Published online Jun 15, 2024. doi: 10.4251/wjgo.v16.i6.2769
Revised: April 25, 2024
Accepted: April 28, 2024
Published online: June 15, 2024
Processing time: 99 Days and 16.8 Hours
Wnt/FZD-mediated signaling pathways are activated in more than 90% of hepatocellular carcinoma (HCC) cell lines. As a well-known secretory gly
To investigate the effect of Wnt3 N-glycosylation on the biological function of HCC cells.
Site-directed mutagenesis was used to verify the Wnt3 N-glycosylation sites, acti
Wnt3 has two N-glycosylation-modified sites (Asn90 and Asn301); when a single site at amino acid 301 is mutated, the stability of Wnt3 is weakened; the binding ability of Wnt3 to FZD7 decreases when both sites are mutated simultaneously; and the level of proteins related to the Wnt/β-catenin signaling pathway is downregulated. Cell proliferation, migration and invasion are also weakened in the case of single 301 site and double-site mutations.
These results indicate that by inhibiting the N-glycosylation of Wnt3, the proliferation, migration, invasion and colony formation abilities of liver cancer cells can be weakened, which might provide new therapeutic strategies for clinical liver cancer in the future.
Core Tip: Our study reveals that Wnt3 undergoes N-glycosylation modification at two specific sites (Asn90 and Asn301). Mutation of these sites impairs the stability and function of Wnt3, reducing its binding ability to FZD7 and downregulating the Wnt/β-catenin signaling pathway. Consequently, cell proliferation, migration, and invasion are attenuated in hepatocellular carcinoma cells. These findings suggest that targeting Wnt3 N-glycosylation could be a potential therapeutic strategy for liver cancer.
- Citation: Zhang XZ, Mo XC, Wang ZT, Sun R, Sun DQ. N-glycosylation of Wnt3 regulates the progression of hepatocellular carcinoma by affecting Wnt/β-catenin signal pathway. World J Gastrointest Oncol 2024; 16(6): 2769-2780
- URL: https://www.wjgnet.com/1948-5204/full/v16/i6/2769.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i6.2769
Wnt/FZD-mediated signaling pathways are activated in more than 90% of hepatocellular carcinoma (HCC) cell lines[1,2]. At present, 19 subtypes of the Wnt gene have been identified in the human genome[3]. Wnt is a secretory glycoprotein that can act as a ligand to interact with the FZD receptor, which is the main receptor of Wnt signal transduction on the cell surface and activates intracellular signaling pathways. At present, 10 FZD subtypes are known to be present in the human genome[3,4]. Compared with those in normal tissues, the protein expression levels of Wnt3/4/5a and FZD3/6/7 were significantly upregulated in more than 95% of HCC tissues[4]. To date, the interactions between 19 Wnt ligands and 10 FZD receptors found in the human proteome have also been specific, among which Wnt3 is the ligand of FZD7, and the upregulation of the Wnt3/FZD7-mediated β-catenin signaling pathway in HCC is more significant[4-6]. When querying the IUPHAR database, the same results were obtained[7]. In summary, Wnt3 is a potential cancer-related protein, but there is a lack of research on the role of Wnt3 N-glycosylation. To explore the role of N-glycosylation of Wnt3, we chose this protein for follow-up research.
Glycosylation is a type of post-translational protein modification that affects more than half of all known proteins[8-10]. A number of experiments and substantial clinical data have shown that there are abnormal glycosylations involved in the occurrence and progression of cancer, among which N-glycosylation is more common in post-translational modifications and occurs on asparagine residues with specific N-X-S/T structures[11]. This modification can affect protein folding, protein stability and other cellular functions[8,12]. Regarding the relationship between N-glycosylation and the Wnt/β-catenin signaling pathway, the binding of Wnt3a to FZD is reportedly affected by N-glycosylation and N-glyc
Therefore, we investigated the effect of N-glycosylation on Wnt3 by treating it with N-glycosylation inhibitors and constructing site-directed mutation vectors. The constructed vectors were transfected into HCC cells to detect changes in cell behaviors and downstream signaling pathways. We hope that this discovery will provide a new treatment strategy for clinical liver cancer.
PLC/PRF/5 cells were purchased from the cell bank of the Shanghai Institute of Biochemistry and Cell Research at the Chinese Academy of Sciences. MHCC97H cells were obtained from the cell bank at Procell Life Science & Technology and were treated with 10% embryonic bovine serum (FBS, TIANHANG, China) and 1% penicillin-streptomycin (C0222, Beyotime, China). Minimum Eagle's medium (MEM, Gibco, United States) was added, and the cells were cultured at 37°C and 5% CO2.
The following compounds were used: DAPI dye solution, RIPA lysis buffer, and protease inhibitor, which were pur
The differences in the Wnt3 expression levels and tumor stages between HCC tissue and normal tissue were determined using the TCGA database (https://www.cancer.gov/ccg/research/genome-sequencing/tcga). The splicing bodies, subcellular localization, possible N-glycosylation sites and interacting proteins of Wnt3 were queried via the HPA database (https://www.proteinatlas.org/) and the UniProt database (https://www.uniprot.org/). The conserved region of Wnt3 was queried using the NCBI database (https://www.ncbi.nlm.nih.gov/).
pLVX-AcGFP1-WNT3, pLVX-AcGFP1-WNT3N90D, pLVX-AcGFP1-WNT3N301D, pLVX-AcGFP1-WNT3N90/301D, and pDsRed1-FZD7 plasmids were constructed by our team. Site-directed mutant vectors were constructed using the KOD-Plus-Mutagenesis Kit (SMK-101, TOYOBO, Japan) according to the manufacturer's instructions. Cells were transfected using LipoFiter 3.0 reagent (HB-TRLF3-1000, HANBIO, China).
Matrigel was added to the upper chamber of the CIM plate (ACEA, United States), and the excess liquid was discarded after 4 h. Transfected cells were inoculated into a CIM plate upper chamber, 150 μL of serum-free MEM was added to the upper chamber, 5 × 104 cells were seeded in the upper chamber, and 165 μL of MEM supplemented with FBS was added to the lower chamber. The detection plate was installed in a real-time cellular analysis (RTCA, ACEA, United States) instrument, and detection was performed every 15 min for a total of 60 h.
The transfected cells were seeded in a 24-well plate and grown to 100% confluence after the cells were attached to the plate. The cells were nicked with the tip of a pipette gun, rinsed with normal saline three times, photographed under an inverted microscope, and recorded at 0, 24 and 48 h respectively.
Fifty microliters of serum-containing MEM was added to an RTCA E-Plate16 (ACEA, United States) for background detection. Then, the transfected cells were spread onto an RTCA E-Plate 16 at a rate of 5000 cells per well and placed in an RTCA for detection, which was set for detection every 20 min for a total of 80 h.
Thirty-six hours after transfection, the cell transfection solution was discarded, and the cells were washed with normal saline three times. Then, MEM culture medium without FBS and ampicillin-streptomycin was added. After 24 h, the cell culture medium was concentrated with Amicon Ultra15 (10 kDa; UFC801096, Millipore, United States), and the amount of protein secreted into the medium was detected by western blotting.
Wnt3 with a GFP tag and FZD7 with an RFP tag were co-transfected into the cells (the site-directed mutant groups were the same as those described above). The transfected cells were placed in a 24-well plate with a sterilized cover slip. After being attached to the wall, the cells were fixed overnight with 4% paraformaldehyde at 4°C. The liquid was discarded, and the nuclei were stained with DAPI dye solution.
Wnt3 with a GFP tag and FZD7 with an RFP tag were co-transfected into cells (the site-directed mutant groups were the same as those described above), and the cells were collected 48 hours after transfection. RIPA buffer (200 μL) was added to the cells, which were then lysed by ultrasonication (200 W for 15 min, working for 3 s, stopping for 5 s). After the samples were crushed and centrifuged at 3000 r/min for 10 minutes, the prepared protein A + G magnetic beads were added, 1 μL of RFP antibody was added, and the mixture was incubated at 4°C overnight. After that, PBS was added three times, and 50 μL of SDS loading buffer was added and heated at 95°C for 10 min to elute and prepare the sample.
SDS-PAGE was performed via gel electrophoresis, after which the proteins were transferred to polyethylene difluoride (PVDF, Millipore, United States) membranes. Then, the PVDF membrane was blocked at 37°C for 40 min with 50 g/L bovine serum albumin (Solarbio, China) and washed with TBST three times. The membrane was incubated with a specific primary antibody at 4°C overnight, after which the appropriate fluorescent secondary antibody was used. The mem
A single dose of DEN (2 mg/kg body weight in PBS) was injected into 3-wk-old male C3H mice intraperitoneally (i.p.) to initiate tumor formation. Next, the mice were injected i.p. with TAA (200 mg/kg body weight, calculated according to PBS) or DEN once a week. After 24 wk, all mouse livers were removed, and the numbers and sizes of the HCC tumors were recorded. Then, an immunohistochemical experiment was performed (No. 2200067).
The tumor mass was isolated from mice, immersed in formalin and embedded in OCT (Solarbio, China). Sections were submerged in EDTA citrate buffer (pH 6.0 or pH 8.0) and microwaved for antigen retrieval. Then, the slides were incubated with the anti-Wnt3 antibody at 4°C overnight and, treated with a goat anti-mouse/rabbit IgG polymer-conjugated secondary antibody for 30 min and developed with diaminobenzidine. The nuclei were counterstained with hematoxylin. Image acquisition was performed using a Nikon camera and software.
SPSS 22.0 and GraphPad Prism 6.0 software were used to analyze the data, and the results are expressed as the means ± SD. Comparisons between groups were first performed for normally distributed data, and one-way analysis of variance was used for normally distributed data. For pairwise comparisons between groups, the homogeneity of variances was tested first. If the variances were homogeneous, the least significant difference method was used; if the variances were not homogeneous, the Dunnett's test (ST3) method was used. When P < 0.05, the difference was considered statistically significant.
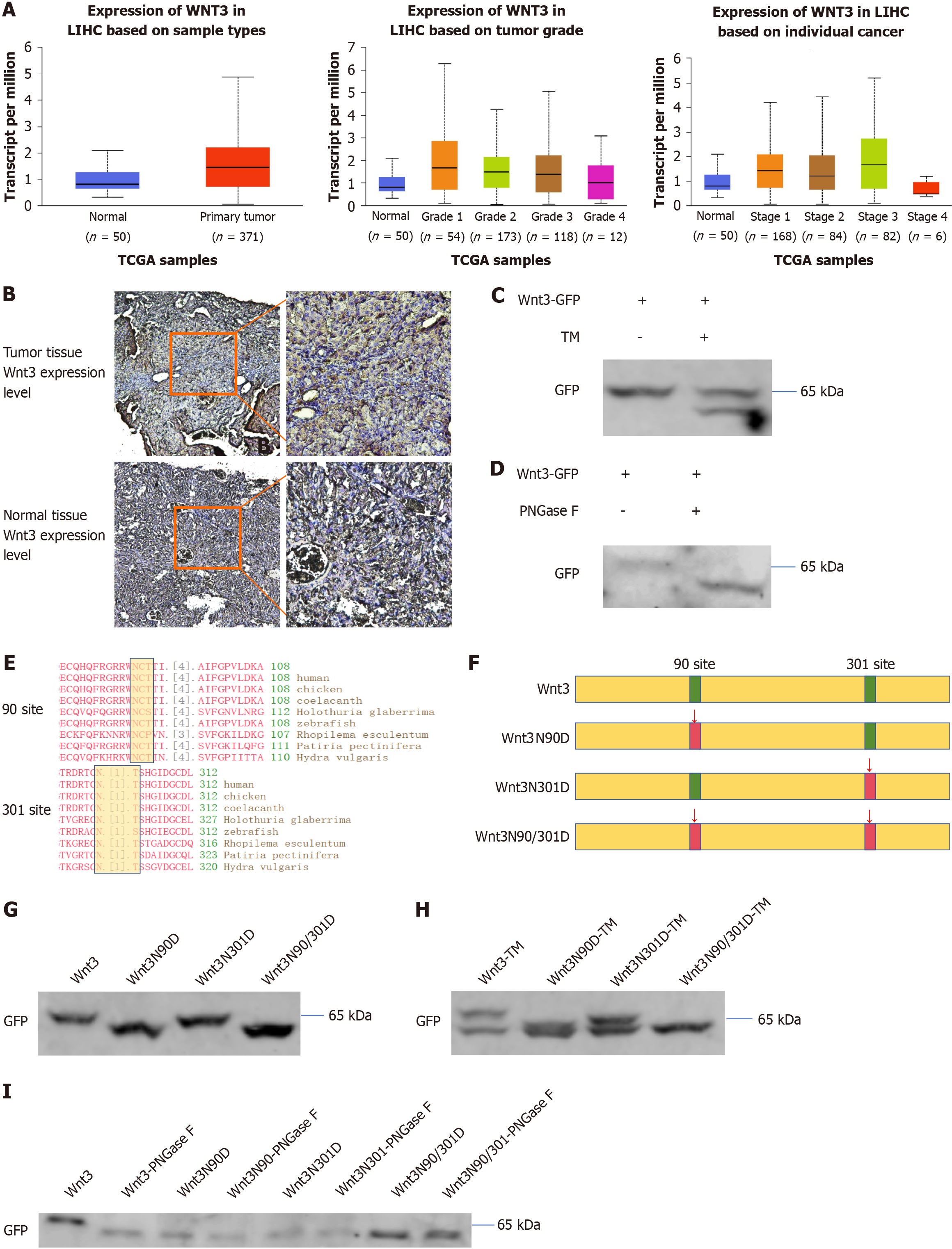
A query of the TCGA database revealed that the protein expression of Wnt3 was upregulated in patients with liver cancer and increased during the early stage of tumorigenesis (Figure 1A). We also found that the protein expression of Wnt3 was significantly increased in mice with primary liver cancer via immunohistochemical staining (Figure 1B). Increased Wnt3 expression may lead to abnormal activation of Wnt/β-catenin signaling, which in turn regulates the occurrence and development of HCC. Therefore, it is very important to find a way to inhibit the effect of Wnt3. According to previous reports, because Wnt3 is highly similar to Wnt3a, the Wnt3 protein is also likely to undergo N-glycosylation. N-glycosylation may also affect the binding of Wnt3 to the FZD protein, regulate the Wnt/β-catenin signaling pathway and affect cell function. To verify the above conjecture, the HCC cell line PLC/PRF/5, which does not express endogenous Wnt3 and was transfected with exogenous Wnt3 with a GFP tag, was treated with the N-glycosylation inhibitor TM. The results showed that the molecular weight of the Wnt3 protein decreased significantly, and the same effect was observed in the cells transfected with Wnt3 and treated with PNGase F (Figure 1C and D). Through a sequence analysis of Wnt3, it was found that there were two possible N-glycosylation sites of Wnt3, at site 90 and site 301, both of which are conserved regions (Figure 1E). Based on this result, we confirmed the existence of N-glycosylation in Wnt3 and adopted site-directed mutagenesis to analyze the specific N-glycosylation site. To test this hypothesis, we constructed site-directed mutation expression vectors for Wnt3 (Figure 1F). Then, PLC/PRF/5 cells were transfected with the site-mutant Wnt3 vector or the wild-type Wnt3 vector, and western blot analysis revealed that the molecular weight of the protein decreased regardless of which site was mutated (Figure 1G). Furthermore, after transfection with TM, Wnt3 was found to have different degrees of N-glycosylation at sites 90 and 301 (Figure 1H). The same result was obtained using PNGase F treatment (Figure 1I). The above experimental results show that the expression of Wnt3 is not only upregulated in HCC but also modified by N-glycosylation at sites 90 and 301.
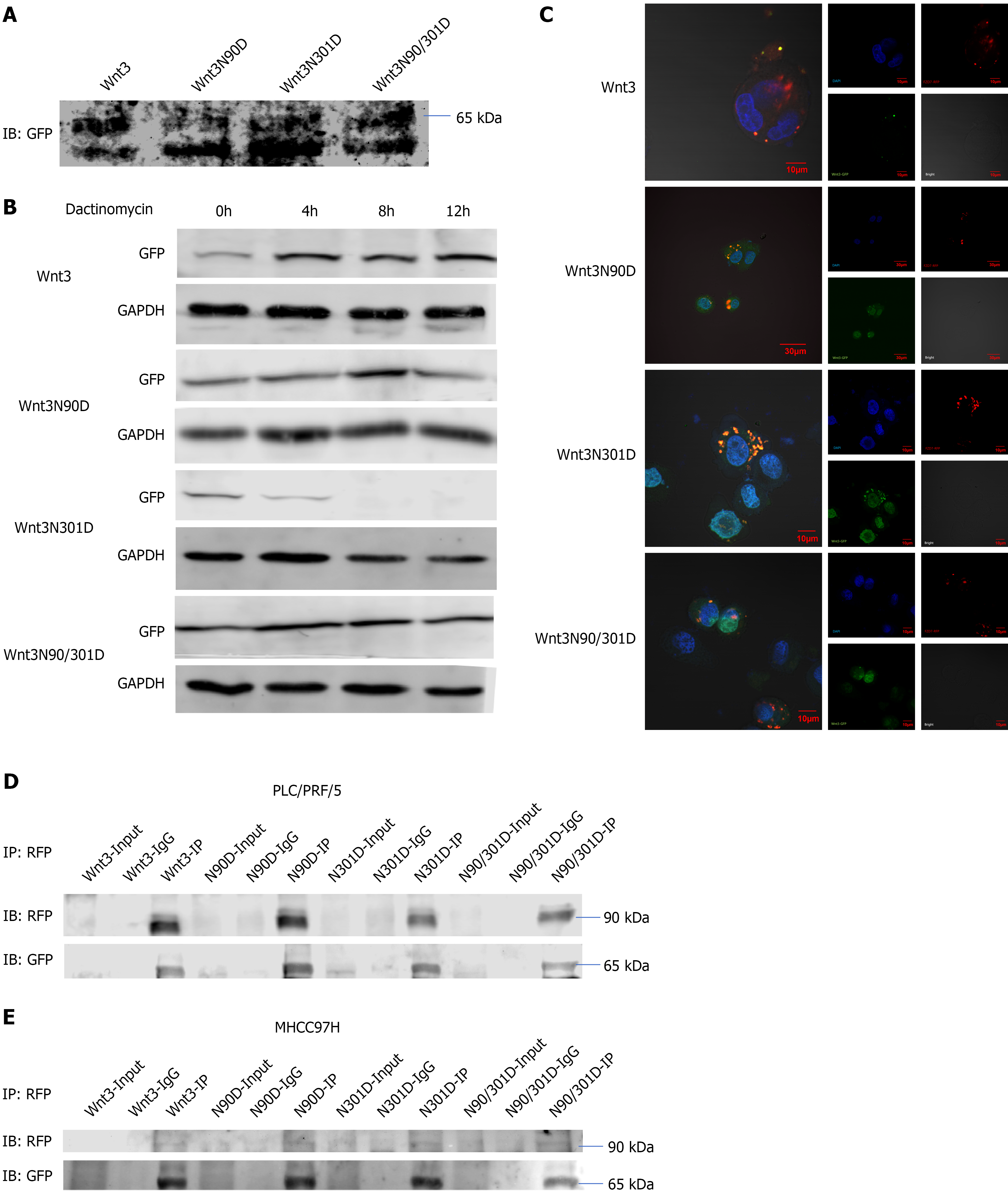
Wnt3 is a secretory protein that usually plays an extracellular biological role. To understand the effect of N-glycosylation on Wnt3, we detected the effect of N-glycosylation site-directed mutations on the secretion of Wnt3 (Figure 2A). Cell culture medium was collected, and extracellular secreted Wnt3 was detected by western blotting analysis. Interestingly, the results of Western blotting analysis showed that Wnt3 that was secreted from the cell exhibited two bands of different molecular weights. Moreover, compared with those of the Wnt3 group, the protein bands of the Wnt3 N-glycosylation-defective mutants migrated more to the low-molecular-weight band (Figure 2A). By querying the HPA database, we learned that the Wnt3 protein has splice variants with different molecular weights. We speculate that the band with a smaller molecular weight might be Wnt3-208, the splice variant of the Wnt3 protein. According to the information obtained from the database, this kind of splice variant is more likely to remain in the cell than to be secreted from the cell, and the specific reason for this phenomenon must still be explored (Table 1). Then, we further examined the effect of N-glycosylation on the stability of Wnt3 and treated PLC/PRF/5 cells transfected with Wnt3 with actinomycin D. The mutation of the N-glycosylation site affected the stability of Wnt3 (Figure 2B). Surprisingly, when Wnt3 was mutated at site 301, the stability of the protein decreased. However, when sites 90/301 were mutated concurrently, the protein stability was enhanced compared with that performed only at site 301 (Figure 2B). Overall, the stability of Wnt3 decreased only when there was a single point mutation at site 301. Based on a previous study showing that Wnt3 exerts its biological effects through interaction with its receptor FZD7, we examined changes in the ability of Wnt3 Lacking N-glycosylation to bind to FZD7. Upon co-transfecting Wnt3 with a GFP tag and FZD7 with an RFP tag into PLC/PRF/5 cells and then observing the cells by laser confocal microscopy, it was found that when both N-glycosylation sites of Wnt3 were mutated, the ability of Wnt3 to bind to FZD7 decreased significantly (Figure 2C). Similarly, the co-IP detection also showed that the binding ability of Wnt3 to FZD7 decreased when both sites were mutated (Figure 2D and E). In summary, site-directed mutations in Wnt3 affect its protein stability and interaction with FZD7. Therefore, we speculate that the loss of N-glycosylation from Wnt3 is likely to lead to changes in the downstream signaling pathway.
| Splice variant | Swissprot | Protein class | Length & mass | Signal peptide | Transmembrane regions |
| Wnt3-201 | P56703 | Predicted secreted proteins Disease related genes Human disease related genes Mapped to neXtProt | 355 aa 39.6 kDa | Yes | 0 |
| Wnt3-205 | Predicted intracellular proteins Human disease related genes | 170 aa 19.7 kDa | No | 0 | |
| Wnt3-207 | Predicted intracellular proteins Human disease related genes | 170 aa 19.7 kDa | No | 0 | |
| Wnt3-208 | Predicted intracellular proteins Human disease related genes | 290 aa 32.5 kDa | No | 0 |
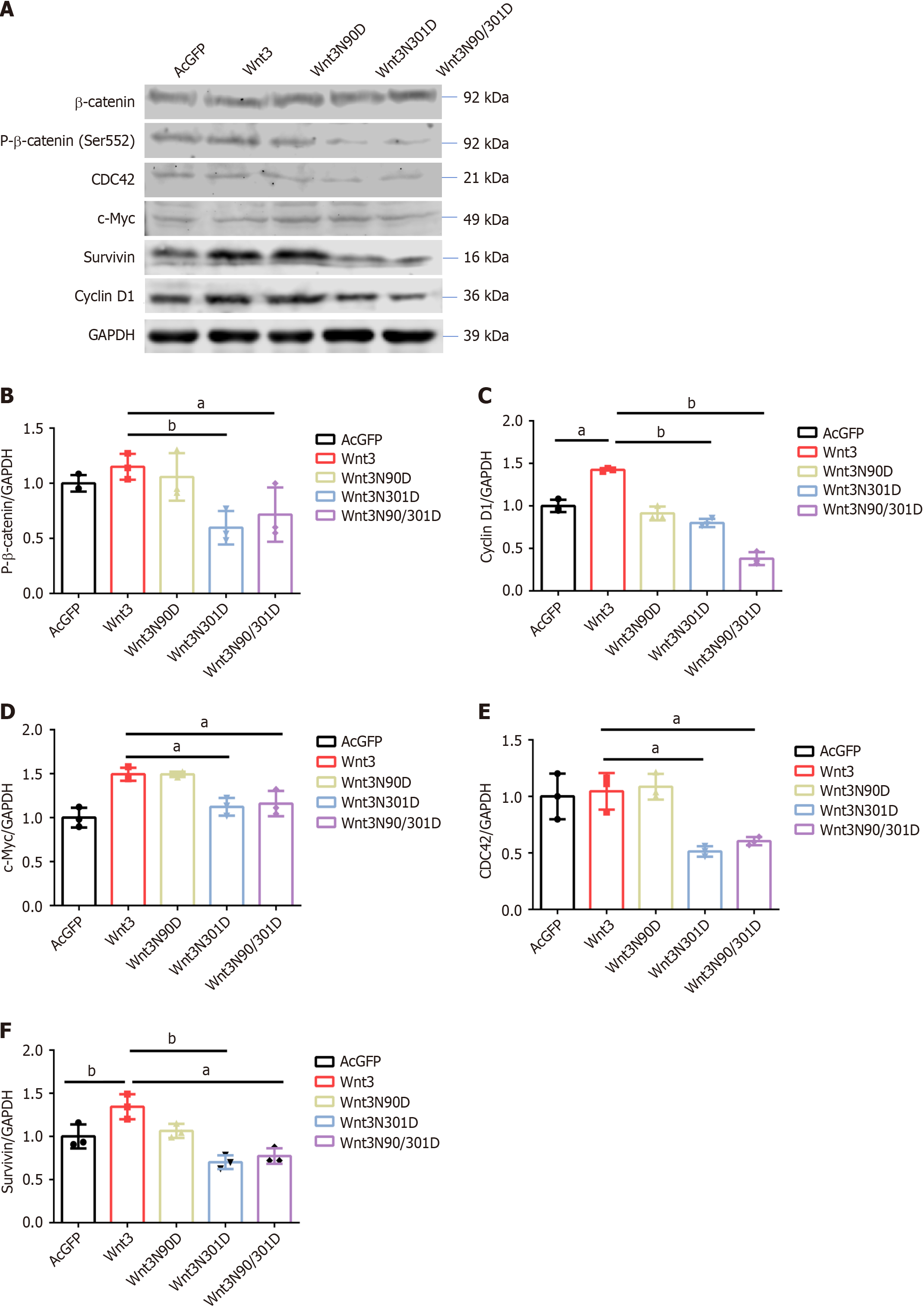
To verify the aforementioned speculation, we transfected PLC/PRF/5 cells with Wnt3 and site-specific mutant vectors to detect downstream protein activation. However, the results of the western blot analysis showed no significant changes in the expression of β-catenin (Figure 3A). However, when a phospho-β-catenin (Ser552) antibody was used to detect the effect of β-catenin on downstream protein transcription, Wnt3N301D and Wnt3N90/301D significantly decreased the levels of β-catenin phosphorylated at position 552 (Figure 3A and B). We speculated that the reduced Wnt3 protein stability caused by the N301D mutation led to the reduced activation of β-catenin. The simultaneous mutation of the two N90/301D sites weakened the ability of Wnt3 to bind to its receptor FZD7, which further decreased the amount of activated β-catenin protein. Next, we examined downstream proteins regulated by the Wnt/β-catenin signaling pathway. The levels of CDC42, C-Myc, Survivin, and Cyclin D1 in the Wnt3N301D group and Wnt3N90/301D group decreased to varying degrees (Figure 3A and C-F). Overall, deleting the N-glycosylation of Wnt3 affects the expression of proteins involved in the Wnt/β-catenin signaling pathway and downstream proteins.
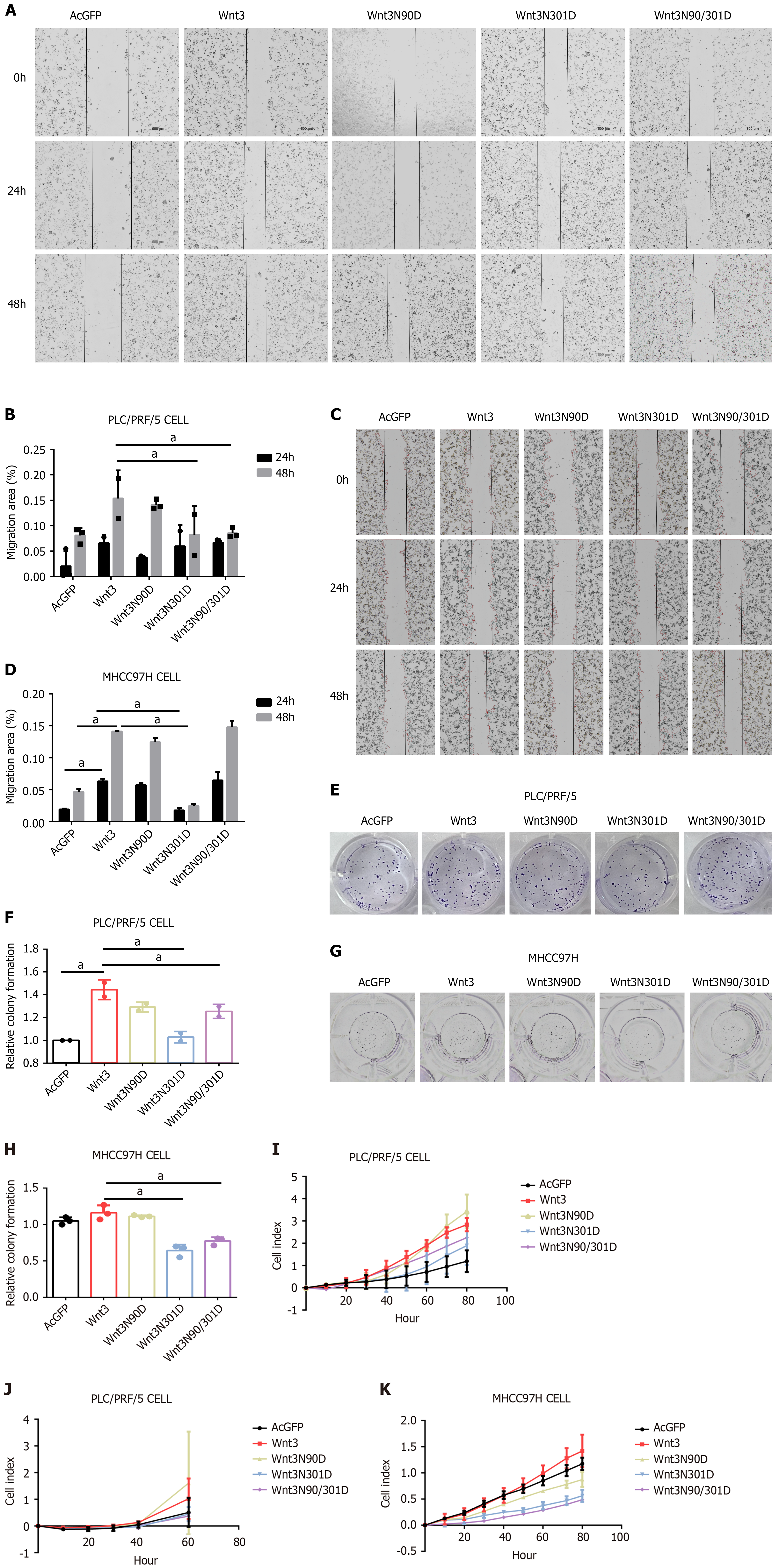
To investigate the effect of N-glycosylation in Wnt3 on cell function, we used wound healing experiments to detect the effect of the Wnt3 mutation and its N-glycosylation site on the migration of the HCC cell lines PLC/PRF/5 and MHCC97H. In the two cell lines, the cell migration ability of the Wnt3N301D group was significantly weakened, while the migration ability of the PLC/PRF/5 cell line decreased in the Wnt3N90/301D group, but there was no obvious difference in the MHCC97H cell line (Figure 4A-D). Subsequently, we tested the effect of site-directed mutations on the clonogenic ability of cells by performing colony formation experiments. In terms of colony formation ability, the two cell lines showed similar results, and the colony formation abilities of the Wnt3N301D group and the Wnt3N90/301D group both decreased (Figure 4E-H). Moreover, RTCA was used to detect the effects on cell proliferation and invasion. Ultimately, we observed that the cell proliferation and migration abilities of the Wnt3N301D and Wnt3N90/301D groups were weakened compared with those of the Wnt3 group (Figure 4I-K). The invasion experiments on the MHCC97H cells failed to confirm complete invasion. In summary, the N-glycosylation of Wnt3 clearly affects cell function. Inhibiting the N-glycosylation of Wnt3 can weaken the proliferation, migration, invasion and colony formation abilities of liver cancer cell lines.
In this study, we found that N-glycosylation of the Wnt3 protein can affect its own stability or its ability to bind to FZD7, thereby affecting the classic Wnt/β-catenin signaling pathway and thus regulating cell migration, proliferation, and invasion. Among these modifications, N-glycosylation at site 301 of Wnt3 affects the Wnt/β-catenin signaling pathway by influencing protein stability. Simultaneous mutations at sites 90 and 301 of Wnt3 affect the binding ability of Wnt3 to FZD7.
Wnt3 is a glycoprotein and belongs to the Wnt superfamily[14]. Its members are upregulated in liver cancer and promote liver cancer progression by activating the classic Wnt/β-catenin signaling pathway[15,16]. Wnt3 can interact with FZD7 and activate signaling pathways[17,18]. According to the HPA database, there are four splicing variants of the Wnt3 protein. Only one Wnt3 with a molecular weight of 35.6 kDa can be secreted from cells, and the remaining three splice variants with molecular weights of 32.5 kDa, 19.7 kDa, and 19.7 kDa are all predicted to be intracellular. In our study, cell culture medium was collected for western blot analysis, which revealed that the Wnt3 protein secreted from cells has two molecular weights. Additionally, changes in N-glycosylation sites also increased the expression of Wnt3, which has a lower molecular weight. We speculate that this phenomenon may be due to the lack of N-glycosylation of Wnt3, which affects the shearing and folding of the Wnt3. This finding also suggested that Wnt3N90D affects downstream signaling pathways and cell functions. Similarly, some studies have shown that the loss of N-glycosylation of Wnt3a weakens the secretion and binding of the protein to FZD8, thereby affecting the Wnt/β-catenin signaling pathway[13]. In our study, the simultaneous mutation of two glycosylation sites also weakened the ability of Wnt3 to bind to its receptor. Immunofluorescence also revealed a reduction in the level of the extracellular portion of Wnt3N90/301D (Figure 2D).
In HCC, clinical evidence has shown that the Wnt/β-catenin signaling pathway is involved in tumor progression and metastasis[19-21]. It is also activated in other cancers, such as adrenocortical carcinoma and colorectal cancer[22-25]. Research has shown that inhibiting Wnt ligands and FZD receptors with monoclonal antibodies or small molecules can promote cell apoptosis and inhibit cell proliferation, which may constitute a therapeutic approach[18,26]. Soluble FZD7 can also bind to Wnt to inhibit Wnt/β-catenin signaling, thereby inhibiting the progression of HCC[27,28]. Overall, there are many reports that inhibiting the activation of the Wnt/β-catenin signaling pathway plays a role in regulating the progression of HCC.
Here, we report the first evidence to date that the stability of Wnt3 is weakened by mutating site 301 of Wnt3. When the 90/301 sites of Wnt3 are mutated, the ability of the Wnt3 protein to bind to the FZD7 protein is weakened. Our study provides a new strategy for inhibiting Wnt3 activity, which inhibits its biological function by inhibiting its N-glycosylation, thereby inhibiting the progression of liver cancer. We have strong hopes that this discovery will provide a new treatment strategy for clinical liver cancer.
| 1. | Rebouissou S, Nault JC. Advances in molecular classification and precision oncology in hepatocellular carcinoma. J Hepatol. 2020;72:215-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 375] [Article Influence: 75.0] [Reference Citation Analysis (1)] |
| 2. | He S, Tang S. WNT/β-catenin signaling in the development of liver cancers. Biomed Pharmacother. 2020;132:110851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 230] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 3. | Pez F, Lopez A, Kim M, Wands JR, Caron de Fromentel C, Merle P. Wnt signaling and hepatocarcinogenesis: molecular targets for the development of innovative anticancer drugs. J Hepatol. 2013;59:1107-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 214] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 4. | Chan KK, Lo RC. Deregulation of Frizzled Receptors in Hepatocellular Carcinoma. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Catalano T, Selvaggi F, Esposito DL, Cotellese R, Aceto GM. Infectious Agents Induce Wnt/β-Catenin Pathway Deregulation in Primary Liver Cancers. Microorganisms. 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 6. | Perugorria MJ, Olaizola P, Labiano I, Esparza-Baquer A, Marzioni M, Marin JJG, Bujanda L, Banales JM. Wnt-β-catenin signalling in liver development, health and disease. Nat Rev Gastroenterol Hepatol. 2019;16:121-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 401] [Article Influence: 66.8] [Reference Citation Analysis (0)] |
| 7. | Dijksterhuis JP, Petersen J, Schulte G. WNT/Frizzled signalling: receptor-ligand selectivity with focus on FZD-G protein signalling and its physiological relevance: IUPHAR Review 3. Br J Pharmacol. 2014;171:1195-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 148] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 8. | Esmail S, Manolson MF. Advances in understanding N-glycosylation structure, function, and regulation in health and disease. Eur J Cell Biol. 2021;100:151186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 121] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 9. | Reily C, Stewart TJ, Renfrow MB, Novak J. Glycosylation in health and disease. Nat Rev Nephrol. 2019;15:346-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1352] [Cited by in RCA: 1357] [Article Influence: 226.2] [Reference Citation Analysis (0)] |
| 10. | Apweiler R, Hermjakob H, Sharon N. On the frequency of protein glycosylation, as deduced from analysis of the SWISS-PROT database. Biochim Biophys Acta. 1999;1473:4-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1406] [Cited by in RCA: 1400] [Article Influence: 53.8] [Reference Citation Analysis (0)] |
| 11. | Cherepanova N, Shrimal S, Gilmore R. N-linked glycosylation and homeostasis of the endoplasmic reticulum. Curr Opin Cell Biol. 2016;41:57-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 190] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 12. | Berthe A, Zaffino M, Muller C, Foulquier F, Houdou M, Schulz C, Bost F, De Fay E, Mazerbourg S, Flament S. Protein N-glycosylation alteration and glycolysis inhibition both contribute to the antiproliferative action of 2-deoxyglucose in breast cancer cells. Breast Cancer Res Treat. 2018;171:581-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 13. | Komekado H, Yamamoto H, Chiba T, Kikuchi A. Glycosylation and palmitoylation of Wnt-3a are coupled to produce an active form of Wnt-3a. Genes Cells. 2007;12:521-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 162] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 14. | Niehrs C. The complex world of WNT receptor signalling. Nat Rev Mol Cell Biol. 2012;13:767-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 950] [Cited by in RCA: 1135] [Article Influence: 87.3] [Reference Citation Analysis (0)] |
| 15. | Zhan T, Rindtorff N, Boutros M. Wnt signaling in cancer. Oncogene. 2017;36:1461-1473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1646] [Cited by in RCA: 1890] [Article Influence: 236.3] [Reference Citation Analysis (0)] |
| 16. | Takahashi-Yanaga F, Kahn M. Targeting Wnt signaling: can we safely eradicate cancer stem cells? Clin Cancer Res. 2010;16:3153-3162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 392] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 17. | Huttlin EL, Bruckner RJ, Paulo JA, Cannon JR, Ting L, Baltier K, Colby G, Gebreab F, Gygi MP, Parzen H, Szpyt J, Tam S, Zarraga G, Pontano-Vaites L, Swarup S, White AE, Schweppe DK, Rad R, Erickson BK, Obar RA, Guruharsha KG, Li K, Artavanis-Tsakonas S, Gygi SP, Harper JW. Architecture of the human interactome defines protein communities and disease networks. Nature. 2017;545:505-509. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 907] [Cited by in RCA: 1059] [Article Influence: 132.4] [Reference Citation Analysis (0)] |
| 18. | Bengochea A, de Souza MM, Lefrançois L, Le Roux E, Galy O, Chemin I, Kim M, Wands JR, Trepo C, Hainaut P, Scoazec JY, Vitvitski L, Merle P. Common dysregulation of Wnt/Frizzled receptor elements in human hepatocellular carcinoma. Br J Cancer. 2008;99:143-150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 175] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 19. | Song J, Xie C, Jiang L, Wu G, Zhu J, Zhang S, Tang M, Song L, Li J. Transcription factor AP-4 promotes tumorigenic capability and activates the Wnt/β-catenin pathway in hepatocellular carcinoma. Theranostics. 2018;8:3571-3583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 20. | Peng YY, He YH, Chen C, Xu T, Li L, Ni MM, Meng XM, Huang C, Li J. NLRC5 regulates cell proliferation, migration and invasion in hepatocellular carcinoma by targeting the Wnt/β-catenin signaling pathway. Cancer Lett. 2016;376:10-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 21. | Gajos-Michniewicz A, Czyz M. WNT/β-catenin signaling in hepatocellular carcinoma: The aberrant activation, pathogenic roles, and therapeutic opportunities. Genes Dis. 2024;11:727-746. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 28] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 22. | Tai Y, Shang J. Wnt/β-catenin signaling pathway in the tumor progression of adrenocortical carcinoma. Front Endocrinol (Lausanne). 2023;14:1260701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 23. | He K, Gan WJ. Wnt/β-Catenin Signaling Pathway in the Development and Progression of Colorectal Cancer. Cancer Manag Res. 2023;15:435-448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 61] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 24. | Disoma C, Zhou Y, Li S, Peng J, Xia Z. Wnt/β-catenin signaling in colorectal cancer: Is therapeutic targeting even possible? Biochimie. 2022;195:39-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 45] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 25. | Manigandan K, Manimaran D, Jayaraj RL, Elangovan N, Dhivya V, Kaphle A. Taxifolin curbs NF-κB-mediated Wnt/β-catenin signaling via up-regulating Nrf2 pathway in experimental colon carcinogenesis. Biochimie. 2015;119:103-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 26. | Xue Y, Chen C, Xu W, Xu H, Zheng J, Gu Y. Downregulation of Frizzled-7 induces the apoptosis of hepatocellular carcinoma cells through inhibition of NF-κB. Oncol Lett. 2018;15:7693-7701. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Xie W, Zhang Y, He Y, Zhang K, Wan G, Huang Y, Zhou Z, Huang G, Wang J. A novel recombinant human Frizzled-7 protein exhibits anti-tumor activity against triple negative breast cancer via abating Wnt/β-catenin pathway. Int J Biochem Cell Biol. 2018;103:45-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 28. | Wei W, Chua MS, Grepper S, So SK. Soluble Frizzled-7 receptor inhibits Wnt signaling and sensitizes hepatocellular carcinoma cells towards doxorubicin. Mol Cancer. 2011;10:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 79] [Article Influence: 5.6] [Reference Citation Analysis (0)] |