Published online May 15, 2024. doi: 10.4251/wjgo.v16.i5.1869
Peer-review started: January 15, 2024
First decision: February 5, 2024
Revised: February 14, 2024
Accepted: March 28, 2024
Article in press: March 28, 2024
Published online: May 15, 2024
Processing time: 115 Days and 6.7 Hours
Paradoxically, patients with T4N0M0 (stage II, no lymph node metastasis) colon cancer have a worse prognosis than those with T2N1-2M0 (stage III). However, no previous report has addressed this issue.
To screen prognostic risk factors for T4N0M0 colon cancer and construct a prognostic nomogram model for these patients.
Two hundred patients with T4N0M0 colon cancer were treated at Tianjin Medical University General Hospital between January 2017 and December 2021, of which 112 patients were assigned to the training cohort, and the remaining 88 patients were assigned to the validation cohort. Differences between the training and validation groups were analyzed. The training cohort was subjected to multi
The 3-year overall survival (OS) rates were 86.2% and 74.4% for the training and validation cohorts, respectively. Enterostomy (P = 0.000), T stage (P = 0.001), right hemicolon (P = 0.025), irregular review (P = 0.040), and carbohydrate antigen 199 (CA199) (P = 0.011) were independent risk factors of OS in patients with T4N0M0 colon cancer. A nomogram model with good concordance and accuracy was constructed.
Enterostomy, T stage, right hemicolon, irregular review, and CA199 were independent risk factors for OS in patients with T4N0M0 colon cancer. The nomogram model exhibited good agreement and accuracy.
Core Tip: Paradoxically, patients with T4N0M0 (stage II, no lymph node metastasis) colon cancer have a worse prognosis than those with T2N1-2M0 (stage III). However, no previous report has addressed this issue. A total of 200 patients underwent radical surgery with pTNM “T4N0M0” were enrolled in this study. The clinical data and outcomes of the 200 patients were analyzed. We confirmed enterostomy, T stage, right hemicolon, irregular review, carbohydrate antigen 199 were independent risk factors of overall survival by using multivariate analysis. A nomogram model based on these factors was established to predict the prognosis of patients with T4N0M0 colon cancer.
- Citation: Liu B, Zhang ZX, Nie XY, Sun WL, Yan YJ, Fu WH. Clinical outcome and prognostic factors of T4N0M0 colon cancer after R0 resection: A retrospective study. World J Gastrointest Oncol 2024; 16(5): 1869-1877
- URL: https://www.wjgnet.com/1948-5204/full/v16/i5/1869.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i5.1869
Colon cancer is one of the most common malignant tumors worldwide[1]. In recent decades, overall survival (OS) has improved, predominantly owing to improved surgical techniques and advances in chemoradiotherapy, accompanied by the advent of targeted therapy and checkpoint blockade immunotherapy[2]. The prognosis of colon cancer mainly depends on the cancer stage as defined by the Union for International Cancer Control and The American Joint Committee on Cancer (AJCC) TNM staging classification, which is the most widely used staging system for colon cancer. Typically, patients with a higher stage have a worse prognosis than those with a lower stage. Paradoxically, it has been observed that patients with T4N0M0 (stage II, no lymph node metastasis) colon cancer have a worse prognosis than those with T2N1-2M0 (stage III)[3-5].
Patients are tentatively staged as IIB/C according to the 8th AJCC consensus guidelines for colon cancer (primary tumor invading the serosa or surrounding adipose tissue without regional lymph node or distant metastasis)[6]. However, 28.5% of patients die within five years owing to tumor recurrence[7]. Therefore, it is important to screen for risk factors affecting the prognosis of T4N0M0 colon cancer and implement stricter treatment measures for these patients.
Herein, we aimed to explore the clinical outcomes and potential prognostic factors of OS in patients with T4N0M0 colon cancer and then utilize the identified factors to build a nomogram model for predicting OS in these patients.
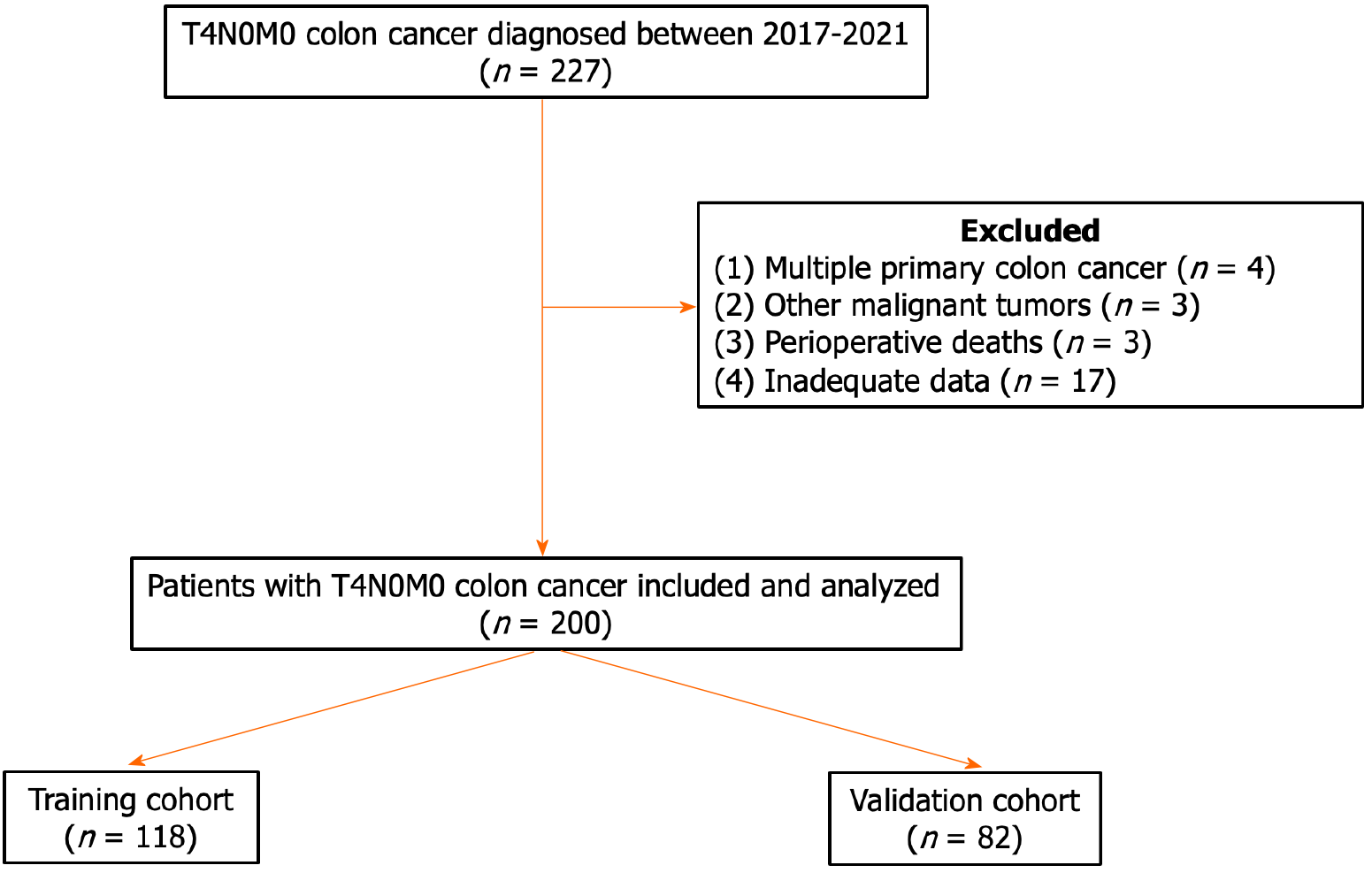
Data were collected from 227 patients with T4N0M0 colon cancer treated at Tianjin Medical University General Hospital between 2017 and 2021. Patients who met the following criteria were included: Primary colon cancer confirmed by postoperative pathology; tumor invasion of the serosa or surrounding adipose tissue without regional lymph node or distant metastasis; and complete clinicopathological data. Patients with (1) multiple primary colon cancers; (2) other types of malignant tumors; (3) perioperative death; or (4) unavailable data were excluded. A total of 200 patients were enrolled in this study (Figure 1) and were subsequently assigned to two groups: The training cohort (n = 112) and the validation cohort (n = 88). This study was approved by The Ethical Committee of Tianjin Medical University General Hospital, No. IRB2023-WZ-205.
Using the inpatient system, the following patient data were collected: Sex, age, preoperative complications, preoperative carcinoembryonic antigen level, preoperative carbohydrate antigen 199 (CA199) level, tumor location, laparotomy/Laparoscopy, anastomosis/enterostomy, tumor size, pathological type, status, and whether regular review.
According to the eighth edition of AJCC TNM classification system (2017)[8], TNM staging was determined by postoperative pathological and preoperative imaging data, such as computed tomography (CT) and magnetic resonance imaging (MRI) (if necessary).
Follow-up included measurement of tumor markers (every 3 months), chest and abdominal CT (every 6 months) or MRI (if necessary), and endoscopy once yearly. OS was calculated as the period from the date of surgery to death from any cause.
Statistical analyses were performed using IBM SPSS Statistics for Windows, version 25.0 (IBM Corp., Armonk, NY, United States). Wilcoxon rank-sum tests, t-tests, and chi-square tests were used to detect differences between the training and validation cohorts. The nomogram model was constructed using the rms package in R Studio version 2022.07.2. The concordance and accuracy of the nomogram model were verified internally and externally. Statistical significance was set at P < 0.05.
Table 1 summarizes the general characteristics of the 200 patients included in the study. No significant differences were observed between training and validation cohorts. The median follow-up time was 32 months (range, 6–55 months) for the training cohort and 30 (7–57) months for the validation cohort. For the training cohort, the 1- and 3-year OS rates were 96.6 and 86.2%, respectively. In the validation cohort, the 1- and 3-year OS rates were 95.1 and 74.4%, respectively.
| Variables | Training cohort, n (%) | Validation cohort, n (%) | P value | |
| All patients | ||||
| 118 (100) | 82 (100) | |||
| Gender | Male | 47 (39.8) | 35 (42.7) | 0.687 |
| Female | 71 (60.2) | 47 (57.3) | ||
| Age | ≥ 75 | 37 (31.4) | 17 (20.7) | 0.096 |
| < 75 | 81 (68.6) | 65 (79.3) | ||
| Obstruction | Yes | 27 (22.9) | 10 (12.2) | 0.056 |
| No | 91 (77.1) | 72 (87.8) | ||
| CEA (ng/mL) | > 5 | 42 (35.6) | 41 (50.0) | 0.042 |
| ≤ 5 | 76 (64.4) | 41 (50.0) | ||
| CA19-9 (U/mL) | > 37 | 15 (12.7) | 13 (15.9) | 0.671 |
| ≤ 37 | 103 (87.3) | 69 (84.1) | ||
| Surgical procedure | Laparotomy | 27 (22.9) | 21 (25.6) | 0.657 |
| Laparoscopy | 91 (77.1) | 61 (74.4) | ||
| Right hemicolon | Yes | 61 (51.7) | 37 (45.1) | 0.360 |
| No | 57 (48.3) | 45 (54.9) | ||
| Enterostomy | Yes | 15 (12.7) | 8 (9.8) | 0.519 |
| No | 103 (87.3) | 74 (90.2) | ||
| Tumor size (cm) | ≥ 6.8 | 25 (21.2) | 25 (30.5) | 0.135 |
| < 6.8 | 93 (78.8) | 57 (69.5) | ||
| Poor differentiated | Yes | 24 (20.3) | 15 (18.3) | 0.719 |
| No | 94 (79.7) | 67 (81.7) | ||
| T stage | T4a | 97 (82.2) | 64 (78.0) | 0.466 |
| T4b | 21 (17.8) | 18 (22.0) | ||
| Lymph node count | ≥ 12 | 101 (85.6) | 70 (85.4) | 0.877 |
| < 12 | 17 (14.4) | 12 (14.6) | ||
| Regular review | Yes | 76 (64.4) | 55 (67.1) | 0.696 |
| No | 42 (35.6) | 27 (32.9) |
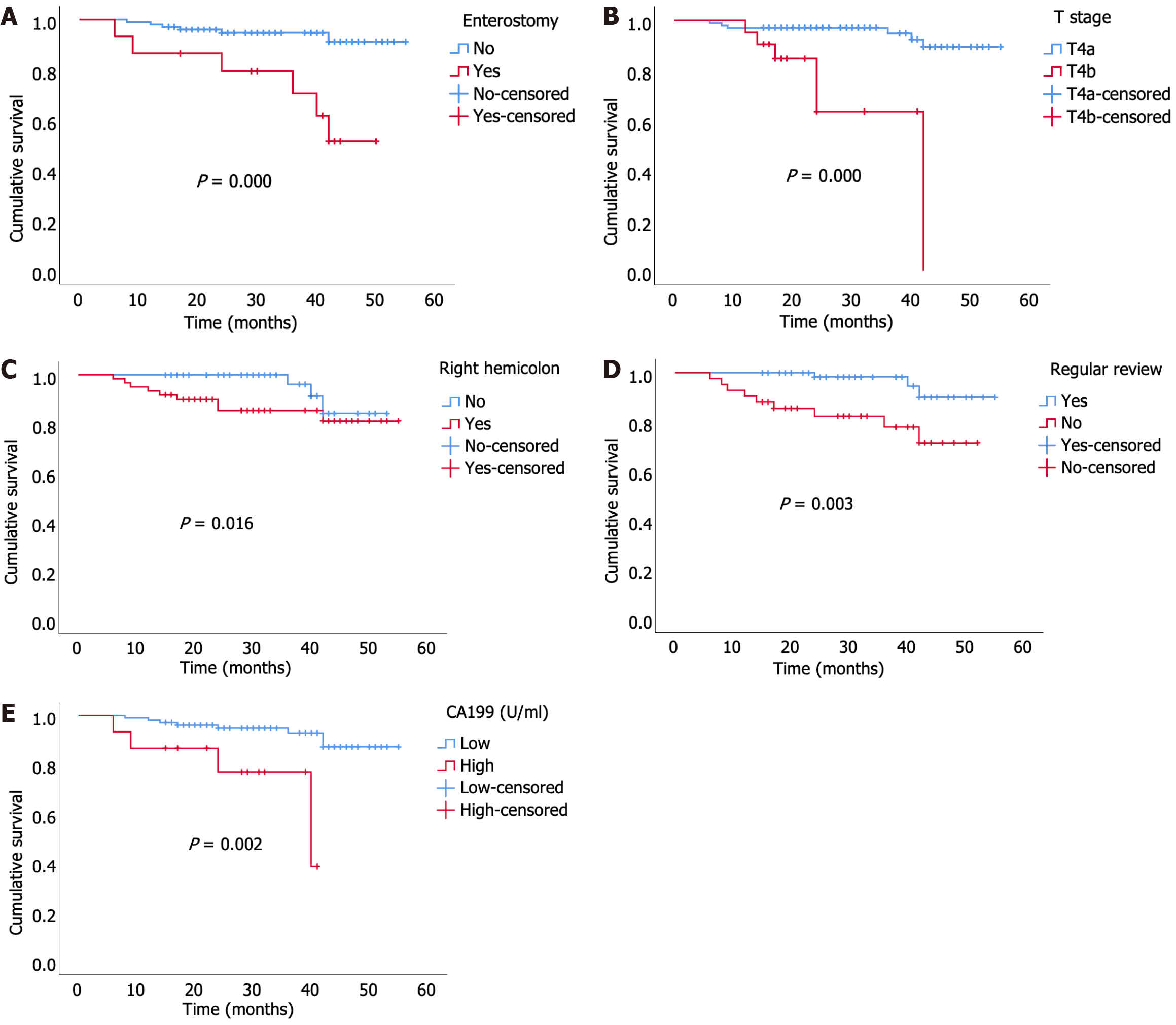
In the univariate analysis, sex (P = 0.033), obstruction (P = 0.014), CA199 (P = 0.002), surgical procedures (P = 0.012), right hemicolon (P = 0.016), enterostomy (P = 0.000), tumor size (P = 0.004), poor differentiation (P = 0.008), T stage (P = 0.000), and irregular review (P = 0.003) were associated with a shorter OS in patients with T4N0M0 colon cancer (Table 2). However, only enterostomy [P = 0.000, hazard ratio (HR) = 13.302 (3.392–52.171)], T stage [P = 0.001, HR = 10.888 (2.809–42.199)], right hemicolon [P = 0.025, HR = 5.236 (1.229–22.308)], irregular review [P = 0.040, HR = 4.626 (1.075–19.905)], and CA199 [P = 0.011, HR = 6.315 (1.520–26.243)] were identified as independent risk factors for OS in the multivariate analysis (Table 2), as shown in the Kaplan-Meier curve in Figure 2.
| Variables | Univariate analysis | P value | Multivariate analysis | P value | |
| HR (95%CI) | HR (95%CI) | ||||
| Gender | Male | 1 | - | - | - |
| Female | 5.755 (0.660, 50.196) | 0.033 | - | - | |
| Age | ≥ 75 | 1.745 (0.198, 15.416) | 0.064 | - | - |
| < 75 | 1 | - | - | - | |
| Obstruction | Yes | 0.785 (0.035, 17.665) | 0.014 | - | - |
| No | 1 | - | - | - | |
| CEA (ng/mL) | > 5 | 1.576 (0.497, 4.994) | 0.434 | - | - |
| ≤ 5 | 1 | - | - | - | |
| CA199 (U/mL) | > 37 | 20.20 (1.881, 216.893) | 0.002 | 6.315 (1.520, 26.243) | 0.011 |
| ≤ 37 | 1 | - | 1 | - | |
| Surgical procedure | Laparotomy | 1.586 (0.130, 19.331) | 0.012 | - | - |
| Laparoscopy | 1 | - | - | - | |
| Right hemicolon | Yes | 2.873 (0.775, 10.656) | 0.016 | 5.236 (1.229, 22.308) | 0.025 |
| No | 1 | - | 1 | - | |
| Enterostomy | Yes | 6.086 (1.949, 19.007) | 0.000 | 13.302 (3.392, 52.171) | 0.000 |
| No | 1 | - | 1 | - | |
| Tumor size (cm) | ≥ 6.8 | 2.252 (0.376, 13.489) | 0.004 | - | - |
| < 6.8 | 1 | - | - | - | |
| Poor differentiated | Yes | 4.311 (1.341, 13.856) | 0.008 | - | - |
| No | 1 | - | - | - | |
| T stage | T4a | 1 | - | 1 | - |
| T4b | 8.241 (2.500, 27.158) | 0.000 | 10.888 (2.809, 42.199) | 0.001 | |
| Lymph node count | ≥ 12 | 1 | - | - | - |
| < 12 | 1.143 (0.250, 5.225) | 0.863 | - | - | |
| Regular review | Yes | 1 | - | 1 | - |
| No | 5.676 (1.534, 21.000) | 0.003 | 4.626 (1.075, 19.905) | 0.040 |
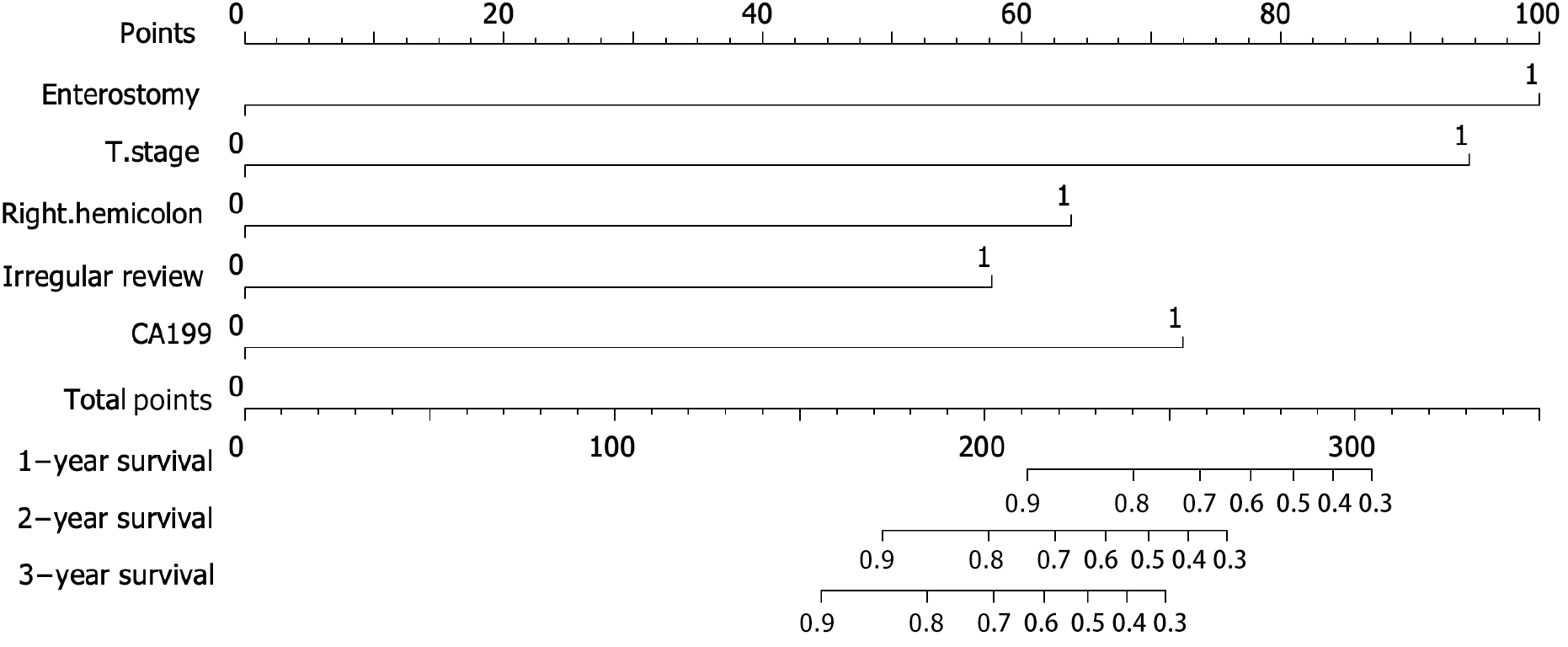
A nomogram model was constructed to predict the prognosis of T4N0M0 colon cancer based on the results of the multivariate Cox regression analyses (Figure 3). The probabilities of 1-, 2- and 3-year OS were predicted by calculating the points of each variable and projecting the total points to the bottom scale.
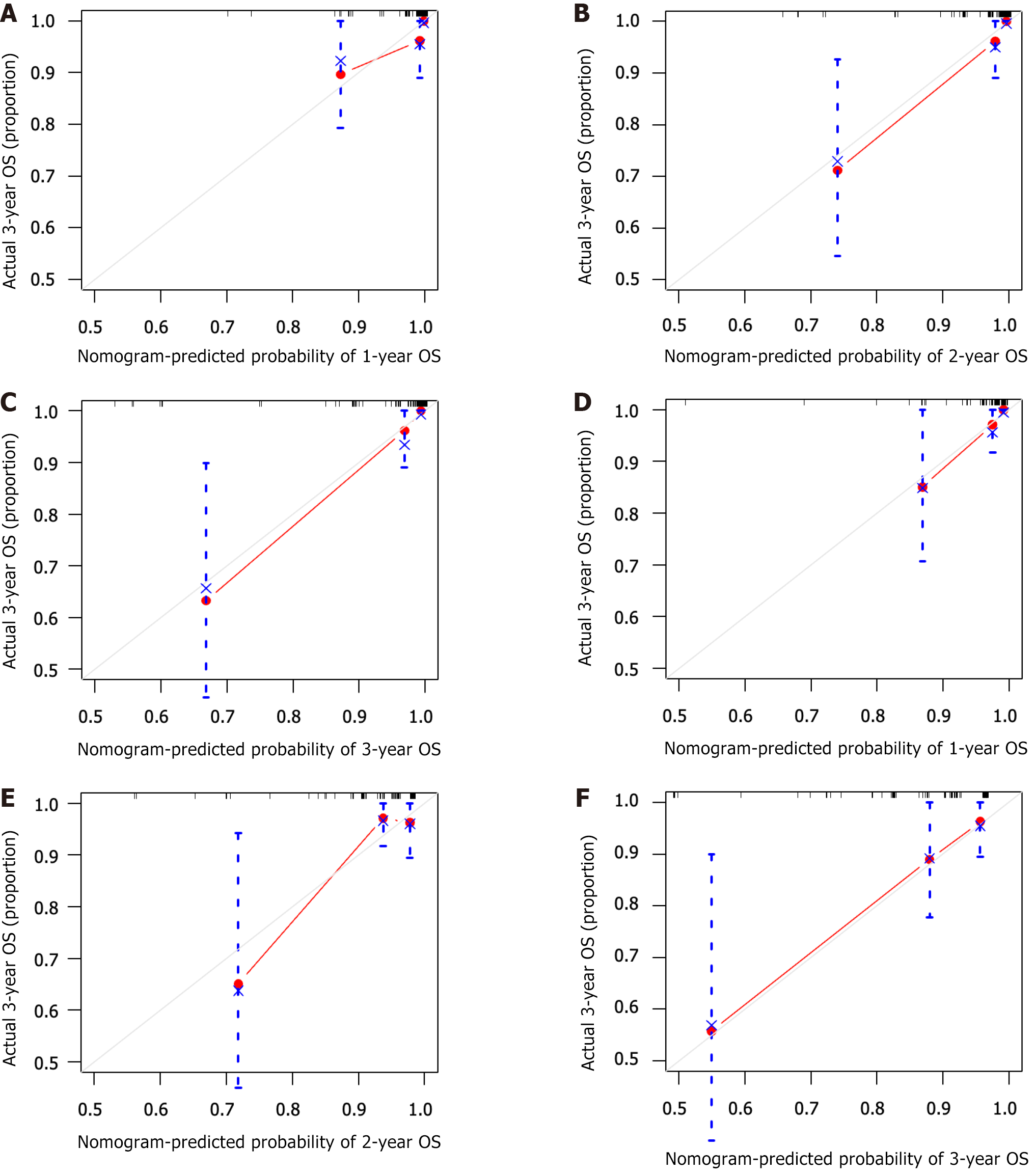
The C-index, representing the predictive ability of the nomogram model for OS, was 0.927 and 0.781 for internal and external validation of the nomogram, indicating good concordance. Both the training and validation cohorts showed good concordance between the predicted and actual 1-, 2- and 3-year OS rates in the calibration curve (Figure 4).
The TNM staging system is widely used to predict the prognosis of colon cancer and has been extensively implemented over the past few years. In general, patients with a higher TNM stage have a worse prognosis than those with a lower stage. Paradoxically, patients with T4N0M0 (stage II, no lymph node metastasis) colon cancer were found to have a worse prognosis than those with T2N1-2M0 (stage III), as shown by data from the Surveillance, Epidemiology, and End Results (SEER) program. Similar results have been reported by the Rectal Cancer Society, Japan Colon Cancer, and other research institutes[9-11]. Therefore, it is crucial to screen for risk factors that can impact the prognosis of T4N0M0 colon cancer and implement stricter treatment measures for these patients. Herein, we found that enterostomy, T stage, right hemicolon, irregular review, and CA199 were independent risk factors for OS in patients with T4N0M0 colon cancer. Moreover, we constructed a nomogram model with good concordance and accuracy using the identified risk factors.
In a study that analyzed 109953 patients with colon cancer from the SEER dataset and End Results dataset, T4a was associated with a more favorable prognosis than T4b[12,13]. Conversely, in 2019, Baguena et al[14] reported that T4a was an independent risk factor for the prognosis of patients with colorectal cancer. Given the paradoxical results for T4a and T4b in different studies, additional factors need to be included in the AJCC TNM staging system[14].
A study from Japan[15] has reported that tumor location was strongly associated with OS in patients who underwent R0 resection for colon cancer. Using data from the National Cancer Database, Narayanan et al[16] identified patients with right- and left-sided colon cancer and revealed that poor OS was associated with right colon cancer at every stage[16]. Taieb et al[17] confirmed that right-sided tumors were more likely to be poorly differentiated, exhibiting more vascular invasion, lymphatic infiltration, microsatellite instability, and BRAF mutations[17], which may contribute to worse OS. As right and left colon cancers differ considerably in terms of clinical and biological characteristics, future clinical trials on colorectal cancer should consider the primary tumor site when determining outcomes[18].
For patients with obstructive colon cancer, enterostomy should be considered when the risk of anastomotic leakage is high, as assessed by the surgeon, or when there is a postoperative anastomotic leak requiring surgical intervention[19-21]. In our study, patients who underwent enterostomy had worse OS than those who underwent anastomosis. However, the adverse effects of enterostomy on patient prognosis have been extensively reported, with dehydration and renal impairment identified as the most common, especially in patients with ileostomy[22]. A meta-analysis has shown that patients with colon cancer who underwent diverting ileostomy and experienced dehydration had worse OS[23]. Furthermore, it has been reported that enterostomies could negatively impact the quality of life, including physical role functioning, social functioning, general health, bodily pain, and vitality[24,25]. Vasilopoulos et al[26] reported that the construction of an ileostomy could impact the patient’s nutritional status, which may deteriorate and result in reduced fluid and food intake[26]. Tripaldi[27] revealed that enterostomy was found to negatively impact sexual function in patients. These adverse outcomes may indirectly result in worse OS.
CA199 is widely used for cancer screening and follow-up in patients with gastrointestinal cancer. Herein, we found that CA199 was an independent risk factor for OS in patients with T4N0M0 colon cancer. Zhou et al[28] found that high preoperative serum CA199 levels were related to worse outcomes in patients with stage III colon cancer[28]. The optimal cutoff value of preoperative CA199 in our study was 37 U/mL, which is consistent with conventional criteria.
Our study found that irregular review was an independent risk factor for OS in patients with T4N0M0 colon cancer. Patients under irregular review had shorter OS than those under regular review, which may be related to the timely detection of risk factors, such as early recurrence of tumors, and taking intervention measures in patients under regular review.
The nomogram model can display independent risk factors that affect the outcome and visually predict survival probability[29,30]. These risk factors were selected through univariate and multivariate analyses[31,32]. Limitations are obvious. This is a single-center study, lacking data from a large multicenter sample. Therefore, more patients with T4N0M0 colon cancer need to be assessed.
Based on our findings, enterostomy, T stage, right hemicolon, irregular review, and CA199 level were identified as independent risk factors of OS. A nomogram model that combines enterostomy, T stage, right hemicolon, irregular review, and CA199 was established to predict the prognosis of patients with T4N0M0 colon cancer. Enterostomy should be performed with strict adherence to the indications.
The authors would like to thank Tianjin Medical University General Hospital for support.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ghannam WM, Egypt; Tangsuwanaruk T, Thailand S-Editor: Fan JR L-Editor: A P-Editor: Zhang XD
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64542] [Article Influence: 16135.5] [Reference Citation Analysis (176)] |
| 2. | Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet. 2019;394:1467-1480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1570] [Cited by in RCA: 3015] [Article Influence: 502.5] [Reference Citation Analysis (3)] |
| 3. | Fang SH, Efron JE, Berho ME, Wexner SD. Dilemma of stage II colon cancer and decision making for adjuvant chemotherapy. J Am Coll Surg. 2014;219:1056-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 4. | Zhang C, Yin S, Tan Y, Huang J, Wang P, Hou W, Zhang Z, Xu H. Patient Selection for Adjuvant Chemotherapy in High-Risk Stage II Colon Cancer: A Systematic Review and Meta-Analysis. Am J Clin Oncol. 2020;43:279-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 5. | Schneider NI, Langner C. Prognostic stratification of colorectal cancer patients: current perspectives. Cancer Manag Res. 2014;6:291-300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | Rahbari NN, Bork U, Motschall E, Thorlund K, Büchler MW, Koch M, Weitz J. Molecular detection of tumor cells in regional lymph nodes is associated with disease recurrence and poor survival in node-negative colorectal cancer: a systematic review and meta-analysis. J Clin Oncol. 2012;30:60-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 127] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 7. | Chen K, Collins G, Wang H, Toh JWT. Pathological Features and Prognostication in Colorectal Cancer. Curr Oncol. 2021;28:5356-5383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 122] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 8. | Tong GJ, Zhang GY, Liu J, Zheng ZZ, Chen Y, Niu PP, Xu XT. Comparison of the eighth version of the American Joint Committee on Cancer manual to the seventh version for colorectal cancer: A retrospective review of our data. World J Clin Oncol. 2018;9:148-161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 46] [Cited by in RCA: 66] [Article Influence: 9.4] [Reference Citation Analysis (1)] |
| 9. | Chu QD, Zhou M, Medeiros K, Peddi P. Positive surgical margins contribute to the survival paradox between patients with stage IIB/C (T4N0) and stage IIIA (T1-2N1, T1N2a) colon cancer. Surgery. 2016;160:1333-1343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 10. | Chu QD, Zhou M, Medeiros KL, Peddi P, Kavanaugh M, Wu XC. Poor survival in stage IIB/C (T4N0) compared to stage IIIA (T1-2 N1, T1N2a) colon cancer persists even after adjusting for adequate lymph nodes retrieved and receipt of adjuvant chemotherapy. BMC Cancer. 2016;16:460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 11. | Huang B, Mo S, Zhu L, Xu T, Cai G. The survival and clinicopathological differences between patients with stage IIIA and stage II rectal cancer: An analysis of 12,036 patients in the SEER database. Oncotarget. 2016;7:79787-79796. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Gunderson LL, Jessup JM, Sargent DJ, Greene FL, Stewart A. Revised tumor and node categorization for rectal cancer based on surveillance, epidemiology, and end results and rectal pooled analysis outcomes. J Clin Oncol. 2010;28:256-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 186] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 13. | Gunderson LL, Jessup JM, Sargent DJ, Greene FL, Stewart AK. Revised TN categorization for colon cancer based on national survival outcomes data. J Clin Oncol. 2010;28:264-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 420] [Cited by in RCA: 437] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 14. | Baguena G, Pellino G, Frasson M, Roselló S, Cervantes A, García-Granero A, Giner F, García-Granero E. Prognostic Impact of pT Stage and Peritoneal Invasion in Locally Advanced Colon Cancer. Dis Colon Rectum. 2019;62:684-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Aoyama T, Kashiwabara K, Oba K, Honda M, Sadahiro S, Hamada C, Maeda H, Mayanagi S, Kanda M, Sakamoto J, Saji S, Yoshikawa T. Clinical impact of tumor location on the colon cancer survival and recurrence: analyses of pooled data from three large phase III randomized clinical trials. Cancer Med. 2017;6:2523-2530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Narayanan S, Gabriel E, Attwood K, Boland P, Nurkin S. Association of Clinicopathologic and Molecular Markers on Stage-specific Survival of Right Versus Left Colon Cancer. Clin Colorectal Cancer. 2018;17:e671-e678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 17. | Taieb J, Kourie HR, Emile JF, Le Malicot K, Balogoun R, Tabernero J, Mini E, Folprecht G, Van Laethem JL, Mulot C, Bouché O, Aparicio T, Michel P, Thaler J, Bridgewater J, Van Cutsem E, Perkins G, Lepage C, Salazar R, Laurent-Puig P; Pan-European Trials in Alimentary Tract Cancer (PETACC)-8 Investigators. Association of Prognostic Value of Primary Tumor Location in Stage III Colon Cancer With RAS and BRAF Mutational Status. JAMA Oncol. 2018;4:e173695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 18. | Lee MS, Menter DG, Kopetz S. Right Versus Left Colon Cancer Biology: Integrating the Consensus Molecular Subtypes. J Natl Compr Canc Netw. 2017;15:411-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 255] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 19. | You YN, Hardiman KM, Bafford A, Poylin V, Francone TD, Davis K, Paquette IM, Steele SR, Feingold DL; On Behalf of the Clinical Practice Guidelines Committee of the American Society of Colon and Rectal Surgeons. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Management of Rectal Cancer. Dis Colon Rectum. 2020;63:1191-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 223] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 20. | Daams F, Luyer M, Lange JF. Colorectal anastomotic leakage: aspects of prevention, detection and treatment. World J Gastroenterol. 2013;19:2293-2297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 107] [Cited by in RCA: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 21. | Rahbari NN, Weitz J, Hohenberger W, Heald RJ, Moran B, Ulrich A, Holm T, Wong WD, Tiret E, Moriya Y, Laurberg S, den Dulk M, van de Velde C, Büchler MW. Definition and grading of anastomotic leakage following anterior resection of the rectum: a proposal by the International Study Group of Rectal Cancer. Surgery. 2010;147:339-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 732] [Cited by in RCA: 1032] [Article Influence: 68.8] [Reference Citation Analysis (4)] |
| 22. | Shabbir J, Britton DC. Stoma complications: a literature overview. Colorectal Dis. 2010;12:958-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 275] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 23. | Borucki JP, Schlaeger S, Crane J, Hernon JM, Stearns AT. Risk and consequences of dehydration following colorectal cancer resection with diverting ileostomy. A systematic review and meta-analysis. Colorectal Dis. 2021;23:1721-1732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 24. | Näsvall P, Dahlstrand U, Löwenmark T, Rutegård J, Gunnarsson U, Strigård K. Quality of life in patients with a permanent stoma after rectal cancer surgery. Qual Life Res. 2017;26:55-64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 145] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 25. | Vonk-Klaassen SM, de Vocht HM, den Ouden ME, Eddes EH, Schuurmans MJ. Ostomy-related problems and their impact on quality of life of colorectal cancer ostomates: a systematic review. Qual Life Res. 2016;25:125-133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 276] [Cited by in RCA: 248] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 26. | Vasilopoulos G, Makrigianni P, Polikandrioti M, Tsiampouris I, Karayiannis D, Margari N, Avramopoulou L, Toulia G, Fasoi G. Pre- and Post-Operative Nutrition Assessment in Patients with Colon Cancer Undergoing Ileostomy. Int J Environ Res Public Health. 2020;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 27. | Tripaldi C. Sexual function after stoma formation in women with colorectal cancer. Br J Nurs. 2019;28:S4-S15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Zhou W, Yang F, Peng J, Wang F, Lin Y, Jiang W, Yang X, Li L, Lu Z, Wan D, Pan Z, Fan W. High pretreatment serum CA19-9 level predicts a poor prognosis for patients with stage III colon cancer after curative resection and adjuvant chemotherapy. J Cancer. 2019;10:3810-3818. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (1)] |
| 29. | Raghav K, Hwang H, Jácome AA, Bhang E, Willett A, Huey RW, Dhillon NP, Modha J, Smaglo B, Matamoros A Jr, Estrella JS, Jao J, Overman MJ, Wang X, Greco FA, Loree JM, Varadhachary GR. Development and Validation of a Novel Nomogram for Individualized Prediction of Survival in Cancer of Unknown Primary. Clin Cancer Res. 2021;27:3414-3421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 30. | Liu S, Yu X, Yang S, Hu P, Hu Y, Chen X, Li Y, Zhang Z, Li C, Lu Q. Machine Learning-Based Radiomics Nomogram for Detecting Extramural Venous Invasion in Rectal Cancer. Front Oncol. 2021;11:610338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 31. | Zhang J, Jin J, Ai Y, Zhu K, Xiao C, Xie C, Jin X. Computer Tomography Radiomics-Based Nomogram in the Survival Prediction for Brain Metastases From Non-Small Cell Lung Cancer Underwent Whole Brain Radiotherapy. Front Oncol. 2020;10:610691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 32. | Zhang W, Gao P, Gao J, Wu X, Liu G, Zhang X. A Clinical Nomogram for Predicting Lymph Node Metastasis in Penile Cancer: A SEER-Based Study. Front Oncol. 2021;11:640036. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |