Published online Mar 15, 2024. doi: 10.4251/wjgo.v16.i3.787
Peer-review started: August 22, 2023
First decision: November 21, 2023
Revised: December 19, 2023
Accepted: January 22, 2024
Article in press: January 22, 2024
Published online: March 15, 2024
Processing time: 203 Days and 2.7 Hours
Patatin like phospholipase domain containing 8 (PNPLA8) has been shown to play a significant role in various cancer entities. Previous studies have focused on its roles as an antioxidant and in lipid peroxidation. However, the role of PNPLA8 in colorectal cancer (CRC) progression is unclear.
To explore the prognostic effects of PNPLA8 expression in CRC.
A retrospective cohort containing 751 consecutive CRC patients was enrolled. PNPLA8 expression in tumor samples was evaluated by immunohistochemistry staining and semi-quantitated with immunoreactive scores. CRC patients were divided into high and low PNPLA8 expression groups based on the cut-off va
PNPLA8 expression was significantly associated with distant metastases in our cohort (P = 0.048). CRC patients with high PNPLA8 expression indicated poor OS (median OS = 35.3, P = 0.005). CRC patients with a higher PNPLA8 expression at either stage I and II or stage III and IV had statistically significant shorter OS. For patients with left-sided colon and rectal cancer, the survival curves of two PN
PNPLA8 is a novel independent prognostic factor for CRC. These findings suggest that PNPLA8 is a potential target in clinical CRC management.
Core Tip: Patatin like phospholipase domain containing 8 (PNPLA8) has been shown to be associated with a variety of cancers, but its role in the progression of colorectal cancer (CRC) is unclear. In this study, 751 consecutive CRC patients were retrospectively analyzed. The results of this study indicate that PNPLA8 is a new independent prognostic factor for CRC. High PNPLA8 expression in CRC leads to impaired survival. These findings suggest that PNPLA8 is a potential target for clinical CRC therapy, providing important insights to help personalize therapy for CRC patients.
- Citation: Zhou PY, Zhu DX, Chen YJ, Feng QY, Mao YH, Zhuang AB, Xu JM. High patatin like phospholipase domain containing 8 expression as a biomarker for poor prognosis of colorectal cancer. World J Gastrointest Oncol 2024; 16(3): 787-797
- URL: https://www.wjgnet.com/1948-5204/full/v16/i3/787.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i3.787
Colorectal cancer (CRC) is among the deadliest tumors[1]. The only curative treatment for localized CRC is surgery, and patients with lymph node metastases are usually advised to undergo adjuvant chemotherapy[2]. The relatively low 5-year survival rate of about 56.9% is further affected by inadequate screening methods and increasing resistance to chemotherapy during the clinical course[3,4]. Currently, several reliable prognostic factors are widely used in clinical practice, such as molecular subtype, therapeutic response to previous adjuvant chemotherapy, time between adjuvant therapy and metastasis development (shorter is associated with poorer prognosis), comorbidities, and frailty[5,6]. There
Patatin like phospholipase domain-containing protein (PNPLA8), also termed Ca2+-independent phospholipase A2γ (iPLA2γ), is localized to the mitochondrial matrix, where it may manifest its unique activity to cleave phospholipid side-chains from both sn-1 and sn-2 positions, consequently releasing either saturated or unsaturated fatty acids, including oxi
In this study, to investigate the potential biomarker value of PNPLA8, 751 cases of tumor samples from a cohort of CRC patients were selected to analyze PNPLA8 protein expression by immunohistochemical staining. Additionally, concentrated analyses on the correlations between PNPLA8 expression and overall survival of CRC patients in this cohort were conducted to unveil the prognostic significance of PNPLA8 in CRC. Our results suggest that higher PNPLA8 ex
A total of 751 patients with CRC that were admitted to Zhongshan Hospital, Fudan University (Shanghai, China) bet
This study was approved by the Clinical Research Ethics Committee of Zhongshan Hospital, Fudan University. In
Formalin-fixed paraffin-embedded surgical specimens were used for tissue microarray (TMA) construction and subsequent immunohistochemistry study as described previously[14]. Standard procedures were used to determine PNPLA8 expression levels in CRC tumor samples. After being dried overnight at 37˚C and deparaffinized in xylene, the TMA slide was rehydrated through graded alcohol and then immersed in 3% hydrogen peroxide to block endogenous peroxidase activity. After that, the sections were pretreated in a microwave oven (14 min in sodium citrate buffer, pH 6) and then incubated with 10% normal goat serum for 30 min. Primary antibody (rabbit anti-human PNPLA8 polyclonal antibody, ab223726, Abcam; diluted 1:150) was applied overnight in a moist chamber at 4˚C. After the primary antibody was washed off with PBS, the secondary goat anti-rabbit antibody (ab6721, Abcam; diluted 1:10000) was applied. Re
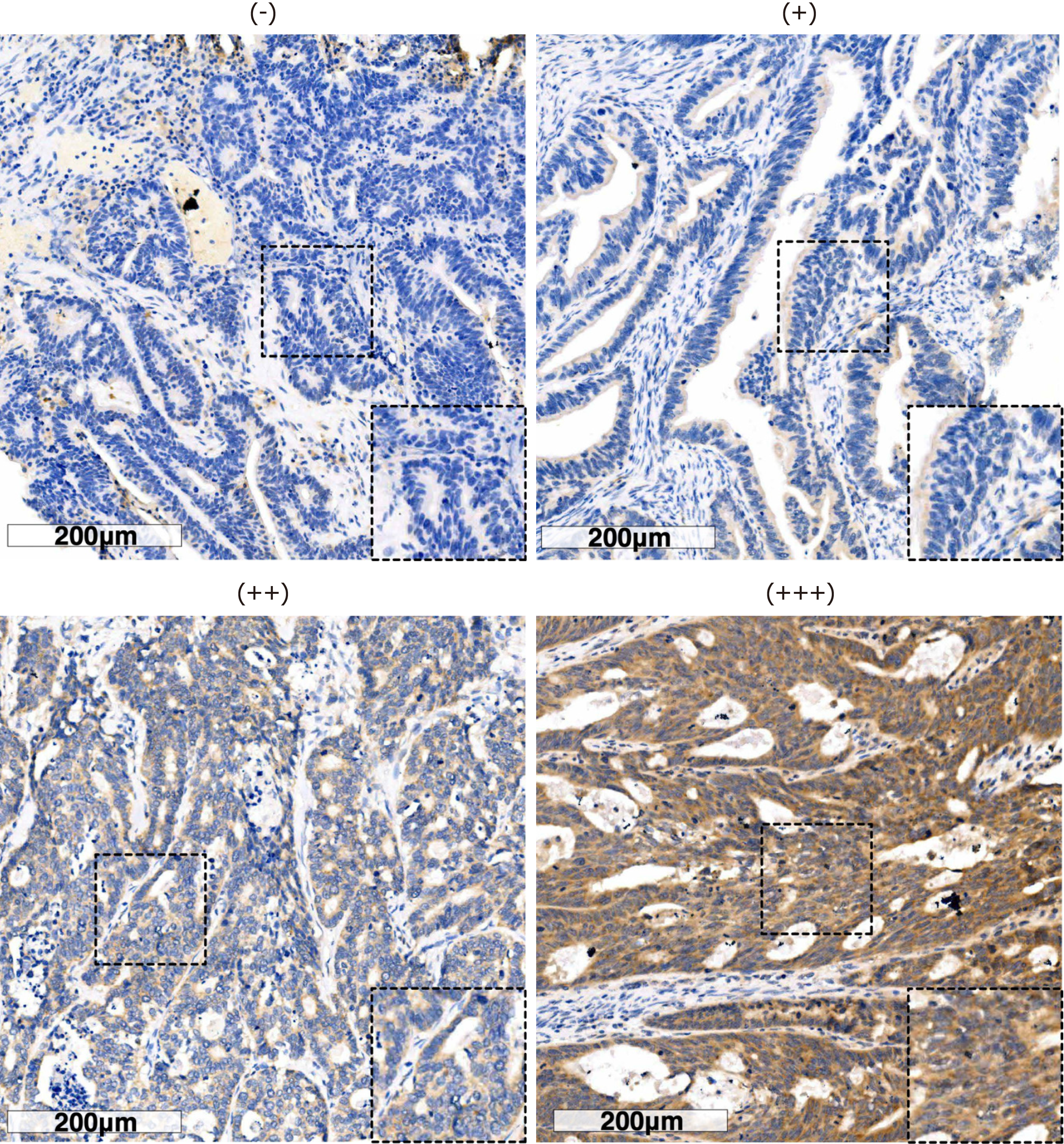
Two independent pathologists who were blinded to the clinical data evaluated the immunostaining and the results were averaged. In case of significant discrepancies, a final score was established by reassessment on a double-headed microscope and a third person was asked to re-score the results and choose the value with the closest score. The scores for PNPLA8 intensity were set as follows: ‘+++’ was 3; ‘++’ was 2; ‘+’ was 1; and ‘−’ was 0. The area scores for PNPLA8 expression were set as follows: ‘1’ (0%-25% positive cells among all tumor cells), ‘2’(25%-50% positive cells), ‘3’ (51%-75% positive cells), and ‘4’ (more than 75% positive cells). The final score for PNPLA8 expression was the intensity score multiplied by the area score, resulting in a final score ranging from 0 to 12. Boundaries were based on the results from X-Tile Software (Yale University, version 3.6.1). A final score of 0-8 was considered low PNPLA8 expression, while 9-12 was considered high PNPLA8 expression.
The statistical analysis was performed using SPSS 23.0 (IBM, Armonk, NY, United States). The association between clinicopathological features and PNPLA8 expression were accessed by Chi-square test or Fisher’s exact test as appro
The clinicopathologic characteristics of the enrolled CRC patients are listed in Table 1. Approximately half of the patients (53.7%) were over 60-years-old, and their ages ranged from 19 years to 90 years with a median age of 62 (SD, 12.3) years. The male to female ratio was 60.3:39.7. The patients with CEA value over 5 ng/mL accounted for 47.3% of total patients, while those with CA199 value more than 37 U/mL accounted for 19.2%. The tumor location was categorized as right-sided colon in 209 cases (27.8%), left-sided colon in 200 cases (26.6%), and rectum in 342 cases (45.6%). There were 323 cases (43%) with tumor size over 4.0 cm, while the majority of all the cases were non-mucinous in terms of primary his
| Clinicopathologic parameters | n | Percentage (%) |
| All patients | 751 | 100.0 |
| Age in yr | ||
| ≤ 60 | 348 | 46.3 |
| > 60 | 403 | 53.7 |
| Sex | ||
| Male | 453 | 60.3 |
| Female | 298 | 39.7 |
| CEA in ng/mL | ||
| ≤ 5 | 396 | 52.7 |
| > 5 | 355 | 47.3 |
| CA199 in U/mL | ||
| ≤ 37 | 607 | 80.8 |
| > 37 | 144 | 19.2 |
| Tumor location | ||
| Right-sided colon | 209 | 27.8 |
| Left-sided colon | 200 | 26.6 |
| Rectum | 342 | 45.6 |
| Tumor size in cm | ||
| ≤ 4.0 | 428 | 57.0 |
| > 4.0 | 323 | 43.0 |
| Primary histological type | ||
| Non-mucinous | 640 | 85.2 |
| Mucinous | 111 | 14.8 |
| Primary differentiation | ||
| Well/moderate | 497 | 66.2 |
| Poor/anaplastic | 254 | 33.8 |
| T stage | ||
| T1 | 24 | 3.2 |
| T2 | 132 | 17.6 |
| T3 | 354 | 47.1 |
| T4 | 241 | 32.1 |
| N stage | ||
| N0 | 410 | 54.6 |
| N1 | 231 | 30.8 |
| N2 | 110 | 14.6 |
| Vascular invasion | ||
| No | 655 | 87.2 |
| Yes | 96 | 12.8 |
| Nerve invasion | ||
| No | 695 | 92.5 |
| Yes | 56 | 7.5 |
| M stage | ||
| M0 | 554 | 73.8 |
| M1 | 197 | 26.2 |
We next examined PNPLA8 expression in tumor samples using immunohistochemistry staining and scored each sample according to the staining intensity (Figure 1) and staining area. Out of 751 stained CRC specimens, 689 (91.7%) showed positive PNPLA8 expression. These 751 samples were categorized into a PNPLA8-low expression group and PNPLA8-high expression group, and the correlations between PNPLA8 expression and the clinicopathological parameters were analyzed (Table 2). A positive correlation was observed between high cytoplasmic PNPLA8 staining and M stage (P = 0.048). However, there were no significant correlations between PNPLA8 staining and other parameters (P > 0.05), including age, sex, CEA, CA199, tumor location, tumor size, primary histological type, primary differentiation, T stage, N stage, vascular invasion, and nerve invasion.
| Variables | PNPLA8 expression | P value | |
| Low (%) | High (%) | ||
| All patients | 331 | 420 | |
| Age in yr | 0.811 | ||
| ≤ 60 | 155 (46.8) | 193 (46.0) | |
| > 60 | 176 (53.2) | 227 (54.0) | |
| Sex | 0.375 | ||
| Male | 199 (60.1) | 239 (56.9) | |
| Female | 132 (39.9) | 181 (43.1) | |
| CEA in ng/mL | 0.821 | ||
| ≤ 5 | 173 (52.3) | 223 (53.1) | |
| > 5 | 158 (47.7) | 197 (46.9) | |
| CA199 in U/mL | 0.900 | ||
| ≤ 37 | 267 (80.7) | 340 (81.0) | |
| > 37 | 64 (19.3) | 80 (19.0) | |
| Tumor location | 0.434 | ||
| Right-sided colon | 97 (29.3) | 112 (26.7) | |
| Left-sided colon | 92 (27.8) | 108 (25.7) | |
| Rectum | 142 (42.9) | 200 (47.6) | |
| Tumor size in cm | 0.589 | ||
| ≤ 4.0 | 185 (55.9) | 243 (57.9) | |
| > 4.0 | 146 (44.1) | 177 (42.1) | |
| Primary histological type | 0.848 | ||
| Non-mucinous | 283 (85.5) | 357 (85.0) | |
| Mucinous | 48 (14.5) | 63 (15.0) | |
| Primary differentiation | 0.160 | ||
| Well/moderate | 210 (63.4) | 287 (68.3) | |
| Poor/anaplastic | 121 (36.6) | 133 (31.7) | |
| T stage | 0.618 | ||
| T1/T2 | 66 (19.9) | 90 (21.4) | |
| T3/T4 | 265 (80.1) | 330 (78.6) | |
| N stage | 0.684 | ||
| N0 | 180 (54.4) | 230 (54.8) | |
| N1 | 106 (32.0) | 125 (29.8) | |
| N2 | 45 (13.6) | 65 (15.4) | |
| Vascular invasion | 0.710 | ||
| No | 287 (86.7) | 368 (87.6) | |
| Yes | 44 (13.3) | 52 (12.4) | |
| Nerve invasion | 0.638 | ||
| No | 308 (93.1) | 387 (92.1) | |
| Yes | 23 (6.9) | 33 (7.9) | |
| M stage | 0.048 | ||
| M0 | 256 (77.3) | 298 (71.0) | |
| M1 | 75 (22.7) | 122 (29.0) | |
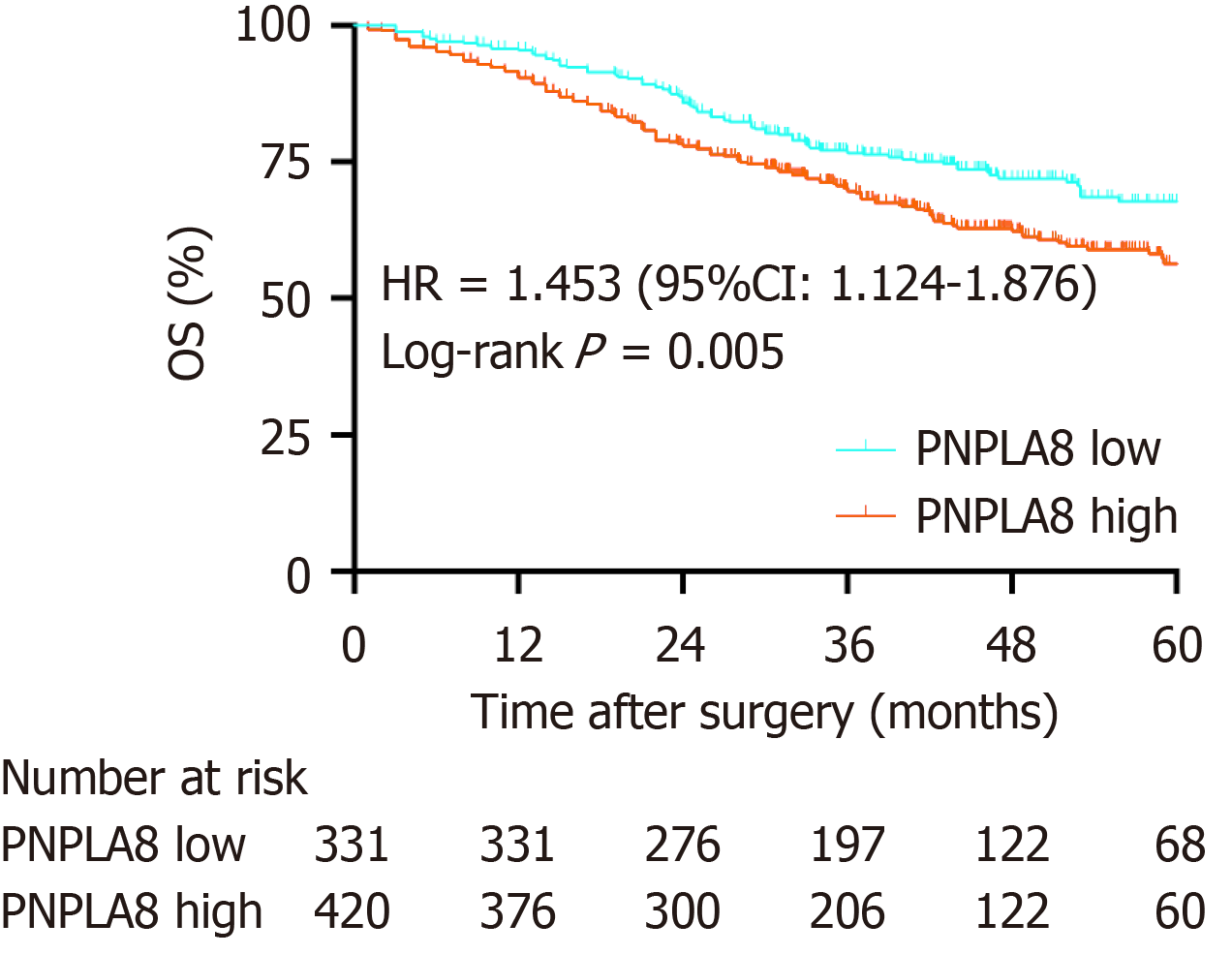
To further substantiate the importance of high PNPLA8 expression in CRC progression, we compared the OS of CRC patients in our study cohort with differential PNPLA8 expression levels. The median follow-up OS of the CRC patients was 46.1 mo (IQR = 36.9-60.9). We found that PNPLA8 expression was statistically significantly associated with a shorter OS (HR 1.445; 43.1 mo for PNPLA8-low group vs 35.4 mo for PNPLA8-high group; P = 0.005) (Figure 2). Therefore, higher PNPLA8 expression could predict poor overall survival in CRC patients, suggesting that PNPLA8 is a prognostic factor of CRC.
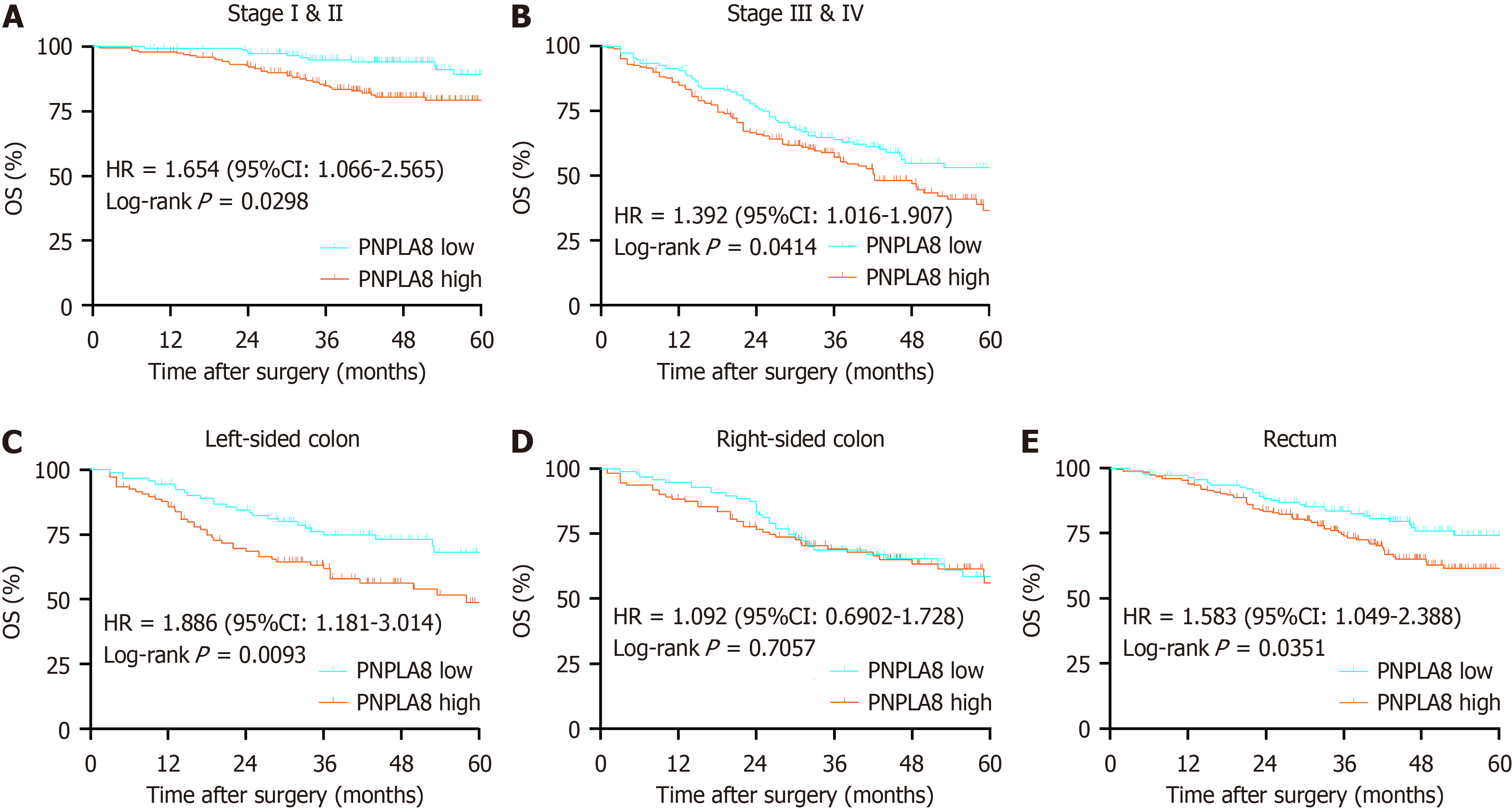
We then conducted further stratified analysis according to the TNM stage of CRC patients. For CRC patients at Stage I and II, PNPLA8 expression was a significant prognostic factor (HR 2.578, P < 0.01; Figure 3A). For CRC patients at Stage III, OS did not show statistical differences among patients with different PNPLA8 expression levels (HR 1.061, P = 0.083; Figure 3B). For CRC patients at Stage IV, patients with a higher PNPLA8 expression also had statistically significantly shorter OS (HR 1.476, P = 0.036; Figure 3C). In stratified analysis according to tumor location, PNPLA8 expression was not a significant prognostic factor for patients with right-sided colon cancer (Figure 3E) (P = 0.7057). However, for patients with left-sided colon (Figure 3D) and rectal cancer (Figure 3F), the survival curves of the two PNPLA8-ex
Using univariate analysis, we found that CRC patients with PNPLA8-high expression showed significant differences compared to PNPLA8-low expression in terms of multiple parameters, including CEA (P < 0.001), CA199 (P < 0.001), primary differentiation (P = 0.02), T stage (P < 0.001), N stage (P < 0.001), M stage (P < 0.001), vascular invasion (P < 0.001), and nerve invasion (P < 0.001) (Table 3). Therefore, multivariate analysis was performed using the Cox proportional hazards model for all of the significant variables examined in the univariate analysis. We found that PNPLA8 expression was a statistically significant independent prognostic factor (HR 1.328, P = 0.038). In addition, CA199 (HR 1.548, P = 0.004), N stage (HR 1.701, P < 0.001), M stage (HR 4.862, P < 0.001) and vascular invasion (HR 1.512, P = 0.017) (Table 3) were also found to be independent factors.
| Variates | Overall survival | |||
| Univariate analysis | Multivariate analysis | |||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| PNPLA8 | 0.004 | 0.038 | ||
| Low | 1 (reference) | 1 (reference) | ||
| High | 1.472 (1.132-1.914) | 1.328 (1.016-1.734) | ||
| Age in yr | 0.561 | |||
| ≤ 60 | 1 (reference) | |||
| > 60 | 1.079 (0.835-1.394) | |||
| Sex | 0.087 | 0.903 | ||
| Male | 1 (reference) | 1 (reference) | ||
| Female | 0.792(0.606-1.304) | 0.903 (0.687-1.187) | ||
| CEA in ng/mL | < 0.001 | 0.811 | ||
| ≤ 5 | 1 (reference) | 1 (reference) | ||
| > 5 | 2.126(1.636-2.763) | 0.964 (0.715-1.300) | ||
| CA199 in U/mL | < 0.001 | 0.004 | ||
| ≤ 37 | 1 (reference) | 1 (reference) | ||
| > 37 | 2.870 (2.191-3.759) | 1.548 (1.150-2.083) | ||
| Tumor location | 0.050 | 0.895 | ||
| Right-sided colon | 1 (reference) | 1 (reference) | ||
| Left-sided colon | 0.952 (0.687-1.320) | 0.933 (0.667-1.305) | ||
| Rectum | 0.706 (0.518-0.962) | 0.934 (0.680-1.284) | ||
| Tumor size in cm | 0.512 | |||
| ≤ 4.0 | 1 (reference) | |||
| > 4.0 | 1.090 (0.843-1.409) | |||
| Primary histological type | 0.371 | |||
| Non-mucinous | 1 (reference) | |||
| Mucinous | 0.854 (0.603-1.208) | |||
| Primary differentiation | 0.002 | 0.162 | ||
| Well/moderate | 1 (reference) | 1 (reference) | ||
| Poor/anaplastic | 1.507 (1.162-1.954) | 1.210 (0.926-1.581) | ||
| T stage | < 0.001 | 0.163 | ||
| T1/T2 | 1 (reference) | 1 (reference) | ||
| T3/T4 | 3.058 (1.934-4.834) | 1.415 (0.869-2.306) | ||
| N stage | < 0.001 | < 0.001 | ||
| N0 | 1 (reference) | 1 (reference) | ||
| N1/N2 | 2.948 (2.852-3.859) | 1.701 (1.272-2.274) | ||
| M stage | < 0.001 | < 0.001 | ||
| M0 | 1 (reference) | 1 (reference) | ||
| M1 | 7.193 (5.520-9.372) | 4.862 (3.608-6.551) | ||
| Vascular invasion | < 0.001 | 0.017 | ||
| No | 1 (reference) | 1 (reference) | ||
| Yes | 2.265 (1.649-3.111) | 1.512 (1.078-2.121) | ||
| Nerve invasion | < 0.001 | 0.218 | ||
| No | 1 (reference) | 1 (reference) | ||
| Yes | 2.901 (1.986-4.236) | 1.291 (0.860-1.939) | ||
In this study, a large patient cohort was evaluated for the prognostic value of PNPLA8 in CRC. Patients with a higher PNPLA8 expression had a significantly impaired OS. Moreover, PNPLA8 expression was identified as a new indepen
PNPLA8 is part of a diverse family of phospholipase A2 enzymes (PLA2s) that hydrolyze the sn-2 substituent from membrane phospholipids to release a free fatty acid and a lysolipid[16,11]. These enzymes are ubiquitously expressed, and in contrast to secretory PLA2s and cytosolic PLA2s, do not require Ca2+ for either translocation or activity. Some of the first descriptions of iPLA2 activity were in the mid- to late-1980s with the identification of a plasmalogen-selective iPLA2 in PNPLA8, when PNPLA8 was found to function as a phospholipase and was better characterized[17]. During the past few years, knockout and transgenic mice for manipulated PNPLA8 expression have been established[18], and studies using these gene-manipulated mice have provided models with which to elucidate the physiological and pathophy
Our findings are in line with previous reports that PNPLA8 expression is increased in CRC[21,12]. The ability of PNPLA8 to promote cell proliferation becomes prominent in the context of tumorigenesis. Several in vitro studies re
However, a couple of limitations of this study must be noted. First, our study is a retrospective one. To further validate our conclusion, a prospective study with data from multiple centers is necessary, especially for patients with left-sided colon and rectal cancer as well as Stage I and II patients. Second, the scores for PNPLA8 protein intensity and area were not determined in a fully automated way, resulting in potential artificial errors due to possible human bias. Third, in-depth in vitro and in vivo experiments are urgently needed to unveil the mechanisms of PNPLA8 in CRC.
In summary, PNPLA8 was identified as a new independent prognostic factor for CRC. CRC with high PNPLA8 ex
The role of patatin like phospholipase domain containing 8 (PNPLA8) in colorectal cancer (CRC) progression is unclear.
The research motivation is to explore the prognostic effects of PNPLA8 expression in CRC.
To evaluate the prognostic value of PNPLA8 expression level for CRC patient survival and its correlation with cli
PNPLA8 expression in tumor samples was evaluated by immunohistochemistry staining and semi-quantitated with immunoreactive scores.
CRC patients with high PNPLA8 expression indicated poor overall survival (OS) (median OS = 35.3, P = 0.005). The multivariate analysis also confirmed that high PNPLA8 expression was a significantly independent prognostic factor for overall survival (hazard ratio HR = 1.328, 95%CI: 1.016-1.734, P = 0.038).
High PNPLA8 expression level is associated with poorer survival outcomes in CRC patients, indicating its prognostic value for predicting patient outcomes.
Further studies are needed to validate the prognostic value of PNPLA8 in multicenter cohorts of CRC patients. The me
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): E
P-Reviewer: Basson MD, United States; Hamaya Y, Japan; Osera S, Japan S-Editor: Gong ZM L-Editor: Filipodia P-Editor: Xu ZH
| 1. | Bao S, Song H, Tan M, Wohltmann M, Ladenson JH, Turk J. Group VIB Phospholipase A(2) promotes proliferation of INS-1 insulinoma cells and attenuates lipid peroxidation and apoptosis induced by inflammatory cytokines and oxidant agents. Oxid Med Cell Longev. 2012;2012:989372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 2. | Biller LH, Schrag D. Diagnosis and Treatment of Metastatic Colorectal Cancer: A Review. JAMA. 2021;325:669-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 1431] [Article Influence: 357.8] [Reference Citation Analysis (0)] |
| 3. | Blanchard H, Taha AY, Cheon Y, Kim HW, Turk J, Rapoport SI. iPLA2β knockout mouse, a genetic model for progressive human motor disorders, develops age-related neuropathology. Neurochem Res. 2014;39:1522-1532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10:7252-7259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1947] [Cited by in RCA: 2936] [Article Influence: 146.8] [Reference Citation Analysis (0)] |
| 5. | Cohen D, Papillon J, Aoudjit L, Li H, Cybulsky AV, Takano T. Role of calcium-independent phospholipase A2 in complement-mediated glomerular epithelial cell injury. Am J Physiol Renal Physiol. 2008;294:F469-F479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet. 2019;394:1467-1480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1570] [Cited by in RCA: 3015] [Article Influence: 502.5] [Reference Citation Analysis (3)] |
| 7. | Dennis EA, Cao J, Hsu YH, Magrioti V, Kokotos G. Phospholipase A2 enzymes: physical structure, biological function, disease implication, chemical inhibition, and therapeutic intervention. Chem Rev. 2011;111:6130-6185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 919] [Cited by in RCA: 874] [Article Influence: 62.4] [Reference Citation Analysis (0)] |
| 8. | Hara S, Yoda E, Sasaki Y, Nakatani Y, Kuwata H. Calcium-independent phospholipase A(2)γ (iPLA(2)γ) and its roles in cellular functions and diseases. Biochim Biophys Acta Mol Cell Biol Lipids. 2019;1864:861-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Hooks SB, Cummings BS. Role of Ca2+-independent phospholipase A2 in cell growth and signaling. Biochem Pharmacol. 2008;76:1059-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Jabůrek M, Průchová P, Holendová B, Galkin A, Ježek P. Antioxidant Synergy of Mitochondrial Phospholipase PNPLA8/iPLA2γ with Fatty Acid-Conducting SLC25 Gene Family Transporters. Antioxidants (Basel). 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Ji M, Ren L, Lv Y, Lao X, Feng Q, Tang W, Zhuang A, Liu T, Zheng P, Xu J. Small Nuclear Ribonucleoprotein Polypeptide N Accelerates Malignant Progression and Poor Prognosis in Colorectal Cancer Transcriptionally Regulated by E2F8. Front Oncol. 2020;10:561287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Khan SA, Ilies MA. The Phospholipase A2 Superfamily: Structure, Isozymes, Catalysis, Physiologic and Pathologic Roles. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 75] [Reference Citation Analysis (0)] |
| 13. | Li N, Lu B, Luo C, Cai J, Lu M, Zhang Y, Chen H, Dai M. Incidence, mortality, survival, risk factor and screening of colorectal cancer: A comparison among China, Europe, and northern America. Cancer Lett. 2021;522:255-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 233] [Article Influence: 58.3] [Reference Citation Analysis (0)] |
| 14. | Liu GY, Moon SH, Jenkins CM, Li M, Sims HF, Guan S, Gross RW. The phospholipase iPLA(2)γ is a major mediator releasing oxidized aliphatic chains from cardiolipin, integrating mitochondrial bioenergetics and signaling. J Biol Chem. 2017;292:10672-10684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 15. | Moon SH, Jenkins CM, Liu X, Guan S, Mancuso DJ, Gross RW. Activation of mitochondrial calcium-independent phospholipase A2γ (iPLA2γ) by divalent cations mediating arachidonate release and production of downstream eicosanoids. J Biol Chem. 2012;287:14880-14895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 16. | Murakami M, Masuda S, Ueda-Semmyo K, Yoda E, Kuwata H, Takanezawa Y, Aoki J, Arai H, Sumimoto H, Ishikawa Y, Ishii T, Nakatani Y, Kudo I. Group VIB Ca2+-independent phospholipase A2gamma promotes cellular membrane hydrolysis and prostaglandin production in a manner distinct from other intracellular phospholipases A2. J Biol Chem. 2005;280:14028-14041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 57] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 17. | Murase R, Taketomi Y, Miki Y, Nishito Y, Saito M, Fukami K, Yamamoto K, Murakami M. Group III phospholipase A(2) promotes colitis and colorectal cancer. Sci Rep. 2017;7:12261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 18. | Nappi A, Nasti G, Romano C, Berretta M, Ottaiano A. Metastatic Colorectal Cancer: Prognostic and Predictive Factors. Curr Med Chem. 2020;27:2779-2791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Peng Z, Chang Y, Fan J, Ji W, Su C. Phospholipase A2 superfamily in cancer. Cancer Lett. 2021;497:165-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 80] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 20. | Ramanadham S, Ali T, Ashley JW, Bone RN, Hancock WD, Lei X. Calcium-independent phospholipases A2 and their roles in biological processes and diseases. J Lipid Res. 2015;56:1643-1668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 151] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 21. | Saunders CJ, Moon SH, Liu X, Thiffault I, Coffman K, LePichon JB, Taboada E, Smith LD, Farrow EG, Miller N, Gibson M, Patterson M, Kingsmore SF, Gross RW. Loss of function variants in human PNPLA8 encoding calcium-independent phospholipase A2 γ recapitulate the mitochondriopathy of the homologous null mouse. Hum Mutat. 2015;36:301-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 22. | Scott KF, Sajinovic M, Hein J, Nixdorf S, Galettis P, Liauw W, de Souza P, Dong Q, Graham GG, Russell PJ. Emerging roles for phospholipase A2 enzymes in cancer. Biochimie. 2010;92:601-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 125] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 23. | Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4235] [Cited by in RCA: 11421] [Article Influence: 3807.0] [Reference Citation Analysis (4)] |
| 24. | Taketo MM, Sonoshita M. Phospolipase A2 and apoptosis. Biochim Biophys Acta. 2002;1585:72-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 106] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 25. | Zhu D, Xia J, Gu Y, Lin J, Ding K, Zhou B, Liang F, Liu T, Qin C, Wei Y, Ren L, Zhong Y, Wang J, Yan Z, Cheng J, Chen J, Chang W, Zhan S, Ding Y, Huo H, Liu F, Sun J, Qin X, Xu J. Preoperative Hepatic and Regional Arterial Chemotherapy in Patients Who Underwent Curative Colorectal Cancer Resection: A Prospective, Multi-center, Randomized Controlled Trial. Ann Surg. 2021;273:1066-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |