Published online Jun 15, 2024. doi: 10.4251/wjgo.v16.i6.2781
Revised: March 6, 2024
Accepted: April 10, 2024
Published online: June 15, 2024
Processing time: 150 Days and 20.8 Hours
Gastric cancer is one of the most common malignant tumors in the world, and its occurrence and development involve complex biological processes. Iron death, as a new cell death mode, has attracted wide attention in recent years. However, the regulatory mechanism of iron death in gastric cancer and its effect on lipid pe
To explore the role of iron death in the development of gastric cancer, reveal its relationship with lipid peroxidation, and provide a new theoretical basis for re
The process of iron death in gastric cancer cells was simulated by cell culture model, and the occurrence of iron death was detected by fluorescence microscopy and flow cytometry. The changes of gene expression related to iron death and lipid peroxidation metabolism were analyzed by high-throughput sequencing technology. In addition, a mouse model of gastric cancer was established, and the role of iron death in vivo was studied by histology and immunohistochemistry, and the level of lipid peroxidation was detected. These methods comprehensively and deeply reveal the regulatory mechanism of iron death on lipid peroxidation metabolism in the occurrence and development of gastric cancer.
Iron death was significantly activated in gastric cancer cells, and at the same time, associated lipid peroxidation levels increased significantly. Through high-throughput sequencing analysis, it was found that iron death re
This study confirmed the important role of iron death in regulating lipid peroxidation metabolism in the occur
Core Tip: As a highly aggressive tumor, the pathophysiological mechanism of gastric cancer has attracted much attention. In recent years, factors such as ferroptosis regulation, lipid peroxidation, and metabolic abnormalities have emerged in the study of gastric cancer, providing a new perspective for understanding the development of gastric cancer. Ferroptosis regu
- Citation: Wang LM, Zhang WW, Qiu YY, Wang F. Ferroptosis regulating lipid peroxidation metabolism in the occurrence and development of gastric cancer. World J Gastrointest Oncol 2024; 16(6): 2781-2792
- URL: https://www.wjgnet.com/1948-5204/full/v16/i6/2781.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i6.2781
As one of the most common malignant tumors in the digestive system, gastric cancer has a high fatality rate year-round[1-4]. According to the global cancer data for 2020, the number of new gastric cancer cases accounted for 5.6%, and the death rate was 7.7%[5]. Cancer statistics in our country also show that gastric cancer is an important cause of tumor death[6-8]. Due to the insidious symptoms of early gastric cancer, treatment is often in the late stages, and the opportunity for surgery is lost[9]. Only other treatment methods can be used to kill cancer cells to improve the quality of life of patients and prolong their survival[10-16]. At present, the main methods of non-surgical cancer treatment are induced tumor cell death based on chemoradiotherapy, immunology, and molecular targeted therapy[17-20]. Ferroptosis is a new pathway of cell death induction with unique characteristics; that is, iron accumulation in cells leads to iron-dependent lipid pe
Iron death is a form of cell death induced by the accumulation of iron-dependent lipid peroxides[26-28]. This accumulation can be caused by a variety of factors, such as inhibition of glutathione peroxidase 4 (GPX4) activity, cysteine defi
The changes are unique features of iron death, including mitochondrial shrinkage, increased double membrane den
The buildup of iron-dependent lipid peroxides causes iron death, a type of cell death. AA peroxidation, cysteine de
The accumulation of iron is the first step in iron death, and free iron (Fe3+) in the blood binds to transferrin. Then, transferrin receptors on the cell membrane take the iron-bound transferrin molecules inside the cell[61-64]. Reductin changes Fe3+ to the highly active ferrous ion (Fe2+) form. Fe2+ is then moved from the body to the cytoplasm and added to the Lithium Ion Polymer (LIP), which is an unstable iron pool. In order to protect cell tissues from the destruction of free iron, the excess Fe2+ in the iron pool is stored in ferritin[65]. Ferritin is an iron storage protein that maintains the balance of iron metabolism within cells by storing and releasing Fe2+. When there is a greater need for iron, nuclear receptor co
Fatty acids participate in energy metabolism and signal pathway transduction and are an important part of the body[68]. However, excess lipid synthesis and oxidation by intracellular ROS to produce lipid peroxides are important features of iron death. Long-chain acyl-CoA synthases (ACSLs) play a key role in lipid anabolism. ACSL activates long-chain fatty acids, which are decomposed into acetyl-CoA (CoA) through a series of processes, such as beta oxidation, and then enter the tricarboxylic acid cycle to produce energy[69]. Long-chain ACSL4 is closely related to the iron death pathway. We found that Erastin-induced iron death was inhibited by deletion of ACSL4 expression in iron death-sensitive cells HepG2 and HL60, while transfection of ACSL4 restored the sensitivity of these cells to Erastin-induced iron death. Also, ACSL4 has a lot of long-chain polyunsaturated ϲ6 fatty acids and is found a lot on cell membranes[70]. Targeting ACSL4 with thiazolidinediones, a type of anti-diabetic compound, made iron death much more common in mouse models. Exogenous monounsaturated fatty acids, activated by long-chain ACSL3, can reduce the sensitivity of the cell membrane to oxidation and thus resist iron death. Long-chain fatty acid protein 5 and fatty acid desaturase 1 are two enzymes involved in the synthesis of oxidizable polyunsaturated fatty acids (PUFA). Studies have found that cells expressing these two enzymes are accompanied by upregulation of AA and adrenal acid and are sensitive to iron death, but not vice versa. The biosynthetic pathway of PUFA plays an important role in cellular iron death.
System Xc is composed of SLC7A11 and SLC3A2 heterodimers, which can transfer cystine into cells in a 1:1 exchange of glutamate and is a bridge connecting amino acid transfer inside and outside cells. The cystine transferred into the cell can be reduced to cysteine over time and used as one of the raw materials for the synthesis of GSH[71]. GSH is a tripeptide sulfhydryl substance composed of glutamic acid, cysteine, and glycine that can remove intracellular excess over time.
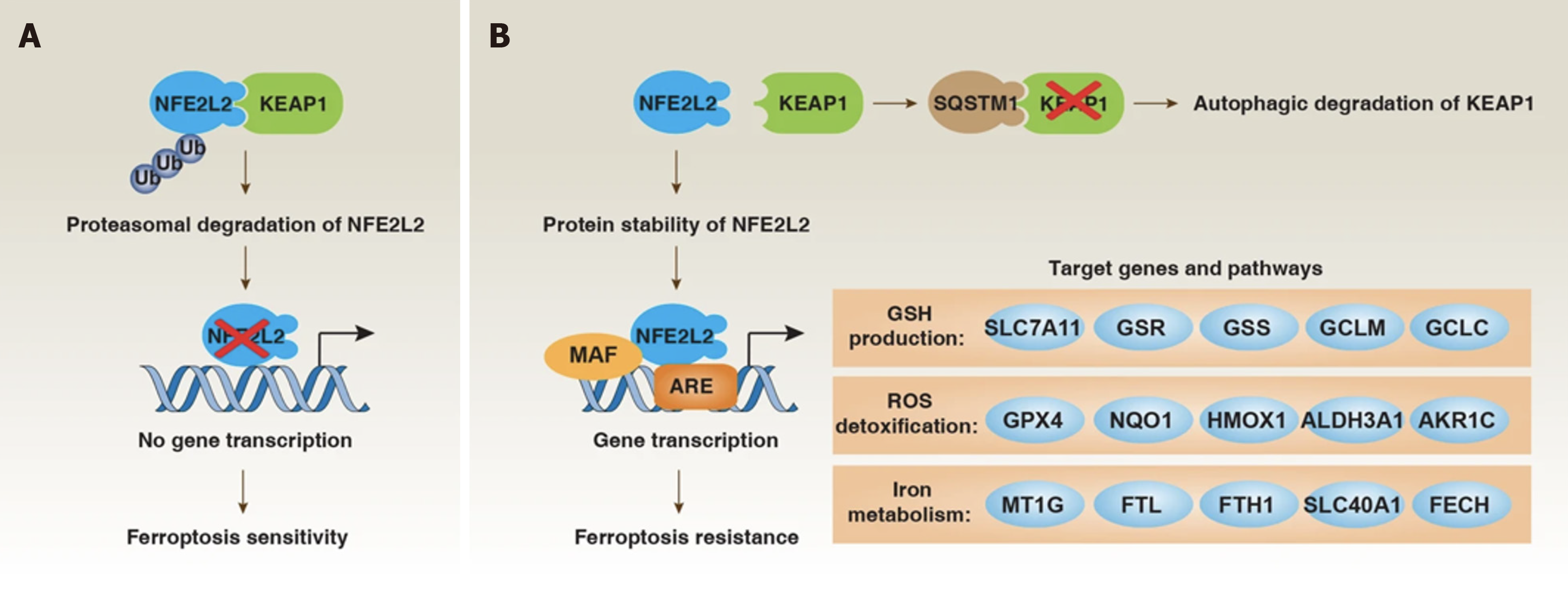
Oxidizing substances maintain the dynamic balance of REDOX. Studies[72-74] have shown that inhibiting System Xc can trigger iron death by depleting GSH. It was found in pancreatic duct adenocarcinoma (PDAC) that GSH cannot induce iron death alone and needs to be regulated in cooperation with CoA, which is related to the cysteine metabolism pathway in the cell. Cystase (e), as a cysteine-consuming drug, can induce iron death in PDAC, confirming that cysteine also plays an important role in iron death. When cysteine is depleted, cysteine can be synthesized from methionine through the sulfur transfer pathway to further synthesize GSH and exert its antioxidant effect[75]. Glutamine also plays an important role in regulating iron death. Under the influence of glutaminase, it is capable of deamination[76]. HIron death is precisely regulated at multiple levels, including epigenetic, transcriptional, post-transcriptional, and post-translational levels. The transcription factor NFE2L2 plays a central role in upregulating anti-ferrofluorescent protein defense, while selective autophagy may promote iron death (Figure 4).
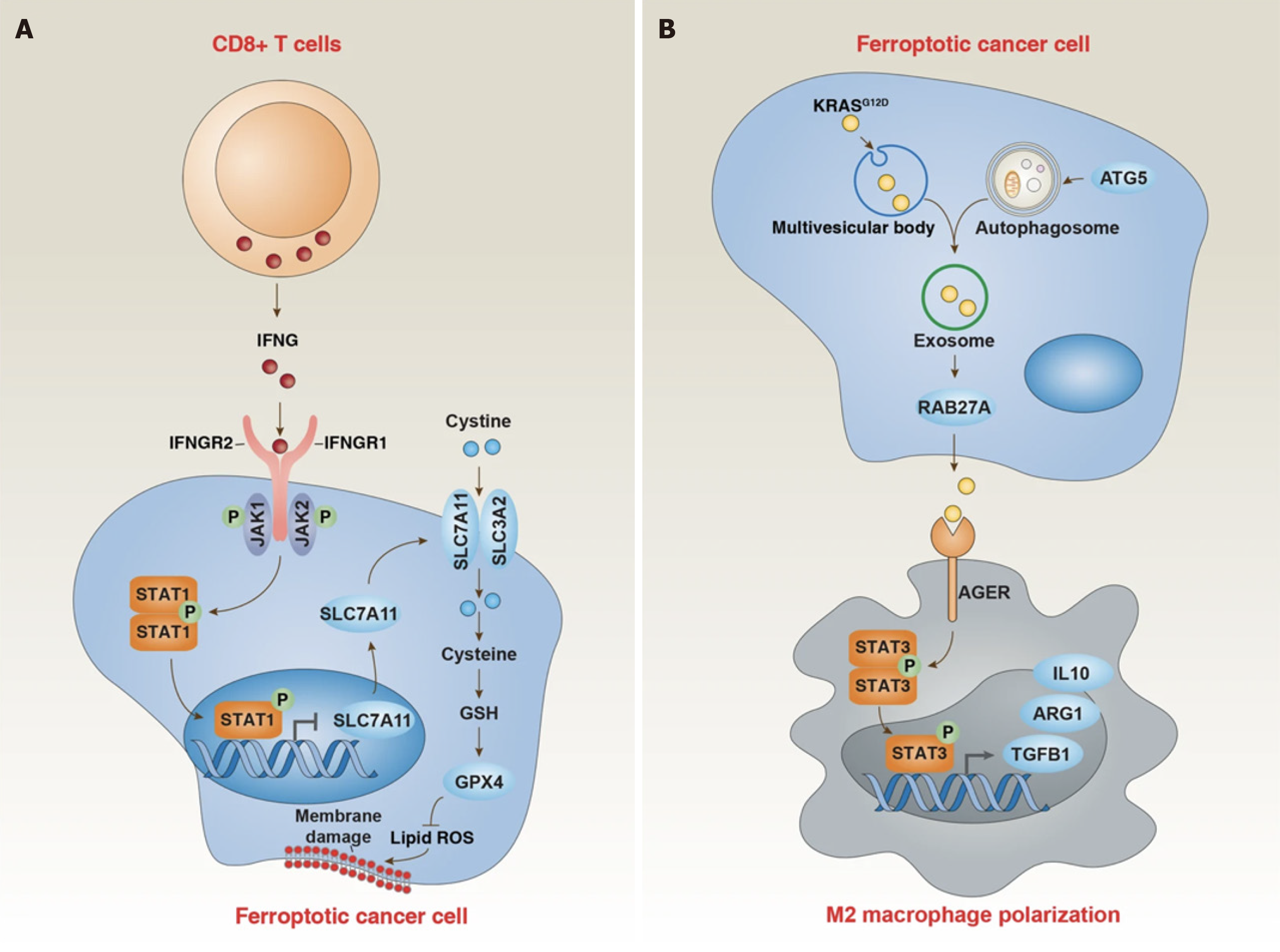
Many studies[77-80] have shown that iron death plays an important role in the occurrence and development of a variety of diseases, such as neurological diseases, heart disease, kidney damage, and so on. The mechanism of tumor cell death is not well studied, and this is a major reason for treatment failure. Tumor cells need to ingestion more iron than non-tumor cells to meet their growth and metabolism needs; this phenomenon becomes "iron addiction". The discovery of iron death provides a new idea for tumor treatment[81]. In gastric cancer, a variety of intracellular substances can participate in the regulation of iron death, including amino acids, non-coding RNA, polypeptides, etc[82-84]. However, the regulatory mechanism of iron death in the development of gastric cancer still needs further study.
Human cysteine dioxygenase 1 (CDO1) can convert cysteine to taurine, thus limiting the production of GSH. In vivo and in vitro experiments have shown that inhibition of CDO1 can restore GSH levels in gastric cancer cells, reduce ROS production and lipid peroxidation, and reduce erastin-induced iron death[85-87]. Obesity and abnormal lipid metabolism are also involved in regulating the process of iron death in gastric cancer[88]. Perilipin2 (PLIN2), also known as fatty phase-related egg white (ADRP), is a protein related to fat drop differentiation. Members of the PLIN family have im
Long non-coding RNA (lncRNA) plays an important role in iron death and the progression of gastric cancer. The role of lncRNA in the tumor microenvironment, treatment response, and prognosis of gastric cancer was systematically eva
miRNAs are associated with malignant phenotypes of various tumors, including proliferation, metastasis, drug resis
CircRNA is a closed-loop non-coding RNA that is involved in the regulation of various signaling pathways and malig
Iron death plays an important role in the occurrence and development of gastric cancer[103]. In the chemotherapy of gastric cancer, it may be possible to achieve the purpose of treating gastric cancer by regulating the sensitivity of che
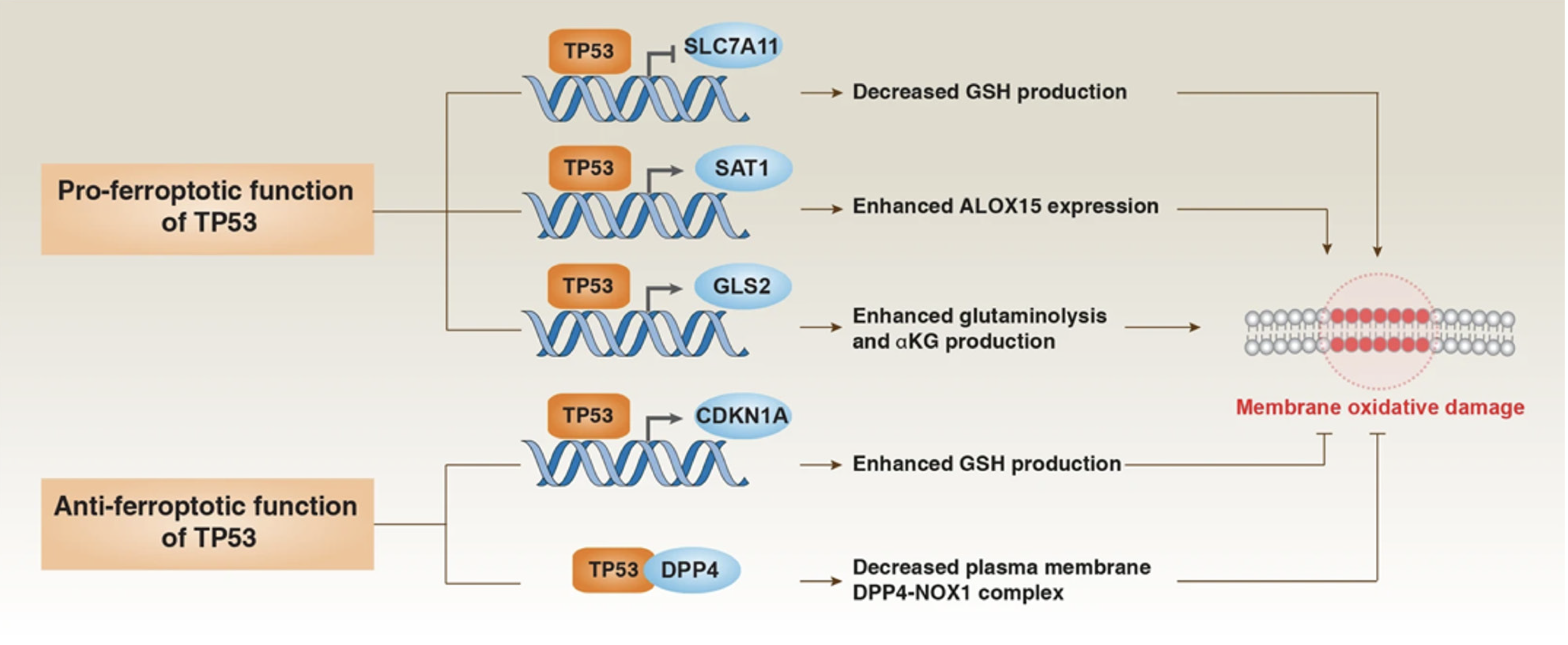
The β-linked albumin/transtranscription 4 (TCF4) transcription complex protects cells from iron death by increasing the expression of GPX4[107]. There is growing evidence that targeting iron metabolism and inducing iron death may provide new avenues for treating drug-resistant cancers. For instance, the transcription factor sterol regulatory element binding protein-1ASREBP-1A in gastric cancer cells mediates apatinib's induction of iron death in gastric cancer cells by down-regulating GPX4 expression. Silenced information regulatory factor 6 (SIRT6) is a member of the Sirtuin family of NAD+-dependent enzymes[107]. Inhibition of SIRT6 can lead to inactivation of the Keap1/Nrf2 signaling pathway and downregulation of GPX4 expression. The study found that going after the SIRT6/Keap1/Nrf2/GPX4 signaling pathway might be a way to get around the fact that gastric cancer is resistant to sorafenib. Studies have shown that transcriptional activation factor 3 (STAT3) can regulate the expression of FNR-related factors (GPX4, SLC7A11, and FTH1), thereby establishing the STAT3 iron death negative regulatory axis. Targeting this pathway can trigger iron death through down
Overexpression of the cysteine protease inhibitor SN significantly promotes the migration and invasion of gastric cancer cells, which may be related to the improvement of GPX4 stability, which may become a potential target for block
As a new pathway to induce cell death, the biological mechanism of iron death has been studied. At the same time, iron death is also an important participant in the occurrence, development, and metastasis of gastric cancer. In the treatment of gastric cancer, iron death plays an important role in revealing new drug resistance mechanisms and regulating the sensitivity of tumor cells to chemotherapy resistance. The regulatory mechanism of the iron death pathway in gastric cancer remains unclear. Lipid oxidation appears to be at the heart of iron death. The high expression of GPX4 in gastric cancer cells seems to be a key molecule to escape iron death, and the induction of iron death may be achieved by targeting certain pathways, thus becoming a therapeutic strategy for advanced drug-resistant gastric cancer. The me
| 1. | Anestis A, Zoi I, Karamouzis MV. Current advances of targeting HGF/c-Met pathway in gastric cancer. Ann Transl Med. 2018;6:247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 2. | Palle J, Hirsch L, Lapeyre-Prost A, Malka D, Bourhis M, Pernot S, Marcheteau E, Voron T, Castan F, Lacotte A, Benhamouda N, Tanchot C, François E, Ghiringhelli F, de la Fouchardière C, Zaanan A, Tartour E, Taieb J, Terme M. Targeting HGF/c-Met Axis Decreases Circulating Regulatory T Cells Accumulation in Gastric Cancer Patients. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Huang KH, Sung IC, Fang WL, Chi CW, Yeh TS, Lee HC, Yin PH, Li AF, Wu CW, Shyr YM, Yang MH. Correlation between HGF/c-Met and Notch1 signaling pathways in human gastric cancer cells. Oncol Rep. 2018;40:294-302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Yuan HH, Zhang XC, Wei XL, Zhang WJ, Du XX, Huang P, Chen H, Bai L, Zhang HF, Han Y. LncRNA UCA1 mediates Cetuximab resistance in Colorectal Cancer via the MiR-495 and HGF/c-MET Pathways. J Cancer. 2022;13:253-267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 5. | Chu PY, Huang WC, Tung SL, Tsai CY, Chen CJ, Liu YC, Lee CW, Lin YH, Lin HY, Chen CY, Yeh CT, Lin KH, Chi HC. IFITM3 promotes malignant progression, cancer stemness and chemoresistance of gastric cancer by targeting MET/AKT/FOXO3/c-MYC axis. Cell Biosci. 2022;12:124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 6. | Marano L, Chiari R, Fabozzi A, De Vita F, Boccardi V, Roviello G, Petrioli R, Marrelli D, Roviello F, Patriti A. c-Met targeting in advanced gastric cancer: An open challenge. Cancer Lett. 2015;365:30-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 7. | Kawakami H, Okamoto I. MET-targeted therapy for gastric cancer: the importance of a biomarker-based strategy. Gastric Cancer. 2016;19:687-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 8. | Dong G, Wang M, Gu G, Li S, Sun X, Li Z, Cai H, Zhu Z. MACC1 and HGF are associated with survival in patients with gastric cancer. Oncol Lett. 2018;15:3207-3213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Ding X, Ji J, Jiang J, Cai Q, Wang C, Shi M, Yu Y, Zhu Z, Zhang J. HGF-mediated crosstalk between cancer-associated fibroblasts and MET-unamplified gastric cancer cells activates coordinated tumorigenesis and metastasis. Cell Death Dis. 2018;9:867. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 82] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 10. | Ahn SY, Kim J, Kim MA, Choi J, Kim WH. Increased HGF Expression Induces Resistance to c-MET Tyrosine Kinase Inhibitors in Gastric Cancer. Anticancer Res. 2017;37:1127-1138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Wang C, Xi W, Ji J, Cai Q, Zhao Q, Jiang J, Zhou C, Shi M, Zhang H, Zhu Z, Zhang J. The prognostic value of HGF-c-MET signaling pathway in Gastric Cancer: a study based on TCGA and GEO databases. Int J Med Sci. 2020;17:1946-1955. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Mihailidou C, Karamouzis MV, Schizas D, Papavassiliou AG. Co-targeting c-Met and DNA double-strand breaks (DSBs): Therapeutic strategies in BRCA-mutated gastric carcinomas. Biochimie. 2017;142:135-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Wu L, Zheng Y, Liu J, Luo R, Wu D, Xu P, Li X. Comprehensive evaluation of the efficacy and safety of LPV/r drugs in the treatment of SARS and MERS to provide potential treatment options for COVID-19. Aging (Albany NY). 2021;13:10833-10852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 14. | Hong SW, Jung KH, Park BH, Zheng HM, Lee HS, Choi MJ, Yun JI, Kang NS, Lee J, Hong SS. KRC-408, a novel c-Met inhibitor, suppresses cell proliferation and angiogenesis of gastric cancer. Cancer Lett. 2013;332:74-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Schmid E, Klotz M, Steiner-Hahn K, Konen T, Frisk AL, Schatz C, Krahn T, von Ahsen O. Detection of MET mRNA in gastric cancer in situ. Comparison with immunohistochemistry and sandwich immunoassays. Biotech Histochem. 2017;92:425-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Yonemura Y, Kaji M, Hirono Y, Fushida S, Tsugawa K, Fujimura T, Miyazaki I, Harada S, Yamamoto H. Correlation between overexpression of c-met gene and the progression of gastric cancer. Int J Oncol. 1996;8:555-560. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 17. | Zeng W, Xing ZT, Tan MY, Wu YW, Zhang CY. Lidocaine suppresses the malignant behavior of gastric cancer cells via the c-Met/c-Src pathway. Exp Ther Med. 2021;21:424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 18. | Zarei B, Javidan Z, Fatemi E, Rahimi Jamnani F, Khatami S, Khalaj V. Targeting c-Met on gastric cancer cells through a fully human fab antibody isolated from a large naive phage antibody library. Daru. 2020;28:221-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 19. | Lu J, Li X, Tu K, Guan Y, Fung KP, Liu F. Verticillin A suppresses HGF-induced migration and invasion via repression of the c-Met/FAK/Src pathway in human gastric and cervical cancer cells. Onco Targets Ther. 2019;12:5823-5833. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Wu Y, Yao X, Zhu M, Qian H, Jiang L, Lan T, Wu M, Pang J, Chen Y. PKG II reverses HGF-triggered cellular activities by phosphorylating serine 985 of c-Met in gastric cancer cells. Oncotarget. 2016;7:34190-34200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Lin X, Peng Z, Wang X, Zou J, Chen D, Chen Z, Li Z, Dong B, Gao J, Shen L. Targeting autophagy potentiates antitumor activity of Met-TKIs against Met-amplified gastric cancer. Cell Death Dis. 2019;10:139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Kasai S, Kuwayama N, Motoo Y, Kawashima A, Matsumoto K, Yano S, Matsushima K, Yasumoto K. Dual blockade of MET and VEGFR2 signaling pathways as a potential therapeutic maneuver for peritoneal carcinomatosis in scirrhous gastric cancer. Biochem Biophys Res Commun. 2022;600:80-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Fushida S, Yonemura Y, Urano T, Yamaguchi A, Miyazaki I, Nakamura T, Shiku H. Expression of hepatocyte growth factor(hgf) and C-met gene in human gastric-cancer cell-lines. Int J Oncol. 1993;3:1067-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 24. | Wu L, Zhong Y, Wu D, Xu P, Ruan X, Yan J, Liu J, Li X. Immunomodulatory Factor TIM3 of Cytolytic Active Genes Affected the Survival and Prognosis of Lung Adenocarcinoma Patients by Multi-Omics Analysis. Biomedicines. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 62] [Reference Citation Analysis (0)] |
| 25. | Kim HJ, Kang SK, Kwon WS, Kim TS, Jeong I, Jeung HC, Kragh M, Horak ID, Chung HC, Rha SY. Forty-nine gastric cancer cell lines with integrative genomic profiling for development of c-MET inhibitor. Int J Cancer. 2018;143:151-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 26. | Kim S, Ahn JM, Bae WJ, Han JH, Lee D. Quantitation of ligand is critical for ligand-dependent MET signalling activation and determines MET-targeted therapeutic response in gastric cancer. Gastric Cancer. 2021;24:577-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 27. | Xiang Y, Liang B, Jiang Y, Sun F, Zhao Y, Wu Q, Hu X, Liu Y, Huang Q, Liao W, Yao Z, Li S, Shi M. MET transcriptional regulator/serine peptidase inhibitor kunitz type 1 panel operating through HGF/c-MET axis as a prognostic signature in pan-cancer. Cancer Med. 2021;10:2442-2460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 28. | Toiyama Y, Yasuda H, Saigusa S, Matushita K, Fujikawa H, Tanaka K, Mohri Y, Inoue Y, Goel A, Kusunoki M. Co-expression of hepatocyte growth factor and c-Met predicts peritoneal dissemination established by autocrine hepatocyte growth factor/c-Met signaling in gastric cancer. Int J Cancer. 2012;130:2912-2921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 29. | Oh HA, Lee G, Kang HJ, Kim YG, Bae SH, Lee JL, Lee KH, Hyun MS, Kim DS. Overexpression of c-met Protein in Gastric Cancer and Role of uPAR as a Therapeutic Target. Cancer Res Treat. 2003;35:9-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | Li S, Zhang H, Wang X, Qu Y, Duan J, Liu R, Deng T, Ning T, Zhang L, Bai M, Zhou L, Ge S, Ying G, Ba Y. Direct targeting of HGF by miR-16 regulates proliferation and migration in gastric cancer. Tumour Biol. 2016;37:15175-15183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 31. | Omote R, Gion Y, Omote S, Tari A, Tanaka T, Nishikori A, Yoshino T, Sato Y. Clinicopathologic analysis of gastric mucosa-associated lymphoid tissue lymphoma with or without c-Met expression. Med Mol Morphol. 2020;53:149-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 32. | Amemiya H, Kono K, Takahashi A, Kamei S, Sugai H, Ichihara F, Fujii H, Matsumoto Y. c-Met expression in a gastric cancer cell line producing alpha-fetoprotein. Surg Today. 2004;34:115-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 33. | Amemiya H, Kono K, Itakura J, Tang RF, Takahashi A, An FQ, Kamei S, Iizuka H, Fujii H, Matsumoto Y. c-Met expression in gastric cancer with liver metastasis. Oncology. 2002;63:286-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 81] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 34. | Inoue T, Kataoka H, Goto K, Nagaike K, Igami K, Naka D, Kitamura N, Miyazawa K. Activation of c-Met (hepatocyte growth factor receptor) in human gastric cancer tissue. Cancer Sci. 2004;95:803-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 35. | Noguchi E, Saito N, Kobayashi M, Kameoka S. Clinical significance of hepatocyte growth factor/c-Met expression in the assessment of gastric cancer progression. Mol Med Rep. 2015;11:3423-3431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 36. | Zhao L, Yasumoto K, Kawashima A, Nakagawa T, Takeuchi S, Yamada T, Matsumoto K, Yonekura K, Yoshie O, Yano S. Paracrine activation of MET promotes peritoneal carcinomatosis in scirrhous gastric cancer. Cancer Sci. 2013;104:1640-1646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 37. | Dong W, Wang L, Shen R. MYO5B is epigenetically silenced and associated with MET signaling in human gastric cancer. Dig Dis Sci. 2013;58:2038-2045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 38. | Catenacci DVT, Tebbutt NC, Davidenko I, Murad AM, Al-Batran SE, Ilson DH, Tjulandin S, Gotovkin E, Karaszewska B, Bondarenko I, Tejani MA, Udrea AA, Tehfe M, De Vita F, Turkington C, Tang R, Ang A, Zhang Y, Hoang T, Sidhu R, Cunningham D. Rilotumumab plus epirubicin, cisplatin, and capecitabine as first-line therapy in advanced MET-positive gastric or gastro-oesophageal junction cancer (RILOMET-1): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18:1467-1482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 284] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 39. | Wu L, Liu Q, Ruan X, Luan X, Zhong Y, Liu J, Yan J, Li X. Multiple Omics Analysis of the Role of RBM10 Gene Instability in Immune Regulation and Drug Sensitivity in Patients with Lung Adenocarcinoma (LUAD). Biomedicines. 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 55] [Reference Citation Analysis (0)] |
| 40. | Chen CT, Kim H, Liska D, Gao S, Christensen JG, Weiser MR. MET activation mediates resistance to lapatinib inhibition of HER2-amplified gastric cancer cells. Mol Cancer Ther. 2012;11:660-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 149] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 41. | Graziano F, Galluccio N, Lorenzini P, Ruzzo A, Canestrari E, D'Emidio S, Catalano V, Sisti V, Ligorio C, Andreoni F, Rulli E, Di Oto E, Fiorentini G, Zingaretti C, De Nictolis M, Cappuzzo F, Magnani M. Genetic activation of the MET pathway and prognosis of patients with high-risk, radically resected gastric cancer. J Clin Oncol. 2011;29:4789-4795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 150] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 42. | Liu SI, Lui WY, Mok KT, Wu CW, Chi CW. Effect of hepatocyte growth factor on cell cycle and c-met expression in human gastric cancer cells. Anticancer Res. 1997;17:3575-3580. [PubMed] |
| 43. | Li L, Jiang X, Zhang Q, Dong X, Gao Y, He Y, Qiao H, Xie F, Xie X, Sun X. Neuropilin-1 is associated with clinicopathology of gastric cancer and contributes to cell proliferation and migration as multifunctional co-receptors. J Exp Clin Cancer Res. 2016;35:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 44. | Graziano F, Catalano V, Lorenzini P, Giacomini E, Sarti D, Fiorentini G, De Nictolis M, Magnani M, Ruzzo A. Clinical impact of the HGF/MET pathway activation in patients with advanced gastric cancer treated with palliative chemotherapy. Pharmacogenomics J. 2014;14:418-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 45. | Amemiya H, Kono K, Mori Y, Takahashi A, Ichihara F, Iizuka H, Sekikawa T, Matsumoto Y. High frequency of c-Met expression in gastric cancers producing alpha- fetoprotein. Oncology. 2000;59:145-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 59] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 46. | Hao NB, Tang B, Wang GZ, Xie R, Hu CJ, Wang SM, Wu YY, Liu E, Xie X, Yang SM. Hepatocyte growth factor (HGF) upregulates heparanase expression via the PI3K/Akt/NF-κB signaling pathway for gastric cancer metastasis. Cancer Lett. 2015;361:57-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 88] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 47. | Kang YK, Muro K, Ryu MH, Yasui H, Nishina T, Ryoo BY, Kamiya Y, Akinaga S, Boku N. A phase II trial of a selective c-Met inhibitor tivantinib (ARQ 197) monotherapy as a second- or third-line therapy in the patients with metastatic gastric cancer. Invest New Drugs. 2014;32:355-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 98] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 48. | Lee JH, Han SU, Cho H, Jennings B, Gerrard B, Dean M, Schmidt L, Zbar B, Vande Woude GF. A novel germ line juxtamembrane Met mutation in human gastric cancer. Oncogene. 2000;19:4947-4953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 245] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 49. | Park WS, Oh RR, Kim YS, Park JY, Shin MS, Lee HK, Lee SH, Yoo NJ, Lee JY. Absence of mutations in the kinase domain of the Met gene and frequent expression of Met and HGF/SF protein in primary gastric carcinomas. APMIS. 2000;108:195-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 50. | Inoue T, Chung YS, Yashiro M, Nishimura S, Hasuma T, Otani S, Sowa M. Transforming growth factor-beta and hepatocyte growth factor produced by gastric fibroblasts stimulate the invasiveness of scirrhous gastric cancer cells. Jpn J Cancer Res. 1997;88:152-159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 51. | Amemiya H, Peña A, Chiurillo M, Moscoso J, Useche A, Baffi R. [Increased expression of the c-Met receptor mRNA in gastric cancer]. Invest Clin. 2013;54:284-298. [PubMed] |
| 52. | Feng Y, Ma PC. Anti-MET targeted therapy has come of age: the first durable complete response with MetMAb in metastatic gastric cancer. Cancer Discov. 2011;1:550-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 53. | Kermorgant S, Cadiot G, Lewin MJ, Lehy T. [Expression of hepatocyte growth factor and its receptor, C-Met in human digestive tissues and different gastric and colonic cancer cell lines]. Gastroenterol Clin Biol. 1996;20:438-445. [PubMed] |
| 54. | Lee KH, Choi EY, Hyun MS, Jang BI, Kim TN, Kim SW, Song SK, Kim JH, Kim JR. Hepatocyte growth factor/c-met signaling in regulating urokinase plasminogen activator in human stomach cancer: A potential therapeutic target for human stomach cancer. Korean J Intern Med. 2006;21:20-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 55. | Wang L, Lin L, Chen X, Sun L, Liao Y, Huang N, Liao W. Metastasis-associated in colon cancer-1 promotes vasculogenic mimicry in gastric cancer by upregulating TWIST1/2. Oncotarget. 2015;6:11492-11506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 56. | Kaji M, Yonemura Y, Harada S, Liu X, Terada I, Yamamoto H. Participation of c-met in the progression of human gastric cancers: anti-c-met oligonucleotides inhibit proliferation or invasiveness of gastric cancer cells. Cancer Gene Ther. 1996;3:393-404. [PubMed] |
| 57. | Catenacci DV, Henderson L, Xiao SY, Patel P, Yauch RL, Hegde P, Zha J, Pandita A, Peterson A, Salgia R. Durable complete response of metastatic gastric cancer with anti-Met therapy followed by resistance at recurrence. Cancer Discov. 2011;1:573-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 58. | Park M, Park H, Kim WH, Cho H, Lee JH. Presence of autocrine hepatocyte growth factor-Met signaling and its role in proliferation and migration of SNU-484 gastric cancer cell line. Exp Mol Med. 2005;37:213-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 59. | Ueda K, Iwahashi M, Matsuura I, Nakamori M, Nakamura M, Ojima T, Naka T, Ishida K, Matsumoto K, Nakamura T, Yamaue H. Adenoviral-mediated gene transduction of the hepatocyte growth factor (HGF) antagonist, NK4, suppresses peritoneal metastases of gastric cancer in nude mice. Eur J Cancer. 2004;40:2135-2142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 60. | Amemiya H, Menolascino F, Peña A. [Role of the expression of c-Met receptor in the progression of gastric cancer]. Invest Clin. 2010;51:369-380. [PubMed] |
| 61. | Tannapfel A, Wittekind C, Tahara E. Effect of hepatocyte growth factor (HGF)/scatter factor (SF) on cell adhesion in gastric cancer. Z Gastroenterol. 1994;32:91-93. [PubMed] |
| 62. | Yu S, Yu Y, Zhao N, Cui J, Li W, Liu T. C-Met as a prognostic marker in gastric cancer: a systematic review and meta-analysis. PLoS One. 2013;8:e79137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 63. | Liu J, Li S, Chen S, Geng Q, Xu D. c-Met-dependent phosphorylation of RhoA plays a key role in gastric cancer tumorigenesis. J Pathol. 2019;249:126-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 64. | Zhang Q, Zhang H, Ning T, Liu D, Deng T, Liu R, Bai M, Zhu K, Li J, Fan Q, Ying G, Ba Y. Exosome-Delivered c-Met siRNA Could Reverse Chemoresistance to Cisplatin in Gastric Cancer. Int J Nanomedicine. 2020;15:2323-2335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 83] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 65. | Wu L, Zheng Y, Ruan X, Wu D, Xu P, Liu J, Li X. Long-chain noncoding ribonucleic acids affect the survival and prognosis of patients with esophageal adenocarcinoma through the autophagy pathway: construction of a prognostic model. Anticancer Drugs. 2022;33:e590-e603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 70] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 66. | Lv WL, Hu YY, Li ZN, Zhang W, Pan Q. PAX3 silencing suppresses gastric cancer proliferation and angiogenesis via MET/PI3K signaling. Neoplasma. 2020;67:304-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 67. | Hou GX, Song BB. Gastric cancer patient with c-MET amplification treated with crizotinib after failed multi-line treatment: A case report and literature review. Math Biosci Eng. 2019;16:5923-5930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 68. | Pereira MA, Ramos MFKP, Dias AR, Cardili L, Ribeiro RRE, de Castria TB, Zilberstein B, Nahas SC, Ribeiro U Jr, de Mello ES. RhoA, Claudin 18, and c-MET in Gastric Cancer: Clinicopathological Characteristics and Prognostic Significance in Curative Resected Patients. Med Sci (Basel). 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 69. | Zhang Z, Miao L, Wang S, Zhao Y, Xie Y, Yun H, Ren Z, Wang G, Teng M, Li Y. Study on the expression of c-Met in gastric cancer and its correlation with preoperative serum tumor markers and prognosis. World J Surg Oncol. 2022;20:204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 70. | Peng Z, Zhu Y, Wang Q, Gao J, Li Y, Ge S, Shen L. Prognostic significance of MET amplification and expression in gastric cancer: a systematic review with meta-analysis. PLoS One. 2014;9:e84502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 71. | Wang C, Li J, Qu L, Tang X, Song X, Yang F, Chen X, Lin Q, Lin W, Zhou Y, Tu Z, Chen Y, Zhang Z, Lu X. Discovery of D6808, a Highly Selective and Potent Macrocyclic c-Met Inhibitor for Gastric Cancer Harboring MET Gene Alteration Treatment. J Med Chem. 2022;65:15140-15164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 27] [Reference Citation Analysis (0)] |
| 72. | Yang Y, Wang C, Dai C, Liu X, Li W, Huang M, Zhao X, Ji D, Li J, Guo W. Amplification and expression of c-MET correlate with poor prognosis of patients with gastric cancer and upregulate the expression of PDL1. Acta Biochim Biophys Sin (Shanghai). 2021;53:547-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 73. | Zhu C, Xu J, Li M, Zhao G, Cao H. Heterogeneity of c-Met expression in Chinese gastric cancer patients. Hum Pathol. 2015;46:1901-1907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 74. | Koustas E, Karamouzis MV, Sarantis P, Schizas D, Papavassiliou AG. Inhibition of c-MET increases the antitumour activity of PARP inhibitors in gastric cancer models. J Cell Mol Med. 2020;24:10420-10431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 75. | Tong G, Cheng B, Li J, Wu X, Nong Q, He L, Li X, Li L, Wang S. MACC1 regulates PDL1 expression and tumor immunity through the c-Met/AKT/mTOR pathway in gastric cancer cells. Cancer Med. 2019;8:7044-7054. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 76. | Guo T, Yang J, Yao J, Zhang Y, Da M, Duan Y. Expression of MACC1 and c-Met in human gastric cancer and its clinical significance. Cancer Cell Int. 2013;13:121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 77. | Grojean M, Schwarz MA, Schwarz JR, Hassan S, von Holzen U, Zhang C, Schwarz RE, Awasthi N. Targeted dual inhibition of c-Met/VEGFR2 signalling by foretinib improves antitumour effects of nanoparticle paclitaxel in gastric cancer models. J Cell Mol Med. 2021;25:4950-4961. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 78. | Gu ML, Zhou XX, Ren MT, Shi KD, Yu MS, Jiao WR, Wang YM, Zhong WX, Ji F. Blockage of ETS homologous factor inhibits the proliferation and invasion of gastric cancer cells through the c-Met pathway. World J Gastroenterol. 2020;26:7497-7512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 79. | Kim BJ, Kim YJ, Sohn SH, Kim B, Sul HJ, Kim HS, Zang DY. Tivantinib inhibits the VEGF signaling pathway and induces apoptosis in gastric cancer cells with c-MET or VEGFA amplification. Invest New Drugs. 2020;38:1633-1640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 80. | Park CH, Cho SY, Ha JD, Jung H, Kim HR, Lee CO, Jang IY, Chae CH, Lee HK, Choi SU. Novel c-Met inhibitor suppresses the growth of c-Met-addicted gastric cancer cells. BMC Cancer. 2016;16:35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 81. | Zheng Z, Yan D, Chen X, Huang H, Chen K, Li G, Zhou L, Zheng D, Tu L, Dong XD. MicroRNA-206: Effective Inhibition of Gastric Cancer Progression through the c-Met Pathway. PLoS One. 2015;10:e0128751. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 82. | Nevisi F, Yaghmaie M, Pashaiefar H, Alimoghaddam K, Iravani M, Javadi G, Ghavamzadeh A. Correlation of HER2, MDM2, c-MYC, c-MET, and TP53 Copy Number Alterations in Circulating Tumor Cells with Tissue in Gastric Cancer Patients: A Pilot Study. Iran Biomed J. 2020;24:47-53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 83. | Sohn SH, Kim B, Sul HJ, Kim YJ, Kim HS, Kim H, Seo JB, Koh Y, Zang DY. INC280 inhibits Wnt/β-catenin and EMT signaling pathways and its induce apoptosis in diffuse gastric cancer positive for c-MET amplification. BMC Res Notes. 2019;12:125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 84. | Xu Z, Hu P, Fang D, Ni L, Xu J. Electrostatic explanation of D1228V/H/N-induced c-Met resistance and sensitivity to type I and type II kinase inhibitors in targeted gastric cancer therapy. J Mol Model. 2019;25:13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 85. | Wu L, Zhong Y, Yu X, Wu D, Xu P, Lv L, Ruan X, Liu Q, Feng Y, Liu J, Li X. Selective poly adenylation predicts the efficacy of immunotherapy in patients with lung adenocarcinoma by multiple omics research. Anticancer Drugs. 2022;33:943-959. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 66] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 86. | Zhao R, Zhang T, Xi W, Sun X, Zhou L, Guo Y, Zhao C, Bao Y. Human chorionic gonadotropin promotes cell proliferation through the activation of c-Met in gastric cancer cells. Oncol Lett. 2018;16:4271-4278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 87. | Erratum: Exosome-Delivered c-Met siRNA Could Reverse Chemoresistance to Cisplatin in Gastric Cancer [Corrigendum]. Int J Nanomedicine. 2022;17:1003-1004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 88. | Fuse N, Kuboki Y, Kuwata T, Nishina T, Kadowaki S, Shinozaki E, Machida N, Yuki S, Ooki A, Kajiura S, Kimura T, Yamanaka T, Shitara K, Nagatsuma AK, Yoshino T, Ochiai A, Ohtsu A. Prognostic impact of HER2, EGFR, and c-MET status on overall survival of advanced gastric cancer patients. Gastric Cancer. 2016;19:183-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 87] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 89. | Wei X, Juan ZX, Min FX, Nan C, Hua ZX, Qing FZ, Zheng L. Recombinant immunotoxin anti-c-Met/PE38KDEL inhibits proliferation and promotes apoptosis of gastric cancer cells. J Exp Clin Cancer Res. 2011;30:67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 90. | Liu M. Combination treatment with trastuzumab and crizotinib in metastatic gastric cancer harboring Her-2 amplification and c-MET amplification: A case report. Medicine (Baltimore). 2021;100:e27017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 91. | Metzger ML, Behrens HM, Böger C, Haag J, Krüger S, Röcken C. MET in gastric cancer--discarding a 10% cutoff rule. Histopathology. 2016;68:241-253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 92. | Seo S, Ryu MH, Ryoo BY, Park Y, Park YS, Na YS, Lee CW, Lee JK, Kang YK. Clinical significance of MET gene amplification in metastatic or locally advanced gastric cancer treated with first-line fluoropyrimidine and platinum combination chemotherapy. Chin J Cancer Res. 2019;31:620-631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 93. | Yang Y, Wu N, Shen J, Teixido C, Sun X, Lin Z, Qian X, Zou Z, Guan W, Yu L, Rosell R, Liu B, Wei J. MET overexpression and amplification define a distinct molecular subgroup for targeted therapies in gastric cancer. Gastric Cancer. 2016;19:778-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 94. | Ha SY, Lee J, Jang J, Hong JY, Do IG, Park SH, Park JO, Choi MG, Sohn TS, Bae JM, Kim S, Kim M, Park CK, Kang WK, Kim KM. HER2-positive gastric cancer with concomitant MET and/or EGFR overexpression: a distinct subset of patients for dual inhibition therapy. Int J Cancer. 2015;136:1629-1635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 95. | Kim HS, Chon HJ, Kim H, Shin SJ, Wacheck V, Gruver AM, Kim JS, Rha SY, Chung HC. MET in gastric cancer with liver metastasis: The relationship between MET amplification and Met overexpression in primary stomach tumors and liver metastasis. J Surg Oncol. 2018;117:1679-1686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 96. | Yashiro M, Nishii T, Hasegawa T, Matsuzaki T, Morisaki T, Fukuoka T, Hirakawa K. A c-Met inhibitor increases the chemosensitivity of cancer stem cells to the irinotecan in gastric carcinoma. Br J Cancer. 2013;109:2619-2628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 97. | Ma JA, Hu C, Li W, Ren J, Zou F, Zhou D, Zou W, Wei Y, Zhou Y. Downregulation of c-Met expression does not enhance the sensitivity of gastric cancer cell line MKN-45 to gefitinib. Mol Med Rep. 2015;11:2269-2275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 98. | Asaoka Y, Tada M, Ikenoue T, Seto M, Imai M, Miyabayashi K, Yamamoto K, Yamamoto S, Kudo Y, Mohri D, Isomura Y, Ijichi H, Tateishi K, Kanai F, Ogawa S, Omata M, Koike K. Gastric cancer cell line Hs746T harbors a splice site mutation of c-Met causing juxtamembrane domain deletion. Biochem Biophys Res Commun. 2010;394:1042-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 99. | Shitara K, Kim TM, Yokota T, Goto M, Satoh T, Ahn JH, Kim HS, Assadourian S, Gomez C, Harnois M, Hamauchi S, Kudo T, Doi T, Bang YJ. Phase I dose-escalation study of the c-Met tyrosine kinase inhibitor SAR125844 in Asian patients with advanced solid tumors, including patients with MET-amplified gastric cancer. Oncotarget. 2017;8:79546-79555. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 100. | Wang XL, Chen XM, Fang JP, Yang CQ. Lentivirus-mediated RNA silencing of c-Met markedly suppresses peritoneal dissemination of gastric cancer in vitro and in vivo. Acta Pharmacol Sin. 2012;33:513-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 101. | Gavine PR, Ren Y, Han L, Lv J, Fan S, Zhang W, Xu W, Liu YJ, Zhang T, Fu H, Yu Y, Wang H, Xu S, Zhou F, Su X, Yin X, Xie L, Wang L, Qing W, Jiao L, Su W, Wang QM. Volitinib, a potent and highly selective c-Met inhibitor, effectively blocks c-Met signaling and growth in c-MET amplified gastric cancer patient-derived tumor xenograft models. Mol Oncol. 2015;9:323-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 103] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 102. | Sunakawa Y, Wakatsuki T, Yang D, Zhang W, Ning Y, Stintzing S, Stremitzer S, Yamauchi S, Sebio A, El-khoueiry R, Iqbal S, Barzi A, Gerger A, Stotz M, Azuma M, Watanabe M, Koizumi W, Lenz HJ. Prognostic impact of the c-MET polymorphism on the clinical outcome in locoregional gastric cancer patients. Pharmacogenet Genomics. 2014;24:588-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 103. | Wang H, Lu J, Tang J, Chen S, He K, Jiang X, Jiang W, Teng L. Establishment of patient-derived gastric cancer xenografts: a useful tool for preclinical evaluation of targeted therapies involving alterations in HER-2, MET and FGFR2 signaling pathways. BMC Cancer. 2017;17:191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 104. | Drebber U, Baldus SE, Nolden B, Grass G, Bollschweiler E, Dienes HP, Hölscher AH, Mönig SP. The overexpression of c-met as a prognostic indicator for gastric carcinoma compared to p53 and p21 nuclear accumulation. Oncol Rep. 2008;19:1477-1483. [PubMed] |
| 105. | Saisana M, Griffin SM, May FE. Importance of the type I insulin-like growth factor receptor in HER2, FGFR2 and MET-unamplified gastric cancer with and without Ras pathway activation. Oncotarget. 2016;7:54445-54462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 106. | Mao SH, Zhu CH, Nie Y, Yu J, Wang L. Levobupivacaine Induces Ferroptosis by miR-489-3p/SLC7A11 Signaling in Gastric Cancer. Front Pharmacol. 2021;12:681338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 107. | Ni H, Qin H, Sun C, Liu Y, Ruan G, Guo Q, Xi T, Xing Y, Zheng L. MiR-375 reduces the stemness of gastric cancer cells through triggering ferroptosis. Stem Cell Res Ther. 2021;12:325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 126] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 108. | Wu C, Hou X, Li S, Luo S. Long noncoding RNA ZEB1-AS1 attenuates ferroptosis of gastric cancer cells through modulating miR-429/BGN axis. J Biochem Mol Toxicol. 2023;37:e23381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 109. | Ye Y, Li X, Feng G, Ma Y, Ye F, Shen H, Sun K, Lu R, Miao S. 3,3'-Diindolylmethane induces ferroptosis by BAP1-IP3R axis in BGC-823 gastric cancer cells. Anticancer Drugs. 2022;33:362-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |