Published online Jun 15, 2024. doi: 10.4251/wjgo.v16.i6.2816
Revised: April 13, 2024
Accepted: April 23, 2024
Published online: June 15, 2024
Processing time: 114 Days and 21.4 Hours
To investigate the relationship between interstitial maturity and prognosis of colorectal cancer.
To examine the correlation between interstitial maturity and the prognosis of colorectal cancer.
The paper database PubMed, EMBASE, Cochranelibrary, Springerlink, CNKI, and Wanfang database were searched until December 2023. "tumor stroma maturity" "desmoplastic stroma reaction" "desmoplastic reaction" "stroma reaction" "degree of stroma reaction "" stroma classification" "stroma density" "colorectal cancer" "colon cancer" "rectal cancer" "prognosis" were searched for the search terms. Two system assessors independently screened the literature quality according to the inclusion exclusion criteria, Quality evaluation and data extraction were perf
Finally, data of 9849 patients with colorectal cancer from 19 cosets in 15 literatures were included, including 4339 patients with mature type (control group), 3048 patients with intermediate type (intermediate group) and 2456 patients with immature type (immature group). The results of meta-analysis showed: Relapse-free survival [hazard ratio (HR) = 2.66, 95% confidence interval (CI): 2.30-3.08; P < 0.00001], disease-free survival (HR = 3.68, 95%CI: 2.33-5.81; P < 0.00001) and overall survival (HR = 1.70, 95%CI: 1.53-1.87; P < 0.00001) were significantly lower than those in mature group (control group); relapse-free survival (HR = 1.36, 95%CI: 1.17-1.59; P < 0.0001) and disease-free survival rate (HR = 1.85, 95%CI: 1.53-2.24; P < 0.0001) was significantly lower than the mature group (control group).
There is the correlation between tumor interstitial maturity and survival prognosis of colorectal cancer, and different degrees of tumor interstitial maturity have a certain impact on the quality of life of colorectal cancer pat
Core Tip: Our study explored the correlation between interstitial maturity and the prognosis of patients with colorectal cancer through meta-analysis. We will collect and analyze data from the literature on interstitial maturity assessment and prognostic indicators in colorectal cancer patients to verify its relevance and evaluate its application prospects in clinical practice. This study will provide a new perspective for understanding the development mechanisms of colorectal cancer and provide a scientific basis for the formulation of clinical treatment strategies.
- Citation: Liu ZJ, Zhang XW, Liu QQ, Wang SZ. Correlation analysis of interstitial maturity and prognosis of colorectal cancer: Meta-analysis. World J Gastrointest Oncol 2024; 16(6): 2816-2825
- URL: https://www.wjgnet.com/1948-5204/full/v16/i6/2816.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i6.2816
The biological behavior of tumor cells is not only related to their own gene mutations but also plays an important role in the tumor microenvironment[1]. The tumor microenvironment includes the structural, functional, and metabolic envi
Numerous factors can affect the prognosis of colorectal cancer, which is a common malignant tumor. Over the past few decades, significant progress has been made in the treatment and management of colorectal cancer, but significant challenges remain. In recent years, more and more studies[2,3] have shown that there is a certain correlation between the interstitial maturity of tumors and the prognosis of patients with colorectal cancer. However, there are differences between the results of different studies, and there is no consistent conclusion. Therefore, a systematic review and meta-analysis of the correlation between interstitial maturity and the prognosis of colorectal cancer are of great significance[4]. With the deepening of cancer research, people gradually realize the importance of the microenvironment around tumors. As an important component of the tumor microenvironment, mesenchymal cells play a key role in tumor progression and therapeutic response. However, there is no comprehensive and consistent understanding of the specific impact of interstitial maturity on the prognosis of patients with colorectal cancer. Some studies have suggested that mature stroma may be associated with tumor development and a good prognosis, while others have argued otherwise. This phenomenon may be due to the heterogeneity of study samples, differences in research methods, and diversity of data analysis.
Given the inconsistent results of current studies on the relationship between interstitial maturity and the prognosis of colorectal cancer, it is important to conduct a systematic review and meta-analysis to integrate the existing research results and reveal the underlying regularities. Through systematic collection, screening, and analysis of existing literature, this study aims to explore the impact of interstitial maturity on the prognosis of patients with colorectal cancer in order to provide more valuable evidence support for clinical practice, provide a scientific basis for the formulation of an individualized treatment plan, and ultimately improve the prognosis and quality of life of patients with colorectal cancer.
In this study, published survival data on DR And colorectal cancer prognosis were meta-analyzed to determine the relationship between tumor interstitial maturity and colorectal cancer prognosis.
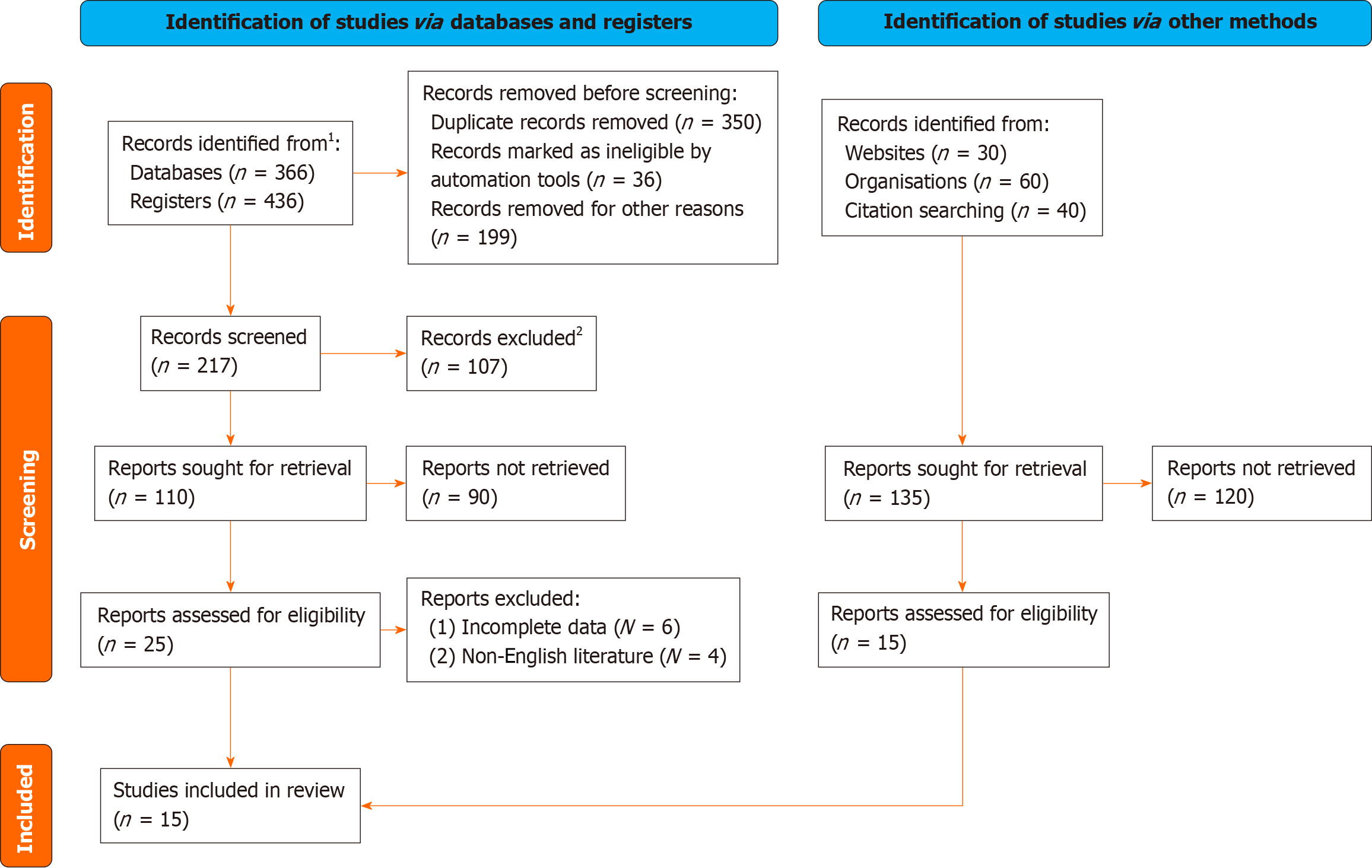
"Tumor interstitial maturity", "pro-fibrostitial reaction", "pro-fibrostitial reaction", "interstitial reaction", "degree of interstitial reaction", "interstitial grade", "interstitial density", "colorectal cancer" and "prognosis" were used as search terms. Chinese databases such as web.com and VIP, as well as foreign language databases such as Cochrane Library, PubMed, SpringerLink, EBSCO, MEDLINE, etc. were included in prospective and retrospective cohort studies from inception to December 2023. In order to avoid bias caused by language limitations, this study searched both English literature. In order to avoid missing relevant studies, relevant references listed in the article and conference abstracts found in the search were traced (Figure 1).
(1) All clinical studies on interstitial maturity and prognosis of colorectal cancer tumors, including clinicopathological characteristics and follow-up data; (2) The age and region of the research are clear; (3) The subjects were patients with colorectal cancer confirmed by pathological results; (4) Patients did not receive preoperative chemoradiotherapy; (5) Different subjects published by the same institution or author in the same year were included; (6) Tumor stromal matu
(1) Duplicate or published reviews or meta-analyses; (2) No literature on control group or non-exposed group; (3) The results of the study did not include literature on the degree of DR in the primary lesion of colorectal cancer; and (4) Unreasonable statistical methods; (5) Literature that did not contain or could not extract prognostic hazard ratio (HR) data from survival curves.
The assessment of heterogeneity among studies was conducted using I2 statistics, with 25%, 50%, and 75% respective reflecting low, medium, and high levels of heterogeneity. The sensitivity analysis involved the sequential removal of the included literature in order to assess the stability and reliability of the pooled effect estimates (Figure 2).
The Cochrane Collaboration Center provided Rewiew Manger 5.2 software (Cochrane Information Management System) for statistical analysis, and the Risk ratio of dichotic variables was adopted. RR and 95% confidence interval (CI) were used as efficacy and side effects analysis statistics in meta analysis. χ² test (P < 0.05 as the test level), hypothesis test adopts the U test expressed by Z value and P value, and sets 0.05 as the significance level, that is, P < 0.05 is used when the difference is statistically significant. The results of hypothesis test are listed in the forest map. Heterogeneity was analyzed by χ² test. P < 0.10, r = 25%, 50% and 75% were considered as low, medium and high heterogeneity, respe
A total of 802 literatures were obtained through search, 107 literatures with obvious inconsistency were excluded by reading the title, and 110 literatures were preliminarily included; 25 literatures were included after irrelevant and non-clinical studies were excluded by reading the abstract; and 15 qualified literatures[5-19] were finally included by reading the full text according to the inclusion and exclusion criteria. Prospective and retrospective cohort studies evaluated by Newcastle-Ottawa Scale were all of high quality, with scores ranging from 6 to 8, as shown in Figure 1 and Table 1.
| Ref. | Time | Research type | Cases | Gender (male/female) | Age (yr) | Stage | Follow-up time (months) | NOS score |
| Ueno et al[5] | 2002 | Cohort study | 627 | 393/234 | 60.3 ± 12 | Ⅰ-Ⅲ | 153 (80-267) | 6 |
| Ueno et al[6] | 2004 | Cohort study | 862 | NA | NA | Ⅰ-Ⅲ | 140 | 6 |
| Wu et al[7] | 2014 | Cohort study | 412 | NA | 61.4 ± 12 | Ⅳ | 61 (11-188) | 7 |
| Ueno et al[8] | 2015 | Cohort study | 880 | 512/368 | 62.9 ± 13 | Ⅱ-Ⅲ | 68 | 8 |
| Ueno et al[8] | 2015 | Cohort study | 474 | 268/206 | 66.3 ± 13 | Ⅱ-Ⅲ | 68 | 6 |
| Ueno et al[9] | 2017 | Cohort study | 821 | 484/337 | 66.2 ± 12 | Ⅱ-Ⅲ | 59 (1-86) | 7 |
| Wu et al[10] | 2018 | Cohort study | 466 | 264/202 | 59.9 ± 13 | Ⅲ | 59 (45-144) | 7 |
| Wu et al[10] | 2018 | Cohort study | 432 | 247/185 | 65.1 ± 12 | Ⅲ | 58 (1-87) | 8 |
| Shin et al[11] | 2019 | Cohort study | 151 | 88/63 | 63.2 ± 11 | Ⅰ-Ⅳ | 56 | 6 |
| Konishi et al[12] | 2018 | Cohort study | 851 | 407/444 | 66 ± 14 | Ⅰ-Ⅲ | 58 | 6 |
| Nearchou et al[13] | 2019 | Cohort study | 283 | 184/99 | 62.3 ± 11 | Ⅱ | NA | 6 |
| Nearchou et al[13] | 2019 | Cohort study | 163 | 87/76 | 63 ± 12 | Ⅱ | NA | 7 |
| Ueno et al[14] | 2019 | Cohort study | 679 | 400/279 | 64.0 ± 12 | Ⅱ | 91 | 8 |
| Ueno et al[14] | 2019 | Cohort study | 446 | 267/179 | 67.0 ± 12 | Ⅱ | 58 | 8 |
| González et al[15] | 2020 | Cohort study | 342 | 179/163 | 65.2 ± 12.6 | Ⅰ-Ⅲ | NA | 8 |
| Hacking et al[16] | 2020 | Cohort study | 234 | 111/118 | 64 ± 13 | I-Ⅲ | NA | 6 |
| Ao et al[17] | 2020 | Cohort study | 363 | 209/154 | 66.6 ± 12 | Ⅲ | 61 (1-136) | 8 |
| Gonzalez et al[18] | 2021 | Cohort study | 372 | 189/183 | 65.7 ± 12.9 | Ⅰ-Ⅲ | 62 (1-170) | 6 |
| Ueno et al[19] | 2021 | Cohort study | 991 | 601/390 | 65.5 ± 12.2 | Ⅱ | 69.7 (2.1-105.6) | 7 |
The 15 literatures included were all foreign literatures, among which 4 literatures included two different cosets of subjects, and a total of 9849 patients with colorectal cancer were included in 19 cosets, including 4339 patients with mature tumor interstitium, 3048 patients with intermediate tumor, and 2456 patients with immature tumor. Two of the studies included patients in stage IV, while the rest were in stage I to III. The characteristic data of each study include publication time, region, study type, sample size, gender, age, stage, tumor site, follow-up time and observation indic
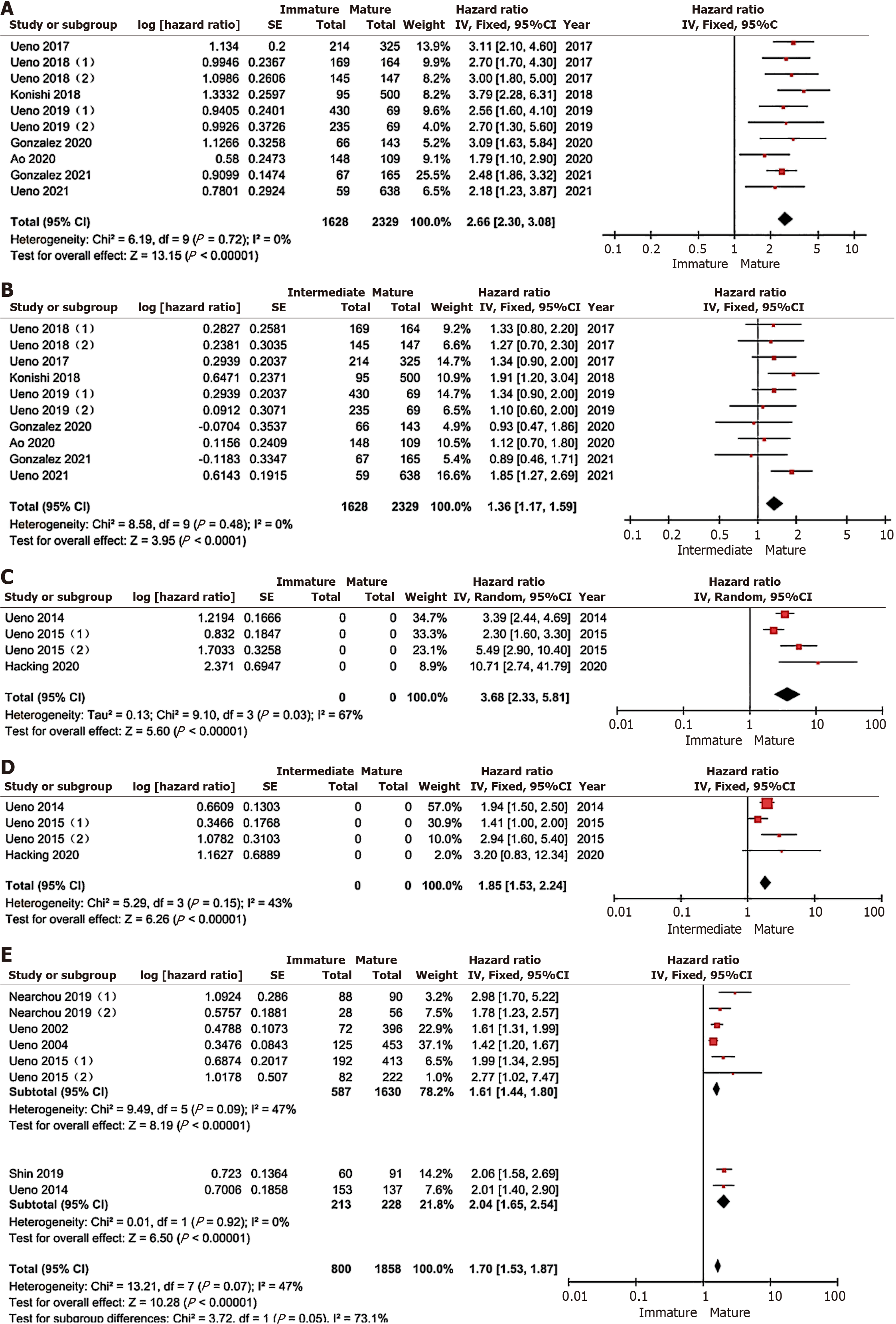
A total of 8 literatures and 10 cohorts were used to study the relationship between tumor interstitial maturity and relapse-free survival. A meta-analysis was conducted using the fixed-effect model, and the results showed that: compared with the mature group, the immature group was better than the mature group. The relapse-free survival rate of patients was significantly lower than that of patients in the mature group (HR = 2.66, 95%CI: 2.30-3.08, P < 0.00001), as shown in Figure 3A. The relapse-free survival rate of patients in the intermediate group was also significantly lower than that of patients in the mature group (HR = 1.36, 95%CI: 1.17-1.59, P < 0.0001). There was no significant heterogeneity in all studies (I2 = 0, P > 0.05), as shown in Figure 3B.
A total of 3 literatures and 4 cohorts were used to study the relationship between tumor interstitial maturity and disease-free survival rate. The tumor stage ranged from stage I to stage IV. Meta-analysis showed that compared with the mature group, the disease-free survival rate of patients in the immature group was significantly lower than that in the mature group (HR = 3.68, 95%CI: 2.33-5.81), there was significant heterogeneity among studies (I2 = 67%), and random effects model was adopted for analysis, as shown in Figure 3C. The disease-free survival rate of patients in the intermediate group was also significantly lower than those in the mature group (HR = 1.85, 95%CI: 1.53-2.24, P < 0.0001), no significant heterogeneity was found in all studies (I2 = 43%, P > 0.05), and the fixed-effect model was used for analysis, as shown in Figure 3D.
A total of 5 literatures and 8 cosets were used to study the difference in OS rate between the immature group and the mature group, with tumor stages ranging from stage I to stage IV. Meta-analysis showed that compared with the mature group, the OS rate of the immature group was significantly lower than that of the mature group (HR = 1.70, 95%CI: 1.53-1.87, P < 0.00001), each study I2 = 47% (P > 0.05), using the fixed effect model analysis, according to subgroup analysis with or without stage IV patients showed that the OS rate of the immature group was significantly lower than that of the mature group (HR = 1.61, 95%CI: 1.44-1.80, P < 0.00001).
Included in the study, I2 = 47% (P > 0.05), including stage IV patients, the OS rate of the immature group was also significantly lower than that of the mature group (HR = 2.04, 95%CI: 1.65-2.54, P < 0.00001), and the study I2 = 0 (P > 0.05). The unstudied total survival rate of patients with intermediate type is shown in Figure 3E.
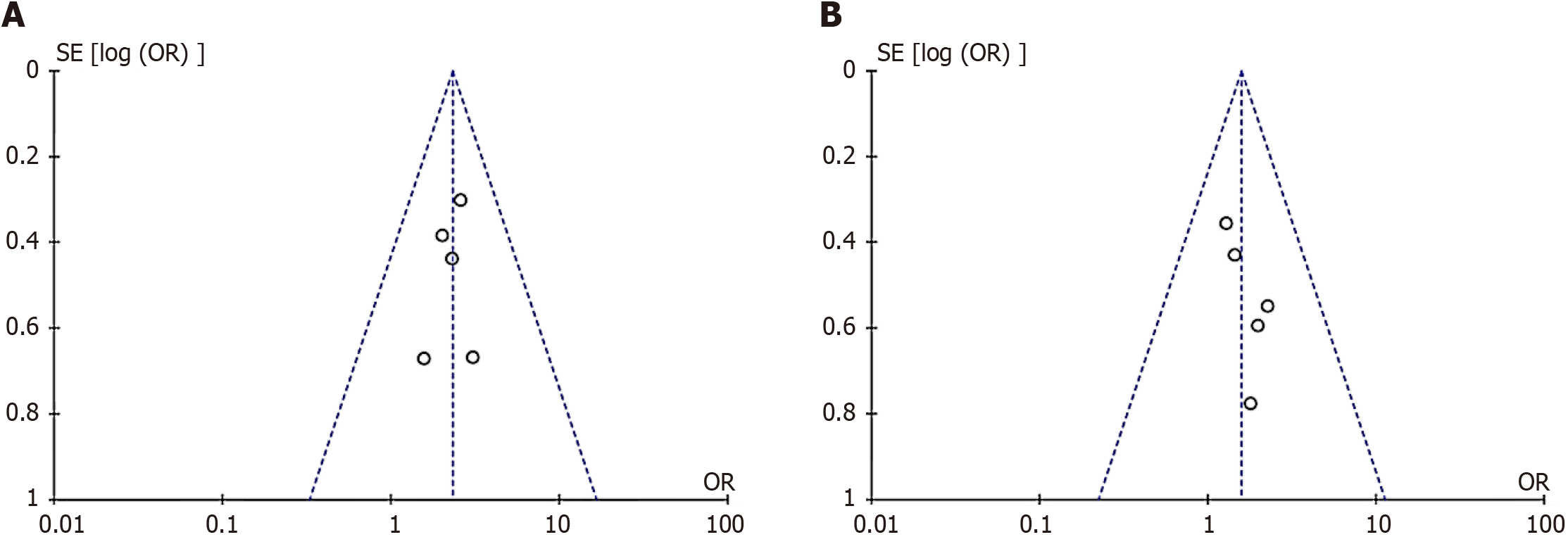
The funnel plot test was adopted, and the funnel plot was basically symmetric with good symmetry. Taking the funnel plot of the included literature on relapse-free survival as an example, the five cohort studies were basically distributed within 95% confidence interval, indicating no significant publication bias (Figure 4).
Regression analysis of age, region, sex, stage, tumor site and degree of differentiation showed that age, region, sex, stage, tumor site and degree of differentiation did not affect relapse-free survival and OS (Table 2).
| Interstitial maturity | Heterogeneous factor | Relapse free survival | Overall survival rate | ||
| Coefficient | P value | Coefficient | P value | ||
| Immaturity | Age | 0.098 | 0.8 | 0.06 | 0.93 |
| Region (Europe and America/Asia) | 0.084 | 0.81 | 0.63 | 0.18 | |
| Gender (male/female) | 0.05 | 0.93 | 0.46 | 0.21 | |
| Stage (Ⅰ-Ⅱ/Ⅲ-Ⅳ) | -0.73 | 0.16 | -0.5 | 0.39 | |
| Tumor site (node/rectum) | 0.68 | 0.14 | 0.46 | 0.54 | |
| Degree of differentiation (high/medium/low) | -0.02 | 0.95 | -0.74 | 0.49 | |
At present, there have been many studies on tumor microenvironment, and the changes in tumor microenvironment, especially the changes in tumor mesenchyma, are closely related to tumor progression[20]. Relevant studies[21-24] have found that tumor mesenchyma is more closely related to survival than tumor cells themselves in breast cancer. The tumor microenvironment, or tumor interstitium, is in a dynamic process of change with the progression of the tumor. There are studies that show the growth and changing of collagen fibers, mainly the growth of thick collagen bundles, at the point where the tumor and interstitium meet is a sign that the tumor interstitium has matured[25]. This dynamic fibrotic process is called DR. And is an important indicator of the tumor interstitium or tumor microenvironment. DR Maturity was divided into mature type, intermediate type, and immature type according to whether the tumor margin interstitial components contained hyaline scar collagen and mucoid interstitial[26-28]. In the early stage of tumor development, the fibrostroma immobilized tumor cells, representing a host response of tumor cells, but as the tumor progressed, tumor cells could break through the fibrostromal restraint and grow infiltratively around the tumor. Studies[29-32] have shown that the interstitial reaction at the tumor edge is closely related to the progression of colorectal cancer. Some in vitro experiments have shown that inhibition of the interstitial reaction in melanoma, for example, may promote the metastasis of tumors. However, Peng et al[33] believe that Colorectal cancer can up-regulate the synthesis of collagen fibers and limit the invasion of tumors. Fibrous interstitial reaction is a dynamic process in tumor progression and plays different roles in different stages of tumor progression.
At present, there have been many studies on the relationship between tumor interstitial maturity and the prognosis of colorectal cancer. This study confirmed, from the perspective of evidence-based medicine, that the lower the degree of tumor interstitial fiber differentiation, the worse the prognosis. TNM staging based on postoperative pathological HE sections is currently the gold standard for the prognostic staging of colorectal cancer. However, for some patients with stage II or III, although the stage is the same, the prognosis is significantly different. Therefore, it is of great significance to study and find more prognostic factors to stratify the risk factors of patients with the same period. The TNM staging is mainly concerned with the tumor cells themselves, but the microenvironment around the tumor is also significantly related to the tumor prognosis[34]. Therefore, DR focuses on the morphological observation of the tumor mesenchyma, which can be an important supplement to the TNM pathological staging of colorectal cancer.
This study systematically and quantitatively analyzed the relationship between tumor interstitial maturity and the prognosis of colorectal cancer[35]. The results of the meta-analysis showed that, compared with patients with mature interstitial, relapse-free survival, disease-free survival, and OS were significantly reduced in patients with immature and intermediate types. All patients included in the relapse-free survival study were in stages I to III, and the results of heterogeneity analysis showed no heterogeneity, indicating that the study results were stable. The heterogeneity of disease-free survival meta-analysis studies may be related to the small number of included studies and the inclusion of stage IV patients. Therefore, more studies on stage IV patients and disease-free survival are needed in the future. Two studies[36,37] involving stage IV patients were included in the meta-analysis of OS, and subgroup analysis showed that primary DR was also significantly associated with OS in patients with stage IV colorectal cancer with metastasis.
Studies[38-40] have shown that DR is closely related to CAFs. CAFs, as an important tumor stromal cell, have both pro- and anti-tumor properties in regulating the tumor microenvironment, and the phenotype of CAFs is closely related to DR. Through interaction with CAFs, tumor cells reconstitute immature fibrostroma, which is conducive to tumor cell invasion and metastasis. Meanwhile, CAFs promote epithelial-mesenchymal transformation of tumor cells by secreting chemical factors and cytokines, which is a recognized mode of tumor cell metastasis. At the same time, there were more tumor buds in the immature interstitial fibers, and tumor buds were a microenvironmental indicator closely related to the poor prognosis of tumors. Studies have shown that an "oligovascular" state in the immature stroma can make it hard for lymphocytes to get in, which lets tumor cells get away from the immune system. Therefore, the interaction between DR and tumor cells involves a variety of molecular mechanisms, and more in-depth studies are still needed.
This study also has some limitations: (1) Relatively few studies included patients in stage IV; (2) there is little literature on disease-free survival; (3) the included studies were mainly from Japan, Europe, and the United States, and there was a lack of large sample studies on Chinese patients; (4) there are differences in the level and mode of surgery in different countries and regions and among different operators, and the differences in postoperative adjuvant treatment plans may affect the results; and (5) DR classification is semi-quantitative, and subjective judgment may also affect the results.
In conclusion, this study shows that the level of tumor interstitial maturity is closely linked to the prognosis of colorectal cancer. This finding can be used in addition to the usual signs found in pathological sections. To be sure, a multicenter prospective cohort study for colorectal cancer patients in China is required.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade B
Novelty: Grade A
Creativity or Innovation: Grade B
Scientific Significance: Grade A
P-Reviewer: Oprea V, Romania S-Editor: Qu XL L-Editor: A P-Editor: Zhao YQ
| 1. | Hewitt RE, Powe DG, Morrell K, Balley E, Leach IH, Ellis IO, Turner DR. Laminin and collagen IV subunit distribution in normal and neoplastic tissues of colorectum and breast. Br J Cancer. 1997;75:221-229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | Kasai H, Nadano D, Hidaka E, Higuchi K, Kawakubo M, Sato TA, Nakayama J. Differential expression of ribosomal proteins in human normal and neoplastic colorectum. J Histochem Cytochem. 2003;51:567-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 83] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 3. | Trauner M, Grygar S, Stauber RE, Brodatsch-Häusler E, Klimpfinger M. Carcinoembryonic antigen, cytokeratin expression and mucin composition in hyperplastic and neoplastic polyps of the colorectum. Z Gastroenterol. 1994;32:626-631. [PubMed] |
| 4. | Grondin MV, Chang WW, Gaskins RD. Crypt alterations and collagen deposition in hyperplastic polyps of colorectum. Dig Dis Sci. 1990;35:12-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Ueno H, Jones A, Jass JR, Talbot IC. Clinicopathological significance of the 'keloid-like' collagen and myxoid stroma in advanced rectal cancer. Histopathology. 2002;40:327-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Ueno H, Konishi T, Ishikawa Y, Shimazaki H, Ueno M, Aosasa S, Saiura A, Hase K, Yamamoto J. Histologic categorization of fibrotic cancer stroma in the primary tumor is an independent prognostic index in resectable colorectal liver metastasis. Am J Surg Pathol. 2014;38:1380-1386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 7. | Wu L, Li X, Qian X, Wang S, Liu J, Yan J. Lipid Nanoparticle (LNP) Delivery Carrier-Assisted Targeted Controlled Release mRNA Vaccines in Tumor Immunity. Vaccines (Basel). 2024;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 59] [Reference Citation Analysis (0)] |
| 8. | Ueno H, Shinto E, Shimazaki H, Kajiwara Y, Sueyama T, Yamamoto J, Hase K. Histologic categorization of desmoplastic reaction: its relevance to the colorectal cancer microenvironment and prognosis. Ann Surg Oncol. 2015;22:1504-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 9. | Ueno H, Sekine S, Oshiro T, Kanemitsu Y, Hamaguchi T, Shida D, Takashima A, Ishiguro M, Ito E, Hashiguchi Y, Kondo F, Shimazaki H, Mochizuki S, Kajiwara Y, Shinto E, Yamamoto J, Shimada Y. Disentangling the prognostic heterogeneity of stage III colorectal cancer through histologic stromal categorization. Surgery. 2018;163:777-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Wu L, Li H, Liu Y, Fan Z, Xu J, Li N, Qian X, Lin Z, Li X, Yan J. Research progress of 3D-bioprinted functional pancreas and in vitro tumor models. Int J Bioprint. 2024;10:1256. [DOI] [Full Text] |
| 11. | Shin N, Son GM, Shin DH, Kwon MS, Park BS, Kim HS, Ryu D, Kang CD. Cancer-Associated Fibroblasts and Desmoplastic Reactions Related to Cancer Invasiveness in Patients With Colorectal Cancer. Ann Coloproctol. 2019;35:36-46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 12. | Konishi T, Shimada Y, Lee LH, Cavalcanti MS, Hsu M, Smith JJ, Nash GM, Temple LK, Guillem JG, Paty PB, Garcia-Aguilar J, Vakiani E, Gonen M, Shia J, Weiser MR. Poorly Differentiated Clusters Predict Colon Cancer Recurrence: An In-Depth Comparative Analysis of Invasive-Front Prognostic Markers. Am J Surg Pathol. 2018;42:705-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 13. | Nearchou IP, Kajiwara Y, Mochizuki S, Harrison DJ, Caie PD, Ueno H. Novel Internationally Verified Method Reports Desmoplastic Reaction as the Most Significant Prognostic Feature For Disease-specific Survival in Stage II Colorectal Cancer. Am J Surg Pathol. 2019;43:1239-1248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 14. | Ueno H, Kanemitsu Y, Sekine S, Ishiguro M, Ito E, Hashiguchi Y, Kondo F, Shimazaki H, Kajiwara Y, Okamoto K, Mochizuki S, Tsujimoto H, Shinto E. A Multicenter Study of the Prognostic Value of Desmoplastic Reaction Categorization in Stage II Colorectal Cancer. Am J Surg Pathol. 2019;43:1015-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | González IA, Bauer PS, Liu J, Chatterjee D. Adenoma-like adenocarcinoma: clinicopathologic characterization of a newly recognized subtype of colorectal carcinoma. Hum Pathol. 2021;107:9-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Hacking S, Ebare K, Angert M, Lee L, Vitkovski T, Thomas R, Chavarria H, Jin C, Nasim M. Immature Stroma and Prognostic Profiling in Colorectal Carcinoma: Development and Validation of Novel Classification Systems. Pathol Res Pract. 2020;216:152970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 17. | Ao T, Kajiwara Y, Yonemura K, Shinto E, Mochizuki S, Okamoto K, Kishi Y, Ueno H. Morphological consistency of desmoplastic reactions between the primary colorectal cancer lesion and associated metastatic lesions. Virchows Arch. 2020;477:47-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | González IA, Bauer PS, Liu J, Chatterjee D. Intraepithelial tumour infiltrating lymphocytes are associated with absence of tumour budding and immature/myxoid desmoplastic reaction, and with better recurrence-free survival in stages I-III colorectal cancer. Histopathology. 2021;78:252-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 19. | Ueno H, Ishiguro M, Nakatani E, Ishikawa T, Uetake H, Murotani K, Matsui S, Teramukai S, Sugai T, Ajioka Y, Maruo H, Kotaka M, Tsujie M, Munemoto Y, Yamaguchi T, Kuroda H, Fukunaga M, Tomita N, Sugihara K. Prognostic value of desmoplastic reaction characterisation in stage II colon cancer: prospective validation in a Phase 3 study (SACURA Trial). Br J Cancer. 2021;124:1088-1097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 64] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 20. | Wong CC, Yu J. Gut microbiota in colorectal cancer development and therapy. Nat Rev Clin Oncol. 2023;20:429-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 272] [Reference Citation Analysis (0)] |
| 21. | Liu Y, Zhang Q, Xing B, Luo N, Gao R, Yu K, Hu X, Bu Z, Peng J, Ren X, Zhang Z. Immune phenotypic linkage between colorectal cancer and liver metastasis. Cancer Cell. 2022;40:424-437.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 237] [Article Influence: 79.0] [Reference Citation Analysis (0)] |
| 22. | Wu L, Zheng Y, Liu J, Luo R, Wu D, Xu P, Li X. Comprehensive evaluation of the efficacy and safety of LPV/r drugs in the treatment of SARS and MERS to provide potential treatment options for COVID-19. Aging (Albany NY). 2021;13:10833-10852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 23. | Jiang SS, Xie YL, Xiao XY, Kang ZR, Lin XL, Zhang L, Li CS, Qian Y, Xu PP, Leng XX, Wang LW, Tu SP, Zhong M, Zhao G, Chen JX, Wang Z, Liu Q, Hong J, Chen HY, Chen YX, Fang JY. Fusobacterium nucleatum-derived succinic acid induces tumor resistance to immunotherapy in colorectal cancer. Cell Host Microbe. 2023;31:781-797.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 155] [Reference Citation Analysis (0)] |
| 24. | Zhong X, He X, Wang Y, Hu Z, Huang H, Zhao S, Wei P, Li D. Warburg effect in colorectal cancer: the emerging roles in tumor microenvironment and therapeutic implications. J Hematol Oncol. 2022;15:160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 153] [Reference Citation Analysis (0)] |
| 25. | Shin AE, Giancotti FG, Rustgi AK. Metastatic colorectal cancer: mechanisms and emerging therapeutics. Trends Pharmacol Sci. 2023;44:222-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 279] [Reference Citation Analysis (0)] |
| 26. | Wu L, Liu Q, Ruan X, Luan X, Zhong Y, Liu J, Yan J, Li X. Multiple Omics Analysis of the Role of RBM10 Gene Instability in Immune Regulation and Drug Sensitivity in Patients with Lung Adenocarcinoma (LUAD). Biomedicines. 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 55] [Reference Citation Analysis (0)] |
| 27. | Lin A, Zhang J, Luo P. Crosstalk Between the MSI Status and Tumor Microenvironment in Colorectal Cancer. Front Immunol. 2020;11:2039. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 241] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 28. | Waldner MJ, Neurath MF. TGFβ and the Tumor Microenvironment in Colorectal Cancer. Cells. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 29. | Foersch S, Glasner C, Woerl AC, Eckstein M, Wagner DC, Schulz S, Kellers F, Fernandez A, Tserea K, Kloth M, Hartmann A, Heintz A, Weichert W, Roth W, Geppert C, Kather JN, Jesinghaus M. Multistain deep learning for prediction of prognosis and therapy response in colorectal cancer. Nat Med. 2023;29:430-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 105] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 30. | Wu L, Zhong Y, Wu D, Xu P, Ruan X, Yan J, Liu J, Li X. Immunomodulatory Factor TIM3 of Cytolytic Active Genes Affected the Survival and Prognosis of Lung Adenocarcinoma Patients by Multi-Omics Analysis. Biomedicines. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 62] [Reference Citation Analysis (0)] |
| 31. | Zhao H, Ming T, Tang S, Ren S, Yang H, Liu M, Tao Q, Xu H. Wnt signaling in colorectal cancer: pathogenic role and therapeutic target. Mol Cancer. 2022;21:144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 457] [Article Influence: 152.3] [Reference Citation Analysis (1)] |
| 32. | Peng Z, Ren Z, Tong Z, Zhu Y, Hu K. Interactions between MFAP5 + fibroblasts and tumor-infiltrating myeloid cells shape the malignant microenvironment of colorectal cancer. J Transl Med. 2023;21:405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 33. | Peng Z, Ye M, Ding H, Feng Z, Hu K. Spatial transcriptomics atlas reveals the crosstalk between cancer-associated fibroblasts and tumor microenvironment components in colorectal cancer. J Transl Med. 2022;20:302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 69] [Reference Citation Analysis (0)] |
| 34. | Nenkov M, Ma Y, Gaßler N, Chen Y. Metabolic Reprogramming of Colorectal Cancer Cells and the Microenvironment: Implication for Therapy. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 103] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 35. | Wu L, Zhong Y, Yu X, Wu D, Xu P, Lv L, Ruan X, Liu Q, Feng Y, Liu J, Li X. Selective poly adenylation predicts the efficacy of immunotherapy in patients with lung adenocarcinoma by multiple omics research. Anticancer Drugs. 2022;33:943-959. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 66] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 36. | Martinez-Ordoñez A, Duran A, Ruiz-Martinez M, Cid-Diaz T, Zhang X, Han Q, Kinoshita H, Muta Y, Linares JF, Kasashima H, Nakanishi Y, Omar M, Nishimura S, Avila L, Yashiro M, Maeda K, Pannellini T, Pigazzi A, Inghirami G, Marchionni L, Sigal D, Diaz-Meco MT, Moscat J. Hyaluronan driven by epithelial aPKC deficiency remodels the microenvironment and creates a vulnerability in mesenchymal colorectal cancer. Cancer Cell. 2023;41:252-271.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 32] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 37. | Wu X, Yan H, Qiu M, Qu X, Wang J, Xu S, Zheng Y, Ge M, Yan L, Liang L. Comprehensive characterization of tumor microenvironment in colorectal cancer via molecular analysis. Elife. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 38. | Gao Y, Wang H, Chen S, An R, Chu Y, Li G, Wang Y, Xie X, Zhang J. Single-cell N(6)-methyladenosine regulator patterns guide intercellular communication of tumor microenvironment that contribute to colorectal cancer progression and immunotherapy. J Transl Med. 2022;20:197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 39. | Wu L, Zheng Y, Ruan X, Wu D, Xu P, Liu J, Li X. Long-chain noncoding ribonucleic acids affect the survival and prognosis of patients with esophageal adenocarcinoma through the autophagy pathway: construction of a prognostic model. Anticancer Drugs. 2022;33:e590-e603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 70] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 40. | Zheng X, Ma Y, Bai Y, Huang T, Lv X, Deng J, Wang Z, Lian W, Tong Y, Zhang X, Yue M, Zhang Y, Li L, Peng M. Identification and validation of immunotherapy for four novel clusters of colorectal cancer based on the tumor microenvironment. Front Immunol. 2022;13:984480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |