Published online Mar 15, 2024. doi: 10.4251/wjgo.v16.i3.844
Peer-review started: October 20, 2023
First decision: December 5, 2023
Revised: December 15, 2023
Accepted: January 17, 2024
Article in press: January 17, 2024
Published online: March 15, 2024
Processing time: 144 Days and 3.6 Hours
Hepatocellular carcinoma (HCC) is one of the most common types of cancers worldwide, ranking fifth among men and seventh among women, resulting in more than 7 million deaths annually. With the development of medical tech
To determine the independent risk factors for CVD death in HCC patients and predict cardiovascular mortality (CVM) in HCC patients.
This study was conducted on the basis of the Surveillance, Epidemiology, and End Results database and included HCC patients with a diagnosis period from 2010 to 2015. The independent risk factors were identified using the Fine-Gray model. A nomograph was constructed to predict the CVM in HCC patients. The nomograph performance was measured using Harrell’s concordance index (C-index), calibration curve, receiver operating characteristic (ROC) curve, and area under the ROC curve (AUC) value. Moreover, the net benefit was estimated via decision curve analysis (DCA).
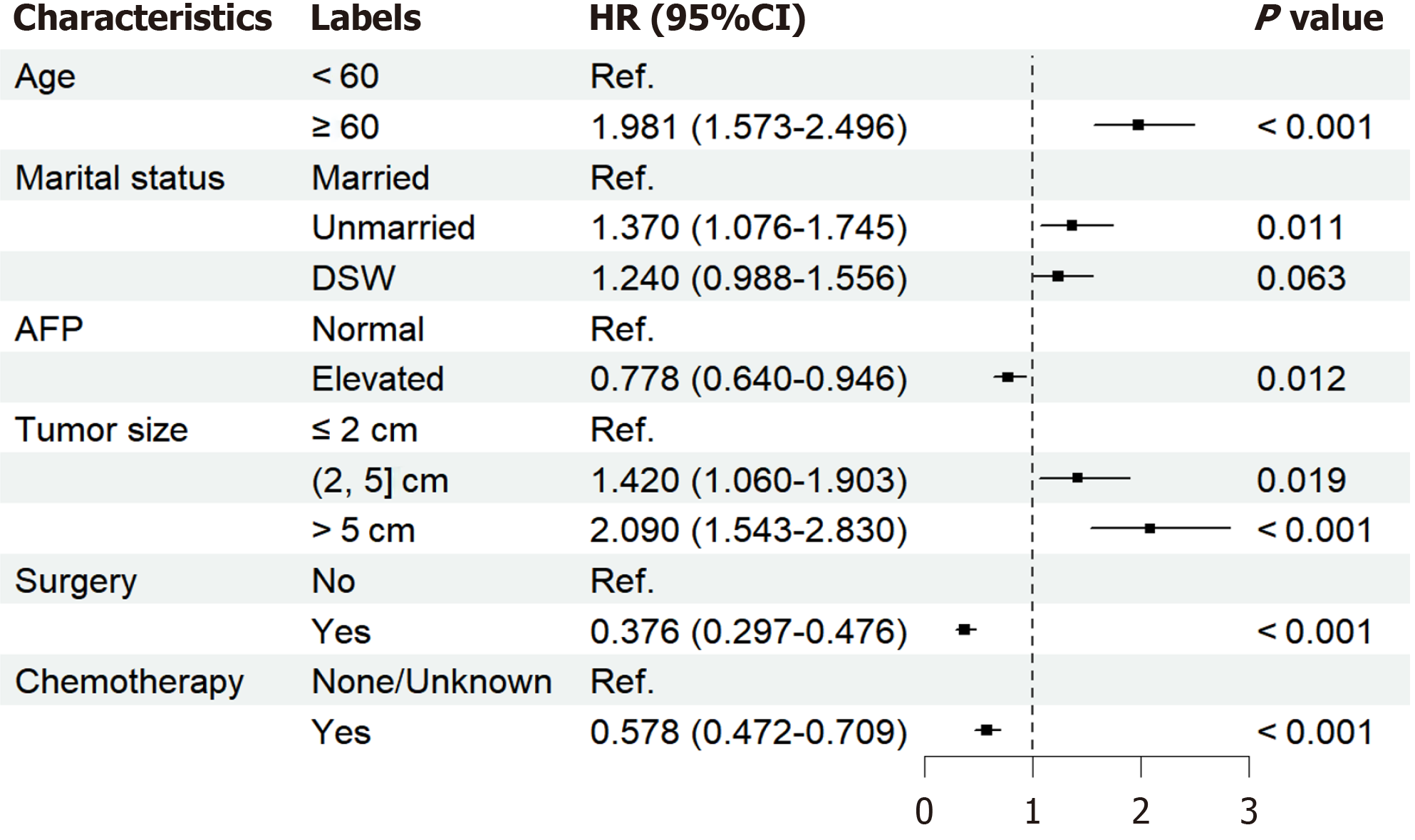
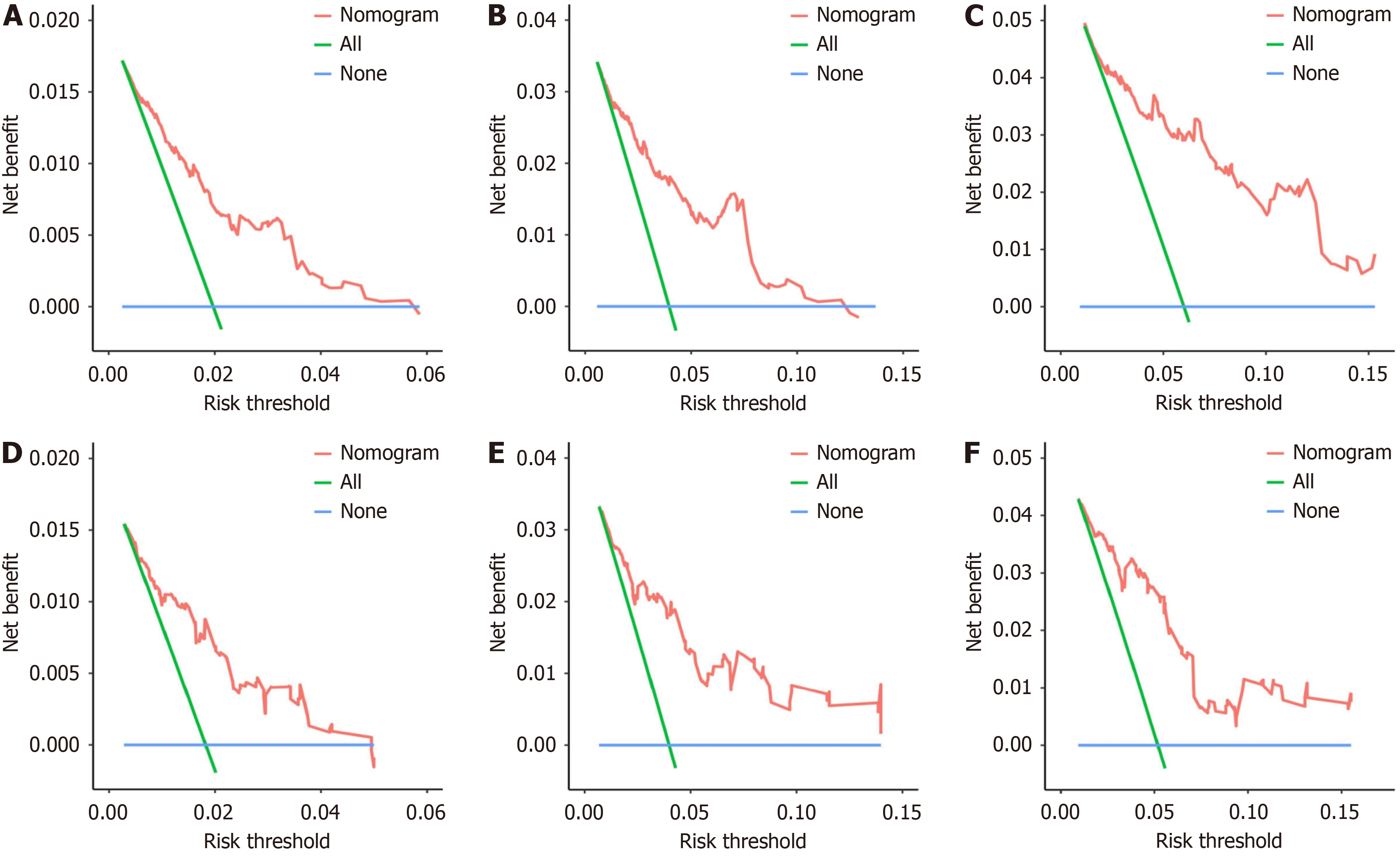
The study included 21545 HCC patients, of whom 619 died of CVD. Age (< 60) [1.981 (1.573-2.496), P < 0.001], marital status (married) [unmarried: 1.370 (1.076-1.745), P = 0.011], alpha fetoprotein (normal) [0.778 (0.640-0.946), P = 0.012], tumor size (≤ 2 cm) [(2, 5] cm: 1.420 (1.060-1.903), P = 0.019; > 5 cm: 2.090 (1.543-2.830), P < 0.001], surgery (no) [0.376 (0.297-0.476), P < 0.001], and chemotherapy(none/unknown) [0.578 (0.472-0.709), P < 0.001] were independent risk factors for CVD death in HCC patients. The discrimination and calibration of the nomograph were better. The C-index values for the training and validation sets were 0.736 and 0.665, respectively. The AUC values of the ROC curves at 2, 4, and 6 years were 0.702, 0.725, 0.740 in the training set and 0.697, 0.710, 0.744 in the validation set, respectively. The calibration curves showed that the predicted probabilities of the CVM prediction model in the training set vs the validation set were largely consistent with the actual probabilities. DCA demonstrated that the prediction model has a high net benefit.
Risk factors for CVD death in HCC patients were investigated for the first time. The nomograph served as an important reference tool for relevant clinical management decisions.
Core Tip: Hepatocellular carcinoma (HCC) is one of the most prevalent cancers worldwide. Studies have shown that HCC patients have chance to improve 5-year survival rate to 70%. How to avoid cardiovascular disease (CVD) death in HCC patients has become a problem worth exploring due to the course of treatment and the manifestation of certain paraneoplastic syndromes. In this study, we used Fine-Gray model to identify the independent risk factors for CVD death in HCC patients and constructed a predictive nomograph with high performance.
- Citation: Zhang YL, Liu ZR, Liu Z, Bai Y, Chi H, Chen DP, Zhang YM, Cui ZL. Risk of cardiovascular death in patients with hepatocellular carcinoma based on the Fine-Gray model. World J Gastrointest Oncol 2024; 16(3): 844-856
- URL: https://www.wjgnet.com/1948-5204/full/v16/i3/844.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i3.844
Hepatocellular carcinoma (HCC) is one of the most common cancers worldwide, ranking fifth among men and seventh among women, resulting in more than 7 million deaths annually[1,2]. Cardiovascular disease (CVD), which includes heart disease and stroke, is the most prevalent noncommunicable disease (NCD)[3]. CVD is also the major cause of mor
In the last decade, CVD has been recognized as one of the most frequent advanced comorbidities of cancer treatment[5]. Advances in therapeutic approaches, especially the advent of immunotherapies, have revolutionized cancer treat
Traditional survival analyses typically focus on only one outcome event, and ignoring observational endpoints in medi
The Surveillance, Epidemiology, and End Results (SEER) database is a publically accessible, federally funded cancer reporting system that represents the collaboration between the Centers for Disease Control and Prevention, the National Cancer Institute, and regional and state cancer registries and serves as the authoritative cancer statistics database in the United States[21]. The SEER database contains data extracted from[18] different geographic populations, representing rural, urban, and regional populations[22]. The aim of this study was to investigate the independent influencing factors of CVD death in HCC patients and to construct a predictive model by analyzing HCC patients (age ≥ 18 years) diagnosed between 2010 and 2015 in. the SEER database to assess the probability of CVD death in HCC patients while effectively avoiding death due to CVD, improving prognosis, and improving the quality of life of HCC patients.
HCC patient information was extracted from the SEER database via SEER stat 8.4.0.1 with the liver site code C22.0, excluding Fibrolamellar histology (8171/3)[23]. The inclusion criteria were as follows: (1) Patients aged 18 years or older and pathologically diagnosed with HCC; (2) diagnosed between 2010 and 2015; and (3) complete follow-up data. The patient information collected includes age, sex, race, marital status, year of diagnosis, pretreatment alpha fetoprotein (AFP) level, American Joint Committee on Cancer (AJCC) stage group, T stage, N stage, M stage, surgery, radiotherapy, and chemotherapy status, survival time, and cause of death. This study used the 7th edition of the AJCC staging. Data on patients with any of the abovementioned missing information were excluded (Supplementary Figure 1).
Death due to CVD was the primary observational endpoint. According to the SEER database, causes of death due to CVD include hypertension without heart disease, heart diseases, cerebrovascular diseases, aortic aneurysm and dissection, atherosclerosis, and other diseases of arteries, arterioles, and capillaries. Death from other causes was considered a competing event, and survival at the end of the study was considered a censored event.
In this study, categorical information was statistically described by number and percentage. The R software was used to divide all the study subjects into two parts in a ratio of 7:3, which were the training set and the validation set. The balance test between the two sets was performance using χ2 test. In the training test, the Fine-Gray model was used for univariate and multivariate analyses. Multivariate analysis of statistically significant indicators in univariate analysis to explore risk factors for CVD death in HCC patients, which was measured as the adjusted hazard ratio (HR) and 95% confidence inter
All statistical analyses for this study were conducted using SPSS 25.0 and the R software (version 4.2.2). The packages used included survival, caret, risk, regression, foreign, state, pROC, ggDCA, and pe. Furthermore, all tests were bilateral, and statistical significance was set at a P value of < 0.05.
In this study, 40401 HCC patients from the SEER database were included. Moreover, 45 patients under the age of 18; 39 patients with a T stage of T0; 5306 patients with missing or zero survival time; and 8966 patients with missing clinical data were excluded. Finally, 21545 HCC patients were included in the statistical analysis. Table 1 shows the detailed cha
| Characteristics | Labels | Participants, n (%) | Alive, n (%) | CVD deaths, n (%) |
| Age | < 60 | 7667 (35.59) | 2142 (39.64) | 143 (23.10) |
| ≥ 60 | 13878 (64.41) | 3262 (60.36) | 476 (76.90) | |
| Sex | Male | 16508 (76.62) | 3955 (73.19) | 487 (78.68) |
| Female | 5037 (23.38) | 1449 (26.81) | 132 (21.32) | |
| Race | White | 14789 (68.64) | 3558 (65.84) | 441 (71.24) |
| Black | 2949 (13.69) | 611 (11.31) | 96 (15.51) | |
| Other | 3807 (17.67) | 1235 (22.85) | 82 (13.25) | |
| Marital status | Married | 11408 (52. 95) | 3315 (61.34) | 292 (47.17) |
| Unmarried | 4778 (22.18) | 1023 (18.93) | 147 (23.75) | |
| DSW | 5359 (24.87) | 1066 (19.73) | 180 (29.08) | |
| Year of diagnosis | 2010-2011 | 6234 (28.93) | 1158 (21.43) | 197 (31.83) |
| 2012-2013 | 7189 (33.37) | 1583 (29.29) | 203 (32.79) | |
| 2014-2015 | 8122 (37.70) | 2663 (49.28) | 219 (35.38) | |
| AFP | Normal | 6324 (29.35) | 2239 (41.43) | 239 (38.61) |
| Elevated | 15221 (70.65) | 3165 (58.57) | 380 (61.39) | |
| Grade | GradeⅠ | 9423 (43.74) | 3384 (62.62) | 340 (54.93) |
| GradeⅡ | 5199 (24.13) | 1532 (28.35) | 139 (22.46) | |
| GradeⅢ | 4153 (19.28) | 362 (6.70) | 101 (16.32) | |
| GradeⅣ | 2770 (12.85) | 126 (2.33) | 39 (6.29) | |
| Tumor size | ≤ 2 cm | 3227 (14.98) | 1420 (26.28) | 88 (14.22) |
| 2-5 cm | 10029 (46.55) | 3038 (56.22) | 290 (46.85) | |
| > 5 cm | 8289 (38.47) | 946 (17.50) | 241 (38.93) | |
| T stage | T1 | 10049 (46.64) | 3429 (63.45) | 353 (57.03) |
| T2 | 5668 (26.31) | 1561 (28.89) | 144 (23.26) | |
| T3 | 5191 (24.09) | 373 (6.90) | 106 (17.12) | |
| T4 | 637 (2.96) | 41 (0.76) | 16 (2.59) | |
| N stage | N0 | 20139 (93.47) | 5330 (98.63) | 599 (96.77) |
| N1 | 1406 (6.53) | 74 (1.37) | 20 (3.23) | |
| M stage | M0 | 19595 (90.95) | 5339 (98.80) | 593 (95.80) |
| M1 | 1950 (9.05) | 65 (1.20) | 26 (4.20) | |
| Surgery | No | 15113 (70.15) | 2177 (40.28) | 460 (74.31) |
| Yes | 6432 (29.85) | 3227 (59.72) | 159 (25.69) | |
| Radiotherapy | None/Unknown | 19400 (90.04) | 5031 (93.10) | 567 (91.60) |
| Yes | 2145 (9.96) | 373 (6.90) | 52 (8.40) | |
| Chemotherapy | None/Unknown | 10566 (49.04) | 2837 (52.50) | 338 (54.60) |
| Yes | 10979 (50.96) | 2567 (47.50) | 281 (45.40) |
As shown in Table 2, no significant differences in basic characteristics were observed between the HCC patients in the training and validation sets (P > 0.05). The results revealed that the distributions of each feature of the HCC patients in the training and validation sets were the same and the resulting nomogram prediction model in the training set could be validated in the validation set.
| Characteristics | Labels | Training set (N1 = 15081) | Validation set (N2 = 6464) | P value |
| Age | 0.835 | |||
| < 60 | 5360 (35.54) | 2307 (35.69) | ||
| ≥ 60 | 9721 (64.46) | 4157 (64.31) | ||
| Sex | 0.994 | |||
| Male | 11555 (76.62) | 4953 (76.62) | ||
| Female | 3526 (23.38) | 1511 (23.38) | ||
| Race | 0.598 | |||
| White | 10340 (68.56) | 4449 (68.83) | ||
| Black | 2087 (13.84) | 862 (13.34) | ||
| Other | 2654 (17.60) | 1153 (17.83) | ||
| Marital status | 0.552 | |||
| Married | 7953 (52.74) | 3455 (53.45) | ||
| Unmarried | 3348 (22.20) | 1430 (22.12) | ||
| DSW | 3780 (25.06) | 1579 (24.43) | ||
| Year of diagnosis | 0.381 | |||
| 2010-2011 | 4395 (29.14) | 1839 (28.45) | ||
| 2012-2013 | 4991 (33.09) | 2198 (34.00) | ||
| 2014-2015 | 5695 (37.77) | 2427 (37.55) | ||
| AFP | 0.231 | |||
| Normal | 4390 (29.11) | 1934 (29.92) | ||
| Elevated | 10691 (70.89) | 4530 (70.08) | ||
| Grade | 0.160 | |||
| GradeⅠ | 6574 (43.59) | 2849 (44.07) | ||
| GradeⅡ | 3692 (24.48) | 1507 (23.31) | ||
| GradeⅢ | 2864 (18.99) | 1289 (19.94) | ||
| GradeⅣ | 1951 (12.94) | 819 (12.68) | ||
| Tumor size | 0.201 | |||
| ≤ 2 cm | 2301 (15.26) | 926 (14.33) | ||
| 2-5 cm | 6986 (46.32) | 3043 (47.08) | ||
| > 5 cm | 5794 (38.42) | 2495 (38.59) | ||
| T stage | 0.193 | |||
| T1 | 7025 (46.58) | 3024 (46.78) | ||
| T2 | 4018 (26.64) | 1650 (25.53) | ||
| T3 | 3585 (23.77) | 1606 (24.85) | ||
| T4 | 453 (3.01) | 184 (2.84) | ||
| N stage | 0.554 | |||
| N0 | 14087 (93.41) | 6052 (93.63) | ||
| N1 | 994 (6.59) | 412 (6.37) | ||
| M stage | 0.680 | |||
| M0 | 13724 (91.00) | 5871 (90.83) | ||
| M1 | 1357 (9.00) | 593 (9.17) | ||
| Surgery | 0.877 | |||
| No | 10574 (70.11) | 4539 (70.22) | ||
| Yes | 4507 (29.89) | 1925 (29.78) | ||
| Radiotherapy | 0.675 | |||
| None/unknown | 13588 (90.10) | 5812 (89.91) | ||
| Yes | 1493 (9.90) | 652 (10.09) | ||
| Chemotherapy | 0.180 | |||
| None/unknown | 7441 (49.34) | 3125 (48.34) | ||
| Yes | 7640 (50.66) | 3339 (51.66) |
As shown in Table 3, the HCC patients in the entire cohort were randomly assigned to the training set (N1 = 15081) vs the validation set (N2 = 6464) in a 7:3 ratio. The univariate analysis of the training set data revealed that age (HR, 2.054; 95%CI: 1.637-2.576), race [other (HR, 0.653; 95%CI: 0.493-0.864)], marital status [unmarried (HR, 1.322; 95%CI: 1.042-1.678); DSW (HR, 1.377; 95%CI: 1.099-1.726)], AFP (HR, 0.786; 95%CI: 0.647-0.954), AJCC stage group [grad Ⅱ (HR, 0.775; 95%CI: 0.611-0.982)], tumor size [(2, 5) cm (HR, 1.361; 95%CI: 1.018-1.821); > 5 cm (HR, 2.254; 95%CI: 1.667-3.048)], T stage [T2 (HR, 0.761; 95%CI: 0.602-0.960); T4 (HR, 1.806; 95%CI: 1.033-3.159)], surgery (HR, 0.447; 95%CI: 0.359-0.557), and chemotherapy (HR, 0.770; 95%CI: 0.637-0.931) were risk factors of CVD death in HCC patients.
| Characteristics | Labels | HR (95%CI) | P value |
| Age | < 60 | Ref. | |
| ≥ 60 | 2.054 (1.637-2.576) | < 0.001 | |
| Sex | Male | Ref. | |
| Female | 0.872 (0.694-1.095) | 0.237 | |
| Race | White | Ref. | |
| Black | 1.089 (0.830-1.428) | 0.539 | |
| Other | 0.653 (0.493-0.864) | 0.003 | |
| Marital status | Married | Ref. | |
| Unmarried | 1.322 (1.042-1.678) | 0.022 | |
| DSW | 1.377 (1.099-1.726) | 0.006 | |
| Year of diagnosis | 2010-2011 | Ref. | |
| 2012-2013 | 0.970 (0.764-1.231) | 0.802 | |
| 2014-2015 | 1.076 (0.846-1.370) | 0.549 | |
| AFP | Normal | Ref. | |
| Elevated | 0.786 (0.647-0.954) | 0.015 | |
| Grade | GradeⅠ | Ref. | |
| GradeⅡ | 0.775 (0.611-0.982) | 0.035 | |
| GradeⅢ | 1.263 (0.961-1.659) | 0.094 | |
| GradeⅣ | 0.860 (0.563-1.314) | 0.486 | |
| Tumor size | ≤ 2 cm | Ref. | |
| 2-5 cm | 1.361 (1.018-1.821) | 0.038 | |
| > 5 cm | 2.254 (1.667-3.048) | < 0.001 | |
| T stage | T1 | Ref. | |
| T2 | 0.761 (0.602-0.960) | 0.022 | |
| T3 | 1.110 (0.844-1.460) | 0.454 | |
| T4 | 1.806 (1.033-3.159) | 0.038 | |
| N stage | N0 | Ref. | |
| N1 | 0.732 (0.400-1.339) | 0.311 | |
| M stage | M0 | Ref. | |
| M1 | 1.039 (0.650-1.662) | 0.872 | |
| Surgery | No | Ref. | |
| Yes | 0.447 (0.359-0.557) | < 0.001 | |
| Radiotherapy | None/unknown | Ref. | |
| Yes | 1.203 (0.877-1.650) | 0.253 | |
| Chemotherapy | None/unknown | Ref. | |
| Yes | 0.770 (0.637-0.931) | 0.007 |
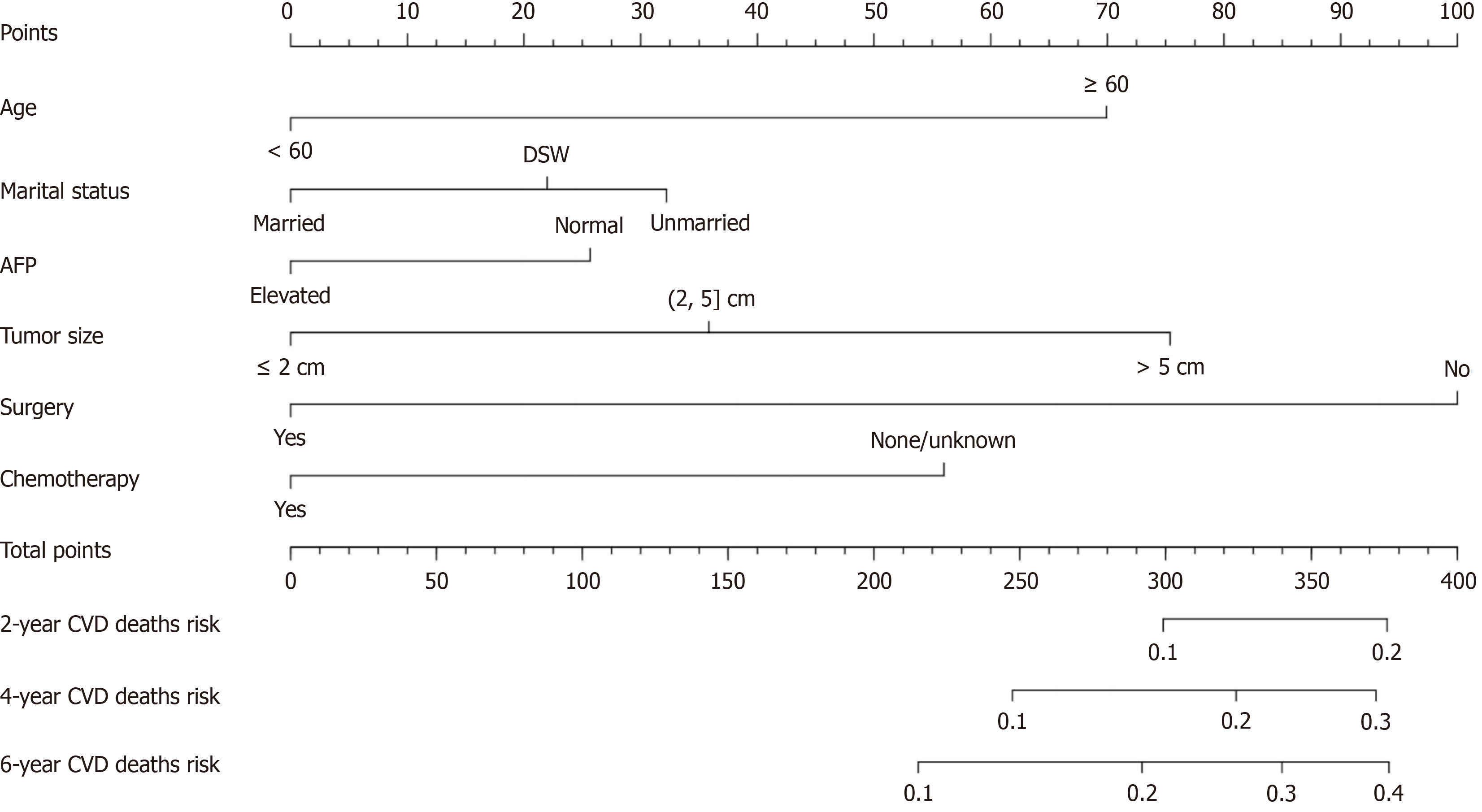
As shown in Figure 1, the variables that were statistically significant in the univariate analysis were included in the multivariate analysis. After adjustment of the model, the following independent risk factors for CVD death in HCC patients were finally obtained, including age (HR, 1.981; 95%CI: 1.573-2.496), marital status [unmarried (HR, 1.370; 95%CI: 1.076-1.745); DSW (HR, 1.240; 95%CI: 0.988-1.556)], AFP (HR, 0.778; 95%CI: 0.640-0.946), tumor size [(2, 5) cm (HR, 1.420; 95%CI: 1.060-1.903); > 5 cm (HR, 2.090; 95%CI: 1.543-2.830), surgery (HR, 0.376; 95%CI: 0.297-0.476), and chemotherapy (HR, 0.578; 95%CI: 0.472-0.709)].
Based on the results of the multifactorial analysis, the six variables of age, marital status, AFP, tumor size, surgery, and chemotherapy were incorporated into the prediction model of CVD death in HCC patients, and a nomograph was con
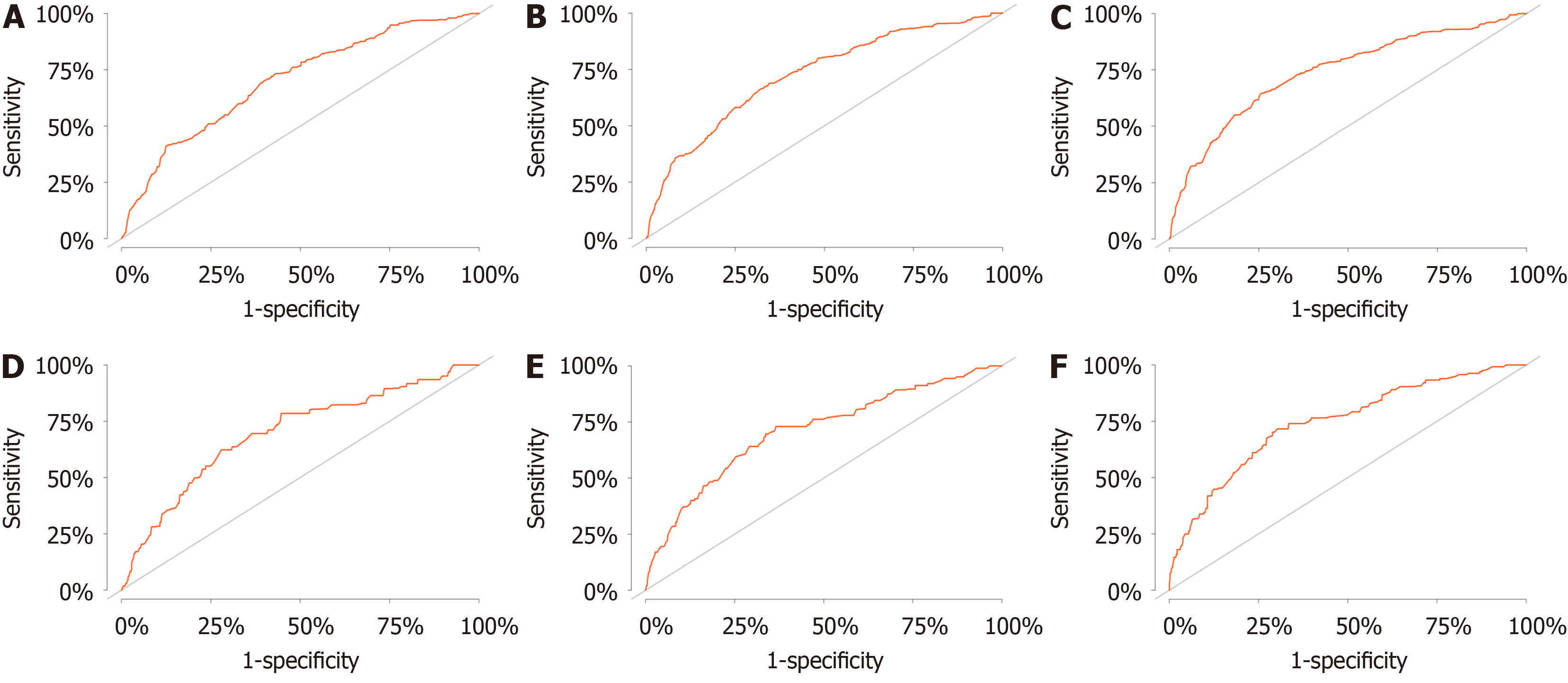
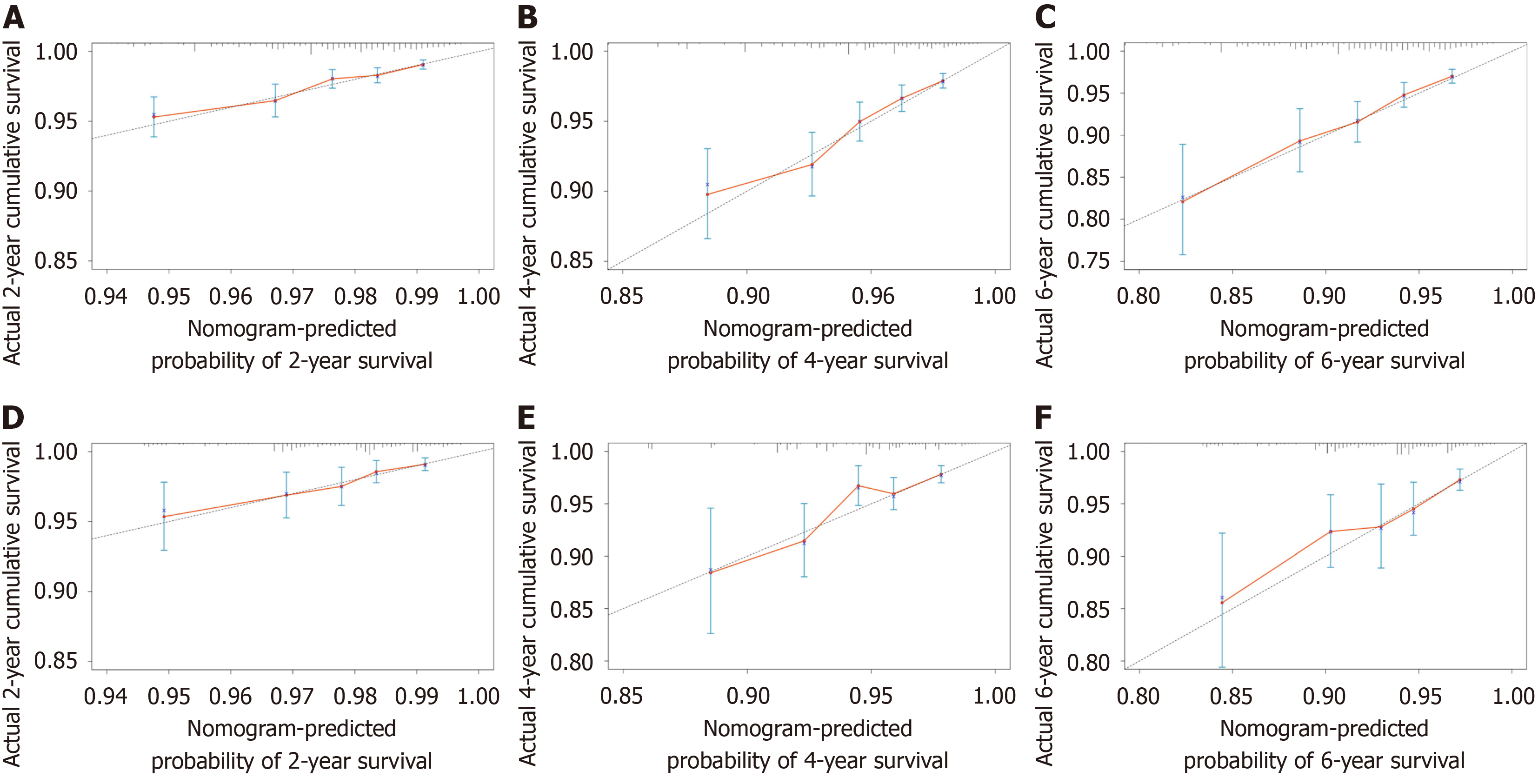
This study used the data from both the training and validation sets to estimate the constructed nomogram model in terms of discrimination and calibration. The evaluation of the degree of discrimination was performed using the C-index obtained from bootstrap resampling, plotting the ROC curve, and calculating the AUC value. The C-index values were 0.736 and 0.665 in the training and development sets, respectively. Figure 3 shows the ROC curves of the nomogram model to predict the 2-, 4-, and 6-year cardiovascular mortalitys (CVMs) in HCC patients, with AUC values of 0.702, 0.725, and 0.740 in the training set and 0.697, 0.710, and 0.744 in the validation set. The AUC values were generally greater than 0.7, which indicated that the discrimination of the nomogram model was good. The calibration was evaluated by plotting the calibration curves of the training and development sets. If the predicted probability is close to the actual pro
Currently, CVD and cancer are the primary causes of premature death in 127 countries[25]. Research has shown that the risk of CVD among cancer survivors is associated with common lifestyles or the toxicity of cancer treatment[26]. For cancer patients, increasingly refined treatment options have greatly extended their survival. Therefore, cardiovascular care for cancer survivors should be emphasized to meet their clinical needs and improve their quality of life. This study is based on the SEER database and used the data of HCC patients with a diagnosis period from 2010 to 2015, which has a high clinical application value.
The factors associated with the CVD outcomes in HCC patients included age, marital status, pretreatment AFP level, tumor size, surgical status, and chemotherapy status. Consistent with the majority of most studies, we observed that the risk of CVD death in HCC patients increased with age[6,27,28]. The American College of Cardiology revealed that advancing age can seriously affect its estimated 10-year CVD event risk[28]. This may be associated with poorer physical fitness and longer acting time of lifestyle risk factors in elderly patients[29]. Moreover, the present study revealed that unmarried people have a significantly increased risk of CVD death compared with married people, which is consistent with previous research results[30-32]. It was revealed that marriage can have a beneficial effect on health by providing social support[33-35]. The higher risk for unmarried individuals compared with married individuals may be due to a combination of lifestyle, body hormones, and stress. Numerous studies have also revealed that unmarried individuals have higher levels of loneliness, lower life satisfaction, and higher mortality from physical illness[36]. Moreover, it is currently well documented that all different unmarried states are associated with an elevated risk of mortality[37]. The findings of the present study revealed that the HCC patients with pretreatment AFP levels above normal had a reduced risk of CVD death. This may be due to the combined effects of interventions taken earlier when AFP positivity is present and the participants’ spontaneous health behavior changes that are effective in protecting their cardiovascular health, which in turn reaches the death-lowering effect of CVD. Studies assessing the link between AFP and CVD are limited, but an inverse association between AFP and CVD prevalence was proven in the study by Bracun et al[38], which is consistent with the results of the present study. Therefore, when the AFP levels are at normal levels in HCC patients, the importance of cardiovascular system care should be increased to avoid the occurrence of CVD death in HCC patients as much as possible. When categorizing tumor size, the risk of CVD death in HCC patients increases as tumor size increases. Recent studies have shown an inverse association between tumor size and CVD death[39,40]. The findings of this analysis suggest that HCC patients undergoing surgery have a significantly lower risk of CVD death. This finding is in agreement with those of previous studies[5,17,41]. It is worth noting that chemotherapy usually increases the risk of CVD because of the cardiotoxicity associated with this treatment modality[42]. Transcatheter arterial chemoembolization (TACE) is the most common first-line treatment, while doxorubicin (DOX) is the most frequently used chemotherapy drug[43]. More
Most previous studies on the relationship between cancer and CVD death have used traditional survival analysis methods, such as Cox proportional-hazards regression models. The model does not well distinguish between the effects of competing events and often overestimates the risk of outcome events. In this study, we used the Fine-Gray model to exploit the independent hazard factors for CVD death in HCC patients and to construct a related predictive model. Based on the literature, the present study is the first to investigate the relationship between HCC and CVD death. The predi
The strengths of this study are as follows: (1) Adequate sample size; (2) less missing information; and (3) its emphasis on the association between HCC and CVD. However, this study has several limitations. First, this study has a retro
Overall, this is the first study to investigate the independent risk factors for CVD death in HCC patients using data from the SEER database and construct a relevant prediction model. With high discrimination, calibration, and net benefit, the model effectively assessed CVMs in HCC patients and was able to serve as an important reference tool for relevant clinical management decisions in HCC patients. However, based on the lack of external data validation, the model remains to be further verified by further research.
Hepatocellular carcinoma (HCC) is one of the most common tumors today. It is known that patients with HCC will have a higher risk of cardiovascular disease (CVD) death compared to non-HCC patients.
CVD is recognized as one of the most common complications of cancer treatment. As medical technology continues to mature, studies have found that the 5-year survival rate for HCC patients can be increased to 70% with early diagnosis and some potential treatments. Just because there are some unique treatment modalities (e.g. Transcatheter arterial che
The aim of this study was to identify the independent risk factors for CVD death in HCC patients, and to further provide a reference tool for the relevant clinical management decisions of HCC patients by constructing a prediction model for CVD death in HCC patients.
In this study, data related to adult HCC patients with diagnosis years 2010-2015 in the Surveillance, Epidemiology, and End Results database were collected. In order to better eliminate the influence of competing events on the study, we utilized the Fine-Gray model to carry out the analysis and constructed a predictive model.
The study included 21545 patients with HCC, of whom 619 died of CVD. Age, marital status, alpha fetoprotein, tumor size, surgery, and chemotherapy were independent risk factors for CVD death in HCC patients. The discrimination as well as the calibration of the nomograph was better. Decision curve analysis demonstrated that the prediction model has a high net benefit.
This study focuses on the cardiovascular risk of HCC patients for the first time. Meanwhile, the independent risk factors for CVD deaths in HCC patients were explored for the first time based on the Fine-Gray model, and a prediction model was constructed, which will serve as a reminder for future clinical work.
Focusing on the burden of CVD in HCC patients and further exploring the impact of different drugs and routes of administration on CVD death in HCC patients.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Shelat VG, Singapore S-Editor: Qu XL L-Editor: A P-Editor: Xu ZH
| 1. | Wang W, Wei C. Advances in the early diagnosis of hepatocellular carcinoma. Genes Dis. 2020;7:308-319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 292] [Article Influence: 58.4] [Reference Citation Analysis (0)] |
| 2. | Sharma SA, Kowgier M, Hansen BE, Brouwer WP, Maan R, Wong D, Shah H, Khalili K, Yim C, Heathcote EJ, Janssen HLA, Sherman M, Hirschfield GM, Feld JJ. Toronto HCC risk index: A validated scoring system to predict 10-year risk of HCC in patients with cirrhosis. J Hepatol. 2017;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 133] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 3. | Silveira EA, Kliemann N, Noll M, Sarrafzadegan N, de Oliveira C. Visceral obesity and incident cancer and cardiovascular disease: An integrative review of the epidemiological evidence. Obes Rev. 2021;22:e13088. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 115] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 4. | Damen JA, Hooft L, Schuit E, Debray TP, Collins GS, Tzoulaki I, Lassale CM, Siontis GC, Chiocchia V, Roberts C, Schlüssel MM, Gerry S, Black JA, Heus P, van der Schouw YT, Peelen LM, Moons KG. Prediction models for cardiovascular disease risk in the general population: systematic review. BMJ. 2016;353:i2416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 597] [Cited by in RCA: 541] [Article Influence: 60.1] [Reference Citation Analysis (0)] |
| 5. | Zhang S, Wang Y, Zhang P, Ai L, Liu T. Cardiovascular Outcomes in the Patients With Colorectal Cancer: A Multi-Registry-Based Cohort Study of 197,699 Cases in the Real World. Front Cardiovasc Med. 2022;9:851833. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 6. | Koene RJ, Prizment AE, Blaes A, Konety SH. Shared Risk Factors in Cardiovascular Disease and Cancer. Circulation. 2016;133:1104-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 924] [Cited by in RCA: 1041] [Article Influence: 115.7] [Reference Citation Analysis (0)] |
| 7. | Poels K, van Leent MMT, Boutros C, Tissot H, Roy S, Meerwaldt AE, Toner YCA, Reiche ME, Kusters PJH, Malinova T, Huveneers S, Kaufman AE, Mani V, Fayad ZA, de Winther MPJ, Marabelle A, Mulder WJM, Robert C, Seijkens TTP, Lutgens E. Immune Checkpoint Inhibitor Therapy Aggravates T Cell-Driven Plaque Inflammation in Atherosclerosis. JACC CardioOncol. 2020;2:599-610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 86] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 8. | Morad G, Helmink BA, Sharma P, Wargo JA. Hallmarks of response, resistance, and toxicity to immune checkpoint blockade. Cell. 2022;185:576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 107] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 9. | Kim DY, Han KH. Epidemiology and surveillance of hepatocellular carcinoma. Liver Cancer. 2012;1:2-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 137] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 10. | Padegimas A, Clasen S, Ky B. Cardioprotective strategies to prevent breast cancer therapy-induced cardiotoxicity. Trends Cardiovasc Med. 2020;30:22-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 11. | Hwang SJ, Luo JC, Li CP, Chu CW, Wu JC, Lai CR, Chiang JH, Chau GY, Lui WY, Lee CC, Chang FY, Lee SD. Thrombocytosis: a paraneoplastic syndrome in patients with hepatocellular carcinoma. World J Gastroenterol. 2004;10:2472-2477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 67] [Cited by in RCA: 75] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 12. | Sohda T, Iwata K, Kitamura Y, Suzuki N, Takeyama Y, Irie M, Anan A, Nakane H, Yoshikane M, Watanabe H, Sakisaka S. Reduced expression of low-density lipoprotein receptor in hepatocellular carcinoma with paraneoplastic hypercholesterolemia. J Gastroenterol Hepatol. 2008;23:e153-e156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Trinder M, Francis GA, Brunham LR. Association of Monogenic vs Polygenic Hypercholesterolemia With Risk of Atherosclerotic Cardiovascular Disease. JAMA Cardiol. 2020;5:390-399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 156] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 14. | Yoshikawa M, Takase O, Tsujimura T, Sano E, Hayashi M, Takato T, Hishikawa K. Long-term effects of low calcium dialysates on the serum calcium levels during maintenance hemodialysis treatments: A systematic review and meta-analysis. Sci Rep. 2018;8:5310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Xu M, Chen R, Liu L, Liu X, Hou J, Liao J, Zhang P, Huang J, Lu L, Chen L, Fan M, Chen X, Zhu X, Liu B, Hu P. Systemic immune-inflammation index and incident cardiovascular diseases among middle-aged and elderly Chinese adults: The Dongfeng-Tongji cohort study. Atherosclerosis. 2021;323:20-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 117] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 16. | Greenlee H, Iribarren C, Rana JS, Cheng R, Nguyen-Huynh M, Rillamas-Sun E, Shi Z, Laurent CA, Lee VS, Roh JM, Santiago-Torres M, Shen H, Hershman DL, Kushi LH, Neugebauer R, Kwan ML. Risk of Cardiovascular Disease in Women With and Without Breast Cancer: The Pathways Heart Study. J Clin Oncol. 2022;40:1647-1658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 91] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 17. | Gaitanidis A, Spathakis M, Tsalikidis C, Alevizakos M, Tsaroucha A, Pitiakoudis M. Risk factors for cardiovascular mortality in patients with colorectal cancer: a population-based study. Int J Clin Oncol. 2019;24:501-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 18. | Forster RB, Engeland A, Kvåle R, Hjellvik V, Bjørge T. Association between medical androgen deprivation therapy and long-term cardiovascular disease and all-cause mortality in nonmetastatic prostate cancer. Int J Cancer. 2022;151:1109-1119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 19. | Cooper H, Wells S, Mehta S. Are competing-risk models superior to standard Cox models for predicting cardiovascular risk in older adults? Analysis of a whole-of-country primary prevention cohort aged ≥65 years. Int J Epidemiol. 2022;51:604-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Austin PC, Putter H, Lee DS, Steyerberg EW. Estimation of the Absolute Risk of Cardiovascular Disease and Other Events: Issues With the Use of Multiple Fine-Gray Subdistribution Hazard Models. Circ Cardiovasc Qual Outcomes. 2022;15:e008368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 21. | Doll KM, Rademaker A, Sosa JA. Practical Guide to Surgical Data Sets: Surveillance, Epidemiology, and End Results (SEER) Database. JAMA Surg. 2018;153:588-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 307] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 22. | Liang W, He J, Shen Y, Shen J, He Q, Zhang J, Jiang G, Wang Q, Liu L, Gao S, Liu D, Wang Z, Zhu Z, Ng CS, Liu CC, Petersen RH, Rocco G, D'Amico T, Brunelli A, Chen H, Zhi X, Liu B, Yang Y, Chen W, Zhou Q. Impact of Examined Lymph Node Count on Precise Staging and Long-Term Survival of Resected Non-Small-Cell Lung Cancer: A Population Study of the US SEER Database and a Chinese Multi-Institutional Registry. J Clin Oncol. 2017;35:1162-1170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 269] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 23. | Ding J, Wen Z. Survival improvement and prognosis for hepatocellular carcinoma: analysis of the SEER database. BMC Cancer. 2021;21:1157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 76] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 24. | Deng Y, Zhang N, Hua W, Cheng S, Niu H, Chen X, Gu M, Cai C, Liu X, Huang H, Cai M, Zhang S. Nomogram predicting death and heart transplantation before appropriate ICD shock in dilated cardiomyopathy. ESC Heart Fail. 2022;9:1269-1278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Bray F, Laversanne M, Weiderpass E, Soerjomataram I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer. 2021;127:3029-3030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 924] [Cited by in RCA: 1232] [Article Influence: 308.0] [Reference Citation Analysis (0)] |
| 26. | Sturgeon KM, Deng L, Bluethmann SM, Zhou S, Trifiletti DM, Jiang C, Kelly SP, Zaorsky NG. A population-based study of cardiovascular disease mortality risk in US cancer patients. Eur Heart J. 2019;40:3889-3897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 470] [Cited by in RCA: 627] [Article Influence: 104.5] [Reference Citation Analysis (0)] |
| 27. | Mehta LS, Watson KE, Barac A, Beckie TM, Bittner V, Cruz-Flores S, Dent S, Kondapalli L, Ky B, Okwuosa T, Piña IL, Volgman AS; American Heart Association Cardiovascular Disease in Women and Special Populations Committee of the Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; and Council on Quality of Care and Outcomes Research. Cardiovascular Disease and Breast Cancer: Where These Entities Intersect: A Scientific Statement From the American Heart Association. Circulation. 2018;137:e30-e66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 502] [Cited by in RCA: 562] [Article Influence: 80.3] [Reference Citation Analysis (0)] |
| 28. | US Preventive Services Task Force, Davidson KW, Barry MJ, Mangione CM, Cabana M, Chelmow D, Coker TR, Davis EM, Donahue KE, Jaén CR, Krist AH, Kubik M, Li L, Ogedegbe G, Pbert L, Ruiz JM, Stevermer J, Tseng CW, Wong JB. Aspirin Use to Prevent Cardiovascular Disease: US Preventive Services Task Force Recommendation Statement. JAMA. 2022;327:1577-1584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 207] [Article Influence: 69.0] [Reference Citation Analysis (0)] |
| 29. | Fang F, Fall K, Mittleman MA, Sparén P, Ye W, Adami HO, Valdimarsdóttir U. Suicide and cardiovascular death after a cancer diagnosis. N Engl J Med. 2012;366:1310-1318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 308] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 30. | Celeng C, Takx RAP, Lessmann N, Maurovich-Horvat P, Leiner T, Išgum I, de Jong PA. The Association Between Marital Status, Coronary Computed Tomography Imaging Biomarkers, and Mortality in a Lung Cancer Screening Population. J Thorac Imaging. 2020;35:204-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Wong CW, Kwok CS, Narain A, Gulati M, Mihalidou AS, Wu P, Alasnag M, Myint PK, Mamas MA. Marital status and risk of cardiovascular diseases: a systematic review and meta-analysis. Heart. 2018;104:1937-1948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 146] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 32. | Wang Y, Jiao Y, Nie J, O'Neil A, Huang W, Zhang L, Han J, Liu H, Zhu Y, Yu C, Woodward M. Sex differences in the association between marital status and the risk of cardiovascular, cancer, and all-cause mortality: a systematic review and meta-analysis of 7,881,040 individuals. Glob Health Res Policy. 2020;5:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 73] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 33. | Waite LJ. Does marriage matter? Demography. 1995;32:483-507. [PubMed] |
| 34. | Hu YR, Goldman N. Mortality differentials by marital status: an international comparison. Demography. 1990;27:233-250. [PubMed] |
| 35. | Wyke S, Ford G. Competing explanations for associations between marital status and health. Soc Sci Med. 1992;34:523-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 148] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 36. | Srivastava S, Debnath P, Shri N, Muhammad T. The association of widowhood and living alone with depression among older adults in India. Sci Rep. 2021;11:21641. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 64] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 37. | Molloy GJ, Stamatakis E, Randall G, Hamer M. Marital status, gender and cardiovascular mortality: behavioural, psychological distress and metabolic explanations. Soc Sci Med. 2009;69:223-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 146] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 38. | Bracun V, Suthahar N, Shi C, de Wit S, Meijers WC, Klip IT, de Boer RA, Aboumsallem JP. Established Tumour Biomarkers Predict Cardiovascular Events and Mortality in the General Population. Front Cardiovasc Med. 2021;8:753885. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 39. | Chen C, Xu F, Yuan S, Zhao X, Qiao M, Han D, Lyu J. Competing risk analysis of cardiovascular death in patients with primary gallbladder cancer. Cancer Med. 2023;12:2179-2186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 40. | Leoce NM, Jin Z, Kehm RD, Roh JM, Laurent CA, Kushi LH, Terry MB. Modeling risks of cardiovascular and cancer mortality following a diagnosis of loco-regional breast cancer. Breast Cancer Res. 2021;23:91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 41. | Du B, Wang F, Wu L, Wang Z, Zhang D, Huang Z, Gao L, Li Y, Liang C, Li P, Yao R. Cause-specific mortality after diagnosis of thyroid cancer: a large population-based study. Endocrine. 2021;72:179-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 42. | Moslehi JJ. Cardiovascular Toxic Effects of Targeted Cancer Therapies. N Engl J Med. 2016;375:1457-1467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 458] [Article Influence: 50.9] [Reference Citation Analysis (0)] |
| 43. | Akada K, Koyama N, Taniguchi S, Miura Y, Aoshima K. Database analysis of patients with hepatocellular carcinoma and treatment flow in early and advanced stages. Pharmacol Res Perspect. 2019;7:e00486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 44. | Park SS, Lee DM, Lim JH, Lee D, Park SJ, Kim HM, Sohn S, Yoon G, Eom YW, Jeong SY, Choi EK, Choi KS. Pyrrolidine dithiocarbamate reverses Bcl-xL-mediated apoptotic resistance to doxorubicin by inducing paraptosis. Carcinogenesis. 2018;39:458-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 45. | Weberpals J, Jansen L, Müller OJ, Brenner H. Long-term heart-specific mortality among 347 476 breast cancer patients treated with radiotherapy or chemotherapy: a registry-based cohort study. Eur Heart J. 2018;39:3896-3903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 46. | Liu F, Hidru TH, Gao R, Lin Y, Liu Y, Fang F, Liu J, Li H, Yang X, Xia Y. Cancer patients with potential eligibility for vascular endothelial growth factor antagonists use have an increased risk for cardiovascular diseases comorbidities. J Hypertens. 2020;38:426-433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |