Published online Apr 15, 2024. doi: 10.4251/wjgo.v16.i4.1268
Peer-review started: December 14, 2023
First decision: January 6, 2024
Revised: January 10, 2024
Accepted: March 4, 2024
Article in press: March 4, 2024
Published online: April 15, 2024
Processing time: 118 Days and 17 Hours
Immunotherapy for advanced gastric cancer has attracted widespread attention in recent years. However, the adverse reactions of immunotherapy and its relationship with patient prognosis still need further study. In order to determine the association between adverse reaction factors and prognosis, the aim of this study was to conduct a systematic prognostic analysis. By comprehensively evaluating the clinical data of patients with advanced gastric cancer treated by immunotherapy, a nomogram model will be established to predict the survival status of patients more accurately.
To explore the characteristics and predictors of immune-related adverse reactions (irAEs) in advanced gastric cancer patients receiving immunotherapy with programmed death protein-1 (PD-1) inhibitors and to analyze the correlation between irAEs and patient prognosis.
A total of 140 patients with advanced gastric cancer who were treated with PD-1 inhibitors in our hospital from June 2021 to October 2023 were selected. Patients were divided into the irAEs group and the non-irAEs group according to whether or not irAEs occurred. Clinical features, manifestations, and prognosis of irAEs in the two groups were collected and analyzed. A multivariate logistic regression model was used to analyze the related factors affecting the occurrence of irAEs, and the prediction model of irAEs was established. The receiver operating characteristic (ROC) curve was used to evaluate the ability of different indicators to predict irAEs. A Kaplan-Meier survival curve was used to analyze the correlation between irAEs and prognosis. The Cox proportional risk model was used to analyze the related factors affecting the prognosis of patients.
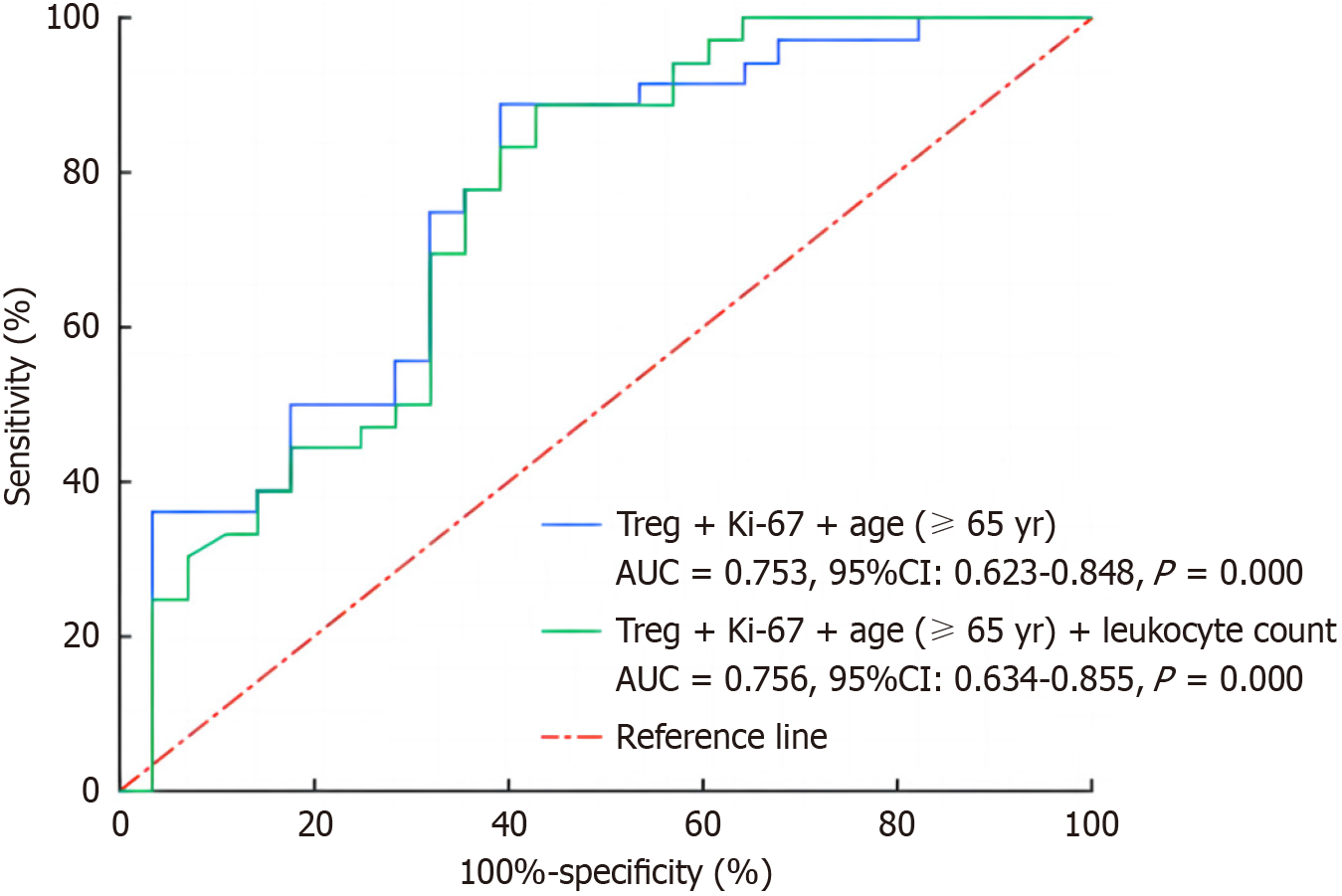
A total of 132 patients were followed up, of whom 63 (47.7%) developed irAEs. We looked at the two groups’ clinical features and found that the two groups were statistically different in age ≥ 65 years, Ki-67 index, white blood cell count, neutrophil count, and regulatory T cell (Treg) count (all P < 0.05). Multivariate logistic regression analysis showed that Treg count was a protective factor affecting irAEs occurrence (P = 0.030). The ROC curve indicated that Treg + Ki-67 + age (≥ 65 years) combined could predict irAEs well (area under the curve = 0.753, 95% confidence interval: 0.623-0.848, P = 0.001). Results of the Kaplan-Meier survival curve showed that progression-free survival (PFS) was longer in the irAEs group than in the non-irAEs group (P = 0.001). Cox proportional hazard regression analysis suggested that the occurrence of irAEs was an independent factor for PFS (P = 0.006).
The number of Treg cells is a separate factor that affects irAEs in advanced gastric cancer patients receiving PD-1 inhibitor immunotherapy. irAEs can affect the patients’ PFS and result in longer PFS. Treg + Ki-67 + age (≥ 65 years old) combined can better predict the occurrence of adverse reactions.
Core Tip: To explore the characteristics and predictors of immune-related adverse reactions (irAEs) in advanced gastric cancer patients receiving immunotherapy with programmed death protein-1 (PD-1) inhibitors, and to analyze the correlation between irAEs and patient prognosis. A total of 132 patients were followed up, of whom 63 (47.7%) developed irAEs. The clinical characteristics of the two groups were compared, and there were statistically significant differences in age ≥ 65 years, Ki-67 index, white blood cell count, neutrophil count, and regulatory T cell (Treg) count between the two groups (all P < 0.05). Multivariate logistic regression analysis showed that Treg count was a protective factor affecting irAEs occurrence (P = 0.030).
- Citation: He XX, Du B, Wu T, Shen H. Prognostic analysis of related factors of adverse reactions to immunotherapy in advanced gastric cancer and establishment of a nomogram model. World J Gastrointest Oncol 2024; 16(4): 1268-1280
- URL: https://www.wjgnet.com/1948-5204/full/v16/i4/1268.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i4.1268
Different to morbidity and mortality worldwide, the incidence of gastric cancer in China ranks second, and mortality due to malignant tumors in China ranks third, which has obvious regional characteristics[1-3]. Most patients with gastric cancer have local or distant metastases at the time of diagnosis, and there is no chance of surgical cure. Their survival can only be prolonged by comprehensive treatment, including palliative chemotherapy or targeted drug therapy[4]. However, the benefits of this type of therapy are limited, with a median survival of only approximately 1 year. In recent years, immunotherapy has achieved good therapeutic effects in many solid tumors, such as lung cancer and kidney cancer, and has also brought new hope for the treatment of patients with stomach cancer[5]. Based on the Attract-02 study, nivolumab has been approved as an indication of third-line treatment for gastric cancer in Japan and South Korea, and the CHECKMATE-649 study will also officially enter the ranks of first-line treatment for gastric cancer chemotherapy combined with immunotherapy[6-8]. Globally, a new supplement for gastric cancer in clinical trials of immunoadjuvant therapy, such as KEYNOTE-585, are ongoing. Programmed death protein-1 (PD-1) inhibitors are mainly used to block the PD-1/programmed death ligand-1 (PD-L1) signaling pathway and exert anti-tumor effects by relieving the inhibition of T cells[9]. The main immune-related adverse reactions (irAEs) of these inhibitors include skin reactions, respiratory system toxicity, gastrointestinal reactions, heart-related reactions, endocrine system toxicity, and so on. At present, irAEs following immunotherapy for gastric cancer is mostly reported in phase I and III clinical studies nationally and internationally[10-12]. However, there is still a certain gap between clinical research and actual clinical application due to strict enrollment conditions. At present, relatively few clinical real-world irAEs data have been reported in the Chinese population, especially in terms of the safety of domestic PD-1 inhibitors.
Advanced gastric cancer is a clinically refractory disease, and traditional treatment has a limited effect on the prognosis of patients. In recent years, immunotherapy as a new treatment method has attracted much attention. However, the relationship between immunotherapy adverse reactions and patient prognosis and its related factors remain a hot topic and a challenge. Studies have demonstrated that immunotherapy has produced specific clinical effects in advanced gastric cancer, but it also results in a number of negative side effects. However, at present, how these adverse reactions affect the prognosis of patients, especially the key factors and mechanisms in the prognosis, has not been fully clarified. In addition, no clinically applicable prognostic assessment model has been established; thus, it is of great clinical significance to explore the influence of factors related to adverse reactions due to immunotherapy on the prognosis of patients with advanced gastric cancer.
Therefore, this study analyzed the occurrence of irAEs in patients with advanced gastric cancer receiving PD-1 inhibitors and its correlation with immune efficacy. A prediction model to analyze the relationship between irAEs and patient prognosis was also created, with a view to providing a theoretical basis for individualized treatment options for such patients.
This retrospective study included 140 patients with advanced gastric cancer who were treated with PD-1 inhibitors in the Oncology Department of our hospital from June 2021 to June 2023. The following clinical characteristics of the patients were recorded: (1) General demographic data, including age (taking into account differences in immune status between older and younger patients); (2) Differences in (with 65 years as the classification limit) sex and number of treatment lines; (3) Basic tumor characteristics, including tumor-node-metastasis (TNM) stage, Ki-67 index, human epidermal growth receptor 2 (Her-2) expression, PD-1/PD-L1 expression, mismatch repair (MMR) protein expression; and (4) Laboratory test indicators before receiving PD-1 inhibitor treatment, tumor markers (alpha-fetoprotein), carcinoembryonic antigen, carbohydrate antigen 19-9 (CA19-9), CA125, CA153, CA724, and cytokeratin fragment antigen 21-1, CA50, CA242, squamous cell carcinoma antigen, neuron-specific enolase, and prostate-specific antigen, endocrine related indicators (thyroid-related hormone, growth hormone, sex hormone 6, cortisol, adrenocorticotropic hormone, amylase), antinuclear antibody expression, routine blood indicators [white blood cell count, neutrophil count, monocyte count, lymphocyte count, C-reactive protein (CRP) level, neutrophil count/lymphocyte count, neutrophil count/CRP, white blood cell count/CRP], lymphocyte subsets [differentiated antiprogenitor 19 (CD19), CD3, CD4, CD8, CD4/CD8, CD56], regulatory T cells (Treg), interferon-α, interleukin-17 (IL-17), tumor necrosis factor-α, IL-2, IL-4, IL-6, IL-8, IL-10.
Follow-up began when the patients received the first immunotherapy and ended on June 30, 2023. During follow-up, irAEs manifestations (including time of occurrence, severity, and prognosis), progression-free survival (PFS), and overall survival (OS) were recorded. Every 2 to 3 treatment cycles, patients were evaluated for immunotreatment-related hematological indicators and imaging of tumor lesions. The patients were divided into the irAEs group and the non-irAEs group according to whether irAEs occurred during the follow-up period.
irAEs were evaluated using the grading and classification criteria of the National Cancer Institute General Adverse Event Terminology (CTCAE) version 4.0. The irAEs were divided into four grades according to severity: (1) Grade 1 was mild, asymptomatic, or mild; no treatment was required, and immunotherapy could be continued; (2) Grade 2 was moderate, requiring local treatment or topical or oral glucocorticoid therapy in the outpatient setting and delayed immunotherapy; (3) Grade 3 was severe or medically important but not life-threatening, requiring hospitalization, systemic glucocorticoid therapy, immunosuppressants if hormone therapy was not satisfactory, and the continuation of immunotherapy should be considered based on the results of the risk/benefit assessment; and (4) Grade 4 was life-threatening and required emergency treatment or permanent discontinuation of immunotherapy, along with systemic use of corticosteroids and other immunosuppressants.
All patients received PD-1 inhibitor immunotherapy via intravenous infusion, with or without chemotherapy or targeted therapy. The Solid Tumor Response Evaluation Criteria (RECIST) version 1.1 was used to rate the outcomes for each patient. These outcomes included the objective response rate (ORR), disease control rate (DCR), partial remission (PR), stable disease (SD), and disease progression (PD).
Possible risk factors for postoperative complications were collected from the preoperative basic data, postoperative course, and postoperative pathological reports of patients, including gender, age, surgical method, resection scope, maximum tumor diameter, pathological grade, and number of lymph node dissections. The χ2 test was used to carry out a single factor analysis for each index. The variables with statistical significance (P < 0.05) were further analyzed by multivariate logistic regression to screen out independent risk factors. The filtering results were introduced into R software (version 3.3.2), and the nomogram model was constructed using the RMS software package.
The nomogram model was evaluated by distinction and calibration curves, respectively. The receiver operating characteristic (ROC) curve was plotted, and the area under the curve (AUC) was calculated to evaluate the differentiation of the model. A calibration curve between the prediction probability of complications and the actual probability of complications was drawn to verify the consistency of the model.
SPSS 23.0 and GraphPad Prism 9.0 were used for data analysis. The normal distribution of quantitative data was tested. If the normal distribution was met, it was expressed as mean ± SD and compared between groups by the Student’s t test. If the normal distribution was not met, it was represented by M (Q1, Q3) and compared between groups by the Mann-Whitney U test. Qualitative data were expressed as frequency (percentage) and analyzed using the χ2 test or Fisher’s exact test.
A multivariate logistic regression model was used to analyze the related factors affecting irAEs, and the prediction model of irAEs was established. The ROC curve was used to verify the predictive ability of the model, and the AUC was calculated. The Hosmer-Lemeshow test was used to evaluate the prediction model’s degree of fit. The Kaplan-Meier survival curve was used to analyze the correlation between irAEs and prognosis. A Cox proportional risk model was used to analyze the prognostic factors. P < 0.05 indicated that the difference was statistically significant.
Of the 140 patients, a total of 132 completed follow-up and were enrolled in the study. During PD-1 inhibitor treatment, 63 patients (47.7%) developed irAEs, and were classified as the irAEs group, and the remaining 69 patients were classified as the non-irAEs group. The clinical characteristics of patients in the 2 groups were analyzed, and the results (Table 1) showed that there were statistically significant differences in age ≥ 65 years, Ki-67 index, white blood cell count, neutrophil count, and Treg count among the groups (P < 0.05).
| Clinical characteristic | irAEs group (n = 63) | Non-irAEs group (n = 69) | P value |
| Age > 65 yr, n | 22 | 37 | 0.030 |
| Gender (male/female) | 43/20 | 46/23 | 0.847 |
| Treatment line (first line/subsequent line), n | 46/17 | 40/29 | 0.071 |
| TNM staging (III/IV) | 6/57 | 4/65 | 0.421 |
| Ki-67 index, % | 49.4 ± 23.3 | 58.9 ± 20.8 | 0.049 |
| Her-2 expression (positive/negative) | 9/54 | 11/58 | 0.791 |
| PD-1/PD-L1 expression (positive/negative), n | 22/21 | 16/17 | 0.818 |
| MMR (pMMR/dMMR), n | 28/2 | 32/4 | 0.535 |
| Tumor marker | |||
| AFP (ng/mL) | 3.3 (2.1, 7.0) | 3.3 (2.5, 4.7) | 0.209 |
| CEA (ng/mL) | 6.0 (2.5, 68.5) | 6.3 (2.3, 21.7) | 0.280 |
| CA19-9 (U/mL) | 20.6 (8.4, 122.9) | 30.0 (9.9, 301.0) | 0.254 |
| CA125 (U/mL) | 31.1 (13.3, 143.5) | 23.3 (10.6, 114.0) | 0.116 |
| CA153 (U/mL) | 12.8 (9.4, 21.2) | 10.9 (7.4, 15.8) | 0.165 |
| CA724 (U/mL) | 5.7 (2.1, 49.7) | 9.1 (4.7, 48.0) | 0.549 |
| CYFRA21-1 (ng/mL) | 5.1 (2.6, 12.4) | 6.7 (3.5, 9.6) | 0.648 |
| CA50 (U/mL) | 17.6 (5.2, 115.6) | 24.6 (6.4, 191.6) | 0.153 |
| CA242 (U/mL) | 6.4 (3.0, 16.4) | 9.9 (4.4, 96.3) | 0.262 |
| SCC (ng/mL) | 1.0 (0.8, 1.7) | 1.2 (0.8, 1.5) | 0.933 |
| NSE (ng/mL) | 11.4 (9.4, 14.6) | 11.5 (9.3, 15.3) | 0.693 |
| PSA (ng/mL) | 0.7 (0.5, 1.5) | 0.8 (0.5, 1.6) | 0.712 |
| Endocrine indicator | |||
| Sex hormone | |||
| Neohombreol (nmol/L) | 7.8 (1.2, 12.0) | 8.5 (1.2, 13.6) | 0.942 |
| Progestin (nmol/L) | 2.0 (0.9, 2.8) | 2.1 (1.4, 3.4) | 0.381 |
| Estradiol (pmol/L) | 84.0 (55.1, 105.0) | 92.0 (72.3, 124.8) | 0.274 |
| Prolactin (ng/mL) | 13.6 (9.7, 19.0) | 13.7 (9.2, 19.7) | 0.812 |
| Folkopoietin (U/L) | 12.8 (7.4, 20.7) | 20.1 (8.1, 39.2) | 0.256 |
| Luteinizing hormone (U/L) | 5.0 (2.8, 11.4) | 6.3 (4.5, 23.3) | 0.456 |
| Cortisol hormone (nmol/L) | 369.5 (313.1, 456.5) | 414.5 (298.6, 465.4) | 0.668 |
| Somatotropic hormone (ng/mL) | 1.5 (0.7, 2.0) | 0.8 (0.2, 2.4) | 0.647 |
| ACTH (pg/mL) | 28.5 (23.5, 39.3) | 29.1 (21.3, 51.7) | 0.269 |
| Amylase (U/L) | 83.5 (63.8, 99.3) | 63.5 (54.8, 75.2) | 0.058 |
| ANA (positive/negative), n | 22/24 | 11/17 | 0.477 |
| Routine blood index | |||
| Leukocyte count (× 109/L) | 5.7 (4.4, 8.3) | 5.4 (4.1, 6.2) | 0.044 |
| Neutrophil count (× 109/L) | 3.8 (2.4, 5.4) | 3.3 (2.3, 4.0) | 0.039 |
| Monocyte count (× 109/L) | 0.5 (0.4, 0.6) | 0.5 (0.4, 0.7) | 0.656 |
| Lymphocyte count (× 109/L) | 1.4 (1.1, 1.6) | 1.3 (1.0, 1.6) | 0.634 |
| CRP (mg/L) | 2.9 (0.5, 11.5) | 1.6 (0.5, 10.3) | 0.569 |
| Neutrophil count/lymphocyte count | 2.8 (2.0, 3.9) | 2.1 (1.6, 3.6) | 0.1 |
| Neutrophil count/CRP | 1.7 (0.4, 4.7) | 2.0 (0.4, 5.0) | 0.712 |
| Leukocyte count/CRP | 2.4 (0.5, 8.5) | 3.3 (0.6, 8.3) | 0.8 |
| Lymphocyte subsets | |||
| CD19, % | 6.5 (4.4, 10.9) | 6.8 (3.9, 9.8) | 0.527 |
| CD3, % | 69.9 (60.6, 76.4) | 67.9 (57.8, 72.8) | 0.175 |
| CD4, % | 37.8 ± 9.1 | 36.1 ± 11.9 | 0.384 |
| CD8, % | 26.1 ± 8.9 | 24.7 ± 10.0 | 0.404 |
| CD4/CD8 | 1.4 (1.1, 2.2) | 1.5 (1.2, 2.4) | 0.37 |
| CD56, % | 19.9 (12.9, 27.1) | 21.0 (14.1, 32.4) | 0.183 |
| Cytokine level | |||
| Treg count, % | 8.3 ± 2.5 | 9.8 ± 3.0 | 0.016 |
| IFN-α (pg/mL) | 1.8 (1.2, 2.3) | 1.5 (0.9, 2.4) | 0.783 |
| IL-17 (pg/mL) | 6.0 (1.3, 9.4) | 3.5 (1.3, 10.0) | 0.666 |
| TNF-α (pg/mL) | 1.8 (1.3, 2.5) | 1.6 (1.0, 3.0) | 0.806 |
| IL-2 (pg/mL) | 1.2 (0.8, 1.5) | 1.2 (0.8, 1.8) | 0.24 |
| IL-4 (pg/mL) | 1.8 (1.0, 3.0) | 1.6 (0.9, 2.5) | 0.349 |
| IL-6 (pg/mL) | 8.1 (4.4, 16.2) | 6.9 (3.8, 12.2) | 0.083 |
| IL-8 (pg/mL) | 46.6 (22.4, 67.3) | 40.0 (19.0, 74.2) | 0.829 |
| IL-10 (pg/mL) | 3.4 (2.2, 4.6) | 2.9 (2.0, 3.9) | 0.299 |
In order to further analyze the relevant factors affecting the occurrence of irAEs, the above five statistically significant variables were included in the multivariate logistic regression analysis, and the results (Table 2) showed that Treg count was a protective factor affecting the occurrence of irAEs (P = 0.030).
| Variable | OR | 95%CI | P value |
| Age ≥ 65 yr | 0.489 | 0.153-1.558 | 0.227 |
| Ki-67 index | 0.985 | 0.958-1.013 | 0.305 |
| Leukocyte count | 0.796 | 0.346-1.799 | 0.574 |
| Neutrophil count | 1.583 | 0.603-4.154 | 0.351 |
| Treg count | 0.796 | 0.647-0.977 | 0.03 |
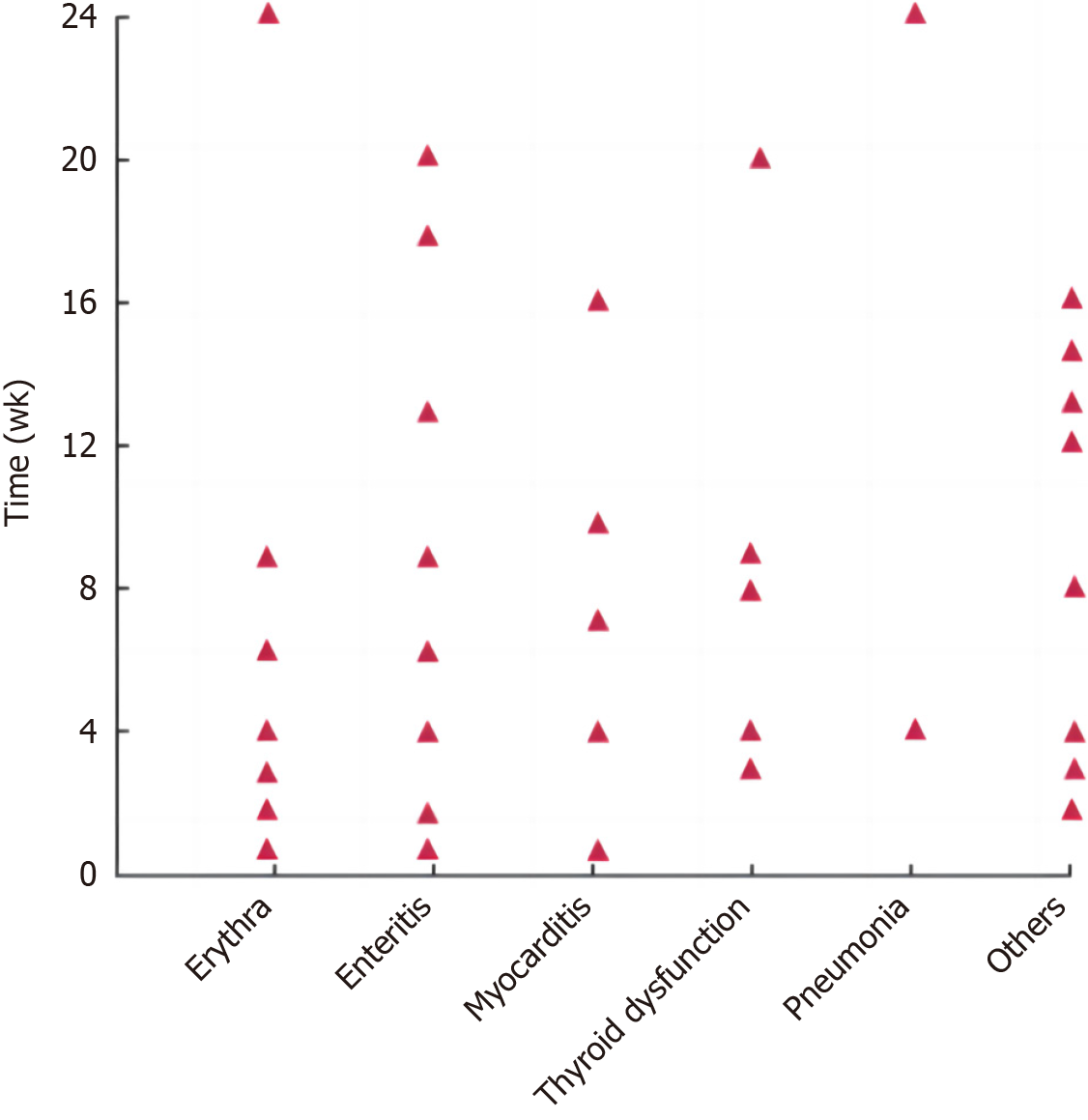
irAEs in patients in the irAEs group occurred more frequently at 1-24 wk after treatment and most frequently at 1-12 wk after treatment. The onset time of different irAEs varied, as shown in Figure 1. In this group, rash (skin reaction) showed the highest incidence. Among them, patients with grade 1 to 2 improved after oral hormone therapy or suspension of related drugs; patients with grade 3 to 4 improved after intravenous hormone therapy; and patients with grade 4 permanently discontinued PD-1 suppressor therapy after treatment remission. The spectrum and classification of adverse reactions in the irAEs group are shown in Table 3.
| irAEs | Incidence, n (%) | Severity, n | |
| Grade 1-2 | Grade 3-4 | ||
| Erythra | 17 (27.0) | 15 | 2 |
| Enteritis | 15 (23.8) | 10 | 5 |
| Myocarditis | 8 (12.7) | 4 | 4 |
| Thyroid dysfunction | 8 (12.7) | 4 | 4 |
| Pneumonia | 3 (4.8) | 3 | 0 |
| Others | 12 (19.0) | 7 | 5 |
Based on the above analysis, we used the ROC curve to verify the predictive power of multiple single factors and their mutual joint indicators in the occurrence of irAEs, and the results are shown in Figure 2. The combination of Treg + age (≥ 65 years) + Ki-67 + white blood cell count and the combination of Treg + age (≥ 65 years) + Ki-67 had good predictive ability (AUC > 0.75), and there was no statistical significance between the two combined indices (P = 0.802). Considering that the combined indicator was composed of fewer factors is more in line with the clinical application needs, and the latter combined indicator was selected to predict the occurrence of irAEs. Subsequently, the Hosmer-Lemeshow test showed that Treg + age (≥ 65 years) + Ki-67, as a joint variable for predicting irAEs, had goodness of fit and could be used as a joint variable for predicting irAEs.
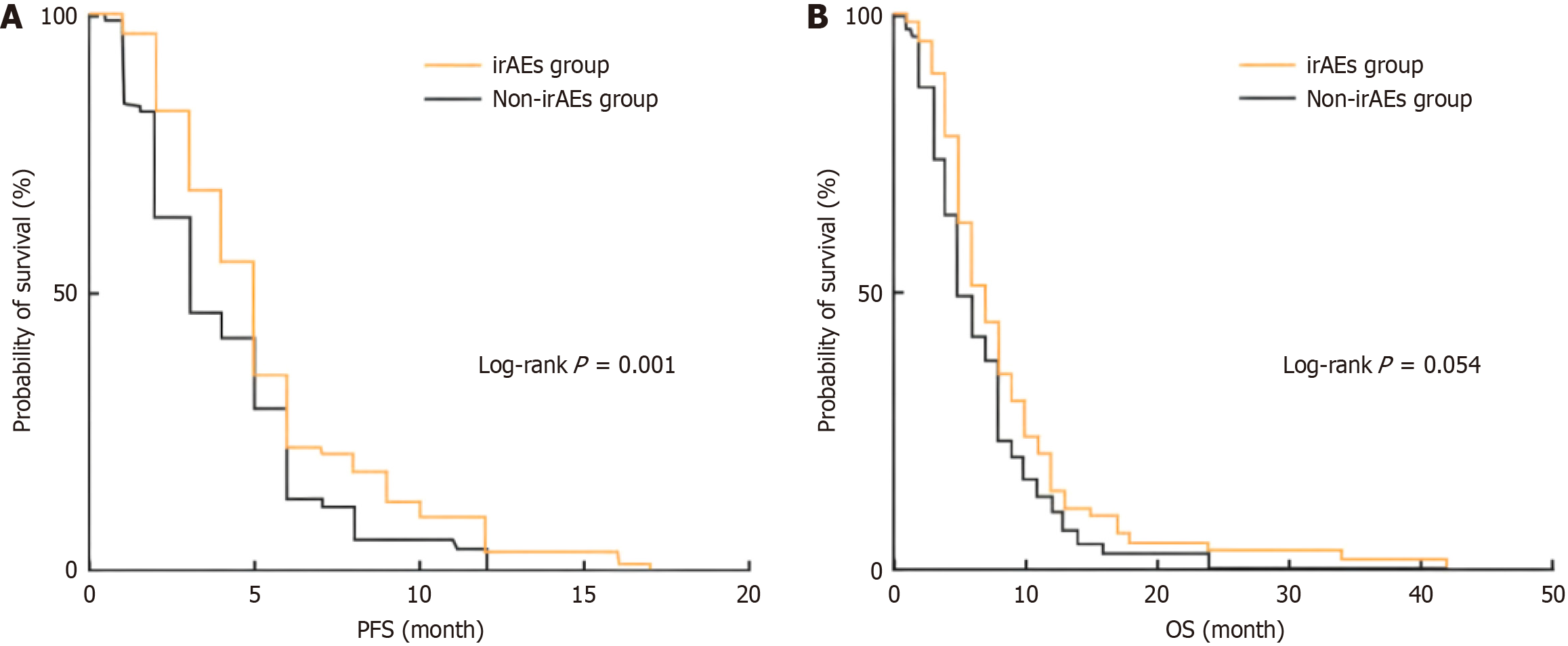
In this study, RECIST version 1.1 was used to evaluate treatment efficacy in all patients, and it was found that 29 patients (22.0%) developed PR, 74 patients (56.0%) developed SD, and 29 patients (22.0%) developed PD. The ORR and DCR of the total population were 22.0% and 78.0%, respectively. The efficacy in the two groups was compared, and the results (Table 4) showed that the differences in PR rate and PD rate between the groups were statistically significant (both P < 0.05). Kaplan-Meier survival analysis was performed for PFS and OS in the two groups, and the results (Figure 3) showed that PFS in the irAEs group was longer than that in the non-irAEs group (P = 0.001), and the median PFS was 5 (5, 6) months and 3 (3, 5) months, respectively. There was no significant difference in OS between the irAEs group and the non-IRAES group (P = 0.054), and the median OS was 7 (6,8) months and 6 (5,7) months, respectively.
| Therapeutic effect | irAEs group (n = 63), n (%) | Non-irAEs group (n = 69), n (%) | χ2/t value | P value |
| PR | 19 (30.1) | 10 (14.5) | 4.679 | 0.031 |
| SD | 35 (55.6) | 39 (56.5) | 0.012 | 0.913 |
| PD | 9 (14.3) | 20 (29.0) | 4.12 | 0.042 |
Subsequently, we selected common factors affecting tumor prognosis (PFS, OS) from the clinical characteristics of patients (such as age ≥ 65 years, gender, number of treatment lines, TNM stage, Her-2 expression and irAEs) for Cox proportional risk analysis. The results (Table 5) showed that irAEs was an influential factor for PFS (P = 0.006). However, no statistically significant factors were found among the influencing factors for OS.
| Variable | Univariate Cox analysis1 | Multivariate Cox analysis1 | Univariate Cox analysis2 | Multivariate Cox analysis2 | ||||
| HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | |
| Age ≥ 65 yr | 0.907 (0.646-1.286) | 0.586 | 0.805 (0.557-1.164) | 0.25 | 1.205 (0.852-1.705) | 0.293 | 1.142 (0.795-1.640) | 0.473 |
| Gender | 0.977 (0.677-1.408) | 0.899 | 0.823 (0.560-1.209) | 0.32 | 0.927 (0.643-1.338) | 0.687 | 0.869 (0.523-1.443) | 0.588 |
| TNM staging | 1.371 (0.715-2.625) | 0.342 | 0.945 (0.651-1.374) | 0.769 | 1.614 (0.816-3.194) | 0.169 | 1.372 (0.666-2.826) | 0.391 |
| Treatment line | 0.794 (0.552-1.141) | 0.213 | 1.591 (0.794-3.186) | 0.19 | 0.992 (0.686-1.433) | 0.965 | 0.924 (0.636-1.341) | 0.677 |
| Her-2 expression | 1.001 (0.620-1.617) | 0.996 | 1.045 (0.634-1.723) | 0.863 | 0.815 (0.495-1.341) | 0.421 | 0.988 (0.678-1.439) | 0.949 |
| irAEs | 0.609 (0.431-0.863) | 0.005 | 0.608 (0.431-0.863) | 0.006 | 0.735 (0.520-1.039) | 0.081 | 0.761 (0.535-1.083) | 0.129 |
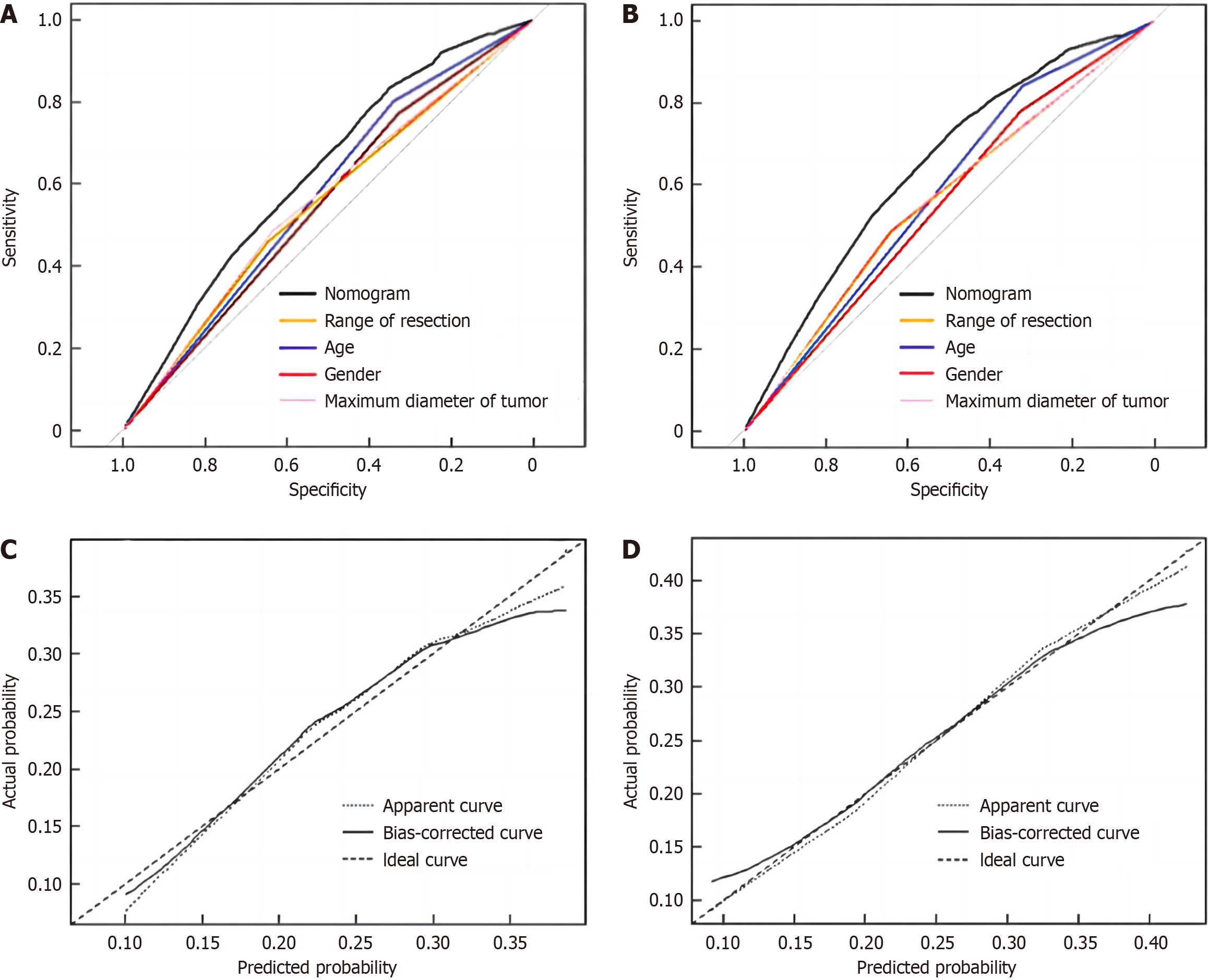
According to the results of multi-factor analysis, R software was used to establish a nomogram model for predicting complications after radical gastrectomy and D2 lymph node dissection (Figure 4). The ROC curve was drawn to evaluate various risk factors and the predictive ability of this model in the training group. The results showed that the AUC of males, age ≥ 60 years, maximum tumor diameter ≥ 5 cm, and total gastrectomy for predicting postoperative complications were 0.550, 0.572, 0.560, and 0.553, respectively, while the AUC of the nomogram model was 0.625, indicating that the nomogram model had better predictive performance than other single-risk factors (Figure 5A).
In the gastric cancer validation group, the nomogram model (AUC = 0.646) also showed better predictive performance and consistency (Figure 5B). The calibration curve showed that the model had good consistency in both the training group and the verification group (Figure 5C and D). The Hosmer-Lemeshow test was used to judge the goodness of fit of the model in the training group and the verification group, respectively. The results showed that the P value of the training group was 0.116 and that of the verification group was 0.961, both P > 0.05, indicating that the nomogram model established in this study had goodness of fit.
Studies have shown that PD-1/PD-L1 inhibitors can block the interaction between the PD-1 receptor on T cells and its ligand, thereby restoring the function of T cells and enhancing their ability to kill tumor cells[13-15]. However, over-activated T cells can cause autoimmune-mediated adverse reactions, resulting in immune damage to various systems and tissues of the body, namely irAEs[16]. At present, there are relatively few studies on irAEs. Based on this, we conducted a retrospective analysis of the occurrence and influencing factors of irAEs in advanced gastric cancer patients receiving PD-1 inhibitors, as well as the relationship between each factor and therapeutic effect[17]. Among the 132 patients analyzed in this study, the incidence of irAEs was 47.7%, which was similar to the results of previous studies, where the incidence of irAEs was about 50%[18-20]. The onset of irAEs is usually associated with the affected organ, ranging from early onset (1 wk after immunotherapy) to delayed event (26 wk after immunotherapy), with the main onset time window ranging from 4 to 12 wk. In this study, it was found that irAEs occurred mostly 1-24 wk after treatment and were most common at 1-12 wk, which was consistent with literature reports. In the irAEs group, rash showed the highest incidence (27.0%), which was similar to that reported in previous studies (30%)[21-23]. The adverse reactions of the patients in this study were mostly grade 1-2 and grade 3 of non-important organs (including rash and thyroid dysfunction), which were alleviated after treatment[24]. Therefore, the toxicity of PD-1 inhibitors in advanced gastric cancer is manageable and safe.
In the comparison of clinical features between groups, we found that age ≥ 65 years, Ki-67, white blood cell count, neutrophil count, and Treg count were related factors affecting the occurrence of irAEs[25]. Multivariate logistic regression analysis showed that Treg count was an independent factor affecting their occurrence. Interestingly, age ≥ 65 years was a protective factor for irAEs, indicating that young people were more likely to develop irAEs[26]. This phenomenon may be related to immune senescence; that is, increased age can lead to increased differentiation of hematopoietic stem cells into myeloid cells, and inhibition of the lymphocyte generation pathway leads to a significant increase in Treg count, thereby reducing the occurrence of irAEs[27-30]. Relevant studies have found that the Ki-67 index is related to tumor infiltrating lymphocytes; that is, the lower the Ki-67 index, the higher tumor infiltrating lymphocytes are, which may increase the occurrence of irAEs. At present, there are few studies on Ki-67 and irAEs, and the relationship between the Ki-67 index and irAEs is not clear; thus, more studies are needed[31]. It is worth noting that in our study, only the white blood cell count and neutrophil count in peripheral blood cells were correlated with the occurrence of irAEs, while lymphocyte count and neutrophil count/lymphocyte count were not correlated with the occurrence of irAEs. This is also related to a term[32]. The results of the study (i.e., increased white blood cell count and neutrophil count in lung and gastrointestinal melanomas were associated with the incidence of irAEs) were consistent. However, some studies have found that the lymphocyte count and neutrophil count/lymphocyte count are also related to the occurrence of irAEs, and the higher the lymphocyte count, the higher the occurrence probability of irAEs[33]. Therefore, the relationship between peripheral blood cells and irAEs still requires further study.
At present, according to CTCAE version 5.0, the severity of irAEs is classified into grades 1 to 4. Most of the patients with mild symptoms of grade 1 to 2 irAEs did not need to terminate immunotherapy, and a small number of patients with severe symptoms of grade 2 irAEs could be treated with oral or intravenous glucocorticoid[34]. For grade 3 to 4 irAEs, especially those with life-threatening grade 4 irAEs, it is recommended that immunotherapy be terminated in accordance with the relevant guidelines. For patients who still have grade 3 to 4 irAEs after systemic treatment, whether they should receive immunotherapy again following recovery to grade 1 is still controversial, and should be used cautiously on the premise of a comprehensive judgment of benefits and risks[35]. A study of patients who had suspended immunotherapy due to the severity of irAEs found that approximately 70% of patients returned to the original grade of irAEs after repeated immunotherapy[36]. Therefore, early detection of irAEs is very important for the treatment strategy and prognosis of patients. Whether irAEs can be predicted early by clinical markers will be a current focus of attention[37]. In the irAEs prediction model constructed in this study, it was found by ROC curve verification that Treg + Ki-67 + age (≥ 65 years) combined had high sensitivity in predicting the occurrence of irAEs[38]. In the future diagnosis and treatment process, this model may be used to assess the risk of patients undergoing immunotherapy; thus, has certain clinical significance. This study analyzed the effect of adverse reactions on the prognosis of patients with advanced gastric cancer during immunotherapy. We found that different types of adverse reactions were significantly correlated with patient prognosis, and some specific adverse reactions were significantly correlated with survival indicators. Most notably, we successfully created a nomogram model based on factors associated with adverse reactions that effectively predicted patient outcomes. The establishment of this model fills the gap in existing research and provides an intuitive and personalized assessment tool for clinical practice, which is expected to help doctors more accurately assess the prognosis of patients and develop more effective treatment strategies[39]. These findings provide an important reference for the practical application of immunotherapy for advanced gastric cancer and provide new ideas and strategies for the treatment and management of patients.
There are some shortcomings in this study: (1) This is a single-center, small-sample retrospective study, and the con
The number of Treg cells is a factor that affects irAEs in advanced gastric cancer patients receiving PD-1 inhibitor immunotherapy. irAEs can affect the patients’ PFS and result in longer PFS. Treg + Ki-67 + age (≥ 65 years) combined can better predict the occurrence of adverse reactions.
Different to morbidity and mortality worldwide, the incidence of gastric cancer in China ranks second, and mortality due to malignant tumors in China ranks third, which has obvious regional characteristics. Most patients with gastric cancer have local or distant metastases at the time of diagnosis, and there is no chance of surgical cure. Their survival can only be prolonged by comprehensive treatment, including palliative chemotherapy or targeted drug therapy. However, the benefits of this type of therapy are limited, with a median survival of only approximately 1 year.
In advanced gastric cancer, traditional treatment has limited effect. However, with the emergence of immunotherapy, there is new hope. Immunotherapy has brought hope of longer survival in advanced gastric cancer patients, but its accompanying adverse reactions have also caused concern. In-depth exploration of the factors related to the adverse reactions of immunotherapy for advanced gastric cancer and prediction of its prognosis will not only help optimize the treatment plan, but also improve the quality of life of patients.
This study aimed to construct a precise nomogram model to analyze the causes of adverse immunotherapy reactions. We believe that this study can provide a strong scientific basis for clinical practice, illuminate the way forward for advanced gastric cancer patients, and allow better treatment outcomes.
This study used a variety of research methods such as a literature review, clinical observation and statistical analysis. First of all, through the literature review, the relevant studies on adverse reactions of immunotherapy for advanced gastric cancer were systematically reviewed to clarify the current status and shortcomings of the research. On this basis, combined with clinical observation, the case data of patients with advanced gastric cancer receiving immunotherapy were collected and the adverse reactions were recorded in detail. Furthermore, statistical analyses were used to examine the collected data and analyze the correlation between adverse reactions and patients’ clinical characteristics and treatment methods. Finally, a nomogram model was established to evaluate the prognostic factors related to adverse reactions.
From the in-depth study of the adverse reactions of immunotherapy for advanced gastric cancer, it was found that the patient’s age, treatment mode, tumor stage and other factors were closely related to the occurrence of adverse reactions. The results of statistical analyses showed that these factors had a significant impact on the prognosis. Based on these results, we successfully constructed a nomogram model to provide an intuitive and quantitative prognostic assessment tool for clinicians.
We constructed a nomogram model to provide a quantitative prognostic assessment tool for clinicians. Therefore, during immunotherapy of advanced gastric cancer, individual differences in patients should be fully considered, and targeted treatment programs should be formulated to reduce the occurrence of adverse reactions and improve the quality of life of patients.
A nomogram model was established to investigate the related factors of adverse reactions to immunotherapy in advanced gastric cancer patients. In the future, we expect to further expand the sample size, optimize the model parameters, and improve the prediction accuracy. At the same time, the research results were combined with clinical practice to provide patients with more personalized and accurate treatment plans. In addition, exploring new immunotherapies to reduce adverse reactions and improve the therapeutic effect in advanced gastric cancer is an important research direction in the future.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Li C, China S-Editor: Wang JJ L-Editor: Webster JR P-Editor: Zheng XM
| 1. | Guan WL, He Y, Xu RH. Gastric cancer treatment: recent progress and future perspectives. J Hematol Oncol. 2023;16:57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 390] [Reference Citation Analysis (5)] |
| 2. | Li K, Zhang A, Li X, Zhang H, Zhao L. Advances in clinical immunotherapy for gastric cancer. Biochim Biophys Acta Rev Cancer. 2021;1876:188615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 226] [Article Influence: 56.5] [Reference Citation Analysis (0)] |
| 3. | Pan H, Pan J, Li P, Gao J. Characterization of PANoptosis patterns predicts survival and immunotherapy response in gastric cancer. Clin Immunol. 2022;238:109019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 86] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 4. | Jin X, Liu Z, Yang D, Yin K, Chang X. Recent Progress and Future Perspectives of Immunotherapy in Advanced Gastric Cancer. Front Immunol. 2022;13:948647. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 67] [Article Influence: 22.3] [Reference Citation Analysis (17)] |
| 5. | Yang H, Zou X, Yang S, Zhang A, Li N, Ma Z. Identification of lactylation related model to predict prognostic, tumor infiltrating immunocytes and response of immunotherapy in gastric cancer. Front Immunol. 2023;14:1149989. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 57] [Reference Citation Analysis (0)] |
| 6. | Qing X, Xu W, Liu S, Chen Z, Ye C, Zhang Y. Molecular Characteristics, Clinical Significance, and Cancer Immune Interactions of Angiogenesis-Associated Genes in Gastric Cancer. Front Immunol. 2022;13:843077. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 68] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 7. | Zhao Q, Cao L, Guan L, Bie L, Wang S, Xie B, Chen X, Shen X, Cao F. Immunotherapy for gastric cancer: dilemmas and prospect. Brief Funct Genomics. 2019;18:107-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 105] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 8. | Wu L, Liu Q, Ruan X, Luan X, Zhong Y, Liu J, Yan J, Li X. Multiple Omics Analysis of the Role of RBM10 Gene Instability in Immune Regulation and Drug Sensitivity in Patients with Lung Adenocarcinoma (LUAD). Biomedicines. 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 51] [Reference Citation Analysis (0)] |
| 9. | Högner A, Moehler M. Immunotherapy in Gastric Cancer. Curr Oncol. 2022;29:1559-1574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 112] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 10. | Li S, Yu W, Xie F, Luo H, Liu Z, Lv W, Shi D, Yu D, Gao P, Chen C, Wei M, Zhou W, Wang J, Zhao Z, Dai X, Xu Q, Zhang X, Huang M, Huang K, Li J, Sheng L, Liu L. Neoadjuvant therapy with immune checkpoint blockade, antiangiogenesis, and chemotherapy for locally advanced gastric cancer. Nat Commun. 2023;14:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 128] [Article Influence: 64.0] [Reference Citation Analysis (0)] |
| 11. | Chen Y, Jia K, Sun Y, Zhang C, Li Y, Zhang L, Chen Z, Zhang J, Hu Y, Yuan J, Zhao X, Gong J, Dong B, Zhang X, Li J, Shen L. Predicting response to immunotherapy in gastric cancer via multi-dimensional analyses of the tumour immune microenvironment. Nat Commun. 2022;13:4851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 124] [Reference Citation Analysis (0)] |
| 12. | Wu L, Zhong Y, Yu X, Wu D, Xu P, Lv L, Ruan X, Liu Q, Feng Y, Liu J, Li X. Selective poly adenylation predicts the efficacy of immunotherapy in patients with lung adenocarcinoma by multiple omics research. Anticancer Drugs. 2022;33:943-959. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 62] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 13. | Zeng D, Wu J, Luo H, Li Y, Xiao J, Peng J, Ye Z, Zhou R, Yu Y, Wang G, Huang N, Rong X, Sun L, Sun H, Qiu W, Xue Y, Bin J, Liao Y, Li N, Shi M, Kim KM, Liao W. Tumor microenvironment evaluation promotes precise checkpoint immunotherapy of advanced gastric cancer. J Immunother Cancer. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 143] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 14. | Bai Y, Xie T, Wang Z, Tong S, Zhao X, Zhao F, Cai J, Wei X, Peng Z, Shen L. Efficacy and predictive biomarkers of immunotherapy in Epstein-Barr virus-associated gastric cancer. J Immunother Cancer. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 67] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 15. | Zeng D, Li M, Zhou R, Zhang J, Sun H, Shi M, Bin J, Liao Y, Rao J, Liao W. Tumor Microenvironment Characterization in Gastric Cancer Identifies Prognostic and Immunotherapeutically Relevant Gene Signatures. Cancer Immunol Res. 2019;7:737-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 666] [Article Influence: 111.0] [Reference Citation Analysis (0)] |
| 16. | Wu L, Zheng Y, Ruan X, Wu D, Xu P, Liu J, Li X. Long-chain noncoding ribonucleic acids affect the survival and prognosis of patients with esophageal adenocarcinoma through the autophagy pathway: construction of a prognostic model. Anticancer Drugs. 2022;33:e590-e603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 68] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 17. | Yuan Q, Deng D, Pan C, Ren J, Wei T, Wu Z, Zhang B, Li S, Yin P, Shang D. Integration of transcriptomics, proteomics, and metabolomics data to reveal HER2-associated metabolic heterogeneity in gastric cancer with response to immunotherapy and neoadjuvant chemotherapy. Front Immunol. 2022;13:951137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 77] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 18. | Xu X, Chen J, Li W, Feng C, Liu Q, Gao W, He M. Immunology and immunotherapy in gastric cancer. Clin Exp Med. 2023;23:3189-3204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 19. | Chen K, Wang X, Yang L, Chen Z. The Anti-PD-1/PD-L1 Immunotherapy for Gastric Esophageal Cancer: A Systematic Review and Meta-Analysis and Literature Review. Cancer Control. 2021;28:1073274821997430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 20. | Wu H, Fu M, Liu J, Chong W, Fang Z, Du F, Liu Y, Shang L, Li L. The role and application of small extracellular vesicles in gastric cancer. Mol Cancer. 2021;20:71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 82] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 21. | Lin Y, Jing X, Chen Z, Pan X, Xu D, Yu X, Zhong F, Zhao L, Yang C, Wang B, Wang S, Ye Y, Shen Z. Histone deacetylase-mediated tumor microenvironment characteristics and synergistic immunotherapy in gastric cancer. Theranostics. 2023;13:4574-4600. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 22. | Wu L, Zhong Y, Wu D, Xu P, Ruan X, Yan J, Liu J, Li X. Immunomodulatory Factor TIM3 of Cytolytic Active Genes Affected the Survival and Prognosis of Lung Adenocarcinoma Patients by Multi-Omics Analysis. Biomedicines. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 59] [Reference Citation Analysis (0)] |
| 23. | Zhang Y, Yang Y, Chen Y, Lin W, Chen X, Liu J, Huang Y, Wang H, Teng L. PD-L1: Biological mechanism, function, and immunotherapy in gastric cancer. Front Immunol. 2022;13:1060497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 24. | Li X, Xu J, Xie J, Yang W. Research progress in targeted therapy and immunotherapy for gastric cancer. Chin Med J (Engl). 2022;135:1299-1313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 25. | Vrána D, Matzenauer M, Neoral Č, Aujeský R, Vrba R, Melichar B, Rušarová N, Bartoušková M, Jankowski J. From Tumor Immunology to Immunotherapy in Gastric and Esophageal Cancer. Int J Mol Sci. 2018;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 97] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 26. | Catanese S, Lordick F. Targeted and immunotherapy in the era of personalised gastric cancer treatment. Best Pract Res Clin Gastroenterol. 2021;50-51:101738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 27. | Faghfuri E, Shadbad MA, Faghfouri AH, Soozangar N. Cellular immunotherapy in gastric cancer: adoptive cell therapy and dendritic cell-based vaccination. Immunotherapy. 2022;14:475-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (36)] |
| 28. | Qi C, Gong J, Li J, Liu D, Qin Y, Ge S, Zhang M, Peng Z, Zhou J, Cao Y, Zhang X, Lu Z, Lu M, Yuan J, Wang Z, Wang Y, Peng X, Gao H, Liu Z, Wang H, Yuan D, Xiao J, Ma H, Wang W, Li Z, Shen L. Claudin18.2-specific CAR T cells in gastrointestinal cancers: phase 1 trial interim results. Nat Med. 2022;28:1189-1198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 317] [Cited by in RCA: 352] [Article Influence: 117.3] [Reference Citation Analysis (36)] |
| 29. | Huang W, Jiang Y, Xiong W, Sun Z, Chen C, Yuan Q, Zhou K, Han Z, Feng H, Chen H, Liang X, Yu S, Hu Y, Yu J, Chen Y, Zhao L, Liu H, Zhou Z, Wang W, Xu Y, Li G. Noninvasive imaging of the tumor immune microenvironment correlates with response to immunotherapy in gastric cancer. Nat Commun. 2022;13:5095. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 54] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 30. | Wu L, Zheng Y, Liu J, Luo R, Wu D, Xu P, Li X. Comprehensive evaluation of the efficacy and safety of LPV/r drugs in the treatment of SARS and MERS to provide potential treatment options for COVID-19. Aging (Albany NY). 2021;13:10833-10852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 31. | Zhang C, Chong X, Jiang F, Gao J, Chen Y, Jia K, Fan M, Liu X, An J, Li J, Zhang X, Shen L. Plasma extracellular vesicle derived protein profile predicting and monitoring immunotherapeutic outcomes of gastric cancer. J Extracell Vesicles. 2022;11:e12209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 32. | Zhao L, Liu Y, Zhang S, Wei L, Cheng H, Wang J. Impacts and mechanisms of metabolic reprogramming of tumor microenvironment for immunotherapy in gastric cancer. Cell Death Dis. 2022;13:378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 53] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 33. | Cui K, Yao S, Liu B, Sun S, Gong L, Li Q, Fei B, Huang Z. A novel high-risk subpopulation identified by CTSL and ZBTB7B in gastric cancer. Br J Cancer. 2022;127:1450-1460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Reference Citation Analysis (0)] |
| 34. | Moehler M, Högner A, Wagner AD, Obermannova R, Alsina M, Thuss-Patience P, van Laarhoven H, Smyth E. Recent progress and current challenges of immunotherapy in advanced/metastatic esophagogastric adenocarcinoma. Eur J Cancer. 2022;176:13-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 35. | Hou W, Zhao Y, Zhu H. Predictive Biomarkers for Immunotherapy in Gastric Cancer: Current Status and Emerging Prospects. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 36. | Niccolai E, Taddei A, Prisco D, Amedei A. Gastric cancer and the epoch of immunotherapy approaches. World J Gastroenterol. 2015;21:5778-5793. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 69] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 37. | Wu W, Zhang F, Zhao J, He P, Li Y. The N6-methyladenosine:mechanisms, diagnostic value, immunotherapy prospec-ts and challenges in gastric cancer. Exp Cell Res. 2022;415:113115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 38. | Dedecker H, Teuwen LA, Vandamme T, Domen A, Prenen H. The Role of Immunotherapy in Esophageal and Gastric Cancer. Clin Colorectal Cancer. 2023;22:175-182. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 39. | Ni L, Tang C, Wang Y, Wan J, Charles MG, Zhang Z, Li C, Zeng R, Jin Y, Song P, Wei M, Li B, Zhang J, Wu Z. Construction of a miRNA-Based Nomogram Model to Predict the Prognosis of Endometrial Cancer. J Pers Med. 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |