Published online Apr 15, 2024. doi: 10.4251/wjgo.v16.i4.1578
Peer-review started: October 8, 2023
First decision: December 15, 2023
Revised: December 24, 2023
Accepted: January 23, 2024
Article in press: January 23, 2024
Published online: April 15, 2024
Processing time: 186 Days and 1 Hours
Heat shock proteins (HSPs) are molecular chaperones that play an important role in cellular protection against stress events and have been reported to be overexpressed in many cancers. The prognostic significance of HSPs and their regulatory factors, such as heat shock factor 1 (HSF1) and CHIP, are poorly understood.
To investigate the relationship between HSP expression and prognosis in esophageal and esophagogastric cancer.
A systematic review was conducted in accordance with PRISMA recommendations (PROSPERO: CRD42022370653), on Embase, PubMed, Cochrane, and LILACS. Cohort, case-control, and cross-sectional studies of patients with eso
The final selection comprised 27 studies, including esophageal squamous cell carcinoma (21), esophagogastric adenocarcinoma (5), and mixed neoplasms (1). The pooled sample size was 3465 patients. HSP40 and 60 were associated with a higher 3-year OS [HSP40: RD = 0.22; 95% confidence interval (CI): 0.09-0.35; HSP60: RD = 0.33; 95%CI: 0.17-0.50], while HSF1 was associated with a poor 3-year OS (RD = -0.22; 95%CI: -0.32 to -0.12). The other HSP families were not associated with long-term survival. HSF1 was associated with a higher probability of lymph node metastasis (RD = -0.16; 95%CI: -0.29 to -0.04). HSP40 was associated with a lower probability of lymph node dissemination (RD = 0.18; 95%CI: 0.03-0.33). The expression of other HSP families was not significantly related to tumor depth and lymph node or distant metastasis.
The expression levels of certain families of HSP, such as HSP40 and 60 and HSF1, are associated with long-term survival and lymph node dissemination in patients with esophageal and esophagogastric cancer.
Core Tip: Heat shock proteins (HSPs) and their regulatory factors, such as heat shock factor 1 (HSF1) and CHIP, play an important role in cellular protection against stress events, and are overexpressed in some types or cancer. However, the prognostic significance of HSPs remains unclear. In the present study, we conducted a systematic review and meta-analysis to investigate the relationship between HSP expression and prognosis in esophageal and esophagogastric cancer that included 27 studies. Our findings demonstrated that the expression levels of some families of HSP, such as HSP40 and 60 and HSF1, are associated with long-term survival and lymph node dissemination in esophageal cancer.
- Citation: Nakamura ET, Park A, Pereira MA, Kikawa D, Tustumi F. Prognosis value of heat-shock proteins in esophageal and esophagogastric cancer: A systematic review and meta-analysis. World J Gastrointest Oncol 2024; 16(4): 1578-1595
- URL: https://www.wjgnet.com/1948-5204/full/v16/i4/1578.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i4.1578
Esophageal cancer (EC) is the sixth most common cancer worldwide, with 600000 new cases reported annually, and is the eighth deadliest cancer[1]. Despite strides in therapeutics and screening methods, the prognosis remains grim, with a 5-year survival rate of approximately 15%-25%[2]. Conventional treatments such as chemotherapy, radiation, and surgery often fall short in halting disease progression and recurrence, leaving patients with limited options[3].
Regrettably, practical prognostic factors for EC, crucial for precise diagnosis and therapy, are underexplored[4]. This underscores the urgent need to develop novel prognostic markers for EC, enabling enhanced risk stratification and targeted therapy. While significant headway has been made in diagnosing EC, the pursuit of reliable prognostic indicators persists.
Numerous studies in cancer biology have unveiled a plethora of potential targets, with heat shock proteins (HSPs) emerging as crucial players in protein folding and apoptotic modulation, reported to be overexpressed in esophageal tumors[5]. Initially discovered as proteins robustly induced in response to heat shock and various stressors, HSPs are highly conserved in all mammalian cells[5]. They contribute to protein quality control by ensuring the accurate folding of newly synthesized proteins and the refolding of denatured proteins under various intracellular and extracellular stressor conditions.
HSPs, categorized into six families based on their relative molecular sizes (HSP 20, HSP 40, HSP 60, HSP 70, and HSP 90)[5,6], are regulated by a complex interplay of factors. The swift induction of HSP expression in response to stressors constitutes the heat shock response (HSR)[7], regulated at the transcriptional level by heat shock factors (HSFs), crucial upstream transcriptional regulators of HSPs[8]. HSF1 is recognized as the primary regulator of the HSR, activated by the denaturation of intracellular proteins due to proteotoxic exposures. States of hypoxia, acidosis, and inflammation, for instance, may trigger proteotoxic effects and HSF1 activation.
Several clinical conditions linked to different families of HSP, such as acute and chronic renal diseases[9], psoriasis[10], and neurodegenerative diseases[11], disrupt normal cell functions. Abnormal expression levels of certain HSPs have been revealed in various cancer types, including prostate, bladder, breast, ovarian, colorectal, and lung cancers[12-14]. Some HSP families exhibit a significant correlation with the prognosis of different cancer types[15,16].
The varying expression levels of HSPs during malignant transformation prompt the question of whether HSPs can serve as prognostic indicators in routine clinical settings for EC[17,18]. The objective of our study was to assess the prognostic value of HSPs and their regulatory factors in the context of esophageal and esophagogastric cancer through a comprehensive meta-analysis.
The systematic review and meta-analysis adhered to the PRISMA guidelines[19]. The research protocol underwent registration on PROSPERO[20] (International Prospective Register of Systematic Reviews) with the registration number CRD42022370653.
This meta-analysis considered studies evaluating HSP in patients with esophageal squamous cell carcinoma (ESCC) or esophagogastric adenocarcinoma. Inclusion criteria comprised cohort, case-control, and cross-sectional studies, while case reports, editorials, abstracts, and those without full-text availability were excluded.
The search spanned PubMed, Embase, LILACS (BVS), Cochrane Library Central, and references from included articles, previous systematic reviews, and meta-analyses. A combination of MeSH terms and keywords, such as “Heat Shock Proteins”, “HSP”, “Heat Shock”, “Esophagus”, “Esophageal”, and others, formed the basis of the search. The period covered was from the inception of the databases to March 2023.
Conducted by two independent authors (Nakamura ET and Park A), the systematic literature search followed predetermined eligibility criteria. Discrepancies in study inclusion were resolved by a third reviewer. No language or publication date restrictions were imposed, and selection filters were not applied.
Reviewers (Nakamura ET and Park A) manually retrieved baseline characteristics and outcomes, including author name, publication year, study design, sample size, histologic cancer type, treatment type, HSP family, age, sex, stage, and follow-up.
The Newcastle-Ottawa Scale (NOS) facilitated bias risk assessment, executed by two authors (Nakamura ET and Kikawa D), with adjudication by a third author (Tustumi F) in the case of disagreements.
The analysis encompassed postoperative mortality, postoperative complications, grade of cellular differentiation, lymph node dissemination, tumor depth, metastasis, and survival.
Meta-analysis was performed using STATA 16.1 software (StataCorp LLC). Categorical variables were reported as risk differences (RD), and continuous variables as mean differences (MD), both with a 95% confidence interval (CI). The I² test assessed statistical heterogeneity, and a random-effect model was employed to balance statistical and clinical heterogeneity. Forest plots were utilized for the meta-analysis compilation. Subgroup analysis was used to control histological cancer type as potential confounding variables. We assessed the subgroup of cohorts that evaluated exclusively ESCC.
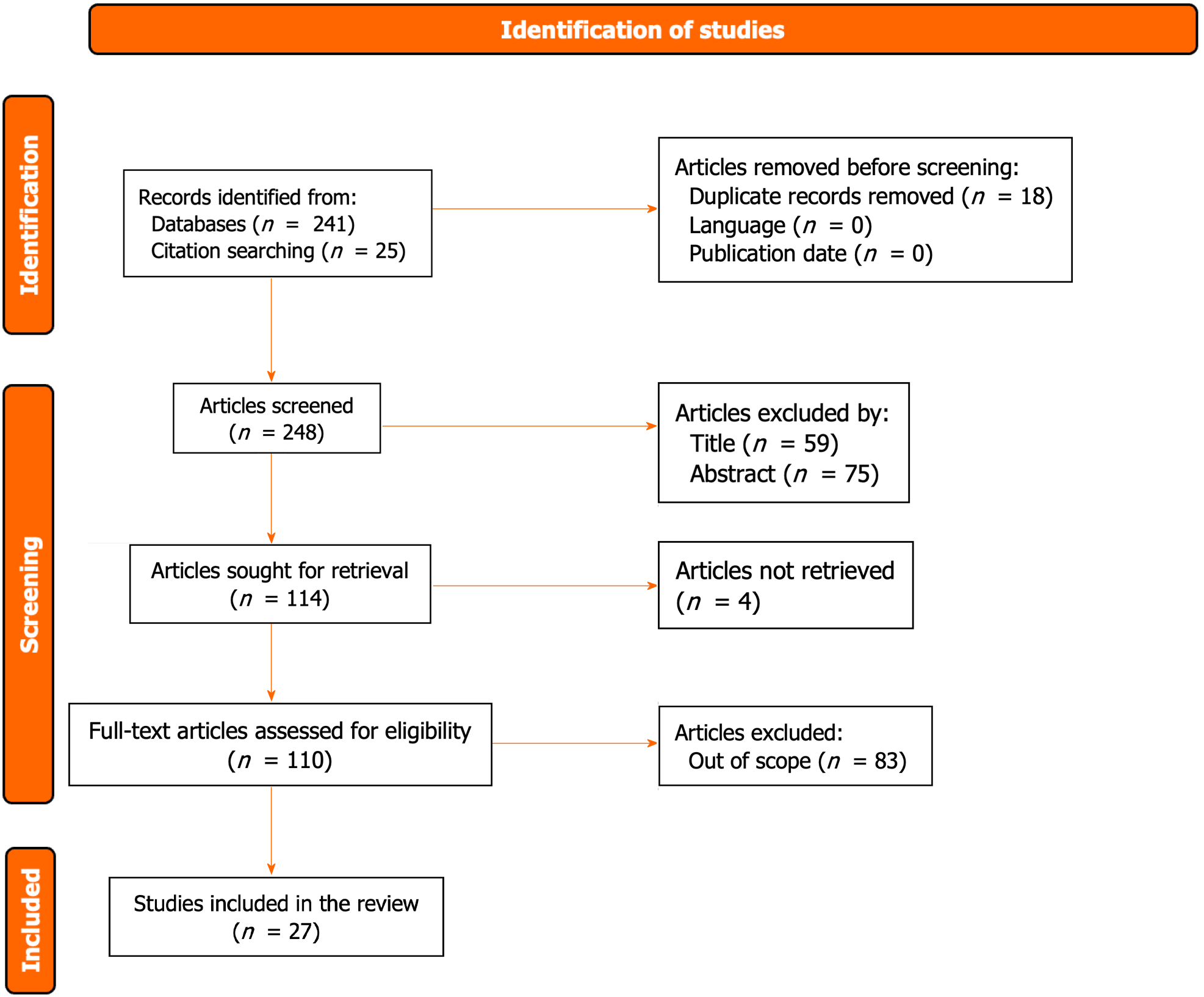
As illustrated in Figure 1, the search flow diagram delineates the identification process, initially yielding 266 primary studies. Following the removal of duplicates and exclusion of articles irrelevant to the meta-analysis, 110 candidate studies underwent full-text review. Subsequently, 27 studies aligning with the inclusion criteria were deemed suitable for the study’s objectives[21-47]. Within this subset, 21 studies focused on ESCC, five on esophagogastric adenocarcinoma, and one on a mixed neoplasm. The cumulative sample size encompassed 3465 patients, with an average age of 60.7 years (range: 46-69) and a predominant male representation (76.9%; range: 38%-91%). A comprehensive summary of the included studies is presented in Table 1.
| Ref. | Cancer type | N | HSP | Age (yr) | Male (%) | Upper third cancer location (%) | Stage III/IV (%) | Follow-up (months) |
| Akutsu et al[21], 2011 | ESCC | 78 | HSP90 | 62 | 89 | 19 | 51 | 24 |
| Berezowska et al[22], 2013 | EA and G | 347 | HSP90 | 69 | 64 | 0 | NI | NI |
| Berg et al[23], 2011 | EA | 87 | HSP20 | 63 | 91 | 0 | NI | NI |
| Doak et al[24], 2004 | EA | 4 | HSP20 | 63 | 83 | 0 | NI | NI |
| Faried et al[25], 2004 | ESCC | 123 | HSP60, 90 | 61 | 86 | 14 | 38 | NI |
| Huang et al[26], 2014 | ESCC | 81 | HSP90 | 58 | 38 | 0 | 30 | NI |
| Iqbal et al[27], 2016 | ESCC | 46 | HSP20, 70, 90 | 58 | 65 | 0 | 16 | NI |
| Kawanishi et al[28], 1999 | ESCC | 102 | HSP20, 70 | 62 | 82 | NI | 36 | 25 |
| Liao et al[29], 2015 | ESCC | 134 | HSF1 | 61 | 81 | NI | 46 | NI |
| Luz et al[30], 2017 | ESCC | 28 | HSP20, 70 | 60 | 82 | NI | NI | 60 |
| Lv et al[31], 2022 | ESCC and EA | 87 | HSP60 | NI | NI | NI | 52 | NI |
| Miyazaki et al[32], 2005 | ESCC | 61 | HSP20, 70 | 65 | 87 | 21 | 49 | 23 |
| Nakajima et al[33], 2002 | ESCC | 62 | HSP20, 70 | 61 | 85 | 13 | 42 | NI |
| Nakajima et al[34], 2009 | EC | 125 | HSP70 | 62 | 86 | 14 | 38 | NI |
| Noguchi et al[35], 2002 | ESCC | 71 | HSP70 | 64 | 89 | 11 | 45 | . |
| Ou et al[36], 2014 | ESCC | 328 | HSP90 | 59 | 82 | NI | NI | 51 |
| Söderström et al[37], 2019 | EA | 151 | HSP20, 70 | 65 | 80 | 0 | 83 | NI |
| Tsukao et al[38], 2017 | ESCC | 212 | HSF1, HSP20, 70, 90 | 65 | 87 | 14 | 54 | NI |
| Wang et al[39], 2007 | G | 60 | HSP70, HSP90 | 46 | 53 | 0 | NI | NI |
| Wang et al[40], 2010 | ESCC | 120 | HSP70, 90 | 57 | 77 | NI | NI | NI |
| Wen et al[41], 2013 | ESCC | 234 | CHIP | 58 | 83 | 10 | NI | 18 |
| Xue et al[42], 2014 | ESCC | 112 | HSP20 | 60 | 54 | NI | NI | NI |
| Yu et al[43], 2015 | ESCC | 72 | HSP40 | 65 | 82 | NI | 66 | NI |
| Zhang et al[44], 2013 | ESCC | 120 | HSP70 | 53 | 75 | 29 | 36 | 60 |
| Zhang et al[45], 2017 | ESCC | 162 | HSP20 | 63 | 67 | NI | NI | NI |
| Zhang et al[46], 2020 | ESCC | 345 | HSP20 | NI | 69 | NI | NI | NI |
| Zhao et al[47], 2015 | ESCC | 113 | HSP70 | 58 | 82 | 20 | 74 | NI |
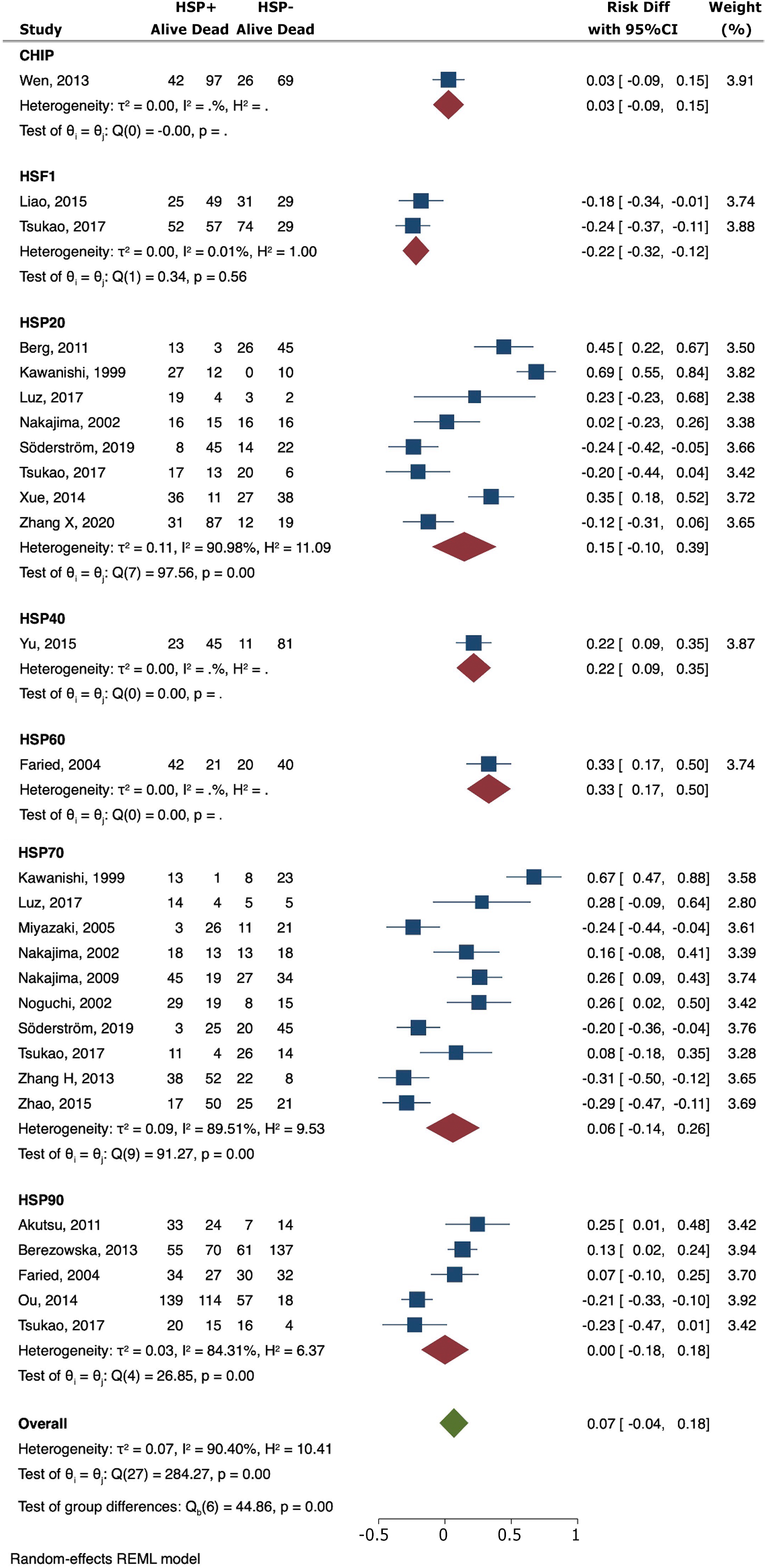
Elevated expression levels of HSP40 (RD = 0.22; 95%CI: 0.09-0.35) and HSP60 (RD = 0.33; 95%CI: 0.17-0.50) were associated with a heightened 3-year overall survival (OS). Conversely, the presence of HSF1 was linked to a poorer 3-year OS (RD = -0.22; 95%CI: -0.32 to -0.12) (Figure 2). Similar findings were observed for ESCC cohorts (Table 2).
| HSP | Overall survivall | Grade of cellular differentiation | T | N | M | |
| CHIP | RD (95%CI) | 0.03 (-0.09 to 0.15) | - | - | 0.00 (0.00-0.00) | - |
| Studies | 1 | - | - | 1 | - | |
| HSF1 | RD (95%CI) | -0.22 (-0.32 to -0.12)1 | 0.04 (-0.09 to 0.17) | -0.22 (-0.51 to 0.06) | -0.16 (-0.29 to -0.04)1 | 0.01 (-0.06 to 0.08) |
| Studies | 2 | 2 | 1 | 2 | 1 | |
| HSP20 | RD (95%CI) | 0.16 (-0.12 to 0.45) | 0.03 (-0.05 to 0.11) | 0.01 (-0.13 to 0.16) | -0.04 (-0.22 to 0.14) | 0.00 (0.15 to -0.15) |
| Studies | 6 | 7 | 6 | 7 | 4 | |
| HSP40 | RD (95%CI) | 0.22 (0.09-0.35)1 | 0.03 (-0.10 to 0.16) | -0.04 (-0.14 to 0.06) | 0.18 (0.03-0.33)1 | 0.03 (-0.05 to 0.11) |
| Studies | 1 | 1 | 1 | 1 | 1 | |
| HSP60 | RD (95%CI) | 0.33 (0.17-0.50)1 | -0.03 (-0.20 to 0.13) | 0.09 (-0.09 to 0.26) | -0.04 (-0.21 to 0.14) | 0.07 (-0.06 to 0.20) |
| Studies | 1 | 1 | 1 | 1 | 1 | |
| HSP70 | RD (95%CI) | 0.07 (-0.18 to 0.31) | -0.08 (-0.17 to 0.00) | -0.07 (-0.25 to 0.11) | -0.17 (-0.45 to 0.12) | -0.10 (-0.33 to 0.12) |
| Studies | 8 | 8 | 6 | 8 | 5 | |
| HSP90 | RD (95%CI) | 0.03 (-0.23 to 0.29) | -0.02 (-0.06 to 0.03) | -0.10 (-0.20 to 0.01) | -0.24 (-0.74 to 0.26) | 0.18 (-0.18 to 0.54) |
| Studies | 3 | 2 | 4 | 3 | 3 |
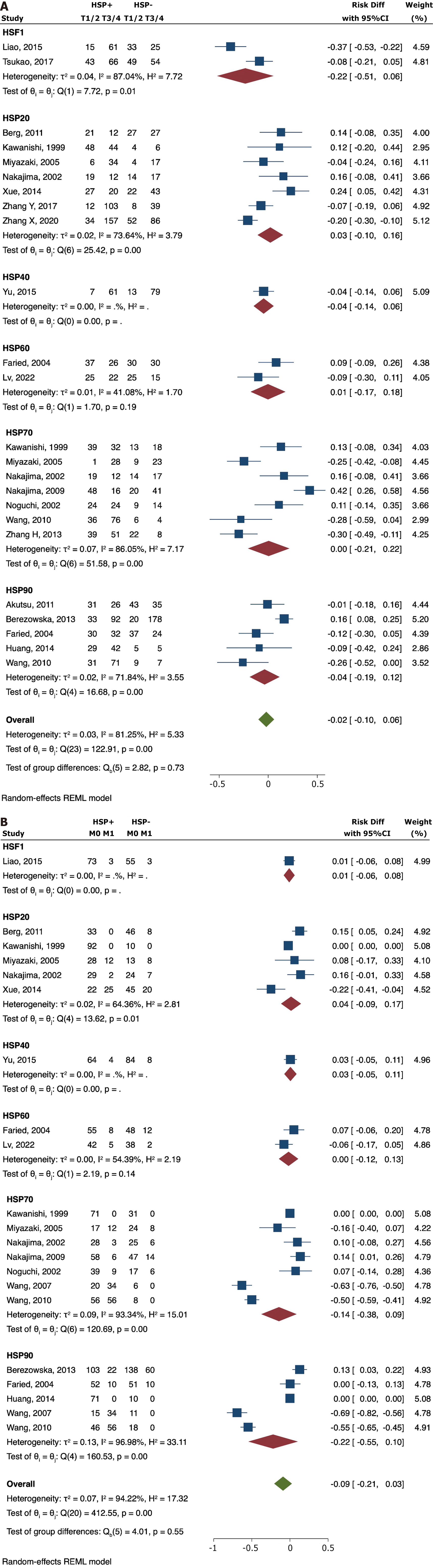
No significant correlation was found between the expression of CHIP (RD = -0.03; 95%CI: -0.15 to 0.09), HSF1 (RD = 0.04; 95%CI: -0.09 to 0.17), HSP20 (RD = -0.09; 95%CI: -0.34 to 0.16), HSP40 (RD = 0.03; 95%CI: -0.10 to 0.16), HSP60 (RD = -0.21; 95%CI: -0.57 to 0.15), HSP70 (RD = -0.14; 95%CI: -0.33 to 0.04), and HSP90 (RD = -0.08; 95%CI: -0.18 to 0.02) with grade of cellular differentiation (Figure 3A).
The results suggested that overexpression of HSF1 (RD = -0.16; 95%CI: -0.29 to -0.04) was significantly associated with positive lymph node metastasis. High HSP40 values were associated with less risk for lymph node dissemination (RD = 0.18; 95%CI: 0.03-0.33). There was no significant difference observed for CHIP (RD = 0.00; 95%CI: 0.00-0.00), HSP20 (RD = 0.05; 95%CI: -0.15-0.24), HSP60 (RD = -0.14; 95%CI: -0.36 to 0.08), HSP70 (RD = -0.20; 95%CI: -0.48 to 0.07), and HSP90 (RD = 0.26; 95%CI: -0.62 to 0.10) (Figure 3B). Similar findings were observed for ESCC cohorts (Table 2).
There was no significant association between tumor depth and HSF1 (RD = -0.22; 95%CI: -0.51 to 0.06), HSP20 (RD =
There was no significant association between distant metastasis and HSF1 (RD = -0.01; 95%CI: -0.06 to 0.08), HSP20 (RD = 0.04; 95%CI: -0.09 to 0.17), HSP40 (RD = 0.03; 95%CI: -0.05 to 0.11), HSP60 (RD = 0.00; 95%CI: -0.12 to 0.13), HSP70 (RD =
All eligible studies underwent a risk of bias assessment with the NOS. Two independent reviewers assessed the quality and risk of bias. In the event of a tie, the decision was determined by a third reviewer after a group discussion in which all sides were taken into account. Points given to each study are shown in Table 3.
| Ref. | Selection of cohorts | Comparability of cohorts | Outcome | |||||
| Representativeness of the exposed cohort | Selection of the non-exposed cohort | Ascertainment of exposure | Demonstration that outcome of interest was not present at start of study | Comparability of cohorts on the basis of the design or analysis | Assessment of outcome | Was follow up long enough for outcomes to occur | Adequacy of follow up of cohorts | |
| Akutsu et al[21], 2011 | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | |
| Berezowska et al[22], 2013 | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | |
| Berg et al[23], 2011 | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | |
| Doak et al[24], 2004 | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | |
| Faried et al[25], 2004 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ||
| Huang et al[26], 2014 | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | ☆ |
| Iqbal et al[27], 2016 | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ||
| Kawanishi et al[28], 1999 | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | ☆ |
| Liao et al[29], 2015 | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | ☆ |
| Luz et al[30], 2017 | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ||
| Lv et al[31], 2022 | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | ☆ |
| Miyazaki et al[32], 2005 | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | ☆ |
| Nakajima et al[33], 2002 | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | ☆ |
| Nakajima et al[34], 2009 | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | ☆ |
| Noguchi et al[35], 2002 | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | ☆ |
| Ou et al[36], 2014 | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | ☆ |
| Söderström et al[37], 2019 | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | ☆ |
| Tsukao et al[38], 2017 | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | ☆ |
| Wang et al[39], 2007 | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ||
| Wang et al[40], 2010 | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ||
| Wen et al[41], 2013 | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | ☆ |
| Xue et al[42], 2014 | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | ☆ |
| Yu et al[43], 2015 | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | ☆ |
| Zhang et al[44], 2013 | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | ☆ |
| Zhang et al[45], 2017 | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ||
| Zhang et al[46], 2020 | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ||
| Zhao et al[47], 2015 | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | ☆ |
The association between HSPs and cancer prognosis has generated significant interest, offering potential implications for clinical decision-making in cancer management. Prognostication, a pivotal aspect of cancer care, can be significantly enhanced through the exploration of HSPs in EC. This investigation holds promise for refining prognostic predictions, tailoring treatment approaches, and ultimately improving patient outcomes[48].
The tumor microenvironment, characterized by conditions such as low glucose, pH, and oxygen levels, induces the expression of HSPs[15]. These molecular chaperones, crucial in apoptosis regulation[49], respond to stressors during carcinogenesis, triggered by the emergence of oncoproteins. However, the diverse functions of HSPs within tumors are influenced by the complex genetic and epigenetic alterations characterizing carcinogenesis[50]. HSPs may play a protective role in the early stages of cancer initiation, such as in chronic esophagitis, yet exhibit different patterns as cancer progresses[5]. For instance, HSP70 exhibits differential expression following thermal injury to the esophageal epithelium, with reduced levels post-injury and subsequent recovery-induced upregulation[51]. This finding highlights the distinct roles of certain HSPs in the context of esophageal injury and recovery in gastroesophageal reflux, known risk factors for Barrett’s esophagus and esophagogastric adenocarcinoma[52]. This nuanced understanding of HSP behavior contributes to the heterogeneous differentiation[53,54] observed within HSPs, with some members associated with aggressive cancer phenotypes and others playing a protective role in cancer development[55].
The present study highlights the overexpression of HSP40 and HSP60, which correlates with higher 3-year OS in EC. Moreover, these HSP families are found overexpressed in various human cancer types beyond EC, including cervical cancer, glioma, skin cancer, lung cancer, colorectal cancer, kidney cancer, and gastric cancer[56-62].
DNAJ, a HSP40 family member[63], plays a crucial role in cellular functions, including stimulating ATPase activity and performing chaperone functions such as protein folding, unfolding, translation, translocation, and degradation[64]. The research conducted by Yu et al[43] on DNAJB6, a nuclear-localized member of the HSP40 family, establishes its independence as a factor associated with better OS in ESCC. Elevated DNAJB6 levels were linked to down regulated AKT signaling and decreased sensitivity to AKT inhibition, providing insights for molecular targeted therapy focusing on oncogene addiction[43]. The prognosis related to HSP40 is, in part, explained by its connection to lymphatic dissemination, as HSP40 overexpression is linked to a lower probability of lymph node metastasis, suggesting a potential association with host immunity and immune-promoting functions[25,33,35].
The HSP60 family serves as an antigen for both B and T-lymphocytes, acting as a ligand for toll-like receptors and playing a pivotal role in immunity[65]. The significance of this family is highlighted by the observation that HSP60 inactivation in mice results in embryonic lethality[66]. Xanthoudakis et al[67] demonstrated that HSP60 facilitates pro-caspase-3 maturation, initiating apoptosis through a Fas-independent pathway. Additionally, HSP60 regulates mitochondrial permeability transition, establishing a cytoprotective network that counters CypD-associated cell death in tumor contexts, where CypD is a component of the mitochondrial permeability transition pore. Furthermore, HSP60 plays a crucial role in protein import and quality control machinery[68,69].
The HSP60 family holds potential as a novel prognostic biomarker in esophageal and esophagogastric cancer[25,31]. The consequences of HSP60 knockdown are substantial, compromising the integrity of respiratory complex I and inducing an excessive production of reactive oxygen species (ROS). This surplus ROS production fuels tumor progression by activating AMP-activated protein kinase, facilitating the acquisition of the Warburg phenotype in HSP60 knockdown cells. Elevated ROS levels may lead to the fragmentation of iron-sulfur clusters, consequently upregulating the expression of ADHFe1. This, in turn, triggers an increase in the production of 2-hydroxyglutarate, impacting DNA methylation and influencing the tumor’s epigenetic landscape[50].
The investigation delved into HSF1 expression and its correlation with lymph node metastasis and diminished 3-year OS in EC. HSF1, a participant in the HSR, plays a multifaceted role in orchestrating molecular changes contributing to malignancy progression[70]. Its activation transforms the tumor microenvironment, promoting processes such as angiogenesis, extracellular matrix (ECM) organization, adhesion, and migration. Elevated HSF1 expression in both tumor and stromal cells significantly correlates with worse disease-free survival and OS in ESCC. Conversely, lower levels of HSF1 activation indicate a more favorable outcome, suggesting its potential as a biomarker for ESCC patient prognosis. In vivo experiments demonstrate that the absence of HSF1 reduces tumor formation, further supporting its role in malignant growth[71]. This transformation occurs through the upregulation of genes promoting the malignant phenotype and the downregulation of genes that might trigger an anticancer immune response[50]. Moreover, HSF1 activation drives specific beneficial pathways within the malignant elements, fostering processes such as angiogenesis, ECM organization, adhesion, and migration[72]. It is plausible that HSF1 is involved in the ESCC microenvironment through the same molecular mechanism. Additionally, stress-damaged proteins, when accumulated in the cytoplasm, recruit HSP70 and HSP90, which bind to HSF1, impeding its activation. Upon activation, HSF1 translocates to the nucleus, binding to the heat shock element sequence in the promoter regions of HSP genes, thereby inducing the expression of inducible HSPs like HSP27, HSP60, HSP70, HSP90, and multidrug resistance 1[73]. Furthermore, HSF1 activation plays a pivotal role in the tumor stroma, especially within cancer-associated fibroblasts. In this context, HSF1 triggers the activation of genes associated with processes such as angiogenesis, ECM remodeling, cellular adhesion, and migration. These molecular alterations collectively contribute to the promotion of malignant growth[74]. In vivo experiments have substantiated this, demonstrating that the absence of HSF1 reduces tumor formation in a mouse model lacking p53[71]. This multifaceted role underscores the potential significance of HSF1 in orchestrating molecular changes contributing to the progression of malignancy, particularly within the context of ESCC. Liao et al[29] demonstrated that the high level of HSF1 expression in both tumor cells and stromal cells was significantly associated with worse disease-free survival and OS of ESCC patients. It was also demonstrated that lower levels of HSF1 activation in both stromal and tumor cells are indicative of a more favorable outcome for patients with ESCC, suggesting the potential of HSF1 activation as a biomarker for ESCC patient prognosis[29]. These findings align with prior research indicating heightened levels of HSF1 expression across diverse cancer types. In a study involving over 1800 participants, nuclear HSF1 levels were elevated in 80% of in situ invasive breast carcinomas[75].
Aligned to the current finding in ESCC, HSF1 has been linked to poor prognosis in various cancer types. Engerud et al[76] established an association between HSF1 overexpression and poor survival after analyzing 823 endometrial cancer lesions. Ishiwata et al[77] similarly demonstrated an association between HSF1 expression and lower OS in oral squamous cell carcinoma. Santagata et al[75] revealed that high HSF1 expression was associated with lymph node invasion in breast cancer patients. Evidence related to cytoskeleton suggests that HSF1 regulates cell motility in esophagogastric adenocarcinoma by binding to the ArgBP2 promoter with the sequence nGAAn[78]. The interaction of HSF1 with MORC2 further mediates invasion and migration in esophagogastric cancer cells by inhibiting ArgBP2, a crucial regulator of cytoskeleton and cellular motility[78]. These findings indicate that the presence of HSF1 influences cell motility, thereby impacting invasion and migration in esophagogastric cancer cells.
The biomarker profile of HSPs has the potential to enhance prognostic stratification accuracy in EC, offering a pathway for personalized medicine and precision therapy-essential components of modern oncology[79]. Targeted therapy, linked to extended OS and reduced treatment costs[80-82], can be optimized by understanding the role of HSPs in cancer development and progression. Breakthroughs in HSP inhibitors and HSP cancer vaccines have been proposed, with studies suggesting their capacity to induce therapeutic resistance against radiotherapy and chemotherapy. HSPs may emerge as crucial targeting molecules for cancer therapy, particularly in esophageal oncology[5]. HSP inhibitors, by targeting key pathways regulated by HSP, have the potential to revolutionize the treatment landscape, inhibiting pro-survival pathways and altering HSP receptor expression[83], thereby reducing malignant transformation and tumor growth[84,85]. Strategies such as genetic removal, stress pathway inhibitors, RNA aptamer insertion, and gene silencing with short hairpin RNA (shRNA) exhibit promising results in inhibiting HSF1 and impeding cancer progression[86,87]. Another practical possibility is the silencing of the HSF1 gene with a shRNA, as shown by Nakamura et al[88], with promising results regarding cancer cell proliferation and activation of apoptotic pathways.
HSPs may also serve as adjuvants for vaccines, as evidenced in experimental models where HSP60-containing exosomes induce a substantial antitumor CD8(+) T cell response[89]. The proinflammatory response elicited by HSP60 in macrophages triggers an adaptive cellular immune reaction, suggesting its potential in enhancing immunotherapy for cancer[72]. Furthermore, HSPs could assist in indicating specific palliative, adjuvant, or neoadjuvant chemotherapy or radiotherapy regimens. Profiling HSPs has the potential to improve precision in EC management, enabling the categorization of patients based on their likelihood of responding to chemotherapy[48]. This knowledge could spare some patients from unnecessary treatment and enhance OS. However, it is crucial to acknowledge that most studies involving HSP inhibitors are limited to preclinical analysis and early-stage trials, and only future research will provide robust evidence for the efficacy of HSP therapies in clinical practice.
Despite these insights, the meta-analysis is subject to limitations. The number of studies conducted for HSP40, HSP60, and HSF1 was relatively small, potentially impacting the overall robustness of the findings. Besides, most of the studies included only ESCC, and few studies assessed the impact of HSP on esophageal adenocarcinoma and esophagogastric junction neoplasms. Although we performed subgroup analysis for ESCC, the same subgroup analysis was not possible for adenocarcinoma due to the small number of articles. Consequently, future studies should investigate the potential value of HSP in cancer prognostication and therapy.
Our systematic review and meta-analysis highlight a significant correlation between the overexpression of HSP40 and 60, and low HSF1 expression, and favorable outcomes, including prolonged survival and diminished lymph node dissemination in individuals with esophageal and esophagogastric cancer. These results underscore the noteworthy prognostic implications of HSPs within the realm of cancer research, suggesting potential avenues for therapeutic interventions. The ongoing exploration of this field offers the prospect of furthering precision medicine and developing targeted strategies for the management of esophageal and esophagogastric cancer.
The association between heat shock proteins (HSPs) and cancer prognosis has generated significant interest, offering potential implications for clinical decision-making in cancer management. HSPs and their regulatory factors, such as heat shock factor (HSF)1 and CHIP, play an important role in cellular protection against stress events, and are overexpressed in some types of cancer.
The prognostic significance of HSPs and their regulatory factors, such as HSF1 and CHIP, are poorly understood in esophageal and esophagogastric cancer.
We conducted a systematic review and meta-analysis to investigate the relationship between HSP expression and prognosis in esophageal and esophagogastric cancer.
A systematic review was conducted in accordance with PRISMA recommendations, on Embase, PubMed, Cochrane, and LILACS. Cohort, case-control, and cross-sectional studies of patients with esophagus or esophagogastric cancer were included. HSP-positive patients were compared with HSP-negative, and the endpoints analyzed were lymph node metastasis, tumor depth, distant metastasis, and overall survival (OS). HSPs were stratified according to the HSP family, and the summary risk difference (RD) was calculated using a random-effect model.
The final selection comprised 27 studies, including esophageal squamous cell carcinoma (21), esophagogastric adenocarcinoma (5), and mixed neoplasms (1). The pooled sample size was 3465 patients. HSP40 and 60 were associated with a higher 3-year OS, while HSF1 was associated with a poor 3-year OS. The other HSP families were not associated with long-term survival. HSF1 was associated with a higher probability of lymph node metastasis. HSP40 was associated with a lower probability of lymph node dissemination. The expression of other HSP families was not significantly related to tumor depth and lymph node or distant metastasis.
Our findings demonstrated that the expression levels of some families of HSP, such as HSP40 and 60 and HSF1, are associated with long-term survival and lymph node dissemination in esophageal and esophagogastric cancer.
The results of this study underscore the noteworthy prognostic implications of HSPs within the realm of cancer research, suggesting potential avenues for therapeutic interventions. The ongoing exploration of this field offers the prospect of furthering precision medicine and developing targeted strategies for the management of esophageal and esophagogastric cancer.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Wang SY, China; Zhang JW, China S-Editor: Wang JJ L-Editor: Webster JR P-Editor: Zhang XD
| 1. | Böhme F, Racz K, Sebesta C Jr, Sebesta C. [Esophageal Cancer]. Wien Med Wochenschr. 2023;173:209-215. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 2. | Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet. 2013;381:400-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1956] [Cited by in RCA: 1957] [Article Influence: 163.1] [Reference Citation Analysis (5)] |
| 3. | Zhou N, Hofstetter WL. Prognostic and therapeutic molecular markers in the clinical management of esophageal cancer. Expert Rev Mol Diagn. 2020;20:401-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Mao Y, Wang Y, Dong L, Zhang Y, Wang C, Zhang Q, Yang S, Cao L, Zhang X, Li X, Fu Z. Hypoxic exosomes facilitate angiogenesis and metastasis in esophageal squamous cell carcinoma through altering the phenotype and transcriptome of endothelial cells. J Exp Clin Cancer Res. 2019;38:389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 92] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 5. | Yun CW, Kim HJ, Lim JH, Lee SH. Heat Shock Proteins: Agents of Cancer Development and Therapeutic Targets in Anti-Cancer Therapy. Cells. 2019;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 191] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 6. | Wu J, Liu T, Rios Z, Mei Q, Lin X, Cao S. Heat Shock Proteins and Cancer. Trends Pharmacol Sci. 2017;38:226-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 482] [Article Influence: 53.6] [Reference Citation Analysis (0)] |
| 7. | Shamovsky I, Nudler E. New insights into the mechanism of heat shock response activation. Cell Mol Life Sci. 2008;65:855-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 130] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 8. | Garrido C, Brunet M, Didelot C, Zermati Y, Schmitt E, Kroemer G. Heat shock proteins 27 and 70: anti-apoptotic proteins with tumorigenic properties. Cell Cycle. 2006;5:2592-2601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 486] [Cited by in RCA: 514] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 9. | Razzaque MS, Taguchi T. Involvement of stress proteins in renal diseases. Contrib Nephrol. 2005;148:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Wang WM, Jin HZ. Heat shock proteins and psoriasis. Eur J Dermatol. 2019;29:121-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 11. | Hunt AP, Minett GM, Gibson OR, Kerr GK, Stewart IB. Could Heat Therapy Be an Effective Treatment for Alzheimer's and Parkinson's Diseases? A Narrative Review. Front Physiol. 2019;10:1556. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 12. | Zou X, Zhang G, Cheng Z, Yin D, Du T, Ju G, Miao S, Liu G, Lu M, Zhu Y. Microvesicles derived from human Wharton's Jelly mesenchymal stromal cells ameliorate renal ischemia-reperfusion injury in rats by suppressing CX3CL1. Stem Cell Res Ther. 2014;5:40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 210] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 13. | Ledford H. Cancer theory faces doubts. Nature. 2011;472:273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 108] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 14. | Calderwood SK, Khaleque MA, Sawyer DB, Ciocca DR. Heat shock proteins in cancer: chaperones of tumorigenesis. Trends Biochem Sci. 2006;31:164-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 663] [Cited by in RCA: 708] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 15. | Ciocca DR, Calderwood SK. Heat shock proteins in cancer: diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones. 2005;10:86-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 962] [Cited by in RCA: 1019] [Article Influence: 53.6] [Reference Citation Analysis (0)] |
| 16. | Lebret T, Watson RW, Molinié V, O'Neill A, Gabriel C, Fitzpatrick JM, Botto H. Heat shock proteins HSP27, HSP60, HSP70, and HSP90: expression in bladder carcinoma. Cancer. 2003;98:970-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 124] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 17. | Wang XW, Shi XH, Tong YS, Cao XF. The Prognostic Impact of Heat Shock Proteins Expression in Patients with Esophageal Cancer: A Meta-Analysis. Yonsei Med J. 2015;56:1497-1502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Kaigorodova EV, Bogatyuk MV. Heat shock proteins as prognostic markers of cancer. Curr Cancer Drug Targets. 2014;14:713-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int J Surg. 2021;88:105906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 728] [Cited by in RCA: 4512] [Article Influence: 1128.0] [Reference Citation Analysis (0)] |
| 20. | Nakamura ET, Tustumi F, Kikawa D, Santos AGE, Silva EIR. Heat-Shock Proteins in Esophageal Cancer: A systematic review and meta-analisis. [cited 27 August 2023]. Available from: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022370653. |
| 21. | Akutsu Y, Matsubara H, Kano M, Usui A, Yoneyama Y, Ikeda N, Komatsu A, Yusup G. Correlation between gp96 expression and the surgical outcome in patients with esophageal squamous cell carcinoma. Ann Surg Oncol. 2011;18:832-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 22. | Berezowska S, Novotny A, Bauer K, Feuchtinger A, Slotta-Huspenina J, Becker K, Langer R, Walch A. Association between HSP90 and Her2 in gastric and gastroesophageal carcinomas. PLoS One. 2013;8:e69098. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 23. | Berg D, Wolff C, Langer R, Schuster T, Feith M, Slotta-Huspenina J, Malinowsky K, Becker KF. Discovery of new molecular subtypes in oesophageal adenocarcinoma. PLoS One. 2011;6:e23985. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Doak SH, Jenkins GJ, Parry EM, Griffiths AP, Baxter JN, Parry JM. Differential expression of the MAD2, BUB1 and HSP27 genes in Barrett's oesophagus-their association with aneuploidy and neoplastic progression. Mutat Res. 2004;547:133-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Faried A, Sohda M, Nakajima M, Miyazaki T, Kato H, Kuwano H. Expression of heat-shock protein Hsp60 correlated with the apoptotic index and patient prognosis in human oesophageal squamous cell carcinoma. Eur J Cancer. 2004;40:2804-2811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 67] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 26. | Huang T, Chen S, Han H, Li H, Huang Z, Zhang J, Yin Q, Wang X, Ma X, Dai P, Duan D, Zou F, Chen X. Expression of Hsp90α and cyclin B1 were related to prognosis of esophageal squamous cell carcinoma and keratin pearl formation. Int J Clin Exp Pathol. 2014;7:1544-1552. [PubMed] |
| 27. | Iqbal MK, Zargar MA, Mudassar S, Lone GN, Yaseen SB, Andrabi KI. Expression Profiling and Cellular Localization of Stress Responsive Proteins in Squamous Cell Carcinoma of Human Esophagus. Cancer Invest. 2016;34:237-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 28. | Kawanishi K, Shiozaki H, Doki Y, Sakita I, Inoue M, Yano M, Tsujinaka T, Shamma A, Monden M. Prognostic significance of heat shock proteins 27 and 70 in patients with squamous cell carcinoma of the esophagus. Cancer. 1999;85:1649-1657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 29. | Liao Y, Xue Y, Zhang L, Feng X, Liu W, Zhang G. Higher heat shock factor 1 expression in tumor stroma predicts poor prognosis in esophageal squamous cell carcinoma patients. J Transl Med. 2015;13:338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 30. | Luz CC, Noguti J, Borges de Araújo L, Gianni MS, Simão Gomes T, Ricardo AN. Hsp27 and Hsp70 Expression in Esophageal Squamous. Asian Pac J Cancer Prev. 2017;18:789-794. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 31. | Lv J, Wang XW, Sun XK, Yang JR, Chen PR. High Expression of Heat Shock Protein Family D Member 1 Predicts Poor Prognosis of Esophageal Cancer. J Clin Med Res. 2022;14:273-281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 32. | Miyazaki T, Kato H, Faried A, Sohda M, Nakajima M, Fukai Y, Masuda N, Manda R, Fukuchi M, Ojima H, Tsukada K, Kuwano H. Predictors of response to chemo-radiotherapy and radiotherapy for esophageal squamous cell carcinoma. Anticancer Res. 2005;25:2749-2755. [PubMed] |
| 33. | Nakajima M, Kuwano H, Miyazaki T, Masuda N, Kato H. Significant correlation between expression of heat shock proteins 27, 70 and lymphocyte infiltration in esophageal squamous cell carcinoma. Cancer Lett. 2002;178:99-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 34. | Nakajima M, Kato H, Miyazaki T, Fukuchi M, Masuda N, Fukai Y, Sohda M, Ahmad F, Kuwano H. Tumor immune systems in esophageal cancer with special reference to heat-shock protein 70 and humoral immunity. Anticancer Res. 2009;29:1595-1606. [PubMed] |
| 35. | Noguchi T, Takeno S, Shibata T, Uchida Y, Yokoyama S, Müller W. Expression of heat shock protein 70 in grossly resected esophageal squamous cell carcinoma. Ann Thorac Surg. 2002;74:222-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 36. | Ou Y, Liu L, Xue L, Zhou W, Zhao Z, Xu B, Song Y, Zhan Q. TRAP1 shows clinical significance and promotes cellular migration and invasion through STAT3/MMP2 pathway in human esophageal squamous cell cancer. J Genet Genomics. 2014;41:529-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 37. | Söderström HK, Kauppi JT, Oksala N, Paavonen T, Krogerus L, Räsänen J, Rantanen T. Overexpression of HSP27 and HSP70 is associated with decreased survival among patients with esophageal adenocarcinoma. World J Clin Cases. 2019;7:260-269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 38. | Tsukao Y, Yamasaki M, Miyazaki Y, Makino T, Takahashi T, Kurokawa Y, Miyata H, Nakajima K, Takiguchi S, Mimori K, Mori M, Doki Y. Overexpression of heat-shock factor 1 is associated with a poor prognosis in esophageal squamous cell carcinoma. Oncol Lett. 2017;13:1819-1825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 39. | Wang XP, Wang QX, Ying XP. Correlation between clinicopathology and expression of heat shock protein 72 and glycoprotein 96 in human gastric adenocarcinoma. Tohoku J Exp Med. 2007;212:35-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 40. | Wang X, Wang Q, Lin H. Correlation between clinicopathology and expression of heat shock protein 72 and glycoprotein 96 in human esophageal squamous cell carcinoma. Clin Dev Immunol. 2010;2010:212537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 41. | Wen J, Luo KJ, Hu Y, Yang H, Fu JH. Metastatic lymph node CHIP expression is a potential prognostic marker for resected esophageal squamous cell carcinoma patients. Ann Surg Oncol. 2013;20:1668-1675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 42. | Xue L, Yang L, Jin ZA, Gao F, Kang JQ, Xu GH, Liu B, Li H, Wang XJ, Liu LJ, Wang BL, Liang SH, Ding J. Increased expression of HSP27 inhibits invasion and metastasis in human esophageal squamous cell carcinoma. Tumour Biol. 2014;35:6999-7007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 43. | Yu VZ, Wong VC, Dai W, Ko JM, Lam AK, Chan KW, Samant RS, Lung HL, Shuen WH, Law S, Chan YP, Lee NP, Tong DK, Law TT, Lee VH, Lung ML. Nuclear Localization of DNAJB6 Is Associated With Survival of Patients With Esophageal Cancer and Reduces AKT Signaling and Proliferation of Cancer Cells. Gastroenterology. 2015;149:1825-1836.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 44. | Zhang H, Chen W, Duan CJ, Zhang CF. Overexpression of HSPA2 is correlated with poor prognosis in esophageal squamous cell carcinoma. World J Surg Oncol. 2013;11:141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 45. | Zhang Y, Feng Z, Wang W, Dong J, Gong X, Pu H, Chen X. Expression of Heat Shock Protein-27 (Hsp27) and P38MAPK in Esophageal Squamous Cell Carcinoma. Med Sci Monit. 2017;23:5246-5253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 46. | Zhang X, Liu T, Zheng S, Liu Q, Shen T, Han X, Zhang Q, Yang L, Lu X. SUMOylation of HSP27 regulates PKM2 to promote esophageal squamous cell carcinoma progression. Oncol Rep. 2020;44:1355-1364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 47. | Zhao G, Kang J, Jiao K, Xu G, Yang L, Tang S, Zhang H, Wang Y, Nie Y, Wu K, Fan D, Zhang D. High Expression of GRP78 Promotes Invasion and Metastases in Patients with Esophageal Squamous Cell Carcinoma. Dig Dis Sci. 2015;60:2690-2699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 48. | Tustumi F, Agareno GA, Galletti RP, da Silva RBR, Quintas JG, Sesconetto LA, Szor DJ, Wolosker N. The Role of the Heat-Shock Proteins in Esophagogastric Cancer. Cells. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 49. | Jolly C, Morimoto RI. Role of the heat shock response and molecular chaperones in oncogenesis and cell death. J Natl Cancer Inst. 2000;92:1564-1572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 756] [Cited by in RCA: 774] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 50. | Tang H, Chen Y, Liu X, Wang S, Lv Y, Wu D, Wang Q, Luo M, Deng H. Downregulation of HSP60 disrupts mitochondrial proteostasis to promote tumorigenesis and progression in clear cell renal cell carcinoma. Oncotarget. 2016;7:38822-38834. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 51. | Dutta SM, Mustafi SB, Raha S, Chakraborty SK. Assessment of thermal stress adaptation by monitoring Hsp70 and MnSOD in the freshwater gastropod, Bellamya bengalensis (Lamark 1882). Environ Monit Assess. 2014;186:8961-8967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 52. | Hamel C, Ahmadzai N, Beck A, Thuku M, Skidmore B, Pussegoda K, Bjerre L, Chatterjee A, Dennis K, Ferri L, Maziak DE, Shea BJ, Hutton B, Little J, Moher D, Stevens A. Screening for esophageal adenocarcinoma and precancerous conditions (dysplasia and Barrett's esophagus) in patients with chronic gastroesophageal reflux disease with or without other risk factors: two systematic reviews and one overview of reviews to inform a guideline of the Canadian Task Force on Preventive Health Care (CTFPHC). Syst Rev. 2020;9:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 53. | Villaseca MA, Roa I, Araya JC, Roa JC, Flores P. Double immunostaining for p53 and molecular chaperone hsp72/73 in gastric carcinoma. Mol Pathol. 1997;50:317-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 54. | Dorsey WC, Tchounwou PB. CYP1a1, HSP70, P53, and c-fos expression in human liver carcinoma cells (HepG2) exposed to pentachlorophenol. Biomed Sci Instrum. 2003;39:389-396. [PubMed] |
| 55. | Uno Y, Kanda M, Miwa T, Umeda S, Tanaka H, Tanaka C, Kobayashi D, Suenaga M, Hattori N, Hayashi M, Yamada S, Nakayama G, Fujiwara M, Kodera Y. Increased Expression of DNAJC12 is Associated with Aggressive Phenotype of Gastric Cancer. Ann Surg Oncol. 2019;26:836-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 56. | Castle PE, Ashfaq R, Ansari F, Muller CY. Immunohistochemical evaluation of heat shock proteins in normal and preinvasive lesions of the cervix. Cancer Lett. 2005;229:245-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 57. | Canamasas I, Debes A, Natali PG, Kurzik-Dumke U. Understanding human cancer using Drosophila: Tid47, a cytosolic product of the DnaJ-like tumor suppressor gene l2Tid, is a novel molecular partner of patched related to skin cancer. J Biol Chem. 2003;278:30952-30960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 58. | Trentin GA, He Y, Wu DC, Tang D, Rozakis-Adcock M. Identification of a hTid-1 mutation which sensitizes gliomas to apoptosis. FEBS Lett. 2004;578:323-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 59. | Oka M, Sato S, Soda H, Fukuda M, Kawabata S, Nakatomi K, Shiozawa K, Nakamura Y, Ohtsuka K, Kohno S. Autoantibody to heat shock protein Hsp40 in sera of lung cancer patients. Jpn J Cancer Res. 2001;92:316-320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 60. | Kanazawa Y, Isomoto H, Oka M, Yano Y, Soda H, Shikuwa S, Takeshima F, Omagari K, Mizuta Y, Murase K, Nakagoe T, Ohtsuka K, Kohno S. Expression of heat shock protein (Hsp) 70 and Hsp 40 in colorectal cancer. Med Oncol. 2003;20:157-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 61. | Teng R, Liu Z, Tang H, Zhang W, Chen Y, Xu R, Chen L, Song J, Liu X, Deng H. HSP60 silencing promotes Warburg-like phenotypes and switches the mitochondrial function from ATP production to biosynthesis in ccRCC cells. Redox Biol. 2019;24:101218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 62. | Isomoto H, Oka M, Yano Y, Kanazawa Y, Soda H, Terada R, Yasutake T, Nakayama T, Shikuwa S, Takeshima F, Udono H, Murata I, Ohtsuka K, Kohno S. Expression of heat shock protein (Hsp) 70 and Hsp 40 in gastric cancer. Cancer Lett. 2003;198:219-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 64] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 63. | Chatterjee S, Burns TF. Targeting Heat Shock Proteins in Cancer: A Promising Therapeutic Approach. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 274] [Cited by in RCA: 330] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 64. | Kaida A, Iwakuma T. Regulation of p53 and Cancer Signaling by Heat Shock Protein 40/J-Domain Protein Family Members. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 65. | Lv LH, Wan YL, Lin Y, Zhang W, Yang M, Li GL, Lin HM, Shang CZ, Chen YJ, Min J. Anticancer drugs cause release of exosomes with heat shock proteins from human hepatocellular carcinoma cells that elicit effective natural killer cell antitumor responses in vitro. J Biol Chem. 2012;287:15874-15885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 351] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 66. | Christensen JH, Nielsen MN, Hansen J, Füchtbauer A, Füchtbauer EM, West M, Corydon TJ, Gregersen N, Bross P. Inactivation of the hereditary spastic paraplegia-associated Hspd1 gene encoding the Hsp60 chaperone results in early embryonic lethality in mice. Cell Stress Chaperones. 2010;15:851-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 67. | Xanthoudakis S, Roy S, Rasper D, Hennessey T, Aubin Y, Cassady R, Tawa P, Ruel R, Rosen A, Nicholson DW. Hsp60 accelerates the maturation of pro-caspase-3 by upstream activator proteases during apoptosis. EMBO J. 1999;18:2049-2056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 231] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 68. | Prangenberg J, Doberentz E, Mawick A, Madea B. Mini Review: The Forensic Value of Heat Shock Proteins. Front Med (Lausanne). 2021;8:800100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 69. | Tang H, Li J, Liu X, Wang G, Luo M, Deng H. Down-regulation of HSP60 Suppresses the Proliferation of Glioblastoma Cells via the ROS/AMPK/mTOR Pathway. Sci Rep. 2016;6:28388. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 70. | Wang G, Cao P, Fan Y, Tan K. Emerging roles of HSF1 in cancer: Cellular and molecular episodes. Biochim Biophys Acta Rev Cancer. 2020;1874:188390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 71. | Min JN, Huang L, Zimonjic DB, Moskophidis D, Mivechi NF. Selective suppression of lymphomas by functional loss of Hsf1 in a p53-deficient mouse model for spontaneous tumors. Oncogene. 2007;26:5086-5097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 131] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 72. | Chen W, Syldath U, Bellmann K, Burkart V, Kolb H. Human 60-kDa heat-shock protein: a danger signal to the innate immune system. J Immunol. 1999;162:3212-3219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Reference Citation Analysis (0)] |
| 73. | Vilaboa NE, Galán A, Troyano A, de Blas E, Aller P. Regulation of multidrug resistance 1 (MDR1)/P-glycoprotein gene expression and activity by heat-shock transcription factor 1 (HSF1). J Biol Chem. 2000;275:24970-24976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 104] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 74. | Scherz-Shouval R, Santagata S, Mendillo ML, Sholl LM, Ben-Aharon I, Beck AH, Dias-Santagata D, Koeva M, Stemmer SM, Whitesell L, Lindquist S. The reprogramming of tumor stroma by HSF1 is a potent enabler of malignancy. Cell. 2014;158:564-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 304] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 75. | Santagata S, Hu R, Lin NU, Mendillo ML, Collins LC, Hankinson SE, Schnitt SJ, Whitesell L, Tamimi RM, Lindquist S, Ince TA. High levels of nuclear heat-shock factor 1 (HSF1) are associated with poor prognosis in breast cancer. Proc Natl Acad Sci U S A. 2011;108:18378-18383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 249] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 76. | Engerud H, Tangen IL, Berg A, Kusonmano K, Halle MK, Oyan AM, Kalland KH, Stefansson I, Trovik J, Salvesen HB, Krakstad C. High level of HSF1 associates with aggressive endometrial carcinoma and suggests potential for HSP90 inhibitors. Br J Cancer. 2014;111:78-84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 77. | Ishiwata J, Kasamatsu A, Sakuma K, Iyoda M, Yamatoji M, Usukura K, Ishige S, Shimizu T, Yamano Y, Ogawara K, Shiiba M, Tanzawa H, Uzawa K. State of heat shock factor 1 expression as a putative diagnostic marker for oral squamous cell carcinoma. Int J Oncol. 2012;40:47-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 78. | Tong Y, Li Y, Gu H, Wang C, Liu F, Shao Y, Li F. HSF1, in association with MORC2, downregulates ArgBP2 via the PRC2 family in gastric cancer cells. Biochim Biophys Acta Mol Basis Dis. 2018;1864:1104-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 79. | Malone ER, Oliva M, Sabatini PJB, Stockley TL, Siu LL. Molecular profiling for precision cancer therapies. Genome Med. 2020;12:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 525] [Cited by in RCA: 538] [Article Influence: 107.6] [Reference Citation Analysis (0)] |
| 80. | Chawla A, Janku F, Wheler JJ, Miller VA, Ryan J, Anhorn R, Zhou Z, Signorovitch J. Estimated Cost of Anticancer Therapy Directed by Comprehensive Genomic Profiling in a Single-Center Study. JCO Precis Oncol. 2018;2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 81. | Zhang Q, Fu Q, Bai X, Liang T. Molecular Profiling-Based Precision Medicine in Cancer: A Review of Current Evidence and Challenges. Front Oncol. 2020;10:532403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 82. | Subbiah V, Kurzrock R. Challenging Standard-of-Care Paradigms in the Precision Oncology Era. Trends Cancer. 2018;4:101-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 83. | Cappello F, Conway de Macario E, Marasà L, Zummo G, Macario AJ. Hsp60 expression, new locations, functions and perspectives for cancer diagnosis and therapy. Cancer Biol Ther. 2008;7:801-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 202] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 84. | Soga S, Akinaga S, Shiotsu Y. Hsp90 inhibitors as anti-cancer agents, from basic discoveries to clinical development. Curr Pharm Des. 2013;19:366-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 85. | Sidera K, Patsavoudi E. HSP90 inhibitors: current development and potential in cancer therapy. Recent Pat Anticancer Drug Discov. 2014;9:1-20. [PubMed] |
| 86. | Cheeseman MD, Chessum NE, Rye CS, Pasqua AE, Tucker MJ, Wilding B, Evans LE, Lepri S, Richards M, Sharp SY, Ali S, Rowlands M, O'Fee L, Miah A, Hayes A, Henley AT, Powers M, Te Poele R, De Billy E, Pellegrino L, Raynaud F, Burke R, van Montfort RL, Eccles SA, Workman P, Jones K. Discovery of a Chemical Probe Bisamide (CCT251236): An Orally Bioavailable Efficacious Pirin Ligand from a Heat Shock Transcription Factor 1 (HSF1) Phenotypic Screen. J Med Chem. 2017;60:180-201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 87. | Salamanca HH, Antonyak MA, Cerione RA, Shi H, Lis JT. Inhibiting heat shock factor 1 in human cancer cells with a potent RNA aptamer. PLoS One. 2014;9:e96330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 88. | Nakamura Y, Fujimoto M, Hayashida N, Takii R, Nakai A, Muto M. Silencing HSF1 by short hairpin RNA decreases cell proliferation and enhances sensitivity to hyperthermia in human melanoma cell lines. J Dermatol Sci. 2010;60:187-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 89. | Chen W, Wang J, Shao C, Liu S, Yu Y, Wang Q, Cao X. Efficient induction of antitumor T cell immunity by exosomes derived from heat-shocked lymphoma cells. Eur J Immunol. 2006;36:1598-1607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 158] [Article Influence: 8.3] [Reference Citation Analysis (0)] |