Published online Nov 15, 2024. doi: 10.4251/wjgo.v16.i11.4326
Revised: June 6, 2024
Accepted: July 15, 2024
Published online: November 15, 2024
Processing time: 212 Days and 15.4 Hours
In this editorial, a comment on the article by Díaz-López et al published in the recent issue of the 2024 is provided. We focus on the practical implications critical for providing a correct and complete diagnosis of mixed neuroendocrine-non-neuroendocrine neoplasm (MiNEN) in the gastrointestinal system. The diagnosis of MiNEN begins with the recognition of neuroendocrine features in one component of a biphasic tumor. The non-neuroendocrine counterpart can be virtually represented by any neoplastic type, even though the most frequent histologies are glandular and squamous. However, qualification of the neuroendocrine component requires histological and immunohistochemical confirmation. Neuroendocrine tumors are characterized by a peculiar architectural organization and bland nuclei with granular “salt and pepper” chromatin. Although neuroendocrine carcinomas have multiple and variable presentations, they typically show a solid or organoid architecture. The histological aspect needs to be confirmed by immunohistochemistry, and a diagnosis is confirmed whenever the expression of keratin and neuroendocrine markers is observed. Once both histopathological and immunohistochemical features of neuroendocrine neoplasms are identified, it is important to consider the three major pitfalls of MiNEN diagnostics: (1) Entrapment of neuroendocrine non-neoplastic cells within the tumor mass; (2) Differential diagnosis with amphicrine neoplasms; and (3) Differential diagnosis of tumors that partially express neuroendocrine markers. According to the current guidelines for diagnosing digestive MiNEN, each component must represent at least 30% of the entire neoplastic mass. Although the high-grade histopathological subtype frequently determines disease prognosis, both components can significantly affect prognosis. Thus, if one of the components, either neuroendocrine or non-neuroendocrine, does not fulfill the volumetric criteria, the guidelines still encourage reporting it. These strict criteria are essential for correctly recognizing and characterizing digestive MiNENs. This task is essential because it has prognostic relevance and substantial potential value for guiding further studies in this field. In the future, systematic analyses should be performed to validate or reconsider the current 30% cutoff value.
Core Tip: Mixed neuroendocrine-non-neuroendocrine neoplasms are a heterogeneous group of neoplastic diseases that share histological and immunohistochemical features. The most important factor is the uncertain presence of a neuroendocrine component. The presence of entrapped neuroendocrine cells, differential diagnosis of amphicrine neoplasms, and neuroendocrine expression in non-neuroendocrine carcinoma can lead to misdiagnosis. Current guidelines require the fulfillment of volumetric criteria, but the prognostic relevance of the current cutoff remains to be proven.
- Citation: Mattiolo P. Practical hints for the diagnosis of mixed neuroendocrine-non-neuroendocrine neoplasms of the digestive system. World J Gastrointest Oncol 2024; 16(11): 4326-4332
- URL: https://www.wjgnet.com/1948-5204/full/v16/i11/4326.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i11.4326
Mixed neuroendocrine-non-neuroendocrine neoplasm (MiNENs) are mixed neoplasms consisting of two components, one neuroendocrine and the other frequently showing an epithelial nature, with features that overlap with those of their pure counterparts in the same region[1]. Neuroendocrine neoplasms represent a particular and heterogeneous group of malignancies that share histological and immunohistochemical profiles and present some site-based specificities[1]. It is crucial to identify some practical features that support the diagnosis of MiNENs in routine clinical practice. These were appropriately presented and discussed by Díaz-López et al[2] in their recent manuscript, however some considerations should be added.
MiNENs refers to a diagnostic category rather than a specific diagnosis. It refers to a neoplastic lesion with two recognizable components, one of which must present neuroendocrine differentiation[1,3], which must be assessed through an examination[1,3]. The typical histomorphology of neuroendocrine differentiation [well-differentiated neuroendocrine tumors (NETs)] includes an architecture showing organoid, nested, ribbon-like, or trabecular growth patterns; neoplastic cells with eosinophilic cytoplasm; and monomorphic nuclei with “salt and pepper” chromatin. A few mitoses and the absence of necrosis are typical features[1-6]. Conversely, high-grade neuroendocrine carcinomas typically harbor two different cellular morphologies (small cell and large cell features), sometimes within the same tumor mass. Small-cell neuroendocrine carcinoma has a distinct histomorphology characterized by a solid and/or sheet-like growth pattern, with atypical round or spindle cells that present scant cytoplasm, hyperchromatic nuclei, inconspicuous nucleoli, and nuclear molding. As suggested by the name, the cell size is small and typically does not exceed three times the dimensions of a resting lymphocyte. Large-cell neuroendocrine carcinomas differ in their larger cell size and different growth patterns; they are often nest-like with peripheral palisading tumor cells and usually exhibit eosinophilic cytoplasm, polymorphous nuclei, prominent nucleoli, and vesicular chromatin[1,3,7-11].
Neuroendocrine differentiation must be confirmed by immunohistochemical analysis. The most sensitive markers are synaptophysin, insulinoma-associated protein 1, and chromogranin A, which sometimes present diminished intensity in poorly differentiated neuroendocrine neoplasia (NEN)[1,3,12,13]. Such markers are beneficial and have reliable staining patterns in a biopsy setting[14]. The epithelial origin of neoplastic cells should also be proven by staining for keratin to rule out the presence of paragangliomas[1]. To complete the final pathology report of NETs, a study of proliferative activity using the Ki-67 index (MIB-1 clone) must be conducted. The mitotic count is now considered poorly reproducible and, thus, is less meaningful than Ki-67 for diagnosis of NENs of the digestive system[1,3]. Ki-67 can be estimated using digital pathology-based tools to improve standardization, even in a biopsy setting[15].
One could ask why immunohistochemical analysis of neuroendocrine differentiation is not widely performed, considering that NENs have a heterogeneous appearance that could be misinterpreted. Immunohistochemical analysis has low specificity in unselected cases, with a risk of overdiagnosis of neuroendocrine neoplasms[11,12,16]. The three main examples of this phenomenon are as follows: (1) Since neuroendocrine cells are typically present in different tissues, normal neuroendocrine cells can be misinterpreted as part of a neoplastic process. A classic example is pancreatic insulae entrapped in pancreatic ductal adenocarcinoma or enterochromaffin-like cells intermingled with neoplastic elements in gastric cancer[17,18]; (2) The second possible misinterpretation in the neuroendocrine context is amphicrine neoplasia. An amphicrine tumor is defined as a neoplastic process in which tumor cells present both exocrine and neuroendocrine phenotypes[19-21]. An example is appendiceal goblet cell adenocarcinoma, in which the cells are both mucinous and positive for neuroendocrine markers[22,23]. Immunohistochemical, genetic, and transcriptome analyses revealed that this histological type significantly differs from adenocarcinoma, neuroendocrine neoplasms, and MiNENs in the same site. Therefore, special attention should be given to identifying this complex entity[24]; and (3) The last example in this context is neoplasms showing aberrant or partial expression of neuroendocrine markers. For example, a solid pseudopapillary neoplasm of the pancreas is a well-known entity that can express synaptophysin and CD56; however, it is not listed as NEN[25-28]. Another example is colorectal adenocarcinoma with synaptophysin expression, a subgroup of BRAF-mutated colorectal adenocarcinomas that does not fulfill the histological criteria for NEN diagnosis[29]. Differentiation of this subgroup is important due to its poor prognosis, as confirmed by other investigators[30].
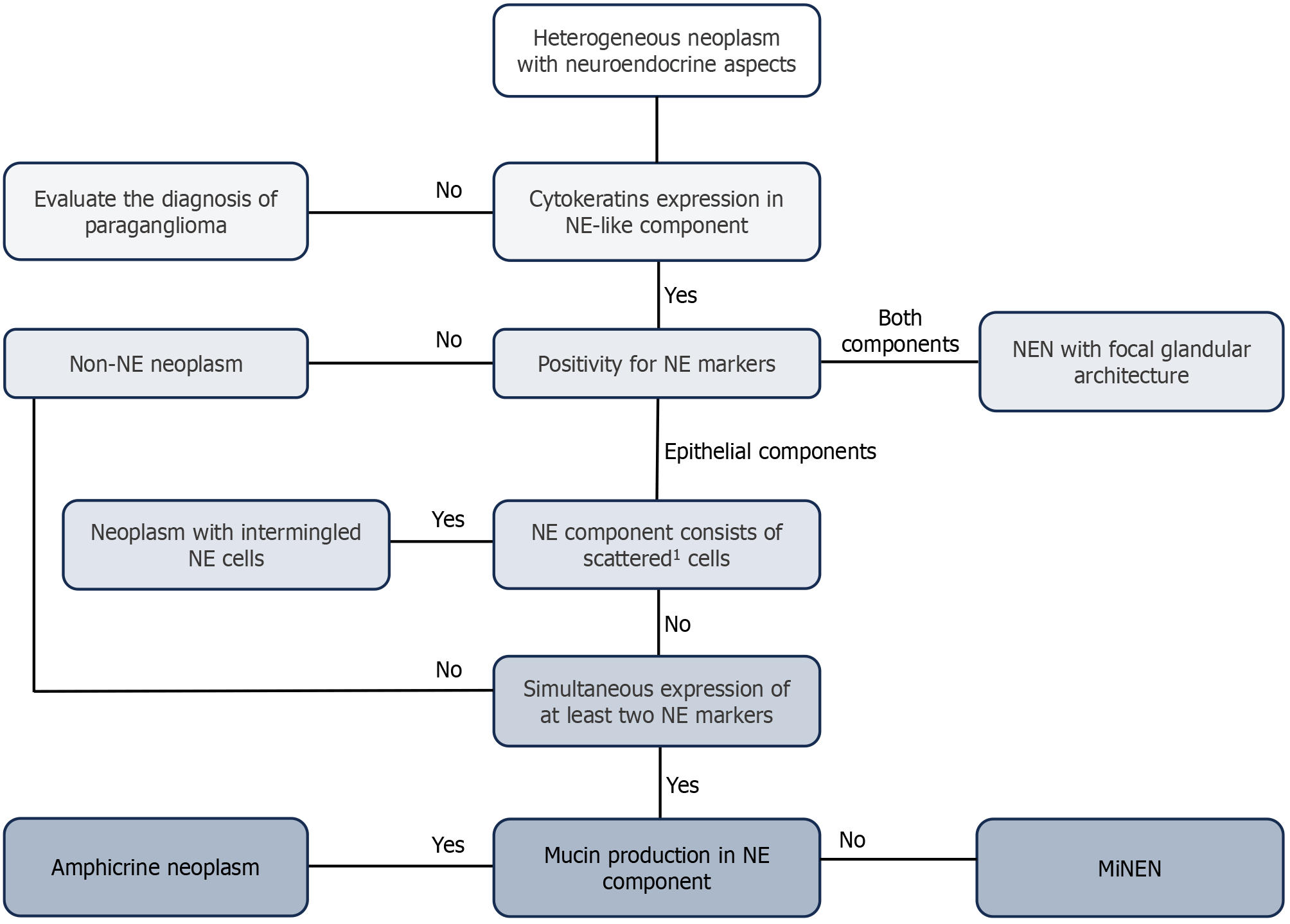
One of the most important topics in the digestive MiNEN landscape is identification of the correct diagnostic algorithm (Figure 1), as highlighted by Díaz-López et al[2]. The first step is to histologically identify a biphasic neoplasm with at least one component that presents both epithelial aspects and a neuroendocrine phenotype[1,3]. The second step concerns the immunohistochemical characterization of both neoplastic components using different staining, including: (1) Keratins to support the epithelial origin[31]; (2) Synaptophysin, chromogranin A, and preferably also INSM-1. In addition, CD56 is recognized as a neuroendocrine marker; nevertheless, the aforementioned markers are used more often for immunohistochemical staining because of their major sensitivity and specificity[1,3,32]; (3) Ki-67 (MIB-1 clone) is also used to investigate the proliferation index of the neoplasm[5,13,33,34]; and (4) Other potentially useful markers are used to identify the site of origin, such as CDX-2 for small bowel NENs[12,31], SATB2 for large bowel NENs and Merkel cell carcinomas[35], and Islet-1 for pancreatic neoplasms[3]. The neuroendocrine component may present challenging features that could hamper the immediate distinction between NET G3 and NEC. In those cases, immunohistochemical assessment of Rb and SSTR2, which are typically lost in NECs and retained in G3 NETs, and p53, which is more frequently altered in NECs than in G3 NETs, is helpful[4,36,37]. Moreover, pancreatic G3 NETs are enriched in DAXX/ATRX mutations, corresponding to a lack of immunohistochemical expression of the homonymous protein, while the expression of these proteins is conserved in NECs[37-40]. In addition, the alternative lengthening of telomeres, ALT, a vital biological mechanism important in pancreatic NETs, is never activated in NEC[39,41]. The presence of necrosis favors the diagnosis of poorly differentiated carcinomas, but it is not listed among the grading parameters of tumors in the gastrointestinal and pancreaticobiliary tracts[1,3]. The diagnosis of a MiNENs is supported whenever neuroendocrine marker expression is identified in one component of the biphasic mass. Notably, NEC can develop a glandular phenotype, but the diagnosis of MiNEN cannot be made in these patients because of the diffuse staining of neuroendocrine markers[42]. The last consideration is tumor staging for MiNEN, which is classified as a site-specific non-neuroendocrine component according to the AJCC/UICC TNM classification[43]. This information is crucial for stratifying patients according to prognosis and guiding clinical management.
The recognition of MiNENs is a critical task because there is much evidence that the two components do not behave like their isolated counterparts[41,44,45]. Generally, the high-grade histological subtype guides the prognosis of the disease; however, both components can progress and metastasize, significantly impacting the clinical history of patients with MiNENs[46].
In most districts, there is no minimum percentage of the two tumor components for establishing a diagnosis of MiNEN. For the gastrointestinal tract, the discussion is less straightforward, and the guidelines indicate that the smaller component must occupy at least 30% of the entire tumor mass[47]. Although this cutoff was arbitrarily determined when there was limited knowledge about these lesions, it has been maintained over time[2,48]. Systematic studies have not yet investigated the possibility of changing the cutoff value. Recent findings suggest that the presence of a high-grade component significantly impacts prognosis, even in cases where the high-grade component represents less than 30% of the entire mass[49]. For gastrointestinal tumors that do not satisfy the current diagnostic criteria for MiNENs, reports of the presence of smaller components (< 30%), both neuroendocrine and non-neuroendocrine, are highly recommended[46,49].
Although first described in 1924[50], MiNENs remain poorly understood. This is partly due to the rarity of these lesions and the different clinical behaviors of MiNENs at distinct sites. In addition, the misdiagnosis or underrecognition of this rare entity could have affected its identification and the overall literature on mixed neoplasms. In this editorial, inspired by the elegant manuscript by Díaz-López et al[2], some controversial lesions that are not real MiNENs have been highlighted, including: (1) Tumors with (scattered) neuroendocrine non-neoplastic cells; (2) Amphicrine neoplasms; and (3) Adenocarcinomas that partially or aberrantly express neuroendocrine markers.
Current guidelines/diagnostic criteria for MiNENs require that the smallest tumor component comprises at least 30% of the entire neoplastic mass. Nevertheless, smaller components (< 30%) should be reported since they can affect prognosis and may represent an additional point of inspiration for further research on MiNEN.
| 1. | Rindi G, Mete O, Uccella S, Basturk O, La Rosa S, Brosens LAA, Ezzat S, de Herder WW, Klimstra DS, Papotti M, Asa SL. Overview of the 2022 WHO Classification of Neuroendocrine Neoplasms. Endocr Pathol. 2022;33:115-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 467] [Article Influence: 155.7] [Reference Citation Analysis (2)] |
| 2. | Díaz-López S, Jiménez-Castro J, Robles-Barraza CE, Ayala-de Miguel C, Chaves-Conde M. Mixed neuroendocrine non-neuroendocrine neoplasms in gastroenteropancreatic tract. World J Gastrointest Oncol. 2024;16:1166-1179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (8)] |
| 3. | WHO. Classification of Tumours. Digestive system tumours. 5th ed. International Agency for Research on Cancer, Lyon. Available from: https://publications.iarc.fr/Book-And-Report-Series/Who-Classification-Of-Tumours/Digestive-System-Tumours-2019. |
| 4. | Luchini C, Pelosi G, Scarpa A, Mattiolo P, Marchiori D, Maragliano R, Sessa F, Uccella S. Neuroendocrine neoplasms of the biliary tree, liver and pancreas: a pathological approach. Pathologica. 2021;113:28-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 5. | Tang LH, Basturk O, Sue JJ, Klimstra DS. A Practical Approach to the Classification of WHO Grade 3 (G3) Well-differentiated Neuroendocrine Tumor (WD-NET) and Poorly Differentiated Neuroendocrine Carcinoma (PD-NEC) of the Pancreas. Am J Surg Pathol. 2016;40:1192-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 270] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 6. | Couvelard A, Cros J. An update on the development of concepts, diagnostic criteria, and challenging issues for neuroendocrine neoplasms across different digestive organs. Virchows Arch. 2022;480:1129-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 7. | Konukiewitz B, Jesinghaus M, Kasajima A, Klöppel G. Neuroendocrine neoplasms of the pancreas: diagnosis and pitfalls. Virchows Arch. 2022;480:247-257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 34] [Reference Citation Analysis (0)] |
| 8. | Cattaneo L, Centonze G, Sabella G, Lagano V, Angerilli V, Pardo C, Bertani E, Spada F, Prinzi N, Pusceddu S, Fassan M, Fazio N, Milione M. Digestive MiNENs: Could histological classification and molecular characterization drive clinical outcome and therapeutic approach? Crit Rev Oncol Hematol. 2023;188:104044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 9. | Tsvetkova V, Luchini C. Mixed Neuroendocrine-Non-Neuroendocrine Neoplasms of the Pancreas. Surg Pathol Clin. 2022;15:555-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Taskin OC, Reid MD, Bagci P, Balci S, Armutlu A, Demirtas D, Pehlivanoglu B, Saka B, Memis B, Bozkurtlar E, Leblebici CB, Birceanu A, Xue Y, Erkan M, Kapran Y, Baygul A, Sokmensuer C, Scarpa A, Luchini C, Basturk O, Adsay V. Infiltration pattern predicts metastasis and progression better than the T-stage and grade in pancreatic neuroendocrine tumors: a proposal for a novel infiltration-based morphologic grading. Mod Pathol. 2022;35:777-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 11. | Vanoli A, Grami O, Klersy C, Milanetto AC, Albarello L, Fassan M, Luchini C, Grillo F, Spaggiari P, Inzani F, Uccella S, Parente P, Nappo G, Mattiolo P, Milione M, Pietrabissa A, Cobianchi L, Schiavo Lena M, Partelli S, Di Sabatino A, Sempoux C, Capella C, Pasquali C, Doglioni C, Sessa F, Scarpa A, Rindi G, Paulli M, Zerbi A, Falconi M, Solcia E, La Rosa S. Ampullary Neuroendocrine Neoplasms: Identification of Prognostic Factors in a Multicentric Series of 119 Cases. Endocr Pathol. 2022;33:274-288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Uccella S, La Rosa S, Volante M, Papotti M. Immunohistochemical Biomarkers of Gastrointestinal, Pancreatic, Pulmonary, and Thymic Neuroendocrine Neoplasms. Endocr Pathol. 2018;29:150-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 13. | Duan K, Mete O. Algorithmic approach to neuroendocrine tumors in targeted biopsies: Practical applications of immunohistochemical markers. Cancer Cytopathol. 2016;124:871-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 14. | Luchini C. Diagnostic Pearls and Pitfalls in the Evaluation of Biopsies of the Pancreas. Arch Pathol Lab Med. 2024;. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 15. | Luchini C, Pantanowitz L, Adsay V, Asa SL, Antonini P, Girolami I, Veronese N, Nottegar A, Cingarlini S, Landoni L, Brosens LA, Verschuur AV, Mattiolo P, Pea A, Mafficini A, Milella M, Niazi MK, Gurcan MN, Eccher A, Cree IA, Scarpa A. Ki-67 assessment of pancreatic neuroendocrine neoplasms: Systematic review and meta-analysis of manual vs. digital pathology scoring. Mod Pathol. 2022;35:712-720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 43] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 16. | Jacob A, Raj R, Allison DB, Soares HP, Chauhan A. An Update on the Management of Mixed Neuroendocrine-Non-neuroendocrine Neoplasms (MiNEN). Curr Treat Options Oncol. 2022;23:721-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Kapran Y, Bauersfeld J, Anlauf M, Sipos B, Klöppel G. Multihormonality and entrapment of islets in pancreatic endocrine tumors. Virchows Arch. 2006;448:394-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Luong TV, Pilozzi E, Bearzi I, Bordi C. Mucosal colonization of gastric endocrine tumors mimicking mixed neoplasms. Virchows Arch. 2008;452:169-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 19. | Chejfec G, Capella C, Solcia E, Jao W, Gould VE. Amphicrine cells, dysplasias, and neoplasias. Cancer. 1985;56:2683-2690. [PubMed] [DOI] [Full Text] |
| 20. | Mastrosimini MG, Mafficini A, Tondulli L, Milella M, Piccoli P, Mattiolo P, Fassan M, Hong SM, Scarpa A, Luchini C. Recurrent gastric amphicrine tumor with neuroendocrine and pancreatic acinar cell differentiation and somatic MEN1 inactivation arisen during immunotherapy. Virchows Arch. 2023;483:415-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 21. | Sciarra A, Uccella S, Hiroz P, Fournier I, Soubeyran V, Finzi G, La Rosa S. Gastric Amphicrine Carcinoma Showing Neuroendocrine and Pancreatic Acinar Cell Differentiation. Lesson from a Challenging Case Opening New Perspectives in the Diagnostic Work-Up of Gastric Neuroendocrine Neoplasms. Endocr Pathol. 2023;34:349-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 22. | Bell PD, Pai RK. Goblet cell adenocarcinoma of the appendix: an update and practical approach to diagnosis and grading. Hum Pathol. 2023;132:183-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 23. | Yozu M, Johncilla ME, Srivastava A, Ryan DP, Cusack JC, Doyle L, Setia N, Yang M, Lauwers GY, Odze RD, Misdraji J. Histologic and Outcome Study Supports Reclassifying Appendiceal Goblet Cell Carcinoids as Goblet Cell Adenocarcinomas, and Grading and Staging Similarly to Colonic Adenocarcinomas. Am J Surg Pathol. 2018;42:898-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 24. | Huang D, Ren F, Ni S, Tan C, Weng W, Zhang M, Xu M, Wang L, Xu Q, Sheng W. Amphicrine carcinoma of the stomach and intestine: a clinicopathologic and pan-cancer transcriptome analysis of a distinct entity. Cancer Cell Int. 2019;19:310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 25. | Mattiolo P, Scarpa A, Luchini C. Hepatoid tumors of the gastrointestinal/pancreatobiliary district: morphology, immunohistochemistry, and molecular profiles. Hum Pathol. 2023;132:169-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 26. | Adsay V, Mino-Kenudson M, Furukawa T, Basturk O, Zamboni G, Marchegiani G, Bassi C, Salvia R, Malleo G, Paiella S, Wolfgang CL, Matthaei H, Offerhaus GJ, Adham M, Bruno MJ, Reid MD, Krasinskas A, Klöppel G, Ohike N, Tajiri T, Jang KT, Roa JC, Allen P, Fernández-del Castillo C, Jang JY, Klimstra DS, Hruban RH; Members of Verona Consensus Meeting, 2013. Pathologic Evaluation and Reporting of Intraductal Papillary Mucinous Neoplasms of the Pancreas and Other Tumoral Intraepithelial Neoplasms of Pancreatobiliary Tract: Recommendations of Verona Consensus Meeting. Ann Surg. 2016;263:162-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 195] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 27. | Reid MD, Bhattarai S, Graham RP, Pehlivanoglu B, Sigel CS, Shi J, Saqi A, Shirazi M, Xue Y, Basturk O, Adsay V. Pancreatoblastoma: Cytologic and histologic analysis of 12 adult cases reveals helpful criteria in their diagnosis and distinction from common mimics. Cancer Cytopathol. 2019;127:708-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 28. | Wang Q, Reid MD. Cytopathology of solid pancreatic neoplasms: An algorithmic approach to diagnosis. Cancer Cytopathol. 2022;130:491-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 29. | Fassan M, Milione M, Maddalena G, Cremolini C, Schirripa M, Pietrantonio F, Pella N, Dell'Aquila E, Sperti E, Zichi C, Bergamo F, Volante M, Boccaccino A, Morano F, Cortiula F, De Maglio G, Rimassa L, Smiroldo V, Calvetti L, Aprile G, Salvatore L, Santini D, Salmaso R, Centonze G, Biason P, Borga C, Lonardi S, Zagonel V, Dei Tos AP, Di Maio M, Loupakis F. Synaptophysin expression in (V600EBRAF-)mutated advanced colorectal cancers identifies a new subgroup of tumours with worse prognosis. Eur J Cancer. 2021;146:145-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Litmeyer AS, Konukiewitz B, Kasajima A, Foersch S, Schicktanz F, Schmitt M, Kellers F, Grass A, Jank P, Lehman B, Gress TM, Rinke A, Bartsch DK, Denkert C, Weichert W, Klöppel G, Jesinghaus M. High expression of insulinoma-associated protein 1 (INSM1) distinguishes colorectal mixed and pure neuroendocrine carcinomas from conventional adenocarcinomas with diffuse expression of synaptophysin. J Pathol Clin Res. 2023;9:498-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 31. | Bellizzi AM. Immunohistochemistry in the diagnosis and classification of neuroendocrine neoplasms: what can brown do for you? Hum Pathol. 2020;96:8-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 130] [Article Influence: 26.0] [Reference Citation Analysis (1)] |
| 32. | Juhlin CC, Zedenius J, Höög A. Clinical Routine Application of the Second-generation Neuroendocrine Markers ISL1, INSM1, and Secretagogin in Neuroendocrine Neoplasia: Staining Outcomes and Potential Clues for Determining Tumor Origin. Endocr Pathol. 2020;31:401-410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 33. | Milione M, Maisonneuve P, Pellegrinelli A, Grillo F, Albarello L, Spaggiari P, Vanoli A, Tagliabue G, Pisa E, Messerini L, Centonze G, Inzani F, Scarpa A, Papotti M, Volante M, Sessa F, Fazio N, Pruneri G, Rindi G, Solcia E, La Rosa S, Capella C. Ki67 proliferative index of the neuroendocrine component drives MANEC prognosis. Endocr Relat Cancer. 2018;25:583-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 74] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 34. | Milione M, Maisonneuve P, Grillo F, Mangogna A, Centonze G, Prinzi N, Pusceddu S, Garzone G, Cattaneo L, Busico A, Bossi P, Spaggiari P, Pellegrinelli A, Del Gobbo A, Ferrero S, Kankava K, Pruneri G, Rolli L, Roca E, Bercich L, Tironi A, Benvenuti MR, Gallazzi MS, Romano R, Berruti A, Pastorino U, Capella C. Ki-67 Index of 55% Distinguishes Two Groups of Bronchopulmonary Pure and Composite Large Cell Neuroendocrine Carcinomas with Distinct Prognosis. Neuroendocrinology. 2021;111:475-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 35. | Hoskoppal D, Epstein JI, Gown AM, Arnold Egloff SA, Gordetsky JB, Shi CJ, Giannico GA. SATB2 protein expression by immunohistochemistry is a sensitive and specific marker of appendiceal and rectosigmoid well differentiated neuroendocrine tumours. Histopathology. 2020;76:550-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 36. | Kasajima A, Konukiewitz B, Schlitter AM, Weichert W, Klöppel G. An analysis of 130 neuroendocrine tumors G3 regarding prevalence, origin, metastasis, and diagnostic features. Virchows Arch. 2022;480:359-368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 37. | Hackeng WM, Brosens LAA, Kim JY, O'Sullivan R, Sung YN, Liu TC, Cao D, Heayn M, Brosnan-Cashman J, An S, Morsink FHM, Heidsma CM, Valk GD, Vriens MR, Nieveen van Dijkum E, Offerhaus GJA, Dreijerink KMA, Zeh H, Zureikat AH, Hogg M, Lee K, Geller D, Marsh JW, Paniccia A, Ongchin M, Pingpank JF, Bahary N, Aijazi M, Brand R, Chennat J, Das R, Fasanella KE, Khalid A, McGrath K, Sarkaria S, Singh H, Slivka A, Nalesnik M, Han X, Nikiforova MN, Lawlor RT, Mafficini A, Rusev B, Corbo V, Luchini C, Bersani S, Pea A, Cingarlini S, Landoni L, Salvia R, Milione M, Milella M, Scarpa A, Hong SM, Heaphy CM, Singhi AD. Non-functional pancreatic neuroendocrine tumours: ATRX/DAXX and alternative lengthening of telomeres (ALT) are prognostically independent from ARX/PDX1 expression and tumour size. Gut. 2022;71:961-973. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 98] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 38. | Singhi AD, Liu TC, Roncaioli JL, Cao D, Zeh HJ, Zureikat AH, Tsung A, Marsh JW, Lee KK, Hogg ME, Bahary N, Brand RE, McGrath KM, Slivka A, Cressman KL, Fuhrer K, O'Sullivan RJ. Alternative Lengthening of Telomeres and Loss of DAXX/ATRX Expression Predicts Metastatic Disease and Poor Survival in Patients with Pancreatic Neuroendocrine Tumors. Clin Cancer Res. 2017;23:600-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 184] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 39. | Luchini C, Lawlor RT, Bersani S, Vicentini C, Paolino G, Mattiolo P, Pea A, Cingarlini S, Milella M, Scarpa A. Alternative Lengthening of Telomeres (ALT) in Pancreatic Neuroendocrine Tumors: Ready for Prime-Time in Clinical Practice? Curr Oncol Rep. 2021;23:106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 40. | Pulvirenti A, Raj N, Cingarlini S, Pea A, Tang LH, Luchini C, Chou JF, Grego E, Marinova I, Capanu M, Landoni L, Scarpa A, Allen PJ, Klimstra DS, Reidy-Lagunes DL. Platinum-Based Treatment for Well- and Poorly Differentiated Pancreatic Neuroendocrine Neoplasms. Pancreas. 2021;50:138-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 41. | Scarpa A, Chang DK, Nones K, Corbo V, Patch AM, Bailey P, Lawlor RT, Johns AL, Miller DK, Mafficini A, Rusev B, Scardoni M, Antonello D, Barbi S, Sikora KO, Cingarlini S, Vicentini C, McKay S, Quinn MC, Bruxner TJ, Christ AN, Harliwong I, Idrisoglu S, McLean S, Nourse C, Nourbakhsh E, Wilson PJ, Anderson MJ, Fink JL, Newell F, Waddell N, Holmes O, Kazakoff SH, Leonard C, Wood S, Xu Q, Nagaraj SH, Amato E, Dalai I, Bersani S, Cataldo I, Dei Tos AP, Capelli P, Davì MV, Landoni L, Malpaga A, Miotto M, Whitehall VL, Leggett BA, Harris JL, Harris J, Jones MD, Humphris J, Chantrill LA, Chin V, Nagrial AM, Pajic M, Scarlett CJ, Pinho A, Rooman I, Toon C, Wu J, Pinese M, Cowley M, Barbour A, Mawson A, Humphrey ES, Colvin EK, Chou A, Lovell JA, Jamieson NB, Duthie F, Gingras MC, Fisher WE, Dagg RA, Lau LM, Lee M, Pickett HA, Reddel RR, Samra JS, Kench JG, Merrett ND, Epari K, Nguyen NQ, Zeps N, Falconi M, Simbolo M, Butturini G, Van Buren G, Partelli S, Fassan M; Australian Pancreatic Cancer Genome Initiative, Khanna KK, Gill AJ, Wheeler DA, Gibbs RA, Musgrove EA, Bassi C, Tortora G, Pederzoli P, Pearson JV, Waddell N, Biankin AV, Grimmond SM. Whole-genome landscape of pancreatic neuroendocrine tumours. Nature. 2017;543:65-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 761] [Cited by in RCA: 680] [Article Influence: 85.0] [Reference Citation Analysis (0)] |
| 42. | Basturk O, Tang L, Hruban RH, Adsay V, Yang Z, Krasinskas AM, Vakiani E, La Rosa S, Jang KT, Frankel WL, Liu X, Zhang L, Giordano TJ, Bellizzi AM, Chen JH, Shi C, Allen P, Reidy DL, Wolfgang CL, Saka B, Rezaee N, Deshpande V, Klimstra DS. Poorly differentiated neuroendocrine carcinomas of the pancreas: a clinicopathologic analysis of 44 cases. Am J Surg Pathol. 2014;38:437-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 186] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 43. | Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR, Sullivan DC, Jessup JM, Brierley JD, Gaspar LE, Schilsky RL, Balch CM, Winchester DP, Asare EA, Madera M, Gress DM, Meyer LR. AJCC Cancer Staging Manual. 8th ed. Springer International Publishing. Available from: https://www.springer.com/gp/book/9783319406176. |
| 44. | Scardoni M, Vittoria E, Volante M, Rusev B, Bersani S, Mafficini A, Gottardi M, Giandomenico V, Malleo G, Butturini G, Cingarlini S, Fassan M, Scarpa A. Mixed adenoneuroendocrine carcinomas of the gastrointestinal tract: targeted next-generation sequencing suggests a monoclonal origin of the two components. Neuroendocrinology. 2014;100:310-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 45. | Konukiewitz B, Jesinghaus M, Steiger K, Schlitter AM, Kasajima A, Sipos B, Zamboni G, Weichert W, Pfarr N, Klöppel G. Pancreatic neuroendocrine carcinomas reveal a closer relationship to ductal adenocarcinomas than to neuroendocrine tumors G3. Hum Pathol. 2018;77:70-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 83] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 46. | Frizziero M, Chakrabarty B, Nagy B, Lamarca A, Hubner RA, Valle JW, McNamara MG. Mixed Neuroendocrine Non-Neuroendocrine Neoplasms: A Systematic Review of a Controversial and Underestimated Diagnosis. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 91] [Article Influence: 18.2] [Reference Citation Analysis (2)] |
| 47. | Lewin K. Carcinoid tumors and the mixed (composite) glandular-endocrine cell carcinomas. Am J Surg Pathol. 1987;11 Suppl 1:71-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 169] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 48. | Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO Classification of Tumours of the Digestive System. 4th ed. Available from: https://publications.iarc.fr/Book-And-Report-Series/Who-Classification-Of-Tumours/WHO-Classification-Of-Tumours-Of-The-Digestive-System-2010. |
| 49. | de Mestier L, Cros J, Neuzillet C, Hentic O, Egal A, Muller N, Bouché O, Cadiot G, Ruszniewski P, Couvelard A, Hammel P. Digestive System Mixed Neuroendocrine-Non-Neuroendocrine Neoplasms. Neuroendocrinology. 2017;105:412-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 111] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 50. | Klappenbach RS, Kurman RJ, Sinclair CF, James LP. Composite carcinoma-carcinoid tumors of the gastrointestinal tract. A morphologic, histochemical, and immunocytochemical study. Am J Clin Pathol. 1985;84:137-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 73] [Article Influence: 1.8] [Reference Citation Analysis (0)] |