Published online Nov 15, 2024. doi: 10.4251/wjgo.v16.i11.4338
Revised: August 24, 2024
Accepted: September 10, 2024
Published online: November 15, 2024
Processing time: 109 Days and 4.5 Hours
The receptor tyrosine kinase encoded by the MET gene plays an important role in various cellular processes such as growth, survival, migration and angiogenesis, and its abnormal activation is closely related to the occurrence and development of various tumors. This article reviews the recent advances in diagnosis and treatment of MET-variant digestive tract tumors. In terms of diagnosis, the application of next-generation sequencing technology and liquid biopsy tech
Core Tip: Our study reviews the progress in the use of MET variation in the diagnosis and treatment of digestive tract tumors and discusses the molecular mechanism of MET variation and its role in the genesis and development of digestive tract tumors. The analysis focused on the potential of MET variants as diagnostic markers and therapeutic targets, covering the latest research results and clinical trial data for MET-based targeted therapies. This paper also summarizes the current challenges and future research directions, aiming to provide new ideas and references for improving the diagnostic accuracy and treatment effectiveness of MET-variant digestive tract tumors.
- Citation: Zhang C, Dong HK, Gao JM, Zeng QQ, Qiu JT, Wang JJ. Advances in the diagnosis and treatment of MET-variant digestive tract tumors. World J Gastrointest Oncol 2024; 16(11): 4338-4353
- URL: https://www.wjgnet.com/1948-5204/full/v16/i11/4338.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i11.4338
Gastrointestinal tumors account for more than 50% of the global incidence and mortality of malignant tumors[1-4]. In addition to conventional surgery, chemotherapy and radiotherapy for the treatment of digestive tract tumors, precise targeted therapy guided by molecular typing has also achieved precise effects in clinical practice[5-8]. In addition to classical RAS, human epidermal growth factor receptor 2 (HER2) and other molecular targets, rare or rare mutation targets such as MET, ROS1, ALK, NTRK and other related drugs have been put on the market one after another and have achieved significant efficacy, becoming one of the current research hotspots in this field[9-12]. This article reviews recent progress in the diagnosis and treatment of MET mutations in patients with digestive tract tumors and explores the application prospects of anti-MET targeted therapy in the field of the digestive tract[13-16].
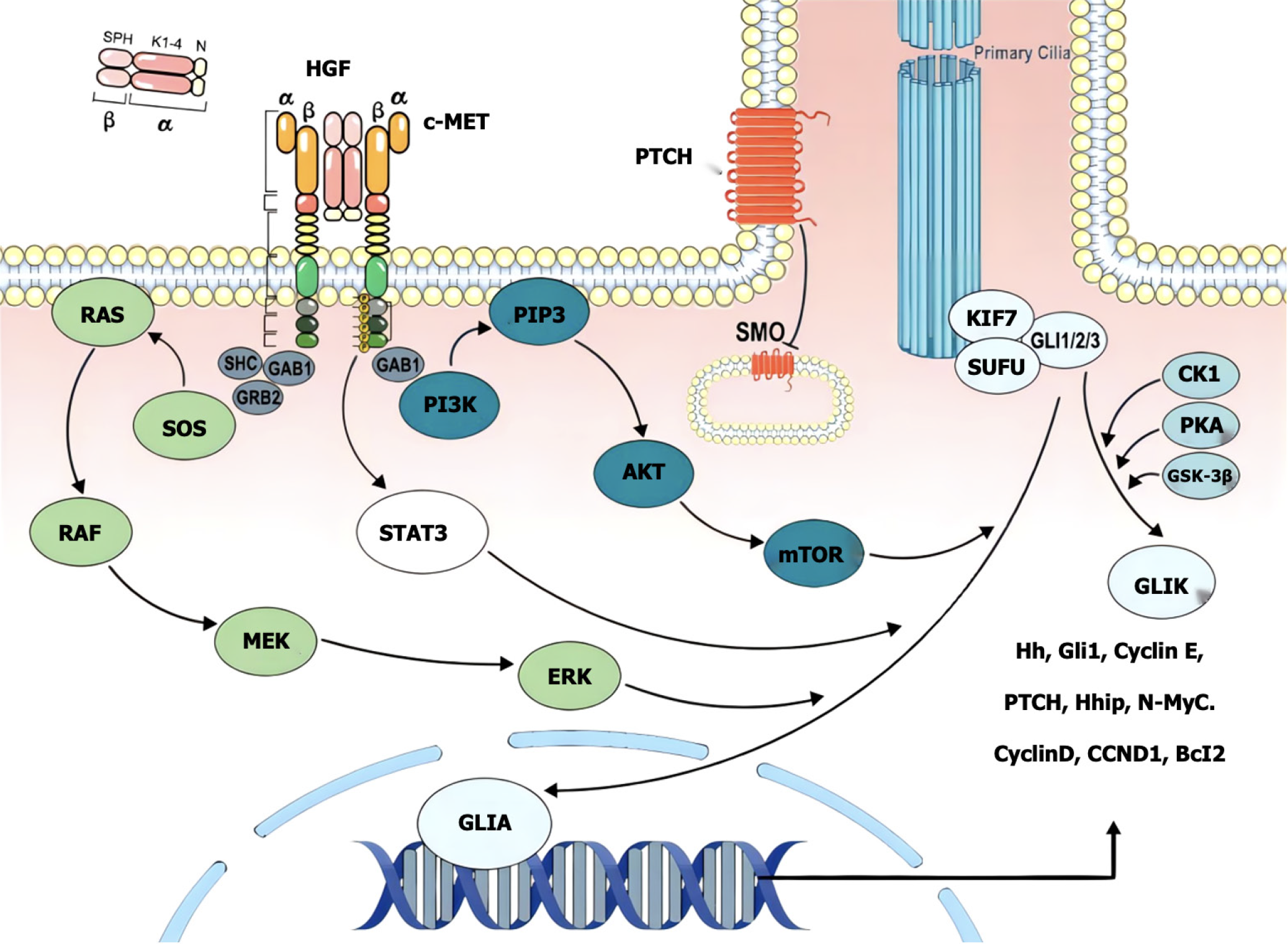
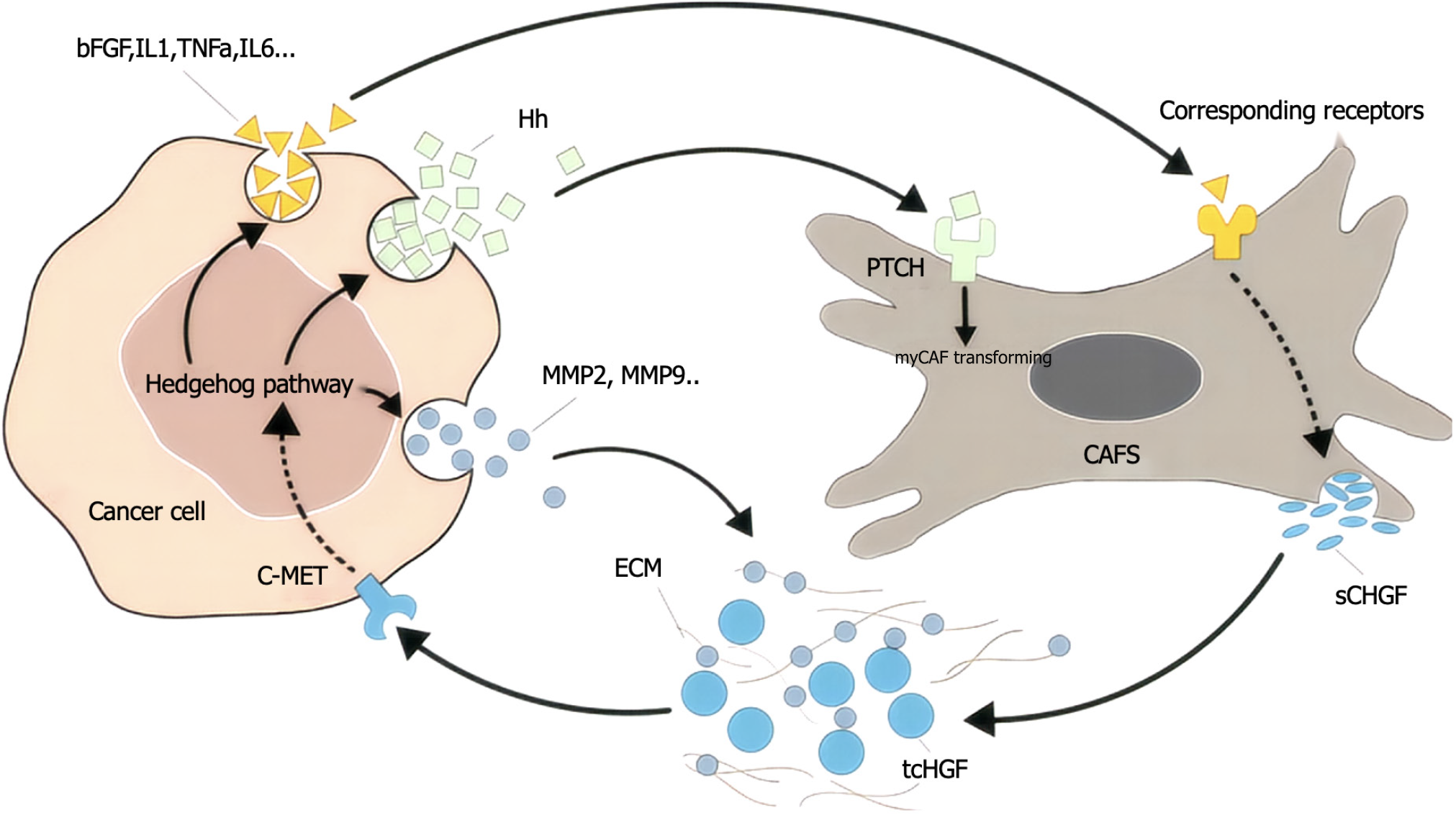
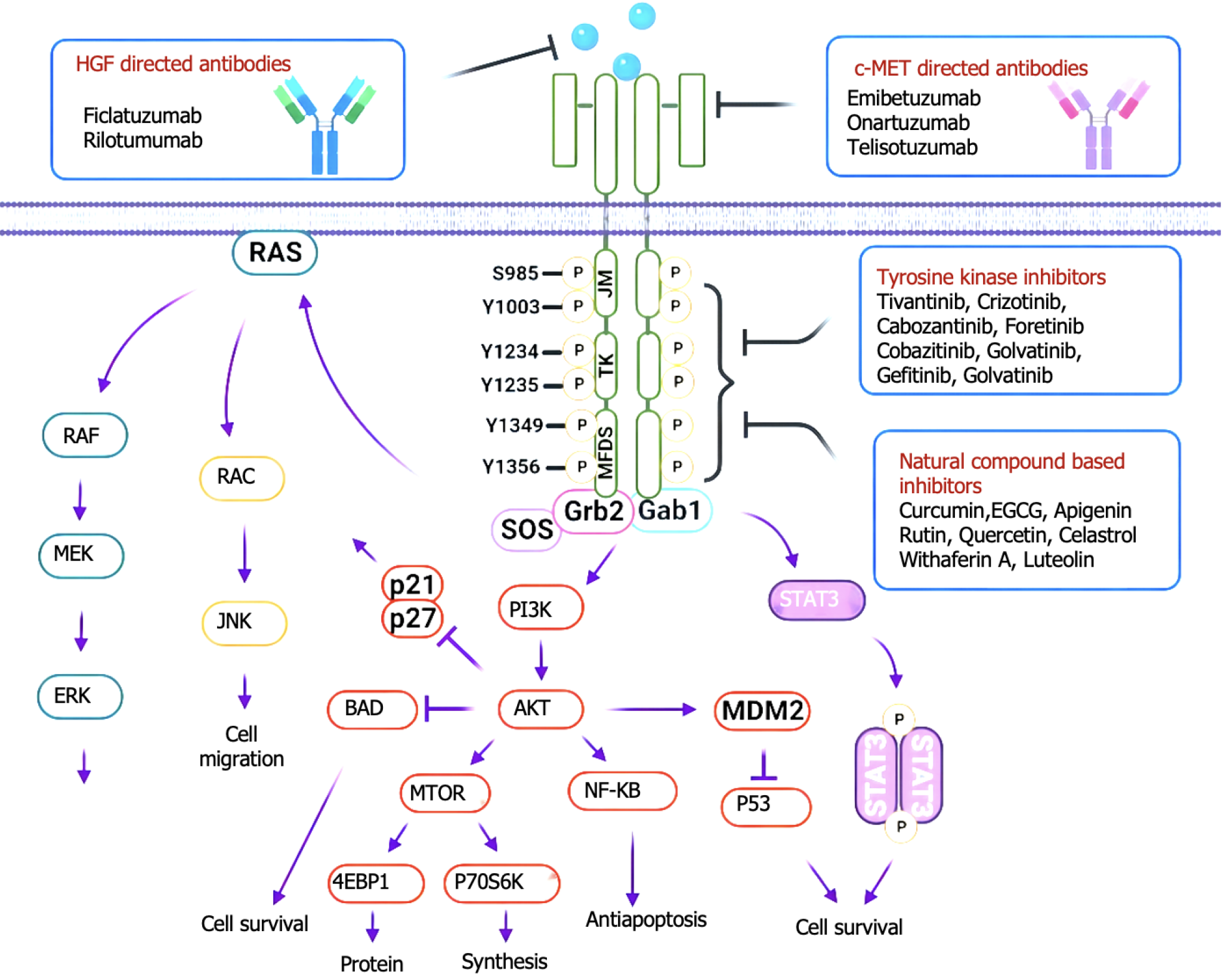
c-MET is a member of the receptor tyrosine kinase family that is expressed mainly in epithelial cells and is structurally divided into an extracellular region, a transmembrane helical domain and an intracellular region, and the extracellular SEMA domain is a key region for ligand binding[17-20]. The hepatocyte growth factor (HGF) synthesized and secreted by mesenchymal cells is the only known ligand of MET[21-24]. HGF and MET receptors bind specifically to the extracellular domain, and the conformation of the MET protein changes to activate the PTK domain of the intracellular tyrosine protein kinase[25-28]. Thus, two phosphorylation sites, Try1234 and Try1235, in the intracellular kinase active region of MET receptors recruit and phosphorylate a variety of effector proteins after phosphorylation[29-32]. Many previous studies have confirmed that the c-MET signaling pathway plays an important role in tumor proliferation, invasion, metastasis, angiogenesis, tumor treatment tolerance, etc., and is abnormally expressed in a variety of digestive tract tumors[33-36] (Figure 1). Therefore, targeted MET therapy may become a new choice for the specific molecular classification of digestive tract tumors[37-40].
The detection rate of MET gene variation in patients with solid tumors in China is low, which conforms to the category of rare mutations[41-44]. Large-scale second-generation sequencing (NGS) analysis revealed MET gene abnormalities in 10445 cancer patients in China; the incidence of MET amplification was 0.9% (141/10445), and that of exon 14 jump mutations was 0.7%[45-48]. MET amplification is most common in hepatocellular carcinoma (1.7%), gastric cancer (1.3%), and non-small cell lung cancer (NSCLC) (0.7%), whereas MET exon 14 jump mutations are most common in NSCLC (0.5%), hepatocellular carcinoma (0.3%), and colorectal cancer (0.2%)[49-52]. The incidence of MET fusion mutations is even lower[53-56]. A retrospective study in China revealed that the detection rate was only 0.15%, and the heterogeneity of fusion partner genes was very strong[57-60]. National and international guidelines currently identify MET14 exon jump mutations and increased MET gene copy number as potential targeted therapeutic molecular features of NSCLC[61-64]. In recent years, major breakthroughs have been made in the precision treatment of digestive tract tumors, including clinical studies on the treatment of rare mutations, including HER2 and MET, which have also achieved satisfactory survival benefits[65-68].
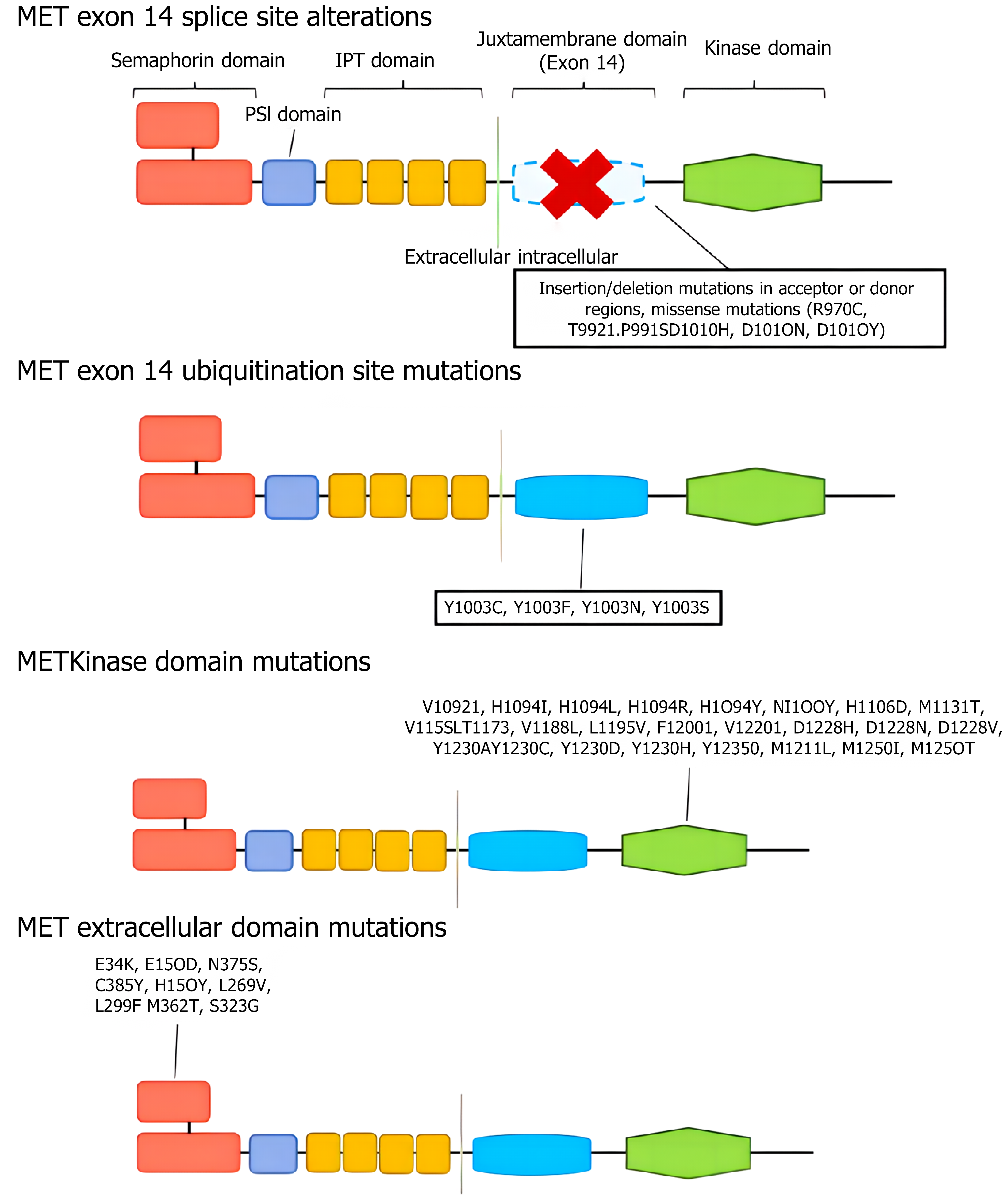
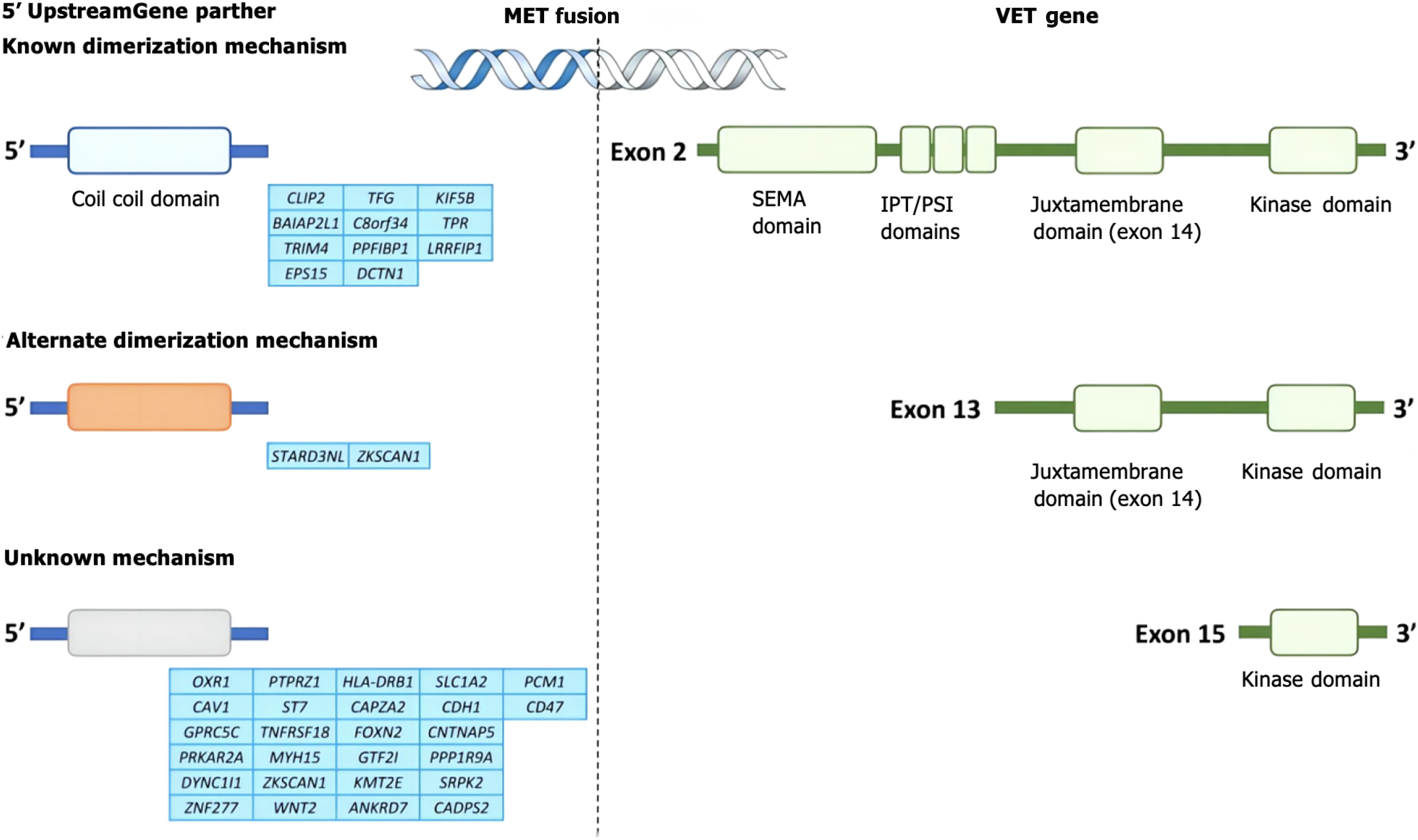
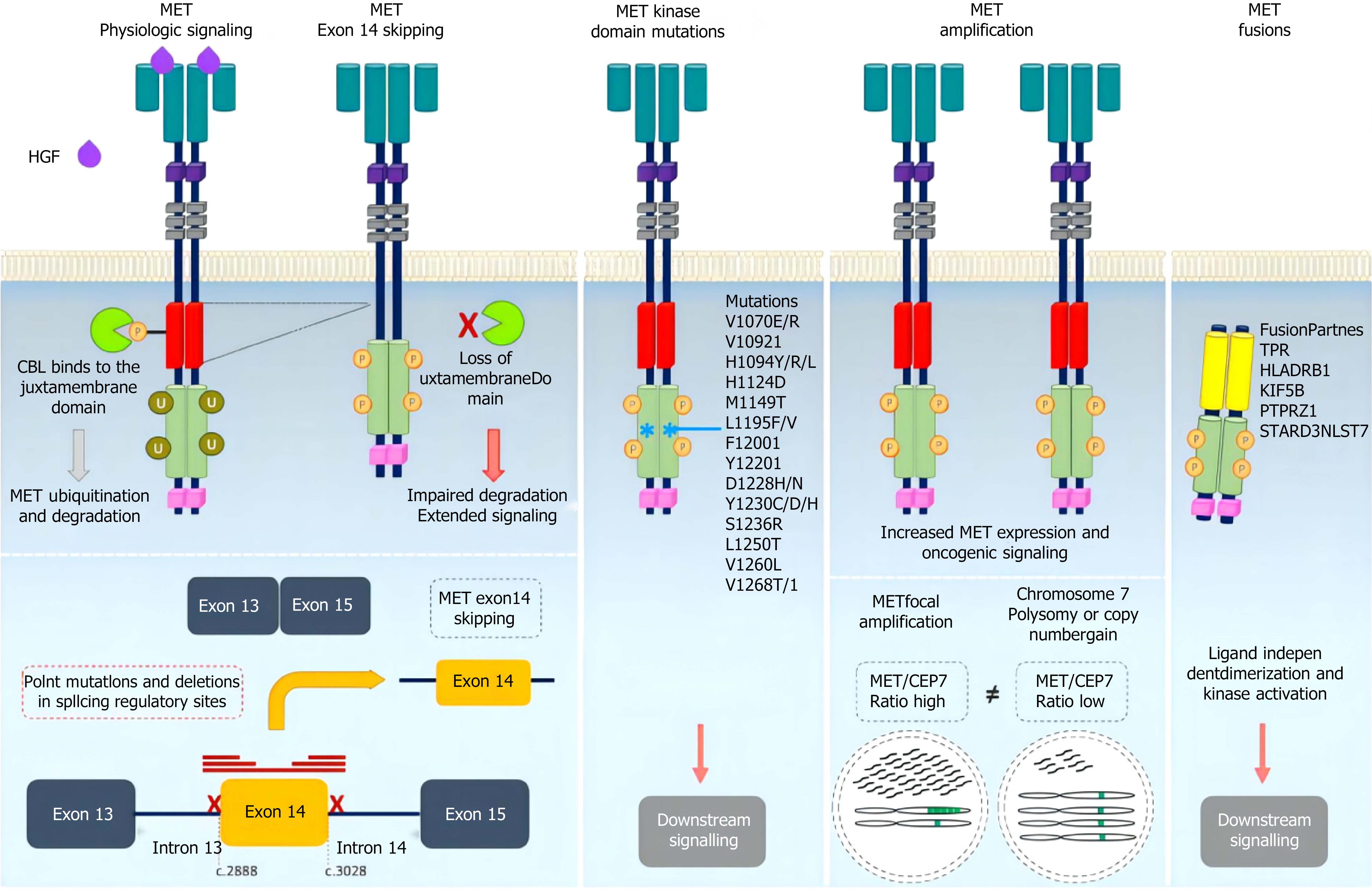
MET variation mainly refers to MET gene mutations (mainly MET exon 14 jump mutations), MET gene amplification and MET protein overexpression[69-72]. MET mutations were first identified in patients with hereditary papillary renal cell carcinoma with germline mutations V1092I, H1094R/Y, M1131T, V1188L, V1220I, M1250T, and D1228H/N/V[73-76]. Mutations in the MET kinase domain increase kinase activity, leading to phenotypic transformation or the formation of tumor foci[77-80]. When exon 14 jumps, the Y1003 and c-CblE3 ubiquitin ligase binding sites are missing, resulting in reduced ubiquitination, increased MET stability and continued activation, and the gene eventually becomes a carcinogenic agent[81]. The incidence of MET14 exon spikes in the general population ranges from 0.9% to 4.0%, but the incidence of MET14 exon spikes in lung sarcomatoid carcinoma is greater, ranging from 5% to 32%[82-85]. At present, the detection methods for MET gene mutations mainly include NGS and reverse transcription polymerase chain reaction[86-89]. MET amplification is a duplication of a gene or a gene in a region that is not replicated on chromosome 7, resulting in increased MET expression and the activation of downstream signaling pathways independent of ligands. Notably, a gene copy number increase refers to chromosome 7 polysomy and MET gene amplification[90-93]. Gene amplification occurs in a variety of tumors, including lung cancer, gastric cancer, bowel cancer, and liver cancer[94-97] (Figure 2). The incidence of primary amplification is approximately 2%-10%, and the prognosis is poor[98-101]. Fluorescence in situ hybridization is the current standard method for detecting MET amplification and can detect local amplification and multisoma; real-time fluorescence quantitative polymerase chain reaction and NGS can also be used for detecting MET amplification[102-106]. In different studies, there were differences in the threshold of MET amplification, and MET/CEP7 was reported to have low expression between 1.8 and 2.2, intermediate expression between 2.2 and 5.0, and high expression > 5[107-111]. In general, the greater the degree of MET amplification and the lower the proportion of other driver mutations are, the stronger the driver[112-116]. MET polysoma was defined as MET polysoma when the gene copy number was ≥ 5 but the MET/CEP7 ratio was < 2[117-120]. The increased copy number of the MET gene caused by polysomy cannot be identified as a driver of gene variation[121-124] (Figure 3). MET protein overexpression may be present regardless of whether the MET gene is abnormal. MET can induce cancer cell proliferation, reduce apoptosis and promote metastasis under hypoxia and/or inflammation[125-128]. Thus, tumors may rely on MET signaling even in the absence of genomic drivers such as MET amplification, mutation, or fusion[129-131]. The incidence of MET protein overexpression is greater than those of MET exon 14 jump mutation and MET gene amplification, which are associated with poor patient prognosis. MET protein expression can be evaluated via immunohistochemistry[132]. We believe that the definition and criteria for MET variation in gastrointestinal tumors can be compared with those for NSCLC, and routine screening for rare mutations, including MET, should be conducted[133-137].
The incidence of MET amplification is approximately 5% to 8% in gastric cancer patients and approximately 15% in diffuse gastric cancer patients[138-140]. MET protein overexpression is highly common in enteric gastric cancer (63% overexpression rate; previous reports vary and may be related to different detection methods). Met-amplified gastric cancer is characterized by low differentiation of tumor cells, easy peritoneal metastasis and malignant lymphangitis[141]. Moreover, a previous study revealed that the MET status of approximately 35% of gastric cancer patients changes with the course of treatment, requiring dynamic detection[142-145]. MET amplification is often accompanied by HER2 overexpression in gastric cancer, and co-expression of MET and HER2 can synergistically enhance tumor invasion, invasion and metastasis, which is an important factor for poor prognosis (Figure 4). According to a retrospective analysis of 233 patients, survival was 24.6 months in the non-MET-amplified group and 9.3 months in the MET-amplified group [hazard ratio (HR) = 1.6, 95% confidence interval (CI): 1.0-2.5, P = 0.049], suggesting shorter survival and a worse prognosis in patients with MET-amplified gastric cancer[146-149]. Abnormal activation of the MET signaling pathway in liver cancer is due mainly to ligand binding, which leads to its overexpression, but its high expression rate is due to differences in detection methods (the subjective influence of immunohistochemistry is relatively large), resulting in a large variation in its incidence rate (25.4%-61.2%)[150]. A meta-analysis evaluating the prognostic value of MET overexpression in 1408 patients who underwent liver resection revealed that progression-free survival (PFS) and overall survival (OS) were significantly worse in patients with high MET expression than in those with low MET expression (HR = 1.26, P = 0.03; HR = 1.16, P = 0.01), suggesting that MET overexpression may be a poor prognostic indicator of liver cancer[151-154]. There are also clinical data showing that patients with high MET expression in liver cancer are more likely to develop intrahepatic metastasis[155-158]. The high expression of MET in patients with early colorectal cancer is related to the depth of intestinal wall lymph node invasion and regional lymph node metastasis, and some studies have shown that the high expression of MET is related to clinical characteristics such as tumor invasion and liver metastasis. Some scholars have analyzed the changes in gene expression in the ctDNA of advanced colorectal cancer patients who previously received anti-epidermal growth factor receptor (EGFR) monoclonal antibody therapy and reported that, after treatment with anti-EGFR monoantibodies, especially in resistant patients, the number of copies of the MET gene significantly increased compared with that in patients who were not exposed to EGFR monoantibodies[159-162]. MET amplification is considered to be one of the main mechanisms of retroline resistance to EGFR monoantibodies in metastatic colorectal cancer[163-166] (Figure 5).
MET inhibitors include highly selective tyrosine kinase inhibitors (TKIs) (sevotinib, tepotinib, camatinib, etc.), pantargeted TKIs (crizotinib), and monoclonal antibodies (TKIs, etc.)[163]. Preclinical studies suggest that the MET inhibitor sevotinib has a dose-dependent killing effect on MET-amplified gastric cancer, and this inhibition is more significant when it is combined with chemotherapy[164-167]. The antitumor efficacy of sevoflurane has been preliminarily demonstrated in a human tumor xenotransplantation (PDX) model of gastric cancer with MET overexpression[168-170]. The VIKTORY clinical trial explored the efficacy of single-agent sevotinib in patients with MET-amplified gastric cancer, and the results revealed that the objective response rate of single-agent sevotinib reached 50%; 10 patients achieved a partial response, and 1 patient achieved a complete response, among which the second-line PFS duration was 4-6 months, and the efficacy was obvious[171-174]. Subgroup analysis revealed that patients with a MET copy number > 10 had a better response rate to sevoflurane, and the MET copy number was strongly associated with PFS duration[175-178]. Crizotinib has also been used to treat advanced gastric cancer patients with MET amplification and liver metastasis who achieved complete remission of liver lesions after 2 months of treatment and a PFS duration of up to 20 months[179,180]. In addition to the effects of sevotinib, the effects of other MET inhibitors, including monoclonal antibodies, have also been explored in patients with MET-variant gastric cancer[148-151] (Figure 6). In general, the efficacy of specific TKIs is greater than that of monoclonal antibodies. However, at present, these are small sample size studies, and more high-level clinical studies are needed to demonstrate its efficacy and safety[181-184].
MET is a therapeutic target for liver cancer, but few monoclonal antibodies and HGF inhibitors have been developed, and related clinical studies have focused on MET TKIs[185-188]. As a highly selective MET TKI, tivantinib significantly improved PFS and OS (2.7 months vs 1.4 months and 7.2 months vs 3.8 months, respectively) compared with placebo in a phase II clinical study of second-line MET TKIs for highly expressed MET liver cancer[189-191]. Therefore, screening the right people or finding new combinations may be effective[192,193]. Therefore, screening the right people or finding new combinations might be useful. Preclinical studies have shown that hypoxia induced by inhibition of the vascular endothelial growth factor signaling pathway induces hypoxia-inducible factor-1 nuclear aggregation, leading to increased MET expression[194]. CELESTIAL clinical studies have shown that cabotinib is a nonselective multitarget inhibitor with anti-MET and angiogenic effects and is effective in patients with liver cancer who have failed sorafenib treatment and received more than 2 systems, with initial results indicating improved OS in patients[195-198]. Although there are no large-scale clinical studies demonstrating the efficacy and safety of MET inhibitors in the field of liver cancer, MET inhibitors may be a promising choice for liver cancer patients with specific molecular types, such as those with high MET expression[199-202].
Crizotinib, camatinib, and tevantinib have been used as selective MET inhibitors in preclinical and in-clinical trials for the treatment of MET-variant colorectal cancer[203-205]. Scholars have reported that crizotinib can effectively improve the sensitivity of cetuximab-resistant cell lines to radiotherapy both in vivo and in vitro[206]. A clinical study of MET-positive metastatic colorectal cancer (NCT02205398) revealed that the combination of carmatinib and cetuximab in four patients with EGFR-resistant MET-positive colorectal cancer achieved a partial response and was well tolerated. However, tivantinib in combination with cetuximab failed to significantly improve PFS in patients with colorectal cancer, and adverse effects persisted. The antitumor activities of SU11274, PHA665752, nororitin and other MET inhibitors have been confirmed in preclinical studies of colorectal cancer, but relevant clinical studies are lacking. The effectiveness of MET inhibitors in colon cancer patients with MET variants still needs to be further explored.
Through a systematic review of the progress in the diagnosis and treatment of MET-variant digestive tract tumors, we found that the molecular mechanisms of MET-variant digestive tract tumors are complex and diverse and show specificity for different tumor types. Therapeutic strategies targeting MET, including small molecule inhibitors and immunotherapy, have shown significant clinical efficacy. However, drug resistance and individual variability remain major challenges. Future research should focus on precision medicine and optimize individualized treatment plans by integrating multilevel data such as genomic and transcriptomic data, thereby improving patient survival and quality of life.
| 1. | Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol. 2006;23:70-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1244] [Cited by in RCA: 1304] [Article Influence: 72.4] [Reference Citation Analysis (33)] |
| 2. | Miettinen M, Lasota J. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med. 2006;130:1466-1478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 827] [Cited by in RCA: 918] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 3. | Gonzalez RS. Diagnosis and Management of Gastrointestinal Neuroendocrine Neoplasms. Surg Pathol Clin. 2020;13:377-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 4. | Mattiolo P, Scarpa A, Luchini C. Hepatoid tumors of the gastrointestinal/pancreatobiliary district: morphology, immunohistochemistry, and molecular profiles. Hum Pathol. 2023;132:169-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 5. | Tam BY, Chiu K, Chung H, Bossard C, Nguyen JD, Creger E, Eastman BW, Mak CC, Ibanez M, Ghias A, Cahiwat J, Do L, Cho S, Nguyen J, Deshmukh V, Stewart J, Chen CW, Barroga C, Dellamary L, Kc SK, Phalen TJ, Hood J, Cha S, Yazici Y. The CLK inhibitor SM08502 induces anti-tumor activity and reduces Wnt pathway gene expression in gastrointestinal cancer models. Cancer Lett. 2020;473:186-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 99] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 6. | Larsson SC, Höijer J, Sun J, Li X, Burgess S, Michaëlsson K. Genome-Wide Association and Two-Sample Mendelian Randomization Analyses of Plasma Ghrelin and Gastrointestinal Cancer Risk. Cancer Epidemiol Biomarkers Prev. 2023;32:1771-1776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Castaño JP, Sundin A, Maecke HR, Villabona C, Vazquez-Albertino R, Navarro E, Oberg K. Gastrointestinal neuroendocrine tumors (NETs): new diagnostic and therapeutic challenges. Cancer Metastasis Rev. 2014;33:353-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Boland CR, Idos GE, Durno C, Giardiello FM, Anderson JC, Burke CA, Dominitz JA, Gross S, Gupta S, Jacobson BC, Patel SG, Shaukat A, Syngal S, Robertson DJ. Diagnosis and Management of Cancer Risk in the Gastrointestinal Hamartomatous Polyposis Syndromes: Recommendations From the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2022;162:2063-2085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 44] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 9. | Disciglio V, Sanese P, Fasano C, Lotesoriere C, Valentini AM, Forte G, Lepore Signorile M, De Marco K, Grossi V, Lolli I, Cariola F, Simone C. Identification and Somatic Characterization of the Germline PTEN Promoter Variant rs34149102 in a Family with Gastrointestinal and Breast Tumors. Genes (Basel). 2022;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 10. | Khalikov DD, Akhmetzyanov FS, Petrov SV. [Clinical and morphological characteristics of gastrointestinal stromal tumors]. Arkh Patol. 2017;79:48-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 11. | Takao A, Koizumi K, Takao M, Inokuchi T, Iijima T, Kojika E, Urushibara M, Horiguchi SI, Yamaguchi T. Upper gastrointestinal tumors are unrelated to the APC genotype in APC-associated polyposis. Jpn J Clin Oncol. 2022;52:554-561. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 12. | Faucz FR, Horvath AD, Assié G, Almeida MQ, Szarek E, Boikos S, Angelousi A, Levy I, Maria AG, Chitnis A, Antonescu CR, Claus R, Bertherat J, Plass C, Eng C, Stratakis CA. Embryonic stem cell factor FOXD3 (Genesis) defects in gastrointestinal stromal tumors. Endocr Relat Cancer. 2023;30. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 13. | Malik A, Berry R, Fung BM, Tabibian JH. Association between chronic inflammatory demyelinating polyneuropathy and gastrointestinal malignancies. Clin J Gastroenterol. 2021;14:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | O'Brien KM, Orlow I, Antonescu CR, Ballman K, McCall L, DeMatteo R, Engel LS. Gastrointestinal stromal tumors, somatic mutations and candidate genetic risk variants. PLoS One. 2013;8:e62119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Mazzoni SM, Fearon ER. AXIN1 and AXIN2 variants in gastrointestinal cancers. Cancer Lett. 2014;355:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 109] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 16. | Fülöp E, Marcu S, Milutin D, Borda A. Gastrointestinal stromal tumors: review on morphology, diagnosis and management. Rom J Morphol Embryol. 2009;50:319-326. [PubMed] |
| 17. | Garg S, Grenier S, Misyura M, Sukhai MA, Thomas M, Kamel-Reid S, Stockley T. Assessing the Diagnostic Yield of Targeted Next-Generation Sequencing for Melanoma and Gastrointestinal Tumors. J Mol Diagn. 2020;22:467-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 18. | Coindre JM, Emile JF, Monges G, Ranchère-Vince D, Scoazec JY. [Gastrointestinal stromal tumors: definition, histological, immunohistochemical, and molecular features, and diagnostic strategy]. Ann Pathol. 2005;25:358-85; quiz 357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Takahashi T, Elzawahry A, Mimaki S, Furukawa E, Nakatsuka R, Nakamura H, Nishigaki T, Serada S, Naka T, Hirota S, Shibata T, Tsuchihara K, Nishida T, Kato M. Genomic and transcriptomic analysis of imatinib resistance in gastrointestinal stromal tumors. Genes Chromosomes Cancer. 2017;56:303-313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | Shi Y, Hou YY, Lu SH, Zhou Y, Xu JF, Ji Y, Hou J, Xu C, Liu YL, Tan YS, Zhu XZ. Clinical and pathological studies of borderline gastrointestinal stromal tumors. Chin Med J (Engl). 2010;123:2514-2520. [PubMed] |
| 21. | Yoshizaki K, Hirata A, Matsushita H, Sakaguchi M, Yoneji W, Owaki K, Sakai H. Molecular epidemiological study of germline APC variant associated with hereditary gastrointestinal polyposis in dogs: current frequency in Jack Russell Terriers in Japan and breed distribution. BMC Vet Res. 2022;18:230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Boland CR, Idos GE, Durno C, Giardiello FM, Anderson JC, Burke CA, Dominitz JA, Gross S, Gupta S, Jacobson BC, Patel SG, Shaukat A, Syngal S, Robertson DJ. Diagnosis and management of cancer risk in the gastrointestinal hamartomatous polyposis syndromes: recommendations from the U.S. Multi-Society Task Force on Colorectal Cancer. Gastrointest Endosc. 2022;95:1025-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Harrold EC, Stadler ZK. Upper Gastrointestinal Cancers and the Role of Genetic Testing. Hematol Oncol Clin North Am. 2024;38:677-691. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 24. | Hatton JN, de Andrade KC, Frone MN, Savage SA, Khincha PP. Spectrum and Excess Risk of Gastrointestinal Tumors in Li-Fraumeni Syndrome. Clin Gastroenterol Hepatol. 2024;22:662-665.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 25. | Lau DK, Fong C, Arouri F, Cortez L, Katifi H, Gonzalez-Exposito R, Razzaq MB, Li S, Macklin-Doherty A, Hernandez MA, Hubank M, Fribbens C, Watkins D, Rao S, Chau I, Cunningham D, Starling N. Impact of pharmacogenomic DPYD variant guided dosing on toxicity in patients receiving fluoropyrimidines for gastrointestinal cancers in a high-volume tertiary centre. BMC Cancer. 2023;23:380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 26. | Oberg K. Neuroendocrine tumors of the gastrointestinal tract: recent advances in molecular genetics, diagnosis, and treatment. Curr Opin Oncol. 2005;17:386-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | Bergholz LM, Grimminger P, Dikeoulia E, Rossmann H, Weinmann A, Möhler M, Zimmermann T, Weber MM, Galle PR, Kaina B, Zimmermann A. Pilot Study on Malnutrition and DNA Damage in Patients with Newly Diagnosed Gastrointestinal Tumors: Is DNA Damage Reversible by Early Individualized Nutritional Support? J Gastrointestin Liver Dis. 2020;29:569-577. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 28. | Mekras A, Krenn V, Perrakis A, Croner RS, Kalles V, Atamer C, Grützmann R, Vassos N. Gastrointestinal schwannomas: a rare but important differential diagnosis of mesenchymal tumors of gastrointestinal tract. BMC Surg. 2018;18:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (1)] |
| 29. | Xu J, Xu L, Li L, You Q, Cha L. HIF-1α C1772T polymorphism and gastrointestinal tract cancer risk: a meta-analysis and meta-regression analysis. Genet Test Mol Biomarkers. 2013;17:918-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 30. | Ravegnini G, Sammarini G, Angelini S, Hrelia P. Pharmacogenetics of tyrosine kinase inhibitors in gastrointestinal stromal tumor and chronic myeloid leukemia. Expert Opin Drug Metab Toxicol. 2016;12:733-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 31. | Proudman D, Miller A, Nellesen D, Gomes A, Mankoski R, Norregaard C, Sullivan E. Financial Implications of Avapritinib for Treatment of Unresectable Gastrointestinal Stromal Tumors in Patients With a PDGFRA Exon 18 Variant or After 3 Previous Therapies in a Hypothetical US Health Plan. JAMA Netw Open. 2020;3:e2025866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 32. | Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol. 2005;29:52-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 852] [Cited by in RCA: 866] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 33. | R OM, J W, T F, C M, A W, N S, Z K. Mismatch Repair Screening of Gastrointestinal Cancers: The Impact on Lynch Syndrome Detection and Immunotherapy. J Gastrointest Cancer. 2023;54:768-775. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 34. | Suster S, Sorace D, Moran CA. Gastrointestinal stromal tumors with prominent myxoid matrix. Clinicopathologic, immunohistochemical, and ultrastructural study of nine cases of a distinctive morphologic variant of myogenic stromal tumor. Am J Surg Pathol. 1995;19:59-70. [PubMed] |
| 35. | Liu Z, Liu S, Zheng G, Yang J, Hong L, Sun L, Fan D, Zhang H, Feng F. Clinicopathological features and prognosis of coexistence of gastric gastrointestinal stromal tumor and gastric cancer. Medicine (Baltimore). 2016;95:e5373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 36. | Körner M, Waser B, Reubi JC, Miller LJ. CCK(2) receptor splice variant with intron 4 retention in human gastrointestinal and lung tumours. J Cell Mol Med. 2010;14:933-943. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 37. | Rasmussen S, Stueck A, Colwell B, Gaston D, Carter M. Gastrointestinal stromal tumors caused by novel germline variants in SDHB and KIT: a report of two cases and literature review. Clin J Gastroenterol. 2022;15:869-875. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 38. | Kang G, Yun H, Sun CH, Park I, Lee S, Kwon J, Do I, Hong ME, Van Vrancken M, Lee J, Park JO, Cho J, Kim KM, Sohn TS. Integrated genomic analyses identify frequent gene fusion events and VHL inactivation in gastrointestinal stromal tumors. Oncotarget. 2016;7:6538-6551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 39. | Basso D, Navaglia F, Fogar P, Zambon CF, Greco E, Schiavon S, Fasolo M, Stranges A, Falda A, Padoan A, Fadi E, Pedrazzoli S, Plebani M. DNA repair pathways and mitochondrial DNA mutations in gastrointestinal carcinogenesis. Clin Chim Acta. 2007;381:50-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 40. | el-Rifai W, Sarlomo-Rikala M, Andersson LC, Miettinen M, Knuutila S. DNA copy number changes in gastrointestinal stromal tumors--a distinct genetic entity. Ann Chir Gynaecol. 1998;87:287-290. [PubMed] |
| 41. | Zhang R, Zhao J, Xu J, Liu F, Xu Y, Bu X, Dai C, Song C. Genetic variations in the TERT and CLPTM1L gene region and gastrointestinal stromal tumors risk. Oncotarget. 2015;6:31360-31367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 42. | Cho YA, Kim J. Association of IL4, IL13, and IL4R polymorphisms with gastrointestinal cancer risk: A meta-analysis. J Epidemiol. 2017;27:215-220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 43. | Xu Z, Huo X, Tang C, Ye H, Nandakumar V, Lou F, Zhang D, Jiang S, Sun H, Dong H, Zhang G, Liu Z, Dong Z, Guo B, Yan H, Yan C, Wang L, Su Z, Li Y, Gu D, Zhang X, Wu X, Wei X, Hong L, Zhang Y, Yang J, Gong Y, Tang C, Jones L, Huang XF, Chen SY, Chen J. Frequent KIT mutations in human gastrointestinal stromal tumors. Sci Rep. 2014;4:5907. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 44. | Kobayashi M, Inaguma S, Raffeld M, Kato H, Suzuki S, Wakasugi T, Mitsui A, Kuwabara Y, Lasota J, Ikeda H, Miettinen M, Takahashi S. Epithelioid variant of gastrointestinal stromal tumor harboring PDGFRA mutation and MLH1 gene alteration: A case report. Pathol Int. 2019;69:541-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 45. | Riera P, Riba M, Bernal S, Virgili AC, Páez D, Moreno ME. Frequency and clinical relevance of DPYD genetic variants in gastrointestinal cancer patients. Farm Hosp. 2021;45:5-10. [PubMed] |
| 46. | Houvast RD, Baart VM, Bhairosingh SS, Cordfunke RA, Chua JX, Vankemmelbeke M, Parsons T, Kuppen PJK, Durrant LG, Vahrmeijer AL, Sier CFM. Glycan-Based Near-infrared Fluorescent (NIRF) Imaging of Gastrointestinal Tumors: a Preclinical Proof-of-Concept In Vivo Study. Mol Imaging Biol. 2020;22:1511-1522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 47. | Paramythiotis D, Kyriakidis F, Karlafti E, Koletsa T, Tsakona A, Papalexis P, Ioannidis A, Malliou P, Netta S, Michalopoulos A. A Rare Case of Multiple Gastrointestinal Stromal Tumors Coexisting with a Rectal Adenocarcinoma in a Patient with Attenuated Familial Adenomatous Polyposis Syndrome and a Mini Review of the Literature. Medicina (Kaunas). 2022;58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 48. | Lou E, Xiu J, Baca Y, Nelson AC, Weinberg BA, Beg MS, Salem ME, Lenz HJ, Philip P, El-Deiry WS, Korn WM. Expression of Immuno-Oncologic Biomarkers Is Enriched in Colorectal Cancers and Other Solid Tumors Harboring the A59T Variant of KRAS. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 49. | Ohmiya N, Matsumoto S, Yamamoto H, Baranovskaya S, Malkhosyan SR, Perucho M. Germline and somatic mutations in hMSH6 and hMSH3 in gastrointestinal cancers of the microsatellite mutator phenotype. Gene. 2001;272:301-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 50. | Lee JR, Joshi V, Griffin JW Jr, Lasota J, Miettinen M. Gastrointestinal autonomic nerve tumor: immunohistochemical and molecular identity with gastrointestinal stromal tumor. Am J Surg Pathol. 2001;25:979-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 81] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 51. | Wang DH, Park JY. Precision Medicine in Gastrointestinal Pathology. Arch Pathol Lab Med. 2016;140:449-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 52. | Díaz-Delgado M, Hernández-Amate A, Sánchez-León M, Pereira-Gallardo S, Prieto-Sánchez E, Jiménez-Sáenz M, González-Cámpora R. Multiple non-metastatic gastrointestinal stromal tumors. Differential features. Rev Esp Enferm Dig. 2010;102:489-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 53. | Agaimy A, Märkl B, Kitz J, Wünsch PH, Arnholdt H, Füzesi L, Hartmann A, Chetty R. Peripheral nerve sheath tumors of the gastrointestinal tract: a multicenter study of 58 patients including NF1-associated gastric schwannoma and unusual morphologic variants. Virchows Arch. 2010;456:411-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 54. | Ben-Aharon I, van Laarhoven HWM, Fontana E, Obermannova R, Nilsson M, Lordick F. Early-Onset Cancer in the Gastrointestinal Tract Is on the Rise-Evidence and Implications. Cancer Discov. 2023;13:538-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 81] [Reference Citation Analysis (0)] |
| 55. | Qi Z, Li Y, Wang Z, Tan X, Zhou Y, Li Z, Zhao W, Zheng X, Yao J, Li F, Wang W, Wang Z, Pang F, Wang G, Gu W. Monitoring of gastrointestinal carcinoma via molecular residual disease with circulating tumor DNA using a tumor-informed assay. Cancer Med. 2023;12:16687-16696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 56. | Li CF, Huang WW, Wu JM, Yu SC, Hu TH, Uen YH, Tian YF, Lin CN, Lu D, Fang FM, Huang HY. Heat shock protein 90 overexpression independently predicts inferior disease-free survival with differential expression of the alpha and beta isoforms in gastrointestinal stromal tumors. Clin Cancer Res. 2008;14:7822-7831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 57. | Torabi K, Erola P, Alvarez-Mora MI, Díaz-Gay M, Ferrer Q, Castells A, Castellví-Bel S, Milà M, Lozano JJ, Miró R, Ried T, Ponsa I, Camps J. Quantitative analysis of somatically acquired and constitutive uniparental disomy in gastrointestinal cancers. Int J Cancer. 2019;144:513-524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 58. | Wardelmann E, Neidt I, Bierhoff E, Speidel N, Manegold C, Fischer HP, Pfeifer U, Pietsch T. c-kit mutations in gastrointestinal stromal tumors occur preferentially in the spindle rather than in the epithelioid cell variant. Mod Pathol. 2002;15:125-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 93] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 59. | Ricci MT, Volorio S, Signoroni S, Mariani P, Mariette F, Sardella D, Pensotti V, Vitellaro M. Development, technical validation, and clinical application of a multigene panel for hereditary gastrointestinal cancer and polyposis. Tumori. 2019;105:338-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 60. | Boland JM, Folpe AL. Oncocytic variant of malignant gastrointestinal neuroectodermal tumor: a potential diagnostic pitfall. Hum Pathol. 2016;57:13-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 61. | Ravegnini G, Nannini M, Zenesini C, Simeon V, Sammarini G, Urbini M, Gatto L, Saponara M, Biasco G, Pantaleo MA, Venturoli N, Hrelia P, Angelini S. An exploratory association of polymorphisms in angiogenesis-related genes with susceptibility, clinical response and toxicity in gastrointestinal stromal tumors receiving sunitinib after imatinib failure. Angiogenesis. 2017;20:139-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 62. | Fisher KE, Zhang L, Wang J, Smith GH, Newman S, Schneider TM, Pillai RN, Kudchadkar RR, Owonikoko TK, Ramalingam SS, Lawson DH, Delman KA, El-Rayes BF, Wilson MM, Sullivan HC, Morrison AS, Balci S, Adsay NV, Gal AA, Sica GL, Saxe DF, Mann KP, Hill CE, Khuri FR, Rossi MR. Clinical Validation and Implementation of a Targeted Next-Generation Sequencing Assay to Detect Somatic Variants in Non-Small Cell Lung, Melanoma, and Gastrointestinal Malignancies. J Mol Diagn. 2016;18:299-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 63. | Wasag B, Debiec-Rychter M, Pauwels P, Stul M, Vranckx H, Oosterom AV, Hagemeijer A, Sciot R. Differential expression of KIT/PDGFRA mutant isoforms in epithelioid and mixed variants of gastrointestinal stromal tumors depends predominantly on the tumor site. Mod Pathol. 2004;17:889-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 72] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 64. | Sigmund G, Buitrago-Téllez CH, Torhorst J, Steinbrich W. [Radiology of gastrointestinal stromal tumors (GIST) and a case of Carney syndrome]. Rofo. 2000;172:287-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 65. | Yen CC, Chen LT, Li CF, Chen SC, Chua WY, Lin YC, Yen CH, Chen YC, Yang MH, Chao Y, Fletcher JA. Identification of phenothiazine as an ETV1targeting agent in gastrointestinal stromal tumors using the Connectivity Map. Int J Oncol. 2019;55:536-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 66. | Pittayanon R, Khongka W, Linlawan S, Thungsuk R, Aumkaew S, Teeratorn N, Maytapa J, Kimtrakool S, Pakvisal P, Kongtub N, Rerknimitr R, Barkun A. Hemostatic Powder vs Standard Endoscopic Treatment for Gastrointestinal Tumor Bleeding: A Multicenter Randomized Trial. Gastroenterology. 2023;165:762-772.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 67. | Balsamo F, Cardoso PAS, do Amaral Junior SA, Theodoro TR, de Sousa Gehrke F, da Silva Pinhal MA, Bianco B, Waisberg J. Birt-Hogg-Dubé syndrome with simultaneous hyperplastic polyposis of the gastrointestinal tract: case report and review of the literature. BMC Med Genet. 2020;21:52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 68. | Huang L, Chen F, Chen Y, Yang X, Xu S, Ge S, Fu S, Chao T, Yu Q, Liao X, Hu G, Zhang P, Yuan X. Thymidine phosphorylase gene variant, platelet counts and survival in gastrointestinal cancer patients treated by fluoropyrimidines. Sci Rep. 2014;4:5697. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 69. | Gauci J, Azzopardi N, Babic D, Cortis K, Axisa B. Neurofibromatosis Type 1: A Rare Predisposition for Gastrinomas and Other Neuroendocrine Tumors. Pancreas. 2022;51:559-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 70. | Koumarianou A, Chatzellis E, Boutzios G, Tsavaris N, Kaltsas G. Current concepts in the diagnosis and management of poorly differentiated gastrointestinal neuroendocrine carcinomas. Endokrynol Pol. 2013;64:60-72. [PubMed] |
| 71. | Haller F, Schulten HJ, Armbrust T, Langer C, Gunawan B, Füzesi L. Multicentric sporadic gastrointestinal stromal tumors (GISTs) of the stomach with distinct clonal origin: differential diagnosis to familial and syndromal GIST variants and peritoneal metastasis. Am J Surg Pathol. 2007;31:933-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 72. | Xie F, Xiao W, Jiang Y, Xia X, Wang Y. Relationship between efficacy of sunitinib and KIT mutation of patients with advanced gastrointestinal stromal tumors after failure of imatinib: A systematic review. Medicine (Baltimore). 2019;98:e15478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 73. | Sarlomo-Rikala M, El-Rifai W, Lahtinen T, Andersson LC, Miettinen M, Knuutila S. Different patterns of DNA copy number changes in gastrointestinal stromal tumors, leiomyomas, and schwannomas. Hum Pathol. 1998;29:476-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 60] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 74. | Sakamaki K, Funasaka K, Miyahara R, Furukawa K, Yamamura T, Ohno E, Nakamura M, Kawashima H, Hirooka Y, Fujishiro M, Goto H. Low ETV1 mRNA expression is associated with recurrence in gastrointestinal stromal tumors. Sci Rep. 2020;10:14767. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 75. | Wong NACS, Taniere P, Walsh S, Wallace A, Nonaka D, Jones T, Gonzalez D. Gastrointestinal Stromal Tumor With Multiple Primary Tyrosine Kinase Mutations-Clinicopathologic and Molecular Characterization. Appl Immunohistochem Mol Morphol. 2019;27:461-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 76. | Angelini S, Ravegnini G, Nannini M, Bermejo JL, Musti M, Pantaleo MA, Fumagalli E, Venturoli N, Palassini E, Consolini N, Casali PG, Biasco G, Hrelia P. Folate-related polymorphisms in gastrointestinal stromal tumours: susceptibility and correlation with tumour characteristics and clinical outcome. Eur J Hum Genet. 2015;23:817-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 77. | Jelsig AM, van Overeem Hansen T, Gede LB, Qvist N, Christensen LL, Lautrup CK, Ljungmann K, Christensen LT, Rønlund K, Tørring PM, Bertelsen B, Sunde L, Karstensen JG. Whole genome sequencing and disease pattern in patients with juvenile polyposis syndrome: a nationwide study. Fam Cancer. 2023;22:429-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 78. | Iwamuro M, Kondo E, Takata K, Yoshino T, Okada H. Diagnosis of follicular lymphoma of the gastrointestinal tract: A better initial diagnostic workup. World J Gastroenterol. 2016;22:1674-1683. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 79. | Flossbach L, Holzmann K, Mattfeldt T, Buck M, Lanz K, Held M, Möller P, Barth TF. High-resolution genomic profiling reveals clonal evolution and competition in gastrointestinal marginal zone B-cell lymphoma and its large cell variant. Int J Cancer. 2013;132:E116-E127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 80. | Liegl B, Hornick JL, Lazar AJ. Contemporary pathology of gastrointestinal stromal tumors. Hematol Oncol Clin North Am. 2009;23:49-68, vii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 81. | Schoppmann SF, Vinatzer U, Popitsch N, Mittlböck M, Liebmann-Reindl S, Jomrich G, Streubel B, Birner P. Novel clinically relevant genes in gastrointestinal stromal tumors identified by exome sequencing. Clin Cancer Res. 2013;19:5329-5339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 82. | Astolfi A, Urbini M, Indio V, Nannini M, Genovese CG, Santini D, Saponara M, Mandrioli A, Ercolani G, Brandi G, Biasco G, Pantaleo MA. Whole exome sequencing (WES) on formalin-fixed, paraffin-embedded (FFPE) tumor tissue in gastrointestinal stromal tumors (GIST). BMC Genomics. 2015;16:892. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 83. | Ishikura H, Kishimoto T, Andachi H, Kakuta Y, Yoshiki T. Gastrointestinal hepatoid adenocarcinoma: venous permeation and mimicry of hepatocellular carcinoma, a report of four cases. Histopathology. 1997;31:47-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 65] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 84. | Antonescu CR, Nafa K, Segal NH, Dal Cin P, Ladanyi M. EWS-CREB1: a recurrent variant fusion in clear cell sarcoma--association with gastrointestinal location and absence of melanocytic differentiation. Clin Cancer Res. 2006;12:5356-5362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 219] [Article Influence: 11.5] [Reference Citation Analysis (36)] |
| 85. | Shibata H, Yamamoto K, Hirose T, Furune S, Kakushima N, Furukawa K, Nakamura M, Honda T, Fujishiro M, Kawashima H. Characteristics of microbiomes of the saliva, duodenal bulb, and descending portion of superficial nonampullary duodenal epithelial tumors. Dig Liver Dis. 2024;56:941-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 86. | Chetty R. Reticular and microcystic schwannoma: a distinctive tumor of the gastrointestinal tract. Ann Diagn Pathol. 2011;15:198-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 87. | Vibert R, Hasnaoui J, Perrier A, Lefebvre A, Colas C, Dhooge M, Basset N, Chansavang A, Desseignes C, Duval A, Farelly S, Hamzaoui N, Laurent-Puig P, Metras J, Moliere D, Muleris M, Netter J, Touat M, Bielle F, Labreche K, Nicolle R, Perkins G, Warcoin M, Coulet F, Benusiglio PR. Lynch syndrome: influence of additional susceptibility variants on cancer risk. Eur J Hum Genet. 2023;31:1078-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 88. | Cordon-Cardo C, Lloyd KO, Sakamoto J, McGroarty ME, Old LJ, Melamed MR. Immunohistologic expression of blood-group antigens in normal human gastrointestinal tract and colonic carcinoma. Int J Cancer. 1986;37:667-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 46] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 89. | Galarza AF, Linden R, Antunes MV, Hahn RZ, Raymundo S, da Silva AC, Staggemeier R, Spilki FR, Schwartsmann G. Endogenous plasma and salivary uracil to dihydrouracil ratios and DPYD genotyping as predictors of severe fluoropyrimidine toxicity in patients with gastrointestinal malignancies. Clin Biochem. 2016;49:1221-1226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 90. | Dong Q, McKee G, Pitman M, Geisinger K, Tambouret R. Epithelioid variant of gastrointestinal stromal tumor: Diagnosis by fine-needle aspiration. Diagn Cytopathol. 2003;29:55-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 91. | Barth TFE, Kraus JM, Lausser L, Flossbach L, Schulte L, Holzmann K, Kestler HA, Möller P. Comparative gene-expression profiling of the large cell variant of gastrointestinal marginal-zone B-cell lymphoma. Sci Rep. 2017;7:5963. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 92. | Ravegnini G, Nannini M, Simeon V, Musti M, Sammarini G, Saponara M, Gatto L, Urbini M, Astolfi A, Biasco G, Pantaleo MA, Venturoli N, Hrelia P, Angelini S. Polymorphisms in DNA repair genes in gastrointestinal stromal tumours: susceptibility and correlation with tumour characteristics and clinical outcome. Tumour Biol. 2016;37:13413-13423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 93. | Hanson JA, Trent JC, Yang D, Cooper K. Small-intestinal rhabdoid gastrointestinal stromal tumor (GIST): mutation analysis and clinical implications of a rare morphological variant. Int J Surg Pathol. 2011;19:653-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 94. | Rinelli M, Agolini E, Milano GM, Russo I, Crocoli A, De Vito R, Di Giannatale A, Di Paolo PL, Novelli A. Pediatric gastrointestinal stromal tumor: Report of two novel patients harboring germline variants in SDHB and SDHC genes. Cancer Genet. 2020;241:61-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 95. | Ravegnini G, Serrano C, Simeon V, Sammarini G, Nannini M, Roversi E, Urbini M, Ferrè F, Ricci R, Tarantino G, Pantaleo MA, Hrelia P, Angelini S. The rs17084733 variant in the KIT 3' UTR disrupts a miR-221/222 binding site in gastrointestinal stromal tumour: a sponge-like mechanism conferring disease susceptibility. Epigenetics. 2019;14:545-557. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 96. | Dasappa L, Suresh Babu MC, Sirsath NT, Suresh TM, Govind Babu K, Sathyanarayna V, Lokesh KN, Lakshmaiah KC. Primary gastrointestinal mantle cell lymphoma: a retrospective study. J Gastrointest Cancer. 2014;45:481-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 97. | Li L, Sheng Y, Lv L, Gao J. The association between two microRNA variants (miR-499, miR-149) and gastrointestinal cancer risk: a meta-analysis. PLoS One. 2013;8:e81967. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 98. | Perry CG, Young WF Jr, McWhinney SR, Bei T, Stergiopoulos S, Knudson RA, Ketterling RP, Eng C, Stratakis CA, Carney JA. Functioning paraganglioma and gastrointestinal stromal tumor of the jejunum in three women: syndrome or coincidence. Am J Surg Pathol. 2006;30:42-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 99. | Barth TF, Barth CA, Kestler HA, Michl P, Weniger MA, Buchholz M, Möller P, Gress T. Transcriptional profiling suggests that secondary and primary large B-cell lymphomas of the gastrointestinal (GI) tract are blastic variants of GI marginal zone lymphoma. J Pathol. 2007;211:305-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 100. | Crofton PM, Smith AF. Regan variant alkaline phosphatase in gastrointestinal carcinoma. Clin Chim Acta. 1978;86:81-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 101. | Liegl B, Bennett MW, Fletcher CD. Microcystic/reticular schwannoma: a distinct variant with predilection for visceral locations. Am J Surg Pathol. 2008;32:1080-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 102. | Liu F, Sun AJ, Sun LP, Chen LR. [Gastrointestinal adenocarcinomas with a micropapillary pattern: a clinicopathologic and immunohistochemical study]. Zhonghua Bing Li Xue Za Zhi. 2011;40:304-309. [PubMed] |
| 103. | Noë M, Pea A, Luchini C, Felsenstein M, Barbi S, Bhaijee F, Yonescu R, Ning Y, Adsay NV, Zamboni G, Lawlor RT, Scarpa A, Offerhaus GJA, Brosens LAA, Hruban RH, Roberts NJ, Wood LD. Whole-exome sequencing of duodenal neuroendocrine tumors in patients with neurofibromatosis type 1. Mod Pathol. 2018;31:1532-1538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 104. | Azuara D, Ginesta MM, Gausachs M, Rodriguez-Moranta F, Fabregat J, Busquets J, Pelaez N, Boadas J, Galter S, Moreno V, Costa J, de Oca J, Capellá G. Nanofluidic digital PCR for KRAS mutation detection and quantification in gastrointestinal cancer. Clin Chem. 2012;58:1332-1341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 105. | Jung M, Park SH, Jeon YK, Won JK, Yang HK, Kim WH. Gastrointestinal stromal tumor of unusual phenotype after imatinib treatment: A case report and diagnostic utility of ETV1 mRNA in situ hybridization. Medicine (Baltimore). 2017;96:e9031. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 106. | Cumsille P, Godoy M, Gerdtzen ZP, Conca C. Parameter estimation and mathematical modeling for the quantitative description of therapy failure due to drug resistance in gastrointestinal stromal tumor metastasis to the liver. PLoS One. 2019;14:e0217332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 107. | Chao C, Goluszko E, Lee YT, Kolokoltsov AA, Davey RA, Uchida T, Townsend CM Jr, Hellmich MR. Constitutively active CCK2 receptor splice variant increases Src-dependent HIF-1 alpha expression and tumor growth. Oncogene. 2007;26:1013-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 108. | Liang M, Wang X, Cai D, Guan W, Shen X. Tissue-resident memory T cells in gastrointestinal tumors: turning immune desert into immune oasis. Front Immunol. 2023;14:1119383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 109. | Passardi A, Scarpi E, Ulivi P. Towards Personalized Treatment and Molecular Research on Gastrointestinal Tumors. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 110. | Zheng K, Feng Y, Li L, Kong F, Gao J, Kong X. Engineered bacterial outer membrane vesicles: a versatile bacteria-based weapon against gastrointestinal tumors. Theranostics. 2024;14:761-787. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 111. | Wahl RL. The Interaction of Genomics, Molecular Imaging, and Therapy in Gastrointestinal Tumors. Semin Nucl Med. 2020;50:471-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 112. | Cohen MM Jr. Molecular dimensions of gastrointestinal tumors: some thoughts for digestion. Am J Med Genet A. 2003;122A:303-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 113. | Wu L, Zheng Y, Liu J, Luo R, Wu D, Xu P, Wu D, Li X. Comprehensive evaluation of the efficacy and safety of LPV/r drugs in the treatment of SARS and MERS to provide potential treatment options for COVID-19. Aging (Albany NY). 2021;13:10833-10852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 114. | Solinas C, Pusole G, Demurtas L, Puzzoni M, Mascia R, Morgan G, Giampieri R, Scartozzi M. Tumor infiltrating lymphocytes in gastrointestinal tumors: Controversies and future clinical implications. Crit Rev Oncol Hematol. 2017;110:106-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 115. | Fassan M, Scarpa A, Remo A, De Maglio G, Troncone G, Marchetti A, Doglioni C, Ingravallo G, Perrone G, Parente P, Luchini C, Mastracci L. Current prognostic and predictive biomarkers for gastrointestinal tumors in clinical practice. Pathologica. 2020;112:248-259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 116. | Zandieh MA, Farahani MH, Rajabi R, Avval ST, Karimi K, Rahmanian P, Razzazan M, Javanshir S, Mirzaei S, Paskeh MDA, Salimimoghadam S, Hushmandi K, Taheriazam A, Pandey V, Hashemi M. Epigenetic regulation of autophagy by non-coding RNAs in gastrointestinal tumors: Biological functions and therapeutic perspectives. Pharmacol Res. 2023;187:106582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 17] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 117. | Capiro N, Flink C, Sai V, Beckett K. Gut wrenching: cases of missed gastrointestinal tumors and their mimics on computed tomography. Emerg Radiol. 2021;28:389-399. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 118. | Zhang J, Fu S, Chen W, Chen H. Exosome as potential biomarkers for gastrointestinal tumors. Medicine (Baltimore). 2021;100:e24509. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 119. | Schimanski CC, Galle PR, Moehler M. Chemokine receptor CXCR4-prognostic factor for gastrointestinal tumors. World J Gastroenterol. 2008;14:4721-4724. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 40] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 120. | Lucchetti D, Colella F, Artemi G, Haque S, Sgambato A, Pellicano R, Fagoonee S. Smart nano-sized extracellular vesicles for cancer therapy: Potential theranostic applications in gastrointestinal tumors. Crit Rev Oncol Hematol. 2023;191:104121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 121. | Cong S, Bai S, Zhang M, Bi Y, Wang Y, Jin S, He H. A study on metabolic characteristics and metabolic markers of gastrointestinal tumors. Cancer Biol Ther. 2023;24:2255369. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 122. | Chida K, Kawazoe A, Suzuki T, Kawazu M, Ueno T, Takenouchi K, Nakamura Y, Kuboki Y, Kotani D, Kojima T, Bando H, Mishima S, Kuwata T, Sakamoto N, Watanabe J, Mano H, Ikeda M, Shitara K, Endo I, Nakatsura T, Yoshino T. Transcriptomic Profiling of MSI-H/dMMR Gastrointestinal Tumors to Identify Determinants of Responsiveness to Anti-PD-1 Therapy. Clin Cancer Res. 2022;28:2110-2117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 45] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 123. | Tullio V, Gasperi V, Catani MV, Savini I. The Impact of Whole Grain Intake on Gastrointestinal Tumors: A Focus on Colorectal, Gastric, and Esophageal Cancers. Nutrients. 2020;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 124. | Wu L, Zhong Y, Wu D, Xu P, Ruan X, Yan J, Liu J, Li X. Immunomodulatory Factor TIM3 of Cytolytic Active Genes Affected the Survival and Prognosis of Lung Adenocarcinoma Patients by Multi-Omics Analysis. Biomedicines. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 62] [Reference Citation Analysis (0)] |
| 125. | Tazawa H, Kagawa S, Fujiwara T. MicroRNAs as potential target gene in cancer gene therapy of gastrointestinal tumors. Expert Opin Biol Ther. 2011;11:145-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 126. | Rey JB, Launay-Vacher V, Tournigand C. Regorafenib as a single-agent in the treatment of patients with gastrointestinal tumors: an overview for pharmacists. Target Oncol. 2015;10:199-213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 127. | Rice SR, Chuong M, Koroulakis A, Siddiqui OM, Sharma AM, Simone CB 2nd, Molitoris JK, Kaiser A. The Utility of PET/Computed Tomography for Radiation Oncology Planning, Surveillance, and Prognosis Prediction of Gastrointestinal Tumors. PET Clin. 2020;15:77-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 128. | Wu L, Liu Q, Ruan X, Luan X, Zhong Y, Liu J, Yan J, Li X. Multiple Omics Analysis of the Role of RBM10 Gene Instability in Immune Regulation and Drug Sensitivity in Patients with Lung Adenocarcinoma (LUAD). Biomedicines. 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 55] [Reference Citation Analysis (0)] |
| 129. | Gervaso L, Ciardiello D, Oliveira RA, Borghesani M, Guidi L, Benini L, Algeri L, Spada F, Zampino MG, Cella CA, Fazio N. Immunotherapy in the neoadjuvant treatment of gastrointestinal tumors: is the time ripe? J Immunother Cancer. 2024;12. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 130. | López-Gómez M, Casado E, Muñoz M, Alcalá S, Moreno-Rubio J, D'Errico G, Jiménez-Gordo AM, Salinas S, Sainz B Jr. Current evidence for cancer stem cells in gastrointestinal tumors and future research perspectives. Crit Rev Oncol Hematol. 2016;107:54-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 131. | Gan Q, Li Y, Li Y, Liu H, Chen D, Liu L, Peng C. Pathways and molecules for overcoming immunotolerance in metastatic gastrointestinal tumors. Front Immunol. 2024;15:1359914. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 132. | Lu G, Li J, Yan X, Sun X, Yin Y, Lu X, Ma F, Ma F, Zheng J, Zhao W, Lv Y, Ren M, He S. Intraoperative localization of gastrointestinal tumors by magnetic tracer technique during laparoscopic-assisted surgery (with video). Scand J Gastroenterol. 2021;56:1442-1449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 133. | Wu L, Zheng Y, Ruan X, Wu D, Xu P, Liu J, Wu D, Li X. Long-chain noncoding ribonucleic acids affect the survival and prognosis of patients with esophageal adenocarcinoma through the autophagy pathway: construction of a prognostic model. Anticancer Drugs. 2022;33:e590-e603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 70] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 134. | Gretschel S, Moesta KT, Hünerbein M, Lange T, Gebauer B, Stroszczinski C, Bembenek A, Schlag PM. New concepts of staging in gastrointestinal tumors as a basis of diagnosis and multimodal therapy. Onkologie. 2004;27:23-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 135. | Zheng H, Li M, Wu L, Liu W, Liu Y, Gao J, Lu Z. Progress in the application of hydrogels in immunotherapy of gastrointestinal tumors. Drug Deliv. 2023;30:2161670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 136. | Märkl B, Arnholdt HM. Prognostic significance of tumor budding in gastrointestinal tumors. Expert Rev Anticancer Ther. 2011;11:1521-1533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 137. | Wu L, Zhong Y, Yu X, Wu D, Xu P, Lv L, Ruan X, Liu Q, Feng Y, Liu J, Li X. Selective poly adenylation predicts the efficacy of immunotherapy in patients with lung adenocarcinoma by multiple omics research. Anticancer Drugs. 2022;33:943-959. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 66] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 138. | Welland S, deCastro T, Bathon M, Wirth TC, Reineke-Plaaß T, Saborowski M, Lehmann U, Saborowski A, Vogel A. Molecular diagnostics and therapies for gastrointestinal tumors: a real-world experience. J Cancer Res Clin Oncol. 2022;148:2137-2144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 139. | Shao DT, Wei WW. [Progress in research of human microbiota for upper gastrointestinal tumors and precancerous lesions]. Zhonghua Liu Xing Bing Xue Za Zhi. 2018;39:382-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 140. | Navrátilová P, Hynková L, Šlampa P. [The Role of Palliative Radiotherapy in Bleeding from Locally Advanced Gastrointestinal Tumors]. Klin Onkol. 2017;30:433-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 141. | Ng L, Poon RT, Pang R. Biomarkers for predicting future metastasis of human gastrointestinal tumors. Cell Mol Life Sci. 2013;70:3631-3656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 142. | Dibra D, Mishra L, Li S. Molecular mechanisms of oncogene-induced inflammation and inflammation-sustained oncogene activation in gastrointestinal tumors: an under-appreciated symbiotic relationship. Biochim Biophys Acta. 2014;1846:152-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 143. | Wu L, Li H, Liu Y, Fan Z, Xu J, Li N, Qian X, Lin Z, Li X, Yan J. Research progress of 3D-bioprinted functional pancreas and in vitro tumor models. Int J Bioprinting. 2024;10:1256. [DOI] [Full Text] |
| 144. | Fan J, Zhu J, Zhu H, Xu H. Potential therapeutic targets in myeloid cell therapy for overcoming chemoresistance and immune suppression in gastrointestinal tumors. Crit Rev Oncol Hematol. 2024;198:104362. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 145. | Lin Y, Jiang M. The Effect of Low-Dose Esketamine on Postoperative Neurocognitive Dysfunction in Elderly Patients Undergoing General Anesthesia for Gastrointestinal Tumors: A Randomized Controlled Trial [Letter]. Drug Des Devel Ther. 2023;17:2859-2860. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 146. | Catalano V, Baldelli AM, Giordani P, Cascinu S. Molecular markers predictive of response to chemotherapy in gastrointestinal tumors. Crit Rev Oncol Hematol. 2001;38:93-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 147. | Zhai JN, Lei XK, Wu AW. [Regarding the selection of individualized therapy after neoadjuvant therapy for gastrointestinal tumors]. Zhonghua Wei Chang Wai Ke Za Zhi. 2024;27:338-347. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 148. | Wu L, Li X, Qian X, Wang S, Liu J, Yan J. Lipid Nanoparticle (LNP) Delivery Carrier-Assisted Targeted Controlled Release mRNA Vaccines in Tumor Immunity. Vaccines (Basel). 2024;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 59] [Reference Citation Analysis (0)] |
| 149. | Esaki M, Maehara R, Nagatomo S, Nishioka K, Minoda Y, Ogino H, Ihara E. Application of traction-method to hybrid endoscopic submucosal dissection for gastrointestinal tumors. Endoscopy. 2022;54:E160-E161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 150. | Ziegler K, Sanft C, Zeitz M, Riecken EO. Preoperative staging of gastrointestinal tumors by endosonography. Surg Endosc. 1990;4:154-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 151. | Zhu X, Li S. Ferroptosis, Necroptosis, and Pyroptosis in Gastrointestinal Cancers: The Chief Culprits of Tumor Progression and Drug Resistance. Adv Sci (Weinh). 2023;10:e2300824. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 152. | Adim SB, Filiz G, Kanat O, Yerci O. Simultaneous occurrence of synchronous and metachronous tumors with gastrointestinal stromal tumors. Bratisl Lek Listy. 2011;112:623-625. [PubMed] |
| 153. | Wu L, Chen X, Zeng Q, Lai Z, Fan Z, Ruan X, Li X, Yan J. NR5A2 gene affects the overall survival of LUAD patients by regulating the activity of CSCs through SNP pathway by OCLR algorithm and immune score. Heliyon. 2024;10:e28282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 41] [Reference Citation Analysis (0)] |
| 154. | Wu GH. [Application of immune nutrition in patients with gastrointestinal tumors]. Zhonghua Wei Chang Wai Ke Za Zhi. 2013;16:1021-1024. [PubMed] |
| 155. | Idowu MO, Laudadio J, Rizzo K. Diagnostic, Prognostic, and Predictive Molecular Biomarkers and the Utility of Molecular Imaging in Common Gastrointestinal Tumors. Biomed Res Int. 2015;2015:890805. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 156. | Igaz I, Topa L. [Significance of microRNA expression in body fluids in the diagnosis of gastrointestinal tumors]. Orv Hetil. 2014;155:11-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 157. | Wu Y, Yang S, Ma J, Chen Z, Song G, Rao D, Cheng Y, Huang S, Liu Y, Jiang S, Liu J, Huang X, Wang X, Qiu S, Xu J, Xi R, Bai F, Zhou J, Fan J, Zhang X, Gao Q. Spatiotemporal Immune Landscape of Colorectal Cancer Liver Metastasis at Single-Cell Level. Cancer Discov. 2022;12:134-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 555] [Article Influence: 138.8] [Reference Citation Analysis (0)] |
| 158. | Wu L, Li X, Yan J. Commentary: Machine learning developed an intratumor heterogeneity signature for predicting prognosis and immunotherapy benefits in cholangiocarcinoma. Transl Oncol. 2024;45:101995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 159. | Zhu H, Wang G, Zhu H, Xu A. ITGA5 is a prognostic biomarker and correlated with immune infiltration in gastrointestinal tumors. BMC Cancer. 2021;21:269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 160. | Streit M, Schmidt R, Hilgenfeld RU, Thiel E, Kreuser ED. Adhesion receptors in malignant transformation and dissemination of gastrointestinal tumors. Recent Results Cancer Res. 1996;142:19-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 161. | Suárez-Velázquez D, Oropeza-Duarte C, Sol-Avalos A, García-Morán K, García-Colunga MF, Buenrostro-Espinosa R, Salazar QLT. Appendiceal mucinous neoplasm, a rare diagnosis within gastrointestinal tumors. Case report. Cir Cir. 2022;90:833-837. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 162. | Jia K, Wang Y, Yang B, Wang H. Revisit the rare clinical cases of upper urinary tract metastases from gastrointestinal tumors. Asian J Surg. 2023;46:1103-1104. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 163. | Gong X, Azhdarinia A, Ghosh SC, Xiong W, An Z, Liu Q, Carmon KS. LGR5-Targeted Antibody-Drug Conjugate Eradicates Gastrointestinal Tumors and Prevents Recurrence. Mol Cancer Ther. 2016;15:1580-1590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 92] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 164. | Zhao Y, Ren M, Jia A, Zhang J, Wang S, Zhao Q, Cai G, He S. The factors influencing the accuracy of pre-operative endoscopic ultrasonography assessment in endoscopic treatments for gastrointestinal tumors. Cancer Med. 2023;12:4321-4331. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 165. | Goldin-Lang P, Kreuser ED, Zunft HJ. Basis and consequences of primary and secondary prevention of gastrointestinal tumors. Recent Results Cancer Res. 1996;142:163-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 166. | Wu L, Yang L, Qian X, Hu W, Wang S, Yan J. Mannan-Decorated Lipid Calcium Phosphate Nanoparticle Vaccine Increased the Antitumor Immune Response by Modulating the Tumor Microenvironment. J Funct Biomater. 2024;15:229. [RCA] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 167. | Xu L, Cai Y, Chen X, Zhu Y, Cai J. Circulating MiR-1290 as a potential diagnostic and disease monitoring biomarker of human gastrointestinal tumors. BMC Cancer. 2021;21:989. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 168. | Patrzyk M, Glitsch A, Beyer K, Lünse S, Höhn J, Heidecke CD, Keßler W. Laparoscopic-endoscopic Rendezvous Procedures for Upper Gastrointestinal Tumors Guided by Laser-supported Reverse Diaphanoscopy: A Modified Technique. Surg Laparosc Endosc Percutan Tech. 2019;29:349-353. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 169. | Kraus MS, Selvam S, Siddiqui I, Reyes JA, Chavhan GB. Imaging of pediatric gastrointestinal tumors: A tertiary center experience over 19 years. Eur J Radiol. 2024;175:111461. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 170. | Wang H, Shou C. [Molecular markers of gastrointestinal tumors and individualized treatment]. Zhonghua Wei Chang Wai Ke Za Zhi. 2014;17:6-9. [PubMed] |
| 171. | Tao F, Wang X, Liu J, Li J, Sui F. Perioperative application of midline catheter and PICC in Patients with gastrointestinal tumors. J BUON. 2019;24:2546-2552. [PubMed] |
| 172. | Wu Y, Li W, Chen X, Wang H, Su S, Xu Y, Deng X, Yang T, Wei M, Li L, Liu Y, Yang J, Li W. DOG1 as a novel antibody-drug conjugate target for the treatment of multiple gastrointestinal tumors and liver metastasis. Front Immunol. 2023;14:1051506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 173. | Chen L, Zheng S, Xie Q, Huang L, Yin G. The Effect of Different Nutritional Nursing Support on the Nutritional Status and Disease Recovery of Elderly Patients with Gastrointestinal Tumors during the Perioperative Period. Comput Math Methods Med. 2022;2022:4977922. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 174. | Lu J, Feng Y, Guo K, Sun L, Ruan S, Zhang K. Association between human blood metabolome and the risk of gastrointestinal tumors. PLoS One. 2024;19:e0304574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 175. | Reubi JC, Häcki WH, Lamberts SW. Hormone-producing gastrointestinal tumors contain a high density of somatostatin receptors. J Clin Endocrinol Metab. 1987;65:1127-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 121] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 176. | Liu Y, Yin C, Deng MM, Wang Q, He XQ, Li MT, Li CP, Wu H. High expression of SHMT2 is correlated with tumor progression and predicts poor prognosis in gastrointestinal tumors. Eur Rev Med Pharmacol Sci. 2019;23:9379-9392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 177. | Takahashi T, Iwama N. Three-dimensional microstructure of gastrointestinal tumors. Gland pattern and its diagnostic significance. Pathol Annu. 1985;20 Pt 1:419-440. [PubMed] |
| 178. | Bansal SC, Bansal BR, Mark R, Rhoads JE Jr. Effect of unblocking therapy and levamisole on primary gastrointestinal tumors in rats: immunologic and histologic correlation. J Natl Cancer Inst. 1978;61:1235-1254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 179. | Hou L, Xu L, Shi Y, Gu F. Effect of electric acupoint stimulation on gastrointestinal hormones and motility among geriatric postoperative patients with gastrointestinal tumors. J Tradit Chin Med. 2016;36:450-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 180. | Ito T, Tanaka M, Sasano H, Osamura YR, Sasaki I, Kimura W, Takano K, Obara T, Ishibashi M, Nakao K, Doi R, Shimatsu A, Nishida T, Komoto I, Hirata Y, Imamura M, Kawabe K, Nakamura K; Neuroendocrine Tumor Workshop of Japan. Preliminary results of a Japanese nationwide survey of neuroendocrine gastrointestinal tumors. J Gastroenterol. 2007;42:497-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 181. | Fruchtenicht AVG, Poziomyck AK, Reis AMD, Galia CR, Kabke GB, Moreira LF. Inflammatory and nutritional statuses of patients submitted to resection of gastrointestinal tumors. Rev Col Bras Cir. 2018;45:e1614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 182. | Snady H. Endoscopic ultrasonography: an effective new tool for diagnosing gastrointestinal tumors. Oncology (Williston Park). 1992;6:63-74; discussion 77. [PubMed] |
| 183. | Li C, Liu Y, Zhou J, Zhang YW. A retrospective analysis of the efficacy of microparticle-mediated chemoembolization in liver metastases arising from gastrointestinal tumors. J Cancer Res Ther. 2017;13:642-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 184. | Dorais J, Marcon N. Endoscopic resection of gastrointestinal tumors: how far can the endoscopist go? Endoscopy. 1997;29:192-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 185. | Lv J, Lv CQ, Wang BL, Mei P, Xu L. Membrane Glycolipids Content Variety in Gastrointestinal Tumors and Transplantable Hepatomas in Mice. Med Sci Monit Basic Res. 2016;22:87-90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 186. | Inui K, Nakazawa S, Yoshino J, Yamao K, Yamachika H, Wakabayashi T, Kanemaki N, Hidano H. Endoscopic MRI: preliminary results of a new technique for visualization and staging of gastrointestinal tumors. Endoscopy. 1995;27:480-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 187. | Isomoto H, Nanashima A, Senoo T, Ogiwara K, Hashisako M, Ohnita K, Yamaguchi N, Kunizaki M, Hidaka S, Fukuda H, Ishii H, Matsushima K, Minami H, Akazawa Y, Takeshima F, Fukuoka J, Nagayasu T, Nakao K. In vivo fluorescence navigation of gastric and upper gastrointestinal tumors by 5-aminolevulinic acid mediated photodynamic diagnosis with a laser-equipped video image endoscope. Photodiagnosis Photodyn Ther. 2015;12:201-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 188. | Liu X, Qian Q, Xu P, Wolf F, Zhang J, Zhang D, Li C, Huang Q. A novel conditionally replicating "armed" adenovirus selectively targeting gastrointestinal tumors with aberrant wnt signaling. Hum Gene Ther. 2011;22:427-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 189. | Rosenberg R, Nekarda H, Thorban S, Siewert JR. [Minimal residual disease in gastrointestinal tumors: tumor detection in bone marrow, blood and lymph nodes]. Acta Med Austriaca Suppl. 2002;59:42-53. [PubMed] |
| 190. | Zhou YB. [Thinking and suggestions on pathway management of perioperative enhanced recovery after surgery in gastrointestinal tumors in China]. Zhonghua Wei Chang Wai Ke Za Zhi. 2022;25:568-574. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 191. | Guo J, Liu Z, Sun S, Wang S, Ge N, Liu X, Wang G, Yang X. Ligation-assisted endoscopic enucleation for the diagnosis and resection of small gastrointestinal tumors originating from the muscularis propria: a preliminary study. BMC Gastroenterol. 2013;13:88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 192. | ElBadre HM, El-Mahdy RI, Mohamed NA, Zakhary MM, Maximous DW. Tissue Indices of Telomere Length and p53 in Patients with Different Gastrointestinal Tumors: Correlation with Clinicopathological Status. Appl Biochem Biotechnol. 2018;186:764-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 193. | D'Errico A, Facchini A, Meliconi R, Grigioni FW, Ferrone S. Reactivity of monoclonal antibody (mAb) GD9 with a cytoplasmic antigen preserved in formalin-fixed, paraffin-embedded gastrointestinal tumors. Hybridoma. 1991;10:113-119. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 194. | Chen WF, Li YL, Ming J, Yu WQ. A study of two different valve types of peripherally inserted central catheters for 5-fluorouracil pumping in chemotherapy patients with gastrointestinal tumors. Asian J Surg. 2024;47:1955-1956. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 195. | Abbrederis K, Lorenzen S, Rothling N, Ihbe-Heffinger A, Schuster T, Peschel C, Lordick F. Chemotherapy-induced nausea and vomiting in the treatment of gastrointestinal tumors and secondary prophylaxis with aprepitant. Onkologie. 2009;32:30-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 196. | Mĺkvy P, Messmann H, Regula J, Conio M, Pauer M, Millson CE, MacRobert AJ, Bown SG. Sensitization and photodynamic therapy (PDT) of gastrointestinal tumors with 5-aminolaevulinic acid (ALA) induced protoporphyrin IX (PPIX). A pilot study. Neoplasma. 1995;42:109-113. [PubMed] |
| 197. | Eriksson BK, Larsson EG, Skogseid BM, Löfberg AM, Lörelius LE, Oberg KE. Liver embolizations of patients with malignant neuroendocrine gastrointestinal tumors. Cancer. 1998;83:2293-2301. [PubMed] |
| 198. | Shen L. [Chemotherapy and target-therapy of gastrointestinal tumors call for development of pathology]. Zhonghua Bing Li Xue Za Zhi. 2007;36:438-439. [PubMed] |
| 199. | Xing N, Liu J, Hou L, Zhao Y, Ma H, Wang F, Guo Z. Clinical biomarkers for thyroid immune-related adverse events in patients with stage III and IV gastrointestinal tumors. Front Immunol. 2024;15:1381061. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 200. | Gencer D, Kästle-Larralde N, Pilz LR, Weiss A, Buchheidt D, Hochhaus A, Hofheinz RD. Presentation, treatment, and analysis of prognostic factors of terminally ill patients with gastrointestinal tumors. Onkologie. 2009;32:380-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 201. | Khan AZ, Miles WF, Singh KK. Initial experience with laparoscopic bypass for upper gastrointestinal malignancy: a new option for palliation of patients with advanced upper gastrointestinal tumors. J Laparoendosc Adv Surg Tech A. 2005;15:374-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 202. | Zhao CH, He B, Yang YF, Liao J. Dietary therapy of qi-yin-reinforcing porridge for the alleviation of chemotherapy related symptoms of gastrointestinal tumors: a single-case randomized controlled study. Chin J Integr Med. 2013;19:418-423. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 203. | Perez-Atayde AR, Shamberger RC, Kozakewich HW. Neuroectodermal differentiation of the gastrointestinal tumors in the Carney triad. An ultrastructural and immunohistochemical study. Am J Surg Pathol. 1993;17:706-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 63] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 204. | Sato H, Suzuki H, Toyota M, Nojima M, Maruyama R, Sasaki S, Takagi H, Sogabe Y, Sasaki Y, Idogawa M, Sonoda T, Mori M, Imai K, Tokino T, Shinomura Y. Frequent epigenetic inactivation of DICKKOPF family genes in human gastrointestinal tumors. Carcinogenesis. 2007;28:2459-2466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 171] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 205. | Nagy J, Sipka S, Kocsis J, Horváth Z. [The standardized mortality numbers of patients with gastrointestinal tumors and cardiovascular diseases in four wine regions and in one not-wine region of Hungary between 2000-2010]. Orv Hetil. 2017;158:992-998. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 206. | Shin DB, Bang SM, Park SH, Kang HG, Jue JI, Han SH, Lee Y, Cho EK, Lee JH. Correlation of quality of life with tumor response in patients receiving palliative chemotherapy for advanced gastrointestinal tumors. Med Oncol. 2008;25:81-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |