Published online Nov 15, 2024. doi: 10.4251/wjgo.v16.i11.4369
Revised: August 14, 2024
Accepted: August 28, 2024
Published online: November 15, 2024
Processing time: 142 Days and 20.6 Hours
Gastric cancer (GC) is the leading diagnosed malignancy worldwide, especially in China. Radical surgery is the cornerstone of GC treatment. We reviewed previous clinical trials and aimed to provide an update on the factors related to the surgical treatment of GC. The number of registered clinical trials in the field of GC surgery is rapidly increasing. With the development and popularization of endoscopic, laparoscopic, and robotic techniques, GC surgery has gradually entered a new era of precise minimally invasive surgery. Postoperative quality of life has become a major issue in addition to surgical oncological safety. Although great progress has been made in clinical research on GC in China, there are still deficiencies. Many studies enrolled large numbers of patients, but the research data were not of high quality. The characteristics of GC in China include a high incidence, large popu
Core Tip: Gastric cancer (GC) is the leading common malignancy in China. This review summarizes the study design and the clinical or translational research focus to explore the state-of-the-art and development trends in Chinese GC cancer surgery studies.
- Citation: Zhang S, Hu RH, Cui XM, Song C, Jiang XH. Current clinical trials on gastric cancer surgery in China. World J Gastrointest Oncol 2024; 16(11): 4369-4382
- URL: https://www.wjgnet.com/1948-5204/full/v16/i11/4369.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i11.4369
The incidence of gastric cancer (GC) has decreased overall, but it is still a significant cancer with high mortality rates globally. Radical surgery is the primary treatment option for completely eradicating GC and forms the basis of GC treatment. Over the years, the evolution of GC surgical treatment has witnessed a transition in the scope of surgery: “from small to large, and then from large to small”[1]. This evolution in surgical approaches reflects advancements in medical practices and the desire for more effective and less invasive treatments.
China accounts for almost 24% of new cases and 30% of cancer-related deaths worldwide[2]. Unfortunately, most patients present with advanced disease because GC is largely asymptomatic in its early stages. Many developments in surgical techniques aim to improve long-term survival while minimizing postoperative complications. Understanding which of these approaches are optimal for patients should be based on robust evidence from well-designed trials. Clinical trials are the gold standard for evaluating the safety and efficacy of therapeutics and generating evidence-based knowledge in the field of medicine. In recent years, the number of clinical trials initiated in China has increased rapidly. Several high-level clinical studies published by Chinese investigators have fulfilled clinical guidance or served as opinion-change references[3]. Clinical trials, which are strictly governed health research investigations, facilitate the transition of medical advancements into everyday clinical applications. These trials present an opportunity for individuals to access cutting-edge treatments and supply essential data for enhancing medical care[4]. Clinical trials, especially randomized clinical trials, can reliably evaluate the true effects of different surgical interventions.
Data on the characteristics of clinical trials about GC surgery in China and how they have changed over time are scarce. Analyzing clinical trials can illuminate important trends over time. Here, we review the content and status of clinical trials for GC surgery in China.
The National Library of Medicine operates ClinicalTrials.gov, a platform that offers details on ongoing clinical research projects across over 200 nations. To find all registered clinical trials involving patients with GC who had surgical procedures (using any technique) as part of their treatment up to April 4, 2022, the ClinicalTrials.gov database was accessed. The criteria for inclusion required “gastric cancer” to be listed as a Medical Subject Headings condition term, along with at least one of the following terms appearing in the keywords or titles: “surgery,” “surgical,” “gastrectomy,” “laparoscopic,” “robot,” or “resection.” This search was confined to randomized trials aimed primarily at treatment, with procedures or devices classified according to the type of intervention. Trials in which the main purpose was related to basic science, diagnostics, health services, prevention, or supportive care were excluded. Subsequently, studies were initially selected through a review of titles and abstracts, followed by a comprehensive review of full texts to confirm that the inclusion criteria were consistently applied.
The number of studies originating from China (271 [31.5%]) ranked first, followed by the United States (151 [17.6%]) and Korea (123 [14.3%]). We excluded trials that did not involve patients who underwent surgery for GC and that were not conducted in mainland China. Finally, 83 studies were further reviewed.
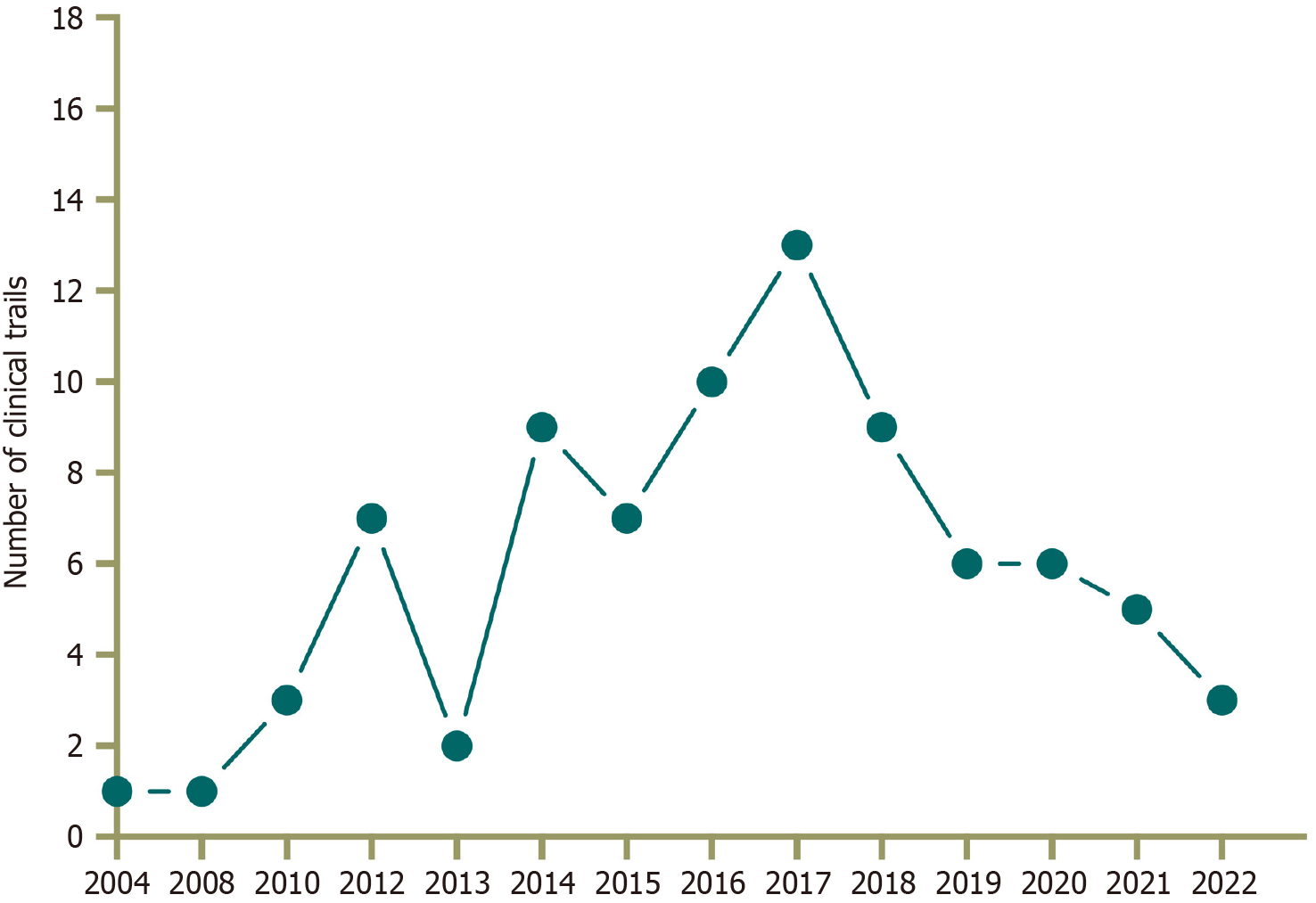
From 2008 to April 2022, a total of 83 GC-related clinical trials were conducted in mainland China, of which 2 were Phase 1 (2.4%), 14 were Phase 2 (16.9%), 2 were Phase 2/3 (2.4%), 14 were Phase 3 (16.9%), 42 were not applicable (50.6%), and the last 9 (10.8%) fell into other categories (Figure 1). From 2008 to 2016, the number of trials conducted increased annually. Since 2014, the number of GC surgery-related clinical trials has increased significantly. Conversely, in 2018, the increase began to slow down. Thirty-two research institutes were responsible for 83 clinical trials, with the most active research institute being Fujian Medical University Union Hospital (15 cases). The majority of the 66 trials included were single-center studies (79.5%), and a large proportion of the clinical trials (89.2%) were interventional studies. Nine protocols were published, and 22 studies presented 28 results.
Regarding research content, surgeries more commonly involved laparoscopic gastrectomy (LG) than open gastrectomy (OG) in 16 trials, robotic gastrectomy (RG) than LG in 12 trials, function-preserving gastrectomy (FPG) and endoscopic treatment in 4 trials, digestive tract reconstruction after gastrectomy in 16 trials, the extent of surgical resection in 14 trials, image-guided minimally invasive treatment in 6 trials, and other methods (e.g., enhanced recovery after surgery, reduced-port surgery, three-dimensional or ultra-high-definition laparoscopic surgery) in 15 trials. The major clinical research interests related to Chinese GC surgery are summarized in Table 1.
| Group | Subgroup | Research interests |
| CLASS | CLASS-01 | LDG for AGC |
| CLASS-02 | LTG for EGC | |
| CLASS-03 | LDG for AGC after NACT | |
| CLASS-04 | Laparoscopic spleen-preserving No. 10 LN dissections for AGC | |
| CLASS-07 | LTG for AGC | |
| CLASS-08 | TLTG vs LATG | |
| CLASS-10 | Laparoscopic vs open lower mediastinal lymphadenectomy for EGJ cancer | |
| CLASS-11 | IGC in LG for AGC | |
| CKLASS01 | TLDG vs LADG | |
| Laparoscopic gastrectomy | LG | LG for AGC |
| LDG | LDG for AGC (especially in the elderly patients) | |
| LTG | LTG with spleen-preserving No. 10 LN dissections for AGC | |
| Robotic gastrectomy | RTG | RTG for EGC, AGC |
| RDG | RDG for AGC | |
| TRG | TRG for GC | |
| Chinese RG | Chinese MIS robot system for GC | |
| Reconstruction | DG | Uncut R-Y, Modified delta-shaped gastroduodenostomy, Modified BII + Braun |
| PG | Double tract reconstruction, gastric tube reconstruction, Cheng's giraffe reconstruction | |
| TG | Functional jejunal interposition, R-Y pouch, Intracorporeal or extracorporeal esophagojejunostomy | |
| The extent of surgical resection | Extended surgery | Bursectomy, type of omentectomy, complete mesogastrium excision, D2+ lymphadenectomy, D2 plus para-aortic nodal dissection, 14v LN dissection |
| Limited surgery | D1 lymphadenectomy for elderly patients in AGC, role of No. 10 LN dissections, length of the proximal resection margin for Adenocarcinomas of the EGJ | |
| Image-guided MIS | Method of ICG injection | |
| Carbon nanoparticles | ||
| Functional preservation and organ preservation | PPG or LAPPG, PG, partial gastrectomy | |
| ESD for EGC, ESD plus laparoscopic regional LN dissection for EGC | ||
| ERAS | NACT for AGC patients in ERAS programs | |
| ERAS in LDG | ||
| Reduced port surgery | 1-3 ports LG | |
| Single-incision plus one-port LG | ||
| Others | Laparoscopic staging | |
| 3D LTG plus spleen-preserving No. 10 LN dissections for AGC | ||
| 4K laparoscopic surgery | ||
| LTG for remnant GC | ||
| Closure of the mesenteric defects | ||
| Intraoperative leak testing in gastrectomy | ||
| Ultrasonic scalpel vs monopolar electrocautery for DG | ||
| Extensive intraoperative peritoneal lavage |
Since its establishment in 2010, the Chinese laparoscopic gastrointestinal surgery (CLASS) group has been devoted to enhancing the quality of life (QoL) of patients through the use of minimally invasive surgery[5]. Prior to commencing numerous prospective trials, the multicenter database was created by integrating the current datasets from the CLASS group members[6].
At that time, the CLASS group performed the largest multicenter retrospective clinical study of laparoscopic advanced GC (AGC) in China. Data from 1184 consecutive patients between February 2003 and December 2009 were collected from the CLASS database and retrospectively analyzed. Postoperative morbidity and mortality after laparoscopic-assisted gastrectomy (LAG) and D2 dissection in AGC patients were 10.1% and 0.1%, respectively. LAG for locally AGC has been proven safe and technically viable, and it may also provide satisfactory short-term oncologic results[7]. Notably, elderly patients with resectable GC should not be denied the possible advantages of LAG, as long as their comorbid conditions are thoroughly considered[8]. Since then, the importance of multicenter clinical studies has been recognized, and high-quality clinical trials for laparoscopic GC surgery have been gradually progressing in China.
The CLASS-01 trial, which was conducted at 14 centers in China between 2012 and 2014, included 1056 patients with clinical stage T2 to T4a GC without bulky nodes or distant metastases. Earlier results were published in 2016 in the Journal of Clinical Oncology[9]. Both morbidity after surgery (15.2% for LAG compared with 12.9% for OG; P = 0.285) and mortality rates (0.4% for LAG compared with 0% for OG; P = 0.249) were found to be similar. These findings suggest that proficient surgeons are able to safely conduct D2 LAG for AGC. The final follow-up of the CLASS-01 trial was December 2017. Three-year survival results published in 2019 in The Journal of the American Medical Association[10] revealed no significant difference in 3-year disease-free survival (DFS; laparoscopic distal gastrectomy [LDG] 76.5% vs open DG [ODG] 77.8%) or overall survival (OS; LDG 83.1% vs ODG 85.2%). Similar to long-term survival with laparoscopy, OG was released in 2022[11]. The OS was 73% in the laparoscopic group and 76% in the open group. For patients with stage I tumors, the 5-year survival rate was 90% with laparoscopy and 89% with open surgery. For stage II and III tumors, the rates were 79% vs 85% and 59% vs 60%. None of these differences were significant after adjusting for age, sex, body mass index, performance status, comorbidities, tumor size, histologic features, or chemotherapy. The CLASS-01 trial concluded that experienced surgeons performing D2 LDG at high-volume specialized institutions achieved comparable long-term survival outcomes to ODG for patients with locally AGC.
Based on the experience of the CLASS-01 trial, the CLASS group has designed and launched several multicenter, prospective, high-quality clinical trials concerning the clinical problems of gastrointestinal surgery. In January 2017, the CLASS group launched the CLASS-02 trial to compare the safety of laparoscopic total gastrectomy (LTG) for clinical stage I GC with that of conventional open TG (OTG)[12]. The JCOG1401[13] and KLASS03[14] trials, which reported the safety of laparoscopic assisted TG (LATG) for patients with clinical stage I proximal GC, were single-arm trials. Single-arm studies, while valuable in certain contexts, do not consistently provide an accurate representation of real-world outcomes. By contrast, the findings from prospective randomized controlled trials (RCTs) offer a higher level of credibility and are generally more persuasive. This CLASS-02 trial was the first prospective randomized two-arm multicenter, open-label, noninferiority RCT to determine the safety of LTG compared with OTG. From January 2017 to September 2018, a total of 227 patients were enrolled in this clinical trial. Its short-term results were published in 2020 in JAMA Oncology[15]. The overall incidence and death rates were 19.1% for the LTG group and 20.2% for the OTG group, with no significant difference observed. Similarly, the overall complication rate post-surgery (17.4% vs 18.1%) and mortality rate (0% vs 1%) were not significantly different between the LTG and OTG groups. Findings from the CLASS-02 trial indicated that LTG can be performed by seasoned surgeons with the same safety level as that for OTG in clinical stage I GC. This research establishes a baseline for subsequent oncological safety studies of LTG in early-stage GC and, more extensively, for AGC.
The safety and efficacy of the laparoscopic procedure in patients who have undergone neoadjuvant chemotherapy remain uncertain. There is a critical question in clinical practice regarding whether reduced surgical trauma is associated with improved postoperative safety, successful completion of chemotherapy, and enhanced survival benefits. To address this, the CLASS-03 trial was conducted from April 2015 to November 2017, aiming to investigate the safety and efficacy of the laparoscopic procedure for these patients (NCT02404753)[16]. The findings revealed that the overall postoperative complication rate was significantly lower in patients who underwent LDG than in those who underwent ODG, with rates of 20% and 46%, respectively (P = 0.007). Additionally, patients in the laparoscopic group experienced less postoperative pain and achieved better completion rates of adjuvant chemotherapy (adjusted odds ratio, 4.39; 95% confidence interval [CI]: 1.63–11.80; P = 0.003)[17]. These short-term results indicate that patients with locally AGC may benefit from LDG because of reduced postoperative complications and improved tolerance to adjuvant chemotherapy.
The rate of No. 10 lymph node (LN) metastasis is high in advanced proximal GC (APGC; 9.8%–27.9%)[18] and is still necessary for a portion of proximal GC[19]. In the past, splenectomy was conducted concurrently to ensure effective No. 10 LN dissection at the splenic hilum. However, various prospective RCTs have indicated that splenectomy should not be routinely performed due to elevated morbidity and mortality rates[20,21]. Because of the unique and intricate anatomy of the spleen, spleen-preserving splenic hilar lymphadenectomy poses challenges, especially during open surgery[22]. As a result, there is an urgent need is to establish an optimal and secure method for laparoscopic spleen-preserving No. 10 LN dissection[23]. CLASS-04 is a single-arm clinical trial conducted in 19 centers in China that enrolled 251 patients. The aim was to provide solid evidence supporting the use of laparoscopic spleen-preserving TG (LSTG) in advanced upper third GC[24]. The mean counts of No. 10 LN dissections and metastases were 2.4 and 0.1, respectively. The overall rate of postoperative complications was 13.6% (33/242). The rates of major complications and mortality were 3.3% (8/242) and 0.4% (1/242), respectively. The 3-year OS was 79.1% (95%CI: 74.0%-84.2%), and the 3-year DFS was 73.1% (95%CI: 67.4%-78.8%). Patients with No. 10 LN metastasis might experience poorer survival outcomes and are more susceptible to recurrence (42.1% vs 20.7%; P = 0.03)[25]. The short- and long-term findings of the CLASS-04 trial demonstrated that LSTG was both safe and efficient when performed by highly skilled surgeons. This approach could be considered for patients requiring splenic hilar LN dissection. Nonetheless, this study lacks information on the reproducibility, oncologic outcomes, and benefits of such endeavors.
Total LDG (TLDG) has several advantages over laparoscopy-assisted DG (LADG), including smaller wounds[26] and easy anastomosis, especially for obese patients. However, evidence is still lacking concerning whether the QoL of patients undergoing TLDG is superior to that of patients undergoing LADG. Therefore, the Korean laparoendoscopic gastrointestinal surgery study (KLASS) group and the CLASS group jointly launched the CKLASS-01 trial for the first time. This CKLASS-01 trial, activated in January 2018, is a prospective, multicenter RCT that compared the QoL of TLDG and LADG patients[27]. The CKLASS-01 trial is a successful attempt of the CLASS group to explore the transnational clinical research cooperation mode of minimally invasive surgery for GC.
After the successful CLASS-01 to CLASS-04 trials, the trials from the CLASS-05 to CLASS-11 series advanced in succession. The CLASS-05 investigated cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy and chemotherapy for patients with GC with peritoneal metastasis (NCT03023436). The CLASS-06 trial focused on the surgical safety of laparoscopic surgery for gastrointestinal stromal tumors in an unfavorable location. Based on previous CLASS-02 results of LTG for early GC (EGC), the prospective CLASS-07 trial is a multicenter RCT (NCT04710758) that aims to evaluate long-term outcomes in patients with locally AGC following either LTG or OTG. Additionally, the CLASS-08 trial seeks to compare the immediate surgical safety and postoperative QoL between total LTG and laparo
The successful implementation of the CLASS series trails has created a new standard in the clinical research of minimally invasive gastrointestinal surgery in China. The CLASS group has cooperated with domestic counterparts, developed guidelines and consensuses[28] based on previous research results, and standardized and established a complete set of standard systems for laparoscopic preoperative evaluation, surgical indications, treatment principles, surgical quality control, oncological efficacy evaluation, and tissue specimen treatment for GC.
While laparoscopy is currently the primary technique in the realm of modern surgical procedures, minimally invasive surgery has been steadily progressing towards robotic-assisted procedures. Early studies conducted in Korea and Japan compared the advantages of RG to LG, with findings suggesting that RG does not show any inferiority[29-31]. Since the first report on RG for GC in 2010[32], several retrospective studies from China have shown that RG is associated with less blood loss[33-36], a shorter hospital stay, more harvested LNs[34-37], and similar long-term oncological outcomes compared with those of LG[33-36,38]. The disadvantages of RG, such as longer operation times[33,35,37,38] and higher total costs[33,34,38], have also been investigated.
However, the findings from a prospective comparative study involving multiple centers revealed that LG is not inferior to RG in terms of immediate surgical results[39]. Additionally, some studies have reported that there were no disparities in long-term cancer-related outcomes between LG and RG. Despite the potential technical advantages of the robotic system over laparoscopy, there was no improvement in cancer-related outcomes following gastrectomy[40]. Criticism of robotic surgery in gastrectomy has focused on the perceived absence of advantages in relation to the cost of the procedure. Consequently, there is an urgent need for additional extensive studies, particularly RCTs, to evaluate the accuracy of these findings.
There were 13 registered clinical trials from China, among which 7 were RCTs including 2 multicenter studies. Eight trials investigated robotic vs LG for DG, proximal gastrectomy (PG) and TG in early to advanced stages (NCT03273920, NCT03524300, NCT03313700, NCT03500471, NCT03524287, NCT03447106, and NCT05235932). One trial compared vagus nerve-preserving robotic-assisted DG (RADG) and conventional RADG for AGC (NCT02806661). One trial is concerned with the learning curve for robot-assisted gastrectomy in GC (NCT03940417), and the other trial is investigating the Chinese domestic surgical robot for GC (NCT02752698).
To date, the results of only two prospective studies comparing RG and LG have been published. One RCT (FUGES-011, NCT03313700) enrolled 300 patients with clinical stages T1–-4a and N0/+ disease between September 2017 and January 2020. The findings indicated that the robotic group experienced quicker postoperative recovery (P < 0.05), a greater number of dissected LNs (17.6 ± 5.8 vs 15.8 ± 6.6; P = 0.018), and decreased postoperative morbidity (9.2% vs 17.6%; P = 0.039) compared with those of the laparoscopic group. Moreover, patients in the robotic DG (RDG) group started ad
Numerous multicenter RCTs conducted in Asia (including KLASS-01, KLASS-02, JCOG0912, JLSSG0901, and CLASS-01) have established that LDG results in improved short-term and long-term outcomes compared with those of open surgery. In addition to the CLASS series trials, eight clinical trials from China have been registered in Clinical Trials. All the studies were RCTs.
Initially, Southwest Hospital, registered in China, conducted an RCT to evaluate the effectiveness of LAG in treating patients with local AGC who underwent proximal, DG, or TG (NCT01043835). From January 2010 to June 2012, a total of 328 patients with preoperative cT2-4aN0-3M0 GC participated in the study. Only 8% of the patients enrolled were in the early stage. Conversely, the percentage of patients with advanced-stage disease exceeded that reported in the CLASS-01 trials. Short-term findings indicated that there was no significant disparity in morbidity rates between the LAG (11.72%) and OG groups (14.38%)[43]. The research confirmed that LAG offered improved short-term results, aligning with that of the prospective CLASS-01 investigations. Notably, there have been no previous prospective RCTs investigating LATG for AGC treatment. The trial included 61 (37.65%) individuals who underwent D2 LATGs. The study reported an overall complication rate of 14.75% in the LATG group, which was within acceptable limits and comparable to OTG outcomes. Nevertheless, limitations included the small patient sample size and low complication rates, potentially impacting the reliability of the subgroup analysis for TG. The long-term outcomes of the trial published in 2019 revealed no significant differences in 5-year OS (LAG 49% vs OG 50.7%) or DFS (LDG 47.2% vs ODG 49.6%)[44]. Similar short-term results from the Beijing Cancer Hospital (Beijing, China) RCT support the same conclusions[45].
Individuals residing in the northern regions of China exhibit a higher prevalence of obesity than their counterparts in the southern areas, primarily attributed to economic disparities, climate variations, and regional distinctions[46]. The presence of obesity plays a significant role in impacting the outcomes of surgical procedures[47]. In contrast to the CLASS-01 study, which encompassed a nationwide RCT, the SWEET trial was specifically designed to assess the comparability of D2 LADG with ODG concerning the safety of operations for locally AGC in northern China (NCT02464215)[48]. The overall incidence of postoperative complications in the SWEET trial (LADG vs ODG: 13.1% vs 17.7%; P = 0.174) mirrored the findings of the CLASS-01 study (LDG vs ODG: 15.2% vs 12.9%; P = 0.285). For the conversion rate from LADG to ODG, the results were also comparable between the two trials (6.3% vs 6.4%). Moreover, the mean number of retrieved LNs was comparable in the SWEET trial (LADG vs ODG: 29.5 ± 10.4 vs 31.4 ± 12.3; P = 0.083), which was slightly lower than that reported in the CLASS-01 trial (LDG vs ODG: 36.1 ± 16.7 vs 36.9 ± 16.1; P = 0.738).
Guo et al[49,50] from the Chinese PLA General Hospital conducted a single-center RCT to compare the short-term surgical outcomes between laparoscopic spleen-preserving splenic hilar lymphadenectomy (LSPL) and open spleen-preserving splenic hilar lymphadenectomy (OSPL; NCT02980861). Unlike the CLASS-04 trial, which focused primarily on the laparoscopic technique for spleen-preserving No. 10 LN dissection, this trial is the first to evaluate the value of laparoscopy specifically for No. 10 LN dissection. The study enrolled a total of 222 patients, with 114 patients assigned to the LSPL group and 108 to the OSPL group. The results revealed no significant difference between the two groups in terms of the number of harvested LNs (P = 0.669), including No. 10 LNs, with averages of 2.1 ± 1.4 in the LSPL group compared with 2.3 ± 1.2 in the OSPL group (P = 0.713). Moreover, there was no statistically significant difference in the operative time (P = 0.152) or postoperative complication rates (18.3% in the LSPL group vs 16.1% in the OSPL group; P = 0.331). However, one notable finding was that the LSPL group experienced significantly less blood loss than the OSPL group, indicating an advantage of the laparoscopic technique. While the assessment of long-term survival outcomes is still ongoing, this trial demonstrated that laparoscopy could offer significant benefits over open surgery in terms of surgical outcomes. Moreover, the radical effects of laparoscopy were found to be noninferior to those of open surgery.
China has experienced a gradual shift towards an aging population. The increasing elderly population has raised significant concerns. Compared with younger individuals, elderly patients typically require more stringent control over surgical injury management. Therefore, local AGC has prioritized research on surgical safety and effectiveness for elderly patients. To date, only one prospective cohort study from Japan revealed that LG can be performed safely in elderly patients and can shorten the length of the postoperative hospital stay[51]. All other currently available evidence supports that the benefits of the laparoscopic approach for elderly patients, such as less trauma and lower systemic morbidities, is from observational studies[52]. There are two ongoing prospective single-center RCTs from China evaluating the safety and efficacy of LG compared with those of OG for AGC in elderly patients (NCT03564834[53]; NCT02246153[54]).
For patients with EGC, increasing postoperative QoL without sacrificing long-term survival is receiving increasing attention. When aiming to maintain a patient's QoL after surgery, FPG, including techniques such as PG and pylorus-preserving gastrectomy, can be considered. Beijing Cancer Hospital is performing multicenter RCTs to evaluate the safety and effectiveness of FPG, including pylorus-preserving DG (PPG), PG, and wedge gastrectomy, for T1 and T2 GC patients (NCT03874871).
In the treatment of middle stomach EGC, either PPG or DG can be conducted. Compared with DGs, PPG has been recognized for its functional advantages, such as nutritional benefits and a reduced risk of dumping syndrome, bile reflux, and gallstone formation[55,56]. Nonetheless, PPG may pose potential risks to oncologic safety because fewer or incompletely dissected LNs are needed compared with that for DGs[57,58]. To date, no RCTs have compared the perioperative outcomes and long-term nutritional status between laparoscopic-assisted PPG (LAPPG) and LADG. One ongoing prospective RCT (NCT02936193) comparing LAPPG to LADG was performed at Shanghai Renji Hospital (Shanghai, China) based on the leading experience of LAPPG in China[56].
Many EGCs with relatively low LN metastasis can be curatively treated with endoscopy. Several retrospective cohort studies have explored the topic of endoscopic submucosal dissection (ESD) vs surgery. Findings from these studies demonstrated that, compared with surgery, ESD resulted in shorter procedure times, quicker recovery, and fewer early and severe complications. Despite these benefits, the ESD group also exhibited a higher recurrence rate[59]. No RCTs have compared the long-term outcomes of ESD and surgery for EGC treatment. Peking University Third Hospital (Beijing, China) designed one RCT to compare the long- and short-term outcomes between ESD and surgery in the treatment of EGC (NCT03857737). The trial enrolled 300 patients.
On the other hand, for some EGC patients with independent risk factors for LN metastasis, such as submucosal tumor invasion, lymphovascular invasion, undifferentiated type, and size larger than 2 cm, standard gastrectomy with LN dissection is usually recommended even if the gastric lesions can be completely removed via endoscopy[19]. Among the new possibilities, the combination of ESD with laparoscopic LN dissection (LLND), the so-called hybrid laparoscopic approach, represents one of the most interesting procedures for EGC patients with a potential risk of LN metastasis[60,61]. The hybrid approach was considered to improve patients’ postoperative QoL. One RCT was conducted at Beijing Friendship Hospital (Beijing, China) to determine whether ESD combined with LLND for EGC can improve long-term outcomes (NCT02325999).
Reconstruction of the digestive tract is a crucial skill in laparoscopic procedures. Nonetheless, there is presently no unanimous agreement on the optimal selection process for the different techniques. There are 16 registered clinical trials from China, among which 14 are RCTs, including one multicenter study.
Billroth-I, Billroth-II, Roux-en-Y, and uncut Roux-en-Y reconstructions are possible for DGs. Delta-shaped gastroduodenostomy (DSG), a method of intracorporeal Billroth-I anastomosis using only endoscopic linear staplers, was first reported in 2002[62]. Huang et al[63] simplified the technique of DSG and reported the safety and advantages of their modified DSG. After that, they performed one prospective RCT that compared modified DSG with the Billroth-I for DGs to explore the clinical application value and long-term oncology results (NCT02289183). Diabetes mellitus is a challenging health issue linked to a greater likelihood of cardiovascular events and diseases. Due to its antidiabetic properties, Roux-en-Y gastric bypass is commonly utilized in bariatric and metabolic surgery. These findings suggest that Roux-en-Y reconstruction during GC surgery could lead to improved glucose metabolism compared with that associated with the Billroth-I and Billroth-II procedures; however, there is still a lack of strong evidence. Two RCTs were designed to separately compare different reconstruction methods in patients with GC comorbid with type 2 diabetes (NCT01528059; NCT01637350). To date, researchers have not published these results.
The Uncut Roux-en-Y gastrojejunostomy modifies the Billroth-II procedure with Braun anastomosis by incorporating a jejunal occlusion to prevent Roux-Stasis Syndrome (RSS)[64]. The number of trials concerning uncut Roux-en-Y anastomosis ranked first among all the registered trials about digestive tract reconstruction. One single-arm RCT evaluated whether the QoL after uncut Roux-en-Y was superior to that after traditional BII + Braun reconstruction (NCT03624725). To analyze the advantages and disadvantages of the Uncut Roux-en-Y and Billroth-II reconstructions, the First Hospital of Jilin University (Jilin, China) performed the first RCT enrolling 158 patients between February 2015 and February 2016. The short-term surgical results revealed that stomach pH values in uncut Roux-en-Y grafts were lower than 7 during the postoperative period and presented a lower incidence of biliary reflux and alkaline gastritis after 1 year of follow-up (NCT02694081)[65]. The lower incidence of bile reflux gastritis and RSS was also confirmed by a multicenter RCT enrolling 124 patients from January 2017 to May 2018[66]. Another ongoing RCT by the Sixth Affiliated Hospital, Sun Yat-sen University, is designed to compare uncut Roux-en-Y with Billroth-II in terms of postoperative nutritional status (NCT02763878)[67]. Two prospective RCTs comparing Uncut Roux-en-Y with conventional Roux-en-Y (NCT03349398; NCT02644148) have also been conducted to investigate the incidence of RSS and QoL.
Several different reconstruction techniques are possible after PG, such as gastric tube reconstruction, jejunal interposition, and jejunal pouch interposition, via laparoscopic or minilaparotomy. Cheng et al[68] from Zhejiang Cancer Hospital created a new reconstruction named Cheng’s GIRAFFE reconstruction. This technique combines the advantages of gastric tube reconstruction with rebuilding the His angle and fundus of the stomach[68,69]. They performed one prospective RCT to investigate the efficacy and safety of PG combined with GIRAFE anastomosis for early adenocarcinoma of the esophagogastric junction (NCT04657848).
There is no agreement on the optimal reconstruction technique following TG. Roux-en-Y anastomosis between the esophagus and jejunum is a straightforward choice for reconstructing the gastrointestinal system[70]. Roux-en-Y OE can be performed via an intracorporeal linear staple and an extracorporeal circular staple. OrVilTM (Covidien, Mansfield, MA, United States) utilizes a transorally inserted anvil along with a circular stapler for conducting intracorporeal esophagojejunostomy[71]. Based on their experience with intracorporeal Roux-en-Y anastomosis via an OrVil, Lu et al[71] designed a prospective RCT to explore the safety of intracorporeal esophagojejunostomy using a transorally inserted anvil (OrVil) compared with extracorporeal circular anastomosis (NCT02085031).
However, some surgeons have noted that the emptying time can be extremely fast, which increases the incidence of dumping syndrome following Roux-en-Y anastomosis. A lack of a stomach can significantly affect a patient’s nutritional status, leading to a reduced QoL[72]. To create a substitute for the stomach, the remaining part of the intestine might be reconstructed into a reservoir, with or without maintaining passage through the duodenum. Functional jejunal interposition (FJI) entails PG reconstruction that includes a reservoir and retains transduodenal passage[73,74]. In one prospective, multicenter RCT, 113 patients were enrolled to compare the nutritional status between those who underwent FJI and those who underwent Roux-en-Y after TG (NCT01996059). After a follow-up period of 12 months, food intake per meal (P = 0.021), the prognostic nutritional index (P = 0.015), weight loss (P = 0.019), and Gastrointestinal Symptom Rating Scale score (P = 0.015) were significantly lower in the FJI group than in the Roux-en-Y group. The results of this trial revealed that the FJI procedure is not superior to Roux-en-Y anastomosis after TG[75]. Roux-en-Y + jejunal pouch anastomosis is another newly designed method that can significantly increase volume to improve postoperative QoL. The Western China Gastric Cancer Collaboration (WCGCC) performed its first RCT to compare the postoperative QoL after traditional Roux-en-Y and Roux-en-Y + jejunal pouch anastomosis for TG (WCGCC-1202, NCT02110628), which began in 2015, and this study is now in the recruitment period[76].
Given the elevated frequencies of morbidities and recurrence after D2 gastrectomy, minimizing surgical risks and enhancing the long-term survival of GC patients presents an ongoing challenge for surgical professionals. Xie et al[77] and Cao et al[78] described the novel notion of integrating D2 lymphadenectomy with total mesogastrium excision (D2 + CME) as a mesentery-based approach in GC surgery. Over the period spanning from September 2014 to June 2018, Gong and colleagues executed a prospective RCT (NCT01978444) to compare D2+ CME with standard D2 dissection in the context of GC management[79]. A cohort of 486 patients receiving DG were randomly allocated to either the D2 arm or the D2 + CME arm[80]. Initial findings revealed that D2 + CME was associated with improvements in surgical outcomes, such as reduced intraoperative blood loss, augmented LN retrieval (34 ± 16 vs 27 ± 13; P < 0.0001), and decreased postoperative flatus duration. The total procedural time was greater for D2 + CME than for D2. The primary endpoint, 3-year DFS, is undergoing evaluation during the ongoing follow-up phase. Notably, the implementation of D2 + CME was limited by its proponent, whereas seven other surgeons carried out D2 procedures, a factor that could introduce potential bias stemming from varying levels of personal experience.
Splenic hilar LN (No. 10) dissection is still controversial for patients in whom APGC does not invade the greater curvature. Huang’s team performed a prospective RCT to evaluate (FUGES-002, NCT02333721) the benefit of LTG combined with spleen-preserving splenic hilar lymphadenectomy (LSTG) for APGC not invading the greater curvature and the characteristics of No. 10 LN metastasis. Between January 2015 and December 2018, 536 patients were enrolled and randomized to receive either LSTG (D2 + No. 10 group) or conventional LTG (D2 group). There were no significant differences in intraoperative or postoperative morbidity between the two groups. The 3-year DFS was not significantly different (70.3% vs 64.3%). However, individuals in whom APGC was positioned behind the gastric wall might gain advantages from No. 10 LN dissection. Stratification analysis revealed that those with advanced posterior GC in the D2 + No. 10 group had superior 3-year DFS (92.9% compared with 39.3%; P < 0.001) and OS (92.9% compared with 42.9%; P < 0.001)[81].
The central controversies include whether bursectomy can reduce local recurrence, improve OS, and increase postoperative complications. Theoretically, both the omentum and bursa omentalis should be resected to prevent peritoneal metastasis. Guangdong Provincial Hospital of Traditional Chinese Medicine published two RCTs to explore the short- and long-term outcomes of LTG with bursectomy (NCT02969148, NCT03117283). However, the sample size of these trials was small. Moreover, the results of a large-scale multi-institutional randomized trial (JCOG 1001) from Japan released in 2019 indicated that bursectomy did not provide a survival advantage over nonbursectomy[82]. Nevertheless, it remains unclear whether omentectomy should be performed. Some meta-analyses have shown that, compared with total omentectomy in GC surgery, partial omentectomy has noninferior oncological outcomes and comparable safety outcomes[83]. Recently, a randomized Phase 2 trial (the TOP-G trial) comparing omentectomy and omentum preservation for GC in Japan reported short-term outcomes, which indicated that the operative risks were generally similar between the two groups[84]. Moreover, Tianjin Medical University Cancer Institute and Hospital (Tianjin, China) successfully conducted single-center (NCT04108494) and multicenter (NCT04843215) prospective RCTs to prove the non-inferiority of omentum preservation over omentectomy in patients with T3–T4a GC. The NCT04843215 trial from China is large scale, and the estimated number of enrolled participants is 950. It is believed that with the publication of the results in the future, more favorable evidence can be provided.
D2 gastrectomy is commonly accepted as the main treatment for AGC. However, there is ongoing debate regarding the scope of LN dissection depending on tumor stage, tumor location, and patient condition. Presently, the disputed regions beyond the typical D2 range include stations No. 13, No. 14v, and No. 16a2/b1 LN[85]. As stated in the Japanese GC treatment guidelines, No. 13 LNs are considered regional LNs in GC patients with duodenal invasion. Optimizing for D2 plus No. 13 lymphadenectomy could be a viable choice in curative gastrectomy for tumors with duodenal involvement. Although 14v is not part of standard D2 lymphadenectomy, it is still classified as a regional gastric LN, and conducting D2+ No. 14v lymphadenectomy could benefit patients with cancer spread to the No. 6 LN[19]. Nevertheless, the guidelines do not address the importance of dissecting No. 8p, 12b, or 12p LNs in cases of duodenal invasion. Due to the lack of RCTs investigating the survival advantages of 14v dissection, no definitive conclusions can be drawn[86]. To further shed light on the necessity of No. 14v node dissection for localized AGC, Tianjin Medical University Cancer Institute and Hospital conducted a multicenter prospective RCT to analyze the potential influence of No. 14v node dissec
JCOG9501 reported that D2 lymphadenectomy plus para-aortic nodal dissection (PAND) did not improve the prog
Despite improvements in surgical methods and patient management, age remains a major factor in the risk of postoperative complications and death. There is ongoing debate regarding the possibility of elderly individuals with GC undergoing less invasive procedures with decreased LN removal. In a study conducted by Huang and colleagues, an RCT was conducted to assess the safety of laparoscopic D1 lymphadenectomy and determine its impact on survival rates for older patients diagnosed with AGC (NCT03290209).
Recently, different tracers and dyes have been utilized in clinical settings to monitor LN drainage from initial tumors. Due to the effective use of ICG fluorescence imaging technology in laparoscopic instruments, the exploration of ICG fluorescence imaging-guided minimally invasive therapy in individuals with GC has emerged as a novel focus[90]. The existing injection methods include the submucosal approach and subserosal approach. There is a lack of RCTs evaluating the safety, efficacy, feasibility, and method of ICG-guided laparoscopic D2 lymphadenectomy. A Phase 3 parallel open-label RCT (FUGES-012, NCT03050879) took place at Fujian Medical University Union Hospital. Between November 2018 and July 2019, a total of 258 patients were enrolled in the study. The number of LNs retrieved in the ICG group (50.5 ± 15.9) was notably greater than that in the non-ICG group (42.0 ± 10.3). The LN retrieval rate was significantly lower in the ICG group (31.8% vs 57.4%; P < 0.001). The postoperative recovery process was not significantly different, with a similar incidence (15.5% vs 16.3%, P = 0.86) and severity of complications within 30 days postsurgery[91]. The researchers indicated that the study included all potential tumor sites, and the unevenly matched tumor location may be a limitation in the trial. Next, they designed another RCT to evaluate ICG in LDG for EGC (FUGES-023, NCT04973475). The same team continued to explore the optimal ICG injection method for LN tracing during LG. The FUGES-019 trial showed that ICG administered by subserosal injection during surgery imposed a lower economic and mental burden on patients and had similar surgical results to those of the preoperative submucosal injection (FUGES-019, NCT04219332)[92].
Carbon nanoparticles (CNPs) are another type of tracer used in laparoscopic surgery. Two RCTs explored the efficacy and safety of CNPs compared with (NCT05229874) or without those of ICG (NCT02123407) during LG.
Thirty patients were enrolled in the NCT02123407 trial conducted by Beijng Cancer Hospital. The mean number of harvested LNs was greater in the CNP group than in the noncoloring tracer group (38.33 vs 28.27; P = 0.041). A smaller diameter of LNs was recorded in the CNP group (3.32 mm vs 4.30 mm; P = 0.023)[93].
The quantity and quality of clinical research on minimally invasive surgery for GC have increased annually. The use of traditional minimally invasive laparoscopic surgery for treating GC has reached a plateau, and various improved new technologies have gradually emerged. Although great progress has been made in clinical research on GC in China, deficiencies still exist. There are more single-center retrospective studies than multi-center prospective studies and more repetitive research than innovative research. Many studies enrolled large numbers of patients, but the research data were not of high quality. However, it should also be noted that Chinese clinical research has distinct advantages and great development opportunities. The large population, high incidence and large proportion of AGC cases are characteristic of GC in China, which provides favorable conditions for studying this disease.
This study had some limitations. First, our review focused solely on the GC surgery trials listed on Clinical Trials.com, which may have led to the omission of some trials that are registered in domestic clinical trial registries in China. Second, the way outcomes are reported in GC surgery trials lacks consistency, showing significant variability in terms of defi
This review highlighted the advancement and increasing adoption of endoscopic, laparoscopic, and robotic methods, marking a transition towards more accurate and less invasive surgical alternatives for the treatment of GC. Additionally, the results emphasized the increasing focus on improving the postoperative QoL of patients, suggesting a comprehensive treatment strategy that extends beyond mere surgical oncological safety. However, there is still a need for well-designed, large randomized clinical trials to improve our knowledge of the surgical treatment of GC.
| 1. | Wang Y, Zhang L, Yang Y, Lu S, Chen H. Progress of Gastric Cancer Surgery in the era of Precision Medicine. Int J Biol Sci. 2021;17:1041-1049. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 2. | Cao W, Chen HD, Yu YW, Li N, Chen WQ. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin Med J (Engl). 2021;134:783-791. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1624] [Cited by in RCA: 1796] [Article Influence: 449.0] [Reference Citation Analysis (1)] |
| 3. | Eom SS, Choi W, Eom BW, Park SH, Kim SJ, Kim YI, Yoon HM, Lee JY, Kim CG, Kim HK, Kook MC, Choi IJ, Kim YW, Park YI, Ryu KW. A Comprehensive and Comparative Review of Global Gastric Cancer Treatment Guidelines. J Gastric Cancer. 2022;22:3-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 48] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 4. | Robinson NB, Fremes S, Hameed I, Rahouma M, Weidenmann V, Demetres M, Morsi M, Soletti G, Di Franco A, Zenati MA, Raja SG, Moher D, Bakaeen F, Chikwe J, Bhatt DL, Kurlansky P, Girardi LN, Gaudino M. Characteristics of Randomized Clinical Trials in Surgery From 2008 to 2020: A Systematic Review. JAMA Netw Open. 2021;4:e2114494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 58] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 5. | Chinese Laparoscopic Gastrointestinal Surgery Study. [Ten years of the CLASS Group: retrospect and prospect]. Zhonghua Weichang Waike Zazhi. 2019;22:916-919. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 6. | Hu YF, Yu J, Zhang C, Wang YN, Cheng X, Huang F, Li GX. [Development and implementation of a clinical data mining system for gastric cancer surgery]. Zhonghua Weichang Waike Zazhi. 2010;13:510-515. [DOI] [Full Text] |
| 7. | Hu Y, Ying M, Huang C, Wei H, Jiang Z, Peng X, Hu J, Du X, Wang B, Lin F, Xu J, Dong G, Mou T, Li G; Chinese Laparoscopic Gastrointestinal Surgery Study (CLASS) Group. Oncologic outcomes of laparoscopy-assisted gastrectomy for advanced gastric cancer: a large-scale multicenter retrospective cohort study from China. Surg Endosc. 2014;28:2048-2056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 8. | Yu J, Hu J, Huang C, Ying M, Peng X, Wei H, Jiang Z, Du X, Liu Z, Liu H, Li G; Chinese Laparoscopic Gastrointestinal Surgery Study (CLASS) Group. The impact of age and comorbidity on postoperative complications in patients with advanced gastric cancer after laparoscopic D2 gastrectomy: results from the Chinese laparoscropic gastrointestinal surgery study (CLASS) group. Eur J Surg Oncol. 2013;39:1144-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 9. | Hu Y, Huang C, Sun Y, Su X, Cao H, Hu J, Xue Y, Suo J, Tao K, He X, Wei H, Ying M, Hu W, Du X, Chen P, Liu H, Zheng C, Liu F, Yu J, Li Z, Zhao G, Chen X, Wang K, Li P, Xing J, Li G. Morbidity and Mortality of Laparoscopic Versus Open D2 Distal Gastrectomy for Advanced Gastric Cancer: A Randomized Controlled Trial. J Clin Oncol. 2016;34:1350-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 536] [Cited by in RCA: 530] [Article Influence: 58.9] [Reference Citation Analysis (0)] |
| 10. | Yu J, Huang C, Sun Y, Su X, Cao H, Hu J, Wang K, Suo J, Tao K, He X, Wei H, Ying M, Hu W, Du X, Hu Y, Liu H, Zheng C, Li P, Xie J, Liu F, Li Z, Zhao G, Yang K, Liu C, Li H, Chen P, Ji J, Li G; Chinese Laparoscopic Gastrointestinal Surgery Study (CLASS) Group. Effect of Laparoscopic vs Open Distal Gastrectomy on 3-Year Disease-Free Survival in Patients With Locally Advanced Gastric Cancer: The CLASS-01 Randomized Clinical Trial. JAMA. 2019;321:1983-1992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 522] [Cited by in RCA: 525] [Article Influence: 87.5] [Reference Citation Analysis (1)] |
| 11. | Huang C, Liu H, Hu Y, Sun Y, Su X, Cao H, Hu J, Wang K, Suo J, Tao K, He X, Wei H, Ying M, Hu W, Du X, Yu J, Zheng C, Liu F, Li Z, Zhao G, Zhang J, Chen P, Li G; Chinese Laparoscopic Gastrointestinal Surgery Study (CLASS) Group. Laparoscopic vs Open Distal Gastrectomy for Locally Advanced Gastric Cancer: Five-Year Outcomes From the CLASS-01 Randomized Clinical Trial. JAMA Surg. 2022;157:9-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 162] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 12. | He H, Li H, Su X, Li Z, Yu P, Huang H, Huang C, Ye J, Li Y, Suo J, Yu J, Li G, Xu Z, Zhao G, Cao H, Hu J, Du X, Liu F, Sun Y; Chinese Laparoscopic Gastrointestinal Surgery Study (CLASS) Group. Study on safety of laparoscopic total gastrectomy for clinical stage I gastric cancer: the protocol of the CLASS02-01 multicenter randomized controlled clinical trial. BMC Cancer. 2018;18:944. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Katai H, Mizusawa J, Katayama H, Kunisaki C, Sakuramoto S, Inaki N, Kinoshita T, Iwasaki Y, Misawa K, Takiguchi N, Kaji M, Okitsu H, Yoshikawa T, Terashima M; Stomach Cancer Study Group of Japan Clinical Oncology Group. Single-arm confirmatory trial of laparoscopy-assisted total or proximal gastrectomy with nodal dissection for clinical stage I gastric cancer: Japan Clinical Oncology Group study JCOG1401. Gastric Cancer. 2019;22:999-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 128] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 14. | Hyung WJ, Yang HK, Han SU, Lee YJ, Park JM, Kim JJ, Kwon OK, Kong SH, Kim HI, Lee HJ, Kim W, Ryu SW, Jin SH, Oh SJ, Ryu KW, Kim MC, Ahn HS, Park YK, Kim YH, Hwang SH, Kim JW, Cho GS. A feasibility study of laparoscopic total gastrectomy for clinical stage I gastric cancer: a prospective multi-center phase II clinical trial, KLASS 03. Gastric Cancer. 2019;22:214-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 114] [Article Influence: 19.0] [Reference Citation Analysis (1)] |
| 15. | Liu F, Huang C, Xu Z, Su X, Zhao G, Ye J, Du X, Huang H, Hu J, Li G, Yu P, Li Y, Suo J, Zhao N, Zhang W, Li H, He H, Sun Y; Chinese Laparoscopic Gastrointestinal Surgery Study (CLASS) Group. Morbidity and Mortality of Laparoscopic vs Open Total Gastrectomy for Clinical Stage I Gastric Cancer: The CLASS02 Multicenter Randomized Clinical Trial. JAMA Oncol. 2020;6:1590-1597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 159] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 16. | Li Z, Shan F, Ying X, Zhang L, Ren H, Li S, Jia Y, Miao R, Xue K, Li Z, Wang Y, Yan C, Zhang Y, Pang F, Ji J. Laparoscopic or open distal gastrectomy after neoadjuvant chemotherapy for advanced gastric cancer: study protocol for a randomised phase II trial. BMJ Open. 2018;8:e021633. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Li Z, Shan F, Ying X, Zhang Y, E JY, Wang Y, Ren H, Su X, Ji J. Assessment of Laparoscopic Distal Gastrectomy After Neoadjuvant Chemotherapy for Locally Advanced Gastric Cancer: A Randomized Clinical Trial. JAMA Surg. 2019;154:1093-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 127] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 18. | Huang CM, Chen T, Lin JX, Chen QY, Zheng CH, Li P, Xie JW, Wang JB, Lu J, Cao LL, Lin M, Tu RH. The effects of laparoscopic spleen-preserving splenic hilar lymphadenectomy on the surgical outcome of proximal gastric cancer: a propensity score-matched, case-control study. Surg Endosc. 2017;31:1383-1392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer. 2021;24:1-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 735] [Cited by in RCA: 1336] [Article Influence: 334.0] [Reference Citation Analysis (2)] |
| 20. | Yu W, Choi GS, Chung HY. Randomized clinical trial of splenectomy versus splenic preservation in patients with proximal gastric cancer. Br J Surg. 2006;93:559-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 187] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 21. | Sano T, Sasako M, Mizusawa J, Yamamoto S, Katai H, Yoshikawa T, Nashimoto A, Ito S, Kaji M, Imamura H, Fukushima N, Fujitani K; Stomach Cancer Study Group of the Japan Clinical Oncology Group. Randomized Controlled Trial to Evaluate Splenectomy in Total Gastrectomy for Proximal Gastric Carcinoma. Ann Surg. 2017;265:277-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 235] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 22. | Schwarz RE. Spleen-preserving splenic hilar lymphadenectomy at the time of gastrectomy for cancer: technical feasibility and early results. J Surg Oncol. 2002;79:73-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Kawamura Y, Satoh S, Suda K, Ishida Y, Kanaya S, Uyama I. Critical factors that influence the early outcome of laparoscopic total gastrectomy. Gastric Cancer. 2015;18:662-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | Zheng CH, Xu YC, Zhao G, Cai LS, Li GX, Xu ZK, Yan S, Wu ZG, Xue FQ, Sun YH, Xu DB, Zhang WB, Jin-Wan, Yu PW, Hu JK, Su XQ, Ji JF, Li ZY, You J, Li Y, Lin-Fan, Jun-Lu, Ping-Li, Huang CM; Chinese Laparoscopic Gastrointestinal Surgery Study (CLASS) Group. Safety and feasibility of laparoscopic spleen-preserving No. 10 lymph node dissection for locally advanced upper third gastric cancer: a prospective, multicenter clinical trial. Surg Endosc. 2020;34:5062-5073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 25. | Zheng C, Xu Y, Zhao G, Cai L, Li G, Xu Z, Yan S, Wu Z, Xue F, Sun Y, Xu D, Zhang W, Wan J, Yu P, Hu J, Su X, Ji J, Li Z, You J, Li Y, Fan L, Lin J, Lin J, Li P, Huang C. Outcomes of Laparoscopic Total Gastrectomy Combined With Spleen-Preserving Hilar Lymphadenectomy for Locally Advanced Proximal Gastric Cancer: A Nonrandomized Clinical Trial. JAMA Netw Open. 2021;4:e2139992. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 26. | Oki E, Sakaguchi Y, Ohgaki K, Saeki H, Chinen Y, Minami K, Sakamoto Y, Toh Y, Kusumoto T, Okamura T, Maehara Y. The impact of obesity on the use of a totally laparoscopic distal gastrectomy in patients with gastric cancer. J Gastric Cancer. 2012;12:108-112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Lee CM, Park JH, In Choi C, Lee HH, Min JS, Jee YS, Jeong O, Chae H, Choi SI, Huang H, Park S. A multi-center prospective randomized controlled trial (phase III) comparing the quality of life between laparoscopy-assisted distal gastrectomy and totally laparoscopic distal gastrectomy for gastric Cancer (study protocol). BMC Cancer. 2019;19:206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 28. | Chinese Laparoscopic Gastrointestinal Surgery Study Group. Endoscopic Surgery, and B. Chinese Medical Association Surgical, [Standard operation procedure of laparoscopic D2 distal gastrectomy for locally advanced gastric cancer: consensus on CLASS-01 trial]. Zhonghua Weichang Waike Zazhi. 2019;22:807-811. [RCA] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 29. | Hashizume M, Sugimachi K. Robot-assisted gastric surgery. Surg Clin North Am. 2003;83:1429-1444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 139] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 30. | Lee J, Kim YM, Woo Y, Obama K, Noh SH, Hyung WJ. Robotic distal subtotal gastrectomy with D2 lymphadenectomy for gastric cancer patients with high body mass index: comparison with conventional laparoscopic distal subtotal gastrectomy with D2 lymphadenectomy. Surg Endosc. 2015;29:3251-3260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 100] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 31. | Park JM, Kim HI, Han SU, Yang HK, Kim YW, Lee HJ, An JY, Kim MC, Park S, Song KY, Oh SJ, Kong SH, Suh BJ, Yang DH, Ha TK, Hyung WJ, Ryu KW. Who may benefit from robotic gastrectomy?: A subgroup analysis of multicenter prospective comparative study data on robotic versus laparoscopic gastrectomy. Eur J Surg Oncol. 2016;42:1944-1949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 32. | Liu FL, Lv CT, Qin J, Shen KT, Chen WD, Shen ZB, Wang C, Sun YH, Qin XY. [Da Vinci robot-assisted gastrectomy with lymph node dissection for gastric cancer: a case series of 9 patients]. Zhonghua Weichang Waike Zazhi. 2010;13:327-329. [DOI] [Full Text] |
| 33. | Li Z, Li J, Li B, Bai B, Liu Y, Lian B, Zhao Q. Robotic versus laparoscopic gastrectomy with D2 lymph node dissection for advanced gastric cancer: a propensity score-matched analysis. Cancer Manag Res. 2018;10:705-714. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 34. | Zhang K, Huang X, Gao Y, Liang W, Xi H, Cui J, Li J, Zhu M, Liu G, Zhao H, Hu C, Liu Y, Qiao Z, Wei B, Chen L. Robot-Assisted Versus Laparoscopy-Assisted Proximal Gastrectomy for Early Gastric Cancer in the Upper Location: Comparison of Oncological Outcomes, Surgical Stress, and Nutritional Status. Cancer Control. 2018;25:1073274818765999. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 35. | Junfeng Z, Yan S, Bo T, Yingxue H, Dongzhu Z, Yongliang Z, Feng Q, Peiwu Y. Robotic gastrectomy versus laparoscopic gastrectomy for gastric cancer: comparison of surgical performance and short-term outcomes. Surg Endosc. 2014;28:1779-1787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 111] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 36. | Li ZY, Zhou YB, Li TY, Li JP, Zhou ZW, She JJ, Hu JK, Qian F, Shi Y, Tian YL, Gao GM, Gao RZ, Liang CC, Shi FY, Yang K, Wen Y, Zhao YL, Yu PW; Robotic, Laparoscopic Surgery Committee of Chinese Research Hospital Association. Robotic Gastrectomy Versus Laparoscopic Gastrectomy for Gastric Cancer: A Multicenter Cohort Study of 5402 Patients in China. Ann Surg. 2023;277:e87-e95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 61] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 37. | Shen W, Xi H, Wei B, Cui J, Bian S, Zhang K, Wang N, Huang X, Chen L. Robotic versus laparoscopic gastrectomy for gastric cancer: comparison of short-term surgical outcomes. Surg Endosc. 2016;30:574-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 38. | Gao Y, Xi H, Qiao Z, Li J, Zhang K, Xie T, Shen W, Cui J, Wei B, Chen L. Comparison of robotic- and laparoscopic-assisted gastrectomy in advanced gastric cancer: updated short- and long-term results. Surg Endosc. 2019;33:528-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 39. | Kim MS, Kim WJ, Hyung WJ, Kim HI, Han SU, Kim YW, Ryu KW, Park S. Comprehensive Learning Curve of Robotic Surgery: Discovery From a Multicenter Prospective Trial of Robotic Gastrectomy. Ann Surg. 2021;273:949-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 97] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 40. | Obama K, Kim YM, Kang DR, Son T, Kim HI, Noh SH, Hyung WJ. Long-term oncologic outcomes of robotic gastrectomy for gastric cancer compared with laparoscopic gastrectomy. Gastric Cancer. 2018;21:285-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 111] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 41. | Lu J, Zheng CH, Xu BB, Xie JW, Wang JB, Lin JX, Chen QY, Cao LL, Lin M, Tu RH, Huang ZN, Lin JL, Zheng HL, Huang CM, Li P. Assessment of Robotic Versus Laparoscopic Distal Gastrectomy for Gastric Cancer: A Randomized Controlled Trial. Ann Surg. 2021;273:858-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 168] [Article Influence: 42.0] [Reference Citation Analysis (1)] |
| 42. | Chen QY, Zhong Q, Liu ZY, Li P, Wang JB, Lin JX, Lu J, Cao LL, Lin M, Tu RH, Huang ZN, Lin JL, Zheng HL, Lin GT, Zheng CH, Huang CM, Xie JW. Surgical Outcomes, Technical Performance, and Surgery Burden of Robotic Total Gastrectomy for Locally Advanced Gastric Cancer: A Prospective Study. Ann Surg. 2022;276:e434-e443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 43. | Shi Y, Xu X, Zhao Y, Qian F, Tang B, Hao Y, Luo H, Chen J, Yu P. Short-term surgical outcomes of a randomized controlled trial comparing laparoscopic versus open gastrectomy with D2 lymph node dissection for advanced gastric cancer. Surg Endosc. 2018;32:2427-2433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 44. | Shi Y, Xu X, Zhao Y, Qian F, Tang B, Hao Y, Luo H, Chen J, Yu P. Long-term oncologic outcomes of a randomized controlled trial comparing laparoscopic versus open gastrectomy with D2 lymph node dissection for advanced gastric cancer. Surgery. 2019;165:1211-1216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 45. | Cui M, Li Z, Xing J, Yao Z, Liu M, Chen L, Zhang C, Yang H, Zhang N, Tan F, Jiang B, Di J, Wang Z, Ji J, Su X. A prospective randomized clinical trial comparing D2 dissection in laparoscopic and open gastrectomy for gastric cancer. Med Oncol. 2015;32:241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 46. | He Y, Pan A, Wang Y, Yang Y, Xu J, Zhang Y, Liu D, Wang Q, Shen H, Zhang Y, Yan D, Peng Z, Hu FB, Ma X. Prevalence of overweight and obesity in 15.8 million men aged 15-49 years in rural China from 2010 to 2014. Sci Rep. 2017;7:5012. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 47. | Inagawa S, Adachi S, Oda T, Kawamoto T, Koike N, Fukao K. Effect of fat volume on postoperative complications and survival rate after D2 dissection for gastric cancer. Gastric Cancer. 2000;3:141-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 79] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 48. | Wang H, Mou T, Chen H, Hu Y, Lin T, Li T, Yu J, Liu H, Li G. Long-term outcomes of laparoscopy-assisted distal gastrectomy versus open distal gastrectomy for gastric cancer: a 10-year single-institution experience. Surg Endosc. 2019;33:135-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 49. | Guo X, Peng Z, Lv X, Cui J, Zhang K, Li J, Jin N, Xi H, Wei B, Chen L. Randomized controlled trial comparing short-term outcomes of laparoscopic and open spleen-preserving splenic hilar lymphadenectomy for advanced proximal gastric cancer: An interim report. J Surg Oncol. 2018;118:1264-1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 50. | Guo X, Bian SB, Peng Z, Wang N, Wei B, Cui JX, Wang XX, Xie TY, Xi HQ, Chen L. [Surgical selection and metastatic warning of splenic lymph node dissection in advanced gastric cancer radical surgery: a prospective, single-center, randomized controlled trial]. Zhonghua Weichang Waike Zazhi. 2020;23:144-151. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 51. | Honda M, Kumamaru H, Etoh T, Miyata H, Yamashita Y, Yoshida K, Kodera Y, Kakeji Y, Inomata M, Konno H, Seto Y, Kitano S, Watanabe M, Hiki N. Surgical risk and benefits of laparoscopic surgery for elderly patients with gastric cancer: a multicenter prospective cohort study. Gastric Cancer. 2019;22:845-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 52. | Zong L, Wu A, Wang W, Deng J, Aikou S, Yamashita H, Maeda M, Abe M, Yu D, Jiang Z, Seto Y, Ji J. Feasibility of laparoscopic gastrectomy for elderly gastric cancer patients: meta-analysis of non-randomized controlled studies. Oncotarget. 2017;8:51878-51887. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 53. | Li Z, Shan F, Ying X, Xue K, Ji J. Laparoscopic versus open gastrectomy for elderly local advanced gastric cancer patients: study protocol of a phase II randomized controlled trial. BMC Cancer. 2018;18:1118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 54. | Luo J, Zhu Y, Liu H, Hu YF, Li TJ, Lin T, Chen T, Chen H, Chen XH, Yu J, Li GX. Morbidity and mortality of elderly patients with advanced gastric cancer after laparoscopy-assisted or open distal gastrectomy: a randomized-controlled trial. Gastroenterol Rep (Oxf). 2018;6:317-319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 55. | Suh YS, Han DS, Kong SH, Kwon S, Shin CI, Kim WH, Kim HH, Lee HJ, Yang HK. Laparoscopy-assisted pylorus-preserving gastrectomy is better than laparoscopy-assisted distal gastrectomy for middle-third early gastric cancer. Ann Surg. 2014;259:485-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 96] [Article Influence: 8.7] [Reference Citation Analysis (2)] |
| 56. | Xia X, Xu J, Zhu C, Cao H, Yu F, Zhao G. Objective evaluation of clinical outcomes of laparoscopy-assisted pylorus-preserving gastrectomy for middle-third early gastric cancer. BMC Cancer. 2019;19:481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 57. | Hiki N, Sano T, Fukunaga T, Ohyama S, Tokunaga M, Yamaguchi T. Survival benefit of pylorus-preserving gastrectomy in early gastric cancer. J Am Coll Surg. 2009;209:297-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 58. | Hiki N, Shimoyama S, Yamaguchi H, Kubota K, Kaminishi M. Laparoscopy-assisted pylorus-preserving gastrectomy with quality controlled lymph node dissection in gastric cancer operation. J Am Coll Surg. 2006;203:162-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (2)] |
| 59. | Abdelfatah MM, Barakat M, Ahmad D, Ibrahim M, Ahmed Y, Kurdi Y, Grimm IS, Othman MO. Long-term outcomes of endoscopic submucosal dissection versus surgery in early gastric cancer: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2019;31:418-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 60. | Abe N, Takeuchi H, Ohki A, Yanagida O, Masaki T, Mori T, Sugiyama M. Long-term outcomes of combination of endoscopic submucosal dissection and laparoscopic lymph node dissection without gastrectomy for early gastric cancer patients who have a potential risk of lymph node metastasis. Gastrointest Endosc. 2011;74:792-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 61. | Li H, Zhao LL, Zhang XC, Liu DX, Wang GY, Huo ZB, Chen SB. Combination of endoscopic submucosal dissection and laparoscopic sentinel lymph node dissection in early mucinous gastric cancer: Role of lymph node metastasis. World J Clin Cases. 2020;8:3474-3482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 62. | Kanaya S, Gomi T, Momoi H, Tamaki N, Isobe H, Katayama T, Wada Y, Ohtoshi M. Delta-shaped anastomosis in totally laparoscopic Billroth I gastrectomy: new technique of intraabdominal gastroduodenostomy. J Am Coll Surg. 2002;195:284-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 237] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 63. | Huang C, Lin M, Chen Q, Lin J, Zheng C, Li P, Xie J, Wang J, Lu J. A modified delta-shaped gastroduodenostomy in totally laparoscopic distal gastrectomy for gastric cancer: a safe and feasible technique. PLoS One. 2014;9:e102736. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 64. | Huang Y, Wang S, Shi Y, Tang D, Wang W, Chong Y, Zhou H, Xiong Q, Wang J, Wang D. Uncut Roux-en-Y reconstruction after distal gastrectomy for gastric cancer. Expert Rev Gastroenterol Hepatol. 2016;10:1341-1347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 65. | Yang D, He L, Tong WH, Jia ZF, Su TR, Wang Q. Randomized controlled trial of uncut Roux-en-Y vs Billroth II reconstruction after distal gastrectomy for gastric cancer: Which technique is better for avoiding biliary reflux and gastritis? World J Gastroenterol. 2017;23:6350-6356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 54] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (1)] |
| 66. | Wang J, Wang Q, Dong J, Yang K, Ji S, Fan Y, Wang C, Ma Q, Wei Q, Ji G. Total Laparoscopic Uncut Roux-en-Y for Radical Distal Gastrectomy: An Interim Analysis of a Randomized, Controlled, Clinical Trial. Ann Surg Oncol. 2021;28:90-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 67. | Chen S, Chen DW, Chen XJ, Lin YJ, Xiang J, Peng JS. Postoperative complications and nutritional status between uncut Roux-en-Y anastomosis and Billroth II anastomosis after D2 distal gastrectomy: a study protocol for a multicenter randomized controlled trial. Trials. 2019;20:428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 68. | Cheng XD, Xu ZY, Du YA, Hu C, Yu JF, Yang LT, Huang L, Yu PF, Dai GG, Zhang YQ. [Preliminary efficacy analysis of Cheng's Giraffe reconstruction after proximal gastrectomy in adenocarcinoma of esophagogastric junction]. Zhonghua Weichang Waike Zazhi. 2020;23:158-162. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 69. | Xu Z, Hu C, Zhang Y, Huang L, Yang L, Yu J, Yu P, Chen J, Du Y, Cheng X. Efficacy analysis of Cheng's GIRAFFE reconstruction after proximal gastrectomy for adenocarcinoma of esophagogastric junction. Chin J Cancer Res. 2022;34:289-297. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 70. | Kumagai K, Shimizu K, Yokoyama N, Aida S, Arima S, Aikou T; Japanese Society for the Study of Postoperative Morbidity after Gastrectomy. Questionnaire survey regarding the current status and controversial issues concerning reconstruction after gastrectomy in Japan. Surg Today. 2012;42:411-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 71. | Lu X, Hu Y, Liu H, Mou T, Deng Z, Wang D, Yu J, Li G. Short-term outcomes of intracorporeal esophagojejunostomy using the transorally inserted anvil versus extracorporeal circular anastomosis during laparoscopic total gastrectomy for gastric cancer: a propensity score matching analysis. J Surg Res. 2016;200:435-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 72. | Lee SS, Chung HY, Kwon OK, Yu W. Long-term Quality of Life After Distal Subtotal and Total Gastrectomy: Symptom- and Behavior-oriented Consequences. Ann Surg. 2016;263:738-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 73. | Pan Y, Li Q, Wang DC, Wang JC, Liang H, Liu JZ, Cui QH, Sun T, Zhang RP, Kong DL, Hao XS. Beneficial effects of jejunal continuity and duodenal food passage after total gastrectomy: a retrospective study of 704 patients. Eur J Surg Oncol. 2008;34:17-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 74. | Zhang L, Pan Y, Liu HM, Zhan HJ, Ding XW, Wang XN, Wang BG, Liu N, Zhang RP. [Interim report of prospective clinical study of two different digestive tract reconstruction after total gastrectomy]. Zhonghua Weichang Waike Zazhi. 2013;16:1159-1163. [DOI] [Full Text] |
| 75. | Wang H, Hu X, Chen S, Xiang J, Yang Z, Zhou Z, Chen Y, Lin Y, Chen Y, Peng J. Functional jejunal interposition versus Roux-en-Y anastomosis after total gastrectomy for gastric cancer: A prospective randomized clinical trial. Surg Oncol. 2020;34:236-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 76. | Hu JK, Zhang WH, and Western China Gastric Cancer Collaboration. [Experience and present situation of Western China Gastric Cancer Collaboration]. Zhonghua Weichang Waike Zazhi. 2017;20:247-250. [DOI] [Full Text] |
| 77. | Xie D, Yu C, Liu L, Osaiweran H, Gao C, Hu J, Gong J. Short-term outcomes of laparoscopic D2 lymphadenectomy with complete mesogastrium excision for advanced gastric cancer. Surg Endosc. 2016;30:5138-5139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 78. | Cao B, Xiao A, Shen J, Xie D, Gong J. An Optimal Surgical Approach for Suprapancreatic Area Dissection in Laparoscopic D2 Gastrectomy with Complete Mesogastric Excision. J Gastrointest Surg. 2020;24:916-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 79. | Shen J, Cao B, Wang Y, Xiao A, Qin J, Wu J, Yan Q, Hu Y, Yang C, Cao Z, Hu J, Yin P, Xie D, Gong J. Prospective randomized controlled trial to compare laparoscopic distal gastrectomy (D2 lymphadenectomy plus complete mesogastrium excision, D2 + CME) with conventional D2 lymphadenectomy for locally advanced gastric adenocarcinoma: study protocol for a randomized controlled trial. Trials. 2018;19:432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 80. | Xie D, Shen J, Liu L, Cao B, Wang Y, Qin J, Wu J, Yan Q, Hu Y, Yang C, Cao Z, Hu J, Yin P, Gong J. Complete mesogastric excision for locally advanced gastric cancer: short-term outcomes of a randomized clinical trial. Cell Rep Med. 2021;2:100217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 81. | Lin JX, Lin JP, Wang ZK, Li P, Xie JW, Wang JB, Lu J, Chen QY, Cao LL, Lin M, Tu RH, Lin GT, Huang ZN, Lin JL, Zheng HL, Lin GS, Huang CM, Zheng CH. Assessment of Laparoscopic Spleen-Preserving Hilar Lymphadenectomy for Advanced Proximal Gastric Cancer Without Invasion Into the Greater Curvature: A Randomized Clinical Trial. JAMA Surg. 2023;158:10-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 82. | Kurokawa Y, Doki Y, Mizusawa J, Terashima M, Katai H, Yoshikawa T, Kimura Y, Takiguchi S, Nishida Y, Fukushima N, Iwasaki Y, Kaji M, Hirao M, Katayama H, Sasako M. Bursectomy versus omentectomy alone for resectable gastric cancer (JCOG1001): a phase 3, open-label, randomised controlled trial. Lancet Gastroenterol Hepatol. 2018;3:460-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 103] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 83. | Chai SW, Wang SH, Wang CY, Chen YC, Soong RS, Huang TS. Partial Versus Total Omentectomy in Patients with Gastric Cancer: A Systemic Review and Meta-Analysis. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 84. | Murakami H, Yamada T, Taguri M, Hasegawa S, Yamanaka T, Rino Y, Mushiake H, Oshima T, Matsukawa H, Tani K, Suzuki Y, Ozawa Y, Tanabe H, Osaragi T, Sato T, Tamagawa H, Yukawa N, Yoshikawa T, Imada T, Masuda M, Yamamoto Y. Short-Term Outcomes from a Randomized Screening Phase II Non-inferiority Trial Comparing Omentectomy and Omentum Preservation for Locally Advanced Gastric Cancer: the TOP-G Trial. World J Surg. 2021;45:1803-1811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 85. | Li JQ, He D, Liang YX. Current status of extended 'D2 plus' lymphadenectomy in advanced gastric cancer. Oncol Lett. 2021;21:467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 86. | Ke B, Liang H. Current status of lymph node dissection in gastric cancer. Chin J Cancer Res. 2021;33:193-202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 87. | Xu ZY, Hu C, Chen S, Du YA, Huang L, Yu PF, Wang LJ, Cheng XD. Evaluation of D2-plus radical resection for gastric cancer with pyloric invasion. BMC Surg. 2019;19:172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 88. | Sasako M, Sano T, Yamamoto S, Kurokawa Y, Nashimoto A, Kurita A, Hiratsuka M, Tsujinaka T, Kinoshita T, Arai K, Yamamura Y, Okajima K; Japan Clinical Oncology Group. D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med. 2008;359:453-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 698] [Cited by in RCA: 754] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 89. | Zhang C, He Y, Schwarz RE, Smith DD, Wang L, Liu F, Zhan W. Evaluation of para-aortic nodal dissection for locoregionally advanced gastric cancer with 1-3 involved para-aortic nodes. Chin Med J (Engl). 2014;127:435-441. [PubMed] [DOI] [Full Text] |
| 90. | Desiderio J, Trastulli S, Gemini A, Di Nardo D, Palazzini G, Parisi A, D'Andrea V. Fluorescence image-guided lymphadenectomy using indocyanine green and near infrared technology in robotic gastrectomy. Chin J Cancer Res. 2018;30:568-570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 91. | Chen QY, Xie JW, Zhong Q, Wang JB, Lin JX, Lu J, Cao LL, Lin M, Tu RH, Huang ZN, Lin JL, Zheng HL, Li P, Zheng CH, Huang CM. Safety and Efficacy of Indocyanine Green Tracer-Guided Lymph Node Dissection During Laparoscopic Radical Gastrectomy in Patients With Gastric Cancer: A Randomized Clinical Trial. JAMA Surg. 2020;155:300-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 212] [Article Influence: 42.4] [Reference Citation Analysis (1)] |
| 92. | Chen QY, Zhong Q, Li P, Xie JW, Liu ZY, Huang XB, Lin GT, Wang JB, Lin JX, Lu J, Cao LL, Lin M, Zheng QL, Tu RH, Huang ZN, Zheng CH, Huang CM. Comparison of submucosal and subserosal approaches toward optimized indocyanine green tracer-guided laparoscopic lymphadenectomy for patients with gastric cancer (FUGES-019): a randomized controlled trial. BMC Med. 2021;19:276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 93. | Li Z, Ao S, Bu Z, Wu A, Wu X, Shan F, Ji X, Zhang Y, Xing Z, Ji J. Clinical study of harvesting lymph nodes with carbon nanoparticles in advanced gastric cancer: a prospective randomized trial. World J Surg Oncol. 2016;14:88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |