which has a negative feedback effect on NF-κB in turn through direct interaction with NOD1, thus promoting persistent H. pylori colonization[28]. Furthermore, NOD1 inhibits gastric IM progression by down-regulating CDX2[27]. The OLFM4/NOD1/CDX2 signaling pathway may exert a regulatory role in gastric IM.
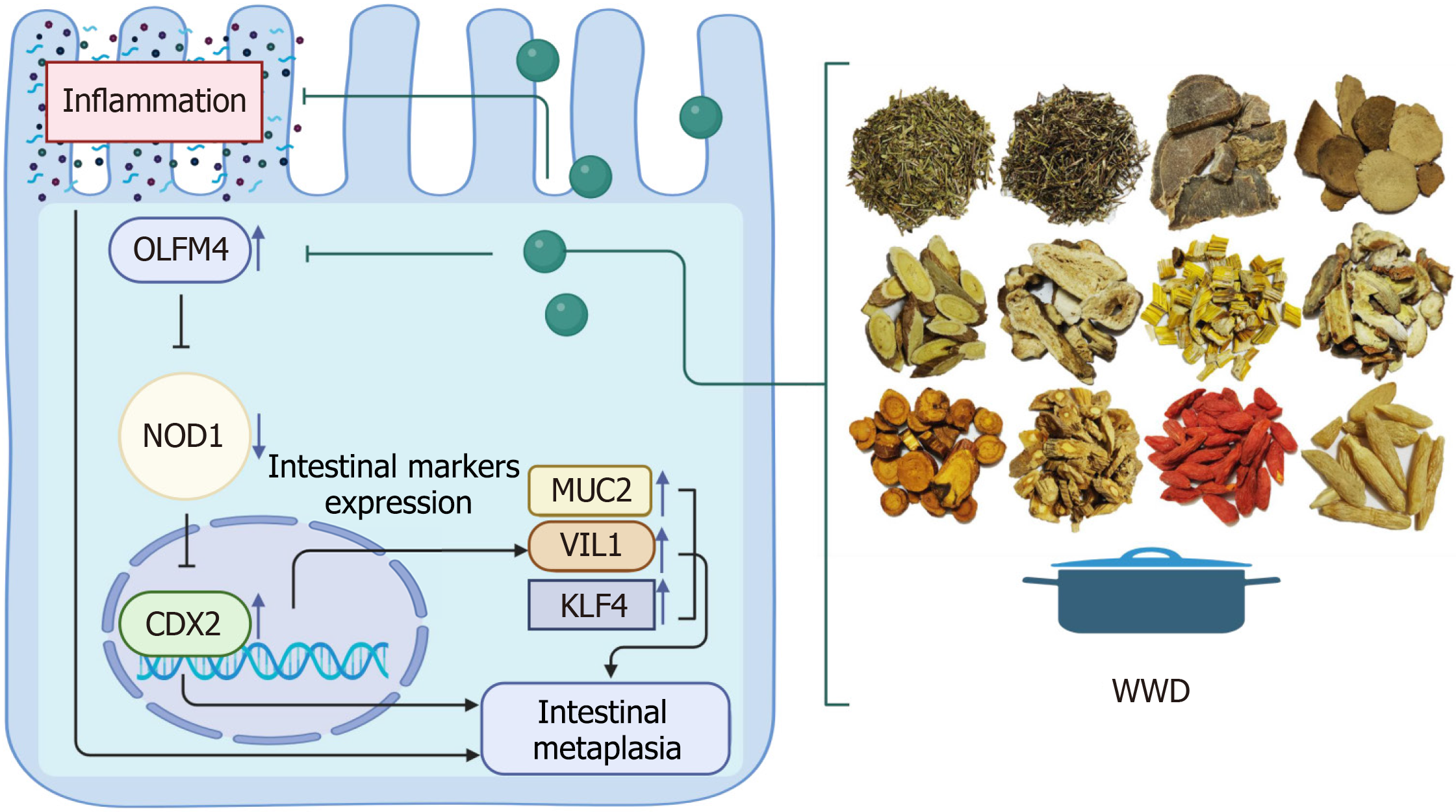
In this study, we performed tissue microarray IHC assays and showed that the OLFM4/NOD1/CDX2 axis is a characteristic of human gastric IM tissues. Further, we indicated that OLFM4 may induce CDX2 through NOD1 and promote the progression of gastric IM. The transfection of IM cell models and AGS cells with shRNAs targeting OLFM4 enhanced NOD1 expression and suppressed CDX2 protein levels. In contrast, the reversed protein alternations were observed in GES-1 cells overexpressing OLFM4. Subsequently, we elucidated that OLFM4 induces CDX2 by interacting with NOD1, rather than activating CDX2 directly. Our Co-IP assays showed that OLFM4 directly interacts with NOD1 in gastric cells. Despite WWD inhibited OLFM4 expression, WWD treatment slightly strengthened OLFM4 and NOD1 interaction. Thus, we confirmed that OLFM4 may play an important regulatory role by interacting with NOD1 in IM progression, whereas WWD attenuates IM by strengthening the interaction of OLFM4 and NOD1. Moreover, we provide further insight into the potential mechanism of OLFM4 regulating CDX2 through the down-regulation of NOD1. The introduction of NOD1 shRNA restored the CDX2 and MUC2 protein down-regulation mediated by OLFM4 shRNA transfection in IM cell models. Taken together, we conclude that OLFM4 exhibits direct binding and subsequent down-regulation of NOD1, thereby sustaining the activation of CDX2 and promoting the progression of IM. In contrast, WWD exerts a therapeutic effect by suppressing OLFM4 expression and simultaneously strengthening the interaction of OLFM4 and NOD1, thereby restoring NOD1 levels and consequently reducing CDX2 in gastric IM. Our study results preliminarily demonstrate that the OLFM4/NOD1/CDX2 signaling pathway is involved in the regulation of IM, and WWD exerts its therapeutic efficacy on IM by modulating this pathway (Figure 8). Results from our research provide valuable insights into the pharmacological mechanisms underlying the effectiveness of WWD in IM treatment. Further offering a new perspective on the treatment of gastric IM and prevention of GC with TCM.
Gastric IM is triggered by chronic inflammatory cell infiltration and repeated epithelial cell damage. Inhibiting the inflammatory actions would effectively limit the transformation of IM and lower the risk of GC accumulation[1]. WWD treatment restrained IL-6, IFN-γ, IL-17, MCP-1, and MIP-1α content in the serum of IM rats. We tested several vital cytokines and chemokines that induce inflammatory injuries in the gastric epithelial cells. WWD-medicated serum could significantly inhibit the transcription of TNF-α, IL-6, and IL-8 in IM cell models. We previously conducted a study that illustrated WWD administration tends to reduce the transcription of cytokines in the gastric mucosa of H. pylori-infected mice, but the differences were not statistically significant. This may be brought on by the more severe inflammatory reactions in gastric IM caused by H. pylori infection compared to those induced by MNNG administration[13]. Taken together, WWD reduces the expression of cytokines and chemokines and limits inflammatory reactions in gastric IM to some extent.
The OLFM4/NOD1/CDX2 signaling pathway is involved in the regulation of IM. WWD exerts its therapeutic efficacy on IM by modulating this pathway. Additionally, WWD attenuates the inflammatory response of IM. These findings provide valuable insights into the pharmacological mechanisms underlying the effectiveness of WWD in IM treatment.
The authors would like to acknowledge Hong-Yue Li, Yu-Sheng Huang, Zi-Ming Zhao, and Sheng-Min Lai for their skillful technical assistance.
| 1. | Goldenring JR, Mills JC. Cellular Plasticity, Reprogramming, and Regeneration: Metaplasia in the Stomach and Beyond. Gastroenterology. 2022;162:415-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 100] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 2. | Du Y, Bai Y, Xie P, Fang J, Wang X, Hou X, Tian D, Wang C, Liu Y, Sha W, Wang B, Li Y, Zhang G, Shi R, Xu J, Huang M, Han S, Liu J, Ren X, Wang Z, Cui L, Sheng J, Luo H, Zhao X, Dai N, Nie Y, Zou Y, Xia B, Fan Z, Chen Z, Lin S, Li ZS; Chinese Chronic Gastritis Research group. Chronic gastritis in China: a national multi-center survey. BMC Gastroenterol. 2014;14:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 105] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 3. | de Vries AC, van Grieken NC, Looman CW, Casparie MK, de Vries E, Meijer GA, Kuipers EJ. Gastric cancer risk in patients with premalignant gastric lesions: a nationwide cohort study in the Netherlands. Gastroenterology. 2008;134:945-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 584] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 4. | Song H, Ekheden IG, Zheng Z, Ericsson J, Nyrén O, Ye W. Incidence of gastric cancer among patients with gastric precancerous lesions: observational cohort study in a low risk Western population. BMJ. 2015;351:h3867. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 182] [Cited by in RCA: 204] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 5. | Li D, Bautista MC, Jiang SF, Daryani P, Brackett M, Armstrong MA, Hung YY, Postlethwaite D, Ladabaum U. Risks and Predictors of Gastric Adenocarcinoma in Patients with Gastric Intestinal Metaplasia and Dysplasia: A Population-Based Study. Am J Gastroenterol. 2016;111:1104-1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 106] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 6. | Jiang JX, Liu Q, Zhao B, Zhang HH, Sang HM, Djaleel SM, Zhang GX, Xu SF. Risk factors for intestinal metaplasia in a southeastern Chinese population: an analysis of 28,745 cases. J Cancer Res Clin Oncol. 2017;143:409-418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Drake JW, Baltz RH. The biochemistry of mutagenesis. Annu Rev Biochem. 1976;45:11-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 292] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 8. | Li T, Guo H, Li H, Jiang Y, Zhuang K, Lei C, Wu J, Zhou H, Zhu R, Zhao X, Lu Y, Shi C, Nie Y, Wu K, Yuan Z, Fan DM, Shi Y. MicroRNA-92a-1-5p increases CDX2 by targeting FOXD1 in bile acids-induced gastric intestinal metaplasia. Gut. 2019;68:1751-1763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 9. | Bao Z, Wu G, Du J, Ye Y, Zheng Y, Wang Y, Ji R. The comparative efficacy and safety of 9 traditional Chinese medicines combined with standard quadruple therapy for Helicobacter pylori-associated gastritis: a systematic review and network meta-analysis. Ann Transl Med. 2022;10:1349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 10. | Lu W, Ni Z, Jiang S, Tong M, Zhang J, Zhao J, Feng C, Jia Q, Wang J, Yao T, Ning H, Shi Y. Resveratrol inhibits bile acid-induced gastric intestinal metaplasia via the PI3K/AKT/p-FoxO4 signalling pathway. Phytother Res. 2021;35:1495-1507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 11. | Xie D, Wu C, Wang D, Nisma Lena BA, Liu N, Ye G, Sun M. Wei-fu-chun tablet halted gastric intestinal metaplasia and dysplasia associated with inflammation by regulating the NF-κB pathway. J Ethnopharmacol. 2024;318:117020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 12. | Xu W, Li B, Xu M, Yang T, Hao X. Traditional Chinese medicine for precancerous lesions of gastric cancer: A review. Biomed Pharmacother. 2022;146:112542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 80] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 13. | Hong X, Li H, Lin Y, Luo L, Xu W, Kang J, Li J, Huang B, Xu Y, Pan H, Guo S. Efficacy and potential therapeutic mechanism of Weiwei decoction on Spasmolytic polypeptide-expressing metaplasia in Helicobacter pylori-infected and Atp4a-knockout mice. J Ethnopharmacol. 2024;319:117062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Deng RN, Huang MH, Liu XL, Mu GP. Clinical study of Weiwei soup on patients with CAG and precancerous change of gastric cancer and effect on expression by PCNA, bcl-2, bax. Zhongguo Zhongxiyi Jiehe Xiaohua Zazhi. 2011;19:10-17. |
| 15. | Kang JY, Guo SJ, Zheng CB, Li JW. Clinical Observation on Weiwei Decoction in the Treatment of Helicobacter pylori-positive Chronic Atrophic Gastritis. Guangming Zhongyi. 2019;34:2605-2608. |
| 16. | Zhang C, Cai T, Zeng X, Cai D, Chen Y, Huang X, Gan H, Zhuo J, Zhao Z, Pan H, Li S. Astragaloside IV reverses MNNG-induced precancerous lesions of gastric carcinoma in rats: Regulation on glycolysis through miRNA-34a/LDHA pathway. Phytother Res. 2018;32:1364-1372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Zeng J, Ma X, Zhao Z, Chen Y, Wang J, Hao Y, Yu J, Zeng Z, Chen N, Zhao M, Tang J, Gong D. Ginsenoside Rb1 Lessens Gastric Precancerous Lesions by Interfering With β-Catenin/TCF4 Interaction. Front Pharmacol. 2021;12:682713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 18. | Zhu J, Wen K. Astragaloside IV inhibits TGF-β1-induced epithelial-mesenchymal transition through inhibition of the PI3K/Akt/NF-κB pathway in gastric cancer cells. Phytother Res. 2018;32:1289-1296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 19. | González-Segovia R, Quintanar JL, Salinas E, Ceballos-Salazar R, Aviles-Jiménez F, Torres-López J. Effect of the flavonoid quercetin on inflammation and lipid peroxidation induced by Helicobacter pylori in gastric mucosa of guinea pig. J Gastroenterol. 2008;43:441-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 72] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 20. | Ekström AM, Serafini M, Nyrén O, Wolk A, Bosetti C, Bellocco R. Dietary quercetin intake and risk of gastric cancer: results from a population-based study in Sweden. Ann Oncol. 2011;22:438-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 21. | Gong Y, Dong R, Gao X, Li J, Jiang L, Zheng J, Cui S, Ying M, Yang B, Cao J, He Q. Neohesperidin prevents colorectal tumorigenesis by altering the gut microbiota. Pharmacol Res. 2019;148:104460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 22. | Li ZL, Yang BC, Gao M, Xiao XF, Zhao SP, Liu ZL. Naringin improves sepsis-induced intestinal injury by modulating macrophage polarization via PPARγ/miR-21 axis. Mol Ther Nucleic Acids. 2021;25:502-514. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 23. | Zhang J, Liu WL, Tang DC, Chen L, Wang M, Pack SD, Zhuang Z, Rodgers GP. Identification and characterization of a novel member of olfactomedin-related protein family, hGC-1, expressed during myeloid lineage development. Gene. 2002;283:83-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 113] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 24. | Oue N, Sentani K, Noguchi T, Ohara S, Sakamoto N, Hayashi T, Anami K, Motoshita J, Ito M, Tanaka S, Yoshida K, Yasui W. Serum olfactomedin 4 (GW112, hGC-1) in combination with Reg IV is a highly sensitive biomarker for gastric cancer patients. Int J Cancer. 2009;125:2383-2392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 81] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 25. | Jang BG, Lee BL, Kim WH. Olfactomedin-related proteins 4 (OLFM4) expression is involved in early gastric carcinogenesis and of prognostic significance in advanced gastric cancer. Virchows Arch. 2015;467:285-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | Jang BG, Lee BL, Kim WH. Intestinal Stem Cell Markers in the Intestinal Metaplasia of Stomach and Barrett's Esophagus. PLoS One. 2015;10:e0127300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 27. | Asano N, Imatani A, Watanabe T, Fushiya J, Kondo Y, Jin X, Ara N, Uno K, Iijima K, Koike T, Strober W, Shimosegawa T. Cdx2 Expression and Intestinal Metaplasia Induced by H. pylori Infection of Gastric Cells Is Regulated by NOD1-Mediated Innate Immune Responses. Cancer Res. 2016;76:1135-1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 28. | Liu W, Yan M, Liu Y, Wang R, Li C, Deng C, Singh A, Coleman WG Jr, Rodgers GP. Olfactomedin 4 down-regulates innate immunity against Helicobacter pylori infection. Proc Natl Acad Sci U S A. 2010;107:11056-11061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 117] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 29. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64034] [Article Influence: 16008.5] [Reference Citation Analysis (174)] |
| 30. | Zhang P, Yang M, Zhang Y, Xiao S, Lai X, Tan A, Du S, Li S. Dissecting the Single-Cell Transcriptome Network Underlying Gastric Premalignant Lesions and Early Gastric Cancer. Cell Rep. 2019;27:1934-1947.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 272] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 31. | Kazumori H, Ishihara S, Takahashi Y, Amano Y, Kinoshita Y. Roles of Kruppel-like factor 4 in oesophageal epithelial cells in Barrett's epithelium development. Gut. 2011;60:608-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 32. | Koide T, Koyanagi-Aoi M, Uehara K, Kakeji Y, Aoi T. CDX2-induced intestinal metaplasia in human gastric organoids derived from induced pluripotent stem cells. iScience. 2022;25:104314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 33. | Manzo BA, Crabtree JE, Fiona Campbell M, Tweedle D, Potten CS, Bajaj-Elliott M, Sanderson IR, Wilson JW. Helicobacter pylori regulates the expression of inhibitors of DNA binding (Id) proteins by gastric epithelial cells. Microbes Infect. 2006;8:1064-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 34. | Zhang T, Zhang B, Xu J, Ren S, Huang S, Shi Z, Guo S, Bian L, Wang P, Wang F, Cai Y, Tang X. Chinese herbal compound prescriptions combined with Chinese medicine powder based on traditional Chinese medicine syndrome differentiation for treatment of chronic atrophic gastritis with erosion: a multi-center, randomized, positive-controlled clinical trial. Chin Med. 2022;17:142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 35. | Pang L, Yan X, Su D, Wu X, Jiang H. Feasibility of olfactomedin 4 as a molecular biomarker for early diagnosis of gastric neoplasia after intestinal metaplasia. Scand J Gastroenterol. 2023;58:133-141. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 36. | Suarez G, Romero-Gallo J, Piazuelo MB, Sierra JC, Delgado AG, Washington MK, Shah SC, Wilson KT, Peek RM Jr. Nod1 Imprints Inflammatory and Carcinogenic Responses toward the Gastric Pathogen Helicobacter pylori. Cancer Res. 2019;79:1600-1611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 37. | Tran LS, Tran D, De Paoli A, D'Costa K, Creed SJ, Ng GZ, Le L, Sutton P, Silke J, Nachbur U, Ferrero RL. NOD1 is required for Helicobacter pylori induction of IL-33 responses in gastric epithelial cells. Cell Microbiol. 2018;20:e12826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 38. | Suarez G, Romero-Gallo J, Piazuelo MB, Wang G, Maier RJ, Forsberg LS, Azadi P, Gomez MA, Correa P, Peek RM Jr. Modification of Helicobacter pylori Peptidoglycan Enhances NOD1 Activation and Promotes Cancer of the Stomach. Cancer Res. 2015;75:1749-1759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 39. | Chen W, Wang J, Luo Y, Wang T, Li X, Li A, Li J, Liu K, Liu B. Ginsenoside Rb1 and compound K improve insulin signaling and inhibit ER stress-associated NLRP3 inflammasome activation in adipose tissue. J Ginseng Res. 2016;40:351-358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 87] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 40. | Cao H, Liu J, Shen P, Cai J, Han Y, Zhu K, Fu Y, Zhang N, Zhang Z, Cao Y. Protective Effect of Naringin on DSS-Induced Ulcerative Colitis in Mice. J Agric Food Chem. 2018;66:13133-13140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 143] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 41. | Song MT, Ruan J, Zhang RY, Deng J, Ma ZQ, Ma SP. Astragaloside IV ameliorates neuroinflammation-induced depressive-like behaviors in mice via the PPARγ/NF-κB/NLRP3 inflammasome axis. Acta Pharmacol Sin. 2018;39:1559-1570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 73] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 42. | Chiang SCC, Owsley E, Panchal N, Chaturvedi V, Terrell CE, Jordan MB, Mehta PA, Davies SM, Akeno N, Booth C, Marsh RA. Quercetin ameliorates XIAP deficiency-associated hyperinflammation. Blood. 2022;140:706-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |