Published online Apr 15, 2024. doi: 10.4251/wjgo.v16.i4.1192
Peer-review started: October 17, 2023
First decision: January 15, 2024
Revised: January 28, 2024
Accepted: February 28, 2024
Article in press: February 28, 2024
Published online: April 15, 2024
Processing time: 176 Days and 13.8 Hours
Indentifying predictive factors for postoperative recurrence of hepatocellular carcinoma (HCC) has great significance for patient prognosis.
To explore the value of gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid (Gd-EOB-DTPA) enhanced magnetic resonance imaging (MRI) combined with clinical features in predicting early recurrence of HCC after resection.
A total of 161 patients with pathologically confirmed HCC were enrolled. The patients were divided into early recurrence and non-early recurrence group based on the follow-up results. The clinical, laboratory, pathological results and Gd-EOB-DTPA enhanced MRI imaging features were analyzed.
Of 161 patients, 73 had early recurrence and 88 were had non-early recurrence. Univariate analysis showed that patient age, gender, serum alpha-fetoprotein level, the Barcelona Clinic Liver Cancer stage, China liver cancer (CNLC) stage, microvascular invasion (MVI), pathological satellite focus, tumor size, tumor number, tumor boundary, tumor capsule, intratumoral necrosis, portal vein tumor thrombus, large vessel invasion, nonperipheral washout, peritumoral enhancement, hepatobiliary phase (HBP)/tumor signal intensity (SI)/peritumoral SI, HBP peritumoral low signal and peritumoral delay enhancement were significantly associated with early recurrence of HCC after operation. Multivariate logistic regression analysis showed that patient age, MVI, CNLC stage, tumor boundary and large vessel invasion were independent predictive factors. External data validation indicated that the area under the curve of the combined predictors was 0.861, suggesting that multivariate logistic regression was a reasonable predictive model for early recurrence of HCC.
Gd-EOB-DTPA enhanced MRI combined with clinical features would help predicting the early recurrence of HCC after operation.
Core Tip: Indentifying predictive factors for postoperative recurrence of hepatocellular carcinoma (HCC) has great significance for patient prognosis. In this retrospective cohort study, we analyzed the clinical, laboratory, pathological results and enhanced magnetic resonance imaging features of 161 HCC patients. Statistical analysis showed that patient age, microvascular invasion, the China live cancer stage, tumor boundary and large vessel invasion were independent predictive factors for predicting early recurrence of HCC. The results were further validated by external data and proved good predictive performance. This study may provide some assistance for the development of personalized treatment plans for HCC patients.
- Citation: Chen JP, Yang RH, Zhang TH, Liao LA, Guan YT, Dai HY. Pre-operative enhanced magnetic resonance imaging combined with clinical features predict early recurrence of hepatocellular carcinoma after radical resection. World J Gastrointest Oncol 2024; 16(4): 1192-1203
- URL: https://www.wjgnet.com/1948-5204/full/v16/i4/1192.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i4.1192
Hepatocellular carcinoma (HCC) is the most common primary malignant tumor of the liver. The morbidity and mortality of the tumor has been increasing over the recent years[1,2]. An early diagnosis and prompt treatment of small HCC is crucial to improving the prognosis and quality of life of patients. In clinics, specific treatment methods are mainly selected based on the size, number, location, invasiveness of the lesions, and liver function of patients[3]. At present, the treatment methods for small HCC mainly include surgical resection and local ablation. However, patients with liver cancer have a high intrahepatic recurrence rate after radical treatment, and the survival rate is still not satisfactory[4]. Studies have reported a 70% recurrence rate of HCC within 5 years after operation, among which early recurrence (< 2 years) accounts for more than 70% of the cases. The clinical and laboratory indicators, pathological and imaging features are helpful to predict the recurrence of HCC after treatment[3-6]. Factors associated with HCC recurrence include the time duration from primary resection to recurrence, serum alpha-fetoprotein (AFP) more than 100 ng/mL at recurrence, tumor larger than 3 cm, the Barcelona Clinic Liver Cancer (BCLC) stage and type of treatment[5,6]. Over the recent years, with the promotion and application of the magnetic resonance imaging (MRI) liver-specific contrast agent gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid (Gd-EOB-DTPA) in clinical practice, studies have revealed that Gd-EOB-DTPA-enhanced MRI is superior compared to enhanced computed tomography (CT) and conventional contrast-enhanced MRI for the diagnosis of small liver lesions and for the differentiation of benign and malignant nodules[7,8]. Gd-EOB-DTPA-enhanced MRI can significantly improve the sensitivity and specificity of the detection of small HCC, indicating the degree of HCC differentiation, and help determine treatment strategies[9-11]. Early detection of recurrent lesions after HCC surgery is important for prolonging the survival time of patients. However, there are inconsistencies in the definition of early recurrence and Gd-EOB-DTPA related MRI and clinical features in different studies[12,13]. Besides, few studies have adopted external data to validate the predicting model of HCC recurrence. The value of Gd-EOB-DTPA-enhanced MRI combined with clinical, laboratory and pathological risk factors for early recurrence of HCC postoperative should to be further investigated.
A total of 161 patients were enrolled from Meizhou People’s Hospital from August 2016 to January 2021 in this retro
The MRI imaging was done using a 3.0 T scanner (Magnetom Skyra, Siemens Healthineers, Erlangen, Germany) with an 18-channel body phased array surface coil. All patients were fasted for > 4 h before examination. The scanning range was from the top of the diaphragm to the lower edge of the liver. The scanning parameters were as follows: (1) T1WI dixon sequence: Positive phase TR = 4.0 ms, TE = 1.3 ms, thickness = 4.5 mm, spacing = 5 mm; reverse phase TR = 4.0 ms, TE = 2.5 ms, thickness = 4.5 mm, spacing = 5.0 mm; (2) T2WI sequence: TR = 3900 ms, TE = 86 ms, thickness = 4.5 mm, spacing = 5.0 mm; (3) DWI sequence: TR = 6900 ms, TE = 59 ms, thickness = 4.5 mm, spacing = 5.0 mm, B values including 50 s/mm2, 800 s/mm2, and 1000 s/mm2, respectively; and (4) Contrast-enhanced scan was performed with intravenous injection of 10 mL Gd-EOB-DTPA (promethazine) contrast medium by a power injector at a rate of 3-4 mL/s followed by a 20 mL saline flush. The axial T1WI images of arterial phase, portal phase, transitional phase and hepatobiliary phase were scanned at the time point of 20 s, 60 s, 180 s, and 20 min respectively after injection. The enhanced scanning parameters were: TR = 4.0 ms, TE = 1.3 ms, slice thickness = 3.0 mm, slice interval = 2.9 mm. All image matrix was 512 × 512.
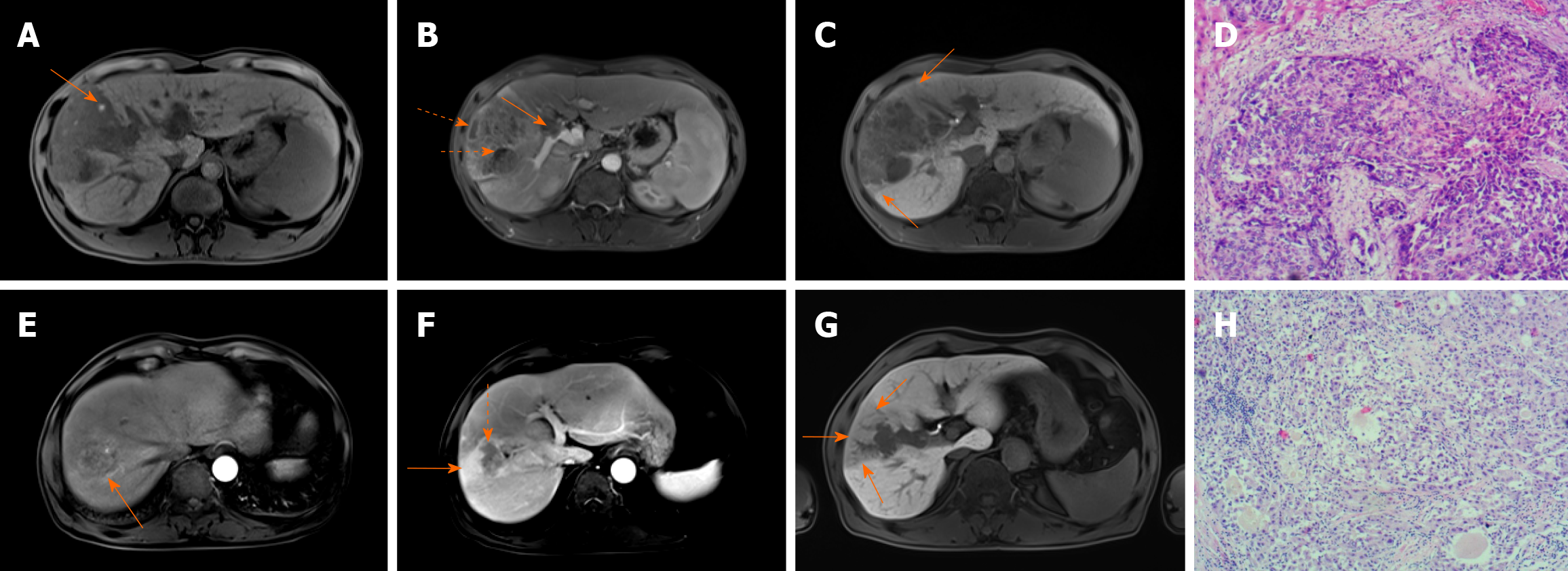
All MRI images were reviewed from the picture archiving and communication system (PACS) by two experienced radiologists (L.A.L. and Y.T.G.) independently, with more than 10 years of experience in diagnostic imaging. The radiologists were blinded to the clinical and pathological information when evaluating the MRI images. Any discrepancies between the two reviewers were resolved by discussion until a consensus was reached. The MRI features analyzed were the major imaging features and ancillary features, in accordance to the guidelines and standards of Liver Imaging Reporting and Data System (LI-RADS) v2018[14], including nonrim arterial phase hyperenhancement (nonrim-like enhancement in arterial phase unequivocally greater in whole or in part than liver), nonperipheral “washout” (nonperipheral visually assessed temporal reduction in enhancement inwhole or in part relative to composite liver tissue from earlier to later phase resulting in hypoenhancement in the extracellular phase), large vessel invasion (filling defect in the portal or hepatic vein), peritumoral enhancement (wedge-shaped or irregular enhancement around tumors in late arterial or early portal vein phase), portal vein phase enhancement (continuous enhancement in portal vein phase), intratumor hemorrhage, intratumor steatosis, and intratumor necrosis. The main signs of hepatobiliary phase (HBP) include: The maximum diameter of tumor in the axial, coronal or sagittal dimension; HBP peritumoral low signal (anomalous wedge-shaped or flame-like area exhibiting low signal intensity in the liver parenchyma outside the tumor margin in the hepatobiliary phase), HBP peritumoral high uptake (high enhancement signal around tumor in the hepatobiliary phase), HBP tumor signal intensity (SI)/liver SI (defined as the ratio of the SI of the tumor to adjacent normal liver parenchyma signal in HBP). Tumor boundary was classified as regular or irregular. The regular boundary was defined as single node or smooth type, while irregular was defined as nodule exogenous type/fusion multinodular type or infiltrating type. Typical examples of MRI imaging features are shown in Figure 1.
Relevant clinical information was recorded from the hospital’s electronic medical record system, including patient’s gender, age, viral hepatitis B status, cirrhosis, hepatitis B virus (HBV) DNA, AFP value, total bilirubin, serum albumin, Child-Pugh score, portal hypertension, the BCLC stage, the China Live Cancer (CNLC) stage, surgical resection method, and whether preventive hepatic artery infusion chemotherapy (HAIC) was performed after operation. The pathological results including tumor pathological differentiation degree, microvascular invasion (MVI) and pathological satellite nodules of all patients were recorded.
All patients received regular follow-ups or telephonic follow-up every 3 to 6 months after the operation. Clinical data included patients’ survival status, and whether tumor recurrence or metastasis were recorded. The follow-up time was calculated from operation to the diagnosis of tumor recurrence. If there was no recurrence, the follow-up was continued till at least 2 years. The last date for follow-up was August 29, 2022. Early recurrence was defined as intrahepatic and/or extrahepatic recurrence in HCC patients within 2 years after surgical resection. The diagnosis of recurrence was based on continuous increase in AFP level and the presence of a suspected recurrence lesion on ultrasound examination, which was further confirmed by contrast enhanced CT or MRI scans. If the imaging evidence was insufficient for diagnosis, additional puncture or surgical pathology was performed.
The clinical and MRI imaging data of the early and non-early recurrence groups are illustrated in Table 1 and Table 2. Quantitative variables were expressed by mean ± SD, and an independent sample t-test was used for comparison the two groups. Univariate analysis was applied to compare the frequency of these features between the early and non-early recurrence groups using the χ2 test. The variables with P < 0.05 were included in to the univariate and multivariate logistic regression analysis using a stepwise regression model (forward LR) to determine the independent predictive factors. The specificity, sensitivity and accuracy of each predictive factor was validated by external data, and the area under the curve (AUC) of receiver operating characteristics (ROC) was used to assess the diagnostic efficiency of each predictive factors and the multivariate logistic regression model. All statistical analyses were performed using SPSS software (version 22.0, SPSS Inc., Chicago, IL, United States). A two-sided P < 0.05 was considered statistically significant.
| Factors | Early recurrence, n = 73 (%) | Non-early recurrence, n = 88 (%) | χ2/t | P value | |
| Age (yr) | - | 53.47 ± 11.07 | 59.88 ± 9.76 | -3.902 | 0.000b |
| Serum albumin | - | 40.43 ± 4.21 | 40.05 ± 5.27 | 0.501 | 0.617 |
| Total bilirubin | - | 17.24 ± 10.52 | 18.62 ± 8.84 | -0.903 | 0.368 |
| Gender | Male | 62 (84.9) | 80 (90.9) | 1.370 | 0.242 |
| Female | 11 (15.1) | 8 (9.1) | - | - | |
| Age (group) | > 50 | 43 (58.9) | 76 (86.4) | 17.679 | 0.000b |
| ≤ 50 | 30 (41.1) | 12 (13.6) | - | - | |
| Hepatitis B | Positive | 72 (98.6) | 81 (92.0) | Fisher | 0.073 |
| Negative | 1 (1.4) | 7 (8.0) | - | - | |
| Cirrhosis | Present | 40 (54.8) | 50 (56.8) | 0.066 | 0.797 |
| Absent | 33 (45.2) | 38 (43.8) | - | - | |
| HBV-DNA | Negative | 12 (16.4) | 19 (21.6) | 0.681 | 0.409 |
| Positive | 61 (83.6) | 69 (78.4) | - | - | |
| AFP | > 100 | 44 (60.3) | 30 (34.1) | 11.013 | 0.001b |
| ≤ 100 | 29 (39.7) | 58 (65.9) | - | - | |
| Child-Pugh score | A | 68 (93.2) | 74 (84.1) | 3.146 | 0.076 |
| B | 5 (6.8) | 14 (15.9) | - | - | |
| Portal hypertension | Present | 23 (31.5) | 27 (30.7) | 0.013 | 0.910 |
| Absent | 50 (68.5) | 61 (69.3) | - | - | |
| Operation mode | Local excision | 21 (28.8) | 34 (38.6) | 2.261 | 0.133 |
| Anatomical excision | 52 (71.2) | 54 (61.4) | - | - | |
| Postoperative HAIC | Present | 13 (17.8) | 11 (12.5) | 0.886 | 0.346 |
| Absent | 60 (82.2) | 77 (87.5) | - | - | |
| BCLC stage | 0 | 3 (4.1) | 9 (10.2) | 26.919 | 0.000b |
| A | 29 (39.7) | 62 (70.5) | - | - | |
| B | 15 (20.5) | 11 (12.5) | - | - | |
| C | 26 (35.6) | 6 (6.8) | - | - | |
| CNLC stage | I | 31 (42.5) | 72 (81.8) | 29.060 | 0.000b |
| II | 16 (21.9) | 10 (11.4) | - | - | |
| III | 26 (35.6) | 6 (6.8) | - | - | |
| Degree of pathological differentiation | Low | 10 (13.7) | 15 (17.0) | 3.397 | 0.183 |
| Middle | 62 (84.9) | 67 (76.1) | - | - | |
| High | 1 (1.4) | 6 (6.8) | - | - | |
| Pathological satellite focus | Present | 15 (20.5) | 6 (6.8) | 6.631 | 0.010b |
| Absent | 58 (79.5) | 82 (93.2) | - | - | |
| MVI | Present | 39 (53.4) | 17 (19.3) | 20.461 | 0.000b |
| Absent | 33 (46.6) | 71 (80.7) | |||
Of the 161 HCC patients, 73 had confirmed early recurrence and 88 had non-early recurrence. The average follow-up time was 26.9 months (1.3-67.7 months). In the early recurrence group, 55 (75.3%) had intrahepatic recurrence, 11 (15.1%) had extrahepatic recurrence, 7 (9.6%) had intrahepatic and extrahepatic recurrence. The average age of early and non-early recurrence group was 53.47 years ± 11.07 years vs 59.88 years ± 9.76 years (t = -3.902, P < 0.01). Compared to the non-recurrence group, terms including serum AFP, pathological satellite lesions, MVI, BCLC stage, and CNLC stage were higher or more frequently seen in the early recurrence group (all P < 0.01), whereas the terms of serum protein value, total bilirubin value, gender, history of hepatitis B, history of liver cirrhosis, HBV DNA quantification, Child-Pugh score, portal hypertension, surgical resection method, postoperative HAIC and pathological differentiation were not statistically significantly different between the two groups (P > 0.05). The clinical data for the cohort of HCC patients after resection are illustrated in Table 1.
The maximum tumor diameter of early recurrence group (6.85 cm ± 3.78 cm) was larger than that of the non-early recurrence group (5.19 cm ± 3.52 cm) (t = 2.918, P = 0.004). The ratio of tumor SI/peritumoral SI in the early recurrence group (50.67 ± 12.20) was smaller than that of the non-early recurrence group (57.63 ± 15.64) (t = -3.097, P = 0.002). Compared to the non-early recurrence group, the presence of multiple tumors, irregular shape, portal vein tumor thrombus, large vessel invasion, intratumoral hemorrhage, intratumoral necrosis, incomplete tumor capsule, nonperipheral washout, peritumoral enhancement and HBP peritumoral low signal were more frequently seen in the early recurrence group (P < 0.05). Peritumoral delay enhancement was more frequently seen in the non-early recurrence group (P < 0.01). There was no significant difference in tumor steatosis, HBP peritumoral high uptake, nonrim arterial phase hyperenhancement between the early and non-early recurrence groups (P > 0.05). The main MRI features for the cohort of HCC patients after resection are illustrated in Table 2.
| Factors | Early recurrence, n = 73 (%) | Non-early recurrence n = 88 (%) | χ2/t | P value | |
| Max-diameter | 6.85 ± 3.78 | 5.19 ± 3.52 | 2.918 | 0.004b | |
| Tumor SI/peritumoral SI | 50.67 ± 12.20 | 57.63 ± 15.64 | -3.097 | 0.002b | |
| Tumor numbers | Single | 51 (69.9) | 75 (85.2) | 5.536 | 0.019a |
| Multiple | 22 (30.1) | 13 (14.8) | |||
| Tumor boundary | Regular | 20 (27.4) | 61 (69.3) | 28.049 | 0.000b |
| Irregular | 53 (72.6) | 27 (30.7) | |||
| Portal vein tumor thrombus | Present | 13 (17.8) | 2 (2.3) | Fisher | 0.001b |
| Absent | 60 (82.2) | 86 (97.7) | |||
| Large vessel invasion | Present | 26 (35.6) | 5 (5.7) | 22.997 | 0.000b |
| Absent | 47 (64.4) | 83 (94.3) | |||
| Intratumoral hemorrhage | Present | 23 (31.5) | 16 (18.2) | 3.860 | 0.049a |
| Absent | 50 (68.5) | 72 (81.8) | |||
| Intratumoral necrosis | Present | 52 (71.2) | 36 (40.9) | 14.804 | 0.000b |
| Absent | 21 (28.8) | 52 (59.1) | |||
| Tumor steatosis | Present | 6 (8.2) | 15 (17.0) | 2.741 | 0.098 |
| Absent | 67 (91.8) | 73 (83.0) | |||
| Tumor capsule | Intact | 20 (27.4) | 55 (62.5) | 20.220 | 0.000b |
| Incomplete | 42 (57.5) | 24 (27.3) | |||
| Lack | 11 (15.1) | 9 (10.2) | |||
| HBP peritumoral high uptake | Present | 0 (0.0) | 5 (5.7) | Fisher | 0.064 |
| Absent | 73 (100.0) | 83 (94.3) | |||
| Nonrim arterial phase hyperenhancement | Present | 73 (100.0) | 86 (97.7) | Fisher | 0.501 |
| Absent | 0 (0.0) | 2 (2.3) | |||
| Nonperipheral washout | Present | 68 (93.2) | 72 (81.8) | 4.518 | 0.034a |
| Absent | 5 (6.8) | 16 (18.2) | |||
| Peritumoral enhancement | Present | 18 (24.7) | 9 (10.2) | 5.953 | 0.015a |
| Absent | 55 (75.3) | 79 (89.9) | |||
| HBP peritumoral low signal | Present | 26 (35.6) | 7 (8.0) | 18.736 | 0.000b |
| Absent | 47 (64.4) | 81 (92.0) | |||
| Peritumoral delay enhancement | Present | 5 (6.8) | 27 (30.7) | 14.231 | 0.000b |
| Absent | 68 (92.2) | 61 (69.3) | |||
Factors with a P < 0.05 were incorporated into the univariate logistic regression analysis. The results showed that patient age, serum AFP level, BCLC stage, CNLC stage, MVI, pathological satellite focus, tumor size, tumor number, tumor boundary, tumor capsule, intratumoral necrosis, portal vein tumor thrombus, large vessel invasion, nonperipheral washout, peritumoral enhancement, HBP tumor SI/peritumoral SI, HBP peritumoral low signal and peritumoral delay enhancement were significantly associated with early recurrence of HCC after surgery. These factors were further included into the multivariate logistic regression analysis. The results showed that patient age, MVI, CNLC stage, tumor boundary and large vessel invasion remained significant as the independent predicting factors. Patients with age ≤ 50 years (HR = 3.722, P = 0.002), MVI (HR = 2.288, P = 0.050), CNLC staging (HR = 2.556, P = 0.059 for stage II; HR = 6.272, P = 0.001 for stage III), irregular tumor shape (HR = 3.638, P = 0.001) and large vessel invasion (HR = 5.675, P = 0.002) were highly indicative of early recurrence of HCC. The univariate and multivariate logistic regression results are shown in Table 3 and Table 4.
| Factors | B | SE | Wald χ2 | P value | HR | 95%CI |
| Age (group) | 1.486 | 0.391 | 14.422 | 0.000b | 4.419 | 2.052, 9.513 |
| AFP | 1.076 | 0.328 | 10.744 | 0.001b | 2.933 | 1.541, 5.582 |
| BCLC stage | 23.108 | 0.000b | ||||
| BCLC stage 1 | 0.339 | 0.704 | 0.232 | 0.6300 | 1.403 | 0.353, 5.572 |
| BCLC stage 2 | 1.409 | 0.776 | 3.297 | 0.069 | 4.091 | 0.894, 18.718 |
| BCLC stage 3 | 2.565 | 0.806 | 10.128 | 0.001a | 13.000 | 2.679, 63.093 |
| CNLC stage | 25.163 | 0.000b | ||||
| CNLC stage 1 | 1.313 | 0.457 | 8.259 | 0.004b | 3.716 | 1.518, 9.097 |
| CNLC stage 1 | 2.309 | 0.501 | 21.218 | 0.000b | 10.065 | 3.768, 26.883 |
| MVI | 1.567 | 0.358 | 19.181 | 0.000b | 4.791 | 2.376, 9.658 |
| Pathological satellite focus | 1.263 | 0.513 | 6.066 | 0.014b | 3.534 | 1.294, 9.653 |
| Tumor size | 0.125 | 0.046 | 7.278 | 0.007b | 1.133 | 1.035, 1.241 |
| Tumor numbers | 0.912 | 0.394 | 5.352 | 0.021a | 2.489 | 1.149, 5.388 |
| Tumor boundary | 1.790 | 0.350 | 26.187 | 0.000b | 5.987 | 3.017, 11.882 |
| Tumor capsule | 0.581 | 0.247 | 5.549 | 0.018a | 1.788 | 1.103, 2.900 |
| Intratumoral hemorrhage | 0.728 | 0.374 | 3.784 | 0.052 | 2.070 | 0.995, 4.308 |
| Intratumoral necrosis | 1.274 | 0.337 | 14.265 | 0.000b | 3.577 | 1.846, 6.930 |
| Portal vein tumor thrombus | 2.232 | 0.778 | 8.230 | 0.004b | 9.317 | 2.028, 42.801 |
| Large vessel invasion | 2.217 | 0.521 | 18.090 | 0.000b | 9.183 | 3.305, 25.515 |
| Nonperipheral washout | 1.106 | 0.540 | 4.202 | 0.040a | 3.022 | 1.050, 8.701 |
| Peritumoral enhancement | 1.055 | 0.444 | 5.638 | 0.018a | 2.873 | 1.202, 6.864 |
| HBP tumor SI/peritumoral SI | -0.035 | 0.012 | 8.688 | 0.003b | 0.965 | 0.943, 0.988 |
| HBP peritumoral low signal | 1.856 | 0.464 | 16.035 | 0.000b | 6.401 | 2.580, 15.882 |
| Peritumoral delay enhancement | -1.795 | 0.518 | 12.017 | 0.001b | 0.166 | 0.060, 0.458 |
| Factors | B | SE | Wald χ2 | P value | HR | 95%CI |
| Age (≤ 50 yr) | 1.314 | 0.430 | 9.356 | 0.002b | 3.722 | 1.603, 8.641 |
| MVI | 0.828 | 0.422 | 3.846 | 0.050a | 2.288 | 1.001, 5.234 |
| CNLC stage | 12.193 | 0.002b | ||||
| Stage II | 0.938 | 0.496 | 3.577 | 0.059 | 2.556 | 0.967, 6.757 |
| Stage III | 1.836 | 0.558 | 10.839 | 0.001b | 6.272 | 2.102, 18.712 |
| Irregular shape | 1.292 | 0.380 | 11.550 | 0.001b | 3.638 | 1.728, 7.663 |
| Large vessel invasion | 1.736 | 0.557 | 9.728 | 0.002b | 5.675 | 1.906, 16.896 |
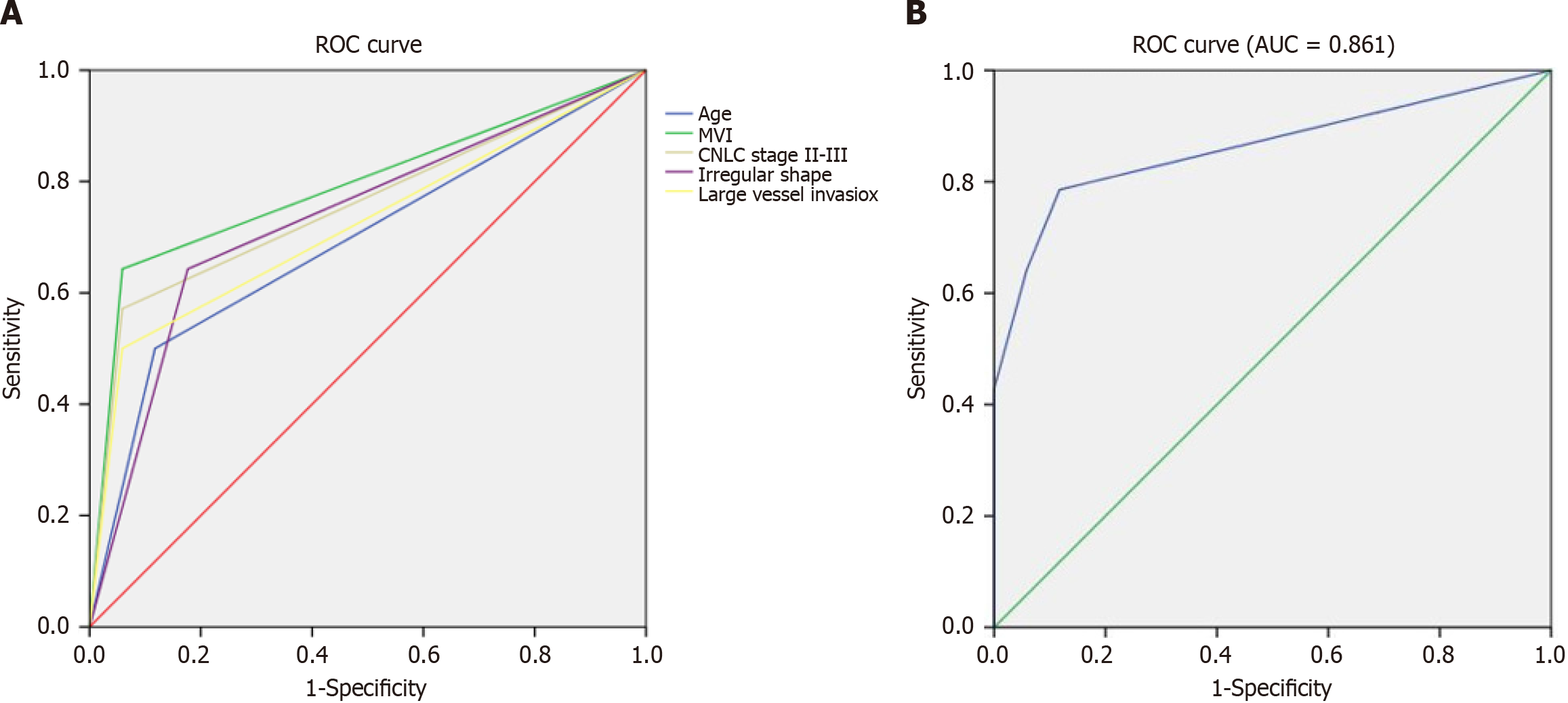
An external data set of 31 patients including 14 early recurrence and 17 non-early recurrence HCC were used to validate the predictive efficiency of the multivariate logistic regression model. The sensitivity, specificity, and accuracy of each predictive factor is illustrated in Table 5. The predictive sensitivity of patient age, MVI, CNLC stage, irregular shape and large vessel invasion ranged from 50.00% to 64.29%. The predictive specificity of patient age, MVI, CNLC stage, irregular shape and large vessel invasion ranged from 82.35% to 94.12%. The predictive accuracy of patient age, MVI, CNLC stage, irregular shape, and large vessel invasion ranged from 70.97% to 80.65%. The ROC analysis showed that the AUC of each predictive factor was approximately 0.70 to 0.80, indicating moderate predictive efficiency (Figure 2A). In the analysis for the predictive factors using the multivariate logistic regression model, the ROC analysis showed an AUC of 0.861 (95% confidence interval: 0.717, 1.000), indicating that the multivariate logistic regression model was a relatively reasonable predictor for early recurrence of HCC (Figure 2B).
| Factors | Sensitivity | Specificity | Accuracy | AUC | P value | 95%CI |
| Age (≤ 50 yr) | 0.500 | 0.882 | 0.710 | 0.691 | 0.071 | 0.496, 0.886 |
| MVI | 0.643 | 0.941 | 0.806 | 0.792 | 0.006 | 0.619, 0.965 |
| CNLC stage (II-III) | 0.571 | 0.941 | 0.774 | 0.756 | 0.015 | 0.574, 0.939 |
| Irregular shape | 0.643 | 0.824 | 0.742 | 0.733 | 0.028 | 0.548, 0.919 |
| Large vessel invasion | 0.500 | 0.941 | 0.742 | 0.721 | 0.037 | 0.530, 0.911 |
| Combined multi-factors | 0.786 | 0.941 | 0.871 | 0.861 | 0.001 | 0.717, 1.000 |
Liver resection is currently recommended as the first-line curative therapy, but the survival of patients with HCC is not satisfactory because of the high recurrence rate after surgery. Therefore, it is important to indentify the risk factors of recurrence after HCC surgery, which can be beneficial in selection of treatment plans and prognosis evaluations. This study comprehensively used the characteristics of Gd-EOB-DTPA-enhanced MRI combined with clinical, laboratory and pathological factors to explore the prediction of early postoperative recurrence of HCC. The results showed that the patient age ≤ 50 years, pathologically proven MVI, CNLC stage II-III, irregular tumor shape and large vessels invasion were the independent predictive factors for early postoperative recurrence in HCC.
The results of this study showed that younger age (≤ 50 years old) was an important clinical factor for predicting early recurrence of HCC after surgery (which is consistent with previous studies). Chen et al[15] reported that age was an independent risk factor for HCC recurrence. Zhang et al[16] reported that the recurrence rate of liver cancer in younger patients was higher after surgery. MVI was found to be one of the most critical factors in predicting recurrence and survival outcomes. Approximately more than 60% of all HCC recurrences are early recurrences which are closely related to presence of MVI[17,18]. The present study confirmed MVI was an important predictive factor for early recurrence of HCC. This finding was consistent with previous studies[19-21]. Tumor staging had an important role in the choice of treatment and prognosis evaluation. There are many staging schemes in HCC at present such as BCLC, CNLC and TNM staging. A higher tumor stage has been reported to be positively correlated with early recurrence of HCC after surgery[22]. However, recent studies have found that in patients undergoing liver transplantation, the BCLC stage does not affect the survival or the recurrence rate[23]. Wei et al[24] also found that BCLC stage is not related to the early recurrence of liver cancer. Our present study revealed that there was no significant correlation between BCLC staging and early recurrence of HCC, whereas CNLC staging was an independent predictive factor for early recurrence, which was consistent with the results of the previous studies. This result may be due to the different causes of HCC between China and other countries. In China, hepatitis B is the main cause of HCC, whereas hepatitis C and alcoholic hepatitis are the main causes of HCC in Europe and the United States. The study from Xie et al[25,26] suggested that the CNLC staging scheme (which was derived from the data of Chinese populations) might be more suitable for the diagnosis and treatment decisions for Chinese patients with HCC.
Several studies have shown that imaging features play important roles in the prognosis evaluation of patients with liver cancer. In the present study, univariate analysis showed that preoperative MRI features including tumor size, tumor number, tumor boundary, tumor capsule, intratumoral hemorrhage, intratumoral necrosis, portal vein tumor thrombus, large vessel invasion, nonperipheral washout, peritumoral enhancement, peritumoral delay enhancement, HBP peritumoral low signal and HBP tumor SI/peritumoral SI were associated with early recurrence of HCC after the operation. Further multivariate logistic analysis confirmed that irregular tumor shape and large vessel invasion as detected in Gd-EOB-DTPA-enhanced MRI were independent predictive factors for early recurrence of HCC. It has been reported that irregular tumor shape and HBP peritumoral low signal are important predictors of MVI[24,27-29], and MVI has been confirmed an effective risk factor for early recurrence of HCC after surgery[30]. Lee et al[28] reported that the specificity of combined HBP peritumoral low signal and irregular tumor boundary to predict MVI was 92.5%. Although HBP peritumoral low signal was not an independent predictive factor in this study, it is important for predicting HCC recurrence and MVI. MVI was shown to be an independent predictive factor in this study. In addition, Huang et al[31] found that HBP peritumoral low signal and irregular tumor boundary were indicators of poor differentiation of HCC. Further studies suggested that poor tumor differentiation was related with early tumor recurrence and patient survival of HCC[32]. The effect of large vessel invasion on recurrence of HCC after resection has been confirmed in previous studies. Wai et al[20] believed that large vessel invasion was related to recurrence free survival. The results of the present study suggested that the large vessel invasion (as detected by MRI) is an independent predictive factor for early recurrence of HCC after resection, which is consistent with previous studies[29].
We analyzed various factors including clinical features, laboratory indicators, and Gd-EOB-DTPA-enhanced MRI features in order to identify the predictive factors for early recurrence of HCC after resection. The predictive model constructed in this study had similar diagnostic efficiency and accuracy compared with other studies[33]. In addition, we introduced an external data set to validate the constructed model, to evaluate the sensitivity, specificity, and accuracy of various predictive factors. ROC analysis showed each independent predictor had moderate diagnostic performance, and combining multiple predictors led to an improvement in the predictive performance. In a clinical setting, it is very important to understand the risk factors for early recurrence of liver cancer. A multicenter retrospective study showed that HCC patients with MVI who underwent R0 resection, 13 months may be a reasonable early recurrence time point, and postoperative adjuvant TACE may result in longer survival compared with surgery alone within this interval[17,21]. And thus, for patients who are predicted to have high risk early recurrence by the established model, subsequent treatment plans such as follow-ups, post-operative adjuvant treatment using TACE or molecular targeted agents should be discussed with the patients.
This study has several limitations. First, this is a single center retrospective study. The data source may be biased, and further prospective multicenter studies are needed. Second, this study did not explore the impact of surgical factors on the prognosis of patients. Some studies showed that surgical related factors, such as intraoperative blood loss, the scope of hepatectomy, anatomical or non-anatomical resection, can affect patient prognosis. Last, the follow-up time of this study was relatively short and the patients with late recurrence were not evaluated. In the future, longer follow-up time should be used to better evaluate the role of various clinical, pathological, and imaging risk factors in predicting the recurrence of HCC.
In conclusion, we retrospectively analyzed the predictive value of Gd-EOB-DTPA-enhanced MRI combined with clinical features in predicting early recurrence of HCC after resection. The results showed that factors including patients age ≤ 50 years old, MVI, CNLC staging II-III, and MRI features including irregular tumor shape and large vessel invasion were the predictive factors for early recurrence of HCC after operation. Our findings might be useful in establishing treatment and follow-up strategies for HCC patients after tumor resection.
Patients with hepatocellular carcinoma (HCC) have a high rate of early recurrence after radical treatment, and the survival rate is not satisfactory.
To understand the risk factors for early recurrence of HCC. For patients who are predicted to have high risk of early recurrence, subsequent treatment plans should be considered and this may result in longer survival compared with surgery alone.
To explore the value of gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid (Gd-EOB-DTPA) enhanced magnetic resonance imaging (MRI) combined with clinical features in predicting early recurrence of HCC after resection, and find out the predictive factors related with early recurrence of HCC.
This retrospective cohort study enrolled 161 patients pathologically confirmed HCC and classified into early recurrence and non-early recurrence group based on the follow-up results. The clinical, laboratory, pathological results and Gd-EOB-DTPA enhanced MRI imaging features were analyzed.
Results showed that patient age, microvascular invasion, China liver cancer stage, tumor boundary and large vessel invasion were independent predictive factors for early recurrence of HCC. This result was further validated by external data and showed good predictive performance.
We found Gd-EOB-DTPA enhanced MRI combined with clinical features would help predicting the early recurrence of HCC after operation.
The outcoming and prognosis of patients with high risk of early recurrence after personalized treatment plans according to our results would be observed.
We would like to thank Gang Xiao (Hanshan Normal University, Chaozhou, China) for his assistance with statistical analysis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Wu JY, China S-Editor: Chen YL L-Editor: A P-Editor: Zhao YQ
| 1. | Bailey A, Shah SA. Screening high risk populations for cancer: Hepatobiliary. J Surg Oncol. 2019;120:847-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Kim TH, Yoon JH, Lee JM. Emerging Role of Hepatobiliary Magnetic Resonance Contrast Media and Contrast-Enhanced Ultrasound for Noninvasive Diagnosis of Hepatocellular Carcinoma: Emphasis on Recent Updates in Major Guidelines. Korean J Radiol. 2019;20:863-879. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 3. | Dimitroulis D, Damaskos C, Valsami S, Davakis S, Garmpis N, Spartalis E, Athanasiou A, Moris D, Sakellariou S, Kykalos S, Tsourouflis G, Garmpi A, Delladetsima I, Kontzoglou K, Kouraklis G. From diagnosis to treatment of hepatocellular carcinoma: An epidemic problem for both developed and developing world. World J Gastroenterol. 2017;23:5282-5294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 228] [Cited by in RCA: 229] [Article Influence: 28.6] [Reference Citation Analysis (4)] |
| 4. | Kim HD, Lim YS, Han S, An J, Kim GA, Kim SY, Lee SJ, Won HJ, Byun JH. Evaluation of early-stage hepatocellular carcinoma by magnetic resonance imaging with gadoxetic acid detects additional lesions and increases overall survival. Gastroenterology. 2015;148:1371-1382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 108] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 5. | Tabrizian P, Jibara G, Shrager B, Schwartz M, Roayaie S. Recurrence of hepatocellular cancer after resection: patterns, treatments, and prognosis. Ann Surg. 2015;261:947-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 652] [Article Influence: 65.2] [Reference Citation Analysis (0)] |
| 6. | He W, Peng B, Tang Y, Yang J, Zheng Y, Qiu J, Zou R, Shen J, Li B, Yuan Y. Nomogram to Predict Survival of Patients With Recurrence of Hepatocellular Carcinoma After Surgery. Clin Gastroenterol Hepatol. 2018;16:756-764.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 7. | Kierans AS, Kang SK, Rosenkrantz AB. The Diagnostic Performance of Dynamic Contrast-enhanced MR Imaging for Detection of Small Hepatocellular Carcinoma Measuring Up to 2 cm: A Meta-Analysis. Radiology. 2016;278:82-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 90] [Article Influence: 9.0] [Reference Citation Analysis (1)] |
| 8. | Lee YJ, Lee JM, Lee JS, Lee HY, Park BH, Kim YH, Han JK, Choi BI. Hepatocellular carcinoma: diagnostic performance of multidetector CT and MR imaging-a systematic review and meta-analysis. Radiology. 2015;275:97-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 391] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 9. | Hu MJ, Yu YX, Fan YF, Jiang YM, Hu S, Wang XM, Hu CH. [The predictive value of gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid enhanced MRI imaging features combined with quantitative parameters for the pathologic grading of hepatocellular carcinoma]. Zhonghua Yi Xue Za Zhi. 2020;100:1299-1304. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 10. | Wang LL, Li JF, Lei JQ, Guo SL, Li JK, Xu YS, Dou Y. The value of the signal intensity of peritumoral tissue on Gd-EOB-DTPA dynamic enhanced MRI in assessment of microvascular invasion and pathological grade of hepatocellular carcinoma. Medicine (Baltimore). 2021;100:e25804. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Li XQ, Wang X, Zhao DW, Sun J, Liu JJ, Lin DD, Yang G, Liu H, Xia ZY, Jia CY, Li HJ. Application of Gd-EOB-DTPA-enhanced magnetic resonance imaging (MRI) in hepatocellular carcinoma. World J Surg Oncol. 2020;18:219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 12. | Ariizumi S, Kitagawa K, Kotera Y, Takahashi Y, Katagiri S, Kuwatsuru R, Yamamoto M. A non-smooth tumor margin in the hepatobiliary phase of gadoxetic acid disodium (Gd-EOB-DTPA)-enhanced magnetic resonance imaging predicts microscopic portal vein invasion, intrahepatic metastasis, and early recurrence after hepatectomy in patients with hepatocellular carcinoma. J Hepatobiliary Pancreat Sci. 2011;18:575-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 135] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 13. | Zhao QY, Liu SS, Fan MX. Prediction of early recurrence of hepatocellular carcinoma after resection based on Gd-EOB-DTPA enhanced magnetic resonance imaging: a preliminary study. J Gastrointest Oncol. 2022;13:792-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Kanmaniraja D, Dellacerra G, Holder J, Erlichman D, Chernyak V. Liver Imaging Reporting and Data System (LI-RADS) v2018: Review of the CT/MRI Diagnostic Categories. Can Assoc Radiol J. 2021;72:142-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Chen YS, Yang SY, Wang PM, Wang CC, Yong CC, Chen DW, Liu YW, Chuang CH, Huang PY, Yao CC, Lin YP, Tsai MC. Concurrent Cholecystectomy Is Associated with a Lower Risk of Recurrence after Curative Resection in Early-Stage Hepatocellular Carcinoma: A 10 Year Observational Single-Center Study. J Pers Med. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Zhang Y, Kuang S, Shan Q, Rong D, Zhang Z, Yang H, Wu J, Chen J, He B, Deng Y, Roberts N, Shen J, Venkatesh SK, Wang J. Can IVIM help predict HCC recurrence after hepatectomy? Eur Radiol. 2019;29:5791-5803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 17. | Yang Y, Dang Z, Lu P, Qian Y, Lin K, Pan Z, Lau WY, Zhou W. Impact of pathological response after preoperative transcatheter arterial chemoembolization (TACE) on incidences of microvascular invasion and early tumor recurrence in hepatocellular carcinoma: a multicenter propensity score matching analysis. Hepatobiliary Surg Nutr. 2022;11:386-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 18. | Wang S, Zheng W, Zhang Z, Zhang GH, Huang DJ. Microvascular invasion risk scores affect the estimation of early recurrence after resection in patients with hepatocellular carcinoma: a retrospective study. BMC Med Imaging. 2022;22:204. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 19. | Wang SY, Chen CL, Hu YC, Chi Y, Huang YH, Su CW, Jeng WJ, Liang YJ, Wu JC. High Expression of MicroRNA-196a is Associated with Progression of Hepatocellular Carcinoma in Younger Patients. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Wai CT, Woon WA, Tan YM, Lee KH, Tan KC. Younger age and presence of macrovascular invasion were independent significant factors associated with poor disease-free survival in hepatocellular carcinoma patients undergoing living donor liver transplantation. Transplant Proc. 2012;44:516-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Liu ZH, Chai ZT, Feng JK, Hou YC, Zhang XP, Chen ZH, Xiang YJ, Guo WX, Shi J, Cheng SQ. A reasonable identification of the early recurrence time based on microvascular invasion for hepatocellular carcinoma after R0 resection: A multicenter retrospective study. Cancer Med. 2023;12:10294-10302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 22. | Xu XF, Xing H, Han J, Li ZL, Lau WY, Zhou YH, Gu WM, Wang H, Chen TH, Zeng YY, Li C, Wu MC, Shen F, Yang T. Risk Factors, Patterns, and Outcomes of Late Recurrence After Liver Resection for Hepatocellular Carcinoma: A Multicenter Study From China. JAMA Surg. 2019;154:209-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 391] [Article Influence: 65.2] [Reference Citation Analysis (0)] |
| 23. | Otto G, Pitton MB, Hoppe-Lotichius M, Weinmann A. Liver transplantation and BCLC classification: Limitations impede optimum treatment. Hepatobiliary Pancreat Dis Int. 2021;20:6-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Wei H, Jiang H, Zheng T, Zhang Z, Yang C, Ye Z, Duan T, Song B. LI-RADS category 5 hepatocellular carcinoma: preoperative gadoxetic acid-enhanced MRI for early recurrence risk stratification after curative resection. Eur Radiol. 2021;31:2289-2302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 25. | Xie D, Shi J, Zhou J, Fan J, Gao Q. Clinical practice guidelines and real-life practice in hepatocellular carcinoma: A Chinese perspective. Clin Mol Hepatol. 2023;29:206-216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 81] [Reference Citation Analysis (0)] |
| 26. | Xie DY, Zhu K, Ren ZG, Zhou J, Fan J, Gao Q. A review of 2022 Chinese clinical guidelines on the management of hepatocellular carcinoma: updates and insights. Hepatobiliary Surg Nutr. 2023;12:216-228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 89] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 27. | Ahn SJ, Kim JH, Park SJ, Kim ST, Han JK. Hepatocellular carcinoma: preoperative gadoxetic acid-enhanced MR imaging can predict early recurrence after curative resection using image features and texture analysis. Abdom Radiol (NY). 2019;44:539-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 73] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 28. | Lee S, Kim SH, Lee JE, Sinn DH, Park CK. Preoperative gadoxetic acid-enhanced MRI for predicting microvascular invasion in patients with single hepatocellular carcinoma. J Hepatol. 2017;67:526-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 346] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 29. | Zhang Z, Jiang H, Chen J, Wei Y, Cao L, Ye Z, Li X, Ma L, Song B. Hepatocellular carcinoma: radiomics nomogram on gadoxetic acid-enhanced MR imaging for early postoperative recurrence prediction. Cancer Imaging. 2019;19:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 98] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 30. | Lei Z, Li J, Wu D, Xia Y, Wang Q, Si A, Wang K, Wan X, Lau WY, Wu M, Shen F. Nomogram for Preoperative Estimation of Microvascular Invasion Risk in Hepatitis B Virus-Related Hepatocellular Carcinoma Within the Milan Criteria. JAMA Surg. 2016;151:356-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 444] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 31. | Huang K, Dong Z, Cai H, Huang M, Peng Z, Xu L, Jia Y, Song C, Li ZP, Feng ST. Imaging biomarkers for well and moderate hepatocellular carcinoma: preoperative magnetic resonance image and histopathological correlation. BMC Cancer. 2019;19:364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 32. | Shen J, Liu J, Li C, Wen T, Yan L, Yang J. The Impact of Tumor Differentiation on the Prognosis of HBV-Associated Solitary Hepatocellular Carcinoma Following Hepatectomy: A Propensity Score Matching Analysis. Dig Dis Sci. 2018;63:1962-1969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 33. | Mo ZY, Chen PY, Lin J, Liao JY. Pre-operative MRI features predict early post-operative recurrence of hepatocellular carcinoma with different degrees of pathological differentiation. Radiol Med. 2023;128:261-273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |